Abstract
A phase I trial of infusing anti-CD3 × anti-CD20 bispecific antibody (CD20Bi) armed activated T cells (aATC) was conducted in high-risk/refractory non-Hodgkin’s lymphoma patients to determine whether aATC infusions are safe, affect immune recovery, and induce an antilymphoma effect. Ex vivo expanded ATC from 12 patients were armed with anti-CD20 bispecific antibody, cryopreserved, and infused after autologous stem cell transplantation (SCT). Patients underwent SCT after high-dose chemotherapy, and aATC infusions were started on day +4. The patients received 1 infusion of aATC per week for 4 weeks after SCT with doses of 5,10,15, and 20 × 109. aATC infusions were safe and did not impair engraftment. The major side effects were chills, fever, hypotension, and fatigue. The mean number of IFN-γ Enzyme-linked Immunosorbent Spots (ElSpots) directed at CD20 positive lymphoma cells (DAUDI, P = .0098) and natural killer cell targets (K562, P < .0051) and the mean specific cytotoxicity directed at DAUDI (P = .037) and K562 (P = .002) from pre-SCT to post-SCT were significantly higher. The increase in IFN-γ EliSpots from pre-SCT to post-SCT in patients who received armed ATC after SCT were significantly higher than those in patients who received SCT alone (P = .02). Serum IL-7, IL-15, Macrophage inflammatory protein (MIP)-1 beta, IP-10, MIP-1α, and Monokine induced by gamma interferone increased within hours after infusion. Polyclonal and specific antibodies were near normal 3 months after SCT. aATC infusions were safe and increased innate and specific antilymphoma cell immunity without impairing antibody recovery after SCT.
Keywords: Non-Hodgkin lymphoma, Activated T cells, Bispecific antibody, Autologous stem cell, transplantation
INTRODUCTION
High-dose chemotherapy (HDC) followed by autologous stem cell transplantation (SCT) induces complete responses in patients with high-risk, refractory, or relapsed non-Hodgkin’s lymphomas (NHLs) [1–6]. In the rituximab era where rituximab added to cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) improves progression-free survival over CHOP [7], only 10% of chemosensitive patients who do not achieve a complete response or who are in relapse remain disease free with salvage chemotherapy alone [8]. In these patients, salvage therapy should be consolidated with HDC followed by SCT [9]. These high-risk refractory or relapsed patients could benefit from targeted T cell immunotherapy approaches that provide anti-lymphoma activity after SCT.
Rituximab (a chimeric monoclonal antibody directed at CD20+ lymphoma cells) kills CD20+ cells by complement-mediated cytotoxicity, antibody-dependent cell-mediated cytotoxicity, and the induction of apoptosis [10–13]. Our approach exploits the none–major histocompatibility complex restricted cytotoxicity of anti-CD3 activated T cells (ATC) [14–17] by redirecting their cytotoxicity to tumor targets by arming ATC with CD20 bispecific antibodies [18]. Arming with anti-CD20 bispecific antibody (CD20Bi) makes every ATC into a CD20-specific cytotoxic lymphocyte.
Our preclinical studies show that CD20Bi armed ATC (aATC) significantly enhanced lysis of CD20 antigen expressing B9C (immortalized normal B cell line), Raji (Burkitt lymphoma line), and ARH-77 (rituximab-resistant multiple myeloma cell line). Mean specific cytotoxicity directed at B9C ranged from 41% to 65% at effector-to-target ratios from 6.25:1 to 25:1, and CD20Bi aATC cultured with lymphoma B9C cells produced significantly more IFN-γ than unarmed ATC cultured with B9C (P =.0103) [18]. The ability of aATC to lyse ARH-77 cells suggested that CD20Bi aATC may be clinically effective for resistant or refractory NHL after HDC and SCT.
In this phase I clinical trial involving multiple infusions of aATC after SCT, we asked whether T cells can be expanded from the peripheral blood mononuclear cells (PBMCs) obtained from heavily pretreated NHL patients to produce ATC that could be infused without dose-limiting toxicities (DLTs) and whether infusions of aATC induce endogenous antilymphoma cytotoxic T lymphocyte responses without impairing immune reconstitution. The primary objective of this study was safety, and secondary objectives included feasibility of the T cell expansions and evaluation of immune responses.
METHODS
Trial Design
We enrolled patients between 18 and 70 years of age with high-risk or refractory, histologically confirmed CD20+NHL at Karmanos Cancer Institute between August 30, 2007 and January 26, 2009. Informed consent was obtained before enrollment on consent forms approved by the Wayne State University institutional review board and the US Food and Drug Administration. Protocol WSU 2007–023 was conducted per guidelines from the Declaration of Helsinki. aATC were produced under IND BB-11746, and the study was monitored by the Karmanos Cancer Institute data safety monitoring committee. Exclusion criteria were forced expiratory volume in 1 second and Carbon monoxide diffusing capacity (DLCO) < 45%, creatinine > 2.0 mg/dL or creatinine clearance < 60 mL/min, direct bilirubin > 2.0 mg/dL, serum glutamic oxaloacetic transminase or serum glutamic pyruvic transminase > 2.5 times the upper limit of normal or history of severe hepatic dysfunction, left ventricular ejection fraction < 40% by Multi-Gated Acquisition Scan (MUGA) or echocardiography at rest, active infection, human immunodeficiency virus antibody positivity, and Eastern Cooperative Oncology Group ( ECOG) performance status >2 or Karnofsky Performance Status (KPS) <60%.
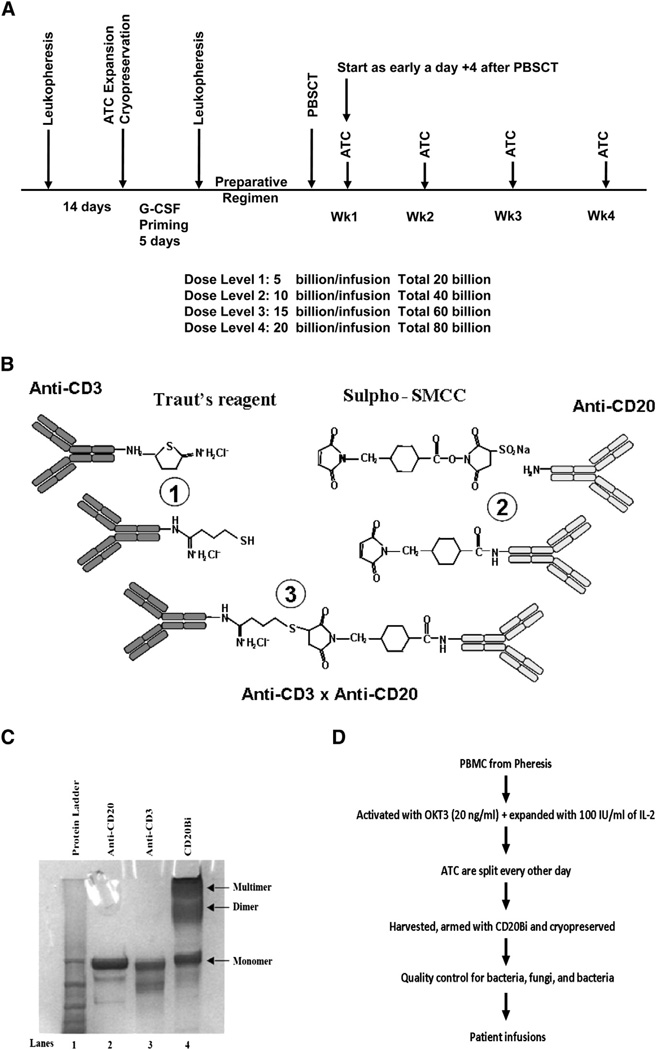
Patients were enrolled in a standard 3+3 dose escalation trial in which doses of 5, 10, 15, and 20 × 109 aATC were given once a week for 4 weeks beginning on day +4 after SCT for total doses of 20, 40, 60, and 80 × 109 (Figure 1A). PBMC from 8 patients (5 NHL and 3 multiple myeloma patients) who received unmanipulated autologous SCT alone on Protocol WSU 2007-046 were tested as a control patient group.
Figure 1.
(A) Treatment schema. The chemotherapy preparative regimen involved BEAM, which consisted of carmustine 300 mg/m2 × 1 dose at day −7, etoposide at 100 mg/m2 every 12 hours and cytarabine 100 mg/m2 every 12 hours on days −6 through −3, and melphalan 140 mg/m2 × 1 dose at day −2, day of rest on day −1, and transplantation on day 0. (B) Process of heteroconjugation of anti-CD3 with rituximab. Step 1 shows cross-linking of Traut’s reagent to anti-CD3 (OKT3) mAb and the cross-linking of Sulpho-SMCC to anti-CD20 (rituximab); step 2 shows the heteroconjugation of the cross-linked anti-CD3 with the cross-linked rituximab; and step 3 shows formation of anti-CD3 × anti-CD20 BiAb (CD20Bi). (C) SDS-PAGE gel of the parent monoclonal antibodies and the heteroconjugated CD20Bi. Lane 1: Protein ladder; lane 2: anti-CD20 mAb; lane 3: anti-CD3 mAb; lane 4: CD20Bi. (D) The schema shows T cell expansion after leukapheresis of the patient, harvesting, and arming of ATC with CD20Bi, cryopreservation in aliquots for infusions after SCT at the designated time points for the treatment schema shown in A.
Leukapheresis and T Cell Expansion
PBMCs were collected by leukapheresis, activated with anti-CD3 (OKT3), and expanded in IL-2 [19,20] (Figure 1D). CD20Bi was produced as previously described (Figure 1B, C) [21] (see Supplemental Appendix).
Mobilization of Stem Cells
Patients received granulocyte colony-stimulating factor (G-CSF) stimulation to obtain a minimum CD34+ cell dose of 2 × 106 cells/kg.
Preparative Regimen and Transplantation
HDC consisted of i.v. carmustine (300 mg/m2 for 1 dose on day −7), etoposide (100 mg/m2 every 12 hours on days −6 through −3), cytarabine (100 mg/m2 every 12 hours on days −6 through −3), and melphalan (140 mg/m2 for 1 dose at day −2) (BEAM) followed by a day of rest on day −1 and transplantation on day 0 (Figure 1A).
Infusions of aATC
On the day of infusion after SCT, cryopreserved aATC were thawed and infused at the bedside. aATC were given over 5 to 15 minutes after premedicating patients with diphenyhydramine and acetaminophen with close monitoring of vital signs and O2 saturations.
Phenotyping, Specific Cytotoxicity, IFN-γ EliSpots, Serum Cytokines, and IgG and Anti-Tetanus Toxoid Antibody Levels
Specific cytotoxicity was performed using fresh PBMCs mixed with 51Cr-labeled DAUDI and K562 cells [18]. IFN-γ Elispots were used to measure CD8-mediated memory cytotoxic T lymphocyte activity and CD4-mediated helper responses [22]. Cytokines were measured by Luminex Array and polyclonal IgG and anti-tetanus toxoid (anti-TT) levels were measured by ELISA (see Supplemental Appendix) [23].
Statistical Analyses
Wilcoxon signed-rank test was used to examine the median of the change at each time point from prestudy in phenotyping, cytotoxicity against DAUDI, and K562. For Elispots against DAUDI and K562, the differences between pre-SCT and the observed peak value within 3 months post-SCT were examined using Wilcoxon signed-rank test. Change in numbers of IFN-γ EliSpots at post-SCT from pre-SCT were compared between patients who received SCT alone with the study patients who received SCT and aATC using the Wilcoxon Mann-Whitney test. Mixed effects models were used to examine the potential trend of phenotyping, cytotoxicity, and EliSpot measurements over time. Detailed methods are provided in the Supplemental Appendix.
RESULTS
Patient Characteristics
Table 1 summarizes the patient characteristics, prior therapy, and disease status at SCT. Most patients had relapsed after first-line chemotherapy with rituximab, cyclophosphamide, adriamycin, vincristine and prednisone (R-CHOP) or did not achieve a complete remission after first-line chemotherapy. All but 1 patient with follicular lymphoma had diffuse large B cell lymphoma who had received 2 regimens before SCT (Table 1). Some patients could not achieve a complete response after salvage therapy with rituximab, ifosphamide, carboplatin and etoposide (RICE) and underwent transplantation with refractory disease. Table 1 shows the disease status at the time of SCT and at 90 days after SCT. All patients had received multiple rounds of rituximab-containing therapy.
Table 1.
Clinical Characteristics
| Age | Dx | Sex | Prior Chemotherapy | Regimens Prior to SCT |
CD 34 × 106/kg | Planned aATC Dose ×109 |
ActualaATC Dose ×109 |
Status at SCT |
Status at 90 days |
C-IT* | Survival (days) (D†/A‡) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 54 | DLBCL | M | RCHOP X4, RICE X2 | 2 | 2.6 | 20 | 18.4 | PRF | PD 56 days | N | 107 (D) |
| 2 | 53 | DLBCL | F | RCHOP X8, RICE X2 | 2 | 8.4 | 20 | 17.15 | PRF | PD 30 days | Y | 111 (D) |
| 3 | 67 | DLBCL | F | RCHOP X6, RICE X2 | 2 | 7.1 | 40 | 38.64 | PR | CR | Y | 1294 (A) |
| 4 | 55 | FCL | F | RCHOP X6, RICE X2 | 2 | 3.2 | 40 | 37.44 | CR2 | PD 60 days | Y | 126 (D) |
| 5 | 61 | DLBCL | M | R-CHOP X8, RICE X2 | 2 | 6.1 | 40 | 37.28 | CR2 | PD 52 days | Y | 112 (D) |
| 6 | 59 | DLBCL | M | RCHOP X6, RCEOP X4 | 2 | 6.0 | 60 | 60 | CR2 | CR | Y | 984 (A) |
| 7 | 56 | DLBCL | F | RCHOP X8, RICE X2 | 2 | 2.6 | 60 | 45 | CR2 | PR | N§ | 987 (A) |
| 8 | 67 | DLBCL | M | RCHOP X6 | 1 | 12.3 | 60 | 61.6 | CR1U | CR | Y | 914 (A) |
| 9 | 47 | DLBCL | M | R X4, RCHOP X6 | 2 | 4 | 60 | 48.92 | PR1 | PD 67 days | Y | 184 (D) |
| 10 | 59 | DLBCL | F | RCHOP X8, RICE X4 | 2 | 9.5 | 80 | 82 | CR2U | CR | Y | 781 (A) |
| 11 | 46 | DLBCL | M | RCHOP X8, RICE X2 | 2 | 6.0 | 80 | 67.64 | CR2 | CR | Y | 718 (A) |
| 12 | 61 | DLBCL | M | RCVP X2, RCHOP X6 | 2 | 2.5 | 80 | 78.04 | PR | CR | Y | 711 (A) |
DLBCL indicates Diffuse large B cell lymphoma; FCL, Follicular lymphoma; R, rituximab; RCHOP, rituximab, cyclophosphamide, adriamycin, vincristine, and prednisone; RCVP, rituximab-cyclophosphamide, vincristine, and prednisone; RICE, rituximab, ifosphamide, carboplatin, and etoposide; CVAD, cyclophosphamide, vincristine, adriamycin, and dexamethasone; RCEOP, rituximab-cyclophosphamide, etoposide, vincristine, and prednisone; CR, clinical remission; PR, partial response; PRF, progressive refractory disease; PD, progressive disease.
C-IT: Immunotherapy completed yes (Y) or no (N).
Alive (A) or dead (D).
Survival days after SCT.
Received 3 out of 4 infusions.
Stem Cell Mobilization
Table 1 shows the doses of CD34+ cells infused into the patients. One patient required G-CSF mobilization twice and a bone marrow harvest to obtain enough CD34+ cells for the SCT.
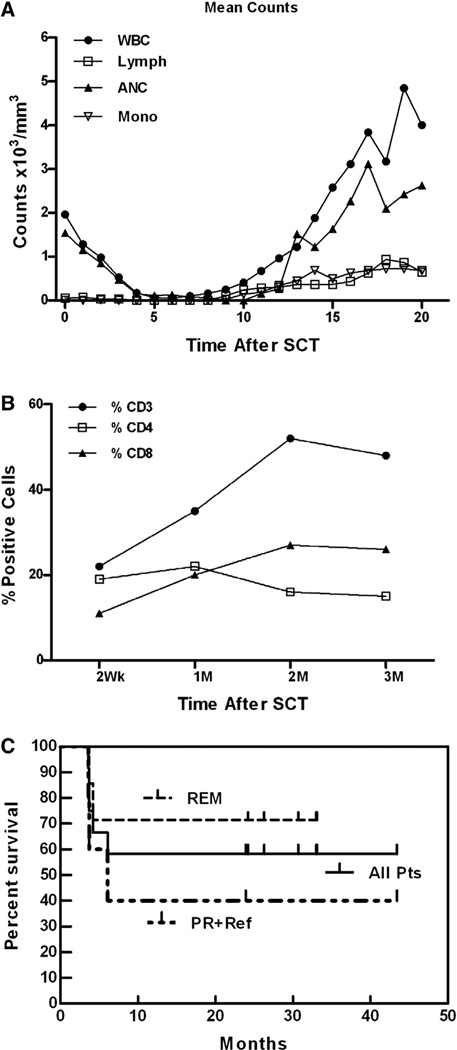
Engraftment of Neutrophils, Lymphocytes, and Monocytes
The median day of neutrophil engraftment (neutrophil count ≥ 500/mm3) was day 13. Figure 2A summarizes the mean absolute white blood cell count and lymphocyte, neutrophil, and monocytes counts for the 12 patients during the first 20 days after SCT. All patients engrafted without G-CSF, except 1 patient who had slow engraftment and engrafted 28 days after SCT with addition of G-CSF.
Figure 2.
Engraftment. (A) Daily mean absolute white blood cell count, lymphocyte, absolute neutrophil, and monocyte counts for 12 patients. (B) Flow cytometry performed on PBMCs from patients at the indicated time points after SCT shows mean proportions of CD3+, CD4+, and CD8+ T cells after SCT. (C) Overall survival of 12 patients transplanted for NHL. The Kaplan-Meier curve is presented for the entire population (All patients), the subgroup of patients who underwent transplantation in remission (REM), and the subgroup of patients who were transplantation inpartial remissionor had refractory disease (PR + Ref).
ATC Characteristics
The planned aATC dose, the actual aATC dose, the percentage of the target dose of aATC dose administered, time to progression, and survival are presented in Table 1. The mean percent (±SD) viability was 91.1% ± 4.18% and the mean proportion (95% confidence interval) of CD3, CD4, and CD8 cells were 96.5% (21.7, 76.8), 78.2% (11.3, 57.1), and 48.2% (9.6, 52.0), respectively (Table 2). The CD20Bi aATC product was tested for the viability and specific cytotoxicity against the target cell line (DAUDI). CD20Bi aATC exhibited a mean (±SD) specific cytotoxicity of 12.1% ± 6.7% (range, 1.5% to 25%) directed at CD20+ DAUDI targets at an effector-to-target ratio of 25:1 by 51Cr release cytotoxicity assay.
Table 2.
Phenotyping of the Harvest Product
| Harvest Product | Mean (%) | Range (%) |
|---|---|---|
| CD3+ | 96.5 | 68.9–98.7 |
| CD3/4+ | 78.2 | 33.3–90.8 |
| CD3/8+ | 48.2 | 17.7–86.8 |
| CD4/25+ | 59.3 | 19.6–88.2 |
| CD8/25+ | 30.9 | 4.1–62.6 |
| CD3+(16/56)+ | 3.3 | .4–16.9 |
| CD3−(16/56)+ | 11.5 | .9–53.3 |
| CD19+ | .4 | .03–5.8 |
| CD20+ | .1 | .01–.5 |
| CD4+(45RA−/45RO+) | 95.2 | 76.1–99.4 |
| CD8+(45RA−/45RO+) | 90.8 | 68.2–98.1 |
| CD4+(45RA+/45RO−) | .4 | .1–2.9 |
| CD8+(45RA+/45RO−) | 1.1 | .3–12.5 |
Infusions of aATC
The median total dose of aATC delivered was 6.7 × 1010 (95% confidence interval of 4.24, 6.73 × 1010). The maximum tolerated dose was not reached, and there were no DLTs. The most frequent side effects included fever, chills, malaise, nausea and/or vomiting, tachycardia, hypotension, headache, transient hypoxia, hypertension, and dyspnea (Table 3).
Table 3.
Toxic Reaction Incidence and Grade by Dose Level
| Dose | Adverse Event | N (%) | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|---|---|
| Level 1*,†(40B‡total) | Nausea and vomiting | 3 (100) | 4 | 3 | ||
| Diarrhea | 1 (33.3) | 2 | ||||
| Malaise | 1 (33.3) | 1 | 1 | |||
| Atrial rhythm | 1 (33.3) | 2 | ||||
| Fever | 3 (100) | 3 | 7 | |||
| Headache | 1 (33.3) | 2 | ||||
| Chills | 2 (66.6) | 7 | ||||
| Hypotension | 2 (66.6) | 4 | ||||
| Hypertension | 1 (33.3) | 1 | ||||
| Sinus tachycardia | 1 (33.3) | 1 | ||||
| Level 2* (60B‡ total) | Chills | 4 (100) | 1§ | |||
| Rhythm, ventricular | 2 (50) | 4§ | 1§ | 9§ | ||
| Hypotension | 1 (25) | 1§ | ||||
| Fever | 3 (100); 4 (100) | 1 | 8§ | 3 | ||
| Headache | 0 (0); 1 (33.3) | 2§ | ||||
| Malaise | 2 (66.6); 2 (66.6) | 7 | ||||
| Nausea | 1 (33.3); 1 (33.3) | 3 | ||||
| Level 3*,†(80B‡ total) | Chills | 2 (66.6) | 5 | 3 | ||
| Malaise | 2 (66.6) | 8 | 4 | |||
| Rhythm, ventricular | 3 (100) | 4 | ||||
| Hypotension | 3 (100) | 3 | 2 | |||
| Fever | 3 (100) | 4 | 8 | |||
| Pulmonary | 1 (33.3) | 1 | 1 | |||
| Headache | 2 (66.6) | 6 | ||||
| Pain | 3 (100) | 4 |
N is the number of patients experiencing adverse event (% of total at the dose level).
Dose level on the new version of the protocol that was approved March 3, 2008.
Total number of episodes by grade at the dose level.
B indicates dose in billions.
These are inclusive of 1 patient, who received only 3 of 4 infusions.
Lymphocyte Recovery
The mean (N=12) proportion of CD3+ and CD4+ cells was 22% and 19% at 2 weeks after SCT, respectively. By 1 month after SCT, the mean proportions of CD3+, CD4+, and CD8+ cells reached 35%, 22%, and 20%, respectively, with a CD4/CD8 ratio of ~1.0. The median percent CD3, CD4, and CD8+ cells are shown in Figure 2B at the indicated time points. The CD8+ population increased between 4 and 8 weeks after SCT. CD19+ B cells were present but low at 0.75% and 0.27% at 2 and 3 months, respectively, and recovered to 3.43% and 3.85% at 6 and 12 months, respectively. The proportions of CD20+ cells followed the same pattern as CD19+ cells at indicated time points.
Clinical Responses
Figure 2C shows the Kaplan-Meier estimates for all patients and for 2 subgroups (patients who were in remission at the time of SCT and patients who had refractory or persistent disease at the time of SCT). The median overall survival for the entire group was not achieved at a median follow-up of 24 months. One patient withdrew after 2 aATC infusions due to progression of disease, and 1 patient withdrew after 3 infusions due to discomfort from the infusions. Both patients were included in Kaplan-Meier estimates because they received aATC.
PBMC-Mediated Cytotoxicity after SCT
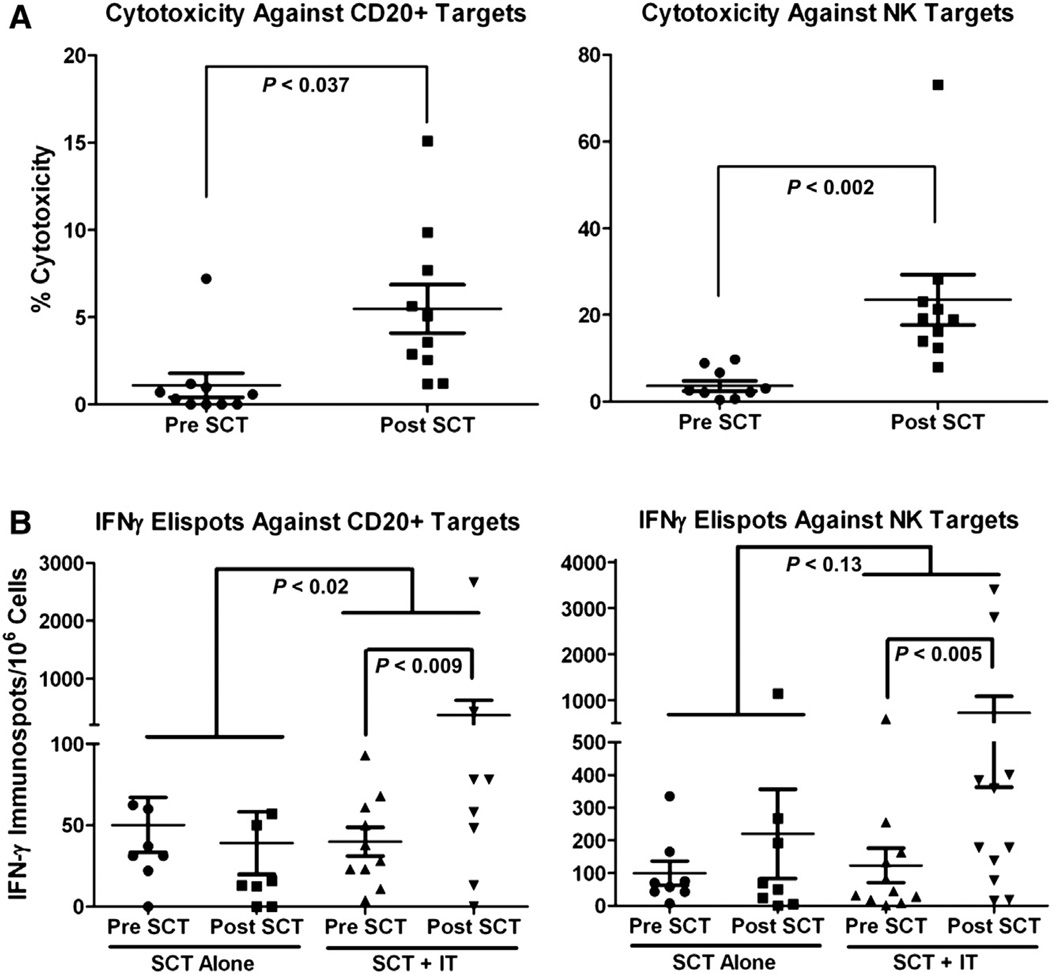
PBMCs from leukapheresis at baseline and from peripheral blood at indicated time points (2 weeks to 3 months post-SCT) were tested in cytotoxicity assays against DAUDI or K562 cells. The highest levels of cytotoxicity by PBMC directed at DAUDI post-SCT (2 weeks to 3 months post-SCT) was significantly higher (P =037, Wilcoxon signed-rank test) compared with pre-SCT PBMCs. Cytotoxicity against K526 targets was also significantly (P =.002, Wilcoxon signed-rank test) higher at 2 weeks to 3 months post-SCT than at baseline PBMCs. These data show that aATC infusions enhanced both antilymphoma and natural killer (NK) activities (Figure 3A).
Figure 3.
(A) Cytotoxicity directed at DAUDI (left) and K562 (right). Fresh PBMCs from patient who received aATC were tested in 51Cr release cytotoxicity assay 2 weeks to 3 months post-SCT. (B) IFN-γ EliSpots against DAUDI (left) and K562 (right) in patients who received aATC and SCT (SCT + IT) and patients who received SCT alone (SCT alone). Fresh PBMCs for patients who received aATC and SCT that were plated onto DAUDI and K562 targets (right two columns in each panel). Fresh PBMCs for patients who received SCT alone were plated onto DAUDI and K562 targets (left two columns in each panel).
IFN-γ EliSpots
Short-term IFN-γ EliSpot (surrogate markers for CD8+ cytotoxic T lymphocytes and CD4+ helper activity) responses to DAUDI or K562 stimulation were studied in 11 patients before and after SCT. Post-SCT data represent the highest levels of IFN-γ–producing T cells between 2 weeks to 3 months post-SCT. IFN-γ Elispots stimulated with DAUDI were significantly higher (P = .0098, Wilcoxon signed-rank test) than that seen in pre-SCT values (Figure 3B, left). Figure 3B, right, shows IFN-γ EliSpot responses to K562 cells that were consistently higher at time points post-SCT over the baseline (P < .005, Wilcoxon signed-rank test). To determine whether the responses post-SCT were due to “immunologic” noise as a result of the SCT process alone, we tested a comparable group of patients who received SCT alone and compared their change in numbers of IFN-γ Eli-Spots obtained pre-SCT and post-SCT with the study patients who received SCT and aATC (Figure 3B). The increases in IFN-γ Elispots from pre-SCT to post-SCT directed at DAUDI was significantly higher in patients who received SCT and aATC (P = .02, Wilcoxon Mann-Whitney Test two-sided exact P value) during the same time interval (Figure 3B). These data show that infusions of aATC induced highly significant increases in antilymphoma cell EliSpots (DAUDI) as well as increases in anti–NK cell activity directed at K562.
Levels of Cytokines and Chemokines
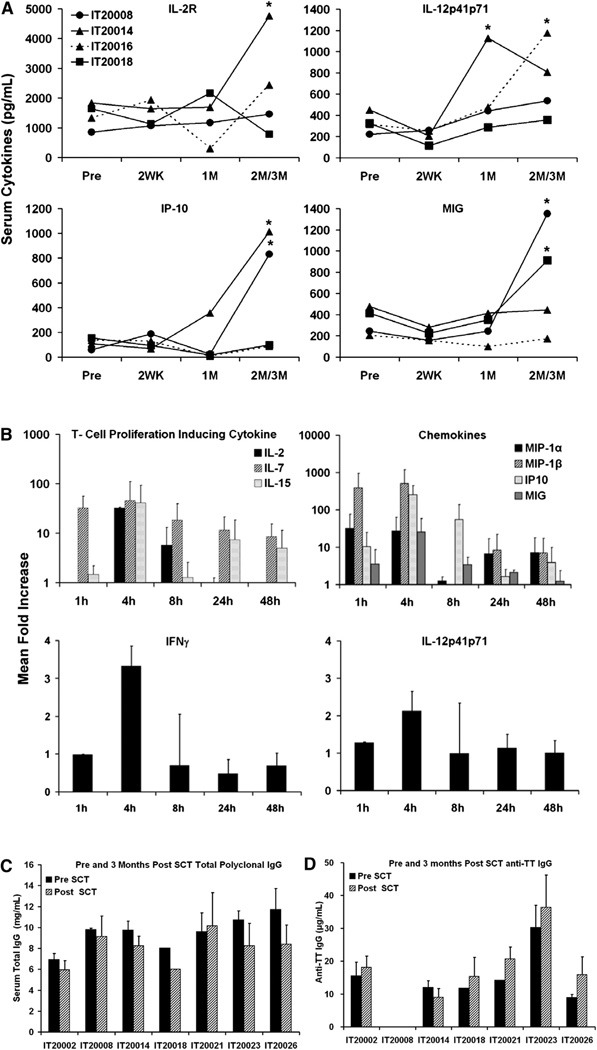
In 4 patients, steady-state serum levels of IL-2R and IL-12 (Figure 4A, top) and IFN-γ–induced chemokines CXCL10 and CXCL9 (Figure 4A, bottom) were elevated above baseline. IL-2R was significantly higher (P < .05) in 1 of 4 patients at 3 months and IL-12 was significantly higher at 1 and 3 months (P < .045 and P < .042, respectively) post-SCT compared with pre-SCT serum levels in 2 of 4 patients, respectively. IFN-γ–induced chemokines CXCL10 (P < .027 and P < .031) and CXCL9 (P < .023 and P < .047) were also significantly higher at 3 months post-SCT compared with pre-SCT serum levels in 2 of 4 patients, respectively. An aATC infusion given 10 days after SCT showed peak levels of Th1 cytokines and chemokines between 1 and 4 hours, (Figure 4B). Levels of IFN-γ and IL-12 increased at 4 hours above baseline (Figure 4B, bottom).
Figure 4.
Changes in serum cytokines and chemokines. (A) Infusions of aATC-induced cytokines and chemokines. aATC infusions enhanced cytokines IL-2R and IL-12 and IFN-γ–induced chemokines CXCL10 (IP-10) and CXCL9 (MIG) in 4 patients at indicated time points (*P < .05). (B) Top: sequential mean (±SD) fold increase in serum levels for T cell proliferation inducing cytokines IL-2, IL-7, and IL-15 (left) and serum chemokines MIP-1β and MIP-1α and IFN-γ–induced chemokines CXCL10 (IP-10) and CXCL9 (MIG). Bottom: sequential serum levels for IFN-γ and IL-12. Serum samples were analyzed in 3 patients after the second infusion at the designated times. (C) Transfer and reconstitution ofIgG and anti-TT. The results for individual patients are presented. Total IgG was tested before and at 3 months after SCT to determine the levels of IgG transferred in the stem cell product aATC infusions did not affect total IgG levels in the serum. (D) Anti-TT before and at 3 months after SCT were analyzed to determine whether the levels of specific anti-TT transferred in the stem cell product.
IgG and anti-TT levels
Figure 4C and D shows serum IgG and anti-TT levels before and 3 months after SCT, respectively. The mean levels of IgG before and after SCT were 9.5 ± 1.5 mg/mL and 8.0 ± 1.5 mg/ mL, respectively. The mean serum levels of anti-TT before and after SCT were 11.4 ± 7.8 µg/mL and 12.8 ± 10.1 µg/mL, respectively.
DISCUSSION
In our phase I trial, patients who received 4 doses of 20 × 109 aATC given in the first 30 days after SCT experienced no DLTs. Infusions of aATC induced a Th1 cytokine pattern in the serum that was associated with increased chemokine levels. There were significantly higher levels of specific cytotoxicity and IFN-γ EliSpots in PBMCs directed at DAUDI and K562 after SCT than at baseline levels before SCT. More importantly, the increase in antilymphoma IFN-γ EliSpots was significantly higher in the phase I group of patients who received aATC than the group of patients who received SCT alone. Although there was a transient decrease in the numbers of CD19+ cells in the circulation, infusions of aATC did not impair the recovery of IgG and anti-TT levels at 100 days after SCT. It is not clear whether the cytokine/chemokine changes observed during the aATC infusions were due to the bispecific antibody aATC or the perturbation induced by infusion of unarmed ATC alone. It is likely that both ATC and the CD20Bi arming of ATC contributed to the enhanced amounts of the serum cytokine/chemokine changes. Further studies are needed to determine which component contributed the most.
Clinical efficacy could not be assessed because of small sample size, short follow-up time, disease status at the time of transplantation, prior chemotherapy, and the conditioning regimen in a heterogeneous patient population. Furthermore, correlates between survival and cytotoxicity, IFN-γ EliSpots, dose of aATC, and product cytotoxicity were not significant.
The clinical picture was consistent with a mild clinically manageable “cytokine storm syndrome.” With the exception of 1 patient who was not adequately hydrated and needed observation in the intensive care unit during i.v. hydration, the patients received their infusions in the bone marrow transplantation unit or in the outpatient bone marrow transplantation clinic.
Because most patients previously received rituximab in initial chemotherapy and salvage regimens, it is likely that CD20-resistant clones might have developed. aATC may not only overcome CD20 resistance but could also kill CD20+ targets in the presence of rituximab [18]. The interactive combination of dosing and tumor burden could lead to more or less interference with CD20Bi aATC targeting. However, the last dose of potentially interfering rituximab would have been given more than 4 weeks before SCT. If there was residual rituximab on the lymphoma or in the circulation, it is likely CD20Bi aATC could still kill the CD20 targets [18]. Therefore, killing by CD20-redirected ATC may further reduce the tumor burden and induce endogenous CD20-specific antilymphoma responses.
The use of rituximab has been encouraging but controversial in terms of overall survival benefit when it has been used in preparative regimens or as consolidation after HDC and SCT to reduce the incidence of relapse [2,24–31]. Rituximab consolidation after SCT may have improved outcomes for patients with aggressive B cell NHL [25,26,28,31]. Studies suggest that immunity directed at pneumococcal conjugate, human telomerase reverse transcriptase (hTERT), and influenzae after autologous SCT can be manipulated before and after SCT by adoptive transfer of T cells [32–34]. Therefore, adoptive transfer aATC may provide a similar antilymphoma effect.
Because ATC could be expanded up to 80 × 109 from a single leukapheresis from heavily pretreated NHL patients, it is clear that ex vivo lymphocyte expansion is feasible. The cytotoxicity by PBMCs after SCT (2 weeks to 3 months) directed at DAUDI cannot be explained by the infused aATC because the mean cytotoxicity of aATC product was 12.1%, the proportion of aATC that would circulate after an infusion of 40 × 109 aATC no more than 4%. Because the amount of cytotoxicity would be very low due to a greater than 20-fold dilution of aATC, the cytotoxicity and IFN-γ EliSpots detected at indicated time points post-SCT were likely derived from endogenous lymphocytes. The Th1 and T cell proliferative cytokines induced by aATC infusions may have provided support for the induction of endogenous antilymphoma activity.
Infusions of a CD4-enriched aATC product likely led to the shift toward a normal CD4/CD8 ratio early after SCT. Infused aATC could be detected up to 7 days, whereas IL-7 and IL-15 known to support T cell growth were detected. There was a significant increase in specific IFN-γ EliSpots to lymphoma targets 100 days after SCT above that seen pre-SCT (P < .0098). The increases in specific cytotoxicity directed at DAUDI targets did not correlate with increases in IFN-γ EliSpots against DAUDI, suggesting a significant proportion of IFN-γ EliSpots may be not be due to IFN-γ–producing cytotoxic CD8+ cells but rather to IFN-γ–secreting CD4+ helper T cells. These data support the argument that armed T cell infusions induced both cytotoxic T lymphocyte and helper antilymphoma responses. Correlations of immune responses or aATC doses with clinical responses could not be assessed because of the small sample size.
NK activity directed at K562 was significantly enhanced over pre-SCT levels in both cytotoxicity and IFN-γ EliSpots, and these increases persisted up to 100 days after SCT. These data suggest that infusions of aATC stimulated recovery of innate immunity. Evaluations of NK activity in contemporaneous NHL and multiple myeloma patients did not show such a pattern of immune recovery.
Although targeting CD20+ B cells decreased the number of circulating CD20+ cells during first 6 months after SCT, there were normal levels of IgG and anti-TT at 3 months after SCT. Previous studies have reported that rituximab consolidation after autologous SCT for NHL may prolong hypogammaglobulinemia, impair B cell reconstitution, and deplete memory B cells [35–40]. In the absence of intravenous gamma globulin supplementation, B cells or CD20 negative plasma cells must produce IgG or anti-TT. The memory B cells or plasma cells in the stem cell graft were capable of reconstituting and producing normal levels of IgG and anti-TT without being depleted by aATC infusions. Because ATC may provide helper activity to B cells for antibody synthesis, aATC infusions may also accelerate hematopoietic recovery [41–43]. These data suggest that aATC infusions may provide helper activity to B cells transferred in the stem cell inoculum despite the apparent transient depletion of B cells.
There are major differences between the approach using gene transduction of chimeric antibody receptors into anti-CD3/anti-CD28 ATCs [44,45] and the approach using anti-CD3/IL-2 ATCs armed with bispecific antibodies [18,21]. Anti-CD3/anti-CD28 coATCs are transduced with scFv and signal transduction elements that are designed to activate upon tumor engagement, rapid expansion, and the development of a sustained antileukemia effect [22,46]. In contrast, aATC are designed to mediate immediate cytotoxicity, undergo short-term proliferation, and release Th1 cytokines and chemokines at the tumor site to recruit endogenous immune cells, leading to in situ vaccination [22]. The complex effort exerted to provide proof-of-principle using chimeric antibody receptor transduced T cells has been labor intensive [47] and costly; however, recent successes suggest the chimeric antibody receptor approach may be successful with liquid tumors [48].
In summary, aATC infusions were safe in patients after SCT and induced significant increases in cytotoxicity and IFN-γ EliSpot anti-DAUDI activity as well as increases in innate immunity above that seen in patients who received SCT alone. The design of this phase I trial and follow-up time did not permit evaluation of clinical efficacy. Although infusions of aATC enhanced antilymphoma activity, T cell helper activity, and NK activity without impairing B cell functions, there were no correlations between clinical efficacy and immune responses. The current study provides a strong rationale for initiating phase II trials to confirm the ability of CD20Bi aATC to enhance antilymphoma effects and accelerate immune reconstitution in patients after SCT for high-risk/refractory NHL.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge Jennifer Wedge, who took a special interest in managing the inpatient infusions.
Financial disclosure: L.G.L. was supported in part by grants from the Leukemia and Lymphoma Society (TRP 6066-06 and TRP 6092-09), the National Cancer Institute, DHHS (R01 CA 092344 and R01 CA 140314), Michigan Life Sciences (grant number 1819), and gifts from the Ruth F. Rattner and the Ann F. & Norman D. Katz Charitable Foundation. Z.A. and A.D. were supported in part by startup funding from the Department Oncology, Wayne State University. The Microscopy, Imaging and Cytometry Resources Core is supported, in part, by NIH Center grant P30CA22453 to The Karmanos Cancer Institute, Wayne State University and the Perinatology Research Branch of the National Institutes of Child Health and Development, Wayne State University.
Footnotes
Conflict of interest statement: L.G.L is founder of Trans-target Inc. M.H.A has received honoraria from Seattle Genetics and Millennium. A.T., Q.L., A.D., Z.A., L.A., C.P., E.N.T., P.A.S., D.L.S., A.M., M.D., H.Y., J.P.U., and V.R. report no conflicts of interest.
SUPPLEMENTARY DATA
Supplementary data related to this article can be found online at http://dx.doi.org/10.1016/j.bbmt.2013.03.010.
REFERENCES
- 1.Anderson DR, Grillo-Lopez A, Varns C, et al. Targeted anti-cancer therapy using rituximab, a chimaeric anti-CD20 antibody (IDEC-C2B8) in the treatment of non-Hodgkin’s B-cell lymphoma. Biochem Soc Trans. 1997;25:705–708. doi: 10.1042/bst0250705. [DOI] [PubMed] [Google Scholar]
- 2.Lambert JF, Rathore R, Lum LG, et al. Immune consolidation with rituximab following autologous stem cell transplantation for non-Hodgkin’s lymphoma: An interim report of a pilot study with historical controls. In: Dicke KA, Keating A, editors. Stem cell and targeted therapy. Charlottesville, NC: Carden Jennings Publishing; 2003. pp. 149–161. [Google Scholar]
- 3.Elfenbein GJ, Lum LG, Rathore R, et al. Interim report of an historically controlled trial of rituximab after autologous stem cell transplanation for non-Hodgkin’s lymphoma [abstract] Blood. 2003;102:295b. [Google Scholar]
- 4.Horwitz SM, Negrin RS, Blume KG, et al. Rituximab as adjuvant to high-dose therapy and autologous hematopoietic cell transplantation for aggressive non-Hodgkin lymphoma. Blood. 2004;103:777–783. doi: 10.1182/blood-2003-04-1257. [DOI] [PubMed] [Google Scholar]
- 5.Kamezaki K, Kikushige Y, Numata A, et al. Rituximab does not compromise the mobilization and engraftment of autologous peripheral blood stem cells in diffuse-large B-cell lymphoma. Bone Marrow Transplant. 2007;39:523–527. doi: 10.1038/sj.bmt.1705649. [DOI] [PubMed] [Google Scholar]
- 6.Maloney DG, Grillo-López AJ, White CA, et al. IDEC-C2B8 (rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood. 1997;90:2188–2195. [PubMed] [Google Scholar]
- 7.Coiffier B, Thieblemont C, Van Den NE, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: A study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116:2040–2045. doi: 10.1182/blood-2010-03-276246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosly A, Coiffier B, Gisselbrecht C, et al. Bone marrow transplantation prolongs survival after relapse in aggressive-lymphoma patients treated with the LNH-84 regimen. J Clin Oncol. 1992;10:1615–1623. doi: 10.1200/JCO.1992.10.10.1615. [DOI] [PubMed] [Google Scholar]
- 9.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333:1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 10.Johnson P, Glennie M. The mechanisms of action of rituximab in the elimination of tumor cells. Semin Oncol. 2003;30:3–8. doi: 10.1053/sonc.2003.50025. [DOI] [PubMed] [Google Scholar]
- 11.Manches O, Lui G, Chaperot L, et al. In vitro mechanisms of action of rituximab on primary non-Hodgkin lymphomas. Blood. 2003;101:949–954. doi: 10.1182/blood-2002-02-0469. [DOI] [PubMed] [Google Scholar]
- 12.Smith MR. Rituximab (monoclonal anti-CD20 antibody): Mechanisms of action and resistance. Oncogene. 2003;22:7359–7368. doi: 10.1038/sj.onc.1206939. [DOI] [PubMed] [Google Scholar]
- 13.Di Gaetano N, Cittera E, Nota R, et al. Complement activation determines the therapeutic activity of rituximab in vivo. J Immunol. 2003;171:1581–1587. doi: 10.4049/jimmunol.171.3.1581. [DOI] [PubMed] [Google Scholar]
- 14.Van Wauwe JP, De Mey JR, Gooseens JG. OKT3: A monoclonal anti-human T lymphocyte antibody with potent mitogenic properties. J Immunol. 1980;124:2708–2713. [PubMed] [Google Scholar]
- 15.Weiss A, Imboden JB. Cell surface molecules and early events involved in human T lymphocyte activation. Adv Immunol. 1987;41:1–38. doi: 10.1016/s0065-2776(08)60029-2. [DOI] [PubMed] [Google Scholar]
- 16.Anderson PM, Bach FH, Ochoa AC. Augmentation of cell number and LAK activity in peripheral blood mononuclear cells activated with anti-CD3 and interleukin-2. Preliminary results in children with acute lymphocytic leukemia and neuroblastoma. Cancer Immunol Immun-other. 1988;27:82–88. doi: 10.1007/BF00205763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ochoa AC, Hasz DE, Rezonzew R, et al. Lymphokine-activated killer activity in long-term cultures with anti-CD3 plus interleukin 2: identification and isolation of effector subsets. Cancer Res. 1989;49:963–968. [PubMed] [Google Scholar]
- 18.Gall JM, Davol PA, Grabert RC, et al. T cells armed with anti-CD3 × anti-CD20 bispecific antibody enhance killing of CD20+ malignant B cells and bypass complement-mediated rituximab resistance in vitro. Exp Hematol. 2005;33:452–459. doi: 10.1016/j.exphem.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Uberti JP, Joshi I, Ueda M, et al. Preclinical studies using immobilized OKT3 to activate human T cells for adoptive immunotherapy: Optimal conditions for the proliferation and induction of non-MHC restricted cytotoxicity. Clin Immunol Immunopathol. 1994;70:234–240. doi: 10.1006/clin.1994.1034. [DOI] [PubMed] [Google Scholar]
- 20.Ueda M, Joshi ID, Dan M, et al. Preclinical studies for adoptive immunotherapy in bone marrow transplantation. II. Generation of anti-CD3 activated cytotoxic T cells from normal donors and autologous bone marrow transplant candidates. Transplantation. 1993;56:351–356. [PubMed] [Google Scholar]
- 21.Sen M, Wankowski DM, Garlie NK, et al. Use of anti-CD3 × anti-HER2/ neu bispecific antibody for redirecting cytotoxicity of activated T cells toward HER2/neu tumors. J Hematother Stem Cell Res. 2001;10:247–260. doi: 10.1089/15258160151134944. [DOI] [PubMed] [Google Scholar]
- 22.Grabert RC, Cousens LP, Smith JA, et al. Human T cells armed with Her2/neu bispecific antibodies divide, are cytotoxic, and secrete cytokines with repeated stimulation. Clin Cancer Res. 2006;12:569–576. doi: 10.1158/1078-0432.CCR-05-2005. [DOI] [PubMed] [Google Scholar]
- 23.Lum LG, Culbertson NJ. The induction and suppression of in vitro IgG anti-tetanus toxoid antibody synthesis by human lymphocytes stimulated with tetanus toxoid in the absence of in vivo booster immunizations. J Immunol. 1985;135:185–191. [PubMed] [Google Scholar]
- 24.Shi YK, Yang S, Han XH, et al. [A prospective multicenter study of rituximab combined with high-dose chemotherapy and autologous peripheral blood stem cell transplantation for aggressive B-cell lymphoma] Zhonghua Zhong.Liu Za Zhi. 2009;31:592–596. [PubMed] [Google Scholar]
- 25.Tsirigotis P, Dray L, Resnick IB, et al. Post-autologous stem cell transplantation administration of rituximab improves the outcome of patients with aggressive B cell non-Hodgkin’s lymphoma. Ann Hematol. 2010;89:263–272. doi: 10.1007/s00277-009-0808-5. [DOI] [PubMed] [Google Scholar]
- 26.Ahmadi T, McQuade J, Porter D, et al. Potential prolongation of PFS in mantle cell lymphoma after R-HyperCVAD: Auto-SCT consolidation or rituximab maintenance. Bone Marrow Transplant. 2012;47:1082–1086. doi: 10.1038/bmt.2011.218. [DOI] [PubMed] [Google Scholar]
- 27.Recher C, Coiffier B, Haioun C, et al. Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B-cell lymphoma (LNH03-2B): An open-label randomised phase 3 trial. Lancet. 2011;378:1858–1867. doi: 10.1016/S0140-6736(11)61040-4. [DOI] [PubMed] [Google Scholar]
- 28.Gisselbrecht C, Mounier N. Rituximab: Enhancing outcome of autologous stem cell transplantation in non-Hodgkin’s lymphoma. Semin Oncol. 2003;30:28–33. doi: 10.1053/sonc.2003.50022. [DOI] [PubMed] [Google Scholar]
- 29.Stover DG, Reddy VK, Shyr Y, et al. Long-term impact of prior rituximab therapy and early lymphocyte recovery on auto-SCT outcome for diffuse large B-cell lymphoma. Bone Marrow Transplant. 2012;47:82–87. doi: 10.1038/bmt.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fitoussi O, Belhadj K, Mounier N, et al. Survival impact of rituximab combined with ACVBP and upfront consolidation autotransplantation in high-risk diffuse large B-cell lymphoma for GELA. Haematologica. 2011;96:1136–1143. doi: 10.3324/haematol.2010.038109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haioun C, Mounier N, Emile JF, et al. Rituximab versus observation after high-dose consolidative first-line chemotherapy with autologous stem-cell transplantation in patients with poor-risk diffuse large B-cell lymphoma. Ann Oncol. 2009;20:1985–1992. doi: 10.1093/annonc/mdp237. [DOI] [PubMed] [Google Scholar]
- 32.Rapoport AP, Stadtmauer EA, Aqui N, et al. Restoration of immunity in lymphopenic individuals with cancer by vaccination and adoptive T-cell transfer. Nat Med. 2005;11:1230–1237. doi: 10.1038/nm1310. [DOI] [PubMed] [Google Scholar]
- 33.Rapoport AP, Aqui NA, Stadtmauer EA, et al. Combination immunotherapy using adoptive T-cell transfer and tumor antigen vaccination on the basis of hTERT and survivin after ASCT for myeloma. Blood. 2011;117:788–797. doi: 10.1182/blood-2010-08-299396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stadtmauer EA, Vogl DT, Luning PE, et al. Transfer of influenza vaccine-primed costimulated autologous T cells after stem cell transplantation for multiple myeloma leads to reconstitution of influenza immunity: results of a randomized clinical trial. Blood. 2011;117:63–71. doi: 10.1182/blood-2010-07-296822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim SH, Esler WV, Zhang Y, et al. B-cell depletion for 2 years after autologous stem cell transplant for NHL induces prolonged hypogammaglobulinemia beyond the rituximab maintenance period. Leuk Lymph. 2008;49:152–153. doi: 10.1080/10428190701742506. [DOI] [PubMed] [Google Scholar]
- 36.Nishio M, Fujimoto K, Yamamoto S, et al. Delayed redistribution of CD27, CD40 and CD80 positive B cells and the impaired in vitro immunoglobulin production in patients with non-Hodgkin lymphoma after rituximab treatment as an adjuvant to autologous stem cell transplantation. Br J Haematol. 2007;137:349–354. doi: 10.1111/j.1365-2141.2007.06584.x. [DOI] [PubMed] [Google Scholar]
- 37.Nishio M, Fujimoto K, Yamamoto S, et al. Hypogammaglobulinemia with a selective delayed recovery in memory B cells and an impaired isotype expression after rituximab administration as an adjuvant to autologous stem cell transplantation for non-Hodgkin lymphoma. Eur J Haematol. 2006;77:226–232. doi: 10.1111/j.1600-0609.2006.00693.x. [DOI] [PubMed] [Google Scholar]
- 38.Nishio M, Endo T, Fujimoto K, et al. Persistent pan-hypogammaglobulinemia with selected loss of memory B cells and impaired isotype expression after rituximab therapy for post-transplant EBV-associated autoimmune hemolytic anemia. Eur J Haematol. 2005;75:527–529. doi: 10.1111/j.1600-0609.2005.00552.x. [DOI] [PubMed] [Google Scholar]
- 39.Shortt J, Spencer A. Adjuvant rituximab causes prolonged hypogammaglobulinaemia following autologous stem cell transplant for non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2006;38:433–436. doi: 10.1038/sj.bmt.1705463. [DOI] [PubMed] [Google Scholar]
- 40.Khouri IF, Saliba RM, Hosing C, et al. Concurrent administration of high-dose rituximab before and after autologous stem-cell transplantation for relapsed aggressive B-cell non-Hodgkin's lymphomas. Journal of Clin Oncol. 2005;23:2240–2247. doi: 10.1200/JCO.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 41.Jin NR, Lum LG, Ratanatharathorn V, Sensenbrenner LL. Anti-CD3-activated splenocytes enhance survival in lethally irradiated mice after transplant of syngeneic hematopoietic stem cells. Exp Hematol. 1995;23:1331–1336. [PubMed] [Google Scholar]
- 42.Hexner EO, net-Desnoyers GA, Zhang Y, et al. Umbilical cord blood xenografts in immunodeficient mice reveal that T cells enhance hematopoietic engraftment beyond overcoming immune barriers by stimulating stem cell differentiation. Biol Blood Marrow Transplant. 2007;13:1135–1144. doi: 10.1016/j.bbmt.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Lum LG. Immunotherapy with activated T cells after high dose chemotherapy and PBSCT for breast cancer. In: Dicke KA, Keating A, editors. Autologous Blood and Marrow Transplantation Proceedings of the Tenth International Symposium. Charlottesville, NC: Carden Jennings; 2000. pp. 95–105. [Google Scholar]
- 44.Porter DL, Kalos M, Zheng Z, et al. Chimeric antigen receptor therapy for B-cell malignancies. J Cancer. 2011;2:331–332. doi: 10.7150/jca.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002842. 95ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lum LG, Thakur A, Rathore R, et al. Phase I clinical trial involving infusions of activated T cells armed with anti-CD3 x anti-Her2neu bispecific antibody in women with metastatic breast cancer: Clinical, immune, and trafficking results. Washington, DC: Presented at the ASCO Breast Cancer Symposium; Oct, 2010. pp. 1–3. [Google Scholar]
- 47.Sadelain M, Brentjens R, Riviere I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21:215–223. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.David L, Porter DL, Levine BL, Kalos M, et al. Chimeric Antigen Receptor-Modified T Cells in Chronic Lymphoid Leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.