Abstract
Carbohydrates in biological systems are often associated with specific recognition and signaling processes leading to important biological functions and diseases. Considerable efforts have been directed toward understanding and mimicking the recognition processes and developing effective agents to control the processes. The pace of discovery research in glycobiology and development of carbohydrate-based therapeutics, however, has been relatively slow due to the lack of appropriate strategies and methods available for carbohydrate-related research. This review summarizes some of the most recent developments in the field, with particular emphasis on work from our laboratories regarding the use of chemoenzymatic strategies to tackle the carbohydrate recognition problem. Highlights include the study of selectin-carbohydrate and aminoglycoside-RNA interactions and development of agents for the intervention of these recognition processes.
Full text
PDF
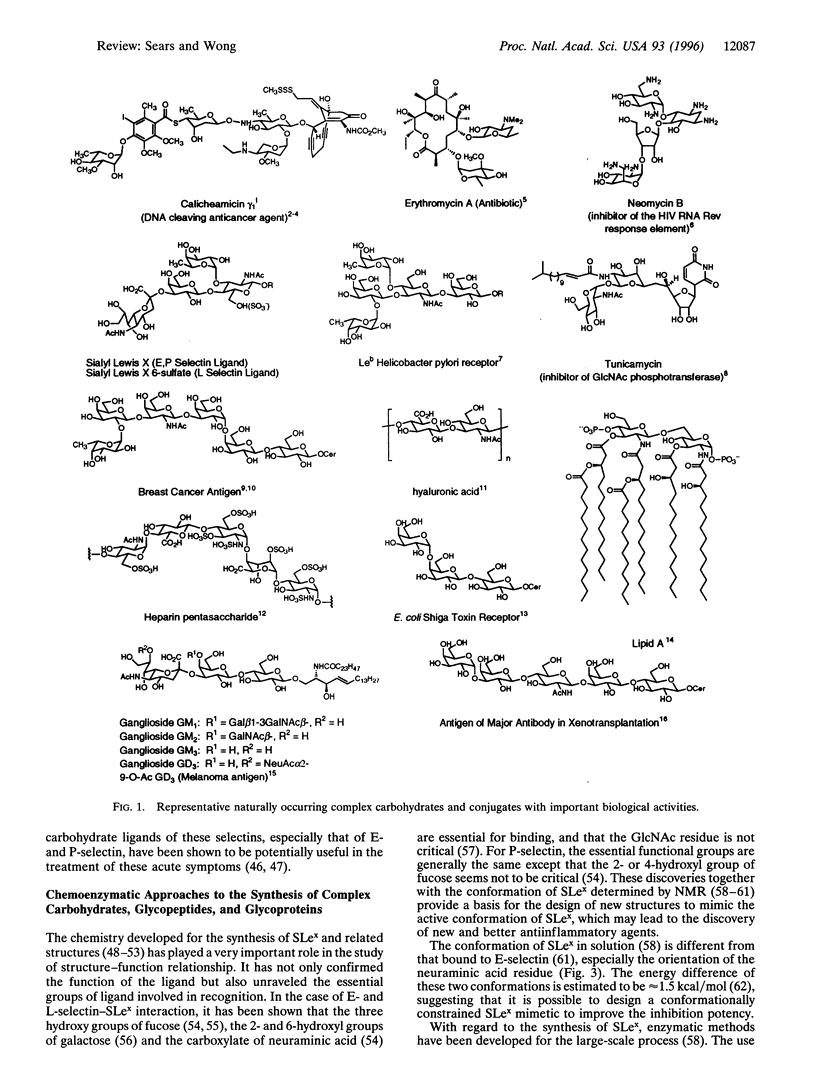
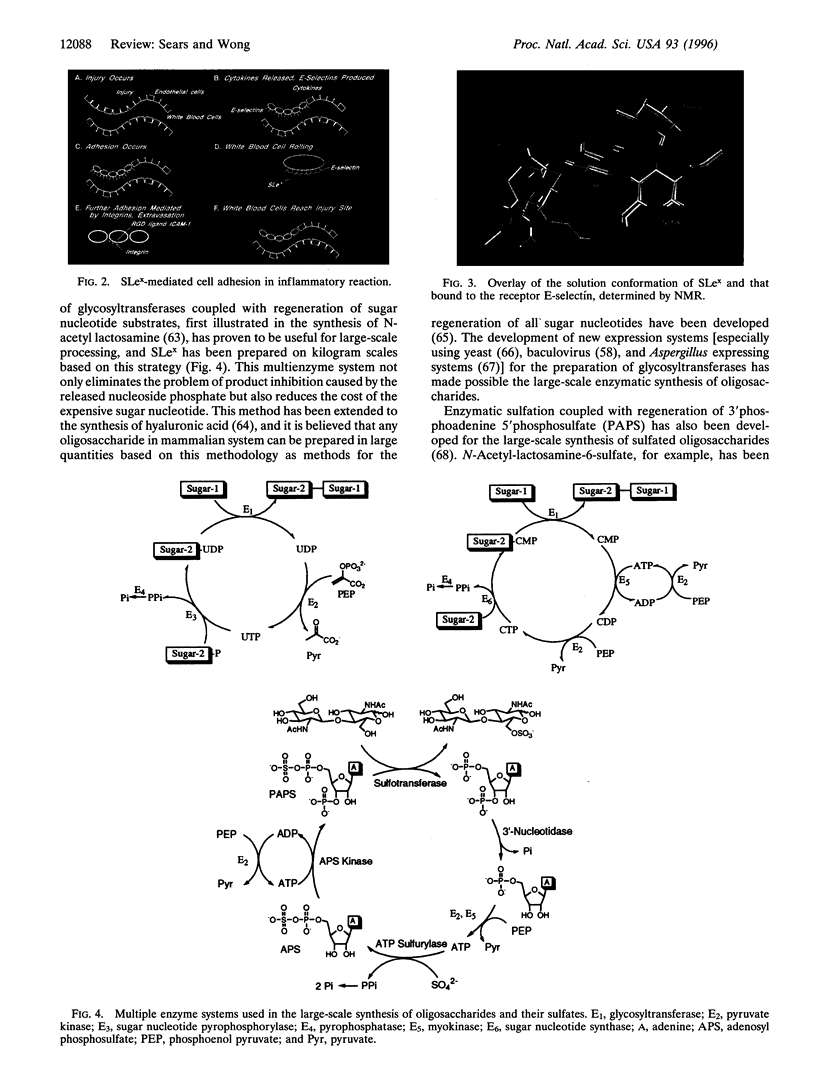
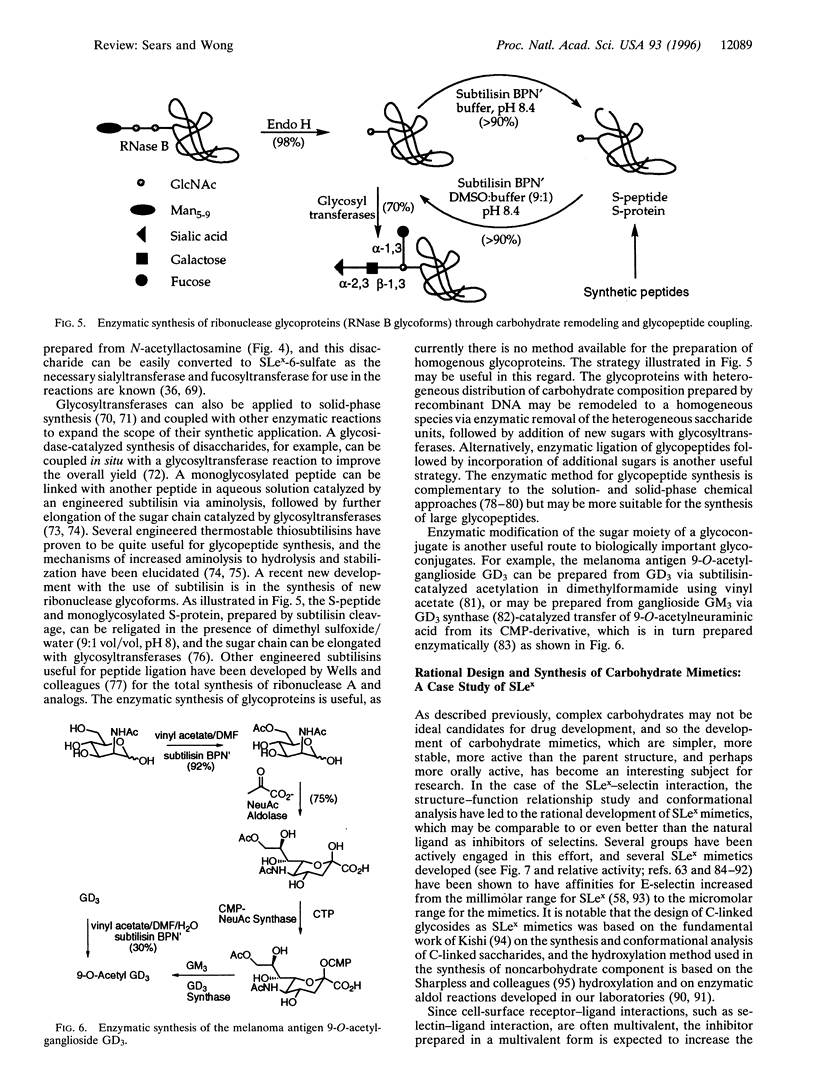


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki S., Kondo H., Wong C. H. Glycosyl phosphites as glycosylation reagents. Methods Enzymol. 1994;247:193–211. doi: 10.1016/s0076-6879(94)47015-4. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Stengelin S., Gimbrone M. A., Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989 Mar 3;243(4895):1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Borén T., Falk P., Roth K. A., Larson G., Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993 Dec 17;262(5141):1892–1895. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- Brandley B. K., Kiso M., Abbas S., Nikrad P., Srivasatava O., Foxall C., Oda Y., Hasegawa A. Structure-function studies on selectin carbohydrate ligands. Modifications to fucose, sialic acid and sulphate as a sialic acid replacement. Glycobiology. 1993 Dec;3(6):633–641. doi: 10.1093/glycob/3.6.633. [DOI] [PubMed] [Google Scholar]
- Bremer E. G., Levery S. B., Sonnino S., Ghidoni R., Canevari S., Kannagi R., Hakomori S. Characterization of a glycosphingolipid antigen defined by the monoclonal antibody MBr1 expressed in normal and neoplastic epithelial cells of human mammary gland. J Biol Chem. 1984 Dec 10;259(23):14773–14777. [PubMed] [Google Scholar]
- Chandrasekaran E. V., Jain R. K., Larsen R. D., Wlasichuk K., Matta K. L. Selectin ligands and tumor-associated carbohydrate structures: specificities of alpha 2,3-sialyltransferases in the assembly of 3'-sialyl-6-sialyl/sulfo Lewis a and x, 3'-sialyl-6'-sulfo Lewis x, and 3'-sialyl-6-sialyl/sulfo blood group T-hapten. Biochemistry. 1995 Mar 7;34(9):2925–2936. doi: 10.1021/bi00009a024. [DOI] [PubMed] [Google Scholar]
- Clark E. A., Brugge J. S. Integrins and signal transduction pathways: the road taken. Science. 1995 Apr 14;268(5208):233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Cooke R. M., Hale R. S., Lister S. G., Shah G., Weir M. P. The conformation of the sialyl Lewis X ligand changes upon binding to E-selectin. Biochemistry. 1994 Sep 6;33(35):10591–10596. doi: 10.1021/bi00201a004. [DOI] [PubMed] [Google Scholar]
- Drak J., Iwasawa N., Danishefsky S., Crothers D. M. The carbohydrate domain of calicheamicin gamma I1 determines its sequence specificity for DNA cleavage. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7464–7468. doi: 10.1073/pnas.88.17.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili U. Evolution and pathophysiology of the human natural anti-alpha-galactosyl IgG (anti-Gal) antibody. Springer Semin Immunopathol. 1993;15(2-3):155–171. doi: 10.1007/BF00201098. [DOI] [PubMed] [Google Scholar]
- Gijsen Harrie J. M., Qiao Lei, Fitz Wolfgang, Wong Chi-Huey. Recent Advances in the Chemoenzymatic Synthesis of Carbohydrates and Carbohydrate Mimetics. Chem Rev. 1996 Feb 1;96(1):443–474. doi: 10.1021/cr950031q. [DOI] [PubMed] [Google Scholar]
- Graves B. J., Crowther R. L., Chandran C., Rumberger J. M., Li S., Huang K. S., Presky D. H., Familletti P. C., Wolitzky B. A., Burns D. K. Insight into E-selectin/ligand interaction from the crystal structure and mutagenesis of the lec/EGF domains. Nature. 1994 Feb 10;367(6463):532–538. doi: 10.1038/367532a0. [DOI] [PubMed] [Google Scholar]
- Han H., Wolfe M. M., Brenner S., Janda K. D. Liquid-phase combinatorial synthesis. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6419–6423. doi: 10.1073/pnas.92.14.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifetz A., Keenan R. W., Elbein A. D. Mechanism of action of tunicamycin on the UDP-GlcNAc:dolichyl-phosphate Glc-NAc-1-phosphate transferase. Biochemistry. 1979 May 29;18(11):2186–2192. doi: 10.1021/bi00578a008. [DOI] [PubMed] [Google Scholar]
- Hemmerich S., Bertozzi C. R., Leffler H., Rosen S. D. Identification of the sulfated monosaccharides of GlyCAM-1, an endothelial-derived ligand for L-selectin. Biochemistry. 1994 Apr 26;33(16):4820–4829. doi: 10.1021/bi00182a010. [DOI] [PubMed] [Google Scholar]
- Hemmerich S., Rosen S. D. 6'-sulfated sialyl Lewis x is a major capping group of GlyCAM-1. Biochemistry. 1994 Apr 26;33(16):4830–4835. doi: 10.1021/bi00182a011. [DOI] [PubMed] [Google Scholar]
- Hensley P., McDevitt P. J., Brooks I., Trill J. J., Feild J. A., McNulty D. E., Connor J. R., Griswold D. E., Kumar N. V., Kopple K. D. The soluble form of E-selectin is an asymmetric monomer. Expression, purification, and characterization of the recombinant protein. J Biol Chem. 1994 Sep 30;269(39):23949–23958. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Itzkowitz S. H., Yuan M., Fukushi Y., Palekar A., Phelps P. C., Shamsuddin A. M., Trump B. F., Hakomori S., Kim Y. S. Lewisx- and sialylated Lewisx-related antigen expression in human malignant and nonmalignant colonic tissues. Cancer Res. 1986 May;46(5):2627–2632. [PubMed] [Google Scholar]
- Jackson D. Y., Burnier J., Quan C., Stanley M., Tom J., Wells J. A. A designed peptide ligase for total synthesis of ribonuclease A with unnatural catalytic residues. Science. 1994 Oct 14;266(5183):243–247. doi: 10.1126/science.7939659. [DOI] [PubMed] [Google Scholar]
- Jackson M. P. Structure-function analyses of Shiga toxin and the Shiga-like toxins. Microb Pathog. 1990 Apr;8(4):235–242. doi: 10.1016/0882-4010(90)90050-z. [DOI] [PubMed] [Google Scholar]
- Jacob G. S., Kirmaier C., Abbas S. Z., Howard S. C., Steininger C. N., Welply J. K., Scudder P. Binding of sialyl Lewis x to E-selectin as measured by fluorescence polarization. Biochemistry. 1995 Jan 31;34(4):1210–1217. doi: 10.1021/bi00004a014. [DOI] [PubMed] [Google Scholar]
- Johnston G. I., Cook R. G., McEver R. P. Cloning of GMP-140, a granule membrane protein of platelets and endothelium: sequence similarity to proteins involved in cell adhesion and inflammation. Cell. 1989 Mar 24;56(6):1033–1044. doi: 10.1016/0092-8674(89)90636-3. [DOI] [PubMed] [Google Scholar]
- Kameyama A., Ishida H., Kiso M., Hasegawa A. Total synthesis of sialyl Lewis X. Carbohydr Res. 1991 Jan 15;209:c1–c4. doi: 10.1016/0008-6215(91)80171-i. [DOI] [PubMed] [Google Scholar]
- Kim S. C., Singh A. N., Raushel F. M. Analysis of the galactosyltransferase reaction by positional isotope exchange and secondary deuterium isotope effects. Arch Biochem Biophys. 1988 Nov 15;267(1):54–59. doi: 10.1016/0003-9861(88)90007-0. [DOI] [PubMed] [Google Scholar]
- Krezdorn C. H., Watzele G., Kleene R. B., Ivanov S. X., Berger E. G. Purification and characterization of recombinant human beta 1-4 galactosyltransferase expressed in Saccharomyces cerevisiae. Eur J Biochem. 1993 Feb 15;212(1):113–120. doi: 10.1111/j.1432-1033.1993.tb17640.x. [DOI] [PubMed] [Google Scholar]
- Lasky L. A. Selectins: interpreters of cell-specific carbohydrate information during inflammation. Science. 1992 Nov 6;258(5084):964–969. doi: 10.1126/science.1439808. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Singer M. S., Yednock T. A., Dowbenko D., Fennie C., Rodriguez H., Nguyen T., Stachel S., Rosen S. D. Cloning of a lymphocyte homing receptor reveals a lectin domain. Cell. 1989 Mar 24;56(6):1045–1055. doi: 10.1016/0092-8674(89)90637-5. [DOI] [PubMed] [Google Scholar]
- Lowe J. B., Stoolman L. M., Nair R. P., Larsen R. D., Berhend T. L., Marks R. M. ELAM-1--dependent cell adhesion to vascular endothelium determined by a transfected human fucosyltransferase cDNA. Cell. 1990 Nov 2;63(3):475–484. doi: 10.1016/0092-8674(90)90444-j. [DOI] [PubMed] [Google Scholar]
- Martens C. L., Cwirla S. E., Lee R. Y., Whitehorn E., Chen E. Y., Bakker A., Martin E. L., Wagstrom C., Gopalan P., Smith C. W. Peptides which bind to E-selectin and block neutrophil adhesion. J Biol Chem. 1995 Sep 8;270(36):21129–21136. doi: 10.1074/jbc.270.36.21129. [DOI] [PubMed] [Google Scholar]
- Masamune S., Bates G. S., Corcoran J. W. Macrolides. Recent progress in chemistry and biochemistry. Angew Chem Int Ed Engl. 1977 Sep;16(9):585–607. doi: 10.1002/anie.197705851. [DOI] [PubMed] [Google Scholar]
- McEver R. P., Moore K. L., Cummings R. D. Leukocyte trafficking mediated by selectin-carbohydrate interactions. J Biol Chem. 1995 May 12;270(19):11025–11028. doi: 10.1074/jbc.270.19.11025. [DOI] [PubMed] [Google Scholar]
- Mulligan M. S., Paulson J. C., De Frees S., Zheng Z. L., Lowe J. B., Ward P. A. Protective effects of oligosaccharides in P-selectin-dependent lung injury. Nature. 1993 Jul 8;364(6433):149–151. doi: 10.1038/364149a0. [DOI] [PubMed] [Google Scholar]
- Muramatsu T. Carbohydrate signals in metastasis and prognosis of human carcinomas. Glycobiology. 1993 Aug;3(4):291–296. doi: 10.1093/glycob/3.4.291. [DOI] [PubMed] [Google Scholar]
- Murohara T., Margiotta J., Phillips L. M., Paulson J. C., DeFrees S., Zalipsky S., Guo L. S., Lefer A. M. Cardioprotection by liposome-conjugated sialyl Lewisx-oligosaccharide in myocardial ischaemia and reperfusion injury. Cardiovasc Res. 1995 Dec;30(6):965–974. [PubMed] [Google Scholar]
- Palcic M. M., Heerze L. D., Srivastava O. P., Hindsgaul O. A bisubstrate analog inhibitor for alpha(1----2)-fucosyltransferase. J Biol Chem. 1989 Oct 15;264(29):17174–17181. [PubMed] [Google Scholar]
- Phillips M. L., Nudelman E., Gaeta F. C., Perez M., Singhal A. K., Hakomori S., Paulson J. C. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex. Science. 1990 Nov 23;250(4984):1130–1132. doi: 10.1126/science.1701274. [DOI] [PubMed] [Google Scholar]
- Polley M. J., Phillips M. L., Wayner E., Nudelman E., Singhal A. K., Hakomori S., Paulson J. C. CD62 and endothelial cell-leukocyte adhesion molecule 1 (ELAM-1) recognize the same carbohydrate ligand, sialyl-Lewis x. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6224–6228. doi: 10.1073/pnas.88.14.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal J. Y., Zheng Z. L., Perez C., Walker L. E., DeFrees S. A., Gaeta F. C. Structure--activity relationships of sialyl Lewis x-containing oligosaccharides. 1. Effect of modifications of the fucose moiety. J Med Chem. 1994 Oct 14;37(21):3459–3463. doi: 10.1021/jm00047a003. [DOI] [PubMed] [Google Scholar]
- Rao B. N., Anderson M. B., Musser J. H., Gilbert J. H., Schaefer M. E., Foxall C., Brandley B. K. Sialyl Lewis X mimics derived from a pharmacophore search are selectin inhibitors with anti-inflammatory activity. J Biol Chem. 1994 Aug 5;269(31):19663–19666. [PubMed] [Google Scholar]
- Sakamoto J., Furukawa K., Cordon-Cardo C., Yin B. W., Rettig W. J., Oettgen H. F., Old L. J., Lloyd K. O. Expression of Lewisa, Lewisb, X, and Y blood group antigens in human colonic tumors and normal tissue and in human tumor-derived cell lines. Cancer Res. 1986 Mar;46(3):1553–1561. [PubMed] [Google Scholar]
- Sasaki K., Kurata K., Kojima N., Kurosawa N., Ohta S., Hanai N., Tsuji S., Nishi T. Expression cloning of a GM3-specific alpha-2,8-sialyltransferase (GD3 synthase). J Biol Chem. 1994 Jun 3;269(22):15950–15956. [PubMed] [Google Scholar]
- Schlaepfer D. D., Hanks S. K., Hunter T., van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994 Dec 22;372(6508):786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- Scudder P. R., Shailubhai K., Duffin K. L., Streeter P. R., Jacob G. S. Enzymatic synthesis of a 6'-sulphated sialyl-Lewisx which is an inhibitor of L-selectin binding to peripheral addressin. Glycobiology. 1994 Dec;4(6):929–932. doi: 10.1093/glycob/4.6.929. [DOI] [PubMed] [Google Scholar]
- Siegelman M. H., van de Rijn M., Weissman I. L. Mouse lymph node homing receptor cDNA clone encodes a glycoprotein revealing tandem interaction domains. Science. 1989 Mar 3;243(4895):1165–1172. doi: 10.1126/science.2646713. [DOI] [PubMed] [Google Scholar]
- Sirott M. N., Bajorin D. F., Wong G. Y., Tao Y., Chapman P. B., Templeton M. A., Houghton A. N. Prognostic factors in patients with metastatic malignant melanoma. A multivariate analysis. Cancer. 1993 Nov 15;72(10):3091–3098. doi: 10.1002/1097-0142(19931115)72:10<3091::aid-cncr2820721034>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Spevak W., Foxall C., Charych D. H., Dasgupta F., Nagy J. O. Carbohydrates in an acidic multivalent assembly: nanomolar P-selectin inhibitors. J Med Chem. 1996 Mar 1;39(5):1018–1020. doi: 10.1021/jm950914+. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993 Apr;3(2):97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz G., Aruffo A., Kolanus W., Bevilacqua M., Seed B. Recognition by ELAM-1 of the sialyl-Lex determinant on myeloid and tumor cells. Science. 1990 Nov 23;250(4984):1132–1135. doi: 10.1126/science.1701275. [DOI] [PubMed] [Google Scholar]
- Weis W. I., Drickamer K., Hendrickson W. A. Structure of a C-type mannose-binding protein complexed with an oligosaccharide. Nature. 1992 Nov 12;360(6400):127–134. doi: 10.1038/360127a0. [DOI] [PubMed] [Google Scholar]
- Winchester B., Fleet G. W. Amino-sugar glycosidase inhibitors: versatile tools for glycobiologists. Glycobiology. 1992 Jun;2(3):199–210. doi: 10.1093/glycob/2.3.199. [DOI] [PubMed] [Google Scholar]
- Zapp M. L., Stern S., Green M. R. Small molecules that selectively block RNA binding of HIV-1 Rev protein inhibit Rev function and viral production. Cell. 1993 Sep 24;74(6):969–978. doi: 10.1016/0092-8674(93)90720-b. [DOI] [PubMed] [Google Scholar]
- Zapp M. L., Stern S., Green M. R. Small molecules that selectively block RNA binding of HIV-1 Rev protein inhibit Rev function and viral production. Cell. 1993 Sep 24;74(6):969–978. doi: 10.1016/0092-8674(93)90720-b. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Moore K. L., Smith D. F., Varki A., McEver R. P., Cummings R. D. The selectin GMP-140 binds to sialylated, fucosylated lactosaminoglycans on both myeloid and nonmyeloid cells. J Cell Biol. 1991 Oct;115(2):557–564. doi: 10.1083/jcb.115.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Echten G., Sandhoff K. Ganglioside metabolism. Enzymology, Topology, and regulation. J Biol Chem. 1993 Mar 15;268(8):5341–5344. [PubMed] [Google Scholar]
- von Ahsen U., Noller H. F. Footprinting the sites of interaction of antibiotics with catalytic group I intron RNA. Science. 1993 Jun 4;260(5113):1500–1503. doi: 10.1126/science.8502993. [DOI] [PubMed] [Google Scholar]