Abstract
Lecithin cholesterol acyl transferase (LCAT) is a plasma enzyme that esterifies cholesterol and raises high-density lipoprotein cholesterol, but its role in atherosclerosis is not clearly established. Studies of various animal models have yielded conflicting results, but studies done in rabbits and non-human primates, which more closely simulate human lipoprotein metabolism, indicate that LCAT is likely atheroprotective. Although suggestive, there are also no biomarker studies that mechanistically link LCAT with cardiovascular disease. Imaging studies of patients with LCAT deficiency have also not yielded a clear answer to the role of LCAT in atherosclerosis. Recombinant LCAT, however, is currently being developed as a therapeutic product for enzyme replacement therapy of patients with genetic disorders of LCAT for the prevention and/or treatment of renal disease, but it may also have value for the treatment of acute coronary syndrome.
Keywords: Lecithin cholesterol acyltransferase, High-density lipoprotein, Fish-eye disease, Cholesterol, Reverse cholesterol transport, Atherosclerosis, Enzyme replacement therapy, Biomarker
Introduction
Lecithin cholesterol acyltransferase (LCAT) (EC2.3.1.43), a lipoprotein-associated enzyme, is a key player in the reverse cholesterol transport (RCT) pathway, which promotes the transfer of excess cellular cholesterol from peripheral tissues to the liver for excretion [1]. Genetic disorders of LCAT are associated with low levels of high-density lipoproteins (HDL) and several pathologic consequences, but interestingly patients with LCAT deficiency do not appear to have a marked increase risk of cardiovascular disease [2]. In this review, we describe recent findings related to the possible anti- or pro-atherogenic roles of LCAT. First, we review LCAT biochemistry and its role in HDL metabolism. Next, we summarize the results of various animal models exploring the physiologic role of LCAT. The association of LCAT activity and protein as a biomarker for cardiovascular risk is also discussed, followed by recent cardiovascular imaging studies in patients with LCAT deficiency. Finally, efforts related to the development of drugs that modulate LCAT expression and activity and the use of recombinant LCAT itself as a therapeutic agent is described.
LCAT Biochemistry and Role in HDL Metabolism
LCAT is approximately a 67-kDa sized secretory protein that is primarily produced in the liver but is also synthesized in the central nervous system [1]. It associates with lipoproteins, with the majority bound to HDL and to a lesser degree to low-density lipoproteins (LDL). It is one of the three known enzymes that esterify cholesterol; the other two are the intracellular acyl-cholesterol transferase (ACAT) enzymes [3]. Based on the level of cholesteryl esters in plasma from patients with LCAT deficiency, LCAT accounts for the majority of circulating cholesteryl esters on plasma lipoproteins [2]. LCAT has two different catalytic activities that account for its ability to esterify cholesterol. The first is a phospholipase A2 activity, which cleaves fatty acids from the sn-2 position of phosphatidylcholine and other phospholipids. It also has a transesterification activity, which transfers the cleaved fatty acid to the hydroxyl group on the A-ring of cholesterol. It does not require any cofactors except for apolipoprotein A-I (apoA-I) and to a lesser degree other apolipoproteins, which most likely activate LCAT by modifying the presentation of its substrates, namely phospholipids and cholesterol, on the surface of lipoproteins.
LCAT has two main impacts on lipoprotein metabolism. First, because cholesteryl esters are significantly more hydrophobic than free cholesterol, cholesteryl esters formed by LCAT partition from the surface of lipoproteins to the hydrophobic core. This transforms the small pre-β HDL (the nascent, discoidal-shaped HDL) into larger, spherical-shaped α-migrating forms of HDL, the major HDL species found in plasma. The increase in size of HDL stabilizes it from removal by renal clearance. The second major effect of LCAT is that the esterification of cholesterol prevents the back exchange of cholesterol by passive diffusion from HDL to peripheral cells, and thus is believed to promote net removal of cholesterol from peripheral cells to HDL [4].
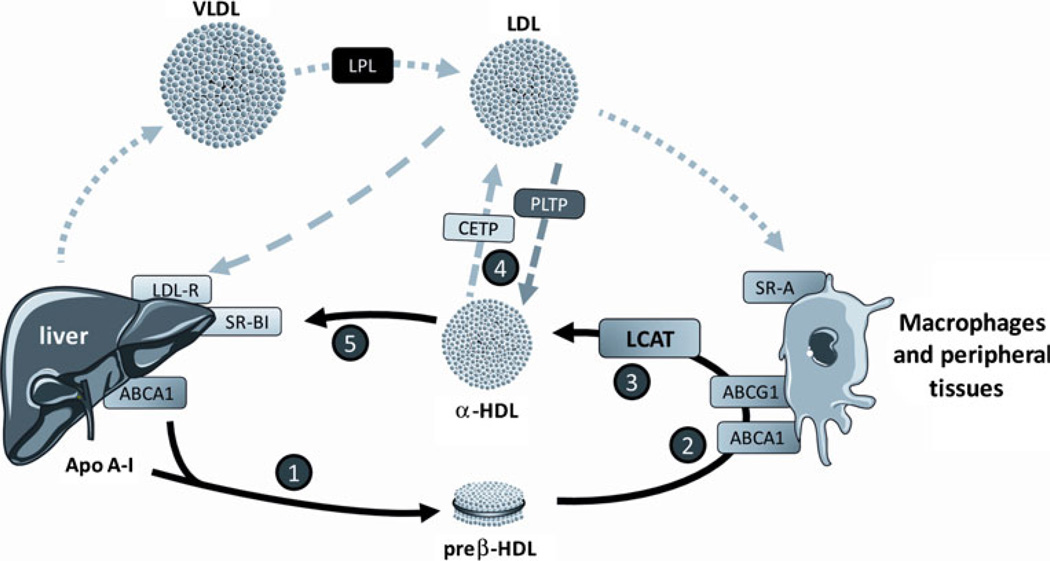
The overall effect of LCAT on the RCT pathway is shown in Fig. 1. The RCT pathway begins with the production of nascent pre-β HDL when apoA-I produced in the liver and small intestine extracts phospholipids and cholesterol from the plasma membrane of the liver and intestine by interacting with the ATP-binding cassette A1 (ABCA1) transporter [5]. Additional cholesterol is removed by pre-β HDL when it interacts with ABCA1 transporters on peripheral cells. ABCA1 is under tight transcriptional control and is induced by oxysterols in cholesterol-loaded cells, such as macrophages. As previously discussed, cholesterol removed by HDL from cells becomes trapped once it is esterified and enters the core of lipoproteins. Recently, it has been shown that LCAT, presumably by preventing its back exchange, facilitates the efflux of cholesterol by another ABC transporter, namely ABCG1 [6]. ABCG1, unlike ABCA1, promotes the efflux of cholesterol to the mature lipid-rich α-HDL. After its esterification by LCAT, cholesteryl esters on HDL are transferred to apoB-containing lipoproteins by cholesteryl ester transfer protein (CETP), although approximately 25% of cholesteryl esters are directly formed on apoB-containing lipoproteins. In the last step of RCT, cholesteryl esters are delivered to the liver for excretion either by the uptake of LDL by the LDL receptor or by selective lipid uptake by scavenger receptor B type I (SR-BI) on the liver.
Fig. 1.
A model of steps in the reverse cholesterol transport pathway. Step 1, Hepatic and intestinal synthesis of apolipoprotein A-I (apoA-I) and its association with phospholipids and cholesterol by the ATP-bindind cassette A1 (ABCA1) transporter forming nascent pre-β high-density lipoprotein (HDL). Step 2, Efflux of cholesterol from peripheral tissues by ABCA1 and ABCG1 transporters. Step 3, Esterification of cholesterol in HDL by lecithin cholesterol acyl transferase (LCAT) and transformation of nascent HDL into spherical α-HDL. Step 4, Cholesteryl ester transfer protein (CETP)-mediated and phoshpholipid transfer protein (PLTP)-mediated exchange of cholesteryl ester and phospholipids between HDL and low-density lipoprotein (LDL). Step 5, Hepatc uptake of cholesterol from HDL by scavenger receptor B type I (SR-BI) and from LDL by LDL receptor. LPL—lipoprotein lipase; VLDL—very low-density lipoprotein
Deficiency of LCAT prevents the formation of mature HDL, which leads to an overall decrease in HDL levels and the relative accumulation of pre-β HDL. In addition, lipoprotein X (Lp-X)–like particles accumulate, which are large multilamellar phospholipid vesicles that contain various apolipoproteins but do not contain a neutral lipid core. Kinetic studies in humans with genetic LCAT deficiency have revealed that they have hypercatabolism of not only HDL but also LDL [7], which along with the decrease transfer of cholesterol from HDL to LDL probably accounts for the fact that familial LCAT deficiency (FLD) patients usually have low LDL cholesterol (LDL-C).
Human deficiency of LCAT can present with two different clinical syndromes [2]. In fish-eye disease (FED), patients have a partial LCAT activity in plasma and are relatively asymptomatic. FED patients usually present with low HDL cholesterol (HDL-C) (typically<10 mg/dL) and corneal opacities, due to the deposition of free cholesterol. Patients with FLD, who have almost complete deficiency of plasma LCAT activity, develop (in addition to corneal opacities and low HDL-C) hypertension, hepatosplenomegaly, and normochromic normocytic anemia. The main cause of morbidity and mortality in these patients is renal disease, which begins as proteinuria in teenage years and variably progresses to end-stage renal disease around the fourth or fifth decade of life. The accumulation of Lp-X– like particles in the glomerulus is believed to be the main mechanism behind the development of renal disease [8]. As discussed below, despite the low HDL levels and perturbations in the RCT pathway, FED or FLD subjects do not appear to have a marked increase risk of cardiovascular disease, which has raised questions about the purported anti-atherogenic role of LCAT [9].
Animal Studies on the Role of LCAT in Atherosclerosis
Animal models of either over- or under-expression of LCAT have been developed in several different species (Table 1). Because this has recently been reviewed [1], we discuss here only recent findings in this area. Past findings have shown conflicting results for the anti- or pro-atherogenic role of LCAT. A major limitation, however, in the interpretation of these animal studies, particularly the mouse studies, are the many differences between mice and humans in lipoprotein metabolism [10, 11]. For example, over-expression of LCAT in mice was found to increase HDL-C but also increased atherosclerosis on an atherogenic diet. This was attributed to the absence of CETP in mice and the generation of apoE-enriched HDL, which was dysfunctional in hepatic delivery of cholesterol [12]. This problem was partly attenuated when LCAT was expressed in mice also expressing CETP [13]. Besides increasing HDL-C, LCAT over-expression in mice on an atherogenic diet also increased the level of apoB-containing lipoproteins [12], which may also have contributed to the increased atherosclerosis observed in mice. A more modest increase in LCAT in another mouse model, however, did not lead to increased atherosclerosis in mice [14]. Over-expression of LCAT in rabbits [15], which express CETP and are a better model for human atherosclerosis, also increased HDL-C, lowered LDL-C (which depended on the expression of the LDL-receptor [16]), and reduced atherosclerosis [17].
Table 1.
Animal models exploring the role of the LCAT gene in cholesterol metabolism
| Animal | Model | Key findings | References |
|---|---|---|---|
| Mice | Transgenic | Hyperalpha-lipoproteinemia, appearance of second HDL peak, rich in apoE. LCAT transgene exacerbated diet-induced atherosclerosis. CETP corrected dysfunctional HDL. | [12, 13, 49] |
| Mice | Transgenic | Increased HDL, efficient apoA1 and LCAT interaction. LCAT transgene did not protect against diet-induced atherosclerosis. | [14] |
| Mice | Transgenic | Increased HDL, decreased VLDL and LDL. LCAT transgene did not protect against diet-induced atherosclerosis. | [50] |
| Mice | AA virus with LCAT gene | LCAT had minimal effects on macrophage reverse cholesterol transport in vivo | [25] |
| Rabbits | Transgenic | Hyperalpha-lipoproteinemia; decreased level of apoB-containing lipoproteins, probably via LDL receptor; protection against diet-induced atherosclerosis | [15, 17] |
| Rabbits | Adenovirus with LCAT gene | Increased HDL, protected heterozygous LDL receptor–deficient rabbits against diet-induced atherosclerosis | [51] |
| Squirrel monkey | Adenovirus with LCAT | Increased HDL, decreased level of apoB-containing lipoproteins | [22] |
| Mice | Knockout | Markedly reduced total and HDL cholesterol; susceptibility to glomerulopathy. Diet-induced atherosclerosis was decreased. | [18–20] |
| Mice | Knockout | High-fat diet without cholate induced atherosclerosis | [21] |
AA adeno-associated, apo apolipoprotein, CETP cholesteryl ester transfer protein, HDL high-density lipoprotein, LCAT lecithin cholesterol acyl transferase, LDL low-density lipoprotein, VLDL very low-density lipoprotein
LCAT-knockout mice had reduced HDL-C and increased pre-β HDL [18], and similar to LCAT-deficient patients, they developed proteinuria and glomerulosclerosis [19]. Unexpectedly, LCAT-knockout mice had reduced diet-induced atherosclerosis when on a high-cholesterol/cholate diet. LCAT deficiency also was atheroprotective in apoE-knockout mice [19, 20]. In all these cases LCAT deficiency was associated with a decrease in apoB-containing particles. In another study, LCAT-knockout/apoE-knockout mice placed on a high-fat diet but without cholate showed instead increased atherosclerosis and elevated apoB-containing lipoproteins [21]. Overall, the results from the various animal models indicate that there is a complex interaction between LCAT and atherosclerosis that depends on diet and other genes, such as CETP and the LDL receptor. It also appears that the anti-atherogenic effect of LCAT in animal models more closely correlates with its ability to lower plasma levels of apoB lipoproteins rather than on its ability to raise HDL-C.
Recently, human LCAT was expressed using adenovirus in squirrel monkeys, which express CETP [22]. LCAT activity was increased 22-fold from the treatment, which increased HDL-C by over 100%. Furthermore, increased LCAT in squirrel monkeys also lowered LDL-C by accelerating its catabolism, which suggests that it has an anti-atherogenic effect in this non-human primate model. In another recent study, the repeated intravenous infusion of human recombinant LCAT (rLCAT) in rabbits over several months was found to markedly reduce atherosclerosis [23] similar to what was observed in LCAT transgenic rabbits [15]. Furthermore, the overall flux of cholesterol was also monitored by an isotope dilutions study, which is a new method for the in vivo monitoring of RCT [24]. Using this technique, rLCAT treatment stimulated the flux of cholesterol from peripheral tissues to the plasma compartment and led to an increase in fecal sterol excretion [23]. However, a similar study in mice recently found a seemingly different result [25]. Macrophages were radiolabeled ex vivo with cholesterol and then implanted into the peritoneal space into various mice. LCAT-null mice were found to have less excretion of the radiolabeled cholesterol in the feces, but over-expression of LCAT with adeno-associated virus in normal mice did not cause a further increase in fecal excretion of the tracer over that observed in untreated mice. The same results were found with CETP transgenic mice. This suggests that LCAT may not be normally rate limiting for RCT, at least in mice. The different outcome from the rabbit study may be that the isotope dilution method used in the rabbit study measures the global flux of cholesterol from all tissues, whereas the experiment done in mice only monitored flux from macrophages, which contain only a small pool of cholesterol, although perhaps the most relevant in regard to atherosclerosis. Because macrophages are known to primarily rely on the ABCA1 transporter for cholesterol efflux, it may be that over-expression of LCAT by esterifying cholesterol decreased the levels of pre-β HDL levels available for efflux by the ABCA1 transporter. The increase of HDL observed in mice with the treatment, however, would likely increase cholesterol efflux by ABCG1 [26] and by passive diffusion in other tissues or cell types besides macrophages, which would have been detected by the isotope dilution study done in the rabbit study.
Plasma LCAT Biomarker Studies in Human Subjects
Several interesting recent studies have examined the relationship between LCAT and cardiovascular disease. In a study of subjects with both high and low HDL-C and cardiovascular disease matched to control groups with the same levels of HDL, the best diagnostic markers were low LCAT activity and high pre-β HDL levels [27••]. Although pre-β HDL is a good ligand for removing excess cholesterol from cells, particularly by the ABCA1 transporter, its accumulation in subjects with cardiovascular disease may suggest that there is an aberration in the RCT pathway preventing the maturation of nascent HDL. One potential factor found in this study was low LCAT activity. Interestingly, although no relationship was observed between LCAT and pre-β HDL levels in healthy subjects, a significant negative correlation was found between LCAT and pre-β HDL in cardiovascular disease patients, which suggests that LCAT is somehow rate limiting for HDL maturation in the presence of disease. The results from this study nicely compare to the many previous studies done by Frohlich and Dobiasova [28] that indirectly measured LCAT activity by the cholesterol esterification rate of plasma, a negative risk biomarker of cardiovascular disease.
However, another recent study found a positive association between an approximately 10% increase in LCAT mass with carotid thickness in patients with metabolic syndrome [29]. LCAT activity levels have also recently been found to be positively correlated with the level of C-reactive protein (CRP) [30]. It is important to note, however, that a causal role of LCAT in the pathogenesis of atherosclerosis cannot be inferred from these types of association studies. An increase in LCAT in patients with cardiovascular disease could be a positive compensatory response or may not be related mechanistically in any way with the protection or development of cardiovascular disease.
Human Imaging Studies on the Role of LCAT in Atherosclerosis
Several recent imaging studies involving the measurement of the intima-media thickness (IMT) of carotid vessels with ultrasound have been used to examine whether LCAT is either anti- or pro-atherogenic (Table 2). Hovingh et al. [31] assessed carotid IMT in nine homozygotes and 47 heterozygotes for LCAT mutations from five Dutch FED families. Heterozygotes had on average a 36% decrease in HDL-C levels, a 23% increase in triglyceride, and a 2.1-fold increase in C-reactive protein (CRP) levels. Mean carotid IMT was 0.623 mm in heterozygotes versus 0.591 mm for control subjects (P<0.005), suggesting that heterozygotes for LCAT are at a higher risk of atherosclerosis. Homozygotes had greater carotid IMT than heterozygotes (0.73 mm vs 0.623 mm) but it did not reach statistical significance because of the small number of homozygous subjects and the different age distribution (mean age of 60 vs 42 years). Further analysis of the carotid thickness was recently reported with a more sensitive and precise technique, involving a 3.0 Tesla MRI in 41 heterozygotes from the Dutch kindred [32]. Carriers clearly had an increased carotid mean wall area (P=0.01) and normalized wall index (P<0.01), which confirms the earlier finding and further supports the anti-atherogenic role of LCAT. In a small study from a Canadian FLD kindred, Ayyobi et al. [33] reported six of eight heterozygotes for LCAT mutations had IMT abnormalities, with four having distinct carotid plaques. Interestingly, the two homozygotes had no plaques and only minimal increases in IMT over time.
Table 2.
Imaging studies of LCAT deficiency
| Study | Year | Study design | Key findings |
|---|---|---|---|
| Hovingh et al. [31] | 2005 | IMT in 9 homozygous and 47 heterozygous FED subjects |
Subjects heterozygous for FED are at higher risk of atherosclerosis |
| Hollenboom et al. [32] | 2010 | MRI of carotid mean wall area in 41 heterozygous subjects from Hovingh et al. [31] trial |
Carriers had increased carotid mean wall area compared to controls |
| Ayyobi et al. [33] | 2004 | IMT in 8 subjects heterozygous for LCAT mutations | Eight heterozygous subjects had IMT abnormalities, 4 had plaques |
| Calabresi et al. [34] | 2009 | IMT, LCAT concentration, and activity in cells | LCAT deficiency did not correlate with atherosclerosis, gene-dose effect observed |
FED fish eye disease, IMT intima-media thickness, LCAT lecithin cholesterol acyl transferase
Another recent IMT study challenged the notion that LCAT was required for atheroprotection. Calabresi et al. [34] measured carotid IMT, maximum IMT, and LCAT activity in 40 carriers of LCAT mutants (12 with two and 28 with one mutant allele) versus 80 controls. LCAT deficiency was not found to be associated with increased atherosclerosis and instead was inversely correlated with IMT (0.07 mm less in carriers vs controls; P=0.0003). Furthermore, by correlating the effect of the number of copies of mutant alleles and activity, a gene-dose effect was suggested.
At this time there is no clear explanation for the seeming contradictory results of the carotid imaging studies, although the most recent MRI study, because of its increased sensitivity, should perhaps be given more credence. Ascertainment bias from the way the patients were recruited in the study or perhaps other differences, such as body mass index, diet, and other cardiovascular risk factors, could possibly have accounted for the different outcomes. The small number of subjects, particularly for homozygous FLD and FED, also contributes to the uncertainty of the overall results. A similar powered study of familial hypercholesterolemia, however, would have likely shown a clear increase in IMT and cardiovascular disease risk. Therefore, a relatively safe conclusion that can be made at this time is that the overall protective effect of LCAT on the development of atherosclerosis is probably small. As already discussed, this may be caused by not only lower HDL-C in homozygous LCAT deficiency, but also lower LDL-C, the main driver for the initiation of atherosclerosis. It is for this reason that it has been proposed that homozygous LCAT-deficient patients may be more protective from atherosclerosis than heterozygotes because of their lower LDL-C levels [1, 33]. As found in the animal studies, the anti- or pro-atherogenic role of LCAT may also be very context dependent and may vary depending on other factors that modulate lipoprotein metabolism and atherogenic risk. It has also been proposed that one product of the LCAT reaction, namely lysophosphatidylcholine, which increases the atherogenicity of lipoproteins [35], may mitigate the beneficial effects of LCAT on the RCT pathway.
LCAT Drug Development
Although a clear role of LCAT as an anti- or pro-atherogenic factor has not yet been definitively established, several drug development efforts have recently been started for modulating LCAT activity. One treatment approach that is being investigated is the use of rLCAT as a therapy for FLD, with the main goal of preventing or reversing renal disease [36•]. This would be analogous to the enzyme replacement therapies that are used to treat various lysosomal storage diseases, such as Gaucher’s disease and Fabry’s disease [37]. Early reports of patients with FLD that received plasma from transfusions have revealed that LCAT has a remarkably long half-life in the circulation, somewhere between 5 and 6 days [38, 39]. The ability of LCAT to associate with HDL probably accounts for its long half-life. These early reports also showed that a relatively small amount of LCAT, less than 10% to 20% of normal, was enough to restore FLD subjects to a normal lipoprotein profile [38, 39]. Infusion of LCAT from plasma in these patients was found to raise HDL-C, increase apoA-I, and restore the ratio of cholesteryl esters to total cholesterol to near normal levels for several days. The fact that FLD patients with partial deficiency of LCAT only develop corneal opacities and not the other manifestations of complete LCAT deficiency [40] also suggests that partial replacement of LCAT in FLD subjects may be enough to prevent to renal disease. The fact that LCAT is a small, single subunit protein of relatively low concentration (5 µg/mL) that acts in the plasma compartment also makes it a good candidate for recombinant enzyme replacement therapy.
A pre-clinical mouse study of human rLCAT was recently reported [36•]. High expression clones secreting rLCAT have been produced in both 293 and CHO cells by AlphaCore Pharma (Ann Arbor, MI). rLCAT injected into LCAT-null mice and was found to reverse their abnormal lipoprotein profile by increasing HDL-C to near normal levels for several days. Interestingly, rLCAT was also found to transform human Lp-X–like particles to HDL-like particles after ex vivo treatment. rLCAT was also found to be equally effective when injected intravenously, intramuscularly, or even subcutaneously, which indicates that it may be possible for patients to self-administer rLCAT Based on the pre-clinical animal studies and plasma infusion studies in FLD subjects, a single weekly subcutaneous injection of rLCAT may be sufficient to prevent the development of renal disease in FLD. Amgen Inc. (Thousand Oaks, CA) has also produced a recombinant form of LCAT in which mutations were introduced to either further lengthen its half-life or increase its activity [23]. Another potential way to deliver LCAT was recently reported in which adipocytes transfected with LCAT were transplanted into mice and were found to raise HDL-C [41, 42•].
A more uncertain but potentially much wider indication of rLCAT would be its use in the treatment of acute coronary syndrome. Patients with unstable angina or a recent non-fatal myocardial infarction have a relatively high risk of having a fatal event in the months following their initial presentation. A new treatment approach called HDL therapy has recently been tried in these patients [43]. It involves the use of reconstituted HDL made with either recombinant apoA-I or apoA-I purified from human serum and complexed to phosphatidylcholine to produce particles similar to pre-β HDL. Short peptide mimics of apoA-I are also being investigated for producing pre-β–like HDL [44]. The rationale for producing this type of phospholipid-rich particle is that it has great capacity for removing excess cellular cholesterol, and hence when given to patients would be expected to mobilize cholesterol from atherosclerotic plaques (Fig. 1). Removal of cholesterol by HDL from specific membrane domains, such as lipid rafts, has also been shown to have a significant anti-inflammatory effect, because it causes the uncoupling of several different cell signaling receptors that affect a wide variety of functions, including cell proliferation [45•].
Based on animal studies [43], even a single infusion of reconstituted HDL has been shown to have beneficial effects on the histology of atherosclerotic plaques. Two small human clinical trials in which HDL was intravenously infused once a week for 4 to 5 weeks in patients with acute coronary syndrome found that the treatment significantly reduced atherosclerotic plaque volume compared to baseline [46], but there has been no reports yet on its impact on clinical events.
Like reconstituted HDL, rLCAT could potentially be used for the treatment of acute coronary syndrome patients, because of its ability to promote cholesterol efflux. Support for this was found in a recent study in which the intravenous infusion of human rLCAT in rabbits was found to raise HDL-C, increase fecal excretion of cholesterol, and reduce atherosclerosis [23]. Besides being used as a standalone therapy, rLCAT could also potentially be used in concert with reconstituted HDL. In human apoA-I transgenic mice, which have a modest increase in HDL-C, the infusion of rLCAT was found to markedly increase HDL-C levels [36]. This suggests that LCAT may be rate limiting when there is excess apoA-I, particularly after the rapid intravenous infusion of reconstituted HDL. This is supported by the studies in which there is a significant increase in the free cholesterol content of HDL and pre-β HDL for several hours following the infusion of reconstituted HDL made with either apoA-I or apolipoprotein mimetic peptides [43]. Co-administration of LCAT along with reconstituted HDL may, therefore, increase the rate of cholesterol esterification and lead to a more effective removal of cholesterol from peripheral cells, although this needs to be tested experimentally.
Only limited studies have been reported to date in developing small molecules to modulate LCAT levels. The proximal 71-base pair LCAT promoter is known to contain at least the five following elements: TATA box, two Sp1 binding site, one LFAI motif [1], and an interleukin-6 response element [47]. LCAT is also known to be partially up-regulated after statin treatment and down-regulated by fibrates [1]. Amgen Inc., with the use of high-throughput screening, identified a small molecule that does not induce the LCAT gene but instead increases its activity by interacting with a free sulfhydryl group near its catalytic site [48]; however, there have been no reports on its effect in animal models. A short apoA-I mimetic peptide that is a potent activator of LCAT has also been developed and was tested in early-stage clinical trials [44], but results from the study have not been published.
Conclusions
LCAT is a key enzyme in HDL metabolism and possibly potentiates RCT, but its role in human atherosclerosis has not been definitively established and is likely to be context dependent. Nevertheless, efforts to develop rLCAT for the treatment of FLD patients are underway and it may also be useful in the case of acute coronary syndrome. The previous small natural history and imaging studies of subjects with genetic deficiency of LCAT are probably inadequate for predicting the potential benefit of the infusion of supraphysiologic levels of rLCAT with or without reconstituted HDL, and this strategy will have to be first tested in animal models. New tools for the in vivo monitoring of cholesterol flux, such as isotope dilution studies [24], may also provide a means for testing the anti-atherogenic role of LCAT in human subjects.
Acknowledgments
All authors were supported by intramural NHLBI funds from the National Institutes of Health.
Footnotes
Disclosure The authors report not potential conflicts of interest relevant to this article.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Rousset X, Vaisman B, Amar M, Sethi AA, Remaley AT. Lecithin: cholesterol acyltransferase–from biochemistry to role in cardiovascular disease. Curr Opin Endocrinol Diabetes Obes. 2009;16(2):163–171. doi: 10.1097/med.0b013e328329233b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santamarina-Fojo S, Hoeg JM, Assmann G, H. Bryan Brewer J. Lecithin Cholesterol Acyltransferase Deficiency and Fish Eye Disease. In: Metabolic & Molecular Bases of Inherited Disease. 2001 [Google Scholar]
- 3.Chang T-Y, Li B-L, Chang CCY, Urano Y. Acyl-coenzyme A: cholesterol acyltransferases. Am J Physiol Endocrinol Metab. 2009;297(1):E1–E9. doi: 10.1152/ajpendo.90926.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czarnecka H, Yokoyama S. Regulation of cellular cholesterol efflux by lecithin:cholesterol acyltransferase reaction through nonspecific lipid exchange. Journal of Biological Chemistry. 1996;271(4):2023–2028. doi: 10.1074/jbc.271.4.2023. [DOI] [PubMed] [Google Scholar]
- 5.Nofer JR, Remaley A. Tangier disease: still more questions than answers. Cellular and Molecular Life Sciences. 2005;62(19):2150–2160. doi: 10.1007/s00018-005-5125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yvan-Charvet L, Kling J, Pagler T, et al. Cholesterol efflux potential and antiinflammatory properties of high-density lipoprotein after treatment with niacin or anacetrapib. Arterioscl Throm Vas. 2010;30(7):U1430–U1405. doi: 10.1161/ATVBAHA.110.207142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishiwaki M, Ikewaki K, Bader G, et al. Human lecithin: cholesterol acyltransferase deficiency: in vivo kinetics of low-density lipoprotein and lipoprotein-X. Arterioscler Thromb Vasc Biol. 2006;26(6):1370–1375. doi: 10.1161/01.ATV.0000217910.90210.99. [DOI] [PubMed] [Google Scholar]
- 8.Lynn EG, Choy PC, Magil A. O K: uptake and metabolism of lipoprotein-X in mesangial cells. Mol Cell Biochem. 1997;175(1–2):187–194. doi: 10.1023/a:1006865420286. [DOI] [PubMed] [Google Scholar]
- 9.Rader DJ. Lecithin: cholesterol acyltransferase and atherosclerosis: another high-density lipoprotein story that doesn’t quite follow the script. Circulation. 2009;120(7):549–552. doi: 10.1161/CIRCULATIONAHA.109.881979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greeve J, Altkemper I, Dieterich JH, Greten H, Windler E. Apolipoprotein B mRNA editing in 12 different mammalian species: hepatic expression is reflected in low concentrations of apoB-containing plasma lipoproteins. J Lipid Res. 1993;34(8):1367–1383. [PubMed] [Google Scholar]
- 11.Paigen B, Ishida BY, Verstuyft J, Winters RB, Albee D. Atherosclerosis susceptibility differences among progenitors of recombinant inbred strains of mice. Arteriosclerosis. 1990;10(2):316–323. doi: 10.1161/01.atv.10.2.316. [DOI] [PubMed] [Google Scholar]
- 12.Berard AM, Foger B, Remaley A, et al. High plasma HDL concentrations associated with enhanced atherosclerosis in transgenic mice overexpressing lecithin-cholesteryl acyltransferase. Nat Med. 1997;3(7):744–749. doi: 10.1038/nm0797-744. [DOI] [PubMed] [Google Scholar]
- 13.Foger B, Chase M, Amar MJ, et al. Cholesteryl ester transfer protein corrects dysfunctional high density lipoproteins and reduces aortic atherosclerosis in lecithin cholesterol acyltransferase transgenic mice. J Biol Chem. 1999;274(52):36912–36920. doi: 10.1074/jbc.274.52.36912. [DOI] [PubMed] [Google Scholar]
- 14.Furbee JW, Jr, Parks JS. Transgenic overexpression of human lecithin: cholesterol acyltransferase (LCAT) in mice does not increase aortic cholesterol deposition. Atherosclerosis. 2002;165(1):89–100. doi: 10.1016/s0021-9150(02)00201-0. [DOI] [PubMed] [Google Scholar]
- 15.Hoeg JM, Vaisman BL, Demosky SJ, Jr, et al. Lecithin: cholesterol acyltransferase overexpression generates hyperalpha-lipoproteinemia and a nonatherogenic lipoprotein pattern in transgenic rabbits. J Biol Chem. 1996;271(8):4396–4402. doi: 10.1074/jbc.271.8.4396. [DOI] [PubMed] [Google Scholar]
- 16.Brousseau ME, Hoeg JM. Transgenic rabbits as models for atherosclerosis research. J Lipid Res. 1999;40(3):365–375. [PubMed] [Google Scholar]
- 17.Brousseau ME, Santamarina-Fojo S, Vaisman BL, et al. Over-expression of human lecithin:cholesterol acyltransferase in cholesterol-fed rabbits: LDL metabolism and HDL metabolism are affected in a gene dose-dependent manner. J Lipid Res. 1997;38(12):2537–2547. [PubMed] [Google Scholar]
- 18.Sakai N, Vaisman BL, Koch CA, et al. Targeted disruption of the mouse lecithin:cholesterol acyltransferase (LCAT) gene. Generation of a new animal model for human LCAT deficiency. J Biol Chem. 1997;272(11):7506–7510. doi: 10.1074/jbc.272.11.7506. [DOI] [PubMed] [Google Scholar]
- 19.Lambert G, Sakai N, Vaisman BL, et al. Analysis of glomerulosclerosis and atherosclerosis in lecithin cholesterol acyltransferase-deficient mice. J Biol Chem. 2001;276(18):15090–15098. doi: 10.1074/jbc.M008466200. [DOI] [PubMed] [Google Scholar]
- 20.Ng DS, Maguire GF, Wylie J, et al. Oxidative stress is markedly elevated in lecithin:cholesterol acyltransferase-deficient mice and is paradoxically reversed in the apolipoprotein E knockout background in association with a reduction in atherosclerosis. J Biol Chem. 2002;277(14):11715–11720. doi: 10.1074/jbc.M112320200. [DOI] [PubMed] [Google Scholar]
- 21.Furbee JW, Jr, Sawyer JK, Parks JS. Lecithin: cholesterol acyltransferase deficiency increases atherosclerosis in the low density lipoprotein receptor and apolipoprotein E knockout mice. J Biol Chem. 2002;277(5):3511–3519. doi: 10.1074/jbc.M109883200. [DOI] [PubMed] [Google Scholar]
- 22.Amar MJ, Shamburek RD, Vaisman B, et al. Adenoviral expression of human lecithin-cholesterol acyltransferase in non-human primates leads to an antiatherogenic lipoprotein phenotype by increasing high-density lipoprotein and lowering low-density lipoprotein. Metabolism. 2009;58(4):568–575. doi: 10.1016/j.metabol.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou M, Sawyer J, Kelley K, et al. Abstract 5920: Lecithin Cholesterol Acyltransferase Promotes Reverse Cholesterol Transport and Attenuates Atherosclerosis Progression in New Zealand White Rabbits. Circulation. 2009;120 (18_MeetingAbstracts): S1 175-b- [Google Scholar]
- 24.Amar MJ, D'Souza W, Turner S, et al. 5A apolipoprotein mimetic peptide promotes cholesterol efflux and reduces atherosclerosis in mice. J Pharmacol Exp Ther. 2010;334(2):634–641. doi: 10.1124/jpet.110.167890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanigawa H, Billheimer JT, Tohyama J, et al. Lecithin: cholesterol acyltransferase expression has minimal effects on macrophage reverse cholesterol transport in vivo. Circulation. 2009;120(2):160–169. doi: 10.1161/CIRCULATIONAHA.108.825109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Collins HL, Ranalletta M, et al. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J Clin Invest. 2007;117(8):2216–2224. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sethi AA, Sampson M, Warnick R, et al. High pre-beta1 HDL concentrations and low lecithin: cholesterol acyltransferase activities are strong positive risk markers for ischemic heart disease and independent of HDL-cholesterol. Clin Chem. 2010;56(7):1128–1137. doi: 10.1373/clinchem.2009.139931. ••This work demonstrates that LCAT and pre-β are good biomarkers for cardiovascular disease
- 28.Frohlich J, Dobiasova M. Fractional esterification rate of cholesterol and ratio of triglycerides to HDL-cholesterol are powerful predictors of positive findings on coronary angiography. Clin Chem. 2003;49(11):1873–1880. doi: 10.1373/clinchem.2003.022558. [DOI] [PubMed] [Google Scholar]
- 29.Dullaart RP, Perton F, Sluiter WJ, de Vries R, van Tol A. Plasma lecithin: cholesterol acyltransferase activity is elevated in metabolic syndrome and is an independent marker of increased carotid artery intima media thickness. J Clin Endocrinol Metab. 2008;93(12):4860–4866. doi: 10.1210/jc.2008-1213. [DOI] [PubMed] [Google Scholar]
- 30.Dullaart RP, Perton F, Kappelle PJ, de Vries R, Sluiter WJ, van Tol A. Plasma lecithin: cholesterol acyltransferase activity modifies the inverse relationship of C-reactive protein with HDL cholesterol in nondiabetic men. Biochim Biophys Acta. 2010;1801(1):84–88. doi: 10.1016/j.bbalip.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 31.Hovingh GK, Hutten BA, Holleboom AG, et al. Compromised LCAT function is associated with increased atherosclerosis. Circulation. 2005;112(6):879–884. doi: 10.1161/CIRCULATIONAHA.105.540427. [DOI] [PubMed] [Google Scholar]
- 32.Holleboom AG, Duivenvoorden R, van den Bogaard B, et al. Carriers of Lcat Gene Mutations Have Increased Atherosclerosis: A 3.0 Tesla Mri Study. Atherosclerosis Supp. 2010;11(2):61–61. [Google Scholar]
- 33.Ayyobi AF, McGladdery SH, Chan S, John Mancini GB, Hill JS, Frohlich JJ. Lecithin: cholesterol acyltransferase (LCAT) deficiency and risk of vascular disease: 25 year follow-up. Atherosclerosis. 2004;177(2):361–366. doi: 10.1016/j.atherosclerosis.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 34.Calabresi L, Baldassarre D, Castelnuovo S, et al. Functional lecithin: cholesterol acyltransferase is not required for efficient atheroprotection in humans. Circulation. 2009;120(7):628–635. doi: 10.1161/CIRCULATIONAHA.108.818143. [DOI] [PubMed] [Google Scholar]
- 35.Wells IC, Peitzmeier G, Vincent JK. Lecithin: cholesterol acyltransferase and lysolecithin in coronary atherosclerosis. Exp Mol Pathol. 1986;45(3):303–310. doi: 10.1016/0014-4800(86)90019-5. [DOI] [PubMed] [Google Scholar]
- 36.Rousset X, Vaisman B, Auerbach B, et al. Effect of Recombinant Human Lecithin-cholesterol:acyltransferase Infusion on Lipoprotein Metabolism in Mice. J Pharmacol Exp Ther. 2010 doi: 10.1124/jpet.110.169540. •This work demonstates the feasibility of LCAT replacement therapy in the case of LCAT deficiency
- 37.Brady RO. Enzyme replacement for lysosomal diseases. Annu Rev Med. 2006;57:283–296. doi: 10.1146/annurev.med.57.110104.115650. [DOI] [PubMed] [Google Scholar]
- 38.Norum KR, Gjone E. The effect of plasma transfusion on the plasma cholesterol esters in patients with familial plasma lecithin: cholesterol acyltransferase deficiency. Scand J Clin Lab Invest. 1968;22(4):339–342. doi: 10.3109/00365516809167071. [DOI] [PubMed] [Google Scholar]
- 39.Murayama N, Asano Y, Kato K, et al. Effects of plasma infusion on plasma lipids, apoproteins and plasma enzyme activities in familial lecithin: cholesterol acyltransferase deficiency. Eur J Clin Invest. 1984;14(2):122–129. doi: 10.1111/j.1365-2362.1984.tb02100.x. [DOI] [PubMed] [Google Scholar]
- 40.Klein HG, Santamarina-Fojo S, Duverger N, et al. Fish eye syndrome: a molecular defect in the lecithin-cholesterol acyltransferase (LCAT) gene associated with normal alpha-LCAT-specific activity. Implications for classification and prognosis. The Journal of Clinical Investigation. 1993;92(1):479–485. doi: 10.1172/JCI116591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asada S, Kuroda M, Aoyagi Y, et al. Disturbed apolipoprotein AI-containing lipoproteins in fish-eye disease are improved by the lecithin:cholesterol acyltransferase produced by gene-transduced adipocytes in vitro. Molecular Genetics and Metabolism. doi: 10.1016/j.ymgme.2010.10.009. In Press. [DOI] [PubMed] [Google Scholar]
- 42.Kuroda M, Aoyagi Y, Asada S, et al. Ceiling culture-derived proliferative adipocytes are a possible delivery vehicle for enzyme replacement therapy in lecithin:cholesterol acyltransferase deficiency. Gene Ther Mol Biol. In press. •This article presents an alternative to LCAT injections for a treatment of the human LCAT deficiency
- 43.Remaley AT, Amar M, Sviridov D. HDL-replacement therapy: mechanism of action, types of agents and potential clinical indications. Expert Rev Cardiovasc Ther. 2008;6(9):1203–1215. doi: 10.1586/14779072.6.9.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sethi AA, Amar M, Shamburek RD, Remaley AT. Apolipoprotein AI mimetic peptides: possible new agents for the treatment of atherosclerosis. Curr Opin Investig Drugs. 2007;8(3):201–212. [PubMed] [Google Scholar]
- 45.Yvan-Charvet L, Pagler T, Gautier EL, et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328(5986):1689–1693. doi: 10.1126/science.1189731. •This article presents new data on the interaction of HDL metabolism and blood cells
- 46.Tardif JC, Heinonen T, Noble S. High-density lipoprotein/ apolipoprotein A-I infusion therapy. Curr Atheroscler Rep. 2009;11(1):58–63. doi: 10.1007/s11883-009-0009-7. [DOI] [PubMed] [Google Scholar]
- 47.Feister HA, Auerbach BJ, Cole LA, Krause BR, Karathanasis SK. Identification of an IL-6 response element in the human LCAT promoter. Journal of Lipid Research. 2002;43(6):960–970. [PubMed] [Google Scholar]
- 48.Zhou M, Fordstrom P, Zhang J, et al. Novel small molecule LCAT activators raise HDL levels in rodent models. Arterioscl Throm Vas. 2008;28(6):E65–E66. [Google Scholar]
- 49.Vaisman BL, Klein HG, Rouis M, et al. Overexpression of human lecithin cholesterol acyltransferase leads to hyperalphalipoproteinemia in transgenic mice. J Biol Chem. 1995;270(20):12269–12275. doi: 10.1074/jbc.270.20.12269. [DOI] [PubMed] [Google Scholar]
- 50.Mehlum A, Staels B, Duverger N, et al. Tissue-specific expression of the human gene for lecithin: cholesterol acyltransferase in transgenic mice alters blood lipids, lipoproteins and lipases towards a less atherogenic profile. Eur J Biochem. 1995;230(2):567–575. doi: 10.1111/j.1432-1033.1995.tb20597.x. [DOI] [PubMed] [Google Scholar]
- 51.Van Craeyveld E, Lievens J, Jacobs F, Feng Y, Snoeys J, De Geest B. Apolipoprotein A-I and lecithin: cholesterol acyltransferase transfer induce cholesterol unloading in complex atherosclerotic lesions. Gene Ther. 2009;16(6):757–765. doi: 10.1038/gt.2009.8. [DOI] [PubMed] [Google Scholar]