Abstract
Allografts remain the clinical “gold standard” for treatment of critical sized bone defects despite minimal engraftment and ~60% long-term failure rates. Therefore, the development of strategies to improve allograft healing and integration are necessary. The periosteum and its associated stem cell population, which are lacking in allografts, coordinate autograft healing. Herein we utilized hydrolytically degradable hydrogels to transplant and localize mesenchymal stem cells (MSCs) to allograft surfaces, creating a periosteum mimetic, termed a ‘tissue engineered periosteum’. Our results demonstrated that this tissue engineering approach resulted in increased graft vascularization (~2.4-fold), endochondral bone formation (~2.8-fold), and biomechanical strength (1.8-fold), as compared to untreated allografts, over 16 weeks of healing. Despite this enhancement in healing, the process of endochondral ossification was delayed compared to autografts, requiring further modifications for this approach to be clinically acceptable. However, this bottom-up biomaterials approach, the engineered periosteum, can be augmented with alternative cell types, matrix cues, growth factors, and/or other small molecule drugs to expedite the process of ossification.
Keywords: bone allografts, periosteum, mesenchymal stem cells, hydrogels, regenerative medicine, tissue engineering
1. Introduction
Critical-sized bone defects are very prevalent and result from skeletal defects, traumatic injuries, and tumor resections [1]. Clinically, >500,000 and >2.2 million bone graft procedures are performed annually in the United States and worldwide, respectively [2, 3]. Reliable and effective techniques for bone processing and infectious disease detection has resulted in allografts becoming the clinical “gold standard” for treatment of critical sized bone defects [3–5]. Unlike autografts, which can be harvested in small volumes from non-load bearing regions of the skeleton, processed cadaveric allografts are readily available and fill the need for large volumes of graft material [3]. Additionally, allografts obviate donor site pain and morbidity, complications often resulting from autograft harvesting [6], and are mechanically superior to alternatives such as morselized bone graft materials, providing a structural advantage in vivo [3]. Despite these attributes, allografts exhibit minimal engraftment and a 60%, 10-year post-implantation failure rate due to fibrotic nonunions (~17%), infections (~8%), and microcrack propagation resulting in secondary fractures (~35%) [7–9].
In contrast to allografts, autografts completely heal, orchestrated by the periosteum, a thin layer of tissue covering the outer surface of bone that is comprised of an inner osteogenic cambrial layer and an outer fibrous layer [4, 5, 10–15]. The cambrial layer houses committed osteoblasts, osteogenic precursors, and periosteal stem cells. The periosteal stem cell is critical for endochondral bone formation in cortical bone healing [16]. However, the role of these cells remains unclear, as they may contribute to healing through a myriad of functions, including proliferation, chondrogenic differentiation, and endochondral ossification, and/or through the release of paracrine signals resulting in recruitment and activation of host osteoprogenitor cells [4, 5, 12–15, 17–22]. Nevertheless, recent literature has demonstrated that intact periosteal tissue, which is lacking in processed allografts, is vital towards vascularization, bone callus formation, and subsequent healing and remodeling of autografts in the context of critical sized defect repair [4, 5, 10–12]. Using a murine defect model wherein a 5 mm segment of femur was resected and replaced with an autologous bone graft, the periosteum has been found to account for greater than 70% of new bone formation during healing [5, 23]. In addition, removal of the periosteum from autografts has been shown to result in a 63% reduction in new bone formation [4, 5, 10].
Many attempts to emulate the healing orchestrated by the periosteum have exploited cell and/or growth factor delivery [4, 5, 14, 15, 19, 20, 23–26]. Towards growth factor mediated healing, delivery of bone morphogenetic protein 2 (BMP-2) [4, 5, 22, 26], basic fibroblast growth factor (FGF-2) [17, 24], parathyroid hormone (PTH) [27] and its peptide fragment teriparatide (PTH1–34) [28], and vascular endothelial growth factor (VEGF) have been most commonly employed [19, 20, 25, 29]. While these approaches have resulted in variable outcomes with respect to allograft revitalization, to date none have matched the success of autograft healing. Furthermore, growth factor delivery is plagued by a host of complications including immunogenic concerns and diffusion and/or degradation of growth factors, which requires delivery of supraphysiologic concentrations, leading to costly clinical translation, and potential off-target pathway activation [20, 27, 30, 31]. Therefore, Food and Drug Administration approval for such approaches remains a significant hurdle [31–33].
Delivery of cells alone or in combination with the aforementioned growth factors is a common approach to improve allograft remodeling. Mesenchymal stem cells (MSCs) are commonly employed as they have been shown to be similar to periosteum stem cells [16, 18, 34–38], can be easily harvested in a patient specific manner, and are non-immunogenic, making their utilization in both allogeneic and autogeneic applications feasible [37]. Numerous preclinical models have demonstrated MSC therapeutic efficacy and regenerative capacity in a variety of musculoskeletal tissues [4, 39–41]. Towards emulation of periosteum function, direct delivery of MSCs in the absence of a biomaterial results in negligible improvements in allograft healing [4, 5, 21]. Without carriers, MSCs exhibit poor graft localization, extensive migration into surrounding tissue, and limited cell survival [4, 5, 21]. To overcome these complications, numerous biomaterials have been investigated as periosteum mimetics. These include naturally derived acellular matricies, such as dermis and intestinal mucosa [4, 21], commercially available collagen-based sponges [5], and synthetic polymers such as poly(lactide-co-glycolide) [29]. While these materials have been shown to improve cell localization to allograft surfaces, they suffer from irreproducible cell seeding and inadequate cell survival [4, 5, 21]. In addition, common scaffolds are poorly hydrated and are not easily modified biochemically or biomechanically to mimic periosteum characteristics [29, 41–44].
Use of cell transplantation, growth factor delivery, and combinations thereof have improved allograft healing. However, they fail to yield adequate chondrocyte differentiation and endochondral ossification within transplanted cell populations, resulting in insufficient healing as compared to autograft controls [4, 5, 19, 21]. In contrast to traditional scaffold materials poly(ethylene glycol) (PEG) hydrogels emulate the mechanical properties and hydration of the native extracellular matrix environment, making them ideal for many tissue engineering applications [41, 43, 45–47]. In addition, PEG hydrogels are easily modified to allow for degradation and inclusion of biomolecules and other cell-adhesion ligands to promote specific cell function [41, 43, 45–47].
In this work, we developed PEG hydrogels, which were designed to have consistent hydration, elastic properties, and provide similar cellular persistence as the periosteum, to transplant and localize MSCs to allograft surfaces. The resulting tissue engineered (T.E.) periosteum only provided signals for cell survival, acting as a ‘blank slate’ to assess MSC-mediated allograft healing. Using live animal fluorescent imaging, micro-computed tomography analysis of vascular and bone callus volume, histological staining, and biomechanical testing, T.E. periosteum-mediated healing of allografts was compared to autografts and untreated allografts, respectively, over 16 weeks.
2. Materials and Methods
All materials were purchased from Sigma-Aldrich unless otherwise specified.
2.1 Synthesis of Poly(ethylene glycol) (PEG) Macromolecular Monomers (Macromers)
Hydrolytically-Degradable PEG Macromers
Degradable, PEG-based tri-block copolymers [methacrylate-poly(lactide)-b-PEG-b-poly(lactide)-methacrylate] (PEGPLADM, Fig. S1A), were synthesized as previously described by functionalizing linear PEG (Alfa Aesar, MW 10 kDa, n=227) with d,l-lactide and performing microwave-assisted methacrylation [48–50]. To determine the number of lactide units and methacrylate functional groups per PEG macromer 1 H-NMR analysis was used (Bruker Avance 400 MHz, CDCl3).
Synthesis of Acrylate-PEG-RGDS
The cell adhesive sequence Arg-Gly-Asp-Ser (RGDS; 433 Da, EMD Chemicals, San Diego CA) was coupled to acrylate-PEG-N-Hydroxysuccinimide (MW 3500 Da, Jenkem Technology, Beijing China) through the amino terminus, as previously described, and allowed for tethering into hydrogels [49]. The product (acrylate-PEG-RGDS, Fig. S1B) was dialyzed against deionized water (molecular weight cutoff = 1000 Da, Spectrum Labs, Rancho Dominguez CA), lyophilized, analyzed via matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF, Bruker AutoFlex III SmartBeam) (solvent: 50% acetonitrile in H2O + 0.1% TFA; matrix: α-cyano-4-hydroxy cinnamic acid (TCI Europe); calibrant: Peptide Calibration Standard (Brucker)) (m/z Na+, 4070 Da), and stored at 4 °C.
2.2 Cell Culture
Mouse MSCs expressing green fluorescent protein (GFP+ mMSCs) isolated from GFP transgenic mice (C57BL/6-Tg(UBC-GFP)30Scha/J) were obtained from the mesenchymal stem cell distribution center at Texas A&M (passage 6) [51]. GFP+ mMSCs were grown at 37 °C and 5% CO2 in growth media consisting of Iscove’s Modified Dulbecco’s Medium (IMDM, Gibco) supplemented with 10% fetal bovine serum, 10% horse serum (Atlanta Biologicals, Lawrenceville, GA, USA), 100 units/ml penicillin (Lonza), 100 µg/ml streptomycin (Lonza), and 0.25 µg/ml amphotericin B (Lonza). GFP+ mMSCs were used prior to passage 10.
2.3 Bone Graft Preparation and Transplantation
Mouse Strains
Female 6–8 week old C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME). Allogeneic bone grafts for implantation into C57BL/6 mice were obtained from freshly euthanized, age-matched wild-type BALB/c mice received from various research groups within the University of Rochester Medical Center.
Murine Segmental Femoral Graft Model
In vivo healing of bone grafts was assessed using a previously established murine segmental femoral graft model [4, 5, 23]. Briefly, 6–8 week old C57BL/6 mice were anesthetized using a combination of ketamine and xylazine (60 mg/kg and 4 mg/kg, respectively) administered via intraperitoneal injections. An 8 mm long incision was made, and blunt dissection of muscle was used to expose the mid-shaft femur. A 5 mm mid-diaphyseal segment was removed from the femur using a Dremel with a diamond blade attachment. A 5 mm cortical bone graft (autografts, allografts, or T.E. periosteum modified allografts) was transplanted into the femur defect and stabilized using a 22-gauge intramedullary pin. For live bone autograft transplantation, the graft was carefully dissected without compromising the periosteum, and immediately transplanted back into the same mouse. For devitalized bone graft transplantation, the grafting procedure was performed between mice with genetically different backgrounds. Briefly, allografts were scraped to physically remove periosteal tissue, flushed repeatedly with phosphate buffered saline (PBS) to remove marrow, sterilized with 70% ethanol, rinsed in PBS to remove residual ethanol, and flash frozen at −80 °C for at least 1 week prior to transplantation. It should be noted that all animal surgery procedures were performed under protocols approved by University Committee of Animal Resources (UCAR).
Photoencapsulation of GFP+ mMSCs in PEG Hydrogels Around Decellularized Allografts (e.g., Tissue Engineered Periosteum)
A 10 wt% solution of PEGPLADM was prepared in PBS with 2.0 mM acrylate-PEG-RGDS to maintain MSC viability through integrin interactions [42, 45, 52, 53]. The photoinitiator lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) was synthesized as previously described [54], and added at a final concentration of 0.05 wt%. Trypsinized MSCs were added to the PEG macromer solution to achieve a final concentration of 25 million cells/mL. As previously described [11], a custom mold was used to form T.E. periosteum modified allografts. Briefly, 20 µL of PEG/cell solution was pipetted into cylindrical molds containing allografts and exposed to long-wavelength 365 nm light (5 mW/cm2 intensity) for 10 min at room temperature to form uniform PEG/cell hydrogel coatings with >95% cell viability [42, 44].
2.4 Analysis of In Vivo Bone Graft Healing
Tracking MSC Localization via Live Animal Imaging
Transplanted MSC localization to allograft surfaces was followed using an IVIS Live Animal Imaging System (Caliper Life Sciences Inc, Hopkinton, MA). T.E. periosteum modified allografts containing GFP+ mMSCs were implanted into murine femur defects and periodically assessed using fluorescent imaging (IVIS). MSC localization was quantified using fluorescent intensity, defined as relative fluorescent units (RFU). GFP+ mMSCs seeded directly on allografts (no PEG, 1×106 cells/graft) were used as a positive control.
Histological Staining and Histomorphometric Analysis
Femur grafts were harvested, fixed in 4% neutral buffered formalin (NBF) for 3 days, washed with PBS, and decalcified in 10% ethylenediaminetetraacetic acid (EDTA) for 14 days. After complete decalcification, samples were embedded in paraffin, sectioned at 5 µm, and stained with alcian blue (blue, glycosaminoglycans/proteoglycans) and orange G (pink, bone/soft tissue). At least three nonconsecutive sections were prepared from each specimen and imaged at 40-times magnification using an Olympus VS110 Virtual Microscopy System for whole-slide scanning. Subsequently, histomorphometric analysis of bridging callus formation was carried out using Visiopharm software (Visiopharm, Denmark). Briefly, a 7 mm region of interest (ROI) was selected spanning the entire graft with 1 mm of host overlap proximally and distally. The graft itself was removed from the selected ROI and only bridging callus on the surface of the graft and host bone was quantified. Mesenchyme, cartilage, and woven bone area were reported based on color threshold intensities and means of 3 nonconsecutive sections were averaged and used to represent area values for each graft.
Immunohistochemical Labeling
To visualize GFP expression from transplanted GFP+ mMSCs, 5 µm sections of paraffin embedded grafted femurs were immunostained using a polyclonal anti-GFP antibody (#ab6673, Abcam Inc., Cambridge, MA). Briefly, slides were deparaffinized, quenched of endogenous peroxidase activity (DAKO Dual Endogenous Enzyme Blocking Reagent; DAKO S2003), and blocked with 5% normal rabbit serum (NRS) (Vectastatin ABC Kit, Vector Laboratories, Burlingame, CA). Slides were then incubated with a 1:1500 dilution of goat polyclonal anti-GFP antibody (#ab6673, Abcam Inc., Cambridge, MA) in 3% NRS in phosphate buffered saline with Tween-20 (PBST) overnight at 4 °C. After overnight incubation, slides were washed and then incubated in a 1:200 dilution of biotinylated rabbit anti-goat antibody (BA-5000, Vector Laboratories) in PBST, washed, and incubated with Vectastain ABC reagent. Slides then developed using a Vector Impact DAB Kit. Developed slides were counter-stained for 1 min using hematoxylin (Invitrogen).
Quantification of Bone Callus Volume using Micro-Computed Tomography (µCT)
Analysis of bone callus volume was preformed using a µCT imaging system (Explore; GE HealthCare) as previously described [5, 55]. Briefly, two-dimensional (2D) images were reconstructed and an appropriate threshold was selected for bone voxels. Both the threshold and volume of interest (VOI) were kept constant throughout the analysis of all femur specimens. To measure new bone callus volume, contour lines were drawn in the 2D slice images to exclude both the transplanted bone graft and the host cortical bone. New bone callus volume in a volume of interest (VOI) spanning the entire length of the transplanted bone graft and 1 mm of the proximal and distal host cortical bone was used to evaluate graft healing.
Union Ratio Calculation
The union between host callus and transplanted bone graft was calculated from µCT images as previously described [56]. For each graft the reconstructed image was split into the proximal and distal halves and the associated graft-host connectivity was calculated for each by dividing the total graft-host connected surface area for the particular half by the total graft surface area for the particular half. The union ratio for the total graft was defined as the lesser of the proximal and distal connectivity ratios.
Quantification of Vascularization using Micro-Computed Tomography
Host-mediated graft vascularization was examined using µCT analysis combined with perfusion of a radiopaque lead chromate silicon rubber compound (Microfil MV-122, Flow Tech; Carver, MA), as previously described [57]. After ketamine/xylazine administration, the thoracic cavity was opened and the inferior vena cava was severed. The vasculature was flushed with excess 0.9% normal saline containing heparin sodium (100 units/mL) using a needle inserted into the left ventricle. The specimens were then pressure fixed with excess 10% NBF and the vasculature was injected with Microfil MV-122 contrast agent. Following perfusion, the femur specimens were isolated and scanned using µCT to image both vascularization and mineralized bone. Samples were subsequently decalcified (10% EDTA, 14 days) and scanned again to image only the remaining vasculature. 2D slices were registered before and after decalcification and contour lines were drawn to isolate a VOI that only included the vasculature within or immediately adjacent to the bone graft or the graft-host junctions.
Assessment of Graft Torsional Biomechanics
The proximal and distal ends of harvested femur specimens were cemented into 6.35 mm square aluminum tubes using poly(methyl methacrylate). A custom jig was used to ensure axial alignment and the maintenance of a 7 mm gauge length for all samples. Samples were mounted on an EnduraTec TestBench system (200 N-mm torque cell; Bose Corp., Minnetonka, MN) and tested in torsion until failure to determine ultimate torques [4, 58].
2.5 Statistical Analysis
Data is presented as mean + standard error of the mean with at least five replicates averaged for each data point. Statistics were assessed with GraphPad Prism Software using two-way ANOVAs with Bonferroni post-hoc analysis or paired Student’s t-tests. A p-value of <0.05 was considered significant.
3. Results
3.1 Hydrogel-mediated MSC Transplantation to Allograft Surfaces
To control MSC transplantation to and prolong their localization at allograft surfaces, hydrolytically degradable PEG-based hydrogels were utilized [59]. In an effort to mimic the ~14 day periosteal stem cell persistence observed during autograft healing [5], the number of degradable repeat units within the hydrogel crosslinks was altered to achieve complete network degradation in ~2 weeks. These hydrogels were subsequently used to encapsulate GFP+ mMSCs around decellularized allografts, thereby creating T.E. periosteum modified allografts (Fig. 1) [11]. Allografts augmented with T.E. periosteum were implanted into murine femur segmental defects and healing was assessed at 3, 6, 9, and 16 weeks post-implantation (Fig. 2A) [4, 5, 23]. Live animal fluorescent imaging was used to monitor in vivo GFP+ mMSC localization to allograft surfaces (Fig. 2B). Subsequent quantification revealed that T.E. periosteum-mediated MSC transplantation prolonged MSC localization for ~12 days at the allograft surface, significantly longer than allografts directly seeded with GFP+ mMSCs, where cells only persisted for ~3 days. Furthermore, MSC localization followed hydrogel degradation kinetics (Fig. S2), as predicted by previously established models and illustrated by the negative exponential decay in normalized MSC allograft localization (Fig. 2C) [48, 60].
Figure 1.
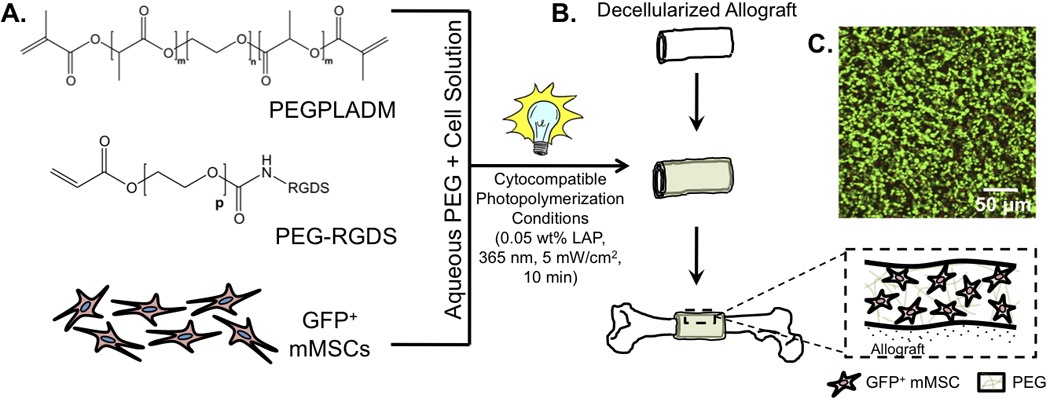
Scheme representing the tissue engineered approach to enhance allograft healing. mMSCs were added to poly(ethylene glycol) macromer solutions (A) and custom molds were used to form hydrogel-cell constructs around decellularized allografts (e.g., tissue engineered periosteum) (B). Encapsulated cells remained >95% viable as illustrated by the live/dead image (of GFP− mMSCs; calcein AM (green = live cells), ethidium homodimer (red – dead cells)) 24 hr after encapsulation (C).
Figure 2.
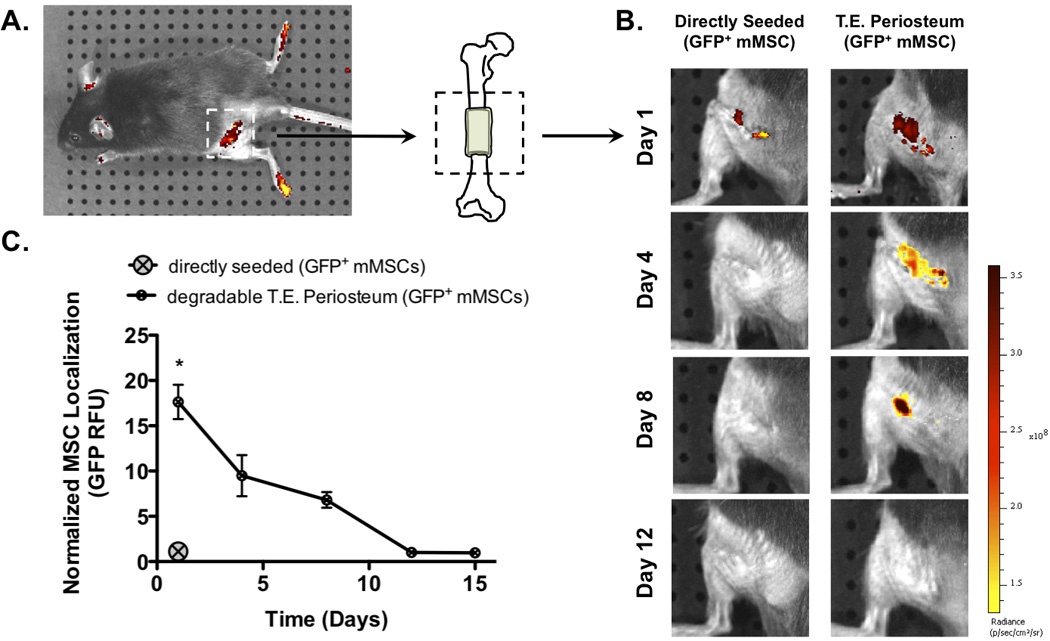
Allografts were modified with PEG hydrogels encapsulating GFP+ mMSCs and implanted into mouse femurs with 5 mm segmental defects (A). In vivo MSC persistence at the allograft surface was followed using live animal fluorescent imaging (B). Compared to control allografts directly seeded with GFP+ mMSCs, T.E. periosteum modified allografts exhibited increased normalized MSC localization through ~12 days as in agreement with hydrogel degradation kinetics (C; Fig. S2) (n=5; error bars represent standard error of the mean; p-value of <0.05 indicates significance compared to directly seeded (*)).
3.2 Tissue Engineered Allograft Vascularization
Having established that PEG hydrogels were able to enhance MSC localization to allograft surfaces as compared to traditional direct seeding methods, we sought to investigate the effect of this approach (a T.E. periosteum) on healing in a murine femur segmental defect model (Fig. 3A) [4, 5, 23]. It is well established that the progression of normal healing and remodeling in autografts is initiated by vascular infiltration, followed by recruitment and activation of host osteoclasts, and finally osteoblast-mediated new bone formation [61]. To examine the efficacy of MSC transplantation on initiating this healing cascade, host-mediated graft vascularization was assessed 3, 6, 9, and 16 weeks post-implantation using lead contrast vascular perfusion techniques [57]. Qualitative analysis of reconstructed µCT scans revealed that T.E. periosteum modified allografts resulted in increased host vascular infiltration as compared to untreated allografts (Fig. 3A). Autografts were fully vascularized 3 weeks post-implantation, exhibiting a vascular volume of 2.1 mm3 (Fig. 3B). Over the course of healing, autograft vascular volume gradually reduced as a result of graft remodeling [62, 63], exhibiting vascular volumes of 1.8, 1.5, and 1.4 mm3 at 6, 9, and 16 weeks respectively. Compared to autografts, untreated allografts exhibited a 12.5- (0.2 mm3), 4.2- (0.4 mm3), 2.4- (0.6 mm3), and 3.4-fold (0.4 mm3) reduction in vascular volume at 3, 6, 9, and 16 weeks post-implantation (Fig. 3B). In contrast to untreated allografts, T.E. periosteum modified allografts exhibited a 4.0- (0.7 mm3), 2.0- (0.9 mm3), 1.4- (0.9 mm3), and 2.3-fold (1.0 mm3) increase in vascular volume at 3, 6, 9, and 16 weeks (Fig. 3B).
Figure 3.
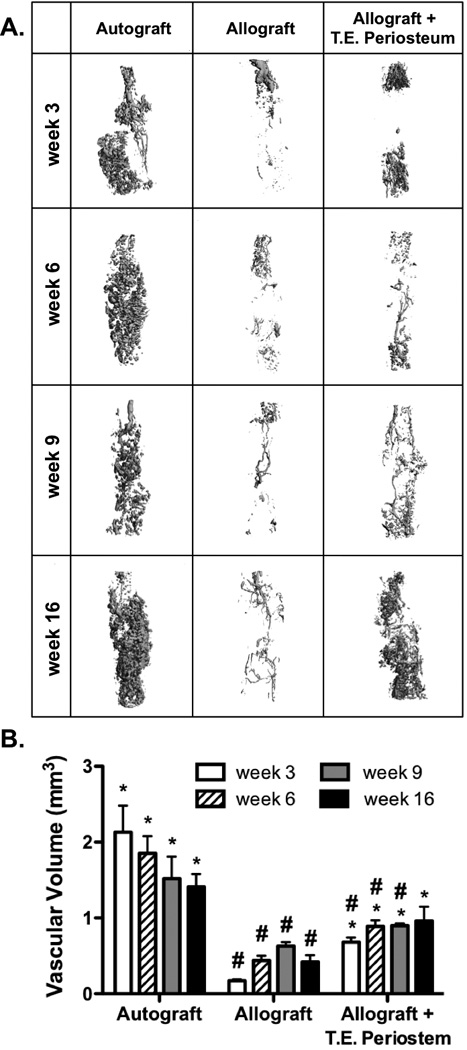
Micro-computed tomography scans were used to assess in vivo graft vascularization (A). Quantification revealed tissue engineered periosteum modified allografts exhibited enhanced vascular volume as compared to allograft controls (B) (n=5; error bars represent standard error of the mean; p-value of <0.05 indicates significance compared to allograft (*) or autograft (#)).
3.3. Tissue Engineered Allograft Bone Callus Formation
To further investigate the role of T.E. periosteum-mediated enhancements in allograft healing, bone callus formation was quantified using µCT analysis 3, 6, 9, and 16 weeks post-implantation. During healing, autografts exhibited uniform graft-mediated callus formation and extensive graft resorption and remodeling (Fig. 4A). Autografts exhibited an initial bone callus volume of 4.6 mm3 3 weeks post-implantation (Fig. 4B). Over the time course of healing, autograft bone callus volume gradually reduced as a result of graft resorption and remodeling, exhibiting values of 3.5, 2.6, and 1.8 mm3 at 6, 9, and 16 weeks. Compared to autografts, untreated allografts exhibited a 3.5- (1.3 mm3), 2.6- (1.4 mm3), 1.3- (2.0 mm3), and 1.3-fold (1.3 mm3) decrease in bone callus volume at 3, 6, 9, and 16 weeks (Fig. 4B). Furthermore, untreated allograft bone callus formation was localized only to the graft host union and complete bridging and integration was not achieved, even after 16 weeks of healing (Fig. 4A). In contrast to untreated allografts, T.E. periosteum modified allografts exhibited a 2.5- (3.3 mm3), 2.8- (3.8 mm3), 1.9- (3.8 mm3), and 4.0-fold (5.3 mm3) increase in bone callus volume compared to untreated allograft controls at 3, 6, 9, and 16 weeks (Fig. 4B). It should be noted that at 6 weeks post-implantation, no increase in bone callus volume was observed when hydrogels lacked MSCs (Fig. S3A). Furthermore, no detectable difference in bone callus volume was observed when compared with untreated allograft controls (Fig. S3B).
Figure 4.
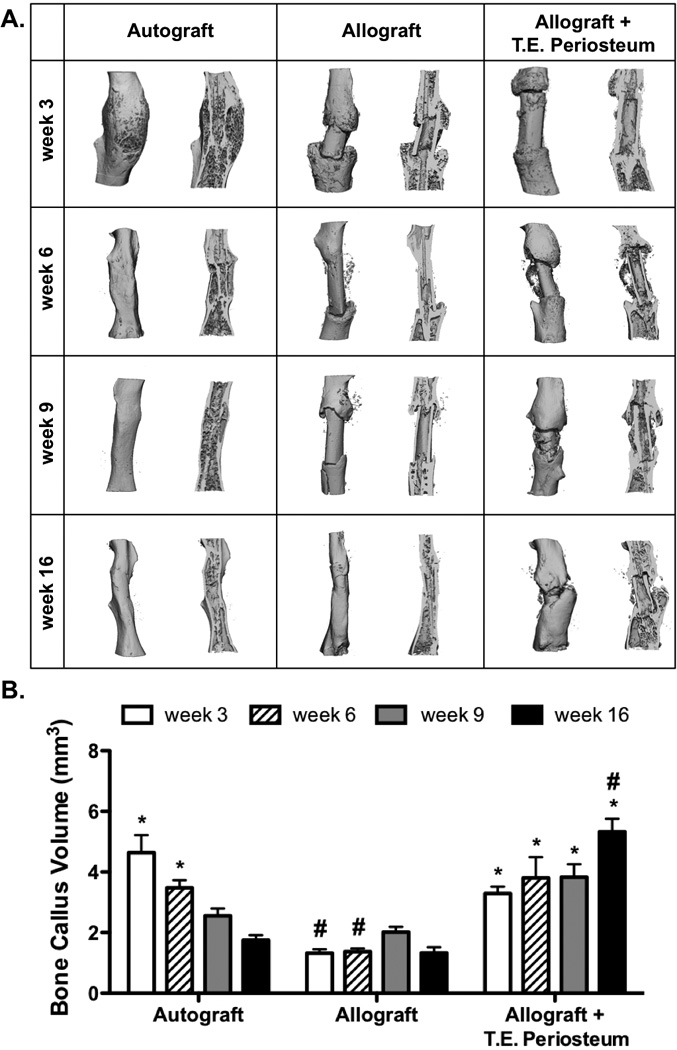
Micro-computed tomography scans were used to assess in vivo bone callus formation. Reconstructed graft calluses show both full intact scans and sagital cut views (A). Subsequent quantification revealed increased bone callus volume in tissue engineered periosteum modified allografts as compared to allograft only controls (B) (n=5; error bars represent standard error of the mean; p-value of <0.05 indicates significance compared to allograft (*) or autograft (#)).
3.4 Tissue Engineered Allograft Bridging Callus and Endochondral Bone Formation
Analysis and subsequent quantification of bone callus formation (Fig. 4) revealed increased, albeit incomplete bridging for T.E. periosteum modified allografts as compared to untreated allograft controls. To further investigate how MSC transplantation via a PEG-based T.E periosteum enhanced bridging callus formation and subsequent allograft healing, histological sections were examined 6, 9, and 16 weeks post-implantation (Fig. 5). As previously observed (Fig. 4A), autograft healing was marked by complete graft resorption and the formation of a uniform rigid bone callus collar after 16 weeks of healing (Fig. 5Ai–iii). Compared to autografts, untreated allografts failed to remodel and exhibited only minimal bone callus formation at the graft-host union (Fig. 5Aiv). Furthermore, the small amounts of callus that did form failed to completely bridge the graft and was resorbed by 16 weeks (Fig. 5Av–vi). In contrast to untreated allografts, T.E. periosteum modified allografts exhibited extensive resorption, qualitatively comparable to autograft controls (Fig. 5Ai; black arrows), as shown at both the proximal and distal ends of the graft (Fig. 5Avii–ix; black arrows). In addition, T.E. periosteum modified allografts exhibited increased bridging cartilaginous matrix production (Fig. 5Avii–ix; black triangles, alcian blue staining).
Figure 5.
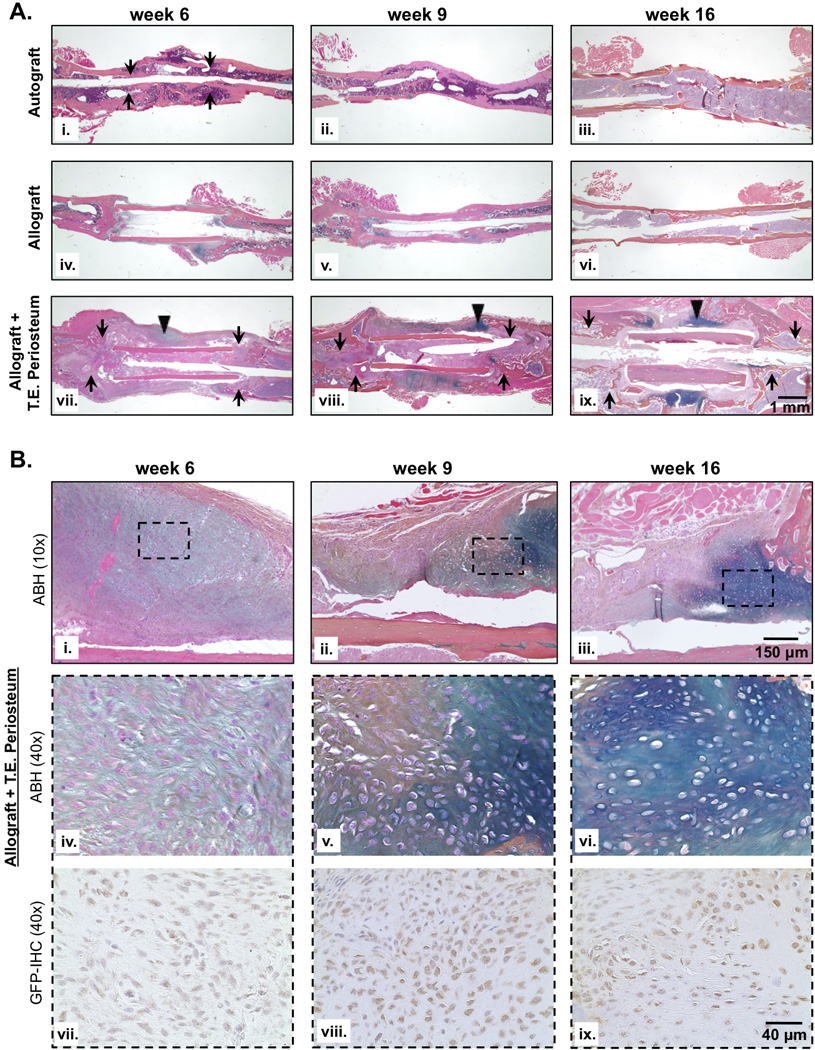
Histological analysis of graft sections revealed extensive cortical bone resorption (black arrows) and endochondral bone formation (black triangles) bridging the defect in tissue engineered periosteum modified allografts as compared to untreated allograft controls (A; i–ix). The presence of glycosaminoglycans and proteoglycans was detected via alcian blue staining (blue), and bone and surrounding soft tissue was stained with orange G (pink). Closer examination of the bridging callus formed across tissue engineered periosteum modified allografts (B (10× magnification of black triangles; i–iii)) revealed a hypertrophic condensation phenotype (B (40× magnification of black dashed squares; iv–vi)) consistent with chondrogenic differentiation of the transplanted GFP+ mMSC population (B (40× magnification of black dashed squares; vii–ix)). Furthermore, GFP+ mMSC colocalized to regions of cartilaginous matrix (alcian blue staining; iv–vi) suggesting that transplanted MSCs directly contributed to endochondral mediated bone formation.
Closer examination of the areas of bridging cartilaginous matrix production (Fig. 5Bi–iii) revealed that cells within these regions exhibited hypertrophic condensation phenotype consistent with MSC chondrogenic differentiation (Fig. 5Biv–vi), particularly at later stages of healing (9 weeks, Fig. 5Bv; 16 weeks, Fig. 5Bvi). To verify the contribution of transplanted GFP+ mMSCs to increased bridging endochondral callus formation, immunohistochemical labeling of GFP was performed. Positively labeled GFP+ mMSCs were found throughout the bridging callus at 6, 9, and 16 weeks, particularly within regions of cartilaginous matrix (Fig. 5Bvii–ix).
To quantify the marked increase in transplanted MSC-mediated bridging callus production, histomorphometric analysis was performed (Fig. 5). Using tissue specific color thresholds, total callus area, as well as mesenchyme, cartilage, and woven bone callus area was quantified (Fig. 6). T.E. periosteum modified allografts exhibited a 2.6- (9.5 mm2), 3.5- (9.5 mm2), and 4.5-fold (7.6 mm2) increase in total bridging callus area compared to untreated allografts at 6, 9, and 16 weeks (Fig. 6A). Compared to untreated allograft controls, T.E. periosteum modified allografts exhibited a 4.9- (4.8 mm2) and 920.9-fold (3.1 mm2) increase in total mesenchyme callus area at 9 and 16 weeks post-implantation (Fig. 6B). Furthermore, T.E. periosteum modified allografts exhibited a 19.9- (0.8 mm2), 31.0- (1.5 mm2), and 152.2-fold (0.7 mm2) increase in total cartilage callus area 6, 9, and 16 weeks post-implantation as compared to untreated allograft controls (Fig. 6C). Finally, T.E. periosteum modified allografts exhibited a 2.2-fold (3.7 mm2) increase in total woven bone callus area compared to untreated allograft controls at 16 weeks post-implantation (Fig. 6D). It should be noted that when MSCs were excluded from the hydrogels in this approach (hydrogels alone), no quantitative difference in total mesenchyme, cartilage, or woven bone callus area was detected as compared to untreated allograft controls (Fig. S3C–D).
Figure 6.
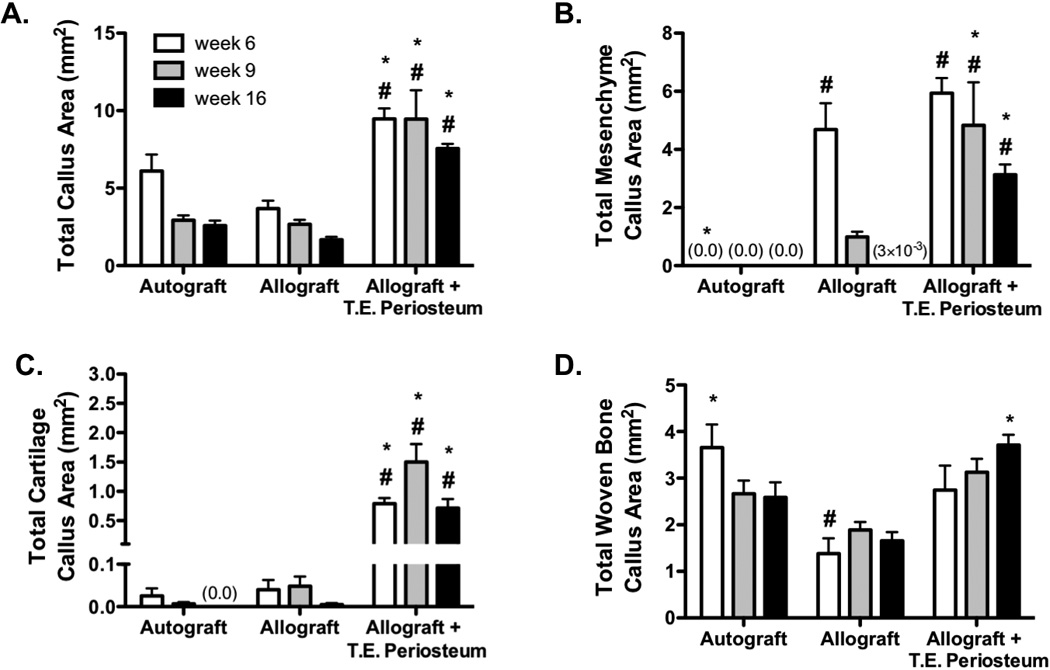
Histomorphometric analysis of alcian blue (blue, glycosaminoglycans/proteoglycans) orange G (pink, bone/soft tissue) stained graft sections (Fig. 5A) revealed a significant increase in total callus (A), total mesenchyme callus (B), total cartilage callus (C), and total woven bone callus (D) area in tissue engineered periosteum modified allografts as compared to allograft only controls. (n=8–10; error bars represent standard error of the mean; p-value of <0.05 indicates significance compared to allograft (*) or autograft (#)).
3.5 Tissue Engineered Allograft Biomechanical Stability
Having demonstrated that T.E. periosteum modified allografts exhibited increased callus bridging and endochondral bone formation, torsion testing was performed at 6, 9, and 16 weeks post-implantation to assess graft-host biomechanical stability. As previously observed [4], autografts exhibited maximal torque values similar to intact control femurs, demonstrating complete healing and full graft-host integration. At 6, 9, and 16 weeks intact femurs exhibited maximal torque values of 31.2, 31.9 and 31.9 N-mm (Fig. 7A). Similarly, autografts exhibited maximal torque values of 26.5, 24.4, and 30.2 N-mm 6, 9, and 16 weeks post-implantation. Untreated allografts exhibited a 7.8- (3.4 N-mm), 7.2- (3.4 N-mm), and 2.9-fold (10.3 N-mm) decrease in maximal torque compared to autograft controls at 6, 9, and 16 weeks (Fig. 7A). In contrast to untreated allografts, T.E. periosteum modified allografts exhibited a 0.1- (0.3 N-mm), 1.2- (4.1 N-mm), and 1.8-fold (18.4 N-mm) increase in maximal torque at 6, 9, and 16 weeks (Fig. 7A). Increased mechanical stability 16 weeks post implantation was further supported by an increasing trend in graft-host connectivity, as determined by µCT mediated union ratio calculations (Fig. 7B) [56]. While not statistically significant, T.E. periosteum modified allografts exhibited a 0.9- (11.2 %), 1.1- (15.9 %), and 1.8-fold (17.9 %) increase in graft-host connectivity at 6, 9, and 16 weeks, as compared to untreated allograft controls.
Figure 7.
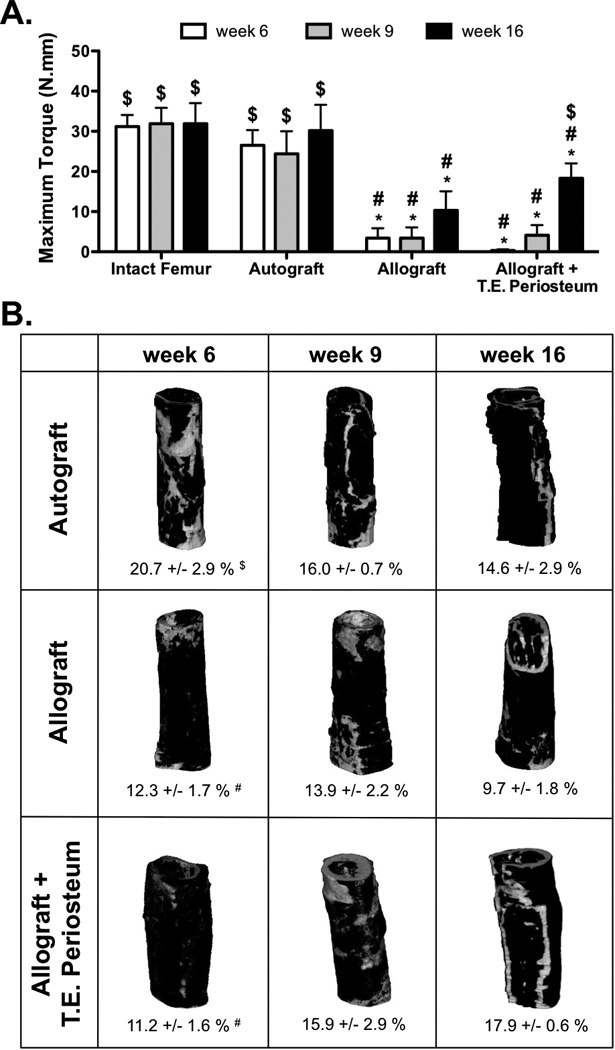
Maximal torsion strength of tissue engineered periosteum modified allograft-host union was significantly increased over untreated allograft controls 16 weeks post-implantation (A) (n=10). Union ratios calculated for graft-host connectivity followed the same trend as those found via histomorphometric analysis of total woven bone callus area (Fig. 6D) and torsional biomechanics (A). While statistical significance was not achieved, qualitative and quantitative analysis revealed increased graft-host union (regions of white) over the healing time course for tissue engineered periosteum modified allografts as compared to untreated allograft controls (B) (n=6; error bars represent standard error of the mean; p-value of <0.05 indicates significance compared to intact femur (*), autograft (#), or allograft ($)).
4. Discussion
Decellularized cortical bone allografts remain the clinical “gold standard” for treatment of critical sized bone defects, largely due to the volume of graft material needed [3–6]. Despite prevalent use, allografts exhibit poor long-term engraftment necessitating the investigation of approaches to improve allograft healing [7–9]. Scaffold materials such as Gelfoam® [5], acellular matrices [4, 21], and poly(lactide-co-glycolide) [29] have previously been used to deliver MSCs in an effort to enhance allograft healing and integration. Unlike native periosteum, these materials lack hydration, three-dimensional hierarchical tissue structure, and the ability to easily incorporate biophysical or biochemical cues to guide transplanted cell function [42, 45, 46, 52, 53, 59]. The focus of this work was to investigate MSC transplantation to the allograft surface via a PEG-based T.E. periosteum and the subsequent effects of this approach on healing and integration within a mouse femoral defect model. PEG-based hydrogels are commonly utilized in tissue engineering applications as they resemble the native extracellular environment with respect to mechanical properties and water content [41, 43, 45–47]. Furthermore, PEG hydrogels are easily modified to incorporate biomolecules, cell-adhesive ligands, or degradable functionalities [41, 43, 45–47]. We aimed to control MSC localization via hydrolytically degradable PEG hydrogels to enhance graft healing through cell-mediated mechanisms, similar to periosteum-mediated healing observed in autografts. Our results demonstrated that MSC localization via T.E. periosteum modified allografts enhanced host vascular ingrowth, bridging callus formation, endochondral bone callus production, and biomechanical strength as compared to untreated allograft controls. Interestingly, however, healing, particularly endochondral bone formation, observed by T.E. periosteum modified allografts was significantly delayed compared to autograft controls, suggesting the need for further modifications to the T.E. periosteum to increase the rate of ossification.
The critical role of the periosteum in autograft healing has been well established [5, 11–14, 16]. However, to date it remains unclear what factors are required to emulate autograft healing for allografts. To our knowledge, the study conducted herein is the first to investigate MSC localization to the allograft surface using PEG hydrogels. Unlike previous attempts to tissue engineer the periosteum using complex combinations of cell and/or growth factor delivery strategies [4, 5, 19, 22, 28, 64], our approach sought to isolate the singular influence of MSC localization on allograft healing and integration. To date, this task has posed difficult as traditional scaffold materials poorly mimic the native extracellular matrix environment and therefore lead to poor MSC graft localization and limited cell survival [4, 5, 21, 29, 41, 43, 44]. Schonmyer et al. seeded acellular dermal matrix with MSCs as an engineered periosteum for treatment of mandibular defects in rats and observed non-uniform cell seeding within their scaffolds [21]. Similarly, Zhang et al. noted poor MSC survival within Gelfoam® collagen sponges when transplanted to allografts [5].
With regards to autograft healing, host vascularization, osteoclast migration and activation, and subsequent osteoblast progenitor cell infiltration are critical steps to achieve complete integration and remodeling [61]. Towards this end, MSC localization to the allograft surface using a T.E. periosteum was shown to significantly enhance host-mediated vascular infiltration as compared to untreated allograft controls (Fig. 3). Periosteal stem cells, as well as MSCs, exhibit robust expression of numerous pro-angiogenic growth factors, including VEGF and FGF-2 [4, 10, 19, 22, 25, 26]. In addition to contributing to host vascular ingrowth, Yang et al. demonstrated that delivery of VEGF enhances osteoclast recruitment and activation, thereby contributing to enhanced bone resorption, as observed at early healing time points (Fig. 5G; black arrows, Fig. S4) [65].
Coupled with vascularization, T.E. periosteum modified allografts exhibited increased bone callus formation as compared to untreated allograft controls (Fig. 4). Closer examination revealed that T.E. periosteum modified allografts exhibited complete bridging callus formation with extensive endochondral ossification, which was absent in untreated allograft controls (Fig. 5–6) [4, 5, 11, 19, 22]. Furthermore, the transplantation of unmodified GFP+ mMSCs directly contributed to bridging cartilage callus formation and subsequent endochondral ossification (Fig. 5B), a result that to date has not been achieved using other MSC transplantation methods [4, 5]. Previous studies have demonstrated the role of recruited cell proliferation in enhanced callus formation. For example, teriparatide (PTH1–34) has been shown to contribute to graft healing through an early proliferative response of chondro- and osteoprogenitor cell populations, thereby enhancing the local cell population able to participate in endochondral ossification [27, 28, 64, 66–68]. Reynolds et al. demonstrated that daily systemic injections of teriparatide (PTH1–34) after allograft transplantation enhanced cartilage-rich bridging callus and subsequent endochondral ossification, as compared to vehicle controls [28]. Similar to PTH1–34 treatment, the T.E. periosteum increases the localized population of cells available to contribute to healing, suggesting that these two approaches may share similar mechanisms for enhancing allograft healing. As previously discussed, this outcome has not been observed in previous MSC transplantation methods due to poor MSC localization, extensive MSC migration into surrounding tissue, and limited MSC survival [4, 5, 21].
While increasing bridging endochondral bone callus formation was a significant achievement with this approach, clinical relevance is greatly reduced without an increase in graft-host biomechanics [11, 62, 63]. Although not statistically significant, the observed trend towards poorer mechanics at 6 weeks by T.E. periosteum modified allografts, as compared to untreated allograft controls may be due to extensive cortical bone remodeling at the proximal and distal graft host unions (Fig. 5Avii; black arrows). Presumably this observed increase in resorption was due to paracrine-mediated osteoclast recruitment and cortical bone remodeling (Fig. S4). As previously discussed, VEGF produced by transplanted MSCs enhances osteoclast activation [65]. In contrast, T.E. periosteum modified allografts exhibited improved maximal torque values at 9 and 16 weeks as compared to untreated allograft controls. Together with the µCT and histology data, these results demonstrated that MSCs transplanted via a PEG-based T.E. periosteum enhanced bridging bone callus formation, thereby increasing graft mechanical stability as compared to untreated allograft controls.
Despite significantly increasing graft-host stability, T.E. periosteum modified allografts biomechanical rescue was significantly delayed compared to autograft controls. The periosteum is comprised of many cells, including committed osteoblasts, differentiated osteogenic progenitors, as well as periosteal stem cells as studied herein [11, 14, 15, 18, 34–36]. Our results demonstrated that while MSC localization alone was able to enhance endochondral bone callus production compared to untreated allograft controls, this approach was unable to fully emulate autograft healing. This suggests that to achieve complete healing within a clinically relevant time course, modifications must be made to enhance the rate of callus ossification [4, 14–16, 22]. By altering the number of transplanted MSCs, paracrine factor release can be regulated to more closely match autograft controls. Doing so may provide more control over the rate of cortical bone resorption, initiation of remodeling, and subsequent ossification. In addition, the incorporation of additional osteogenic signals (e.g., cells, matrix cues, soluble factors) may mitigate the delay in complete ossification. Recently, Ma et al. demonstrated that rabbit MSCs grown in sheets and subjected to osteogenic predifferentiation are able to significantly enhance bone formation within a rabbit mandibular fracture model, as compared to undifferentiated MSCs [69, 70]. Using our bottom-up T.E. periosteum design we can easily alter the delivered cell type [69, 70], incorporate functional groups to promote osteogenic differentiation [53], or deliver growth factors or small molecule drugs to expedite the process of osteogenesis [46, 47] to positively affect ossification.
5. Conclusion
In this study, we provided evidence that MSC transplantation to the allograft surface via hydrogel-based T.E. periosteum increased allograft healing as compared to untreated allograft controls. We demonstrated that T.E. periosteum modified allografts exhibited increased vascular infiltration and bone callus formation. In addition, MSCs transplanted using T.E. periosteum were shown to directly contribute to bridging callus formation with greater amounts of mesenchyme, cartilage, and woven bone than untreated allografts. Finally, this approach provided enhanced biomechanical stability as compared to untreated allograft controls at time points greater than 9 weeks. In moving forward, it is necessary to more closely examine the mechanisms by which transplanted MSCs modulate allograft healing while simultaneously improving functional outcomes to match autograft healing over a clinically relevant time period. The work described herein has established a strategy for enhancing callus bridging and ossification. As a result, hydrogel based T.E. periosteum-mediated MSC transplantation may be a viable first step towards the realization of more effective and reproducible clinical strategies for treatment of critical sized bone defects.
Supplementary Material
Acknowledgments
Funding for this research was received from the Orthopaedic Research and Education Foundation /Musculoskeletal Transplant Foundation (OREF/MTF), the Rochester/Finger Lakes Eye & Tissue Bank (RETB/FLETB), and the NIH (R01-AR064200). MDH is supported in part by the NIH (T32-AR053459). CX is supported by the OREF/MTF (055951-002). XZ is supported by the NIH (RC1-AR058435) and NYSTEM (N08G495, N09G346). Equipment, including the IVIS Live Animal Imaging System, Visiopharm software, and whole-slide scanner were purchased through NIH funds (S10-RR026542-01, P30-AR061307, and S10-RR027340-01). The GFP+ mMSCs were provided by the Texas A&M Health Science Center College of Medicine Institute for Regenerative Medicine at Scott & White through a grant from the NIH (P40-RR017447). The authors also wish to thank Mike Thullen, Sarah Mack, Kathy Maltby, Ashish Thomas, Eric Yehling, and Dr. Longze Zhang for their assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: all authors state that they have no conflicts of interest
Contributor Information
Michael D. Hoffman, Email: hoffman@bme.rochester.edu, University of Rochester, Department of Biomedical Engineering, University of Rochester Medical Center, Center for Musculoskeletal Research, 207 Robert B. Goergen Hall, Box 270168, Rochester, NY 14627-0168.
Chao Xie, Email: chao_xie@urmc.rochester.edu, University of Rochester Medical Center, Department of Orthopaedics, and Center for Musculoskeletal Research, 601 Elmwood Ave, Box 665, Rochester, NY 14642.
Xinping Zhang, Email: xinping_zhang@urmc.rochester.edu, University of Rochester Medical Center, Department of Orthopaedics, and Center for Musculoskeletal Research, 601 Elmwood Ave, Box 665, Rochester, NY 14642.
Danielle S.W. Benoit, Email: benoit@bme.rochester.edu, University of Rochester, Departments of Biomedical Engineering, Chemical Engineering, University of Rochester Medical Center, Department of Orthopaedics, and Center for Musculoskeletal Research, 207 Robert B. Goergen Hall, Box 270168, Rochester, NY 14627-0168.
References
- 1.Farfalli GL, Aponte-Tinao L, Lopez-Millan L, Ayerza MA, Muscolo DL. Clinical and functional outcomes of tibial intercalary allografts after tumor resection. Orthopedics. 2012;35(3):e391–e396. doi: 10.3928/01477447-20120222-25. [DOI] [PubMed] [Google Scholar]
- 2.Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005;36(Suppl 3):S20–S27. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 3.Greenwald AS, Boden SD, Goldberg VM, Khan Y, Laurencin CT, Rosier RN. Bone-graft substitutes: facts, fictions, and applications. J Bone Joint Surg Am. 2001;83-A(Pt 2) Suppl 2:98–103. doi: 10.2106/00004623-200100022-00007. [DOI] [PubMed] [Google Scholar]
- 4.Xie C, Reynolds D, Awad H, Rubery PT, Pelled G, Gazit D, et al. Structural bone allograft combined with genetically engineered mesenchymal stem cells as a novel platform for bone tissue engineering. Tissue Eng. 2007;13(3):435–445. doi: 10.1089/ten.2006.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X, Xie C, Lin AS, Ito H, Awad H, Lieberman JR, et al. Periosteal progenitor cell fate in segmental cortical bone graft transplantations: implications for functional tissue engineering. J Bone Miner Res. 2005;20(12):2124–2137. doi: 10.1359/JBMR.050806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sasso RC, LeHuec JC, Shaffrey C. Iliac crest bone graft donor site pain after anterior lumbar interbody fusion: a prospective patient satisfaction outcome assessment. J Spinal Disord Tech. 2005;18(Suppl):S77–S81. doi: 10.1097/01.bsd.0000112045.36255.83. [DOI] [PubMed] [Google Scholar]
- 7.Hornicek FJ, Gebhardt MC, Tomford WW, Sorger JI, Zavatta M, Menzner JP, et al. Factors affecting nonunion of the allograft-host junction. Clin Orthop Relat Res. 2001;(382):87–98. doi: 10.1097/00003086-200101000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Wheeler DL, Enneking WF. Allograft bone decreases in strength in vivo over time. Clin Orthop Relat Res. 2005;(435):36–42. doi: 10.1097/01.blo.0000165850.58583.50. [DOI] [PubMed] [Google Scholar]
- 9.Mankin HJ, Hornicek FJ, Raskin KA. Infection in massive bone allografts. Clin Orthop Relat Res. 2005;(432):210–216. doi: 10.1097/01.blo.0000150371.77314.52. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Awad HA, O'Keefe RJ, Guldberg RE, Schwarz EM. A perspective: engineering periosteum for structural bone graft healing. Clin Orthop Relat Res. 2008;466(8):1777–1787. doi: 10.1007/s11999-008-0312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman MD, Benoit DS. Emerging ideas: engineering the periosteum: revitalizing allografts by mimicking autograft healing. Clin Orthop Relat Res. 2013;471(3):721–726. doi: 10.1007/s11999-012-2695-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res. 2009;24(2):274–282. doi: 10.1359/jbmr.081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malizos KN, Papatheodorou LK. The healing potential of the periosteum molecular aspects. Injury. 2005;36(Suppl 3):S13–S19. doi: 10.1016/j.injury.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 14.Chang H, Knothe Tate ML. Concise review: the periosteum: tapping into a reservoir of clinically useful progenitor cells. Stem Cells Transl Med. 2012;1(6):480–491. doi: 10.5966/sctm.2011-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans SF, Chang H, Knothe Tate ML. Elucidating multiscale periosteal mechanobiology: a key to unlocking the smart properties and regenerative capacity of the periosteum? Tissue Eng Part B Rev. 2013;19(2):147–159. doi: 10.1089/ten.teb.2012.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutmacher DW, Sittinger M. Periosteal cells in bone tissue engineering. Tissue Eng. 2003;9(Suppl 1):S45–S64. doi: 10.1089/10763270360696978. [DOI] [PubMed] [Google Scholar]
- 17.Bielby R, Jones E, McGonagle D. The role of mesenchymal stem cells in maintenance and repair of bone. Injury. 2007;38(Suppl 1):S26–S32. doi: 10.1016/j.injury.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Dwek JR. The periosteum: what is it, where is it, and what mimics it in its absence? Skeletal Radiol. 2010;39(4):319–323. doi: 10.1007/s00256-009-0849-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito H, Koefoed M, Tiyapatanaputi P, Gromov K, Goater JJ, Carmouche J, et al. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat Med. 2005;11(3):291–297. doi: 10.1038/nm1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodrigues M, Griffith LG, Wells A. Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cell Res Ther. 2010;1(4):32. doi: 10.1186/scrt32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schonmeyr B, Clavin N, Avraham T, Longo V, Mehrara BJ. Synthesis of a tissue-engineered periosteum with acellular dermal matrix and cultured mesenchymal stem cells. Tissue Eng Part A. 2009;15(7):1833–1841. doi: 10.1089/ten.tea.2008.0446. [DOI] [PubMed] [Google Scholar]
- 22.Yazici C, Takahata M, Reynolds DG, Xie C, Samulski RJ, Samulski J, et al. Self-complementary AAV2.5-BMP2-coated femoral allografts mediated superior bone healing versus live autografts in mice with equivalent biomechanics to unfractured femur. Mol Ther. 2011;19(8):1416–1425. doi: 10.1038/mt.2010.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tiyapatanaputi P, Rubery PT, Carmouche J, Schwarz EM, O'Keefe RJ, Zhang X. A novel murine segmental femoral graft model. J Orthop Res. 2004;22(6):1254–1260. doi: 10.1016/j.orthres.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 24.Devescovi V, Leonardi E, Ciapetti G, Cenni E. Growth factors in bone repair. Chir Organi Mov. 2008;92(3):161–168. doi: 10.1007/s12306-008-0064-1. [DOI] [PubMed] [Google Scholar]
- 25.Han SK, Yoon TH, Lee DG, Lee MA, Kim WK. Potential of human bone marrow stromal cells to accelerate wound healing in vitro. Ann Plast Surg. 2005;55(4):414–419. doi: 10.1097/01.sap.0000178809.01289.10. [DOI] [PubMed] [Google Scholar]
- 26.Pluhar GE, Manley PA, Heiner JP, Vanderby R, Jr, Seeherman HJ, Markel MD. The effect of recombinant human bone morphogenetic protein-2 on femoral reconstruction with an intercalary allograft in a dog model. J Orthop Res. 2001;19(2):308–317. doi: 10.1016/S0736-0266(00)90002-0. [DOI] [PubMed] [Google Scholar]
- 27.Takahata M, Awad HA, O'Keefe RJ, Bukata SV, Schwarz EM. Endogenous tissue engineering: PTH therapy for skeletal repair. Cell Tissue Res. 2012;347(3):545–552. doi: 10.1007/s00441-011-1188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynolds DG, Takahata M, Lerner AL, O'Keefe RJ, Schwarz EM, Awad HA. Teriparatide therapy enhances devitalized femoral allograft osseointegration and biomechanics in a murine model. Bone. 2011;48(3):562–570. doi: 10.1016/j.bone.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy WL, Peters MC, Kohn DH, Mooney DJ. Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2000;21(24):2521–2527. doi: 10.1016/s0142-9612(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 30.Derner R, Anderson AC. The bone morphogenic protein. Clin Podiatr Med Surg. 2005;22(4):607–618. vii. doi: 10.1016/j.cpm.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Awad HA, Zhang X, Reynolds DG, Guldberg RE, O'Keefe RJ, Schwarz EM. Recent advances in gene delivery for structural bone allografts. Tissue Eng. 2007;13(8):1973–1985. doi: 10.1089/ten.2006.0107. [DOI] [PubMed] [Google Scholar]
- 32.Bonewald LF, Dallas SL. Role of active and latent transforming growth factor beta in bone formation. J Cell Biochem. 1994;55(3):350–357. doi: 10.1002/jcb.240550312. [DOI] [PubMed] [Google Scholar]
- 33.Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64(2):278–294. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 34.Ellender G, Feik SA, Carach BJ. Periosteal structure and development in a rat caudal vertebra. J Anat. 1988;158:173–187. [PMC free article] [PubMed] [Google Scholar]
- 35.Ham AW. A histological study in the early phases of bone repair. J Bone Joint Surg Am. 1930;12:827–844. [Google Scholar]
- 36.Squier CA, Ghoneim S, Kremenak CR. Ultrastructure of the periosteum from membrane bone. J Anat. 1990;171:233–239. [PMC free article] [PubMed] [Google Scholar]
- 37.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 38.Choi YS, Noh SE, Lim SM, Lee CW, Kim CS, Im MW, et al. Multipotency and growth characteristic of periosteum-derived progenitor cells for chondrogenic, osteogenic, and adipogenic differentiation. Biotechnol Lett. 2008;30(4):593–601. doi: 10.1007/s10529-007-9584-2. [DOI] [PubMed] [Google Scholar]
- 39.Banfi A, Muraglia A, Dozin B, Mastrogiacomo M, Cancedda R, Quarto R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp Hematol. 2000;28(6):707–715. doi: 10.1016/s0301-472x(00)00160-0. [DOI] [PubMed] [Google Scholar]
- 40.Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48(12):3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 41.Elisseeff J, Puleo C, Yang F, Sharma B. Advances in skeletal tissue engineering with hydrogels. Orthod Craniofac Res. 2005;8(3):150–161. doi: 10.1111/j.1601-6343.2005.00335.x. [DOI] [PubMed] [Google Scholar]
- 42.Benoit DS, Tripodi MC, Blanchette JO, Langer SJ, Leinwand LA, Anseth KS. Integrin-linked kinase production prevents anoikis in human mesenchymal stem cells. J Biomed Mater Res A. 2007;81(2):259–268. doi: 10.1002/jbm.a.31292. [DOI] [PubMed] [Google Scholar]
- 43.Lin CC, Anseth KS. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm Res. 2009;26(3):631–643. doi: 10.1007/s11095-008-9801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nuttelman CR, Tripodi MC, Anseth KS. Synthetic hydrogel niches that promote hMSC viability. Matrix Biol. 2005;24(3):208–218. doi: 10.1016/j.matbio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 45.Benoit DSW, Collins SD, Anseth KS. Multifunctional hydrogels that promote osteogenic human mesenchymal stem cell differentiation through stimulation and sequestering of bone morphogenic protein 2. Adv Funct Mater. 2007;17(13):2085–2093. doi: 10.1002/adfm.200700012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benoit DSW, Nuttelman CR, Collins SD, Anseth KS. Synthesis and characterization of a fluvastatin-releasing hydrogel delivery system to modulate hMSC differentiation and function for bone regeneration. Biomaterials. 2006;27(36):6102–6110. doi: 10.1016/j.biomaterials.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 47.Nuttelman CR, Tripodi MC, Anseth KS. Dexamethasone-functionalized gels induce osteogenic differentiation of encapsulated hMSCs. J Biomed Mater Res A. 2006;76(1):183–195. doi: 10.1002/jbm.a.30537. [DOI] [PubMed] [Google Scholar]
- 48.Sawhney AS, Pathak CP, Hubbell JA. Bioerodible hydrogels based on photopolymerized poly(ethylene glycol)-co-poly(alpha-hydroxy acid) diacrylate macromers. Macromolecules. 1993;26(4):581–587. [Google Scholar]
- 49.Hoffman MD, Benoit DS. Agonism of Wnt-beta-catenin signalling promotes mesenchymal stem cell (MSC) expansion. J Tissue Eng Regen Med. 2013 doi: 10.1002/term.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin-Gibson S, Bencherif S, Cooper JA, Wetzel SJ, Antonucci JM, Vogel BM, et al. Synthesis and characterization of PEG dimethacrylates and their hydrogels. Biomacromolecules. 2004;5(4):1280–1287. doi: 10.1021/bm0498777. [DOI] [PubMed] [Google Scholar]
- 51.Benoit DS, Boutin ME. Controlling mesenchymal stem cell gene expression using polymer-mediated delivery of siRNA. Biomacromolecules. 2012;13(11):3841–3849. doi: 10.1021/bm301294n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benoit DSW, Durney AR, Anseth KS. The effect of heparin-functionalized PEG hydrogels on three-dimensional human mesenchymal stem cell osteogenic differentiation. Biomaterials. 2007;28(1):66–77. doi: 10.1016/j.biomaterials.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 53.Benoit DSW, Schwartz MP, Durney AR, Anseth KS. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat Mater. 2008;7(10):816–823. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fairbanks BD, Schwartz MP, Bowman CN, Anseth KS. Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: polymerization rate and cytocompatibility. Biomaterials. 2009;30(35):6702–6707. doi: 10.1016/j.biomaterials.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reynolds DG, Hock C, Shaikh S, Jacobson J, Zhang X, Rubery PT, et al. Micro-computed tomography prediction of biomechanical strength in murine structural bone grafts. J Biomech. 2007;40(14):3178–3186. doi: 10.1016/j.jbiomech.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 56.Reynolds DG, Shaikh S, Papuga MO, Lerner AL, O'Keefe RJ, Schwarz EM, et al. muCT-based measurement of cortical bone graft-to-host union. J Bone Miner Res. 2009;24(5):899–907. doi: 10.1359/JBMR.081232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duvall CL, Taylor WR, Weiss D, Guldberg RE. Quantitative microcomputed tomography analysis of collateral vessel development after ischemic injury. Am J Physiol Heart Circ Physiol. 2004;287(1):H302–H310. doi: 10.1152/ajpheart.00928.2003. [DOI] [PubMed] [Google Scholar]
- 58.Brodt MD, Ellis CB, Silva MJ. Growing C57Bl/6 mice increase whole bone mechanical properties by increasing geometric and material properties. J Bone Miner Res. 1999;14(12):2159–2166. doi: 10.1359/jbmr.1999.14.12.2159. [DOI] [PubMed] [Google Scholar]
- 59.Benoit DS, Durney AR, Anseth KS. Manipulations in hydrogel degradation behavior enhance osteoblast function and mineralized tissue formation. Tissue Eng. 2006;12(6):1663–1673. doi: 10.1089/ten.2006.12.1663. [DOI] [PubMed] [Google Scholar]
- 60.Metters AT, Anseth KS, Bowman CN. Fundamental studies of biodegradable hydrogels as cartilage replacement materials. Biomed Sci Instrum. 1999;35:33–38. [PubMed] [Google Scholar]
- 61.Henriksen K, Karsdal M, Delaisse JM, Engsig MT. RANKL and vascular endothelial growth factor (VEGF) induce osteoclast chemotaxis through an ERK1/2-dependent mechanism. J Biol Chem. 2003;278(49):48745–48753. doi: 10.1074/jbc.M309193200. [DOI] [PubMed] [Google Scholar]
- 62.Burchardt H. The biology of bone graft repair. Clin Orthop Relat Res. 1983;(174):28–42. [PubMed] [Google Scholar]
- 63.Einhorn TA. The cell and molecular biology of fracture healing. Clin Orthop Relat Res. 1998;(355 Suppl):S7–S21. doi: 10.1097/00003086-199810001-00003. [DOI] [PubMed] [Google Scholar]
- 64.Takahata M, Schwarz EM, Chen T, O'Keefe RJ, Awad HA. Delayed short-course treatment with teriparatide (PTH(1–34)) improves femoral allograft healing by enhancing intramembranous bone formation at the graft-host junction. J Bone Miner Res. 2012;27(1):26–37. doi: 10.1002/jbmr.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang Q, McHugh KP, Patntirapong S, Gu X, Wunderlich L, Hauschka PV. VEGF enhancement of osteoclast survival and bone resorption involves VEGF receptor-2 signaling and beta3-integrin. Matrix Biol. 2008;27(7):589–599. doi: 10.1016/j.matbio.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 66.Barnes GL, Kakar S, Vora S, Morgan EF, Gerstenfeld LC, Einhorn TA. Stimulation of fracture-healing with systemic intermittent parathyroid hormone treatment. J Bone Joint Surg Am. 2008;90(Suppl 1):120–127. doi: 10.2106/JBJS.G.01443. [DOI] [PubMed] [Google Scholar]
- 67.Nakazawa T, Nakajima A, Shiomi K, Moriya H, Einhorn TA, Yamazaki M. Effects of low-dose, intermittent treatment with recombinant human parathyroid hormone (1–34) on chondrogenesis in a model of experimental fracture healing. Bone. 2005;37(5):711–719. doi: 10.1016/j.bone.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 68.Nakajima A, Shimoji N, Shiomi K, Shimizu S, Moriya H, Einhorn TA, et al. Mechanisms for the enhancement of fracture healing in rats treated with intermittent low-dose human parathyroid hormone (1–34) J Bone Miner Res. 2002;17(11):2038–2047. doi: 10.1359/jbmr.2002.17.11.2038. [DOI] [PubMed] [Google Scholar]
- 69.Ma D, Yao H, Tian W, Chen F, Liu Y, Mao T, et al. Enhancing bone formation by transplantation of a scaffold-free tissue-engineered periosteum in a rabbit model. Clin Oral Implants Res. 2011;22(10):1193–1199. doi: 10.1111/j.1600-0501.2010.02091.x. [DOI] [PubMed] [Google Scholar]
- 70.Ma D, Ren L, Liu Y, Chen F, Zhang J, Xue Z, et al. Engineering scaffold-free bone tissue using bone marrow stromal cell sheets. J Orthop Res. 2010;28(5):697–702. doi: 10.1002/jor.21012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


