Abstract
Objective
In addition to extensively characterized role of high density lipoprotein (HDL) in reverse cholesterol transport, bioactive lipids bound to HDL can also exert diverse vascular effects. Despite this, integration of HDL action in the vasculature with pathways that metabolize HDL and release bioactive lipids has been much less explored. The effects of HDL on endothelial cells (ECs) are mediated in part by HDL-associated sphingosine 1-phosphate (S1P), which binds to S1P1 receptors and promotes activation of eNOS and the kinase Akt. In these studies, we characterized the role of endothelial lipase (EL) in the control of endothelial signaling and biology, including those mediated by HDL-associated S1P.
Approach and Results
HDL-induced angiogenesis in aortic rings from EL-deficient (EL−/−) mice was markedly decreased versus wild-type controls. In cultured ECs, siRNA-mediated knockdown of EL abrogated HDL-promoted EC migration and tube formation. siRNA-mediated EL knockdown also attenuated HDL-induced phosphorylation of eNOS1179 and Akt473. S1P stimulation restored HDL-induced endothelial migration and Akt/eNOS phosphorylation that had been blocked by siRNA-mediated EL knockdown. HDL-induced EC migration and Akt/eNOS phosphorylation were completely inhibited by the S1P1 antagonist W146 but not by the S1P3 antagonist CAY10444.
Conclusions
Endothelial lipase is a critical determinant of the effects of HDL on S1P-mediated vascular responses and acts on HDL to promote activation of S1P1, leading to Akt/eNOS phosphorylation and subsequent endothelial migration and angiogenesis. The role of EL in HDL-associated S1P effects provides new insights into EL action, the responses seen through EL and HDL interaction, and S1P signaling.
Keywords: HDL, endothelial lipase, endothelial cells, sphingosine 1-phosphate, angiogenesis
Plasma levels of high-density lipoprotein cholesterol (HDL-C) are inversely associated with the risk of cardiovascular diseases.1 The anti-atherogenic effect of HDL is attributed at least in part to reverse cholesterol transport.2 In addition, multiple lines of evidence indicate that other salutary effects of HDL on endothelial cells include anti-oxidative, anti-inflammatory, anti-thrombotic and pro-angiogenic effects.3–5 HDL is the most quantitatively important plasma carrier of sphingosine-1-phosphate (S1P), which is a bioactive lipid molecule. HDL-associated S1P has been demonstrated to be involved in a broad range of vascular responses6–9 modulated by a family of at least five different S1P receptor subtypes identified in mammals; endothelial cells express most prominently the S1P1 and S1P3 receptor isoforms.9, 10 It was recently reported that HDL-induced Akt activation and endothelial responses are mediated by S1P1 in human umbilical vein endothelial cells.11 In contrast, other studies suggest that HDL-induced Akt activation is mediated by S1P3 receptors.12 The identity of the S1P receptor subtype that modulates HDL responses in the endothelium remains unsettled, and the mechanisms whereby S1P is released from HDL is unclear. The studies presented here explore the roles of endothelial lipase (EL) and the identity of the S1P receptor subtype in determining HDL-dependent responses in the vascular wall.
EL is a member of the triglyceride lipase family, which includes lipoprotein lipase and hepatic lipase. EL is principally a phospholipase, with nominal triglyceride lipase activity.13, 14 Endothelial lipase is synthesized principally by EC15 and hydrolyzes HDL much more efficiently than other lipoproteins.16 Plasma HDL levels are increased in EL knockout (EL−/−) mice and are decreased in EL transgenic mice.17 Furthermore, the N396S variant in the human EL gene (LIPG) shows reduced lipase activity and is associated with in elevated HDL-C levels.18 Another EL variant, G26S EL shows reduced plasma levels of EL protein.19 Yet the role of EL in atherosclerosis remains controversial. Inactivation of EL increases the plasma HDL-C levels and inhibits atherosclerosis in ApoE−/− mice.20 In contrast, another study reported that a deficiency of EL expression does not affect atherosclerosis in either ApoE−/− mice or LDLR−/− mice, even though plasma HDL-C levels are elevated.21 Broedl et al. have shown that EL overexpression results in reduced VLDL/LDL cholesterol and phospholipid levels.22 In addition, we have previously reported that HDL hydrolysis by EL activates PPARα and represses VCAM1 expression in endothelial cells, which may contribute to the anti-inflammatory effects of HDL.23 These multiple lines of evidence suggest EL action might limit atherosclerosis. Several genetic association studies have shown conflicting results regarding association between common genetic variants in LIPG gene and the risk of cardiovascular diseases. Vergeer et al. reported that the T111I variant in the LIPG gene is associated with higher HDL-C levels but is not associated with increased cardiovascular disease risk.24 Moreover, recent Mendelian randomization analysis studies in 20,913 myocardial infarction (MI) cases versus 95,407 controls found that a single nucleotide polymorphism in the EL gene significantly increased HDL-C levels but conferred no protection against myocardial infarction. These findings raise questions about the connection between plasma HDL-C levels per se and protection against atherosclerosis and the connection between EL’s enzymatic function and HDL’s beneficial effects.25
Sphingosine 1-phosphate (S1P) is a bioactive lipid that binds to family of G protein-coupled receptors that modulate signaling responses in a broad range of cells and tissues.26 S1P1 receptors in the vascular endothelium are reversibly targeted to plasmalemmal caveolae and promote the activation of kinase Akt and of the endothelial isoform of nitric oxide synthase (eNOS), leading to vasorelaxation.27 The EC50 for S1P-promoted eNOS phosphorylation is at least one order of magnitude lower than the plasma concentration of S1P, reflecting the fact that plasma S1P is mostly bound to plasma proteins. HDL particles represent the predominant S1P binding proteins in plasma, with recent studies revealing apolipoprotein M (apoM) in HDL as a specific S1P binding protein.28, 29
The roles of EL in modulating HDL-dependent signaling responses via S1P have not been well characterized. The current studies use experiments in ex vivo vascular preparations as well as in cultured endothelial cells to test the hypothesis that HDL hydrolysis by EL induces angiogenesis and stimulates endothelial signaling responses via S1P1 receptors in the vascular endothelium.
Materials and Methods
See online supplement.
Results
Endothelial lipase is involved in HDL-induced endothelial proliferation, tube formation, and angiogenesis
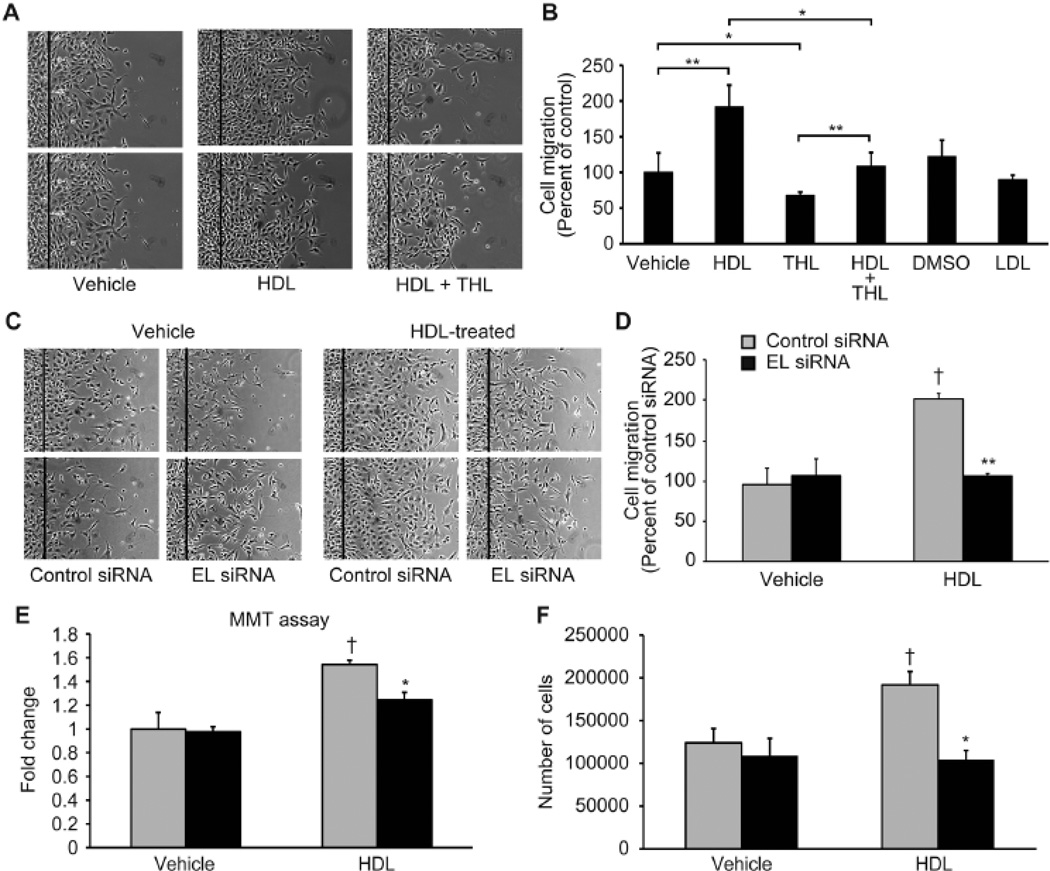
Given EL as the predominant lipase expressed by endothelial cells, we first studied the effects of the general lipase inhibitor tetrahydrolipstatin (THL) on HDL-induced endothelial cell migration. Addition of HDL (100 µg/ml) approximately doubled endothelial cell migration as compared to vehicle stimulation, an effect blocked by THL-mediated lipase inhibition (Figure 1A, B). To more directly investigate the role of EL in HDL- induced cell migration, bovine aortic EC (BAEC) were transfected with a duplex siRNA construct targeting EL. BAEC transfection with EL-siRNA reduced EL mRNA by 90% and EL protein abundance by 50% reduction (Supplement Figure 1A), but had no effect on eNOS, Akt, AMPK, ERK1/2, p38MAPK or PTEN protein levels (Supplement Figure 1B). siRNA-mediated EL knockdown suppressed HDL-promoted EC migration (Figure 1C, D); control siRNA had no effect on the HDL-induced migration response. Viability assays (MMT) revealed that HDL increased endothelial cell viability but not after siRNA-mediated EL knockdown (Figure 1E). Similarly, HDL stimulation increased endothelial cell proliferation (Figure 1F) but not after EL siRNA exposure.
Figure 1. Inhibition of lipase activity attenuates HDL-induced endothelial cell (EC) migration and proliferation.
(A) EC migration assay after treatment with HDL (100 µg/ml) in the presence or absence of the lipase inhibitor tetrahydrolipstatin (THL) (10 µM). DMSO (0.1%) and LDL (100 µg/ml) were used as controls. (B) Quantification of EC migration was determined by the number of the cells that populated the area (n = 4 per condition). *p < 0.05 vehicle versus THL and HDL versus THL + HDL, **p < 0.01 vehicle versus THL and HDL versus THL + HDL. (C) EC migration assays were analyzed in BAEC transfected with control or EL siRNA. (D) Quantification of EC migration as determined by the number of the cells that populated the area (n = 4 per condition). **p < 0.01 versus control siRNA with the same treatment, †p < 0.05 versus control siRNA with vehicle. (E) MTT viability assay after transfection with control siRNA or EL siRNA and treatment with HDL (75 µg/ml, n = 4 per condition) is shown as fold change versus cells exposed to control siRNA/vehicle. *p < 0.05, †p < 0.05 versus control siRNA/vehicle. (F) Effect of EL deficiency on the proliferative response to HDL by counting the number of cells after a 24 hour treatment (n = 3 per condition). *p < 0.05 versus control siRNA with the same treatment, †p < 0.05 versus control siRNA with vehicle.
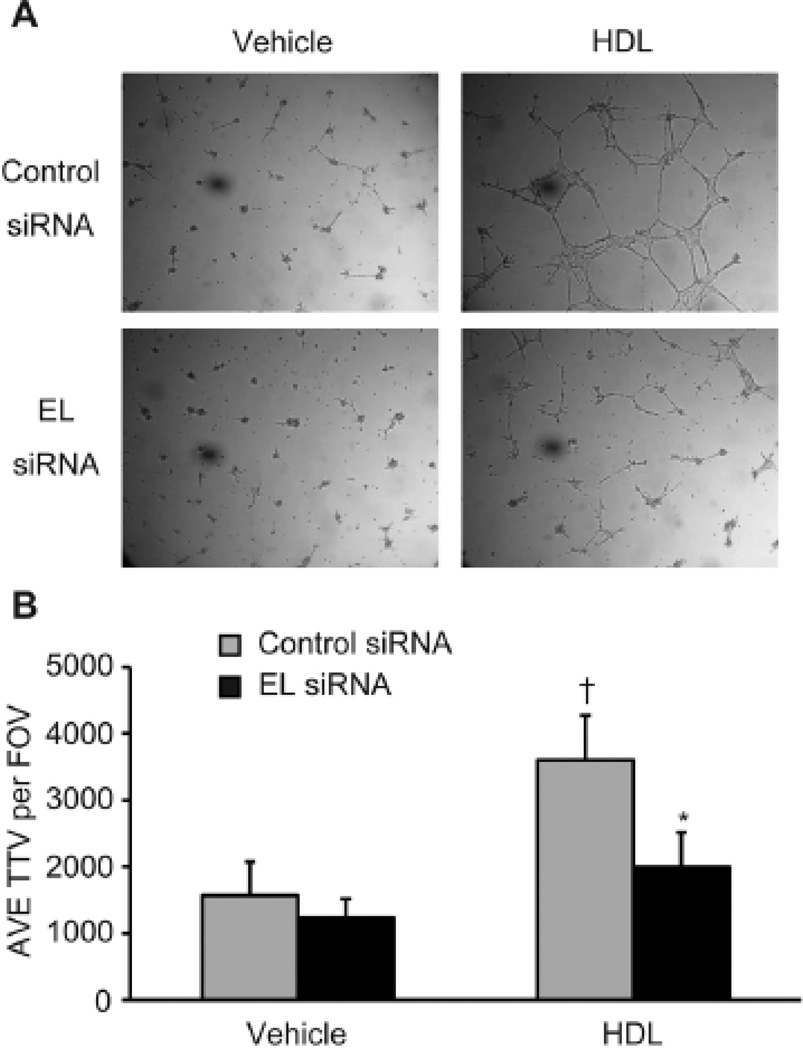
HDL stimulation of endothelial cells promoted endothelial tube formation, which was also inhibited by siRNA-mediated EL knockdown, as seen on microscopy (Figure 2A) and after quantification of the average total tube length (AVE TTL) per field of view (FOV) (Figure 2B).
Figure 2. siRNA-mediated knockdown of EL attenuates HDL-induced tube formation.
(A) Effect of EL knockdown on tube formation induced by HDL (75 µg/ml). (B) Quantification of tube formation as determined by average total tube length (TTL) per field of view (FOV) (n = 5 per condition). *p < 0.05 versus control siRNA with the same treatment, †p < 0.05 versus control siRNA with vehicle. Data represent mean values ± SEM.
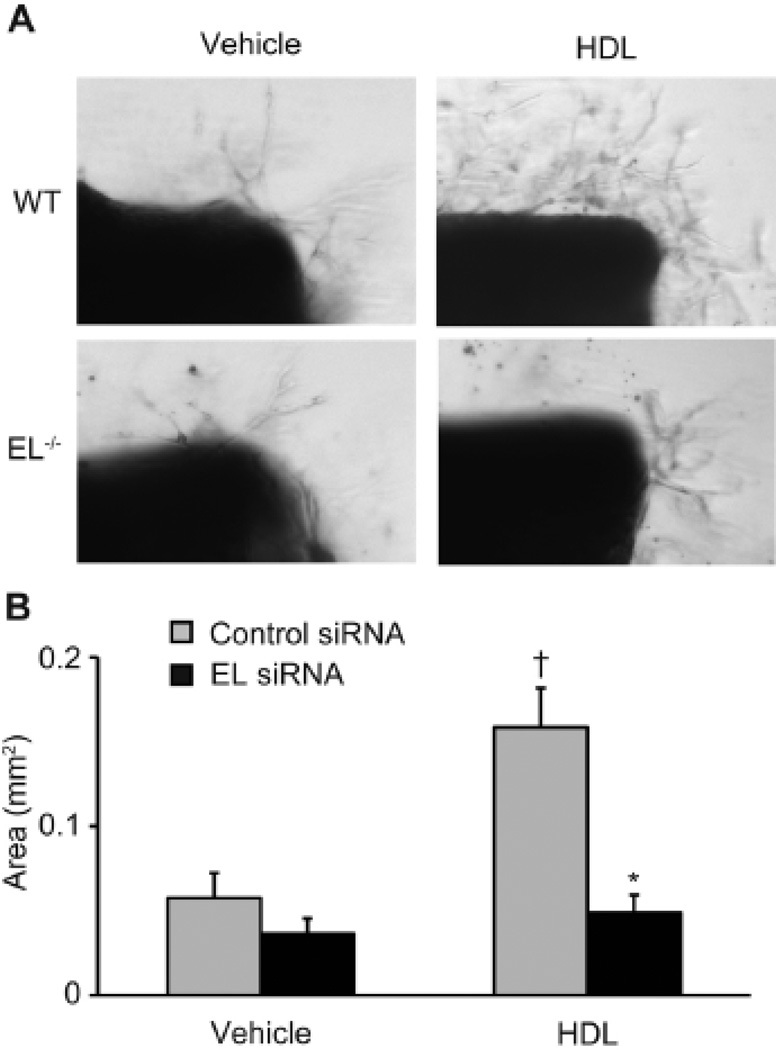
We next utilized an ex vivo angiogenesis in aortic rings model to evaluate the role of EL in HDL-induced angiogenesis. HDL markedly stimulated angiogenesis in aortic rings isolated from wild-type but not EL−/− knockout mice (Figure 3A), as quantified using the total area of new vessel formation (Figure 3B).
Figure 3. HDL-induced angiogenesis is impaired in aortic preparations from the EL−/− knockout mouse.
(A) Angiogenesis from the aortic rings. (B) vessel growth area quantification (n = 11–12 per condition). *p < 0.05 versus control siRNA with the same condition, †p < 0.05 versus WT with vehicle. Data represent means ± SEM.
siRNA-mediated endothelial lipase knockdown inhibits HDL-induced phosphorylation responses
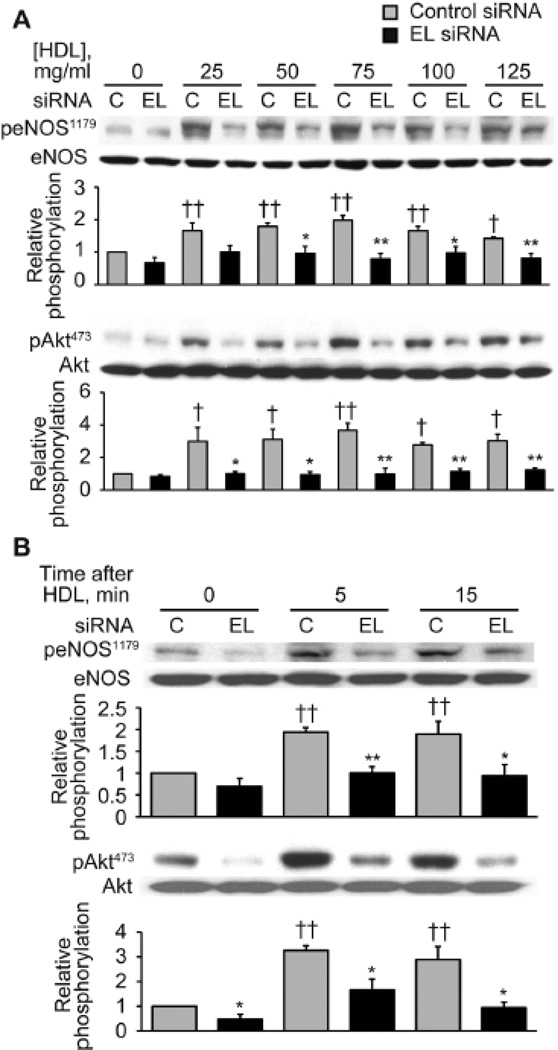
To elucidate the signaling pathways responsible for these HDL-mediated EL-dependent migration and angiogenesis responses, we examined the phosphorylation pattern of several key endothelial signaling proteins in response to HDL in the presence or absence of EL RNA interference approaches. As shown in Figure 4, HDL treatment promoted the time- and dose-dependent phosphorylation of eNOS Ser1179 and of kinase Akt, yielding a 2.0-fold (n = 3, p < 0.01) increase in eNOS phosphorylation and a 3.7-fold (n = 3, p < 0.01) increase in Akt phosphorylation. siRNA-mediated EL knockdown attenuated HDL-induced eNOS and Akt phosphorylation, as assessed both in dose-response (Figure 4A) and time course (Figure 4B) experiments.
Figure 4. siRNA-mediated EL knockdown inhibits HDL-induced phosphorylation of eNOS and Akt.
(A) Immunoblots were analyzed in either vehicle- or HDL-treated BAEC transfected with control or EL siRNA. Cell lysates were harvested at 5 min following addition of different amounts of HDL as indicated and analyzed in immunoblots probed with specific antibodies against Ser1179-phospho-eNOS (peNOS1179), Ser473-phospho-Akt (pAkt473) and total eNOS and Akt. The experiment shown is a representative of three independent experiments. *p < 0.05, **p < 0.01 versus control siRNA with the same dose of HDL, †p < 0.05, ††p < 0.01 versus control siRNA without HDL treatment. (B) Immunoblots were analyzed in BAEC transfected with control or EL siRNA. Cell lysates were harvested at the indicated times following addition of HDL (75 µg/ml) and analyzed in immunoblots probed with specific antibodies against Ser1179-phospho-eNOS (peNOS1179), Ser473-phospho-Akt (pAkt473) and total eNOS and Akt. The experiment shown is a representative of three independent experiments. *p < 0.05, **p < 0.01 versus control siRNA with the same dose of HDL, ††p < 0.01 versus control siRNA without HDL treatment (0 min). Data represent means ± SEM.
HDL-stimulated endothelial lipase-dependent signaling responses are mediated by S1P/S1P1
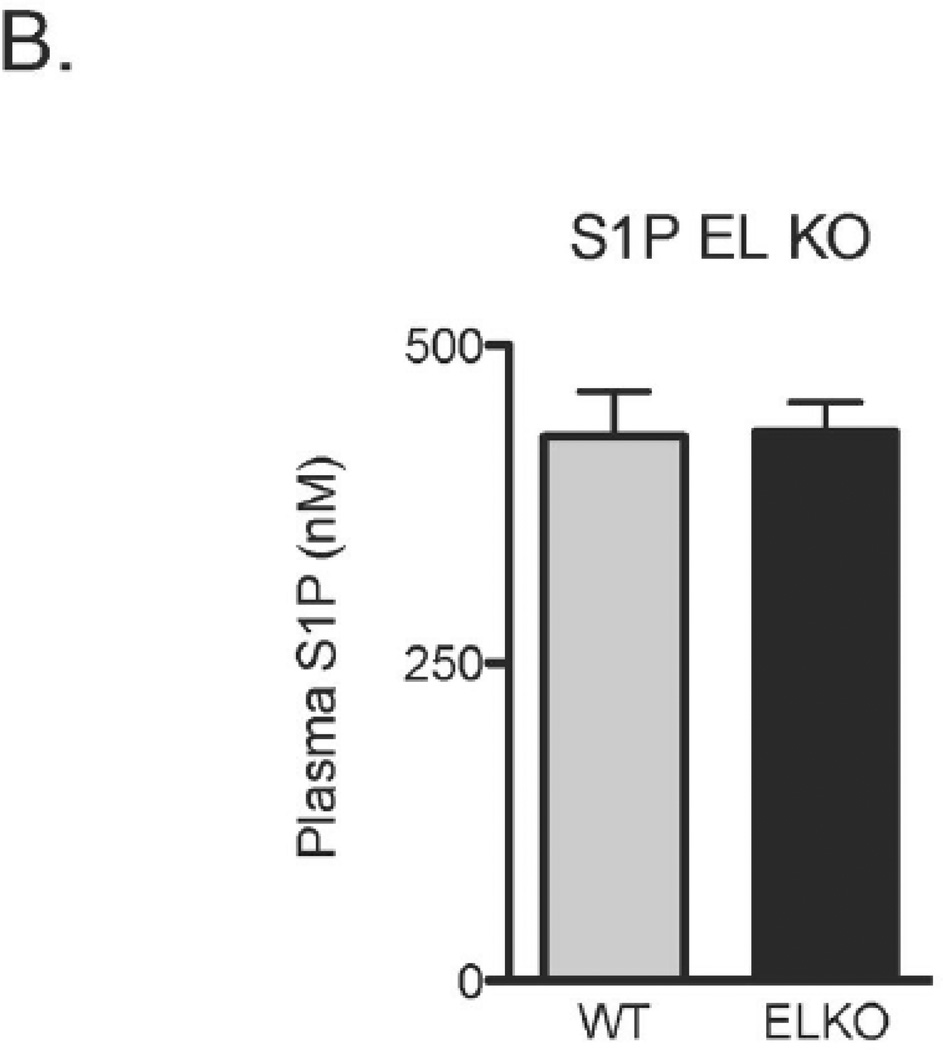
S1P signals through distinct S1P receptors. We used S1P receptor subtype-selective antagonists to assess the role of HDL-bound S1P in HDL-induced EC migration (Figure 5) and Akt/eNOS phosphorylation (Figure 6A) responses mediated by endothelial lipase. The S1P1 antagonist W146 blocked HDL-induced EC migration and Akt/eNOS phosphorylation. In contrast, the S1P3 antagonist CAY10444 did not alter either HDL-induced EC migration or Akt/eNOS phosphorylation. Direct stimulation of cells with S1P itself reversed the inhibition seen with EL siRNA on both HDL-induced EC migration (Figure 5) and Akt/eNOS phosphorylation (Figure 6). Given these findings that endothelial lipase can modulate HDL-induced endothelial responses via S1P, we sought to investigate differences in S1P levels in plasma from wildtype and EL-deficient mice. After developing and validating a liquid chromatography, tandem mass spectroscopy (LC/MS/MS) method in our laboratory for detecting S1P levels in murine plasma, we documented S1P concentrations in murine plasma in the high nanomolar range (Supplemental Figure 2). S1P plasma levels did not differ between wildtype (n = 4) and EL-deficient male mice (n = 6, Figure 6B).
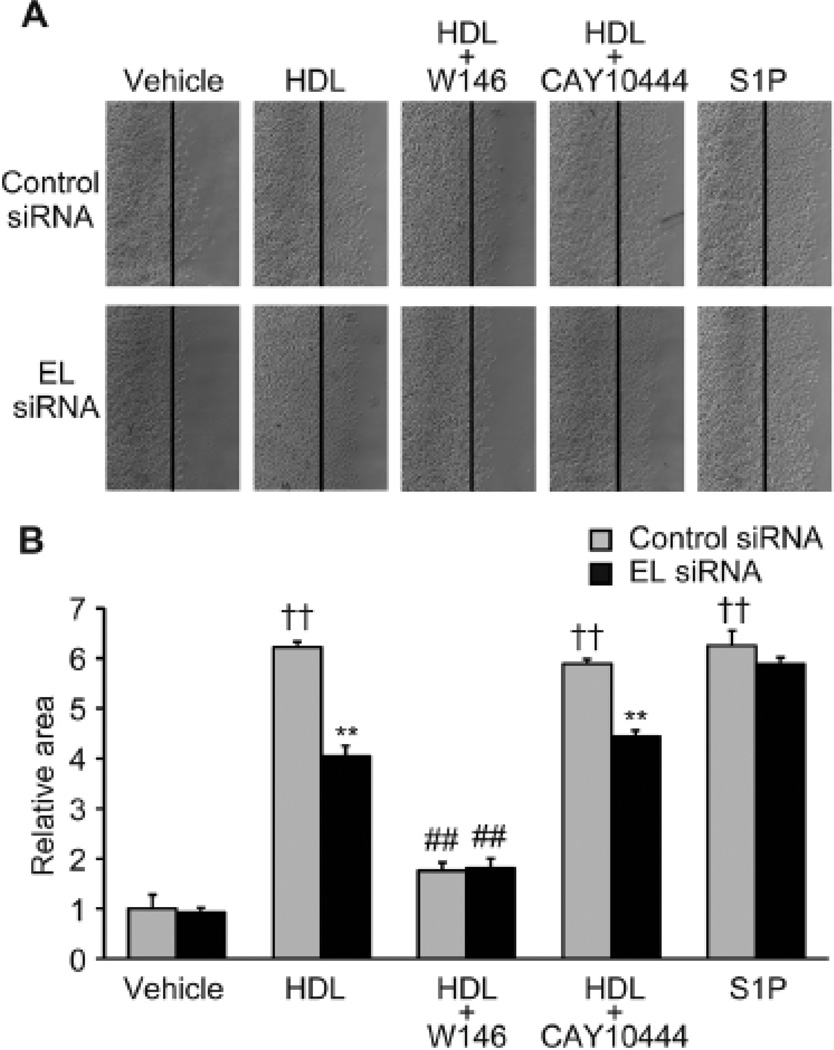
Figure 5. S1P/S1P1 is involved in HDL-induced EC migration mediated by endothelial lipase.
(A) EC migration assay after treatment with vehicle, HDL (75 µg/ml), HDL (75 µg/ml) + W146 (10 µM), HDL (75 µg/ml) + CAY10444 (10 µM) or S1P (100 nM) in BAEC transfected with control or EL siRNA. (B) Quantification of EC migration as determined by the area of migrated cells (n = 4 per condition). **p < 0.01 versus control siRNA with the same treatment, ††p < 0.01 versus control siRNA with vehicle, ##p < 0.01 versus HDL with the same siRNA. Data represent means ± SEM.
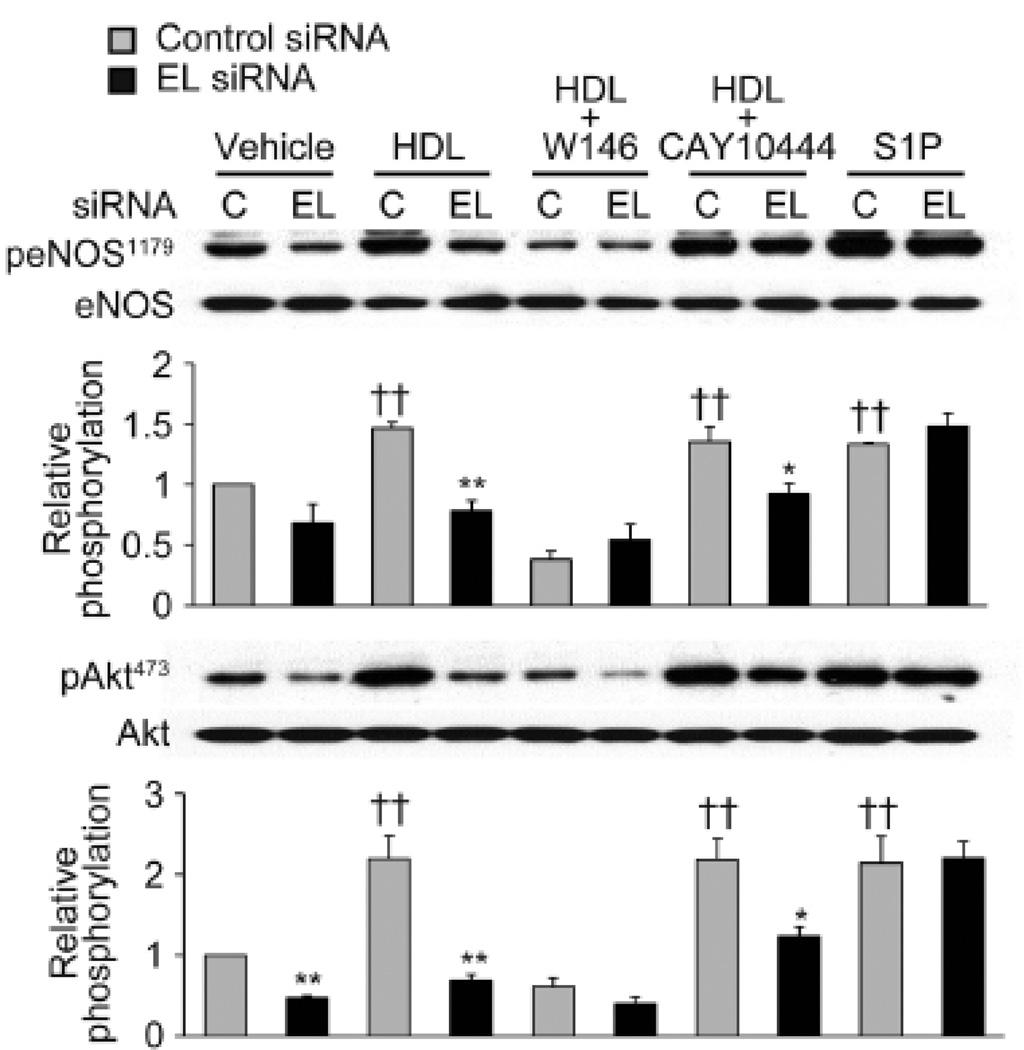
Figure 6. S1P/S1P1 is involved in HDL-induced phosphorylation of eNOS and Akt mediated by endothelial lipase.
A. Immunoblots were analyzed in BAEC transfected with control or EL siRNA after treatment with vehicle, HDL (75 µg/ml), HDL (75 µg/ml) + W146 (10 µM), HDL (75 µg/ml) + CAY10444 (10 µM) or S1P (100 nM). Vehicle or W146 was added to the cells 1 h prior to addition of HDL or S1P. Cell lysates were harvested 5 min after adding HDL or S1P and analyzed in immunoblots probed with specific antibodies against Ser1179-phospho-eNOS (peNOS1179), Ser473-phospho-Akt (pAkt473) and total eNOS and Akt. The experiment shown is a representative of three independent experiments. *p < 0.05, **p < 0.01 versus control siRNA with the same treatment, ††p < 0.01 versus control siRNA with vehicle. Data represent means ± SEM. B. S1P plasma levels from wildtype (n=4) and EL deficient mice (n=6) do not differ on LC/MS/MS measurement (also see Supplemental Fig. 2).
Discussion
We have used siRNA and pharmacological approaches in cultured endothelial cells, as well as analyses using knockout mouse models to study the roles of endothelial lipase and S1P in HDL-induced angiogenesis and signaling responses. We found that endothelial lipase loss of function, through either the lipase inhibitor tetrahydrolipstatin, or through siRNA-mediated EL knockdown, markedly attenuated HDL-induced EC migration (Figure 1A–D), proliferation (Figure 1E, F) and tube formation (Figure 2). HDL-induced angiogenesis was also inhibited in ex vivo studies of aortic rings from EL-deficient mice (Figure 3). HDL-promoted Akt and eNOS phosphorylation was markedly inhibited following siRNA-mediated EL knockdown (Figure 4). The effects of EL/HDL on EC migration (Figure 5) as well as Akt and eNOS phosphorylation (Figure 6) were blocked by an antagonist to S1P1 but not S1P3, while S1P stimulation reversed the inhibition of these endothelial responses seen with EL siRNA exposure. Taken together, these experiments using lipase inhibitors, siRNA approaches, and a genetic mouse model strongly implicate endothelial lipase as a key determinant of HDL-mediated physiological responses in the endothelium.
HDL elicits a broad range of physiological and signaling responses in the endothelium.30, 31 Importantly, HDL may induce substantively different responses in different vascular beds and experimental models. For example, HDL promotes tube formation through Ras/ERK1/2 signaling pathway in human coronary artery endothelial cells,3 while Akt-dependent pathways appear to modulate HDL responses in human umbilical vein endothelial cells32. Other studies implicate AMP-activated protein kinase in HDL responses, including eNOS phosphorylation.33 The role of endothelial lipase in mediating these and other HDL effects has not been well explored. Our findings suggest differential expression and activity of endothelial lipase may be an under-recognized variable influencing HDL responses seen in b=various experimental models and perhaps in vivo as well.
The roles of EL-mediated HDL hydrolysis in atherosclerosis remain incompletely understood, with unresolved controversies in the field. Hara et al. reported that HDL from EL knockout mice retained the anti-inflammatory features seen in HDL isolated from WT mice.34 Moreover, Riederer et al. reported that lysophosphatidylcholine generated by the action of EL on HDL induced the expression of IL-8, a pro-inflammatory and pro-adhesive chemokine.24 These results lend support to the view that hydrolysis of HDL by EL has pro-inflammatory properties. In contrast, we previously reported that EL overexpression decreased TNFα-induced VCAM1 expression and promoter activity through PPARα activation in EC, thus limiting vascular inflammation.23 Interestingly, individuals with EL-loss of function variants have higher HDL but no apparent protection against atherosclerosis or its complications.25 In the present study, we have shown that HDL exposure to EL induces angiogenesis and promotes Akt/eNOS activation. Angiogenic therapies have been explored for treating cardiovascular diseases, yet clearly a complex, dynamic balance of pro- and anti-angiogenic pathways exist and may vary in different physiologic and pathologic settings.35 Our data suggests HDL and changes in EL expression and function as potential contributors to these patterns. Further elucidation about mechanisms through which HDL and HDL interaction with EL exert biologic effects may provide insight into divergent datasets regarding the impact of HDL, HDL-modulating therapy, and genetic EL variants on atherosclerosis.
The data provided here implicates S1P as a possible mediator of responses induced by HDL through endothelial lipase action. The S1P1 receptor antagonist W146 blocked HDL-induced EC migration as well as Akt/eNOS phosphorylation, while the S1P3 antagonist CAY10444 did not affect either HDL-induced EC migration or Akt/eNOS phosphorylation. Decerased EL levels, as achieved with siRNA, inhibited HDL-induced EC migration and Akt/eNOS phosphorylation, responses that were restored by adding back S1P (Figure 5, 6). Our results support the hypothesis of S1P as a key HDL constituent generated by endothelial lipase that can induce angiogenesis and elicit Akt/eNOS signaling responses. S1P and its receptors have been implicated in HDL-stimulated Akt/eNOS phosphorylation.11, 32, 35 We previously reported that free S1P activates eNOS through PI3-K/Akt signaling pathways,27, 36 a finding consistent with the current studies showing that HDL-associated S1P elicits similar effects. It is quite likely that other bioactive lipids in addition to S1P are liberated following hydrolysis of HDL by endothelial lipase. Indeed, the precise mechanism(s) through which EL promotes HDL-dependent S1P release and signaling remains to be determined. EL reportedly catalyzes the conversion of large HDL2 particles to small HDL3 particles, which are more highly enriched in S1P relative to large HDL2 particles.38–40 In addition, EL may also augment S1P effects by reducing S1P catabolism, inhibiting S1P lyase, and/or facilitating S1P delivery.
Based on our findings, it will be of particular interest to consider how apoM, as a recently identified S1P binding partner in HDL28, 29, might be involved in the EL-mediated effects reported here. One recent studied analyzed S1P plasma levels in relationship to apoM in different monogenic disorders of HDL metabolism.37 In that work, considerable variability in S1P levels was seen among the 10 Dutch patients with LIPG (EL) gene variations, which the authors speculated could have resulted from various issues. Our inability to detect changes in S1P levels in wildtype versus EL-deficient mouse plasma may have been influenced by multiple factors. Total plasma S1P reflects heterogenous protein-bound S1P pools. The levels of S1P actually bound to isolated murine HDL might be a more relevant measure, but has not been routinely done in the field and remains technically challenging (data not shown). The total plasma S1P concentration in mice does far exceed the EC50 for S1P-promoted Akt phosphorylation or eNOS activation (2–10 nM)36, arguing for the biologic plausibility of this mechanism. The possibility that EL might be involved in S1P release from HDL, either directly or indirectly, has been raised in various models38 but to our knowledge, the results presented here are the first that directly support this prospect. Of note, ABCA1, ABCG1 and SR-B1, important transporter proteins in HDL pathways, have all been implicated in S1P handling.39
We also provide evidence that S1P released from HDL by endothelial lipase activates S1P1 receptors that promote Akt/eNOS activation. Although other model systems have implicated both S1P3 and S1P1 receptors in these responses,39, 40 we found an S1P3 antagonist did not inhibit HDL-dependent effects in our system, while a S1P1 antagonist completely blocked responses to HDL. BAEC do express both S1P3 and S1P1.41, 42 Our experiments using S1P receptor subtype-selective antagonists identify the S1P1 receptor subtype as the principal receptor mediating the HDL response in these ECs.
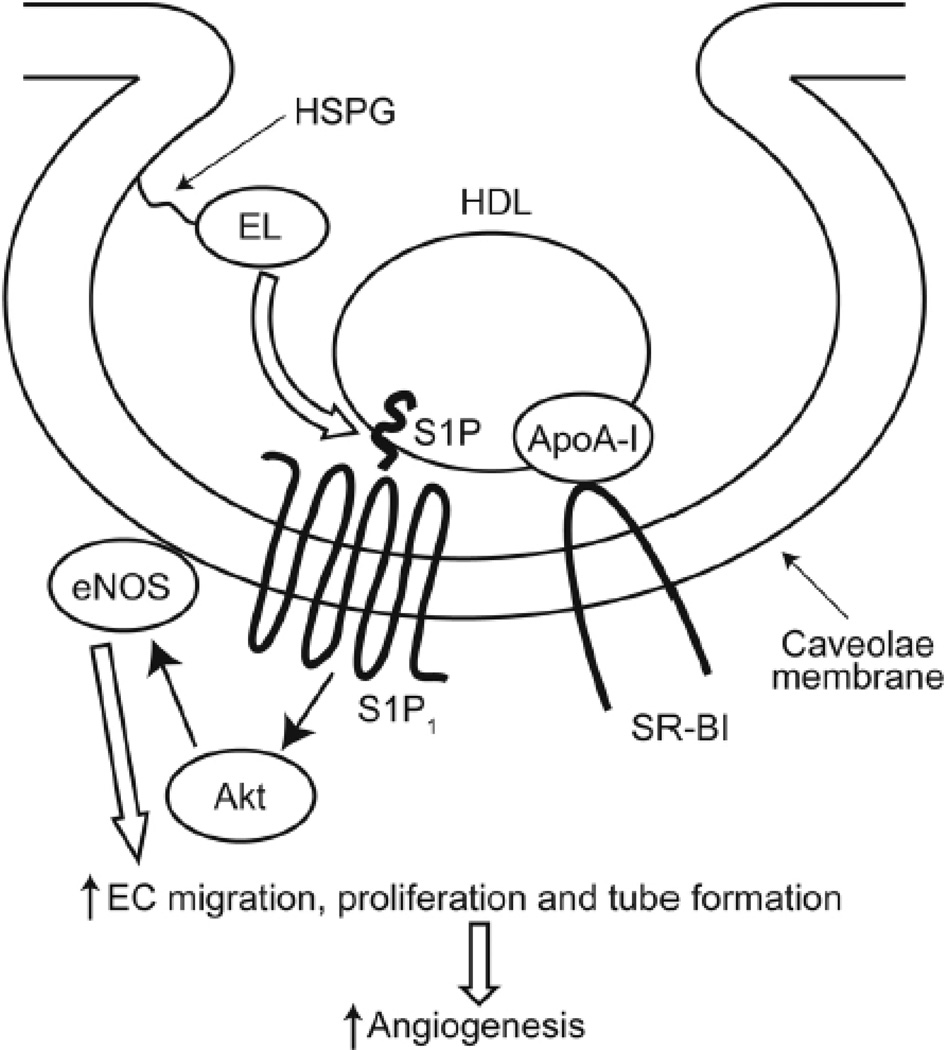
Taken together, these findings reveal endothelial lipase as a key player in facilitating the activation of HDL-dependent signals that lead to S1P1 receptor activation as well as Akt and eNOS phosphorylation, with functional effects on endothelial cell migration, tube formation and angiogenesis (Figure 7). These results provide new insights into the molecular mechanisms through which HDL, endothelial lipase, and their interaction may modulate angiogenesis and vascular responses.
Figure 7. Model of endothelial lipase-mediated HDL-dependent signaling via S1P/S1P1 pathways.
The data presented here suggests that HDL hydrolysis by endothelial lipase can release biologically active molecules that promote Akt and eNOS phosphorylation in endothelial cells, with functional effects promoting EC migration, tube formation and angiogenesis. These EL/HDL responses appear to involve HDL-bound S1P, which then binds to S1P1 receptors, inducing these endothelial effects. Key proteins involved in HDL biology, including SR-B1, ABC1, and ABCG8, have all been implicated in S1P transport. ApoA-I, apolipoprotein A-I; HSPG, heparan sulfate proteoglycan; SR-BI, scavenger receptor class B type I, ABCA1, ABCG1, ATP-binding cassette transporters.
Supplementary Material
Significance.
Controversies persist regarding the relationship between HDL and atherosclerosis. Despite a well-validated inverse relationship between HDL levels and cardiovascular events in epidemiologic studies, HDL-raising therapies levels have not shown clear clinical benefit in prospective, randomized clinical trials. Moreover, recent data reveals that genetic loss of function endothelial lipase (EL) variants have increased HDL levels without any obvious protection against cardiovascular events. Extensive studies now establish that specific lipases can exert many distinct and important biologic effects. We provide evidence here that EL interacts with HDL to promote angiogenesis, increase endothelial proliferation, and endothelial cell signaling via eNOS and aKT phosphorylation. These EL/HDL appear to involve the release of sphingosine-1 phosphate and signaling through the S1P1 receptor. This data provides new perspectives on how EL interaction with HDL may modulate endothelial responses through S1P release and offers an example of how EL deficiency might result in a loss of potentially beneficial effects.
Acknowledgments
We thank Hong Wang for technical assistance, and Takashi Shiroto and Ruqin Kou for helpful discussions.
Sources of Funding
This study was supported by NIH grants HL48743 (TM, JP) and HL46457 (TM), and the Mitsubishi Pharma Research Foundation and the Keio University Medical Science Fund (ST), and b.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
None.
References
- 1.Gordon DJ, Rifkind BM. High-density lipoprotein--the clinical implications of recent studies. N Engl J Med. 1989;321:1311–1316. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- 2.Rader DJ, Alexander ET, Weibel GL, et al. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res. 2009;50(Suppl):189–194. doi: 10.1194/jlr.R800088-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miura S-i, Fujino M, Matsuo Y, et al. High density lipoprotein-induced angiogenesis requires the activation of Ras/MAP kinase in human coronary artery endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:802–808. doi: 10.1161/01.ATV.0000066134.79956.58. [DOI] [PubMed] [Google Scholar]
- 4.Barter PJ, Nicholls S, Rye K-A, et al. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 5.Viswambharan H, Ming X-F, Zhu S, et al. Reconstituted high-density lipoprotein inhibits thrombin-induced endothelial tissue factor expression through inhibition of RhoA and stimulation of phosphatidylinositol 3-kinase but not Akt/endothelial nitric oxide synthase. Circ Res. 2004;94:918–925. doi: 10.1161/01.RES.0000124302.20396.B7. [DOI] [PubMed] [Google Scholar]
- 6.Sattler K, Levkau B. Sphingosine-1-phosphate as a mediator of high-density lipoprotein effects in cardiovascular protection. Cardiovasc Res. 2009;82:201–211. doi: 10.1093/cvr/cvp070. [DOI] [PubMed] [Google Scholar]
- 7.Argraves KM, Argraves WS. HDL serves as a S1P signaling platform mediating a multitude of cardiovascular effects. J Lipid Res. 2007;48:2325–2333. doi: 10.1194/jlr.R700011-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez C, Gonzalez-Diez M, Badimon L, et al. Sphingosine-1-phosphate: A bioactive lipid that confers high-density lipoprotein with vasculoprotection mediated by nitric oxide and prostacyclin. Thromb Haemost. 2009;101:665–673. [PubMed] [Google Scholar]
- 9.Rosen H, Gonzalez-Cabrera PJ, Sanna MG, et al. Sphingosine 1-phosphate receptor signaling. Annu Rev Biochem. 2009;78:743–768. doi: 10.1146/annurev.biochem.78.072407.103733. [DOI] [PubMed] [Google Scholar]
- 10.Lee MJ, Thangada S, Claffey KP, et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- 11.Argraves KM, Gazzolo PJ, Groh EM, et al. High density lipoprotein-associated sphingosine 1-phosphate promotes endothelial barrier function. J Biol Chem. 2008;283:25074–25081. doi: 10.1074/jbc.M801214200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nofer J-R, van der Giet M, Tolle M, et al. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J Clin Invest. 2004;113:569–581. doi: 10.1172/JCI18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirata K, Dichek HL, Cioffi JA, et al. Cloning of a unique lipase from endothelial cells extends the lipase gene family. J Biol Chem. 1999;274:14170–14175. doi: 10.1074/jbc.274.20.14170. [DOI] [PubMed] [Google Scholar]
- 14.Jaye M, Lynch KJ, Krawiec J, et al. A novel endothelial-derived lipase that modulates HDL metabolism. Nat Genet. 1999;21:424–428. doi: 10.1038/7766. [DOI] [PubMed] [Google Scholar]
- 15.Yu KCW, David C, Kadambi S, et al. Endothelial lipase is synthesized by hepatic and aorta endothelial cells and its expression is altered in apoE-deficient mice. J Lipid Res. 2004;45:1614–1623. doi: 10.1194/jlr.M400069-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.McCoy MG, Sun G-S, Marchadier D, et al. Characterization of the lipolytic activity of endothelial lipase. J Lipid Res. 2002;43:921–929. [PubMed] [Google Scholar]
- 17.Ishida T, Choi S, Kundu RK, et al. Endothelial lipase is a major determinant of HDL level. J Clin Invest. 2003;111:347–355. doi: 10.1172/JCI16306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edmondson AC, Brown RJ, Kathiresan S, et al. Loss-of-function variants in endothelial lipase are a cause of elevated HDL cholesterol in humans. J Clin Invest. 2009;119:1042–1050. doi: 10.1172/JCI37176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown RJ, Edmondson AC, Griffon N, et al. A naturally occurring variant of endothelial lipase associated with elevated HDL exhibits impaired synthesis. J Lipid Res. 2009;50:1910–1916. doi: 10.1194/jlr.P900020-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishida T, Choi SY, Kundu RK, et al. Endothelial lipase modulates susceptibility to atherosclerosis in apolipoprotein-E-deficient mice. J Biol Chem. 2004;279:45085–45092. doi: 10.1074/jbc.M406360200. [DOI] [PubMed] [Google Scholar]
- 21.Ko KWS, Paul A, Ma K, et al. Endothelial lipase modulates HDL but has no effect on atherosclerosis development in apoE−/− and LDLR−/− mice. J Lipid Res. 2005;46:2586–2594. doi: 10.1194/jlr.M500366-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Broedl UC, Maugeais C, Millar JS, et al. Endothelial lipase promotes the catabolism of ApoB-containing lipoproteins. Circ Res. 2004;94:1554–1561. doi: 10.1161/01.RES.0000130657.00222.39. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed W, Orasanu G, Nehra V, et al. High-density lipoprotein hydrolysis by endothelial lipase activates PPARalpha: a candidate mechanism for high-density lipoprotein-mediated repression of leukocyte adhesion. Circ Res. 2006;98:490–498. doi: 10.1161/01.RES.0000205846.46812.be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vergeer M, Cohn DM, Boekholdt SM, et al. Lack of association between common genetic variation in endothelial lipase (LIPG) and the risk for CAD and DVT. Atherosclerosis. 2010;211:558–564. doi: 10.1016/j.atherosclerosis.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skoura A, Hla T. Regulation of vascular physiology and pathology by the S1P2 receptor subtype. Cardiovasc Res. 2009;82:221–228. doi: 10.1093/cvr/cvp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Igarashi J, Michel T. Sphingosine 1-phosphate and isoform-specific activation of phosphoinositide 3-kinase beta. Evidence for divergence and convergence of receptor-regulated endothelial nitric-oxide synthase signaling pathways. J Biol Chem. 2001;276:36281–36288. doi: 10.1074/jbc.M105628200. [DOI] [PubMed] [Google Scholar]
- 28.Sevvana M, Ahnstrom J, Egerer-Sieber C, et al. Serendipitous fatty acid binding reveals the structural determinants for ligand recognition in apolipoprotein M. J Mol Biol. 2009;393:920–936. doi: 10.1016/j.jmb.2009.08.071. [DOI] [PubMed] [Google Scholar]
- 29.Christoffersen C, Obinata H, Kumaraswamy SB, et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc Natl Acad Sci U S A. 2011;108:9613–9618. doi: 10.1073/pnas.1103187108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saddar S, Mineo C, Shaul PW. Signaling by the high-affinity HDL receptor scavenger receptor B type I. Arterioscler Thromb Vasc Biol. 2010;30:144–150. doi: 10.1161/ATVBAHA.109.196170. [DOI] [PubMed] [Google Scholar]
- 31.O'Connell BJ, Genest J., Jr High-density lipoproteins and endothelial function. Circulation. 2001;104:1978–1983. doi: 10.1161/hc3901.096667. [DOI] [PubMed] [Google Scholar]
- 32.Kimura T, Tomura H, Sato K, et al. Mechanism and role of high density lipoprotein-induced activation of AMP-activated protein kinase in endothelial cells. J Biol Chem. 2010;285:4387–4397. doi: 10.1074/jbc.M109.043869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drew BG, Fidge NH, Gallon-Beaumier G, et al. High-density lipoprotein and apolipoprotein AI increase endothelial NO synthase activity by protein association and multisite phosphorylation. Proc Natl Acad Sci U S A. 2004;101:6999–7004. doi: 10.1073/pnas.0306266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hara T, Ishida T, Kojima Y, et al. Targeted deletion of endothelial lipase increases HDL particles with anti-inflammatory properties both in vitro and in vivo. J Lipid Res. 2011;52:57–67. doi: 10.1194/jlr.M008417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khurana R, Simons M, Martin JF, et al. Role of angiogenesis in cardiovascular disease: a critical appraisal. Circulation. 2005;112:1813–1824. doi: 10.1161/CIRCULATIONAHA.105.535294. [DOI] [PubMed] [Google Scholar]
- 36.Igarashi J, Bernier SG, Michel T. Sphingosine 1-phosphate and activation of endothelial nitric-oxide synthase. differential regulation of Akt and MAP kinase pathways by EDG and bradykinin receptors in vascular endothelial cells. J Biol Chem. 2001;276:12420–12426. doi: 10.1074/jbc.M008375200. [DOI] [PubMed] [Google Scholar]
- 37.Karuna R, Park R, Othman A, et al. Plasma levels of sphingosine-1-phosphate and apolipoprotein M in patients with monogenic disorders of HDL metabolism. Atherosclerosis. 2011;219:855–863. doi: 10.1016/j.atherosclerosis.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 38.Kontush A, Therond P, Zerrad A, et al. Preferential sphingosine-1-phosphate enrichment and sphingomyelin depletion are key features of small dense HDL3 particles: relevance to antiapoptotic and antioxidative activities. Arterioscler Thromb Vasc Biol. 2007;27:1843–1849. doi: 10.1161/ATVBAHA.107.145672. [DOI] [PubMed] [Google Scholar]
- 39.Liu X, Xiong SL, Yi GH. ABCA1, ABCG1, and SR-BI: Transit of HDL-associated sphingosine-1-phosphate. Clin Chim Acta. 2012;413:384–390. doi: 10.1016/j.cca.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Igarashi J, Miyoshi M, Hashimoto T, et al. Statins induce S1P1 receptors and enhance endothelial nitric oxide production in response to high-density lipoproteins. Br J Pharmacol. 2007;150:470–479. doi: 10.1038/sj.bjp.0707114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang F, Van Brocklyn JR, Hobson JP, et al. Sphingosine 1-phosphate stimulates cell migration through a G(i)-coupled cell surface receptor. Potential involvement in angiogenesis. J Biol Chem. 1999;274:35343–35350. doi: 10.1074/jbc.274.50.35343. [DOI] [PubMed] [Google Scholar]
- 42.Lee H, Goetzl EJ, An S. Lysophosphatidic acid and sphingosine 1-phosphate stimulate endothelial cell wound healing. Am J Physiol Cell Physiol. 2000;278:C612–C618. doi: 10.1152/ajpcell.2000.278.3.C612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.