Abstract
Atypical meningiomas have poor local control with emerging literature indicating the use of radiosurgery in treatment. The purpose of this study was to evaluate clinical outcomes including local control and failure pattern after Gamma Knife radiosurgery (GKRS) and factors that may affect these outcomes. Between 1999 and 2008, 24 patients were treated with GKRS as either primary or salvage treatment for pathologically proven atypical meningiomas. Treatment failures were determined by serial magnetic resonance imaging. A median marginal dose of 14 Gy was used (range 10.5–18 Gy). Overall local control rates at 1, 2, and 5 years were 75, 51, and 44%, respectively. With median follow-up time of 42.5 months, 14 of 24 patients experienced a treatment failure at time of last follow-up. Eight recurrences were in-field, four were marginal failures, and two were distant failures. Wilcoxon analysis revealed that the conformality index (CI) was a significant predictor of local recurrence (P = 0.04). CI did not predict for distant recurrences (P = 0.16). On multivariate analysis evaluating factors predicting progression free survival, dose >14 Gy was found to be statistically significant (P = 0.01). There appears to be a dose response using GKRS beyond 14 Gy but given the suboptimal local control rates in this study, higher doses may still be needed to obtain better local control.
Keywords: Atypical meningioma, Conformality index, Gamma Knife radiosurgery
Introduction
Atypical meningiomas comprise approximately 4–7% of meningiomas [1]. Local control of these tumors is suboptimal and has been reported in the range of 38–68% [2–9]. The standard therapy for atypical meningiomas has been maximal safe resection followed by possible adjuvant external beam radiotherapy (EBRT). Salvage therapy, primarily including re-operation and stereotactic radiosurgery are often used. There is an emerging literature on the use of radiosurgery in the treatment of atypical meningiomas with local control rates ranging from 29 to 83% [3, 10–12].
Radiosurgical management of benign meningiomas has limited target volume to enhancing disease alone, without treatment of a dural margin or dural tail [13, 14]. This targeting of a limited volume with radiosurgery has been substantiated with excellent local control in multiple series for benign meningiomas. Conversely, atypical meningiomas have a higher failure rate after radiosurgery than benign histology [4, 9], and it has been unclear as to whether this is because of increased resistance to radiation, or because of insufficient targeting of the tumor [6]. Due to the relative rarity of atypical meningiomas, they have often been combined with malignant meningiomas in series reported in the scientific literature [15]. However, atypical histology is known to have better local control and survival than the malignant counterpart [16].
We present a single institution retrospective review of our use of Gamma Knife radiosurgery (GKRS) in the treatment of atypical meningiomas. We sought to evaluate the patterns of failure of patients with atypical meningioma treated with GKRS and to determine factors such as conformality index (CI) or marginal dose which might affect clinical outcomes.
Methods
Patient characteristics
This study was approved by the Wake Forest University Institutional Review Board. Between 1999 and 2008, 214 patients were treated with GKRS at Wake Forest University Baptist Medical Center for a diagnosis of meningioma. Of these, 38 patients were treated for atypical meningioma. Fourteen patients who did not have pathology reviewed within our institution or without imaging follow-up were excluded from the analysis. Patients with hemangiopericytomas or meningeal sarcomas were excluded from analysis. In total, 24 patients treated for pathologically proven atypical meningioma had sufficient follow-up for analysis. The patient data were retrospectively reviewed from the electronic medical record. Patient demographics, tumor locations, and tumor volumes (TVs) are summarized in Table 1.
Table 1.
Patient and treatment characteristics
| Patient, sex, age at GK |
Tumor location |
TV (cm3) |
Prescription dose |
CI (treatment volume/TV) |
Recurrence type (in-field, marginal, distant) |
Time to first recurrence after GK |
Prior fractionated radiation (Y/N) |
|---|---|---|---|---|---|---|---|
| 1, F, 68 | Frontal lobe | 6.6 | 12.5 Gy to 48% isodose line | 1.68 | Marginal | 64 months | N |
| 2, M, 30 | Frontal lobe | 0.168 | 15 Gy to 50% isodose line | 3.51 | Marginal | 20 months | Y |
| 3, F, 73 | Frontal lobe | 7 | 15 Gy to 50% isodose line | 2.01 | Distant | 25 months | Y |
| 4, M, 78 | Frontal lobe | 11.3 | 13.5 Gy to 45% isodose line | 1.72 | In-field | 2 months | Y |
| 5, F, 69 | Frontal lobe | 13.4 | 12 Gy to 45% isodose line | 1.04 | In-field | 2 months | Y |
| 6, F, 63 | Frontal lobe | 1.8 | 18 Gy to 50% isodose line | 1.56 | In-field | 94 months | N |
| 7, F, 51 | Cavernous sinus | 8.35 | 14 Gy to 50% isodose line | 1.2 | In-field | 19 months | N |
| 8, M, 70 | Frontal lobe | 8.9 | 14 Gy to 50% isodose line | 1.12 | In-field | 11 months | N |
| 9, F, 57 | Frontal lobe | 6.3 | 15 Gy to 50% isodose line | 1.43 | No recurrence | N/A | N |
| 10, F, 55 | Frontal lobe | 0.32 | 12.5 Gy to 45% isodose line | 16.5 | No recurrence | N/A | N |
| 11, F, 68 | Temporal lobe | 1.8 | 16 Gy to 50% isodose line | 2.83 | No recurrence | N/A | N |
| 12, F, 57 | Frontal lobe | 25.4 | 10.5 Gy to 50% isodose line | 1.39 | In-field | 5 months | N |
| 13, M, 63 | Frontal lobe | 0.629 | 18 Gy to 50% isodose line | 2.38 | Marginal | 26 months | Y |
| 14, M, 74 | Frontal lobe | 44.08 | 11.5 Gy to 44% isodose line | 1.91 | No recurrence | N/A | N |
| 15, F, 58 | Frontal lobe | 1.8 | 15 Gy to 47% isodose line | 1.39 | In-field | 15 months | Y |
| 16, F, 58 | Parietal lobe | 0.23 | 17 Gy to 50% isodose line | 12.85 | In-field | 12 months | Y |
| 17, F, 76 | Cavernous sinus | 4.7 | 12.7 Gy to 50% isodose line | 1.38 | In-field | 12 months | N |
| 18, F, 83 | Parietal lobe | 0.32 | 12 Gy to 50% isodose line | 1.84 | No recurrence | N/A | Y |
| 19, F, 67 | Parietal lobe | 23.3 | 12 Gy to 50% isodose line | 1.46 | No recurrence | N/A | N |
| 20, F, 70 | Temporal lobe | 8 | 12 Gy to 50% isodose line | 1.63 | No recurrence | N/A | N |
| 21, M, 66 | Parietal lobe | 1.8 | 18 Gy to 50% isodose line | 1.56 | No recurrence | N/A | N |
| 22, F, 69 | Parietal lobe | Not available | 14 Gy to 50% isodose line | Not available | Marginal | 6 months | Y |
| 23, F, 58 | Frontal lobe | 6.2 | 16 Gy to 50% isodose line | 1.65 | No recurrence | N/A | Y |
| 24, M, 6 | Frontal lobe | 0.21 | 18 Gy to 50% isodose line | 2.78 | No recurrence | N/A | N |
Histological classification
Tumors were classified as atypical if they had any of the following features: (1) high mitotic index (≥4 mitosis per 10 high-power fields); (2) 3 or 4 of the following histologic characteristics: sheeting, hypercellularity, macronuclei, or small cell formation; or (3) brain invasion. Pathology for all patients in the analysis was re-reviewed by our pathologist to ensure tumors met the 2007 WHO definition for grade II meningioma.
Radiosurgery and dosimetry
All patients were initially evaluated by a radiation oncologist and neurosurgeon. Prior to radiosurgery, patients underwent a high-resolution stereotactic magnetic resonance imaging (MRI) study of the brain using a 1.5 T MRI until 2005, after which a 3T MRI was used. Our institutional standard MRI sequences used in treatment planning were T1 with contrast and spoiled gradient recall acquisition in steady state (SPGR) using slice thickness between 1.0 and 3.0 mm with no gap. Conformal radiosurgery treatment plans were generated using the Leksell GammaPlan treatment planning system (Elekta AB, Stockholm, SE). All patients were treated with the Leksell Model B or C Gamma Knife units (Elekta AB, Stockholm, SE). Patients were treated with a median marginal prescription dose of 14 Gy, with a range of 10.5–18 Gy, targeting enhancing gross TV without margin. The wide dose range that was used in this series was based upon a wide range of TVs, and the fact that six radiation oncologists and three neurosurgeons treated this patient group over a 9-year period. Because of toxicity data published by Kondziolka et al. [17] citing a 5% incidence of new neurologic deficits with median marginal doses of 16 Gy, and a trend towards dose de-escalation for meningiomas in general, the median doses used in the current series are lower than has been previously reported for atypical meningiomas [18]. A simple CI was calculated for each tumor, defined as the prescription isodose volume (PIV) divided by the TV: CI = PIV/TV [19]. Additional dosimetric factors that were recorded and included in the analysis were the number of isocenters and the maximum dose.
Patient follow-up
Patient outcomes were determined by their electronic medical records. Performance status was determined by the Eastern Cooperative Oncology Group grading scale. Patients were followed after GKRS by the treating neurosurgeon both clinically, and with serial MRI. In general, post-GKRS MRI was performed 3 months after GKRS, and then every 6 months for the first 2 years, and then annually after that unless the patient experienced a recurrence or new symptoms.
Treatment failures were determined by serial imaging. Local recurrence of meningioma was defined as a tumor recurrence within 2 cm of the tumor margin on diagnostic MRI. Local recurrences were further divided into central and marginal recurrences. A central, or “in-field”, recurrence was defined as occurring within the radiosurgical prescription volume, whereas a marginal recurrence was defined as occurring less than 2 cm from the edge of the prescription volume. Distant recurrence was defined as a tumor recurrence greater than 2 cm from the edge of prescription volume.
Toxicity was graded based on the SOMA/LENT scale [20].
Statistics
Kaplan–Meier analysis was used to estimate local control, progression free survival, and overall survival. Univariate analysis was performed on the Kaplan–Meier curves using the log-rank statistic with a P < 0.05 set as significant. Multivariate analysis using the variables marginal dose, maximum dose, TV, isocenter number, CI, and tumor location was performed using non-parametric (Wilcoxon) tests.
Results
Tumor control and survival
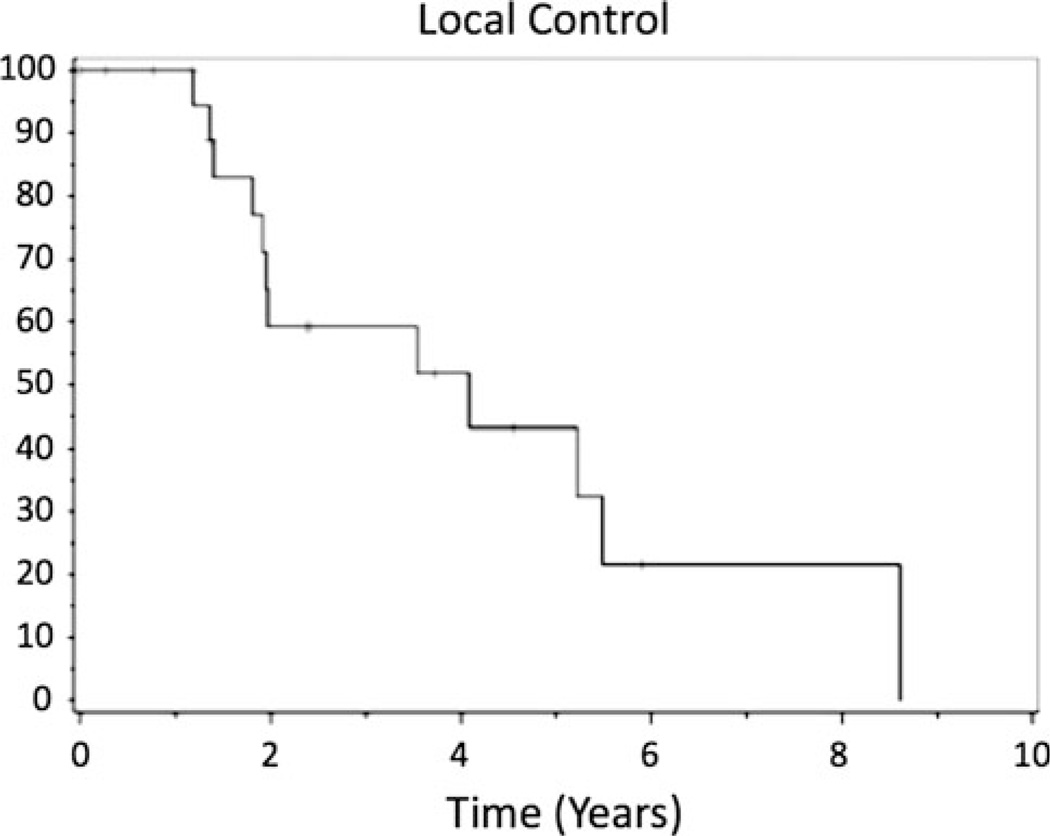
With a median follow-up time of 42.5 months (range 3–103 months), local control rates were 75, 51, and 44% at 1, 2, and 5 years, respectively (Fig. 1). Nine of 24 patients (38%) experienced local failure, either within the treatment volume or within 2 cm of the treatment volume. The median time to local recurrence (in-field or marginal recurrence) was 24.8 months. Median progression free survival time (in-field, marginal, or distant recurrence) was 23.7 months. Progression free survival was 40% at 2 years and 25% at 5 years.
Fig. 1.
Kaplan–Meier plot of time to local failure
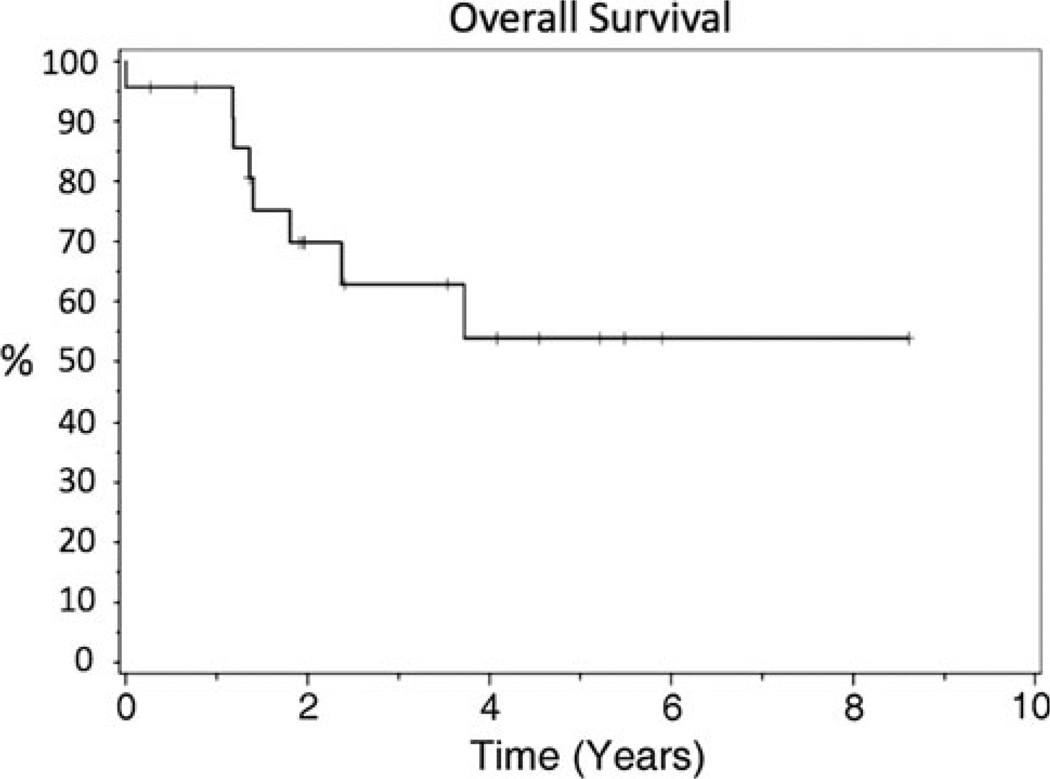
Fourteen of 24 patients (58%) were alive at last follow-up. Overall survival rates at 1, 2, and 5 years was 92, 67, and 52%, respectively (Fig. 2).
Fig. 2.
Kaplan–Meier plot of overall survival
Patterns of failure
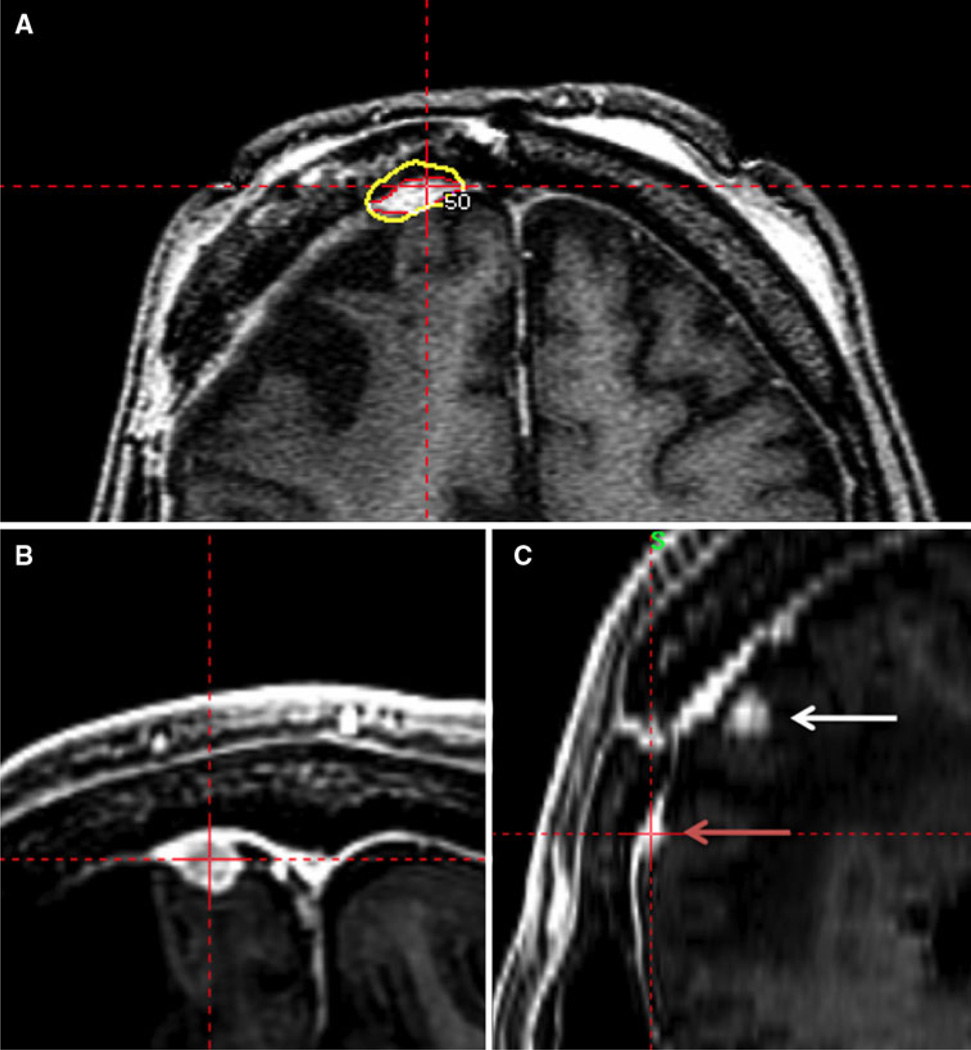
Out of a total of 24 patients, 14 experienced treatment failure. Of those who recurred, 8 recurrences were in-field (within radiosurgical treatment volume), 4 recurrences were marginal failures (out of radiosurgical treatment volume but less than 2 cm from volume; i.e., adjacent to radiosurgical treatment volume), and 2 recurred distantly (>2 cm from treatment volume). Of the 4 marginal failures, 2 of these were attached to the original tumor, and the other 2 arose from adjacent dura but were not attached to the index lesion. All marginal recurrences were dural; no recurrences were parenchymal. Figure 3 depicts an example of a marginal recurrence. Of the 24 patients treated with GKRS, 11 had previous fractionated EBRT. The median dose of EBRT was 5,580 cGy (range 1,800–6,480 cGy). The mean time to failure and subsequent GKRS was 3 years. Among patients who had previous EBRT, there were 3 in-field failures, 4 marginal failures, and 2 distant failures in patients previously receiving EBRT. Thirteen patients had no previous fractionated EBRT. Of these, all five treatment failures were in-field failures with a mean time to subsequent failure of 2.6 years.
Fig. 3.
a Targeting of atypical meningioma. Axial SPGR MRI sequence with radiosurgical dose targeting enhancing tumor. The yellow line represents the 50% isodose line. The red line represents tumor delineation. b, c Follow-up MRI demonstrating marginal recurrence. b An axial SPGR MRI sequence. c Sagittal SPGR MRI sequence
Of the 14 GKRS treatment failures, 5 underwent surgery, 7 SRS, 2 EBRT, and 1 hydroxyurea.
Predictors of treatment outcome
Multivariate analysis using Wilcoxon analysis was performed to determine if any patient-related or treatment-related factors predicted for local control, progression free survival, or overall survival. The CI was found to be statistically significant for predicting local or marginal recurrence (P = 0.04). Approximately, 65% of recurrences occurred in patients who had a CI <2. Patients who recurred had a mean CI of 1.7 versus 4.6 in the patients who did not recur. None of age, sex, TV, maximum dose, or marginal dose predicted for either local control.
On multivariate analysis evaluating factors for time to failure, dose was found to be statistically significant (P = 0.01). The median dose of those who failed in-field or marginally was 14 Gy. The median dose of those who did not recur was 15 Gy. CI was not found to be statistically significant as predictor of time to failure. None of age, sex, TV, maximum dose, or tumor location predicted for treatment failure.
Multivariate analysis was unable to determine any patient-related or treatment-related factors that predicted for overall survival.
Toxicity
Of the 24 patients, two developed treatment-related toxicity (≥grade II). One of the patients, with a 4 cm left parasagittal tumor, was treated with a marginal dose of 10.5 Gy, and subsequently developed symptomatic edema (grade II). This was successfully treated with dexamethasone. This patient also had previously received fractionated EBRT prior to GKRS. The remaining patient, who had a 3 cm tumor in the right temporal convexity, was treated to a marginal dose of 13.5 Gy and also developed symptomatic edema (grade III). Symptoms resolved with dexamethasone.
Discussion
Surgery has generally been considered as first line treatment for atypical meningioma with the intention of maximal safe resection. Adjuvant radiotherapy is recommended in patients with residual disease and frequently in patients with totally resected tumors, although this is somewhat debatable. The role for radiosurgery has been controversial for atypical meningiomas, as there have been emerging series suggesting adequate local control rates, but with limited follow-up. In 1998, Hakim et al. [10] published a review of 127 patients with all types of meningiomas treated with linear accelerator-based SRS to a median marginal dose of 15 Gy. Of these, 26 had histologically proven atypical meningiomas. The 4-year local control rate was found to be 83% with median progression free survival of 24.4 months and a 3-year overall survival rate of 83%. The University of Pittsburgh also published results of 30 patients with non-benign meningiomas, which included 18 atypical meningioma patients [3]. With a mean marginal dose was 15 Gy, 5- and 10-year overall survival rates were 59% and the 5-year progression free survival rate was 83%. Because of the encouraging results, the authors recommended immediate radiosurgery for residual tumor after surgery and any recurrence found during follow-up. In our series, there was no significant difference in the local control rate between patients who have had previous radiotherapy versus those who were treated with radiosurgery in the absence of previous EBRT.
Historical local control rates for atypical meningiomas range from 38 to 68%, and are therefore significantly worse than for their benign counterparts [4, 21, 22]. As such, salvage therapy is often necessary, and in spite of multiple local therapies, these tumors can continue to fail locally. Our findings demonstrated a higher rate of local recurrences in the patients who had more conformal treatment volumes. This suggests that the pattern of failure is often one caused by a marginal miss of microscopic disease that is involving normal-appearing dura. A Japanese radiosurgical series recently reported 19 treatment failures for 30 tumors treated with linear accelerator-based radiosurgery. Of these failures, 6 occurred outside of the radiosurgical volume suggesting that highly focal radiotherapy does not encompass adjacent microscopic disease [6].
An implication of the higher marginal failure rate of atypical meningiomas is the use of a larger target volume during the treatment planning process. Tumor locations in close proximity to the optic structures or brainstem may provide a greater challenge during treatment planning. One must also be mindful that increased TVs, especially if greater than 4 cm3, will predict for greater likelihood of treatment-related edema [23].
When EBRT is used in the adjuvant setting for atypical meningiomas, the treatment volume is expanded in order to account for microscopic spread of disease. In the current RTOG phase II trial, a 1 cm volume expansion beyond enhancing tumor is used to define the CTV in order to account for microscopic spread of WHO grade II tumors. Recently, a series from Massachusetts General Hospital examined a series of patients who underwent gross total resection of atypical meningiomas. In the patients who received post-operative radiotherapy, 1 cm CTV margins were used with eight of eight patients who received post-operative radiotherapy achieving long-term local control of disease [21]. While evidence for radiosurgery targeting solely the enhancing tumor for WHO grade I meningiomas is abounding, our series adds to the evidence that for atypical meningiomas, a CTV expansion may reduce the rate of marginal/local failures. Addition of CTV margin on the order of what is used with EBRT would significantly increase the treated volume with radiosurgery, and thus the risk of toxicity. Addition of a 2–3 mm margin with radiosurgical treatment may be feasible, though the volumes would likely need to be customized to avoid parenchymal brain as well as critical structures such as the optics. Such techniques as hypofractionated stereotactic radiotherapy could be considered where addition of a 2–3 mm margin may be better tolerated given the use of fractionation to decrease toxicity risk. Alternatively, increasing the radiation dose delivered to the tumor with radiosurgery would also increase the dose delivered to the peritumoral tissue.
Because of the patterns of failure seen in the current series, radiosurgery appears to be a treatment modality best reserved as a salvage regimen for tumors that have failed prior external beam irradiation. Surgery with adjuvant EBRT allows for safer treatment of a CTV margin such that the entire dural base and possible extent of microscopically occult disease can be adequately covered in the radiation volume. With the advent of techniques such as intensity modulated radiation therapy, doses can be delivered to larger volumes while sparing dose to the optics and brainstem.
In the series by Kano et al. [6], the authors described a dose response for tumors that received greater than a 20 Gy marginal dose, though the majority of the tumors in this series were recurrent. In our series, we were also able to detect a dose response. These two series were different in that our series used doses in the range of 10.5–18 Gy with a median dose of 14 Gy. Our data suggests that the dose response may also exist in the dose range less than 20 Gy. With atypical meningiomas treated with EBRT, a dose response has been reported in multiple series. Goldsmith et al. demonstrated improved local control in patients receiving doses exceeding 53 Gy in a series including tumors that under the WHO 2007 definition would be considered grade II. A recent report of mixed photon and proton beam radiotherapy showed improved cause-specific and overall survival in patients receiving greater than 60 cobalt Gray equivalent. In the present series, multivariate analysis for time to treatment failure showed that dose was more important than CI. It is possible that with the pattern of failure analysis, lower conformality was causing delivery of a higher dose within the treatment volume. Given the suboptimal local control rate seen in the current series, higher doses may be warranted to obtain better control. Our current practice is to use doses between 15 and 18 Gy for non-recurrent meningiomas, and to consider doses as high as 20 Gy in atypical meningiomas that have recurred.
There are several limitations to this study. As a retrospective study, there is possibility for significant patient selection bias. As such, results are limited to hypothesis generation. One of the greatest sources of potential bias is the fact that 14 total patients were excluded from analysis because of either lack of follow-up or lack of confirmation of WHO grade II pathology within our institution. It is possible that the exclusion of this number of patients could have biased the ultimate results of this series. Prospective trials such as the RTOG 0539 study will be necessary to confirm the best treatment options for grade II meningiomas.
Conclusion
In conclusion, there appears to be a dose response using GKRS beyond 14 Gy but given the lower control rates in this study, higher doses may still be needed to obtain better local control.
Footnotes
Conflict of Interest None of the authors has any personal, financial, or professional conflicts of interest to report.
Portions of this were presented in poster/abstract form at the ASTRO 2009 National Meeting in Chicago, IL in November 2009.
Contributor Information
Albert Attia, Email: aattia@wakehealth.edu, Department of Radiation Oncology, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157, USA.
Michael D. Chan, Department of Radiation Oncology, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157, USA
Ryan T. Mott, Department of Pathology, Wake Forest University School of Medicine, Winston-Salem, NC, USA
Greg B. Russell, Department of Biostatistical Sciences, Wake Forest University School of Medicine, Winston-Salem, NC, USA
David Seif, Department of Anesthesiology, Wake Forest University School of Medicine, Winston-Salem, NC, USA.
J. Daniel Bourland, Department of Radiation Oncology, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157, USA.
Allan F. Deguzman, Department of Radiation Oncology, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157, USA
Thomas L. Ellis, Department of Neurosurgery, Wake Forest University School of Medicine, Winston-Salem, NC, USA
Kevin P. McMullen, Department of Radiation Oncology, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157, USA
Michael T. Munley, Department of Radiation Oncology, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157, USA
Stephen B. Tatter, Department of Neurosurgery, Wake Forest University School of Medicine, Winston-Salem, NC, USA
Edward G. Shaw, Department of Radiation Oncology, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157, USA
References
- 1.Whittle IR, Smith C, Navoo P, Collie D. Meningiomas. Lancet. 2004;363:1535–1543. doi: 10.1016/S0140-6736(04)16153-9. [DOI] [PubMed] [Google Scholar]
- 2.Jaaskelainen J. Seemingly complete removal of histologically benign intracranial meningioma: late recurrence rate and factors predicting recurrence in 657 patients. A multivariate analysis. Surg Neurol. 1986;26:461–469. doi: 10.1016/0090-3019(86)90259-4. [DOI] [PubMed] [Google Scholar]
- 3.Harris AE, Lee JY, Omalu B, Flickinger JC, Kondziolka D, Lunsford LD. The effect of radiosurgery during management of aggressive meningiomas. Surg Neurol. 2003;60:298–305. doi: 10.1016/s0090-3019(03)00320-3. (discussion 305) [DOI] [PubMed] [Google Scholar]
- 4.Goyal LK, Suh JH, Mohan DS, Prayson RA, Lee J, Barnett GH. Local control and overall survival in atypical meningioma: a retrospective study. Int J Radiat Oncol Biol Phys. 2000;46:57–61. doi: 10.1016/s0360-3016(99)00349-1. [DOI] [PubMed] [Google Scholar]
- 5.Hug EB, Devries A, Thornton AF, et al. Management of atypical and malignant meningiomas: role of high-dose, 3D-conformal radiation therapy. J Neurooncol. 2000;48:151–160. doi: 10.1023/a:1006434124794. [DOI] [PubMed] [Google Scholar]
- 6.Kano H, Takahashi JA, Katsuki T, et al. Stereotactic radiosurgery for atypical and anaplastic meningiomas. J Neurooncol. 2007;84:41–47. doi: 10.1007/s11060-007-9338-y. [DOI] [PubMed] [Google Scholar]
- 7.Winkler C, Dornfeld S, Schwarz R, Friedrich S, Baumann M. The results of radiotherapy in meningiomas with a high risk of recurrence. A retrospective analysis. Strahlenther Onkol. 1998;174:624–628. doi: 10.1007/BF03038510. [DOI] [PubMed] [Google Scholar]
- 8.Condra KS, Buatti JM, Mendenhall WM, Friedman WA, Marcus RB, Jr, Rhoton AL. Benign meningiomas: primary treatment selection affects survival. Int J Radiat Oncol Biol Phys. 1997;39:427–436. doi: 10.1016/s0360-3016(97)00317-9. [DOI] [PubMed] [Google Scholar]
- 9.Stafford SL, Pollock BE, Foote RL, et al. Meningioma radiosurgery: tumor control, outcomes, and complications among 190 consecutive patients. Neurosurgery. 2001;49:1029–1037. doi: 10.1097/00006123-200111000-00001. (discussion 1027–1037) [DOI] [PubMed] [Google Scholar]
- 10.Hakim R, Alexander E, III, Loeffler JS, et al. Results of linear accelerator-based radiosurgery for intracranial meningiomas. Neurosurgery. 1998;42:446–453. doi: 10.1097/00006123-199803000-00002. (discussion 444–453) [DOI] [PubMed] [Google Scholar]
- 11.Huffmann BC, Reinacher PC, Gilsbach JM. Gamma knife surgery for atypical meningiomas. J Neurosurg. 2005;102(Suppl):283–286. doi: 10.3171/jns.2005.102.s_supplement.0283. [DOI] [PubMed] [Google Scholar]
- 12.Ojemann SG, Sneed PK, Larson DA, et al. Radiosurgery for malignant meningioma: results in 22 patients. J Neurosurg. 2000;93(Suppl 3):62–67. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 13.Henzel M, Gross MW, Hamm K, et al. Stereotactic radiotherapy of meningiomas: symptomatology, acute and late toxicity. Strahlenther Onkol. 2006;182:382–388. doi: 10.1007/s00066-006-1535-7. [DOI] [PubMed] [Google Scholar]
- 14.Debus J, Wuendrich M, Pirzkall A, et al. High efficacy of fractionated stereotactic radiotherapy of large base-of-skull meningiomas: long-term results. J Clin Oncol. 2001;19:3547–3553. doi: 10.1200/JCO.2001.19.15.3547. [DOI] [PubMed] [Google Scholar]
- 15.Boskos C, Feuvret L, Noel G, et al. Combined proton and photon conformal radiotherapy for intracranial atypical and malignant meningioma. Int J Radiat Oncol Biol Phys. 2009;75:399–406. doi: 10.1016/j.ijrobp.2008.10.053. [DOI] [PubMed] [Google Scholar]
- 16.Pasquier D, Bijmolt S, Veninga T, et al. Atypical and malignant meningioma: outcome and prognostic factors in 119 irradiated patients. A multicenter, retrospective study of the Rare Cancer Network. Int J Radiat Oncol Biol Phys. 2008;71:1388–1393. doi: 10.1016/j.ijrobp.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 17.Kondziolka D, Lunsford LD, McLaughlin MR, Flickinger JC. Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med. 1998;339:1426–1433. doi: 10.1056/NEJM199811123392003. [DOI] [PubMed] [Google Scholar]
- 18.Kondziolka D, Mathieu D, Lunsford LD, et al. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery. 2008;62:53–58. doi: 10.1227/01.NEU.0000311061.72626.0D. (discussion 58–60) [DOI] [PubMed] [Google Scholar]
- 19.Shaw E, Scott C, Souhami L, et al. Radiosurgery for the treatment of previously irradiated recurrent primary brain tumors and brain metastases: initial report of radiation therapy oncology group protocol (90-05) Int J Radiat Oncol Biol Phys. 1996;34:647–654. doi: 10.1016/0360-3016(95)02106-x. [DOI] [PubMed] [Google Scholar]
- 20.Pavy JJ, Denekamp J, Letschert J, et al. EORTC Late Effects Working Group. Late effects toxicity scoring: the SOMA scale. Radiother Oncol. 1995;35:11–15. doi: 10.1016/0167-8140(95)97448-m. [DOI] [PubMed] [Google Scholar]
- 21.Aghi MK, Carter BS, Cosgrove GR, et al. Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery. 2009;64:56–60. doi: 10.1227/01.NEU.0000330399.55586.63. (discussion 60) [DOI] [PubMed] [Google Scholar]
- 22.Goldsmith BJ, Wara WM, Wilson CB, Larson DA. Post-operative irradiation for subtotally resected meningiomas. A retrospective analysis of 140 patients treated from 1967 to 1990. J Neurosurg. 1994;80:195–201. doi: 10.3171/jns.1994.80.2.0195. [DOI] [PubMed] [Google Scholar]
- 23.Ganz JC, Backlund EO, Thorsen FA. The results of gamma knife surgery of meningiomas, related to size of tumor and dose. Stereotact Funct Neurosurg. 1993;61(Suppl 1):23–29. doi: 10.1159/000100656. [DOI] [PubMed] [Google Scholar]