Abstract
We are expected to live longer than if we had been born 100 years ago however, the additional years are not necessarily spent in good health or free from disability. Body composition changes dramatically over the course of life. There is a gradual increase in body weight throughout adult life until the age of about 60–65 years. In contrast, body weight appears to decrease with age after the age of 65–75 years, even in those demonstrating a previous healthy body weight. This age related decrease in body weight, often called unintentional weight loss or involuntary weight loss can be a significant problem for the elderly. This has been shown to be related to decline in appetite and food intake is common amongst the elderly and is often referred to the anorexia of aging. Underlying mechanisms regulate energy homeostasis and appetite may change as people age. In this review, peripheral factors regulating appetite have been summarized in regards to their age-dependent changes and role in the etiology of anorexia of aging. Understanding the alterations in the mechanisms regulating appetite and food intake in conjunction with aging may help inform strategies that promote healthy aging and promote health and wellbeing in the elderly years, with the end goal to add life to the years and not just years to our lives.
Keywords: Aging, anorexia, gut, hormones
During the aging process, changes occur that have significant impact on our general health and wellbeing. Over the past century it appears that there have been significant changes to the human life cycle. We are now expected to live longer than ever before, with estimated life expectancy at birth in the United States now 79 years, opposed to 47 years in 1900 (www.aoa.gov/Aging_Statistics/Profile/2012). This increase in life expectancy can be explained by the improvements in healthcare provision, causing a drop in mortality rates, rather an extension in the maximum life span per se. The increase in life expectancy has caused a shift in the population demographic, with the number of people aged 65 years and older predicted to increase by 135% between 2010 and 2050 (quickfacts.census.gov/qfd/states/00000.html). Moreover, the fastest growth is seen in the ‘oldest’ old; those aged 85 years and older, which is the group most likely to require healthcare services (esa.un.org/wpp/Excel-Data/population.htm). This shift can be partially explained by the slower growth in the proportion of the non-elderly in the US population. However, largely as a result of higher fertility rates and immigration, America’s population remains younger than other developed countries [1]. Nevertheless, like the rest of the world, the US is an ageing society; Americans are living longer in the period of their lives which they are considered ‘elderly’, the population average age is rising, and the proportion of the population classed as ‘elderly’ is increasing each year (www.aoa.gov/Aging_Statistics/Profile/2012). These demographic changes have profound implications for public health, the economy, social care systems, healthcare policies, and more importantly for human health and well-being. However, although based on these reports showing that the life expectancy has increased, the additional years are not necessarily spent in good health or free from disability. The risk of developing at least one non-communicable disease increases with age [2]. This is not so much of a function of age in years, but rather reflects an accumulation of risk factors during the course of life. Investigating physiological changes that occur in conjunction with aging may help inform strategies that promote healthy aging and promote health and wellbeing in the elderly years, with the end goal to add life to the years and not just years to our lives.
Body composition changes dramatically over the course of life. There is a gradual increase in body weight throughout adult life until the age of about 60–65 years. In contrast, body weight appears to decrease with age after the age of 65–75 years, even in those demonstrating a previous healthy body weight [3–5]. This age related decrease in body weight, often called unintentional weight loss [6] or involuntary weight loss [7] can be a significant problem for the elderly. It is estimated that the prevalence of unintentional weight loss and involuntary weight loss could be as high as 27% in high risk populations. More specifically, this weight loss occurs in the form of lean muscle tissue loss, also called sarcopenia[8, 9]. This loss of muscle mass may result in loss of strength [10, 11], frailty [12], bone fractures [13], higher risk of falls [14, 15], greater injury from falling [16, 17], higher rates of hospital admissions (www.cdc.gov/HomeanRecreationalSafety/Falls/adultfalls.html), delayed recovery [18]and premature mortality [19–21]. Furthermore, the relative stability of BMI during this period often masks the true severity of loss of lean tissues [22], since in some cases there is an increase in body fat which accompanies the loss of lean tissue. The weight loss in older individuals [23] occurs mainly as the result of a progressive decrease in energy intake [24–26] as well as reductions in energy expenditure from both reduced physical activity [27] and reduced basal metabolic rate due to less metabolically active tissue mass [28, 29]. Several studies in rats have shown that there is an age related decrease in body weight, and this decrease is often preceded by a decreased food intake [30–32]. Similarly, data from human studies using dietary surveys report low energy intake in elderly, suggesting the elderly population may be malnourished, which despite being one of the greatest threats to health and well being of older adults, is increasingly common among the elderly [33]. It is estimated that 4.3% community-living individuals aged over 65 years are malnourished, and as high as 37% of the institutionalized elderly demonstrate “protein-energy malnutrition” [34]. However, despite strong evidence to suggest that the changes in food intake are responsible for the age related changes in body weight and body composition, the mechanisms responsible for the age related changes in dietary intake are unclear.
The decline in appetite and food intake is common amongst the elderly and is often referred to the anorexia of aging [34, 35]. In a study which focused on the ability to perform specific activities of daily living, over 27% of community-resident and institutionalized Medicare beneficiaries aged over 65 years reported that among all daily activities (e.g. walking, dressing, showering, using toilet, getting out of bed/chair), the activity that showed no significant increases in age-related limitations was ‘eating behavior’ [36]. These findings suggest that the anorexia of aging cannot directly be explained by age-related limitations of daily activity. On the other hand, the age-related limitations on more strenuous physical activity [37, 38] may lead to decrease in energy expenditure which may cause a reduction in appetite. Although one study showed that acute food intake regulation was not attenuated by physical activity status [39], reduced physical activity is an important factor contributing to reduced energy requirement in the elderly [40, 41]. Future studies need to address age-related effects of energy homeostasis while controlling for physical activity levels in older individuals. The mechanisms responsible for the reduction in energy intake that lead to the anorexia of aging are not clearly understood, but are of increasing interest and have been the focus of many recent reviews [42]. Identifying the mechanisms responsible for this age related anorexia, is a key step to develop future treatment and preventions strategies to prevent age related weight loss and adverse changes in body weight and composition which represent such a risk of malnutrition, frailty and falls in the aging population.
Unlike energy expenditure, energy intake occurs in distinct bouts and fluctuates considerably from day to day in response to individual demands [43]. The fact that food intake does vary considerably indicates that it is a controlled process. This control is achieved by regulating the initiation and termination of eating occasions; hunger triggers the meal initiation which determines the daily number of meals (meal frequency) whereas satiation terminates a meal and determines the size of a particular meal [44]. Overall, hunger and satiation are tightly regulated by peripheral and central systems as well as the environmental factors interact to control energy homeostasis. Studies have reported that the systems involved in regulating energy homeostasis may become increasingly ineffective as we age. Roberts et al [45]investigated the effects of overfeeding (energy intake > energy requirements), and underfeeding (energy intake < energy requirements) on subsequent energy intake and body weight in young and elderly men. Following the period of underfeeding, young men effectively compensated for the energy deficit by increasing their subsequent voluntary energy intake. Furthermore, following the period of overfeeding the young men decreased their subsequent meal intake to accurately compensate for the excess energy intake. Conversely, the elderly men were unable to adjust their subsequent meal intakes in both of the conditions. Resultantly, the young men were able to re-gain the weight they had lost during the underfeeding intervention, and lose the weight they had gained during the overfeeding intervention. However, the older men re-gained just over 60% weight they lost during underfeeding, and were only able to lose about 30% of the weight they had gained during the overfeeding intervention [45]. In another study, Rolls et al [46] assessed energy intake following a standard fixed energy yogurt preload in young (aged 18–35 yrs) and older (age 60–84 yrs) men. The older men were unable to compensate for the yogurt preload by reducing intake at a subsequent test meal as precisely as younger men. Although the older men reported higher fullness ratings following the yogurt preload, they consistently overate at the subsequent test meal [46]. Together these findings support the notion the mechanisms controlling energy intake are impaired in the elderly.
However, the precise mechanism underlying the impaired regulation on energy homeostasis in the elderly is unclear; partially because the mechanisms involved in the successful control of energy intake in healthy, young adults are not fully understood, but also because these mechanisms may also be dysregulated, resulting the excess energy intake and development of overweight and obesity in the younger population. It is also important to note that as with the majority of human physiology studies, selected study participants are mostly without health-related issues which may not be representative of the general population, particularly the elderly population who are struggling with various major and multiple health problems. Thus, the results from these studies may even underestimate the true extent and severity of the energy balance dysregulation in elderly individuals with (multiple) chronic diseases.
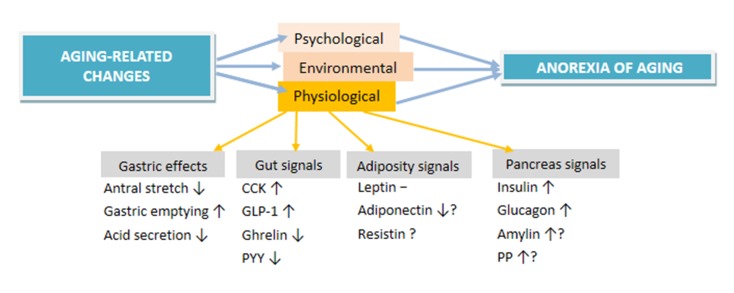
The complexity of anorexia in the elderly is also related to the fact that energy balance is a multifactorial phenomenon that is undeniable regulated by complex peripheral and central mechanisms [47]. Meal size (satiation) is affected by various factors include olfactory stimuli [48], stretch receptor signals originating in the stomach and proximal small intestine [49–51], nutrients (glucose, amino acids), metabolites (lactate, pyruvate and ketones) and alterations in gut hormones including cholecystokinin (CCK), glucagon like peptide -1 (GLP-1) and ghrelin in response to nutrient ingestion. These signals have indirect effects on the central mechanisms via activation of afferent vagal fibres, or direct effects through the blood to release neurotransmitters. However, these mechanisms are also affected by the environment context specifically the obesogenic environment that we live in [52], it is clear that the etiology of anorexia of aging involves multiple failures in the physiology (Figure 1)[6]. Elucidating the age-related alterations in the mechanisms associated with the energy homeostasis may lead novel strategies for prevention and intervention methods for anorexia of aging.
Figure 1.
The factors involved in the etiology of anorexia of aging
Gastric effects
One of the factors that has been shown to play a role in the etiology of anorexia in the elderly is antral stretch is [53]. Impaired relaxation of proximal stomach in the elderly might also cause rapid antral filling and earlier antral stretch [54] which may contribute to early satiation [55]. Several studies have reported a decreased gastric acid secretion, and slower rate of gastric emptying in the elderly (age 70–84 year) compared to young (age 23–50 year) persons[56, 57]. On the other hand, Madsen et al (2004) found no changes in gastric emptying associated with age are small in young (20–30 years) versus (74–85 years) participants. One possible speculation would be that the effects of smoking in the older population may explain the inconsistency in the results from different studies, as it has been shown that smoking delays gastric emptying [58, 59]. Thus, studies measuring gastric emptying need to control for the smoking habits of the participants. Moreover, the studies which demonstrate a negative effect of age on gastric acid secretion often fail to exclude study participants who are suffering from atrophic gastritis or the presence of helicobacter pylori (H.pylori) -its causative bacterium [60]. In contrast, studies that have excluded study participants with gastric disease have reported that no age-related decrease in gastric acid secretion [61]. Furthermore, although the presence of H.pylori could explain a lower gastric acid secretion in elderly persons, when the presence of the bacterium was controlled for the negative effect of age on pepsin output persisted [61]. Moreover, there is a large variability in gastric emptying rate in both between- and within-subject [62]. Therefore it is possible that the differences that have been shown in gastric emptying in older versus younger persons may need to be reconsidered based on these concerns. In addition to several age-associated changes in gut physiology, altered synthesis, secretion and impaired responsiveness to signals from both brain and gut are part of the main factors underlying the anorexia of aging. There is increasing evidence showing the importance of the hormones from the gastrointestinal tract (cholecystokinin, glucagon-like peptide, ghrelin, Peptide-YY), pancreatic signals (e.g. insulin), and adipokines (e.g. leptin) in producing the anorexia of aging. In the next sections, we will review the evidence for potential role in the anorexia of aging.
Signals from the gut
Cholecystokinin (CCK)
CCK is released predominately from the I cells in the duodenum and jejunum in response to the presence of nutrients in the intestinal lumen [63–65]. Higher concentrations are associated with a reduction in meal size, the duration of a meal, and enhanced perceptions of fullness and reduced hunger during the course of a meal in both humans and animal models [66–68]. CCK co-ordinates the release of digestive enzymes from the pancreas, stimulates gall bladder contraction, increases intestinal motility and inhibits gastric emptying [63, 69]. Fatty acids and proteins are particularly potent stimuli for CCK secretion, although fat in the triacylglyceride form does not cause postprandial elevation of CCK [70–72], and fatty acids with chain lengths of 12 or more carbons are the most potent stimuli for CCK. Studies with animals showed that vagotomised rats do not show the lipid induced delay in gastric emptying or suppression in subsequent food intake [73, 74], and furthermore administration of dexoxyglumide; a selective CCK-1 receptor antagonist blocks the fatty acid induced slowing of gastric emptying, and abolishes the associated reduction in the size of a subsequent meal [74]. These findings suggest that the CCK gut-brain signalling is a vagally mediated process, and this hypothesis is supported by reports that the CCK-1 receptor expression in human vagal afferent neurons [75, 76].
Some studies have suggested an increase in CCK signaling with age, which ultimately suppresses food intake and resultantly causes weight loss. MacIntosh et al [35] reported that elderly persons had higher fasting plasma CCK concentrations, and demonstrated greater CCK response following intraduodenal lipid infusion. However, despite these higher circulating concentrations, the older men did not report any change in subjective hunger following the nutrient infusion. In another study by the same authors, an intravenous CCK-8 infusion, caused greater suppression in food intake and higher plasma CCK-8 concentrations in the older persons[77]. Thus, older individuals have higher fasting concentrations of CCK, and they appear to be more sensitive to the effects of elevated CCK than younger persons suggesting a possibility for an aging-related resistance to the appetite suppressing effects of CCK [77].
Glucagon like peptide-1 (GLP-1)
GLP-1 is mainly released by the L cells of the distal small intestine in response to nutrient ingestion [78–80]. Following a meal GLP-1 is secreted in two phases; the first short rapid release occurs 5–10 min postprandially[78, 81], followed by a second delayed and extended release occurring 30 to 60 min following the ingestion of a meal. Thus, it is hypothesized that there are two distinct mechanisms of GLP-1 secretion. The initial phase, where GLP-1 is secreted rapidly into the blood stream, occurs before the GLP-1-secreting L cells are in direct contact with the nutrients. Therefore it is likely that a proximal to distal gut signalling pathway exists to regulate the response of the L cells during this initial phase [82]. The second, delayed GLP-1 secretion phase may be associated with the direct stimulation of the L cells by the ingested nutrients.
Central administration of GLP-1 reduced food intake, slows gastric emptying and reduces body weight in rats [83]. Furthermore intravenous administration of GLP-1 is reported to suppress appetite and reduce energy intake in some [84–86], but not all studies [87, 88]. There is consistent evidence that GLP-1 can slow the rate of gastric emptying, even within a physiological range [89–91], which occurs via activation of vagal afferent pathways [92]. MacIntosh et al [93] showed that there was no difference between young and older persons in their plasma GLP-1 following glucose infusion via intraduodenal feeding tube which bypassed the antrum. However, several others reported increased GLP-1 responses after a preload meal [94] and to oral glucose in older compared to young women [95], suggesting that increased GLP-1 may be contributing to suppressed appetite and lower energy intake in older individuals.
Ghrelin
Ghrelin is predominately synthesized by the oxyntic cells in the stomach in response to a period of fasting [96]. Unlike many of the other gut peptides plasma ghrelin concentrations rise in the fasted state [97], and ghrelin concentrations are suppressed, rather than stimulated by nutrient exposure in the stomach, duodenum, jejunum [98], with carbohydrates and proteins more potent suppressors of ghrelin than fats [99]. Ghrelin stimulates food intake when administered peripherally or directly into the brain [100]. Central administration of ghrelin in rats has been shown to increase the number of meals without causing significant changes to the size of each eating occasion [101], resultantly rats ate more and gained weight.
In humans peripheral ghrelin infusion to achieve physiological plasma concentrations is a potent stimulator of energy intake [102]. The pre-prandial rise in plasma ghrelin is associated with an increase in subjective hunger ratings [103], suggesting that ghrelin is an important signal in meal initiation, opposed to controlling the size of meals. Long term infusion of ghrelin in rats creates hyperphagia, and results in increased adiposity. It is reported that older persons have ghrelin concentrations that are a third lower than those observed in younger persons [104]. Postprandial ghrelin concentrations were higher in undernourished elderly than well nourished elderly and young group [105]. However others have failed to demonstrate similar results [106]. It is possible that differences in BMI between the groups may confound the results as BMI is reported to be associated with plasma ghrelin concentrations [107]. Moreover, using total, acylated and desacylated forms of ghrelin may confound the results; for instance Di Francesco and colleagues [108] reported that acylated ghrelin –that is the active component of ghrelin, was lower in elderly in fasting conditions. Similarly, Bauer et al [109] reported that basal and postprandial levels of active and total ghrelin declined postprandially (carbohydrate-rich test meal) only in the younger (N=15; age=35.4 years) than the older (N=19; age=80.7 years) persons. In line with this, they also showed that older participants had higher fullness and lower hunger self-ratings postprandially in the absence of a postprandial decline in ghrelin levels. This indicates a clear manifestation of anorexia specific for the elderly. Interestingly although both anorexia nervosa and anorexia of aging are marked with reduced food intake, the fasting and circulating levels of the hunger gut hormone ghrelin levels -when compared with the controls, are higher in AN patients (vs. healthy controls) whereas lower in anorexia of aging group (vs. young persons). This may indicate that in AN, the high levels of ghrelin is a consequence of reduced food intake whereas in anorexia of aging, the low ghrelin levels may be the contributing factor for reduced food intake.
Peptide YY (PYY)
PYY is produced in the small intestine and colon, released from the gastrointestinal tract after eating a meal, and inhibits energy intake in rats and humans [110]. Although PYY concentrations have been shown to decrease with age in rats and humans which might influence energy intake [111], its role in unintentional weight loss has yet to be studied.
Signals from the adipose tissue
Leptin
Leptin is another possible factor that plays a role in the development of aging anorexia. Leptin is an anorexigenic hormone secreted by the adipose tissue proportionally to the adiposity levels in the body influencing long-term energy balance status [112]. In a study with healthy elderly (age = 67–78 years; BMI = 18–36 kg/m2) women, fasting plasma leptin levels were found to be higher than the young persons, even after adjusting for body fat mass [113]. They also found that leptin and insulin levels were significantly correlated in elderly women [113]. In another study [114]with an older group of elderly individuals (age = 74–82 years; 77.9 ± 1 year (m ± SD), postprandial (from 30 minutes to 4 hours) serum leptin was also significantly higher compared with the young individuals (age = 25–38 years; 29.5 ± 1 year). Di Francesco et al [114](2006) also reported that older group also had higher levels of serum leptin prior to the meal intake, thus leptin concentrations did not change significantly postprandially in both groups. They showed no time x age interaction effect which indicates that leptin did not changes significantly short after a meal [114] which is in line with the leptin’s role in long-term rather than short-term energy balance [47]. On the other hand, Bauer et al [109] reported no difference between old and young persons in basal leptin levels and following meal ingestion as well as over time in young (N = 15; age = 35.4 years) and older (N = 19; age = 80.7) groups.
Adiponectin
Adiponectinor adipocyte complement–related protein of 30 kDa is a circulating protein produced and secreted from adipose tissue into the bloodstream. It is involved in glucose regulation and fatty acid oxidation [115] and inversely correlated with body fat percentage in adults [116]. Circulating adiponectin levels were found to show steep increase by the third week of postnataly and became relatively stable therafter in mice [117]. An association between insulin sensitivity and circulating levels of adiponectin has also been shown in adult mice [118] and humans [119]. Cnop et al [120] showed that adiponectin positively correlated with age, and negatively correlated with BMI. Thus, age-dependent decrease in insulin sensitivity, it may be possible that adiponectin levels may show age-dependent increases.
Resistin
Resistin an adipocyte-secreted factor and FIZZ3 [121, 122], is a 12.5-kDa cysteine-rich protein that is specifically expressed in white adipose tissue and when released into the circulation it reduces the energy intake by a central mechanism affecting the NPY neurons in animals and humans and suppresses insulin-stimulated glucose uptakein adipocytes [123, 124]. Energy restriction reduces resistin gene expression and insulin resistance in rodents [125]. To date, there has been no study investigating the alterations in resistin or its effects have been reported.
Signals from the pancreas
Insulin
Insulin is one of the principal hormones involved in glucose metabolism and one of the satiety signals that acts on the arcuate nucleus of lateral hypothalamus to reduce food intake [126]. Several studies assessed fasting levels of insulin in response to various stimuli such as food intake, intraduodenal food administration or intravenous CCK, and results varied in different protocols. Several studies found that basal levels of insulin elderly and young individuals did not show any difference [93, 94, 109, 127], whereas significantly higher basal insulin levels in elderly compared with young individuals were reported by others [104, 105, 114].
Di Francesco and colleagues [114] showed that fasting plasma insulin was higher in the elderly than in the young persons. They also reported a significant time x age interaction effect showing a higher peak of plasma insulin at 30min postprandially in the elderly [114]. When the elderly group is divided as under and well nourished, insulin levels were found to be higher in well nourished elderly than in both undernourished elderly and well nourished young individuals [105]. Greater rise in blood glucose and insulin were shown in elderly compared to the young persons following intraduodenal glucose infusions [105]. Another study using intraduodenal glucose and lipid infusions (on separate days) for 120 minutes showed that although basal plasma insulin levels did not differ, increased significantly in older than younger individuals following glucose intraduodenal infusions [93]. Interestingly in a similar study with the age-matched groups, MacIntosh et al. [77] observed statistically significantly higher basal levels of insulin in young than the elderly persons (5.6 ± 0.4 vs. 4.6 ± 0.3 mU/liter; p < 0.05) and blood glucose concentrations were statistically significantly lower in young than in older persons at baseline (4.9 ± 0.1 vs. 5.2 ± 0.1 mmol/L; p < 0.05).
Glucagon
Glucagon is produced by α-cells in the pancreatic islets of Langerhans. Researchers postulated that glucagon might contribute to satiety in older adults [23]. Glucagon decreases energy intake by reducing meal size via a mechanism causing increases in the glucose oxidation for energy [128]. In an animal study, there was no significant effect of glucagon on food intake as a function of age in mice [129, 130]. Melanson et al [130]compared older women (N = 8; age = 72 ± 2 yrs) and young women (N = 8; age = 25 ± 2 yrs) in their ability to maintain normal blood glucose homeostasis following a meal consumption. Although young and older women did not differ in their hormone and metabolite responses to fasting and consumption of a small meal (snacks), following consumption of large meals (2092 and 4148 kj), older individuals showed higher levels of blood glucagon [130].
Amylin
Islet amyloid polypeptide (IAPP, amylin), like insulin, is produced in the β-cells within the pancreatic islets of Langerhans in response to blood glucose levels. Amylin inhibits insulin-stimulated glucose metabolism and muscle glycogen synthesis. It slows gastric emptying and reduces energy intake through effects on the hypothalamus [131]. When administered as an anti-obesity agent, amylin reduces food intake and lead to weight loss in obese patients [132]. However there is limited evidence from either animal [128, 129], or human studies [133, 134] that aging alters amylin secretion or effect. Intraperitoneal administration of amylin has been shown to be slightly but not significantly more potent in suppressing food intake in young than older rats [135]. In humans, basal amylin concentration and increase 120 minutes following a oral glucose load did not differ between older (from 5.3 ± 0.5 to 16.4 ± 2.3 pmol/l) and young group (from 4.9 ± 0.5 to 14.1 ± 1.5 pmol/l) [133]. However, another study comparing, elderly and young study participants amylin responses following an oral glucose tolerance test (OGTT) were found to be proportional to insulin levels indicating reduced levels of amylin (impaired amylin release) in the older compared to the younger persons[136, 137]. However, based on the insulin sensitivity changes in the elderly, amylin may be merely be acting as a marker of impaired glucose metabolism [136]. Further studies needed to systematically investigate the age-based amylin alterations in individuals controlling for insulin sensitivity and glucose mechanisms.
Pancreatic polypeptide (PP)
PP is a 36-amino acid peptide discovered in the early 1970s. It is synthesized by endocrine F cells of the pancreatic islets and secreted from the pancreas postprandiallyin proportion to the amount of calories ingested [138]. It reduces energy intake and body weight by slowing gastric emptying [139]. In addition, PP is expressed in a sparse number of endocrine cells in the small and large intestine [140]. It is a satiety signal that is released 10–20 min following a meal [141] which increases satiety and decreases test meal intake [142]. PP also inhibits gastric emptying through centrally mediated mechanism that involves the vagal nerves which may also be part of the contributors to its appetite reduction role [143, 144]. To our knowledge, there are very few studies to date investigating the change in PP levels with aging. Plasma PP is secreted in a diurnal pattern and shown to be higher in the adults older than 35 years [145]. It has also been shown that, on the intestinal epithelium, PP can also inhibit intestinal electrolyte and water secretion [146] which may be a mechanism that can explain the constipation that is seen in the elderly [147]. Thus, one may speculate that PP may contribute to unintentional or involuntary weight loss in elderly people due to their anorectic effects.
Conclusion
Underlying mechanisms regulate energy homeostasis may change as people age. It is possible that variations in peripheral satiety signals including GLP-1, CCK, leptin and ghrelin may be affect the central control mechanisms responsible for co-ordination of energy intake control signals. In healthy old individuals, hunger signals prevail over satiety signals, and this is perhaps an adopted response to the age related increasing of BMI up until the ages 70 to 75 [6]. It is possible that this adopted response may then contribute to the prolonged satiety and inhibition of hunger after age 70–75. This condition may lead to a calorie deficit and finally to malnutrition in the elderly and may be one of the explanations for the anorexia of aging in the older individuals. Thus the increases in the satiety signals (i.e. GLP-1) due to relatively large amounts of fat and the reductions in the acylated to desacylated ratio of ghrelin [108], would lead to decreased hunger in the elderly. This condition may lead to a reduction in energy intake which results in anorexia of aging. A similar interplay between hunger signal ghrelin and short term satiety hormone CCK has been proposed as an explanation for anorexia of aging [126]. Energy homeostasis is complex mechanism with multiple central and peripheral signals leading hunger and satiety. To date, there are signals related to energy homeostasis that are yet to be studied [42] such as; bombesin, glucose-dependent insulinotropic polypeptide, glucagon-like peptide 1, obestatin, orexins, and resistin. Moreover, central factors also have highly complex pivotal roles in energy homeostasis; however age related alterations in central appetite signals in humans are very limited, thus we have not included that literature in this review.
References
- [1].United Nations Department of Economics and Social Affairs 2011. World population prospects, the 2010 revision Vol. 2013.
- [2].Beaglehole R, Yach D. Globalisation and the prevention and control of non-communicable disease: the neglected chronic diseases of adults. Lancet. 2003;362:903–908. doi: 10.1016/S0140-6736(03)14335-8. [DOI] [PubMed] [Google Scholar]
- [3].Steen B. Body composition and aging. Nutr Rev. 1988;46:45–51. doi: 10.1111/j.1753-4887.1988.tb05386.x. [DOI] [PubMed] [Google Scholar]
- [4].Shimokata H, Tobin JD, Muller DC, Elahi D, Coon PJ, Andres R. Studies in the distribution of body fat: I. Effects of age, sex, and obesity. J Gerontol. 1989;44:M66–73. doi: 10.1093/geronj/44.2.m66. [DOI] [PubMed] [Google Scholar]
- [5].Chumlea WC, Garry PJ, Hunt WC, Rhyne RL. Distributions of serial changes in stature and weight in a healthy elderly population. Hum Biol. 1988;60:917–925. [PubMed] [Google Scholar]
- [6].Wernette CM, White BD, Zizza CA. Signaling proteins that influence energy intake may affect unintentional weight loss in elderly persons. J Am Diet Assoc. 2011;111:864–873. doi: 10.1016/j.jada.2011.03.011. [DOI] [PubMed] [Google Scholar]
- [7].Wallace JI, Schwartz RS. Involuntary weight loss in elderly outpatients: recognition, etiologies, and treatment. Clin Geriatr Med. 1997;13:717–735. [PubMed] [Google Scholar]
- [8].Rosenberg IH. Summary comments. Am J Clin Nutr. 1989;50:1231–1233. [Google Scholar]
- [9].Hao R, Hong G. Anorexia, undernutrition, weight loss, sarcopenia, and cachexia of aging. Eur Rev Aging Phys Act. 2012;9:119–127. [Google Scholar]
- [10].Perry HM, 3rd, Miller DK, Patrick P, Morley JE. Testosterone and leptin in older African-American men: relationship to age, strength, function, and season. Metabolism. 2000;49:1085–1091. doi: 10.1053/meta.2000.7710. [DOI] [PubMed] [Google Scholar]
- [11].Zoico E, Di Francesco V, Guralnik JM, Mazzali G, Bortolani A, Guariento S, Sergi G, Bosello O, Zamboni M. Physical disability and muscular strength in relation to obesity and different body composition indexes in a sample of healthy elderly women. Int J Obes Relat Metab Disord. 2004;28:234–241. doi: 10.1038/sj.ijo.0802552. [DOI] [PubMed] [Google Scholar]
- [12].Gobbens RJ, van Assen MA, Luijkx KG, Wijnen-Sponselee MT, Schols JM. Determinants of frailty. J Am Med Dir Assoc. 2010;11:356–364. doi: 10.1016/j.jamda.2009.11.008. [DOI] [PubMed] [Google Scholar]
- [13].Lang T, Cauley JA, Tylavsky F, Bauer D, Cummings S, Harris TB. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res. 2010;25:513–519. doi: 10.1359/jbmr.090807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: A review of the literature. Maturitas. 2013;75:51–61. doi: 10.1016/j.maturitas.2013.02.009. [DOI] [PubMed] [Google Scholar]
- [15].Wang C, Bai L. Sarcopenia in the elderly: basic and clinical issues. Geriatr Gerontol Int. 2012;12:388–396. doi: 10.1111/j.1447-0594.2012.00851.x. [DOI] [PubMed] [Google Scholar]
- [16].Stevens JA, Dellinger AM. Motor vehicle and fall related deaths among older Americans 1990–98: sex, race, and ethnic disparities. Inj Prev. 2002;8:272–275. doi: 10.1136/ip.8.4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stevens JA, Corso PS, Finkelstein EA, Miller TR. The costs of fatal and non-fatal falls among older adults. Inj Prev. 2006;12:290–295. doi: 10.1136/ip.2005.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Demling RH, DeSanti L. Involuntary weight loss and the nonhealing wound: the role of anabolic agents. Adv Wound Care. 1999;12:1–14. quiz 15–16. [PubMed] [Google Scholar]
- [19].Cornali C, Franzoni S, Frisoni GB, Trabucchi M. Anorexia as an independent predictor of mortality. J Am Geriatr Soc. 2005;53:354–355. doi: 10.1111/j.1532-5415.2005.53126_4.x. [DOI] [PubMed] [Google Scholar]
- [20].Landi F, Liperoti R, Lattanzio F, Russo A, Tosato M, Barillaro C, Bernabei R, Onder G. Effects of anorexia on mortality among older adults receiving home care: an observation study. J Nutr Health Aging. 2012;16:79–83. doi: 10.1007/s12603-011-0064-y. [DOI] [PubMed] [Google Scholar]
- [21].Serra-Prat M, Palomera E, Roca M, Puig-Domingo M. Long-term effect of ghrelin on nutritional status and functional capacity in the elderly: a population-based cohort study. Clin Endocrinol (Oxf) 2010;73:41–47. doi: 10.1111/j.1365-2265.2009.03730.x. [DOI] [PubMed] [Google Scholar]
- [22].Kimyagarov S, Klid R, Levenkrohn S, Fleissig Y, Kopel B, Arad M, Adunsky A. Body mass index (BMI), body composition and mortality of nursing home elderly residents. Arch Gerontol Geriatr. 2010;51:227–230. doi: 10.1016/j.archger.2009.10.013. [DOI] [PubMed] [Google Scholar]
- [23].Roberts SB, Rosenberg I. Nutrition and aging: changes in the regulation of energy metabolism with aging. Physiol Rev. 2006;86:651–667. doi: 10.1152/physrev.00019.2005. [DOI] [PubMed] [Google Scholar]
- [24].de Groot CP, van Staveren WA, de Graaf C. Determinants of macronutrient intake in elderly people. Eur J Clin Nutr. 2000;54(Suppl 3):S70–76. doi: 10.1038/sj.ejcn.1601028. [DOI] [PubMed] [Google Scholar]
- [25].Hallfrisch J, Muller D, Drinkwater D, Tobin J, Andres R. Continuing diet trends in men: the Baltimore Longitudinal Study of Aging (1961–1987) J Gerontol. 1990;45:M186–191. doi: 10.1093/geronj/45.6.m186. [DOI] [PubMed] [Google Scholar]
- [26].Koehler KM. The New Mexico Aging Process Study. Nutr Rev. 1994;52:S34–37. doi: 10.1111/j.1753-4887.1994.tb01446.x. [DOI] [PubMed] [Google Scholar]
- [27].Centers for Disease Control and Prevention (CDC) Prevalence of physical activity, including lifestyle activities among adults--United States, 2000–2001. MMWR Morb Mortal Wkly Rep. 2003;52:764–769. [PubMed] [Google Scholar]
- [28].Elia M, Ritz P, Stubbs RJ. Total energy expenditure in the elderly. Eur J Clin Nutr. 2000;54(Suppl 3):S92–103. doi: 10.1038/sj.ejcn.1601030. [DOI] [PubMed] [Google Scholar]
- [29].Roberts SB, Fuss P, Heyman MB, Young VR. Influence of age on energy requirements. Am J Clin Nutr. 1995;62:1053S–1058S. doi: 10.1093/ajcn/62.5.1053S. [DOI] [PubMed] [Google Scholar]
- [30].Chapman IM, MacIntosh CG, Morley JE, Horowitz M. The anorexia of ageing. Biogerontology. 2002;3:67–71. doi: 10.1023/a:1015211530695. [DOI] [PubMed] [Google Scholar]
- [31].McDonald RB, Florez-Duquet M, Murtagh-Mark C, Horwitz BA. Relationship between cold-induced thermoregulation and spontaneous rapid body weight loss of aging F344 rats. Am J Physiol. 1996;271:R1115–1122. doi: 10.1152/ajpregu.1996.271.5.R1115. [DOI] [PubMed] [Google Scholar]
- [32].Wolden-Hanson T. Mechanisms of the anorexia of aging in the Brown Norway rat. Physiol Behav. 2006;88:267–276. doi: 10.1016/j.physbeh.2006.05.032. [DOI] [PubMed] [Google Scholar]
- [33].Margetts BM, Thompson RL, Elia M, Jackson AA. Prevalence of risk of undernutrition is associated with poor health status in older people in the UK. Eur J Clin Nutr. 2003;57:69–74. doi: 10.1038/sj.ejcn.1601499. [DOI] [PubMed] [Google Scholar]
- [34].Morley JE. Protein-energy malnutrition in older subjects. Proc Nutr Soc. 1998;57:587–592. doi: 10.1079/pns19980085. [DOI] [PubMed] [Google Scholar]
- [35].MacIntosh CG, Andrews JM, Jones KL, Wishart JM, Morris HA, Jansen JB, Morley JE, Horowitz M, Chapman IM. Effects of age on concentrations of plasma cholecystokinin, glucagon-like peptide 1, and peptide YY and their relation to appetite and pyloric motility. Am J Clin Nutr. 1999;69:999–1006. doi: 10.1093/ajcn/69.5.999. [DOI] [PubMed] [Google Scholar]
- [36].Administration on Aging 2012. A profile of older Americans 2012.
- [37].Dallosso HM, Morgan K, Bassey EJ, Ebrahim SB, Fentem PH, Arie TH. Levels of customary physical activity among the old and the very old living at home. J Epidemiol Community Health. 1988;42:121–127. doi: 10.1136/jech.42.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bassey EJ, Harries UJ. Normal values for handgrip strength in 920 men and women aged over 65 years, and longitudinal changes over 4 years in 620 survivors. Clin Sci (Lond) 1993;84:331–337. doi: 10.1042/cs0840331. [DOI] [PubMed] [Google Scholar]
- [39].Van Walleghen EL, Orr JS, Gentile CL, Davy KP, Davy BM. Habitual physical activity differentially affects acute and short-term energy intake regulation in young and older adults. Int J Obes (Lond) 2007;31:1277–1285. doi: 10.1038/sj.ijo.0803579. [DOI] [PubMed] [Google Scholar]
- [40].Durnin JV. Food intake and energy expenditure of elderly people. Gerontol Clin (Basel) 1962;4:128–133. doi: 10.1159/000244723. [DOI] [PubMed] [Google Scholar]
- [41].Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- [42].Malafarina V, Uriz-Otano F, Gil-Guerrero L, Iniesta R. The anorexia of ageing: Physiopathology, prevalence, associated comorbidity and mortality. A systematic review Maturitas. 2013;74:293–302. doi: 10.1016/j.maturitas.2013.01.016. [DOI] [PubMed] [Google Scholar]
- [43].Consolazio CF, Johnson HL, Daws TA, Nelson RA. Energy requirement and metabolism during exposure to extreme environments. World Rev Nutr Diet. 1973;18:177–194. doi: 10.1159/000394231. [DOI] [PubMed] [Google Scholar]
- [44].Burton-Freeman B, Gietzen DW, Schneeman BO. Meal pattern analysis to investigate the satiating potential of fat, carbohydrate, and protein in rats. Am J Physiol. 1997;273:R1916–1922. doi: 10.1152/ajpregu.1997.273.6.R1916. [DOI] [PubMed] [Google Scholar]
- [45].Roberts SB, Fuss P, Heyman MB, Evans WJ, Tsay R, Rasmussen H, Fiatarone M, Cortiella J, Dallal GE, Young VR. Control of food intake in older men. JAMA. 1994;272:1601–1606. doi: 10.1001/jama.1994.03520200057036. [DOI] [PubMed] [Google Scholar]
- [46].Rolls BJ, Dimeo KA, Shide DJ. Age-related impairments in the regulation of food intake. Am J Clin Nutr. 1995;62:923–931. doi: 10.1093/ajcn/62.5.923. [DOI] [PubMed] [Google Scholar]
- [47].Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- [48].Rolls ET. Sensory processing in the brain related to the control of food intake. Proc Nutr Soc. 2007;66:96–112. doi: 10.1017/S0029665107005332. [DOI] [PubMed] [Google Scholar]
- [49].Paintal AS. A study of gastric stretch receptors; their role in the peripheral mechanism of satiation of hunger and thirst. J Physiol. 1954;126:255–270. doi: 10.1113/jphysiol.1954.sp005207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Iggo A. Tension receptors in the stomach and the urinary bladder. J Physiol. 1955;128:593–607. doi: 10.1113/jphysiol.1955.sp005327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Iggo A. Gastro-intestinal tension receptors with unmyelinated afferent fibres in the vagus of the cat. Q J Exp Physiol Cogn Med Sci. 1957;42:130–143. doi: 10.1113/expphysiol.1957.sp001228. [DOI] [PubMed] [Google Scholar]
- [52].Atalayer D, Rowland NE. Structure of motivation using food demand in mice. Physiol Behav. 2011;104:15–19. doi: 10.1016/j.physbeh.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Jones KL, Doran SM, Hveem K, Bartholomeusz FD, Morley JE, Sun WM, Chatterton BE, Horowitz M. Relation between postprandial satiation and antral area in normal subjects. Am J Clin Nutr. 1997;66:127–132. doi: 10.1093/ajcn/66.1.127. [DOI] [PubMed] [Google Scholar]
- [54].Morley JE. Anorexia of aging: physiologic and pathologic. Am J Clin Nutr. 1997;66:760–773. doi: 10.1093/ajcn/66.4.760. [DOI] [PubMed] [Google Scholar]
- [55].Morley JE. Decreased food intake with aging. J Gerontol A Biol Sci Med Sci. 2001;56(Spec No 2):81–88. doi: 10.1093/gerona/56.suppl_2.81. [DOI] [PubMed] [Google Scholar]
- [56].Cave DR. Transmission and epidemiology of Helicobacter pylori. Am J Med. 1996;100:12S–17S. doi: 10.1016/s0002-9343(96)80224-5. discussion 17S–18S. [DOI] [PubMed] [Google Scholar]
- [57].Clarkston WK, Pantano MM, Morley JE, Horowitz M, Littlefield JM, Burton FR. Evidence for the anorexia of aging: gastrointestinal transit and hunger in healthy elderly vs. young adults. Am J Physiol. 1997;272:R243–248. doi: 10.1152/ajpregu.1997.272.1.R243. [DOI] [PubMed] [Google Scholar]
- [58].Scott AM, Kellow JE, Shuter B, Nolan JM, Hoschl R, Jones MP. Effects of cigarette smoking on solid and liquid intragastric distribution and gastric emptying. Gastroenterology. 1993;104:410–416. doi: 10.1016/0016-5085(93)90408-5. [DOI] [PubMed] [Google Scholar]
- [59].Rausch T, Beglinger C, Alam N, Gyr K, Meier R. Effect of transdermal application of nicotine on colonic transit in healthy nonsmoking volunteers. Neurogastroenterol Motil. 1998;10:263–270. doi: 10.1046/j.1365-2982.1998.00105.x. [DOI] [PubMed] [Google Scholar]
- [60].Portnoi VA. Helicobacter pylori infection and anorexia of aging. Arch Intern Med. 1997;157:269–272. [PubMed] [Google Scholar]
- [61].Feldman M, Cryer B, McArthur KE, Huet BA, Lee E. Effects of aging and gastritis on gastric acid and pepsin secretion in humans: a prospective study. Gastroenterology. 1996;110:1043–1052. doi: 10.1053/gast.1996.v110.pm8612992. [DOI] [PubMed] [Google Scholar]
- [62].Brophy CM, Moore JG, Christian PE, Egger MJ, Taylor AT. Variability of gastric emptying measurements in man employing standardized radiolabeled meals. Dig Dis Sci. 1986;31:799–806. doi: 10.1007/BF01296046. [DOI] [PubMed] [Google Scholar]
- [63].Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest. 1985;75:1144–1152. doi: 10.1172/JCI111809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Lieverse RJ, Jansen JB, Masclee AM, Lamers CB. Satiety effects of cholecystokinin in humans. Gastroenterology. 1994;106:1451–1454. doi: 10.1016/0016-5085(94)90397-2. [DOI] [PubMed] [Google Scholar]
- [65].Parker BA, Doran S, Wishart J, Horowitz M, Chapman IM. Effects of small intestinal and gastric glucose administration on the suppression of plasma ghrelin concentrations in healthy older men and women. Clin Endocrinol (Oxf) 2005;62:539–546. doi: 10.1111/j.1365-2265.2005.02254.x. [DOI] [PubMed] [Google Scholar]
- [66].Gibbs J, Young RC, Smith GP. Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol. 1973;84:488–495. doi: 10.1037/h0034870. [DOI] [PubMed] [Google Scholar]
- [67].Kissileff HR, Pi-Sunyer FX, Thornton J, Smith GP. C-terminal octapeptide of cholecystokinin decreases food intake in man. Am J Clin Nutr. 1981;34:154–160. doi: 10.1093/ajcn/34.2.154. [DOI] [PubMed] [Google Scholar]
- [68].Kissileff HR, Carretta JC, Geliebter A, Pi-Sunyer FX. Cholecystokinin and stomach distension combine to reduce food intake in humans. Am J Physiol Regul Integr Comp Physiol. 2003;285:R992–998. doi: 10.1152/ajpregu.00272.2003. [DOI] [PubMed] [Google Scholar]
- [69].Moran TH, Kornbluh R, Moore K, Schwartz GJ. Cholecystokinin inhibits gastric emptying and contracts the pyloric sphincter in rats by interacting with low affinity CCK receptor sites. Regul Pept. 1994;52:165–172. doi: 10.1016/0167-0115(94)90050-7. [DOI] [PubMed] [Google Scholar]
- [70].Guimbaud R, Moreau JA, Bouisson M, Durand S, Escourrou J, Vaysse N, Frexinos J. Intraduodenal free fatty acids rather than triglycerides are responsible for the release of CCK in humans. Pancreas. 1997;14:76–82. doi: 10.1097/00006676-199701000-00012. [DOI] [PubMed] [Google Scholar]
- [71].McLaughlin JT, Lomax RB, Hall L, Dockray GJ, Thompson DG, Warhurst G. Fatty acids stimulate cholecystokinin secretion via an acyl chain length-specific, Ca2+-dependent mechanism in the enteroendocrine cell line STC-1. J Physiol. 1998;513(Pt 1):11–18. doi: 10.1111/j.1469-7793.1998.011by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].McLaughlin J, Grazia Luca M, Jones MN, D’Amato M, Dockray GJ, Thompson DG. Fatty acid chain length determines cholecystokinin secretion and effect on human gastric motility. Gastroenterology. 1999;116:46–53. doi: 10.1016/s0016-5085(99)70227-1. [DOI] [PubMed] [Google Scholar]
- [73].Holzer HH, Turkelson CM, Solomon TE, Raybould HE. Intestinal lipid inhibits gastric emptying via CCK and a vagal capsaicin-sensitive afferent pathway in rats. Am J Physiol. 1994;267:G625–629. doi: 10.1152/ajpgi.1994.267.4.G625. [DOI] [PubMed] [Google Scholar]
- [74].Lal S, McLaughlin J, Barlow J, D’Amato M, Giacovelli G, Varro A, Dockray GJ, Thompson DG. Cholecystokinin pathways modulate sensations induced by gastric distension in humans. Am J Physiol Gastrointest Liver Physiol. 2004;287:G72–79. doi: 10.1152/ajpgi.00351.2003. [DOI] [PubMed] [Google Scholar]
- [75].Corp ES, McQuade J, Moran TH, Smith GP. Characterization of type A and type B CCK receptor binding sites in rat vagus nerve. Brain Res. 1993;623:161–166. doi: 10.1016/0006-8993(93)90024-h. [DOI] [PubMed] [Google Scholar]
- [76].Moriarty P, Dimaline R, Thompson DG, Dockray GJ. Characterization of cholecystokininA and cholecystokininB receptors expressed by vagal afferent neurons. Neuroscience. 1997;79:905–913. doi: 10.1016/s0306-4522(96)00675-6. [DOI] [PubMed] [Google Scholar]
- [77].MacIntosh CG, Morley JE, Wishart J, Morris H, Jansen JB, Horowitz M, Chapman IM. Effect of exogenous cholecystokinin (CCK)-8 on food intake and plasma CCK, leptin, and insulin concentrations in older and young adults: evidence for increased CCK activity as a cause of the anorexia of aging. J Clin Endocrinol Metab. 2001;86:5830–5837. doi: 10.1210/jcem.86.12.8107. [DOI] [PubMed] [Google Scholar]
- [78].Herrmann C, Goke R, Richter G, Fehmann HC, Arnold R, Goke B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion. 1995;56:117–126. doi: 10.1159/000201231. [DOI] [PubMed] [Google Scholar]
- [79].Feinle C, O’Donovan D, Doran S, Andrews JM, Wishart J, Chapman I, Horowitz M. Effects of fat digestion on appetite, APD motility, and gut hormones in response to duodenal fat infusion in humans. Am J Physiol Gastrointest Liver Physiol. 2003;284:G798–807. doi: 10.1152/ajpgi.00512.2002. [DOI] [PubMed] [Google Scholar]
- [80].Naslund E, King N, Mansten S, Adner N, Holst JJ, Gutniak M, Hellstrom PM. Prandial subcutaneous injections of glucagon-like peptide-1 cause weight loss in obese human subjects. Br J Nutr. 2004;91:439–446. doi: 10.1079/BJN20031064. [DOI] [PubMed] [Google Scholar]
- [81].Ghatei MA, Uttenthal LO, Christofides ND, Bryant MG, Bloom SR. Molecular forms of human enteroglucagon in tissue and plasma: plasma responses to nutrient stimuli in health and in disorders of the upper gastrointestinal tract. J Clin Endocrinol Metab. 1983;57:488–495. doi: 10.1210/jcem-57-3-488. [DOI] [PubMed] [Google Scholar]
- [82].Roberge JN, Brubaker PL. Regulation of intestinal proglucagon-derived peptide secretion by glucose-dependent insulinotropic peptide in a novel enteroendocrine loop. Endocrinology. 1993;133:233–240. doi: 10.1210/endo.133.1.8319572. [DOI] [PubMed] [Google Scholar]
- [83].Tang-Christensen M, Larsen PJ, Goke R, Fink-Jensen A, Jessop DS, Moller M, Sheikh SP. Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. Am J Physiol. 1996;271:R848–856. doi: 10.1152/ajpregu.1996.271.4.R848. [DOI] [PubMed] [Google Scholar]
- [84].Gutzwiller JP, Goke B, Drewe J, Hildebrand P, Ketterer S, Handschin D, Winterhalder R, Conen D, Beglinger C. Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut. 1999;44:81–86. doi: 10.1136/gut.44.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Naslund E, Barkeling B, King N, Gutniak M, Blundell JE, Holst JJ, Rossner S, Hellstrom PM. Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. Int J Obes Relat Metab Disord. 1999;23:304–311. doi: 10.1038/sj.ijo.0800818. [DOI] [PubMed] [Google Scholar]
- [86].Verdich C, Flint A, Gutzwiller JP, Naslund E, Beglinger C, Hellstrom PM, Long SJ, Morgan LM, Holst JJ, Astrup A. A meta-analysis of the effect of glucagon-like peptide-1 (7–36) amide on ad libitum energy intake in humans. J Clin Endocrinol Metab. 2001;86:4382–4389. doi: 10.1210/jcem.86.9.7877. [DOI] [PubMed] [Google Scholar]
- [87].Long SJ, Sutton JA, Amaee WB, Giouvanoudi A, Spyrou NM, Rogers PJ, Morgan LM. No effect of glucagon-like peptide-1 on short-term satiety and energy intake in man. Br J Nutr. 1999;81:273–279. [PubMed] [Google Scholar]
- [88].Brennan IM, Feltrin KL, Horowitz M, Smout AJ, Meyer JH, Wishart J, Feinle-Bisset C. Evaluation of interactions between CCK and GLP-1 in their effects on appetite, energy intake, and antropyloroduodenal motility in healthy men. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1477–1485. doi: 10.1152/ajpregu.00732.2004. [DOI] [PubMed] [Google Scholar]
- [89].Naslund E, Gutniak M, Skogar S, Rossner S, Hellstrom PM. Glucagon-like peptide 1 increases the period of postprandial satiety and slows gastric emptying in obese men. Am J Clin Nutr. 1998;68:525–530. doi: 10.1093/ajcn/68.3.525. [DOI] [PubMed] [Google Scholar]
- [90].Flint A, Raben A, Ersboll AK, Holst JJ, Astrup A. The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity. Int J Obes Relat Metab Disord. 2001;25:781–792. doi: 10.1038/sj.ijo.0801627. [DOI] [PubMed] [Google Scholar]
- [91].Meier JJ, Kemmeries G, Holst JJ, Nauck MA. Erythromycin antagonizes the deceleration of gastric emptying by glucagon-like peptide 1 and unmasks its insulinotropic effect in healthy subjects. Diabetes. 2005;54:2212–2218. doi: 10.2337/diabetes.54.7.2212. [DOI] [PubMed] [Google Scholar]
- [92].Andrews CN, Bharucha AE, Camilleri M, Low PA, Seide BM, Burton DD, Nickander KK, Baxter KL, Zinsmeister AR. Effects of glucagon-like peptide-1 and sympathetic stimulation on gastric accommodation in humans. Neurogastroenterol Motil. 2007;19:716–723. doi: 10.1111/j.1365-2982.2007.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].MacIntosh CG, Horowitz M, Verhagen MA, Smout AJ, Wishart J, Morris H, Goble E, Morley JE, Chapman IM. Effect of small intestinal nutrient infusion on appetite, gastrointestinal hormone release, and gastric myoelectrical activity in young and older men. Am J Gastroenterol. 2001;96:997–1007. doi: 10.1111/j.1572-0241.2001.03684.x. [DOI] [PubMed] [Google Scholar]
- [94].Di Francesco V, Barazzoni R, Bissoli L, Fantin F, Rizzotti P, Residori L, Antonioli A, Graziani MS, Zanetti M, Bosello O, Guarnieri G, Zamboni M. The quantity of meal fat influences the profile of postprandial hormones as well as hunger sensation in healthy elderly people. J Am Med Dir Assoc. 2010;11:188–193. doi: 10.1016/j.jamda.2009.08.004. [DOI] [PubMed] [Google Scholar]
- [95].Ranganath L, Sedgwick I, Morgan L, Wright J, Marks V. The ageing entero-insular axis. Diabetologia. 1998;41:1309–1313. doi: 10.1007/s001250051070. [DOI] [PubMed] [Google Scholar]
- [96].Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- [97].Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- [98].Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- [99].Overduin J, Frayo RS, Grill HJ, Kaplan JM, Cummings DE. Role of the duodenum and macronutrient type in ghrelin regulation. Endocrinology. 2005;146:845–850. doi: 10.1210/en.2004-0609. [DOI] [PubMed] [Google Scholar]
- [100].Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, Batterham RL, Taheri S, Stanley SA, Ghatei MA, Bloom SR. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50:2540–2547. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- [101].Faulconbridge LF, Cummings DE, Kaplan JM, Grill HJ. Hyperphagic effects of brainstem ghrelin administration. Diabetes. 2003;52:2260–2265. doi: 10.2337/diabetes.52.9.2260. [DOI] [PubMed] [Google Scholar]
- [102].Callahan HS, Cummings DE, Pepe MS, Breen PA, Matthys CC, Weigle DS. Postprandial suppression of plasma ghrelin level is proportional to ingested caloric load but does not predict intermeal interval in humans. J Clin Endocrinol Metab. 2004;89:1319–1324. doi: 10.1210/jc.2003-031267. [DOI] [PubMed] [Google Scholar]
- [103].Cummings DE, Frayo RS, Marmonier C, Aubert R, Chapelot D. Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues. Am J Physiol Endocrinol Metab. 2004;287:E297–304. doi: 10.1152/ajpendo.00582.2003. [DOI] [PubMed] [Google Scholar]
- [104].Rigamonti AE, Pincelli AI, Corra B, Viarengo R, Bonomo SM, Galimberti D, Scacchi M, Scarpini E, Cavagnini F, Muller EE. Plasma ghrelin concentrations in elderly subjects: comparison with anorexic and obese patients. J Endocrinol. 2002;175:R1–5. doi: 10.1677/joe.0.175r001. [DOI] [PubMed] [Google Scholar]
- [105].Sturm K, MacIntosh CG, Parker BA, Wishart J, Horowitz M, Chapman IM. Appetite, food intake, and plasma concentrations of cholecystokinin, ghrelin, and other gastrointestinal hormones in undernourished older women and well-nourished young and older women. J Clin Endocrinol Metab. 2003;88:3747–3755. doi: 10.1210/jc.2002-021656. [DOI] [PubMed] [Google Scholar]
- [106].Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- [107].Atalayer D, Gibson C, Konopacka A, Geliebter A. Ghrelin and eating disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2013;40:70–82. doi: 10.1016/j.pnpbp.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Di Francesco V, Fantin F, Residori L, Bissoli L, Micciolo R, Zivelonghi A, Zoico E, Omizzolo F, Bosello O, Zamboni M. Effect of age on the dynamics of acylated ghrelin in fasting conditions and in response to a meal. J Am Geriatr Soc. 2008;56:1369–1370. doi: 10.1111/j.1532-5415.2008.01732.x. [DOI] [PubMed] [Google Scholar]
- [109].Bauer JM, Haack A, Winning K, Wirth R, Fischer B, Uter W, Erdmann J, Schusdziarra V, Sieber CC. Impaired postprandial response of active ghrelin and prolonged suppression of hunger sensation in the elderly. J Gerontol A Biol Sci Med Sci. 2010;65:307–311. doi: 10.1093/gerona/glp174. [DOI] [PubMed] [Google Scholar]
- [110].Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- [111].Ali-Rachedi A, Varndell IM, Adrian TE, Gapp DA, Van Noorden S, Bloom SR, Polak JM. Peptide YY (PYY) immunoreactivity is co-stored with glucagon-related immunoreactants in endocrine cells of the gut and pancreas. Histochemistry. 1984;80:487–491. doi: 10.1007/BF00495439. [DOI] [PubMed] [Google Scholar]
- [112].Rosenbaum M, Leibel RL. The role of leptin in human physiology. N Engl J Med. 1999;341:913–915. doi: 10.1056/NEJM199909163411211. [DOI] [PubMed] [Google Scholar]
- [113].Zamboni M, Zoico E, Fantin F, Panourgia MP, Di Francesco V, Tosoni P, Solerte B, Vettor R, Bosello O. Relation between leptin and the metabolic syndrome in elderly women. J Gerontol A Biol Sci Med Sci. 2004;59:396–400. doi: 10.1093/gerona/59.4.m396. [DOI] [PubMed] [Google Scholar]
- [114].Di Francesco V, Zamboni M, Zoico E, Mazzali G, Dioli A, Omizzolo F, Bissoli L, Fantin F, Rizzotti P, Solerte SB, Micciolo R, Bosello O. Unbalanced serum leptin and ghrelin dynamics prolong postprandial satiety and inhibit hunger in healthy elderly: another reason for the “anorexia of aging”. Am J Clin Nutr. 2006;83:1149–1152. doi: 10.1093/ajcn/83.5.1149. [DOI] [PubMed] [Google Scholar]
- [115].Diez JJ, Iglesias P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol. 2003;148:293–300. doi: 10.1530/eje.0.1480293. [DOI] [PubMed] [Google Scholar]
- [116].Ukkola O, Santaniemi M. Adiponectin: a link between excess adiposity and associated comorbidities? J Mol Med (Berl) 2002;80:696–702. doi: 10.1007/s00109-002-0378-7. [DOI] [PubMed] [Google Scholar]
- [117].Combs TP, Berg AH, Rajala MW, Klebanov S, Iyengar P, Jimenez-Chillaron JC, Patti ME, Klein SL, Weinstein RS, Scherer PE. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes. 2003;52:268–276. doi: 10.2337/diabetes.52.2.268. [DOI] [PubMed] [Google Scholar]
- [118].Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- [120].Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46:459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- [121].Holcomb IN, Kabakoff RC, Chan B, Baker TW, Gurney A, Henzel W, Nelson C, Lowman HB, Wright BD, Skelton NJ, Frantz GD, Tumas DB, Peale FV, Jr, Shelton DL, Hebert CC. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J. 2000;19:4046–4055. doi: 10.1093/emboj/19.15.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Kim KH, Lee K, Moon YS, Sul HS. A cysteine-rich adipose tissue-specific secretory factor inhibits adipocyte differentiation. J Biol Chem. 2001;276:11252–11256. doi: 10.1074/jbc.C100028200. [DOI] [PubMed] [Google Scholar]
- [123].Singhal NS, Lazar MA, Ahima RS. Central resistin induces hepatic insulin resistance via neuropeptide Y. J Neurosci. 2007;27:12924–12932. doi: 10.1523/JNEUROSCI.2443-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Steppan CM, Lazar MA. The current biology of resistin. J Intern Med. 2004;255:439–447. doi: 10.1111/j.1365-2796.2004.01306.x. [DOI] [PubMed] [Google Scholar]
- [125].Chiba T, Yamaza H, Komatsu T, Nakayama M, Fujita S, Hayashi H, Higami Y, Shimokawa I. Pituitary growth hormone suppression reduces resistin expression and enhances insulin effectiveness: relationship with caloric restriction. Exp Gerontol. 2008;43:595–600. doi: 10.1016/j.exger.2008.03.003. [DOI] [PubMed] [Google Scholar]
- [126].Moss C, Dhillo WS, Frost G, Hickson M. Gastrointestinal hormones: the regulation of appetite and the anorexia of ageing. J Hum Nutr Diet. 2012;25:3–15. doi: 10.1111/j.1365-277X.2011.01211.x. [DOI] [PubMed] [Google Scholar]
- [127].Serra-Prat M, Palomera E, Clave P, Puig-Domingo M. Effect of age and frailty on ghrelin and cholecystokinin responses to a meal test. Am J Clin Nutr. 2009;89:1410–1417. doi: 10.3945/ajcn.2008.27076. [DOI] [PubMed] [Google Scholar]
- [128].Geary N, Le Sauter J, Noh U. Glucagon acts in the liver to control spontaneous meal size in rats. Am J Physiol. 1993;264:R116–122. doi: 10.1152/ajpregu.1993.264.1.R116. [DOI] [PubMed] [Google Scholar]
- [129].Silver AJ, Flood JF, Morley JE. Effect of gastrointestinal peptides on ingestion in old and young mice. Peptides. 1988;9:221–225. doi: 10.1016/0196-9781(88)90254-9. [DOI] [PubMed] [Google Scholar]
- [130].Melanson KJ, Greenberg AS, Ludwig DS, Saltzman E, Dallal GE, Roberts SB. Blood glucose and hormonal responses to small and large meals in healthy young and older women. J Gerontol A Biol Sci Med Sci. 1998;53:B299–305. doi: 10.1093/gerona/53a.4.b299. [DOI] [PubMed] [Google Scholar]
- [131].Brunetti L, Recinella L, Orlando G, Michelotto B, Di Nisio C, Vacca M. Effects of ghrelin and amylin on dopamine, norepinephrine and serotonin release in the hypothalamus. Eur J Pharmacol. 2002;454:189–192. doi: 10.1016/s0014-2999(02)02552-9. [DOI] [PubMed] [Google Scholar]
- [132].Smith SR, Blundell JE, Burns C, Ellero C, Schroeder BE, Kesty NC, Chen KS, Halseth AE, Lush CW, Weyer C. Pramlintide treatment reduces 24-h caloric intake and meal sizes and improves control of eating in obese subjects: a 6-wk translational research study. Am J Physiol Endocrinol Metab. 2007;293:E620–627. doi: 10.1152/ajpendo.00217.2007. [DOI] [PubMed] [Google Scholar]
- [133].Mitsukawa T, Takemura J, Nakazato M, Asai J, Kanagawa K, Matsuo H, Matsukura S. Effects of aging on plasma islet amyloid polypeptide basal level and response to oral glucose load. Diabetes Res Clin Pract. 1992;15:131–134. doi: 10.1016/0168-8227(92)90016-k. [DOI] [PubMed] [Google Scholar]
- [134].Edwards BJ, Perry HM, Kaiser FE, Morley JE, Kraenzle D, Kreutter DK, Stevenson RW. Age-related changes in amylin secretion. Mech Ageing Dev. 1996;86:39–51. doi: 10.1016/0047-6374(95)01664-3. [DOI] [PubMed] [Google Scholar]
- [135].Morley JE, Morley PM, Flood JF. Anorectic effects of amylin in rats over the life span. Pharmacol Biochem Behav. 1993;44:577–580. doi: 10.1016/0091-3057(93)90169-t. [DOI] [PubMed] [Google Scholar]
- [136].Dechenes CJ, Verchere CB, Andrikopoulos S, Kahn SE. Human aging is associated with parallel reductions in insulin and amylin release. Am J Physiol. 1998;275:E785–791. doi: 10.1152/ajpendo.1998.275.5.E785. [DOI] [PubMed] [Google Scholar]
- [137].Scheen AJ. Diabetes mellitus in the elderly: insulin resistance and/or impaired insulin secretion? Diabetes Metab. 2005;31(Spec No 2):5S27–25S34. doi: 10.1016/s1262-3636(05)73649-1. [DOI] [PubMed] [Google Scholar]
- [138].Ekblad E, Sundler F. Distribution of pancreatic polypeptide and peptide YY. Peptides. 2002;23:251–261. doi: 10.1016/s0196-9781(01)00601-5. [DOI] [PubMed] [Google Scholar]
- [139].Katsuura G, Asakawa A, Inui A. Roles of pancreatic polypeptide in regulation of food intake. Peptides. 2002;23:323–329. doi: 10.1016/s0196-9781(01)00604-0. [DOI] [PubMed] [Google Scholar]
- [140].Cox HM. Neuropeptide Y receptors; antisecretory control of intestinal epithelial function. Auton Neurosci. 2007;133:76–85. doi: 10.1016/j.autneu.2006.10.005. [DOI] [PubMed] [Google Scholar]
- [141].Adrian TE, Bloom SR, Bryant MG, Polak JM, Heitz PH, Barnes AJ. Distribution and release of human pancreatic polypeptide. Gut. 1976;17:940–944. doi: 10.1136/gut.17.12.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Batterham RL, Le Roux CW, Cohen MA, Park AJ, Ellis SM, Patterson M, Frost GS, Ghatei MA, Bloom SR. Pancreatic polypeptide reduces appetite and food intake in humans. J Clin Endocrinol Metab. 2003;88:3989–3992. doi: 10.1210/jc.2003-030630. [DOI] [PubMed] [Google Scholar]
- [143].Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature. 2006;444:854–859. doi: 10.1038/nature05484. [DOI] [PubMed] [Google Scholar]
- [144].Field BC, Chaudhri OB, Bloom SR. Bowels control brain: gut hormones and obesity. Nature reviews Endocrinology. 2010;6:444–453. doi: 10.1038/nrendo.2010.93. [DOI] [PubMed] [Google Scholar]
- [145].Johns CE, Newton JL, Westley BR, May FE. Human pancreatic polypeptide has a marked diurnal rhythm that is affected by ageing and is associated with the gastric TFF2 circadian rhythm. Peptides. 2006;27:1341–1348. doi: 10.1016/j.peptides.2005.11.002. [DOI] [PubMed] [Google Scholar]
- [146].Tough IR, Holliday ND, Cox HM. Y(4) receptors mediate the inhibitory responses of pancreatic polypeptide in human and mouse colon mucosa. J Pharmacol Exp Ther. 2006;319:20–30. doi: 10.1124/jpet.106.106500. [DOI] [PubMed] [Google Scholar]
- [147].Gras-Miralles B, Cremonini F. A critical appraisal of lubiprostone in the treatment of chronic constipation in the elderly. Clin Interv Aging. 2013;8:191–200. doi: 10.2147/CIA.S30729. [DOI] [PMC free article] [PubMed] [Google Scholar]