Abstract
Thrombotic disease is a leading cause of death and disability worldwide. The development of magnetic resonance molecular imaging provides potential promise for early disease diagnosis. In this study, we explore the preparation and characterization of gadolinium (Gd)-loaded poly (lactic-co-glycolic acid) (PLGA) particles surface modified with the Arg-Gly-Asp-Ser (RGDS) peptide for the detection of thrombus. PLGA was employed as the carrier-delivery system, and a double emulsion solvent-evaporation method (water in oil in water) was used to prepare PLGA particles encapsulating the magnetic resonance contrast agent Gd diethylenetriaminepentaacetic acid (DTPA). To synthesize the Gd-PLGA/chitosan (CS)-RGDS particles, carbodiimide-mediated amide bond formation was used to graft the RGDS peptide to CS to form a CS-RGDS film that coated the surface of the PLGA particles. Blank PLGA, Gd-PLGA, and Gd-PLGA/CS particles were fabricated using the same water in oil in water method. Our results indicated that the RGDS peptide successfully coated the surface of the Gd-PLGA/CS-RGDS particles. The particles had a regular shape, smooth surface, relatively uniform size, and did not aggregate. The high electron density of the Gd-loaded particles and a translucent film around the particles coated with the CS and CS-RGDS films could be observed by transmission electron microscopy. In vitro experiments demonstrated that the Gd-PLGA/CS-RGDS particles could target thrombi and could be imaged using a clinical magnetic resonance scanner. Compared with the Gd-DTPA solution, the longitudinal relaxation time of the Gd-loaded particles was slightly longer, and as the Gd-load concentration increased, the longitudinal relaxation time values decreased. These results suggest the potential of the Gd-PLGA/CS-RGDS particles for the sensitive and specific detection of thrombus at the molecular level.
Keywords: poly (lactic-co-glycolic acid), Arg-Gly-Asp-Ser peptide, magnetic resonance imaging, thrombus, particle
Introduction
Thrombotic disease is a direct cause of myocardial infarction, ischemic stroke, pulmonary embolism, and various other disorders. Recently, the mortality and disability rates due to thrombi have increased worldwide, and the morbidity associated with thrombi is greater than that caused by cancers, infectious diseases, and respiratory diseases.1 However, as conventional imaging modalities are still focused on the examination of anatomical structure and physiological function, the early detection of thrombus is limited. In recent years, with the development of nanotechnology and the continuing application of molecular probes, molecular imaging has experienced rapid development and achieved satisfactory results in targeting thrombi.2 Compared with other imaging modalities, molecular magnetic resonance imaging (MRI), with the advantages of nonionizing radiation, deep tissue penetration, and higher spatial resolution, has great potential for thrombus characterization. Moreover, the molecular information obtained from a contrast agent can be overlaid onto the inherent anatomical image to provide context.3
Thrombus targeted magnetic resonance (MR) molecular imaging can potentially identify thrombi in the presence of atherosclerotic plaques. Depending on the choice of imaging target, it may be possible to distinguish active, forming clots from older constituted thrombi. For example, fresh thrombi may be visualized by targeting activated platelets or thrombin factor XIIIa. Accordingly, the choice of imaging target in the design of the molecular probe is paramount. To date, numerous studies have been focused on the design of MR probes with high relaxation efficiency to improve MR thrombus depiction.4–9 However, such probes are becoming more and more complicated, are not readily available for other researchers, and are not yet approved for clinical applications. In this study, we introduce a relatively simple method to prepare an MR probe for targeting activated platelets.
Poly (lactic-co-glycolic acid) (PLGA) has attracted considerable attention due to its attractive properties: (1) it is biodegradable and biocompatible, (2) the US Food and Drug Administration and European Medicine Agency have approved it for use in drug delivery systems for parenteral administration, (3) it has well described formulations and methods of production adapted to various types of drugs, eg, hydrophilic or hydrophobic small molecules or macromolecules, (4) it protects drugs from degradation, (5) it has the possibility of sustained release, (6) the surface properties can be modified to provide better interaction with biological materials, and (7) it can possibly target particles to specific organs or cells.10,11 PLGA is widely used for the preparation of intravenous drug delivery systems and biomimetic materials and has extensive application prospects in drug delivery, disease diagnosis, treatment, medical imaging, and tissue engineering.12–14
In recent years, many studies have reported that MR contrast agents, such as gadolinium diethylenetriaminepentaacetic acid (Gd-DTPA) and superparamagnetic iron oxide, have been successfully encapsulated into PLGA particles to prepare new types of contrast agents. These preparations have been confirmed to have low biological toxicity via the study of their physical and chemical characteristics, and distribution and degradation in vivo in animal models.15–17 They can be imaged using 1.5 T MR scanners, and their imaging capabilities have been demonstrated to be similar to those associated with the separate use of Gd-DTPA or superparamagnetic iron oxide. Faranesh et al18 reported that vascular endothelial growth factor (VEGF) and Gd-DTPA were encapsulated into PLGA particles in the preparation of a multifunctional contrast agent which was intended to allow the simultaneous detection and treatment of ischemic disease. In addition, in our preliminary studies, we utilized PLGA microbubbles containing halothane gas and Gd-DTPA to prepare multimodal contrast agents that could be imaged using both ultrasound and MR.19
PLGA can be polymerized by lactic acid and glycolic acid. By changing the ratio of these two monomers, the physical and chemical properties can be adjusted to meet different demands for different loaded drugs.20 In addition, PLGA particles completely maintain their original nature after encapsulating the drugs. The drugs encapsulated into the PLGA particles, such as Gd-DTPA or VEGF, would be released via the biodegradation of the PLGA particles. However, due to the lack of active functional groups on the molecular chain and the poor adhesion of cells, PLGA has been limited in further biomedical applications. Therefore, to increase the concentration of drugs in specific areas in a sustained manner, to enhance the efficacy of the drugs, and to reduce the side effects of normal tissues, many studies have focused on the surface modification of PLGA particles. The most common method is to introduce a large number of active groups through chemical modification of the surface of the PLGA particles.10 Recently, it was shown that these surfaces can be modified through a coating procedure by depositing a few atomic layers of biocompatible polymers.10,21 The polymer coating not only leads to the creation of more hydrophilic nanostructures but also provides a variety of surface functional groups to bind drug molecules, inhibit aggregation, and increase stability. For example, poly ethylene glycol has been covalently bound to the surface of PLGA particles to prevent recognition by mononuclear phagocyte systems thus increasing their circulation time.10 Chitosan (CS), an amino poly-saccharide (poly 1, 4-D-glucoamine), has been extensively investigated in drug delivery systems and tissue engineering because of its low toxicity, low immunogenicity, excellent biodegradability, and biocompatibility as well as its high positive charge that easily forms polyelectrolyte complexes with negatively charged entities.17 Experiments suggest that PLGA particles coated with CS can significantly shorten clot lysis times, a potential beneficial effect of CS on blood clot lysis.21
The specific binding of activated platelets and fibrinogen is part of the final pathway of thrombosis. The Arg-Gly-Asp-Ser (RGDS) peptide is a receptor antagonist of platelet membrane glycoprotein GP IIb/IIIa, and RGDS has a tendency to bind activated platelets on the thrombus site and adhere to the thrombus surface through competition with fibrinogen to restrain the platelets from accumulation.22 Previous studies have reported that the RGDS peptide binds to the surface of the liposome microbubbles to target activated platelets.22,23 By surface conjugating RGDS peptide to liposome microbubbles loaded with tissue plasminogen activator could enhance in vitro thrombolysis.24 Urokinase and RGDS peptide have been successfully connected to the surface of microbubbles for in vitro targeted thrombolysis.25 RGDS peptide grafted CS has also been used as a surface modifier for thrombolytic drug loaded PLGA particles to investigate the possible effects on thrombolysis targeting.21
However, the design of Gd-loaded PLGA particles used for the early detection of thrombus on MRI has not been reported. PLGA has a relatively long circulatory half-life which offers adequate time to reach and saturate targets directly accessible to the vasculature. Therefore, in this study, we aimed to explore the preparation and characterization of Gd-loaded PLGA particles that are surface modified with RGDS peptide for the detection of thrombus. PLGA was employed as a carrier-delivery system. A double emulsion solvent-evaporation method (water in oil in water [W/O/W]) was used to prepare PLGA particles encapsulating Gd-DTPA. Using carbodiimide-mediated amide bond formation, the RGDS peptide was grafted to the surface of CS to form a CS-RGDS film coating on the surface of the PLGA particles during the synthesis of the Gd-PLGA/CS-RGDS particles. Blank PLGA, Gd-PLGA, and Gd-PLGA/CS particles were also fabricated using the W/O/W method. Their characteristics, such as the RGDS carrying rate, particle sizes, zeta potentials, encapsulation efficiencies of Gd-DTPA, targeting ability, MR imaging ability, and longitudinal relaxation time (T1), were determined. We expect to provide a new, relatively simple, effective, and feasible technique and molecular modality for the early detection of thrombus.
Materials and methods
Materials
PLGA, in a lactide/glycolide molar ratio of 75:25, with a molecular weight of 20,000 Dalton (Da), was purchased from Jinan Daigang Biological Material Limited Company (Shangdong, People’s Republic of China). The RGDS peptide was specially synthesized by the Chinese Peptide Company (Hangzhou, People’s Republic of China). CS, of molecular weight 100,000–300,000 Da, was provided by Sangon Biotech (Shanghai, People’s Republic of China). Gd-DTPA, at a concentration of 469 mg/mL, was obtained from Kangchen Pharmaceutical Company Ltd (Guangzhou, People’s Republic of China). Polyvinyl alcohol (PVA), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), 2-morpholinoethanesulfonic acid (MES) buffer solution (0.1 M), N-hydroxysuccinimide (NHS) were obtained from Sigma-Aldrich Corporation (St Louis, MO, USA). All other reagents used were at least of analytical grade.
Preparation of CS film and CS-RGDS film
First, 100 mg of CS was dissolved in 10 mL of 1% acetic acid solution, stirred until fully dissolved, ultrasonically degassed, and poured into a polytetrafluoroethylene mold. The mold was placed in an oven at 40°C to dry after the cast became fat, and then the CS film was retrieved and soaked in 5% sodium hydroxide solution for half an hour, flushed repeatedly using distilled water until pH 7 was reached, and air dried for storage. The CS-RGDS film was prepared using carbodiimide-mediated amide bond formation.26 In general, an appropriate amount of EDC and NHS with a molar ratio of 1.5:1 were added to 0.1 M MES buffer solution. The pH was adjusted to 7 using a sodium hydroxide solution, and 0.05 millimoles (mM) of RGDS peptide and CS film were added and allowed to react for 24 hours at 4°C to form the CS-RGDS film. Finally, the CS-RGDS film was rinsed with water to remove the unreacted materials and air dried at room temperature.
Preparation of PLGA particles
The W/O/W method was used to prepare the PLGA particles. To fabricate the Gd-PLGA/CS-RGDS particles, the CS-RGDS film was first dissolved in 0.5% acetic acid solution. Next, 100 mg of PLGA was dissolved in 2 mL of dichloromethane, and then 0.1 mL Gd-DTPA solution was added as an inner aqueous phase. After acoustic vibration for 60 seconds via the use of an ultrasonic processor (VCX130, Sonics and Materials, Inc., Newtown, CT, USA), the resultant first emulsification (W/O emulsion) was slowly poured into the external aqueous phase, which was mixed with 5 mL of CS-RGDS solution and 5 mL of aqueous solution containing 5% sodium chloride and 3% PVA before being homogenized at 10,000 rpm for 5 minutes with a high-speed homogenization dispersion machine (C10, Shanghai HENC Mechanical Equipment Company, Shanghai, People’s Republic of China) to allow the particles to become a uniform size. Then, 20 mL of 2% isopropanol solution was added into the double emulsion solution (W/O/W emulsion) and stirred continuously at room temperature for 1 hour until particle surface solidification and dichloromethane volatilization occurred. The pH value of the emulsion was adjusted to 6–7 during solvent volatilization to enhance the CS-RGDS coating onto the PLGA particles. The Gd-PLGA/CS-RGDS particles were collected by centrifugation using a high-speed refrigerated centrifuge (centrifuge 5804R, Eppendorf, Germany) at 5,000 rpm for 5 minutes and rinsed with double distilled water to remove the unloaded Gd-DTPA.
To prepare Gd-PLGA/CS particles, 100 mg of CS was dissolved in 10 mL of 1% acetic acid solution. Then, following the method used to prepare the Gd-PLGA/CS-RGDS particles, 5 mL of CS solution and 5 mL of aqueous solution were mixed with 5% sodium chloride and 3% PVA as the external aqueous phase, and the aforementioned W/O emulsion was added with continuous homogenization. To prepare Gd-PLGA particles, 10 mL of aqueous solution mixed with 5% sodium chloride and 3% PVA was used as the external aqueous phase, and the aforementioned W/O emulsion was added following the same procedure used for the preparation of the Gd-PLGA/CS-RGDS particles. To fabricate blank PLGA particles, 0.1 mL of double distilled water instead of 0.1 mL of Gd-DTPA was used as the inner aqueous phase, and the next steps were conducted following the procedures used to prepare the Gd-PLGA particles.
Physical and chemical characteristics
Confirmation of RGDS coating and measurement of the RGDS carrying rate
An X-ray photoelectron spectrometer ([XPS] XSAM800, Kratos, UK) was used to confirm whether RGDS was covalently bound to the surface of the PLGA particles. In general, the Gd-PLGA, Gd-PLGA/CS, and Gd-PLGA/CS-RGDS particles bound at 2 × 10−7 Pa. An aluminum target with a photon energy of 1,486.6 eV was used to measure the surface element composition of the Gd-loaded particles (power 12 kV × 15 mA).
The Gd-PLGA/CS particles were considered the control group, and the Gd-PLGA/CS-RGDS particles were considered the experimental group (RGDS were labeled using fluorescein isothiocyanate). We randomly counted 10,000 particles and measured and calculated the RGDS carrying rate using flow cytometry (FACSVantage SE, Becton Dickinson, San Jose, CA, USA).
Size, zeta potential, and morphology of Gd-loaded PLGA particles
An appropriate amount of particles was dissolved in double distilled water, and the sizes and zeta potentials of the different particle types were determined at 25°C using a laser particle size analyzer (Zetasizer Nano ZS90, Malvern Instruments, Malvern, UK). The morphology, surface, and dispersion were observed with an optical microscope (Olympus CKX41, Olympus Corporation, Tokyo, Japan), and the internal structures were observed using a transmission electron microscope ([TEM] Hitachi7500, Hitachi Ltd, Tokyo, Japan).
Encapsulation efficiency of Gd-loaded PLGA particles
The concentration of Gd-DTPA was measured using a direct-reading inductively coupled plasma optical emission spectrometer ([ICP-OES]Optima8000, PerkinElmer Inc., Waltham, MA, USA). A standard Gd-DTPA sample was formulated using standard solutions with different concentrations, and the standard curve was measured and plotted using the ICP-OES method. All the supernatant samples were collected after centrifugation in the preparation process of the particles and then the Gd ion spectral line intensity in the solution was measured using the ICP method. Using a comparison with the standard curve, the free Gd ion content of the solution was obtained and recorded as Cf. The total amount of added Gd was recorded as Ct, and then the encapsulation efficiency was calculated using the following formula: EE (%) = (1 − Cf/Ct) × 100%. A blood cell counting chamber was used to count the particles prepared in 100 mg of PLGA material, and then the values were divided by Ct to obtain the Gd content per particle.
In vitro thrombus targeting experiment
Whole blood (10 mL) was collected in a test tube (no anticoagulant) from healthy volunteers according to a protocol approved by our institutional ethical committee, after obtaining informed consent. Small blood clots were formed by combining citrated plasma, 100 mM calcium chloride (3:1, v/v), and 5 U thrombin before incubating in a 37°C water bath for 2 hours. The blank PLGA, Gd-PLGA, Gd-PLGA/CS, and Gd-PLGA/CS-RGDS particles were dissolved in phosphate buffer saline (PBS), and a blood clot with a diameter of 7 mm was added to each solution. After incubation at 37°C in a water bath for 30 minutes, the blood clots were rinsed repeatedly with PBS buffer at a flow rate of 20 cm/second to remove the unbound particles. The solution was absorbed using filter paper, placed on the supporting platform, and embedded in optimal cutting temperature (O.C.T) compound (Sakura Finetek USA, Inc., Torrance, CA, USA) at −25°C, and was then separated using a cryostat microtome to create frozen sections with a thickness of 8 μm. Finally, the sections were placed on glass slides to observe their targeting ability to thrombus using an optical microscope.
In vitro MRI
MRI characterization of Gd-loaded PLGA particles
To assess the contrast enhancement of the particles for MRI, six groups, including 8% gelatin solution, blank PLGA, Gd-DTPA, Gd-PLGA/CS-RGDS, Gd-PLGA/CS, and Gd-PLGA solutions, were created. Because the PLGA particles were prone to sinking in water, each group containing particles was embedded in an 8% gelatin solution with the same concentrations of Gd-DTPA (2.4 mM). The total volume of the solution was 5 mL. MRI was performed using a 1.5 T MR scanner (Excite-II, Echospeed GE Medical System, Fairfield CT, USA) with a head coil and fast spin echo-T1 weighted imaging sequence with the following parameters: repetition time (TR) = 400 ms, echo time (TE) = 10.3 ms, matrix 320 × 192, and number of excitations (NEX) = 1. The MRI in vitro experiments were performed three times for each solution. The mean signal intensities (SIs) for the particles were measured in the central section of the imaging volume using regions of interest with a minimum of 10 pixels. The SI data were divided by the background noise to yield the signal to noise ratio (SNR) where SNR = SI/noise. The differences in the SNR of the different particles were analyzed. The role of paramagnetically chelated Gd-DTPA was calculated only for T1. Gd-DTPA and Gd-loaded particles were dissolved in an 8% gelatin solution, and the suspended particles containing Gd-DTPA concentrations ranging from 0.8 to 2.4 mM were scanned and compared to the same concentrations of Gd-DTPA solution. An inversion recovery (IR) sequence was performed using the following parameters: TR = 4,500 ms; inversion time (TI) = 50 ms, 75 ms, 100 ms, 150 ms, 200 ms, 250 ms, 300 ms, 400 ms, 500 ms, 600 ms, 700 ms, and 800 ms; slice thickness of 3.0 mm; layer of 0.5 mm; matrix 192 × 160; and NEX = 1. Data obtained from the IR sequence were transferred to an image workstation (ADW4.2, GE Medical System). The research T1 mapping software package (Functool 2.6.0, GE Medical System) was used to measure the T1 values. Each group was measured three times, and an average of the three measurements was used as the final value.
In vitro MRI for thrombus targeting
MRI of the targeted thrombi was performed before and after incubation with different particles using a 3.0 T MRI scanner (Achieva 3.0T TX, Philips Healthcare, Best, the Netherlands). A T1-weighted turbo spin echo sequence was acquired at higher resolution using a 3.0 T rat experiment coil (CG-MUC 18-H300-AP, Shanghai Chenguang Medical Technologies Co., Ltd., Shanghai, People’s Republic of China). The imaging parameters were as follows: TR = 330 ms, TE = 10 ms, matrix 140 × 136, slice thickness of 3.0 mm, and NEX = 8. The SNR was measured and calculated as described above. The SIs for the thrombi were measured in the peripheral regions. To characterize the deposition of the targeted particles, the blood clots were formed in 5.0 mm diameters (3 groups). The control group was placed into PBS, and the other groups were soaked in Gd-PLGA and Gd-PLGA/CS-RGDS for 20 minutes and washed repeatedly with PBS. Subsequently, these thrombi were incubated in gelatin solution for the MRI scans.
Statistical analysis
Data were analyzed using the Statistical Program for Social Sciences (SPSS for Windows, version 17.00, Chicago, IL, USA). Continuous variables are presented as the mean ± standard deviation, and categorical variables are reported numerically and as percentages. One-way ANOVA and independent sample t-tests were used to compare the differences of the SNR and T1 values among the different particles. Statistical significance was defined as P < 0.05.
Results
Confirmation of RGDS coating and measurement of the RGDS carrying rate
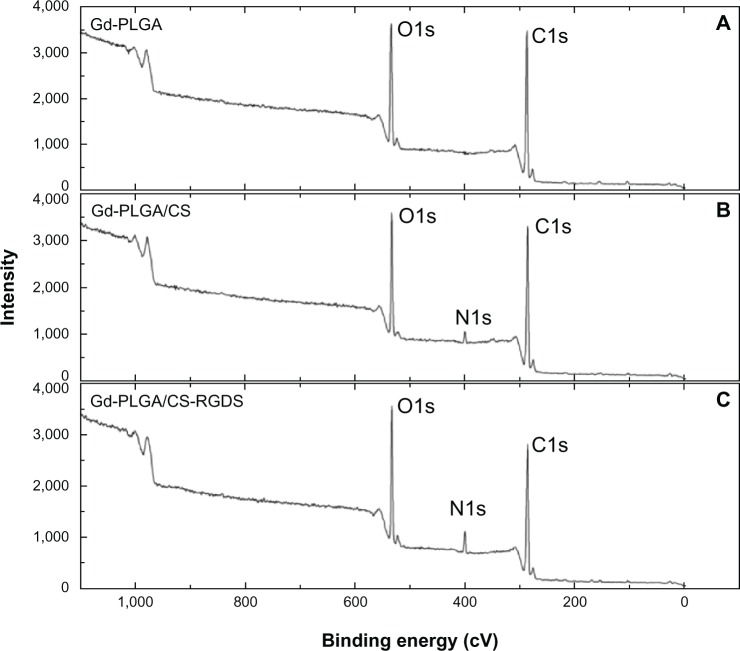
The characteristics of PLGA particles with or without CS-RGDS film obtained from XPS are presented in Figure 1 and Table 1. Figure 1 shows the changes of the surface elemental compositions of the Gd-PLGA, Gd-PLGA/CS, and Gd-PLGA/CS-RGDS particles. The three peaks at 286.85 eV, 401.25 eV, and 534.50 eV in this figure represent elemental carbon (C), nitrogen (N), and oxygen (O), respectively. Changes in the relative C, N, and O particles were observed (Table 1). When compared with Gd-PLGA/CS particles, the relative content of Gd-PLGA/CS-RGDS particles increased from 3.34% to 6.86% for N, which was related to the higher content of N in the RGDS peptides. The relative C content was reduced from 75.65% to 67.10% due to the relatively lower C content in the RGDS peptides. The relative O content also increased. The above results indicate that the RGDS peptide was successfully coated onto the surface of the PLGA particles.
Figure 1.
XPs spectra of PLGA particles.
Notes: (A) The surface elemental composition of the gadolinium (Gd)-loaded poly (lactic-co-glycolic acid) (PLGA) particles. (B) The surface elemental composition of the Gd-PLGA/chitosan (CS) particles. (C) The surface elemental composition of the Gd-PLGA/CS-Arg-Gly-Asp-Ser peptide particles.
Abbreviations: CS, chitosan; Gd-PLGA, gadolinium-loaded poly (lactic-co-glycolic acid); RGDS, Arg-Gly-Asp-Ser peptide; XPS, X-ray photoelectron spectrometer.
Table 1.
Comparison of elements among gadolinium-loaded poly (lactic-co-glycolic acid) (Gd-PLGA), Gd-PLGA/chitosan (CS), and Gd-PLGA/CS-Arg-Gly-Asp-Ser peptide particles
| Particles | C (%) | N (%) | O (%) | N/C |
|---|---|---|---|---|
| Gd-PLGA | 75.28 | 0.00 | 24.72 | 0.000 |
| Gd-PLGA/CS | 75.65 | 3.34 | 21.01 | 0.044 |
| Gd-PLGA/CS-RGDS | 67.10 | 6.86 | 26.04 | 0.102 |
Abbreviations: C, elemental carbon; CS, chitosan; Gd-PLGA, gadolinium-loaded poly (lactic-co-glycolic acid); N, elemental nitrogen; O, elemental oxygen; RGDS, Arg-Gly-Asp-Ser peptide.
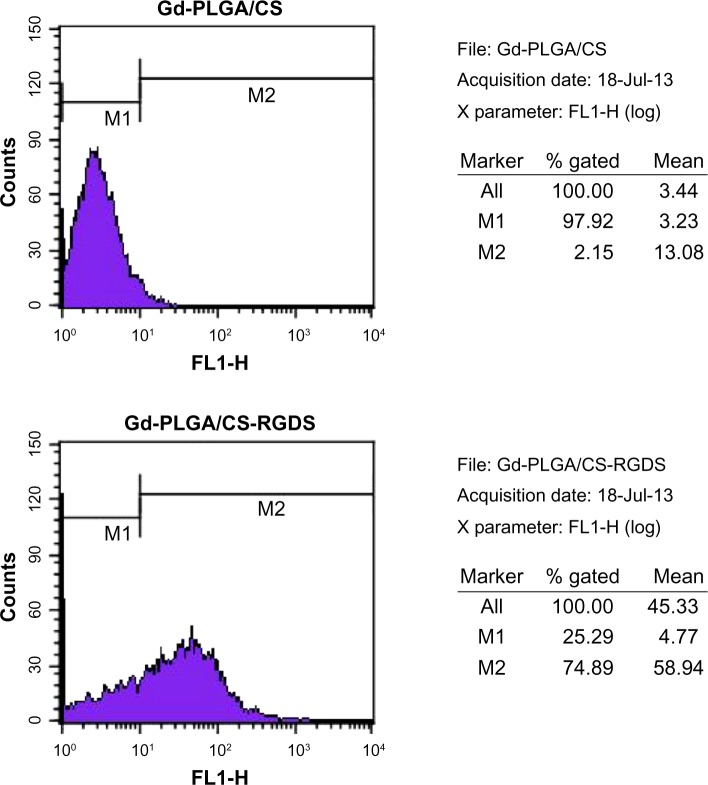
When compared with the control group, the shell wavelength of the Gd-PLGA/CS-RGDS particles was changed due to covalent binding of the RGDS peptide. In a random count of 10,000 particles, there were 7,489 particles different from the control group, so the RGDS carry rate was 74.89% (Figure 2).
Figure 2.
Flow cytometry.
Notes: In comparison with the gadolinium (Gd)-poly (lactic-co-glycolic acid) (PLGA)/chitosan (CS) particles, the shell wavelength of the Gd-PLGA/CS-Arg-Gly-Asp-Ser (RGDS) peptide particles was changed, and the RGDS carrying rate was 74.89%.
Abbreviations: CS, chitosan; Gd-PLGA, gadolinium-loaded poly (lactic-co-glycolic acid); RGDS, Arg-Gly-Asp-Ser peptide.
Characteristics of Gd-loaded PLGA particles
The Gd-loaded PLGA particles prepared by the W/O/W method possessed a regular shape, smooth surface, relatively uniform size, and good dispersion (Figure 3). Several characteristics of the Gd-loaded PLGA particles are presented in Table 2. The sizes of the coated particles were slightly larger than the Gd-PLGA particles. After CS or CS-RGDS was coated onto the PLGA particles, the zeta potentials of the Gd-PLGA/CS and Gd-PLGA/CS-RGDS particles were changed from negative to positive when compared with the Gd-PLGA particles. The encapsulation efficiencies of the Gd-loaded particles were only slightly affected by the CS and CS-RGDS film coating. In addition, there were 3.7 × 1010 particles prepared by 100 mg PLGA, so the Gd content per Gd-PLGA, Gd-PLGA/CS, and Gd-PLGA/CS-RGDS particle was 6.98 × 10−10 mg, 6.41 × 10−10 mg, and 6.27 × 10−10 mg, respectively. Compared with blank PLGA particles, the light transmittance of the Gd-loaded particles was reduced. As observed by TEM, the center of the Gd-loaded particles was filled with a high electron density and low light transmittance, but no electron density was observed in the center of the blank PLGA particles, and a translucent film could be observed around the Gd-PLGA/CS and Gd-PLGA/CS-RGDS particles (Figure 4).
Figure 3.
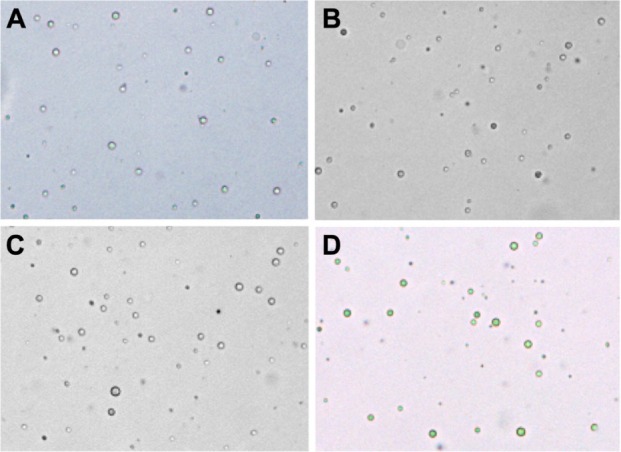
Optical microscope images (×400).
Notes: (A) Blank poly (lactic-co-glycolic acid) (PLGA); (B) gadolinium (Gd)-PLGA; (C) Gd-PLGA/chitosan (CS); and (D) Gd-PLGA/CS-Arg-Gly-Asp-Ser peptide.
Table 2.
Characteristics of gadolinium-loaded poly (lactic-co-glycolic acid) particles
| Particles | Size (nm) | Zeta potential (mV) | Encapsulation efficiency (%) |
|---|---|---|---|
| Gd-PLGA | 824 ± 46.7 | −35.0 ± 7.1 | 55.1 ± 12.2 |
| Gd-PLGA/CS | 870 ± 51.4 | 10.1 ± 3.4 | 50.6 ± 14.3 |
| Gd-PLGA/CS-RGDS | 893 ± 63.7 | 7.2 ± 3.8 | 49.5 ± 11.5 |
Abbreviations: CS, chitosan; Gd-PLGA, gadolinium-loaded poly (lactic-co-glycolic acid); RGDS, Arg-Gly-Asp-Ser peptide.
Figure 4.
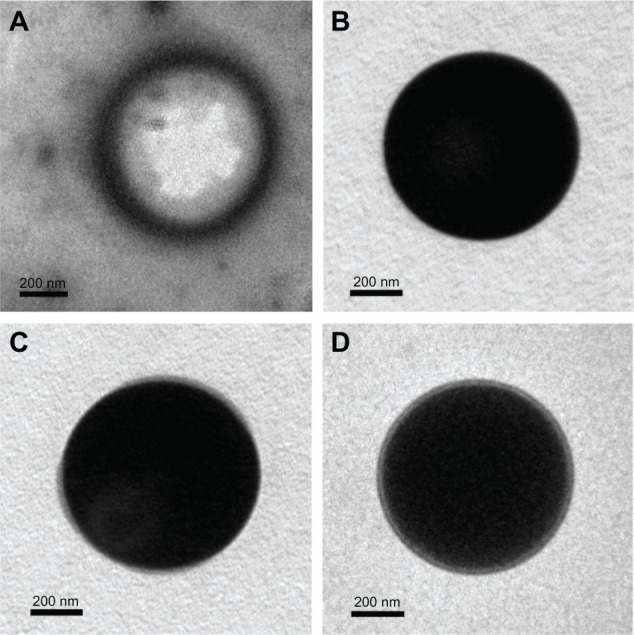
Transmission electron microscope images.
Notes: (A) Blank poly (lactic-co-glycolic acid) (PLGA); (B) gadolinium (Gd)-PLGA; (C) Gd-PLGA/chitosan (CS); and (D) Gd-PLGA/CS-Arg-Gly-Asp-Ser (RGDS) peptide. In comparison with blank PLGA, the light transmittance of the Gd-loaded particles was reduced. A translucent film is visible around the Gd-PLGA/CS and Gd-PLGA/CS-RGDS particles.
Evaluation of thrombus targeting with pathological frozen sections
The pathological frozen sections revealed that a large number of particles were present on the surface of thrombi in the Gd-PLGA/CS-RGDS particle group, but there were no similar observations in the blank PLGA, Gd-PLGA, and Gd-PLGA/CS particle groups (Figure 5).
Figure 5.
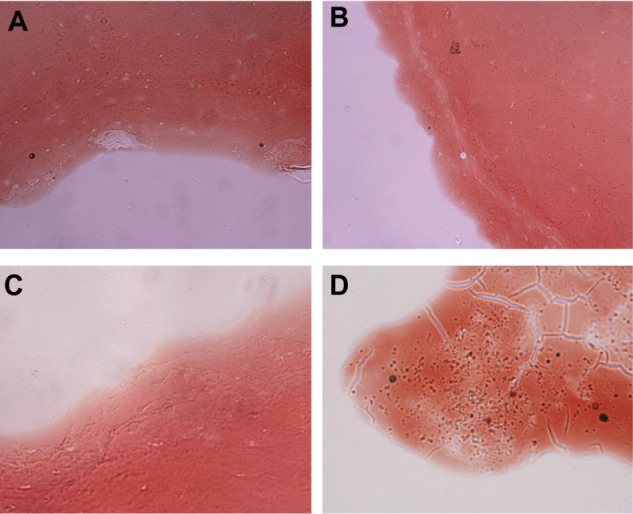
Pathological frozen sections (×400).
Notes: (A) Blank poly (lactic-co-glycolic acid) (PLGA); (B) gadolinium (Gd)-PLGA; (C) Gd-PLGA/chitosan (CS); and (D) Gd-PLGA/CS-Arg-Gly-Asp-Ser (RGDS) peptide. There were a large number of particles on the surface of the thrombi in the Gd-PLGA/CS-RGDS particle groups. The other groups had no similar level of accumulation of particles on the surface.
In vitro MRI
MR characterization of Gd-loaded PLGA particles
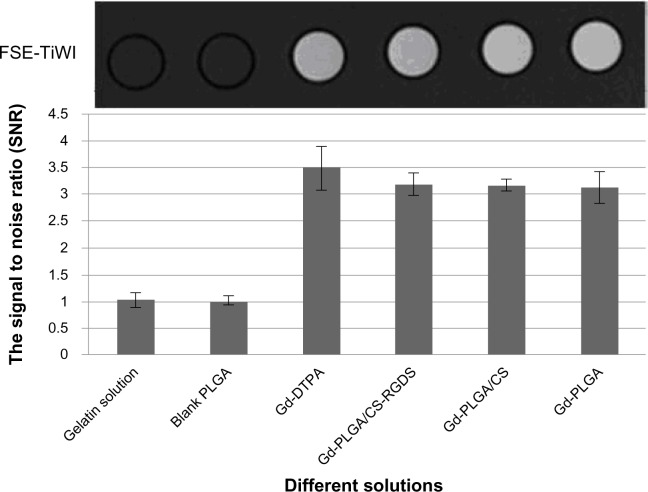
The Gd-loaded particles could be imaged using a 1.5 T MRI scanner, and the SNR on T1 weighted imaging was significantly higher than the values of the gelatin solution and the blank PLGA particles, but was similar to the value of the Gd-DTPA solution (Figure 6). There was no significant difference between the SNR of the gelatin solution and the blank PLGA particles (t = −1.639, P > 0.05). When compared with the Gd-DTPA solution, the SNR slightly decreased for the Gd-loaded particles, but the differences were not significant (F = 3.418, P > 0.05). When compared with the blank PLGA particles, the SNR significantly increased for the Gd-loaded particles (F = 4325.578, P < 0.05).
Figure 6.
In vitro fast spin echo-longitudinal relaxation time weighted imaging and the signal to noise ratio of different solutions.
Notes: The gadolinium (Gd)-loaded particles could be imaged using a 1.5 T magnetic resonance imaging scanner, and the signal to noise ratio values on longitudinal relaxation time weighted imaging were significantly higher than those of the gelatin solution and the blank poly (lactic-co-glycolic acid) particle values and were similar to the Gd-diethylenetriaminepentaacetic acid solution values.
Abbreviations: CS, chitosan; DTPA, diethylenetriaminepentaacetic acid; Gd, gadolinium; PLGA, poly (lactic-co-glycolic acid); RGDS, Arg-Gly-Asp-Ser peptide; SNR, signal to noise ratio.
In this study, the Gd-loaded particles caused a change in contrast on MR images as characterized by their T1 values, which are shown in Figure 7. The increased concentration of Gd-DTPA led to a reduction of T1 values. Gd-loaded particles, including Gd-PLGA, Gd-PLGA/CS, and Gd-PLGA/CS-RGDS, demonstrated slightly longer T1 values than a Gd-DTPA solution at the same concentration (F = 28.55, 35.25, 9.39, 9.49, and 8.84, P < 0.05), but there were no significant differences among the Gd-loaded particles at the same concentration (F = 1.33, 2.94, 1.64, 0.76, and 2.54, P > 0.05).
Figure 7.
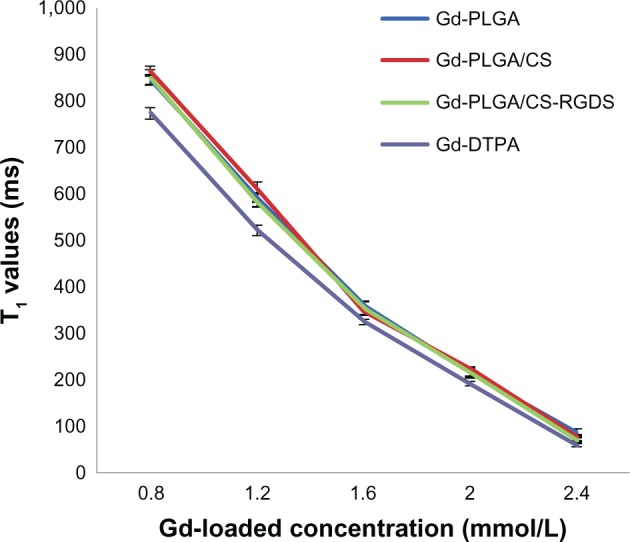
Measurement of the longitudinal relaxation time values.
Notes: The graph shows the longitudinal relaxation time values for concentrations of gadolinium (Gd)-loaded particles and Gd-diethylenetriaminepentaacetic acid solution, which were measured with an inversion recovery magnetic resonance sequence.
Abbreviations: CS, chitosan; DTPA, diethylenetriaminepentaacetic acid; Gd, gadolinium; PLGA, poly (lactic-co-glycolic acid); RGDS, Arg-Gly-Asp-Ser peptide; T1, longitudinal relaxation time.
In vitro MRI for evaluation of thrombus targeting
The high resolution MR images revealed that the binding of the targeted particles exhibited a ring enhancement, ie, the targeting contrast agent was deposited in a thin layer on the clot surface. There were no abnormal signal changes in the blood clots after treatment with PBS and Gd-PLGA particles (Figure 8). The SNR of the blood clots treated with PBS, Gd-PLGA, and Gd-PLGA/CS-RGDS were 9.91 ± 0.39, 9.64 ± 0.12, and 16.79 ± 0.29, respectively (F = 598.77, P < 0.05).
Figure 8.
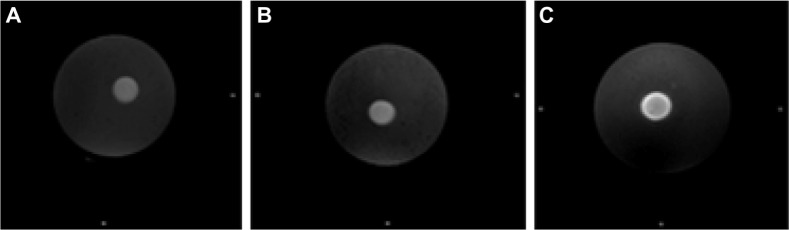
High resolution magnetic resonance images.
Notes: The blood clots were treated with (A) phosphate buffered saline; (B) Gadolinium (Gd)-loaded poly (lactic-co-glycolic acid) (PLGA); and (C) Gd-PLGA/chitosan (CS)-Arg-Gly-Asp-Ser (RGDS) peptide particles. The Gd-PLGA/CS-RGDS particles were deposited in a thin layer on the clot surface and exhibited ring enhancement. There were no abnormal signal changes in the blood clots treated with phosphate buffered saline and Gd-PLGA particles.
Discussion
PLGA is typically used in the form of particles encapsulating contrast agents for molecular imaging. The W/O/W method is a commonly used preparation method and is suitable for encapsulating hydrophilic drugs.27 Gd-DTPA is an intravenous injection aqueous solution. Thus, the W/O/W method could be used to prepare Gd-PLGA particles, and this was confirmed by previous studies.15,16,18,19,28 We can control the sizes of the particles and achieve their optimal characteristics by changing the parameters of the preparation procedure.29 Accordingly, we believe that the W/O/W method is easy to perform, and the preparation procedure is simple when compared with other methods. Our results demonstrate that the Gd-loaded PLGA particles have a regular shape, good dispersion, and are small and relatively uniform in size, which made the particles suitable for MR molecular imaging.
To prepare Gd-loaded PLGA particles that were surface modified with RGDS peptide, we attempted to first prepare Gd-loaded PLGA particles and then use carbodiimide-mediated amide bond formation to covalently bond RGDS to the surface of the particles. However, inevitably there will be a burst release of Gd-DTPA from the PLGA particles during the covalent binding. As a result, the encapsulation efficiency of Gd-DTPA decreased significantly. Doiron et al15 confirmed that Gd-DTPA appears to suffer a burst release in the first 1 hour due to the hydrophilic nature of the Gd-DTPA solution, and in their experiments, more than 90% of the Gd-DTPA solution was released within 1 hour and was almost completely released within 5 hours. During the construction of the thrombus targeted MR molecular probes, the RGDS peptide bonding procedure would take more than 2–3 hours. Thus, no sufficient amount of Gd-DTPA solution encapsulated in the PLGA particles could achieve a good MR contrast enhancement because of the burst release. Therefore, in this study, a CS-RGDS film was prepared before the fabrication of the PLGA particles. XPS and in vitro thrombus targeting experiments in this study confirmed that the CS-RGDS film was successfully coated on the surface of Gd-PLGA particles. The results indicate that the size of the PLGA particles surface modified with CS-RGDS film was slightly larger than the Gd-PLGA particles. The negative zeta potential of the Gd-PLGA particles is consistent with other reported work.15,21 The positive zeta potential of the Gd-PLGA/CS and Gd-PLGA/CS-RGDS particles and the translucent film around the particles revealed the presence of CS or CS-RGDS film on the surface of the PLGA particles, which is consistent with an earlier study.21 A previous study suggested that the electrostatic force or ligand-receptor interactions between the positive zeta potentials of PLGA/CS or PLGA/CS-RGDS particles and the components of blood clots, such as fibrin substrates or GP IIb/IIIa receptors of the platelet membranes, would be a main factor driving the particles to permeate intrablood clots and release the loaded drugs.21 In this study, there were no significant differences in the encapsulation efficiencies among the different Gd-loaded particles. The encapsulation efficiencies of Gd-DTPA were generally unrelated to the sizes and zeta potentials of the particles, both with or without coating.
The MR in vitro experiments with the PLGA particles indicated that there was no significant difference in the SNR between the blank PLGA particle group and the 8% gelatin solution group, indicating that the effect on the signal of the polymer itself was negligible. When compared with blank PLGA particles and the 8% gelatin solution, the SNR significantly increased in Gd-loaded particles, but there were no significant differences among the latter. Compared with the Gd-DTPA solution, the SNR slightly decreased in Gd-loaded particles, but the differences were not significant. This is because once the Gd ions were loaded, the interaction between the Gd ions and the surrounding water molecules was restricted. The measurement of T1 values using an IR sequence also demonstrated that the T1 values of Gd-loaded PLGA particles were slightly longer than the values obtained with the same concentration of Gd-DTPA solution, and there were no significant differences among the Gd-loaded PLGA particles. There were many pores on the surface of the PLGA particles prepared using the W/O/W method, but the pore size can be controlled by changing the preparation conditions, such as PLGA solution concentration, stirring speed, time, and output power of the sonic pulses. Their optimal characteristic is that they restrict the free access of Gd-DTPA instead of water molecules. Therefore, despite the fact that the relaxation properties of the Gd-loaded particles slightly declined, the particles still produced satisfactory images when used with a clinical MR scanner, and increases in the Gd-loaded concentration led to increases in the relaxation rate.
The pathological frozen sections revealed that a large number of thrombus targeted particles were accumulated on the surface of thrombi, but untargeted particle groups did not display similar accumulation. We observed that the molecular probes had a strong thrombus targeting property in vitro. After repeated washes with PBS, the targeted particles could still be observed gathering at the surface of the thrombus, which demonstrated that its combination with the thrombus was stable. These findings also indicate that Gd-PLGA particles were successfully coated with the CS-RGDS film, and RGDS could identify the GP IIb/IIIa receptors on the activated platelets for thrombus targeting. With additional time, there were increasingly fewer particles observed in the thrombus interior. The high resolution MR image revealed that the binding of the targeted particles was restricted to a thin layer on the surface of the clot, and there were no abnormal signal changes in blood clots before and after treatment with PBS and the Gd-PLGA particles. These results suggest that thrombus targeted PLGA particles can be bound to the surface of the thrombus and imaged using a clinical MR scanner. Previous reports5,6,30 have demonstrated the same findings using other thrombus targeted MR probes. The MR findings are supported by the analysis of the pathological frozen sections in our study.
There are still several limitations to this study. First, it is only a preliminary study of the Gd-loaded PLGA particles for thrombus targeting and in vivo experiments still need to be performed to verify our hypothesis. In vivo circulation is much more complicated than in vitro experiments, so whether thrombus targeted PLGA particles can accurately target thrombi in MR monitoring of such a system remains uncertain and will be the emphasis of our follow-up work. Secondly, the particle construction method needs to be further improved in some aspects. For example, although the Gd-loaded particles can be imaged using a clinical MR scanner, the encapsulation efficiency of Gd-DTPA needs to be improved, and the relaxation efficiency is slightly decreased. The size of the particles was slightly larger with the CS-RGDS film coating. Thirdly, many previous studies have reported on the release profile of the Gd-PLGA particles,15,18 so we did not replicate the research to determine the in vitro release because we presume that the CS-RGDS film would not affect the release of Gd-DTPA and because drug release characterization in vitro correlates poorly with in vivo release. Accordingly, drug release will be characterized by our in vivo experiment in future studies.
Conclusion
The Gd-PLGA/CS-RGDS particles could be successfully prepared using the W/O/W method. The particles could be imaged using a clinical MR scanner due to a longitudinal relaxation rate similar to a Gd-DTPA solution, and they have a strong ability to target thrombi in vitro. These results suggest the potential of the particles to specifically detect thrombi at the molecular level.
Acknowledgments
Dr Yu Zhang and Dr Jun Zhou contributed equally to this work and should be considered first coauthors. The authors are grateful to MRI technologists Wei Wu and Yindeng Luo for their assistance with MRI technical support and American Journal Experts for assistance with language editing. This study was supported by the National Natural Science Foundation of China (Grant No 81171332, No 81130025), the Research Funds of the Chongqing Bureau of Health (Grant No 2011-1-052), and the Program of Chongqing University Innovation Team (Grant No KJTD201303).
Footnotes
Disclosure
The authors report no conflict of interest in this work.
References
- 1.Whinna HC. Overview of murine thrombosis models. Thromb Res. 2008;122(Suppl 1):S64–S69. doi: 10.1016/S0049-3848(08)70022-7. [DOI] [PubMed] [Google Scholar]
- 2.Ciesienski KL, Caravan P. Molecular MRI of thrombosis. Curr Cardiovasc Imaging Rep. 2010;4(1):77–84. doi: 10.1007/s12410-010-9061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gore JC, Manning HC, Quarles CC, Waddell KW, Yankeelov TE. Magnetic resonance in the era of molecular imaging of cancer. Magn Reson Imaging. 2011;29(5):587–600. doi: 10.1016/j.mri.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Overoye-Chan K, Koerner S, Looby RJ, et al. EP-2104R: a fibrin-specific gadolinium-based MRI contrast agent for detection of thrombus. J Am Chem Soc. 2008;130(18):6025–6039. doi: 10.1021/ja800834y. [DOI] [PubMed] [Google Scholar]
- 5.Pan D, Caruthers SD, Hu G, et al. Ligand-directed nanobialys as theranostic agent for drug delivery and manganese-based magnetic resonance imaging of vascular targets. J Am Chem Soc. 2008;130(29):9186–9187. doi: 10.1021/ja801482d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miserus RJ, Herías MV, Prinzen L, et al. Molecular MRI of early thrombus formation using a bimodal alpha2-antiplasmin-based contrast agent. JACC Cardiovasc Imaging. 2009;2(8):987–996. doi: 10.1016/j.jcmg.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 7.McCarthy JR, Patel P, Botnaru I, Haghayeghi P, Weissleder R, Jaffer FA. Multimodal nanoagents for the detection of intravascular thrombi. Bioconjug Chem. 2009;20(6):1251–1255. doi: 10.1021/bc9001163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klink A, Lancelot E, Ballet S, et al. Magnetic resonance molecular imaging of thrombosis in an arachidonic acid mouse model using an activated platelet targeted probe. Arterioscler Thromb Vasc Biol. 2010;30(3):403–410. doi: 10.1161/ATVBAHA.109.198556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von zur Muhlen C, von Elverfeldt D, Moeller JA, et al. Magnetic resonance imaging contrast agent targeted toward activated platelets allows in vivo detection of thrombosis and monitoring of thrombolysis. Circulation. 2008;118(3):258–267. doi: 10.1161/CIRCULATIONAHA.107.753657. [DOI] [PubMed] [Google Scholar]
- 10.Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161(2):505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 11.Faisant N, Akiki J, Siepmann F, Benoit JP, Siepmann J. Effects of the type of release medium on drug release from PLGA-based microparticles: experiment and theory. Int J Pharm. 2006;314(2):189–197. doi: 10.1016/j.ijpharm.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 12.Kocbek P, Obermajer N, Cegnar M, Kos J, Kristl J. Targeting cancer cells using PLGA nanoparticles surface modified with monoclonal antibody. J Control Release. 2007;120(1–2):18–26. doi: 10.1016/j.jconrel.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Doiron AL, Homan KA, Emelianov S, Brannon-Peppas L. Poly(lactic-co-glycolic) acid as a carrier for imaging contrast agents. Pharm Res. 2009;26(3):674–682. doi: 10.1007/s11095-008-9786-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan H, Wu J, Lao L, Gao C. Gelatin/chitosan/hyaluronan scaffold integrated with PLGA microspheres for cartilage tissue engineering. Acta Biomater. 2009;5(1):328–337. doi: 10.1016/j.actbio.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 15.Doiron AL, Chu K, Ali A, Brannon-Peppas L. Preparation and initial characterization of biodegradable particles containing gadolinium-DTPA contrast agent for enhanced MRI. Proc Natl Acad Sci U S A. 2008;105(45):17232–17237. doi: 10.1073/pnas.0710205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onuki Y, Jacobs I, Artemov D, Kato Y. Noninvasive visualization of in vivo release and intratumoral distribution of surrogate MR contrast agent using the dual MR contrast technique. Biomaterials. 2010;31(27):7132–7138. doi: 10.1016/j.biomaterials.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee PW, Hsu SH, Wang JJ, et al. The characteristics, biodistribution, magnetic resonance imaging and biodegradability of superparamagnetic core-shell nanoparticles. Biomaterials. 2010;31(6):1316–1324. doi: 10.1016/j.biomaterials.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Faranesh AZ, Nastley MT, Perez de la Cruz C, et al. In vitro release of vascular endothelial growth factor from gadolinium-doped biodegradable microspheres. Magn Reson Med. 2004;51(6):1265–1271. doi: 10.1002/mrm.20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ao M, Wang Z, Ran H, et al. Gd-DTPA-loaded PLGA microbubbles as both ultrasound contrast agent and MRI contrast agent – a feasibility research. J Biomed Mater Res B Appl Biomater. 2010;93(2):551–556. doi: 10.1002/jbm.b.31614. [DOI] [PubMed] [Google Scholar]
- 20.Yu L, Zhang Z, Ding J. Influence of LA and GA sequence in the PLGA block on the properties of thermogelling PLGA-PEG-PLGA block copolymers. Biomacromolecules. 2011;12(4):1290–1297. doi: 10.1021/bm101572j. [DOI] [PubMed] [Google Scholar]
- 21.Chung TW, Wang SS, Tsai WJ. Accelerating thrombolysis with chitosan-coated plasminogen activators encapsulated in poly-(lactide-co-glycolide) (PLGA) nanoparticles. Biomaterials. 2008;29(2):228–237. doi: 10.1016/j.biomaterials.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 22.Huang G, Zhou Z, Srinivasan R, et al. Affinity manipulation of surface-conjugated RGD peptide to modulate binding of liposomes to activated platelets. Biomaterials. 2008;29(11):1676–1685. doi: 10.1016/j.biomaterials.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srinivasan R, Marchant RE, Gupta AS. In vitro and in vivo platelet targeting by cyclic RGD-modified liposomes. J Biomed Mater Res A. 2010;93(3):1004–1015. doi: 10.1002/jbm.a.32549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hua X, Liu P, Gao YH, et al. Construction of thrombus-targeted microbubbles carrying tissue plasminogen activator and their in vitro thrombolysis efficacy: a primary research. J Thromb Thrombolysis. 2010;30(1):29–35. doi: 10.1007/s11239-010-0450-z. [DOI] [PubMed] [Google Scholar]
- 25.Mu Y, Li L, Ayoufu G. Experimental study of the preparation of targeted microbubble contrast agents carrying urokinase and RGDS. Ultrasonics. 2009;49(8):676–681. doi: 10.1016/j.ultras.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Ho MH, Wang DM, Hsieh HJ, et al. Preparation and characterization of RGD-immobilized chitosan scaffolds. Biomaterials. 2005;26(16):3197–3206. doi: 10.1016/j.biomaterials.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 27.Lassalle V, Ferreira ML. PLA nano- and microparticles for drug delivery: an overview of the methods of preparation. Macromol Biosci. 2007;7(6):767–783. doi: 10.1002/mabi.200700022. [DOI] [PubMed] [Google Scholar]
- 28.Ito F, Fujimori H, Makino K. Factors affecting the loading efficiency of water-soluble drugs in PLGA microspheres. Colloids Surf B Biointerfaces. 2008;61(1):25–29. doi: 10.1016/j.colsurfb.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 29.Tu F, Lee D. Controlling the stability and size of double-emulsion-templated poly(lactic-co-glycolic) acid microcapsules. Langmuir. 2012;28(26):9944–9952. doi: 10.1021/la301498f. [DOI] [PubMed] [Google Scholar]
- 30.Yu X, Song SK, Chen J, et al. High-resolution MRI characterization of human thrombus using a novel fibrin-targeted paramagnetic nanoparticle contrast agent. Magn Reson Med. 2000;44(6):867–872. doi: 10.1002/1522-2594(200012)44:6<867::aid-mrm7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]