Abstract
The predominant mechanism of drug resistance in African trypanosomes is decreased drug uptake due to loss-of-function mutations in the genes for the transporters that mediate drug import. The role of transporters as determinants of drug susceptibility is well documented from laboratory-selected Trypanosoma brucei mutants. But clinical isolates, especially of T. b. gambiense, are less amenable to experimental investigation since they do not readily grow in culture without prior adaptation. Here we analyze a selected panel of 16 T. brucei ssp. field isolates that (i) have been adapted to axenic in vitro cultivation and (ii) mostly stem from treatment-refractory cases. For each isolate, we quantify the sensitivity to melarsoprol, pentamidine, and diminazene, and sequence the genomic loci of the transporter genes TbAT1 and TbAQP2. The former encodes the well-characterized aminopurine permease P2 which transports several trypanocides including melarsoprol, pentamidine, and diminazene. We find that diminazene-resistant field isolates of T. b. brucei and T. b. rhodesiense carry the same set of point mutations in TbAT1 that was previously described from lab mutants. Aquaglyceroporin 2 has only recently been identified as a second transporter involved in melarsoprol/pentamidine cross-resistance. Here we describe two different kinds of TbAQP2 mutations found in T. b. gambiense field isolates: simple loss of TbAQP2, or loss of wild-type TbAQP2 allele combined with the formation of a novel type of TbAQP2/3 chimera. The identified mutant T. b. gambiense are 40- to 50-fold less sensitive to pentamidine and 3- to 5-times less sensitive to melarsoprol than the reference isolates. We thus demonstrate for the first time that rearrangements of the TbAQP2/TbAQP3 locus accompanied by TbAQP2 gene loss also occur in the field, and that the T. b. gambiense carrying such mutations correlate with a significantly reduced susceptibility to pentamidine and melarsoprol.
Author Summary
Human African Trypanosomiasis, or sleeping sickness, is a fatal disease restricted to sub-Saharan Africa, caused by Trypanosoma brucei gambiense and T. b. rhodesiense. The treatment relies on chemotherapy exclusively. Drug resistance in T. brucei was investigated mainly in laboratory-selected lines and found to be linked to mutations in transporters. The adenosine transporter TbAT1 and the aquaglyceroporin TbAQP2 have been implicated in sensitivity to melarsoprol and pentamidine. Mutations in these transporters rendered trypanosomes less susceptible to either drug. Here we analyze T. brucei isolates from the field, focusing on isolates from patients where melarsoprol treatment has failed. We genotype those isolates to test for mutations in TbAQP2 or TbAT1, and phenotype for sensitivity to pentamidine and melarsoprol. Six T. b. gambiense isolates were found to carry mutations in TbAQP2. These isolates stemmed from relapse patients and exhibited significantly reduced sensitivity to pentamidine and melarsoprol as determined in cell culture. These findings indicate that mutations in TbAQP2 are present in the field, correlate with loss of sensitivity to pentamidine and melarsoprol, and might be responsible for melarsoprol treatment failures.
Introduction
The chemotherapy of human African trypanosomiasis (HAT, also known as sleeping sickness) currently relies on suramin or pentamidine for the first, haemolymphatic stage and on melarsoprol or eflornithine/nifurtimox combination therapy (NECT) for the second stage, when the trypanosomes have invaded the central nervous system (CNS) [1]. All five drugs have unfavorable pharmacokinetics and adverse effects. Melarsoprol is particularly toxic, causing severe encephalopathies in over 5% of the treated patients [2]. And yet, melarsoprol is the only treatment for late-stage T. b. rhodesiense infections. New and safer drugs are at various stages of (pre)clinical development, thanks largely to the Drugs for Neglected Diseases initiative (www.dndi.org). Two molecules that have successfully passed clinical Phase I trials are now being tested in patients: the nitroimidazole fexinidazole [3], [4] and the benzoxaborole SCYX-7158 [5], [6]. Both are orally available and cure 2nd stage T. b. brucei infections in a mouse model [7]. However, until new drugs for HAT are on the market, the current ones – problematic as they are – need to be used in a sustainable way. This requires an understanding of the mechanisms of drug resistance.
The mechanisms of drug resistance in African trypanosomes have been studied in the lab for over 100 years [8]. Two observations were made recurrently, namely (i) reduced drug uptake by drug resistant trypanosomes [9]–[14] and (ii) cross-resistance between melarsoprol and pentamidine [15], [16]. Both phenomena were attributed to the fact that melarsoprol and pentamidine are taken up by trypanosomes via the same transporters, which appeared to be lacking in drug-resistant mutants. The first transporter identified was called P2 since it was one of two purine nucleoside transporters identified [17], [18]. It is encoded by the gene TbAT1 for adenine/adenosine transporter 1 [19]. Homozygous genetic deletion of TbAT1 in bloodstream-form T. b. brucei resulted in pentamidine and melarsoprol cross-resistance, albeit only by a factor of about 2.5 [20]. This weak phenotype, together with the fact that the TbAT1−/− mutants still exhibited saturable drug import [21], indicated that further transporters are involved in melarsoprol-pentamidine cross-resistance [16], [21], [22]. One such transporter was recently identified, the aquaglyceroporin TbAQP2 [23], [24]. Aquaporins and aquaglyceroporins belong to the major intrinsic protein (MIP) family and form channels that facilitate transmembrane transport of water and small non-ionic solutes such as glycerol and urea [25]. The three aquaporins of T. brucei (TbAQP1-3) are thought to physiologically function as osmoregulators and are involved in glycerol transport [26]. Aquaporins were described to mediate uptake of arsenite in mammalian cells [27] and in Leishmania, and loss of aquaporin function was implicated in heavy metal resistance [28]. Homozygous genetic deletion of TbAQP2 in bloodstream-form T. b. brucei increased the IC50 towards melarsoprol and pentamidine by about 2- and 15- fold, respectively [24]. Moreover, a T. b. brucei lab mutant selected for high-level pentamidine resistance [21] carried a chimeric TbAQP2 gene, where 272 nucleotides had been replaced by the corresponding sequence from a neighboring, very similar gene TbAQP3 [24]. Differences in the TbAQP2/TbAQP3 tandem locus on chromosome 10 were also observed between the reference genome sequences of T. b. gambiense DAL972 [29] and T. b. brucei TREU927 [23], [30]. They possess identical versions of TbAQP2 but differ in TbAQP3 [31]. More recent field isolates of T. brucei ssp. have so far not been genotyped regarding their TbAQP2/TbAQP3 locus.
The genotypic status of TbAT1, located proximal to a telomere on chromosome 5 [32], has been more intensely investigated. Point mutations in TbAT1 were described, both in selected lab strains and in clinical T. brucei ssp. isolates, which rendered the gene non-functional when expressed in yeast [19]. The occurrence of these mutations correlated to a certain degree with melarsoprol treatment failure in 2nd stage T. b. gambiense HAT patients [33]–[36]. However, the relationship between polymorphisms in TbAT1, drug susceptibility, and treatment failure in patients is not fully resolved as the TbAT1 mutant T. b. gambiense were not analyzed phenotypically. Such investigations are notoriously difficult since clinical T. b. gambiense isolates are hard to obtain (given the inaccessibility of HAT foci and the poor success rate of isolation and adaptation in rodents) and cannot readily be propagated in axenic culture. Here we concentrate on clinical T. brucei ssp. isolates from drug refractory cases that have been adapted to axenic in vitro cultivation, aiming to investigate whether mutations at the known melarsoprol and pentamidine transporter loci also occur in the field – and if so, whether such mutations are accompanied by loss of drug susceptibility.
Materials and Methods
Trypanosoma brucei ssp. isolates
The 16 analyzed isolates are described in Table 1 (origin) and Table 2 (clinical outcome). For more details on the recent isolates from the DRC please refer to Table S4 of Pyana et al (2011) [37]. All have previously been adapted to axenic cultivation. T. b. brucei and T. b. rhodesiense isolates were cultured in minimum essential medium (MEM) with Earle's salts with the addition of 0.2 mM 2-mercaptoethanol, 1 mM Na-pyruvate, 0.5 mM hypoxanthine, and 15% heat-inactivated horse serum as described by Baltz et al (1985) [38]. T. b. gambiense strains were cultured in IMDM medium supplemented according to Hirumi and Hirumi (1989) [39], plus 0.2 mM 2-mercaptoethanol, 15% heat-inactivated fetal calf serum and 5% human serum. The cultures were maintained under a humidified 5% CO2 atmosphere at 37°C and were subpassaged 3 times a week to ensure growth in the exponential (log) phase.
Table 1. Origin of the analyzed T. brucei isolates.
| Isolate | Species | Origin | Reference |
| STIB 930 | Tbg | Republic of Côte d'Ivoire, 1978 | [49] |
| ITMAP 141267 | Tbg | Democratic Republic of the Congo, 1960 | [50] |
| STIB 756 | Tbg | Liberia, 1981 | [51] |
| STIB 891 | Tbg | Uganda, 1995 | [33] |
| DAL 870R | Tbg | Republic of Côte d'Ivoire, 1985 | [52] |
| DAL 898R | Tbg | Republic of Côte d'Ivoire, 1985 | [52] |
| K03048 | Tbg | South Sudan, 2003 | [53] |
| 45 BT (MHOM/CD/INRB/2006/1) | Tbg | Democratic Republic of the Congo, 2006 | [37] |
| 130 BT (MHOM/CD/STI/2006/02) | Tbg | Democratic Republic of the Congo, 2006 | [37] |
| 349 BT (MHOM/CD/INRB/2006/16) | Tbg | Democratic Republic of the Congo, 2006 | [37] |
| 349 AT (MHOM/CD/INRB/2006/19) | Tbg | Democratic Republic of the Congo, 2006 | [37] |
| 40 AT (MHOM/CD/INRB/2006/07) | Tbg | Democratic Republic of the Congo, 2006 | [37] |
| STIB 900 | Tbr | Tanzania, 1982 | [52] |
| STIB 871 | Tbr | Uganda, 1994 | [54] |
| STIB 940 | Tbb | Somalia, 1985 | [42], [55] |
| STIB 950 | Tbb | Somalia, 1985 | [41] |
Table 2. Drug sensitivity (IC50 ± SD in nM), genotypic status of TbAT1 and TbAQP2, and clinical outcome of melarsoprol treatment of the patients.
| Isolate | MelB | Pentamidine | Diminazene | TbAT1 | TbAQP2 | Clinics |
| STIB 930 | 9.6±4.5 | 1.9±0.7 | 21.0±8.5 | Ref | Ref | Cure |
| ITMAP 141267 | 15.0±8.1 | 8.3±3.4 | 9.9±4.4 | WT | WT | Cure |
| STIB 756 | 6.2±1.1 | 1.3±0.7 | 24.7±7.9 | WT | WT | Unknown |
| STIB 891 | 5.3±0.9 | 1.7±1.4 | 23.3±2.7 | WT | WT | Unknown |
| DAL 870R | 4.4±1.7 | 1.1±1.0 | 5.3±2.2 | WT | WT | Relapse |
| DAL 898R | 8.9±5.9 | 1.7±1.2 | 22.7±16.8 | WT | WT | Relapse |
| K03048 | 24.8±9.2 | 81.2±21.9 | 58.0±33.6 | WT | deletion/chimeric | Relapse |
| 45 BT | 25.9±8.6 | 91.8±29.7 | 37.5±10.8 | WT | chimeric | Relapse |
| 130 BT | 42.3±17.6 | 76.9±22.3 | 12.3±4.5 | WT | chimeric | Probable relapse |
| 349 BT | 26.2±11.3 | 71.9±12.4 | 20.0±3.2 | WT | chimeric | Relapse |
| 349 AT | 25.6±11.8 | 81.9±31.8 | 15.4±1.0 | WT | chimeric | Relapse |
| 40 AT | 22.0±8.0 | 72.2±21.1 | 39.9±16.7 | WT | chimeric | Relapse |
| STIB 900 | 4.6±2.6 | 3.2±0.9 | 3.8±1.5 | Ref | Ref | Cure |
| STIB 871 | 4.4±1.3 | 2.5±1.0 | 201±163 | R allele | WT | Cure |
| STIB 940 | 13.6±7.0 | 3.4±2.0 | 340±218 | R allele | WT | n.a. |
| STIB 950 | 27.6±9.4 | 1.8±0.4 | 102±53.6 | R allele | WT | n.a. |
WT = identical to reference (Ref) strain, being STIB 930 for T. b. gambiense isolates and STIB 900 for T. b. brucei and T. b. rhodesiense strains.
Phenotyping
Drug sensitivity was determined with the Alamar blue assay as described by Räz et al (1997) [40], using the redox-sensitive dye resazurin as an indicator of cell number and viability. The trypanosomes were cultivated in 96-well microtiter plates in serial dilutions of drugs for 70 h. 10 ul of resazurin (125 ug/ml (Sigma) dissolved in PBS pH 7.2) was added to each well. The plates were further incubated for 2–4 hours for T. b. rhodesiense and T. b. brucei, and 6–8 hours for T. b. gambiense, before being read with a SpectraMax Gemini XS microplate fluorescence scanner (Molecular Devices) at an excitation wavelength of 536 nm and an emission wavelength of 588 nm. IC50 values were calculated by non-linear regression to a sigmoidal inhibition curve using SoftMax Pro software (V. 5.2). The IC50 values given in Table 2 are averages ± standard deviation of at least 3 independent assays (n = 3–12), each determined in duplicate. Melarsoprol (Sanofi-Aventis) was obtained from WHO. Pentamidine isothionate and diminazene aceturate were purchased from Sigma.
Genotyping
Genomic DNA was isolated from 10 ml dense trypanosome cultures. The cells were spun down and the pellets resuspended in 300 µl 10 mM TrisHCl pH 8, 1 mM EDTA and 3 µl 10% SDS was added before incubating for 10–15 min at 55°C. After 5 min incubation 3 µl of pronase mix (20 mg/ml, Sigma) was added to increase the stability of the extracted DNA. 90 µl of ice cold 5 M potassium acetate was added and the mixture was incubated for 5 min on ice. After spinning down for 5 minutes at max speed in a microfuge, the supernatant was transferred to a new tube and DNA was precipitated in 2–2.5 volumes of absolute ethanol, washed in 70% ethanol and dissolved in 20 µl ddH2O. PCR was performed with Taq polymerase (Solis BioDyne, Estonia); the primers and annealing temperatures are summarized in Table S1. PCR products were run on a 0.8% agarose gel and purified on a silica membrane column (Nucleospin gel and PCR clean up, Macherey Nagel, Germany). The purified PCR products were directly sequenced (Microsynth, Switzerland or GATC, Germany) with the same primers as used for PCR amplification. Only the TbAQP2/TbAQP3 locus of T. b. gambiense K03048 produced two PCR products, which were cloned in pCR2.1-TOPO (Invitrogen). The assembled sequences were submitted to GenBank; accession numbers are listed in Table S2.
Results
A panel of Trypanosoma brucei ssp. field isolates
To be able to compare – and possibly correlate – genotype and phenotype of T. brucei ssp., we assembled a set of 16 isolates that had been adapted to axenic in vitro cultivation as blood-stream forms. These included 5 recent T. b. gambiense isolates from the Democratic Republic of the Congo (DRC), 2 older isolates from the Republic of Côte d'Ivoire and one isolate from South Sudan, which were all isolated from patients who had relapsed after melarsoprol chemotherapy. Other T. b. gambiense isolates from the DRC, northwestern Uganda, and Liberia were from patients who were successfully treated with melarsoprol or the treatment outcome is unknown. T. b. gambiense STIB 930 is a fully drug-susceptible lab strain that was used as a reference strain. We further included the field isolates T. b. brucei STIB 940, T. b. brucei STIB 950 and T. b. rhodesiense STIB 871, which are multidrug-resistant to isometamidium, diminazene and tubercidin. The fully drug-susceptible reference strain T. b. rhodesiense STIB 900 was included as a reference. The different isolates and their origin are summarized in Table 1. All isolates were genotyped regarding TbAQP2 and TbAT1.
Naturally occurring mutations in TbAQP2
When the TbAQP2/TbAQP3 genomic locus was amplified by PCR from the 16 T. brucei ssp. isolates, all the recent T. b. gambiense isolates from the DRC (40 AT, 45 BT, 130 BT, 349 BT and 349 AT) exhibited a smaller band than expected for the wild-type locus. Direct sequencing of the PCR product in each of the five isolates revealed only one gene at the locus: a chimeric version of TbAQP2 and TbAQP3. The first 813 bp of the open reading frame perfectly matched TbAQP2 while the remaining 126 bp derived from TbAQP3 (Figure 1C). These 126 bp perfectly matched to TbAQP3 of T. b. rhodesiense STIB 900 but this exact sequence is not found in the published genome of T. b. gambiense DAL 972. Note that the present TbAQP2-TbAQP3 chimeric gene (Figure 1C) differs from the one described by Baker et al. from a pentamidine-selected T. b. brucei lab mutant (Figure 1B; [24]). T. b. gambiense K03048 from the South Sudan also gave rise to an abnormal pattern upon PCR amplification of the TbAQP2/TbAQP3 locus from genomic DNA: a distinctly smaller double band instead of the expected product, indicative of heterozygosity. The smaller band contained the upstream region of TbAQP2 followed by the open reading frame of TbAQP3 while the TbAQP2 open reading frame was missing (Figure 1D). The larger band contained a TbAQP2/3 chimera similar to that encountered in the T. b. gambiense isolates of the DRC (Figure 1C). Point mutations in TbAQP2 were encountered in the multidrug-resistant field isolates T. b. brucei STIB 940, T. b. brucei STIB 950 and T. b. rhodesiense STIB 871, all of which had the same 4 SNPs in TbAQP2 compared to the T. b. brucei 927 reference gene (Tb927.10.14170), leading to the amino acid change threonine159 to alanine (Figure 1E). However, the same 4 SNPs also occurred in our drug-susceptible reference strain T. b. rhodesiense STIB 900, so they are not likely to be involved in the mdr phenotype [41], [42] of these isolates. All other isolates analyzed had a wild-type copy of TbAQP2. The identified sequence polymorphisms are summarized in Table 2, GenBank accession numbers are in Table S2.
Figure 1. Schematic view of the TbAQP2/TbAQP3 locus on chromosome 10.

A) Reference locus of T. b. brucei TREU927, T. b. gambiense STIB 930 and T. b. gambiense DAL972 (minor differences in TbAQP3 are not highlighted). B) Chimera of TbAQP2 and TbAQP3 as described by Baker et al. (2012) [24] for the in vitro selected, pentamidine-resistant T. b. brucei line B48. C) Chimera of TbAQP2 and TbAQP3 plus loss of TbAQP3 in T. b. gambiense 40 AT, 45 BT, 130 BT, 349 BT, and 349 AT, and in one K03048 allele. D) Deletion of the TbAQP2 ORF in the other T. b. gambiense K03048 allele. E) TbAQP2 polymorphisms (C474A, G475A, C477T, T480C) in several T. b. rhodesiense and T. b. brucei isolates from East Africa (STIB 900, STIB 950, STIB 940, and STIB 871).
Naturally occurring mutations in TbAT1
All of the 12 analyzed T. b. gambiense isolates were identical in TbAT1 sequence to the reference STIB 930 as well as to the genome strain DAL972. The previously described TbAT1R allele [19], [33] was found in the 3 mdr lines T. b. brucei STIB 940, T. b. brucei STIB 950 and T. b. rhodesiense STIB 871. TbAT1R carries 5 coding and 4 silent mutations and a codon deletion as compared to the reference sequence (STIB 900), and the resultant protein appeared to be non-functional when expressed in Saccharomyces cerevisiae [19] or re-expressed in a tbat1 null T. b. brucei (De Koning, unpublished results). The remainder of the isolates did not possess mutations in TbAT1 when compared to the respective reference isolate. The GenBank accession numbers of all the sequences are in Table S2.
Correlating TbAQP2 and TbAT1 genotype to drug susceptibility
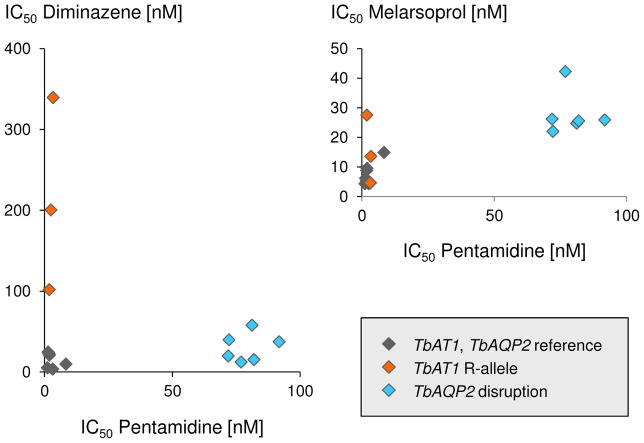
Drug sensitivities of the bloodstream-forms of all isolates were determined in vitro regarding melarsoprol, pentamidine, and diminazene. The five T. b. gambiense that possessed the chimeric TbAQP2/3 gene (45 BT, 130 BT, 349 BT, 349 AT, 40 AT), as well as K03048 which carries a deletion of TbAQP2 in one allele, in addition to one chimeric TbAQP2/3 allele, all showed a similar drug sensitivity profile with markedly increased IC50 values towards pentamidine and, to a lesser extent, also melarsoprol (Figure 2). IC50 values were in the range of 70–92 nM for pentamidine and 22–42 nM for melarsoprol (Table 2); compared to the median of the four drug sensitive T. b. gambiense lines STIB 930, STIB 891, STIB 756 and ITMAP 141267, this corresponds to a 40- to 52-fold decrease in susceptibility to pentamidine and a 2.8- to 5.3-fold decrease for melarsoprol. The higher IC50 values of the isolates that carried a mutation in TbAQP2 (n = 6) compared to the remainder (n = 10) were statistically significant both with respect to pentamidine (p = 0.0002, two-tailed Mann-Whitney test) and melarsoprol (p = 0.0047); no association was observed regarding TbAQP2 status and sensitivity to diminazene. However, the isolates that carried the known resistance allele TbAT1R (i.e. STIB 940, STIB 950 and STIB 871) exhibited strongly increased IC50 values to diminazene (p = 0.01, two-tailed Mann-Whitney test) but not to pentamidine (Figure 2, Table 2). T. b. brucei STIB 950 also had an elevated IC50 against melarsoprol (Figure 2), but over all three TbAT1R isolates there was no significant effect on melarsoprol susceptibility.
Figure 2. In vitro drug sensitivities.
50% inhibitory concentrations (IC50) as determined with the Alamar blue assay. Susceptibility to pentamidine correlates with that to melarsoprol but not diminazene. TbAT1 and TbAQP2 genotypes are indicated.
Across all 16 T. brucei isolates, pentamidine sensitivity positively correlated with that to melarsoprol (Spearman's rank correlation coefficient of 0.67, p = 0.005) while there was no correlation between the two structurally related diamidines, pentamidine and diminazene (Figure 2).
Discussion
It is an intriguing phenomenon with African trypanosomes that drug resistance is predominantly linked to reduced drug import, typically arising from loss of function mutation of a non-essential transporter [12], . Here we investigated the aminopurine transporter TbAT1 and the aquaglyceroporin TbAQP2, two proteins known to be involved in uptake of – and susceptibility to – melarsoprol and diamidines in bloodstream-form T. brucei. While there is evidence for a link between TbAT1 mutations and melarsoprol treatment failure in the field [33]–[36], the more recently identified gene TbAQP2 has so far not been analyzed in a clinical setting. TbAQP2 is dispensable for growth in culture [24] and partial gene replacement of TbAQP2 with TbAQP3 was observed in a pentamidine-selected T. b. brucei lab mutant [24] that displayed reduced infectivity to rodents [21]. However, it was unknown whether similar mutations also occur in the field, as they might bear a fitness cost in patients or during transmission by the tsetse fly. Concentrating on a panel of clinical T. brucei ssp. isolates that (i) derived from treatment-refractory cases and (ii) had been adapted to axenic in vitro culture, we have genotyped their TbAT1 and TbAQP2 loci, and phenotyped their in vitro sensitivity towards melarsoprol, pentamidine and diminazene. Our aim was to explore whether TbAQP2 mutations occur in the field and if so, whether mutant isolates exhibit reduced drug susceptibility.
Five of the analyzed T. b. gambiense isolates, all from melarsoprol relapse patients of Dipumba Hospital in Mbuji-Mayi, DRC, carried only one gene at the TbAQP2/TbAQP3 tandem locus, an unprecedented TbAQP2/3 chimera. The high degree of sequence similarity between TbAPQ2 and TbAQP3 allows for homologous recombination between the two genes, leading to chimerization and gene loss. TbAQP2 has a unique selectivity filter with unusual NSA/NPS motifs instead of the characteristic NPA/NPA that occur in the vast majority of MIP family members [43] including TbAQP1 and TbAQP3 [24]. The published, pentamidine-resistant T. b. brucei lab mutant possessed a TbAQP2/3 chimera whose C-terminal filter triplet was from TbAQP3, suggesting that the unusual NPS triplet may be involved in pentamidine transport. However, the presently described pentamidine-resistant T. b. gambiense isolates carry a TbAQP2/3 chimera encoding a predicted protein with both selectivity filter triplets from TbAQP2. We hypothesize that the TbAQP2/3 chimera observed in the T. b. gambiense isolates fails to contribute to pentamidine and melarsoprol susceptibility despite having the proposed selectivity filter residues of TbAQP2. Functional expression of the chimeric gene in tbaqp2 null cells will be necessary to test this hypothesis.
The occurrence of rearrangements at the TbAQP2/TbAQP3 locus correlated with reduced susceptibility to pentamidine and, to a lesser extent, melarsoprol. Thus field isolates also exhibit the well known cross-resistance between melarsoprol and pentamidine 15,16,31, while no cross-resistance was observed to diminazene aceturate. This is in agreement with TbAT1 being the primary uptake route for diminazene [44], [45] and consistent with results obtained using TbAQP2 −/− cells, which showed no resistance to the rigid diamidines diminazene or DB75 [24], as opposed to pentamidine which has a highly flexible structure. It is also noteworthy that T. b. rhodesiense STIB 871 and T. b. brucei STIB 940 are susceptible to melarsoprol and pentamidine in vitro although both carry the TbAT1r allele. Loss of TbAT1 function has been described without mutations in the open reading frame of the gene [32]. However, since in the present study all isolates with a ‘wild-type’ TbAT1 ORF were fully susceptible to diminazene, we conclude that they possess a functional TbAT1 (i.e. P2) transporter. Trypanosoma congolense and T. vivax appear to lack an AT1 orthologue [46], therefore diminazene transport and resistance must have a different mechanism in these livestock parasites.
The plasma levels of pentamidine in treated patients peak about 1 hour after injection and vary extensively from 0.42 µM to 13 µM, while the mean elimination half-life after multiple applications is approximately 12 days [47]. Thus, since pentamidine is very potent, even a 50-fold increase in IC50 of pentamidine as observed here for the T. b. gambiense isolates with mutations in TbAQP2, is unlikely to jeopardize the success of treatment. With melarsoprol, however, the obtainable drug levels are more critical. Only 1–2% of the maximal plasma levels are seen in the CSF [48], and a 5-fold reduced sensitivity to melarsoprol might allow trypanosomes to survive in the CSF during melarsoprol therapy. Thus mutations in TbAQP2 might indeed be responsible for melarsoprol treatment failures with T. b. gambiense. However, two of the T. b. gambiense isolates from relapse patients (DAL 870R and DAL 898 R) were sensitive to melarsoprol and pentamidine, and they possessed wild-type copies of TbAT1 and TbAQP2, indicating that factors other than drug resistance can contribute to treatment failures. Larger sample sizes will be required to test the significance of TbAQP2 for successful treatment. We show here for the first time that a TbAQP2/3 chimera as well as loss of TbAQP2 occurs in T. b. gambiense clinical isolates, and that the presence of such rearrangements at the TbAQP2/TbAQP3 locus is accompanied by a 40- to 50-fold loss in pentamidine sensitivity and a 3- to 5-fold loss in melarsoprol sensitivity. We recommend genotyping of the TbAQP2/TbAQP3 locus to be integrated into larger field trials such as clinical studies with drug candidates.
Supporting Information
Primers used for PCR, their target gene, annealing temperature and sequence (5′ to 3′).
(PDF)
GenBank accession numbers of the sequenced genes.
(PDF)
Acknowledgments
We are grateful to Christina Kunz, Monica Cal and Eva Greganova for help in the lab, Simon Hänni for the quick DNA isolation protocol, and Christian Burri for comments on the manuscript.
Funding Statement
This work was supported by the Swiss National Science Foundation (31003A_135746). PPP received a PhD grant from the Institute of Tropical Medicine; PL received fellowships from the Emilia Guggenheim-Schnurr Foundation, the Mathieu-Stiftung, and the Freiwillige Akademische Gesellschaft Basel; DH is funded by a Wellcome Trust Senior Investigator Award (100320/Z/12/Z). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Brun R, Blum J, Chappuis F, Burri C (2010) Human African trypanosomiasis. Lancet 375: 148–159 doi:10.1016/S0140-6736(09)60829-1 [DOI] [PubMed] [Google Scholar]
- 2. Kennedy PGE (2008) The continuing problem of human African trypanosomiasis (sleeping sickness). Ann Neurol 64: 116–126 doi:10.1002/ana.21429 [DOI] [PubMed] [Google Scholar]
- 3. Torreele E, Bourdin Trunz B, Tweats D, Kaiser M, Brun R, et al. (2010) Fexinidazole–a new oral nitroimidazole drug candidate entering clinical development for the treatment of sleeping sickness. PLoS Negl Trop Dis 4: e923 doi:10.1371/journal.pntd.0000923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaiser M, Bray MA, Cal M, Bourdin Trunz B, Torreele E, et al. (2011) Antitrypanosomal activity of fexinidazole, a new oral nitroimidazole drug candidate for treatment of sleeping sickness. Antimicrob Agents Chemother 55: 5602–5608 doi:10.1128/AAC.00246-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nare B, Wring S, Bacchi C, Beaudet B, Bowling T, et al. (2010) Discovery of novel orally bioavailable oxaborole 6-carboxamides that demonstrate cure in a murine model of late-stage central nervous system african trypanosomiasis. Antimicrob Agents Chemother 54: 4379–4388 doi:10.1128/AAC.00498-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jacobs RT, Nare B, Wring SA, Orr MD, Chen D, et al. (2011) SCYX-7158, an orally-active benzoxaborole for the treatment of stage 2 human African trypanosomiasis. PLoS Negl Trop Dis 5: e1151 doi:10.1371/journal.pntd.0001151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mäser P, Wittlin S, Rottmann M, Wenzler T, Kaiser M, et al. (2012) Antiparasitic agents: new drugs on the horizon. Curr Opin Pharmacol 12: 562–566 doi:10.1016/j.coph.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 8. Ehrlich P (1907) Chemotherapeutische trypanosomen-studien. Berl Klin Wochenzeitschrift 44. [Google Scholar]
- 9. Hawking F (1937) Studies on Chemotherapeutic Action I. the Absorption of Arsenical Compounds and Tartar Emetic by Normal and Resistant Trypanosomes and Its Relation to Drugresistance. J Pharmacol Exp Ther 59: 123–156. [Google Scholar]
- 10. Frommel TO, Balber AE (1987) Flow cytofluorimetric analysis of drug accumulation by multidrug-resistant Trypanosoma brucei brucei and T. b. rhodesiense. Mol Biochem Parasitol 26: 183–191. [DOI] [PubMed] [Google Scholar]
- 11. Mäser P, Lüscher A, Kaminsky R (2003) Drug transport and drug resistance in African trypanosomes. Drug Resist Updat Rev Comment Antimicrob Anticancer Chemother 6: 281–290. [DOI] [PubMed] [Google Scholar]
- 12. Vincent IM, Creek D, Watson DG, Kamleh MA, Woods DJ, et al. (2010) A molecular mechanism for eflornithine resistance in African trypanosomes. PLoS Pathog 6: e1001204 doi:10.1371/journal.ppat.1001204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baker N, Alsford S, Horn D (2011) Genome-wide RNAi screens in African trypanosomes identify the nifurtimox activator NTR and the eflornithine transporter AAT6. Mol Biochem Parasitol 176: 55–57 doi:10.1016/j.molbiopara.2010.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schumann Burkard G, Jutzi P, Roditi I (2011) Genome-wide RNAi screens in bloodstream form trypanosomes identify drug transporters. Mol Biochem Parasitol 175: 91–94 doi:10.1016/j.molbiopara.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 15. ROLLO IM, WILLIAMSON J (1951) Acquired resistance to “Melarsen”, tryparsamide and amidines in pathogenic trypanosomes after treatment with “Melarsen” alone. Nature 167: 147–148. [DOI] [PubMed] [Google Scholar]
- 16. De Koning HP (2008) Ever-increasing complexities of diamidine and arsenical crossresistance in African trypanosomes. Trends Parasitol 24: 345–349 doi:10.1016/j.pt.2008.04.006 [DOI] [PubMed] [Google Scholar]
- 17. Carter NS, Fairlamb AH (1993) Arsenical-resistant trypanosomes lack an unusual adenosine transporter. Nature 361: 173–176 doi:10.1038/361173a0 [DOI] [PubMed] [Google Scholar]
- 18. Carter NS, Berger BJ, Fairlamb AH (1995) Uptake of diamidine drugs by the P2 nucleoside transporter in melarsen-sensitive and -resistant Trypanosoma brucei brucei. J Biol Chem 270: 28153–28157. [DOI] [PubMed] [Google Scholar]
- 19. Mäser P, Sütterlin C, Kralli A, Kaminsky R (1999) A nucleoside transporter from Trypanosoma brucei involved in drug resistance. Science 285: 242–244. [DOI] [PubMed] [Google Scholar]
- 20. Matovu E, Stewart ML, Geiser F, Brun R, Mäser P, et al. (2003) Mechanisms of arsenical and diamidine uptake and resistance in Trypanosoma brucei. Eukaryot Cell 2: 1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bridges DJ, Gould MK, Nerima B, Mäser P, Burchmore RJS, et al. (2007) Loss of the high-affinity pentamidine transporter is responsible for high levels of cross-resistance between arsenical and diamidine drugs in African trypanosomes. Mol Pharmacol 71: 1098–1108 doi:10.1124/mol.106.031351 [DOI] [PubMed] [Google Scholar]
- 22. De Koning HP (2001) Uptake of pentamidine in Trypanosoma brucei brucei is mediated by three distinct transporters: implications for cross-resistance with arsenicals. Mol Pharmacol 59: 586–592. [DOI] [PubMed] [Google Scholar]
- 23. Alsford S, Eckert S, Baker N, Glover L, Sanchez-Flores A, et al. (2012) High-throughput decoding of antitrypanosomal drug efficacy and resistance. Nature 482: 232–236 doi:10.1038/nature10771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baker N, Glover L, Munday JC, Aguinaga Andrés D, Barrett MP, et al. (2012) Aquaglyceroporin 2 controls susceptibility to melarsoprol and pentamidine in African trypanosomes. Proc Natl Acad Sci U S A 109: 10996–11001 doi:10.1073/pnas.1202885109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Uzcategui NL, Szallies A, Pavlovic-Djuranovic S, Palmada M, Figarella K, et al. (2004) Cloning, heterologous expression, and characterization of three aquaglyceroporins from Trypanosoma brucei. J Biol Chem 279: 42669–42676 doi:10.1074/jbc.M404518200 [DOI] [PubMed] [Google Scholar]
- 26. Bassarak B, Uzcátegui NL, Schönfeld C, Duszenko M (2011) Functional characterization of three aquaglyceroporins from Trypanosoma brucei in osmoregulation and glycerol transport. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol 27: 411–420 doi:10.1159/000327968 [DOI] [PubMed] [Google Scholar]
- 27. Liu Z, Shen J, Carbrey JM, Mukhopadhyay R, Agre P, et al. (2002) Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proc Natl Acad Sci U S A 99: 6053–6058 doi:10.1073/pnas.092131899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gourbal B, Sonuc N, Bhattacharjee H, Legare D, Sundar S, et al. (2004) Drug uptake and modulation of drug resistance in Leishmania by an aquaglyceroporin. J Biol Chem 279: 31010–31017 doi:10.1074/jbc.M403959200 [DOI] [PubMed] [Google Scholar]
- 29. Jackson AP, Sanders M, Berry A, McQuillan J, Aslett MA, et al. (2010) The genome sequence of Trypanosoma brucei gambiense, causative agent of chronic human african trypanosomiasis. PLoS Negl Trop Dis 4: e658 doi:10.1371/journal.pntd.0000658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berriman M, Ghedin E, Hertz-Fowler C, Blandin G, Renauld H, et al. (2005) The genome of the African trypanosome Trypanosoma brucei. Science 309: 416–422 doi:10.1126/science.1112642 [DOI] [PubMed] [Google Scholar]
- 31. Baker N, de Koning HP, Mäser P, Horn D (2013) Drug resistance in African trypanosomiasis: the melarsoprol and pentamidine story. Trends Parasitol 29: 110–118 doi:10.1016/j.pt.2012.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stewart ML, Burchmore RJS, Clucas C, Hertz-Fowler C, Brooks K, et al. (2010) Multiple genetic mechanisms lead to loss of functional TbAT1 expression in drug-resistant trypanosomes. Eukaryot Cell 9: 336–343 doi:10.1128/EC.00200-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matovu E, Geiser F, Schneider V, Mäser P, Enyaru JC, et al. (2001) Genetic variants of the TbAT1 adenosine transporter from African trypanosomes in relapse infections following melarsoprol therapy. Mol Biochem Parasitol 117: 73–81. [DOI] [PubMed] [Google Scholar]
- 34. Nerima B, Matovu E, Lubega GW, Enyaru JCK (2007) Detection of mutant P2 adenosine transporter (TbAT1) gene in Trypanosoma brucei gambiense isolates from northwest Uganda using allele-specific polymerase chain reaction. Trop Med Int Heal TM IH 12: 1361–1368 doi:10.1111/j.1365-3156.2007.01918.x [DOI] [PubMed] [Google Scholar]
- 35. Maina N, Maina KJ, Mäser P, Brun R (2007) Genotypic and phenotypic characterization of Trypanosoma brucei gambiense isolates from Ibba, South Sudan, an area of high melarsoprol treatment failure rate. Acta Trop 104: 84–90 doi:10.1016/j.actatropica.2007.07.007 [DOI] [PubMed] [Google Scholar]
- 36. Kazibwe AJN, Nerima B, de Koning HP, Mäser P, Barrett MP, et al. (2009) Genotypic status of the TbAT1/P2 adenosine transporter of Trypanosoma brucei gambiense isolates from Northwestern Uganda following melarsoprol withdrawal. PLoS Negl Trop Dis 3: e523 doi:10.1371/journal.pntd.0000523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pyana PP, Ngay Lukusa I, Mumba Ngoyi D, Van Reet N, Kaiser M, et al. (2011) Isolation of Trypanosoma brucei gambiense from cured and relapsed sleeping sickness patients and adaptation to laboratory mice. PLoS Negl Trop Dis 5: e1025 doi:10.1371/journal.pntd.0001025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baltz T, Baltz D, Giroud C, Crockett J (1985) Cultivation in a semi-defined medium of animal infective forms of Trypanosoma brucei, T. equiperdum, T. evansi, T. rhodesiense and T. gambiense. EMBO J 4: 1273–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hirumi H, Hirumi K (1989) Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J Parasitol 75: 985–989. [PubMed] [Google Scholar]
- 40. Räz B, Iten M, Grether-Bühler Y, Kaminsky R, Brun R (1997) The Alamar Blue assay to determine drug sensitivity of African trypanosomes (T.b. rhodesiense and T.b. gambiense) in vitro. Acta Trop 68: 139–147. [DOI] [PubMed] [Google Scholar]
- 41. Kaminsky R, Chuma F, Zweygarth E (1989) Trypanosoma brucei brucei: expression of drug resistance in vitro. Exp Parasitol 69: 281–289. [DOI] [PubMed] [Google Scholar]
- 42. Zweygarth E, Röttcher D (1989) Efficacy of experimental trypanocidal compounds against a multiple drug-resistant Trypanosoma brucei brucei stock in mice. Parasitol Res 75: 178–182. [DOI] [PubMed] [Google Scholar]
- 43. Gupta AB, Verma RK, Agarwal V, Vajpai M, Bansal V, et al. (2012) MIPModDB: a central resource for the superfamily of major intrinsic proteins. Nucleic Acids Res 40: D362–369 doi:10.1093/nar/gkr914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. De Koning HP, Anderson LF, Stewart M, Burchmore RJS, Wallace LJM, et al. (2004) The trypanocide diminazene aceturate is accumulated predominantly through the TbAT1 purine transporter: additional insights on diamidine resistance in african trypanosomes. Antimicrob Agents Chemother 48: 1515–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Teka IA, Kazibwe AJN, El-Sabbagh N, Al-Salabi MI, Ward CP, et al. (2011) The diamidine diminazene aceturate is a substrate for the high-affinity pentamidine transporter: implications for the development of high resistance levels in trypanosomes. Mol Pharmacol 80: 110–116 doi:10.1124/mol.111.071555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Munday JC, Rojas López KE, Eze AA, Delespaux V, Van Den Abbeele J, et al. (2013) Functional expression of TcoAT1 reveals it to be a P1-type nucleoside transporter with no capacity for diminazene uptake. Int J Parasitol Drugs Drug Resist 3: 69–76 doi:10.1016/j.ijpddr.2013.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burri C, Stich A, Brun R (2004) Chemotherapy of Human African Trypanosomiasis. In: Maudlin I, Holmes PH, Miles MA, editors. The Trypanosomiasis. Wallingford, UK; Cambridge, MA, USA: CABI Pub. pp. 403–419.
- 48. Burri C, Baltz T, Giroud C, Doua F, Welker HA, et al. (1993) Pharmacokinetic properties of the trypanocidal drug melarsoprol. Chemotherapy 39: 225–234. [DOI] [PubMed] [Google Scholar]
- 49. Felgner P, Brinkmann U, Zillmann U, Mehlitz D, Abu-Ishira S (1981) Epidemiological studies on the animal reservoir of gambiense sleeping sickness. Part II. Parasitological and immunodiagnostic examination of the human population. Tropenmed Parasitol 32: 134–140. [PubMed] [Google Scholar]
- 50. Likeufack ACL, Brun R, Fomena A, Truc P (2006) Comparison of the in vitro drug sensitivity of Trypanosoma brucei gambiense strains from West and Central Africa isolated in the periods 1960–1995 and 1999–2004. Acta Trop 100: 11–16 doi:10.1016/j.actatropica.2006.09.003 [DOI] [PubMed] [Google Scholar]
- 51. Richner D, Brun R, Jenni L (1988) Production of metacyclic forms by cyclical transmission of west African Trypanosoma (T.) brucei isolates from man and animals. Acta Trop 45: 309–319. [PubMed] [Google Scholar]
- 52. Brun R, Schumacher R, Schmid C, Kunz C, Burri C (2001) The phenomenon of treatment failures in Human African Trypanosomiasis. Trop Med Int Heal TM IH 6: 906–914. [DOI] [PubMed] [Google Scholar]
- 53. Maina NWN, Oberle M, Otieno C, Kunz C, Maeser P, et al. (2007) Isolation and propagation of Trypanosoma brucei gambiense from sleeping sickness patients in south Sudan. Trans R Soc Trop Med Hyg 101: 540–546 doi:10.1016/j.trstmh.2006.11.008 [DOI] [PubMed] [Google Scholar]
- 54. Matovu E, Iten M, Enyaru JC, Schmid C, Lubega GW, et al. (1997) Susceptibility of Ugandan Trypanosoma brucei rhodesiense isolated from man and animal reservoirs to diminazene, isometamidium and melarsoprol. Trop Med Int Heal TM IH 2: 13–18. [DOI] [PubMed] [Google Scholar]
- 55. Kaminsky R, Zweygarth E (1989) Effect of in vitro cultivation on the stability of resistance of Trypanosoma brucei brucei to diminazene, isometamidium, quinapyramine, and Mel B. J Parasitol 75: 42–45. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers used for PCR, their target gene, annealing temperature and sequence (5′ to 3′).
(PDF)
GenBank accession numbers of the sequenced genes.
(PDF)