Antivirulence Therapy as an Alternative to Antibiotics
Antibiotics are still critically important as a first line therapy for the treatment of various bacterial infections in the clinic. In addition to their use in human medicine, these compounds have also been used for decades in animal production, for both growth promotion and veterinary purposes [1], [2]. Because of the development and spread of antibiotic resistance, there is a growing awareness that antibiotics should be used with more care [3], and as a consequence, the development of alternative methods to control pathogenic bacteria in animal production will be important to ensure good productivity in the future.
Infection of both terrestrial and aquatic animals by bacterial pathogens requires the production of different virulence factors, i.e. gene products that allow the pathogenic bacteria to enter and damage the host. Major virulence factors include gene products involved in motility, adhesion, host tissue degradation, iron acquisition, secretion of toxins, and protection from host defense [4]. As virulence factors are required for infection, preventing pathogens from producing them constitutes an interesting alternative strategy for disease control, a strategy that has been termed antivirulence therapy [5]. Antivirulence therapy is based on a thorough understanding of the mechanisms by which bacterial pathogens cause disease. In this respect, studies aimed at understanding how bacteria cause disease have identified (and will probably continue to do so) targets for therapeutics with completely novel modes of action. Inhibitors of specific virulence factors, such as secretion systems, have been reported in literature [6]. However, considerably more research effort is being directed towards interference with regulatory mechanisms that control the expression of (multiple) virulence factors, such as bacterial cell-to-cell communication (quorum sensing) and host-pathogen signalling ( Fig. 1 ). The following paragraphs will focus on interference with these mechanisms as a novel strategy to control animal pathogens, using Escherichia coli and Salmonella spp. as examples of pathogens for terrestrial animals, and Aeromonas spp. and Vibrio spp. as examples of aquatic pathogens.
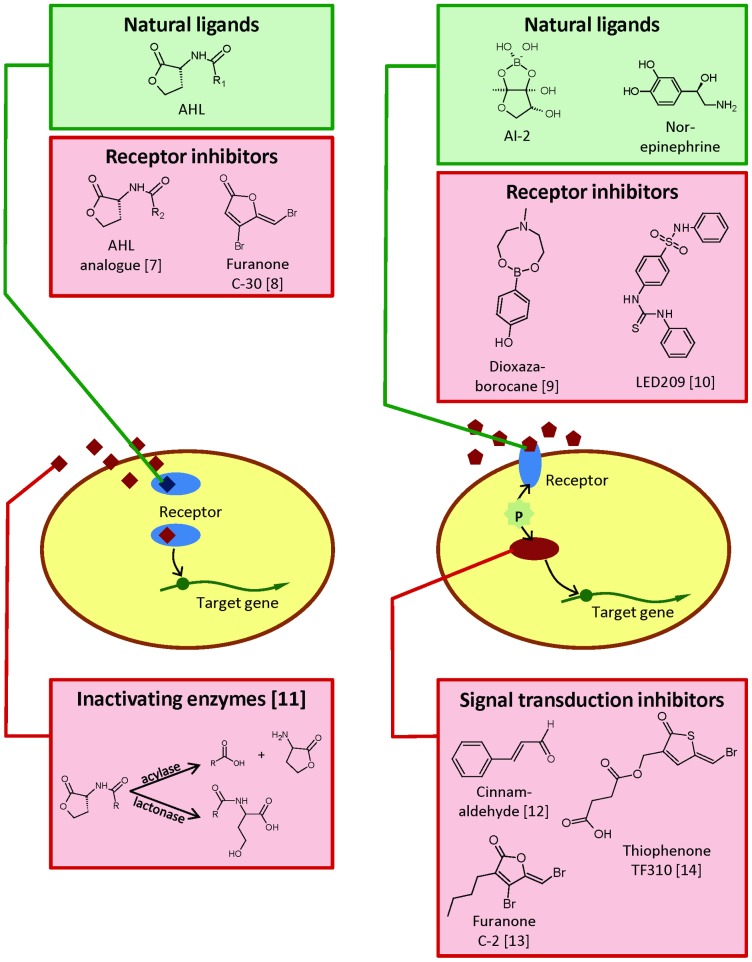
Figure 1. Simplified schematic representation of virulence regulatory systems based on detection of signal molecules in animal pathogenic bacteria.
These include (left) quorum sensing based on acylhomoserine lactones (AHL) and (right) quorum sensing in vibrios and catecholamine stress hormone sensing. For each type of system, examples of natural ligands, receptor inhibitors, and other inhibiting agents are shown. Dioxazaborocane is an inhibitor of AI-2 sensing in V. harveyi and LED209 is an inhibitor of catecholamine sensing in E. coli. The signal transduction inhibitors are inhibitors of quorum sensing signal transduction in vibrios.
Interfering with Bacterial Cell-to-Cell Communication in Animal Pathogens
Quorum sensing, or bacterial cell-to-cell communication, is a mechanism of gene regulation in which bacteria coordinate the expression of certain genes in response to the presence of small signal molecules. This regulatory mechanism has been shown to control virulence gene expression in many different pathogens, and a wide range of molecules (both of natural and synthetic origin) able to interfere with quorum sensing systems have been reported (for a recent review see [15]). Quorum sensing has been documented to be required for full virulence of Aeromonas spp. and vibrios towards different aquatic hosts, including fish and crustaceans [16]–[18]; moreover, different quorum sensing-disrupting agents have been proven effective in controlling disease. Effective compounds include cinnamaldehyde, brominated furanones and brominated thiophenones [14], antagonistic acylhomoserine lactones [7], and signal molecule-degrading enzymes [11]. Virulence-related phenotypes (including motility and adhesion) of E. coli and Salmonella spp. have also been reported to be controlled by quorum sensing molecules [19], [20], and the signal molecule indole has been shown to affect killing of the nematode C. elegans [21]. However, to the best of my knowledge, no reports have been published thus far mentioning the successful use of inhibitors of these types of bacterial cell-to-cell communication to protect terrestrial farmed animals from disease caused by these pathogens. The evaluation of these kind of compounds in terrestrial animals should be rather straightforward, as many inhibitors have been isolated and/or synthesised [15]. Although the peptide quorum sensing systems of Gram-positive bacteria thus far have received much less attention than acylhomoserine lactone systems in Gram-negative bacteria, some inhibitors of these systems have been documented as well (e.g. cyclic peptide inhibitors of quorum sensing in staphylococci [22]), and these kind of compounds might also prove effective in controlling animal diseases caused by Gram-positive pathogens.
Interfering with Host-Pathogen Signalling in Animal Pathogens
In addition to bacterial signals, E. coli and Salmonella spp. can also sense and respond to host cues such as the catecholamine stress hormones adrenaline and noradrenaline. These hormones are an integral part of the acute “fight or flight” stress response in animals and are conserved among vertebrates and invertebrates. Catecholamines can facilitate the removal of iron from host iron-binding proteins, thereby making it available to the bacteria and increasing their growth under iron-limited conditions [23]. In addition to their growth-stimulatory effect, catecholamines also increase virulence gene expression of pathogenic bacteria. In different pathogenic E. coli strains, the compounds have been reported to affect the production of virulence-related phenotypes such as motility and type III secretion [24], Shiga toxin expression [25], and expression of pilus and fimbrial adhesins [26]. In Salmonella spp., they have been reported to affect motility [27], hemolysin production [28], type III secretion [10], and intestinal colonization in chicks, pigs, and calves [29], [30]. Different bacterial adrenergic sensors have recently been described (with the best-described one being QseC), showing different susceptibilities to blocking with eukaryotic α- and β- adrenergic receptors, respectively [31], [32]. An inhibitor of bacterial catecholamine sensing, LED209, has also been described [10]. It needs to be noted that (at least in Salmonella spp.) different research groups have reported conflicting effects of catecholamines, which may reflect differences in host species, bacterial strains, routes of infection, and nature of mutations [23], [31], [32]. Interestingly, vibrios and Aeromonas spp. also respond to catecholamines, and QseC homologues have been reported in these bacteria as well [33].
Advantages of this Strategy
When compared to the use of antibiotics, a major advantage of antivirulence therapy is that there will be less interference with non-target organisms (i.e. the commensal microbiota), as it specifically targets virulence gene expression or virulence gene regulation; in the latter case there might be some interference with regulatory mechanisms in non-target organisms. Moreover, because such a strategy will pose selective pressure only under conditions in which the virulence genes are required, the tendency towards resistance development and spread will probably also be lower (though not absent) [34]. It should be noted, however, that some of the resistance mechanisms that bacteria have acquired during exposure to antibiotics can also render them resistant to antivirulence agents. This was recently demonstrated in Pseudomonas aeruginosa, in which clinical isolates showing an increased expression of a multidrug efflux pump were also resistant to a quorum sensing-disrupting brominated furanone [35]. A major advantage of targeting the regulatory mechanisms described above is that agents can be used that do not need to enter the cells to exert their activity (e.g., signal molecule-degrading enzymes or compounds that interfere with cell surface receptors). Consequently, pre-existing nonspecific resistance mechanisms (e.g. multidrug efflux pumps and decreased cell membrane permeability) will not alter the effectiveness of such agents.
Conclusion
It is of significant interest to further develop antivirulence therapy as a novel biocontrol strategy for animal production. Further research is needed to document the impact of such a strategy in different host-pathogen settings and to continue the quest for novel antivirulence agents, i.e. inhibitors of either natural or synthetic origin, or microorganisms able to interfere with virulence (regulatory) mechanisms.
Funding Statement
The author acknowledges financial support from the Fund for Scientific Research - Flanders (FWO). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Heuer H, Schmitt H, Smalla K (2011) Antibiotic resistance gene spread due to manure application on agricultural fields. Curr Opin Microbiol 14: 236–243 doi: 10.1016/j.mib.2011.04.009 [DOI] [PubMed] [Google Scholar]
- 2. Defoirdt T, Sorgeloos P, Bossier P (2011) Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr Opin Microbiol 14: 251–258 doi: 10.1016/j.mib.2011.03.004 [DOI] [PubMed] [Google Scholar]
- 3. Cabello FC (2006) Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environ Microbiol 8: 1137–1144. [DOI] [PubMed] [Google Scholar]
- 4. Donnenberg MS (2000) Pathogenic strategies of enteric bacteria. Nature 406: 768–774. [DOI] [PubMed] [Google Scholar]
- 5. Clatworthy AE, Pierson E, Hung DT (2007) Targeting virulence: a new paradigm for antimicrobial therapy. Nat Chem Biol 3: 541–548. [DOI] [PubMed] [Google Scholar]
- 6. Baron C (2010) Antivirulence drugs to target bacterial secretion systems. Curr Opin Microbiol 13: 100–105 doi: 10.1016/j.mib.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 7. Natrah FMI, Alam MdI, Harzevili AS, Sorgeloos P, Bossier P, et al. (2012) The impact of quorum sensing on the virulence of Aeromonas hydrophila and Aeromonas salmonicida towards burbot (Lota lota L.) larvae. Vet Microbiol 159: 77–82 doi: 10.1016/j.vetmic.2012.03.014 [DOI] [PubMed] [Google Scholar]
- 8. Manefield M, Rasmussen TB, Hentzer M, Andersen JB, Steinberg P, et al. (2002) Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology 148: 1119–1127. [DOI] [PubMed] [Google Scholar]
- 9. Brackman G, Al Quntar AAA, Enk CD, Karalic I, Nelis HJ, et al. Synthesis and evaluation of thiazolidinedione and dioxazaborocane analogues as inhibitors of AI-2 quorum sensing in Vibrio harveyi . Bioorg Med Chem 21: 660–667 doi: 10.1016/j.bmc.2012.11.055 [DOI] [PubMed] [Google Scholar]
- 10. Rasko DA, Moreira CG, Li DR, Reading NC, Ritchie JM, et al. (2008) Targeting QseC signaling and virulence for antibiotic development. Science 321: 1078–1080 doi: 10.1126/science.1160354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cao YA, He SX, Zhou ZG, Zhang MC, Mao W, et al. (2012) Orally administered thermostable N-acyl homoserine lactonase from Bacillus sp strain AI96 attenuates Aeromonas hydrophila infection in zebrafish. Appl Environ Microbiol 78: 1899–1908 doi: 10.1128/AEM.06139-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brackman G, Defoirdt T, Miyamoto C, Bossier P, Van Calenbergh S, et al. (2008) Cinnamaldehyde and cinnamaldehyde derivatives reduce virulence in Vibrio spp. by decreasing the DNA-binding activity of the quorum sensing response regulator LuxR. BMC Microbiol 8: 149 doi: 10.1186/1471-2180-8-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Defoirdt T, Miyamoto CM, Wood TK, Meighen EA, Sorgeloos P, et al. (2007) The natural furanone (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone disrupts quorum sensing-regulated gene expression in Vibrio harveyi by decreasing the DNA-binding activity of the transcriptional regulator protein luxR. Environ Microbiol 9: 2486–2495. [DOI] [PubMed] [Google Scholar]
- 14. Defoirdt T, Benneche T, Brackman G, Coenye T, Sorgeloos P, et al. (2012) A quorum sensing-disrupting brominated thiophenone with a promising therapeutic potential to treat luminescent vibriosis. PLOS One 7 (7) e41788 doi: 10.1371/journal.pone.0041788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kalia VC (2013) Quorum sensing inhibitors: an overview. Biotechnol Adv 31: 224–245 doi: 10.1016/j.biotechadv.2012.10.004 [DOI] [PubMed] [Google Scholar]
- 16. Schwenteit J, Gram L, Nielsen KF, Fridjonsson OH, Bornscheuer UT, et al. (2011) Quorum sensing in Aeromonas salmonicida subsp. achromogenes and the effect of the autoinducer synthase AsaI on bacterial virulence. Vet Microbiol 147: 389–397 doi: 10.1016/j.vetmic.2010.07.020 [DOI] [PubMed] [Google Scholar]
- 17. Bjelland AM, Sorum H, Tegegne DA, Winter-Larsen HC, Willassen NP, et al. (2012) LitR of Vibrio salmonicida is a salinity-sensitive quorum-sensing regulator of phenotypes involved in host interaction and virulence. Infect Immun 80: 1681–1689 doi: 10.1128/IAI.06038-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Defoirdt T, Sorgeloos P (2012) Monitoring of Vibrio harveyi quorum sensing activity in real time during infection of brine shrimp larvae. ISME J 6: 2314–2319 doi: 10.1038/ismej.2012.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Han X, Bai H, Liu L, Dong H, Liu R, et al. (2013) The luxS gene functions in the pathogenesis of avian pathogenic Escherichia coli. Microb Pathog 55: 21–27 doi: 10.1016/j.micpath.2012.09.008 [DOI] [PubMed] [Google Scholar]
- 20. Hirakawa H, Inazumi Y, Masaki T, Hirata T, Yamaguchi A (2005) Indole induces the expression of multidrug exporter genes in Escherichia coli. . Mol Microbiol 55: 1113–1126. [DOI] [PubMed] [Google Scholar]
- 21. Anyanful A, Dolan-Livengood JM, Lewis T, Sheth S, Dezalia MN, et al. (2005) Paralysis and killing of Caenorhabditis elegans by enteropathogenic Eschrichia coli requires the bacterial tryptophanase gene. Mol Microbiol 57: 988–1007. [DOI] [PubMed] [Google Scholar]
- 22. George EA, Novick RP, Muir TW (2008) Cyclic peptide inhibitors of staphylococcal virulence prepared by Fmoc-based thiolactone peptide synthesis. J Am Chem Soc 130: 4914–4924 doi: 10.1021/ja711126e [DOI] [PubMed] [Google Scholar]
- 23. Lyte M, Vulchanova L, Brown DR (2011) Stress at the intestinal surface: catecholamines and mucosa-bacteria interactions. Cell Tissue Res 343: 23–32 doi: 10.1007/s00441-010-1050-0 [DOI] [PubMed] [Google Scholar]
- 24. Sperandio V, Torres AG, Jarvis B, Nataro JP, Kaper JB (2003) Bacteria-host communication: the language of hormones. Proc Natl Acad Sci USA 100: 8951–8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lyte M, Arulanandam BP, Frank CD (1996) Production of Shiga-like toxins by Escherichia coli O157:H7 can be influenced by the neuroendocrine hormone norepinephrine. J Lab Clin Med 128: 392–398. [DOI] [PubMed] [Google Scholar]
- 26. Lyte M, Erickson AK, Arulanandam BP, Frank CD, et al. (1997) Norepinephrine-induced expression of the K99 pilus adhesin of enterotoxigenic Escherichia coli . Biochem Biophys Res Commun 232: 682–686. [DOI] [PubMed] [Google Scholar]
- 27. Bearson BL, Bearson SMD (2008) The role of the QseC quorum-sensing sensor kinase in colonization and norepinephrine-enhanced motility of Salmonella enterica serovar Typhimurium. Microb Pathog 44: 271–278. [DOI] [PubMed] [Google Scholar]
- 28. Karavolos MH, Bulmer DM, Spencer H, Rampioni G, Schmalen I, et al. (2011) Salmonella Typhi sense host neuroendocrine stress hormones and release the toxin haemolysin E. EMBO Rep 12: 252–258 doi: 10.1038/embor.2011.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pullinger GD, Carnell SC, Sharaff FF, van Diemen PM, Dziva F, et al. (2010) Norepinephrine augments Salmonella enterica-induced enteritis in a manner associated with increased net replication but independent of the putative adrenergic sensor kinases QseC and QseE. Infect Immun 78: 372–380 doi: 10.1128/IAI.01203-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Methner U, Rabsch W, Reissbrodt R, Williams PH (2008) Effect of norepinephrine on colonisation and systemic spread of Salmonella enterica in infected animals: role of catecholate siderophore precursors and degradation products. Int J Med Microbiol 298: 429–439. [DOI] [PubMed] [Google Scholar]
- 31. Hughes DT, Sperandio V (2008) Inter-kingdom signalling: communication between bacteria and their hosts. Nat Rev Microbiol 6: 111–120 doi: 10.1038/nrmicro1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karavolos MH, Winzer K, Williams P, Khan CMA (2013) Pathogen espionage: multiple bacterial adrenergic sensors eavesdrop on host communication systems. Mol Microbiol 87: 455–465 doi: 10.1111/mmi.12110 [DOI] [PubMed] [Google Scholar]
- 33. Defoirdt T (2013) Virulence mechanisms of bacterial aquaculture pathogens and antivirulence therapy for aquaculture. Rev Aquaculture in press. doi: 10.1111/raq.12030 [Google Scholar]
- 34. Defoirdt T, Boon N, Bossier P (2010) Can bacteria evolve resistance to quorum sensing disruption? PLoS Pathog 6 (7) e1000989 doi: 10.1371/journal.ppat.1000989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maeda T, García-Contreras R, Pu M, Sheng L, Garcia LR, et al. (2012) Quorum quenching quandary: resistance to antivirulence compounds. ISME J 6: 493–501 doi: 10.1038/ismej.2011.12 [DOI] [PMC free article] [PubMed] [Google Scholar]