Abstract
Human uveal melanoma is the most common primary intraocular tumor, and brachytherapy is one of the most common and effective treatment strategies. In order to find a safer and more effective way to increase the radio sensitivity of the tumor, we tried to use the dendrimer nanoparticle performing coexpression gene radiotherapy. In this study, we constructed recombinant DNA plasmids (early growth response-1 tumor necrosis factor-α [pEgr1-TNFα], pEgr1 thymidine kinase [TK], and pEgr1-TNFα-TK) according to the Egr1 promoter sequence. The sequences of human TNFα and herpes simplex virus (HSV) TK that were published by GenBank. Agarose gel electrophoresis and DNA sequencing had proven that we constructed the double-gene recombined plasmids pEgr1-TNF-TK correctly, as well as the plasmids pEgr1-TNFα and pEgr1-TK. The dendrimer nanoparticles combined with plasmid DNA as dendriplexes were verified with agarose gel electrophoresis and observed by transmission electron microscopy (TEM) and scanning electron microscopy to define size and shape. Zeta potential was measured using a Zetasizer analyzer. Optimal size and neutral zeta-potential characteristics of dendriplexes were achieved for the transfection studies. DNase I examination proved that the dendriplexes could protect plasmid DNA for at least 6 hours. The recombinant plasmids were transfected with dendrimer nanoparticles into the human choroidal melanoma OCM-1 cell line, followed by exposure to iodine-125 (125I) after transfection. After transfection with dendrimer nanoparticles and the irradiation of 125I, the gene expressions of TNFα and HSV1-TK were significantly increased at the protein level by enzyme-linked immunosorbent assay and Western blot analysis in OCM-1 cells. The cellular morphology of OCM-1 cells altering was observed by TEM, and a decrease in cell proliferation was revealed in cell-growth curves. Flow cytometry of annexin V/propidium iodide double-dyeing apoptosis and caspase-3 fluorescence staining showed that this treatment method could turn transfected OCM-1 cells into apoptosis and necrosis by the effects of the gene expression. This study indicated that the dendrimer nanoparticles with coexpression of TNF-α and HSV1-TK gene therapy are effective and safe and can provide us with a novel strategy to treat human uveal melanoma in the future.
Keywords: recombinant plasmid, human uveal melanoma, gene radiotherapy, gene expression, dendrimer nanoparticle
Introduction
Uveal melanoma is the primary malignant tumor of the adult eye, with an incidence rate, especially in the choroid, of 78%–85% of all cases, followed by the ciliary body (9%–12%), and the iris (6%–9.5%).1,2 The tumor can metastasize to the liver via a hematogenous pathway, and approximately 50% of patients show metastasis within 15 years of initial diagnosis and treatment. Once it has metastasized, the mortality rate is high.3–5 Although current clinical practice involves ophthalmectomy, localized tumor resection, radiotherapy, and laser treatment, none of these treatments effectively inhibits tumor metastasis or improves postoperative life quality. The dilemma of treating melanoma and improving the success rate is still an important research subject.
With advances in molecular biology and molecular genetic technology, gene therapy for malignant tumor had become the main issue for researchers, in which the herpes simplex virus thymidine kinase (HSV-TK) suicide-gene system has been considered the most promising treatment.6 Moolten was the initiator who proposed this method.7 The combination of the HSV-1-TK suicide-gene system and ganciclovir (GCV), not only killed the infected cells, but through a bystander effect also eliminated surrounding uninfected cells, which in turn effectively reduced the stress induced by the tumor cells. However, it could not sufficiently mobilize all bodily immune responses to fight the tumor. Tumor necrosis factor (TNF)-α is known as a central signaling molecule in natural antitumor mechanisms.8 It is sometimes referred to as cachectin, and has a direct killing effect and sensitizes tumor cells to radiation. At high doses, TNFα can cause hemorrhage and necrosis in tumor tissues, while the activation of the immune response could synergistically play an antitumor role.9 The combinatory use is often recommended to overcome the inadequacy of either treatment when used alone.
Dendrimer nanoparticles are a nonviral vector composed of a polymer of nanoparticles of diameter less than 100 nm. Dendrimer nanoparticles contain many amino groups, which protonize under physiological pH. The protonized amino groups can then neutralize the electric charge on the surface of the DNA, allowing DNA molecules to be compacted into relatively smaller structures to prevent the nuclease from degradation. The transfection complex primarily passes the DNA into a cell via endocytosis and forms an endosome vesicle. DNA is then released from the vesicle into cytoplasm before entering the nucleus for transcription and translation. The nanometer-scale transfection reagent shows unique characteristics, such as provision of stronger protection to DNA and lower cytotoxicity.10,11 Many studies have shown the combination of early growth response-1 (Egr-1) promoter, and the target gene could prove to be an effective gene radiotherapy for tumor treatment.12–16
To find a more feasible and less harmful treatment, we used dendrimeric nanoparticle as a vector to transfect into the OCM-1 human uveal melanoma cell strain with recombinant plasmid involving double-gene expression of Egr-1 promoter-controlled TNFα and HSV1-TK. The OCM-1 human uveal melanoma cell strain would then be exposed to 2 Gy iodine-125 (125I) radiation and the expression level of these two genes measured as well as the impact of radiation on recombinant plasmid expression being investigated and the cellular morphology, proliferation, and apoptosis observed in order to understand the efficacy and feasibility of an in vitro method for killing tumor cells.
Materials and methods
Construction of plasmid pEgr-TNFα-TK, plasmid pEgr-TNFα, and plasmid pEgr-HSV-TK
The recombinant DNA plasmids were constructed and sequenced by Life Technologies (Shanghai, People’s Republic of China). According to the Egr-1 promoter sequence, the sequence of human TNFα and HSV-TK of GenBank, chemically synthesized the recombinant plasmids were restored in the Escherichia coli competent cells DH5α. These plasmids were extracted from the E. coli DH5α strain and subjected to agarose gel electrophoresis to ensure their correction.
Human choroidal melanoma OCM-1 cell line
The human choroidal melanoma (OCM-1) cell line (American Type Culture Collection, Manassas, VA, USA) was cultured in Roswell Park Memorial Institute 1640 medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Cells were maintained at 37°C and 5% CO2 in an incubator with 95% humidity. The cell-culture medium was replaced every second day, and cells were passaged at 85%–90% confluence.
Polyplex formation
Dendrimer nanoparticles were purchased from Engreen Biosystem (Beijing, People’s Republic of China). Following the protocol, 3 μg each of the recombinant DNA plasmids was incubated with 9 μL dendrimeric nanoparticles in 200 μL pH 7–8 culture medium without serum, protein, and antibiotics for 15–30 minutes, and verified with agarose gel electrophoresis. The dendriplexes were observed on transmission electron microscopy (TEM) and scanning electron microscopy (SEM). Zeta-potential analysis was performed on a Zetasizer Nano ZS90 (Malvern Instruments, Malvern, UK). DNase I sensitivity was examined by the following methods. All dendriplexes and the plasmid DNA were incubated with 1 U DNase I in a 37°C water bath for 30 minutes and 1, 2, 4, and 6 hours. After adding ethylenediaminetetraacetic acid (EDTA; 5 mmol/L), the samples were placed in a 65°C water bath for 10 minutes, then the appropriate concentration of heparin solution (final concentration of 5 mg/mL) was added, before placing them back in a 37°C water bath for 2 hours and subjecting them to agarose gel electrophoresis.
Transient transfections
For in vitro transfection experiments, OCM-1 cells were grown to about 80% confluence. Cells were incubated for 8 hours with dendriplexes in the absence of serum and antibiotics, followed by incubation with growth medium for 24 hours. The OCM-1 cells were transfected with the recombinant plasmid pEgr-TNFα-TK, hereafter referred to as TNF-TK, pEgr-TNFα, hereafter referred to as TNF, and pEgr-HSV-TK, hereafter referred to as TK. The negative-control group was cells incubated only with polyplexes without plasmids. The blank-control group was treated with phosphate-buffered saline (PBS).
125I radiation
In order to excite the recombinant DNA plasmids in cells, the transfected OCM-1 cells were exposed to 2 Gy 125I radiation and cultivated in normal condition (37°C, 5% CO2). After transfection, at the appropriate time, the expression products were tested and analyzed. The timing depended on the type of test and the target transfection gene.
Measuring the protein-expression level of the target gene
ELLSA
At 24 hours after transfection, the supernatant of the OCM-1 cells was collected and exposed to 2 Gy radiation. The cellular supernatant was collected again at 0, 2, 4, 8, 12, 24, and 48 hours after radiation. Enzyme-linked immunosorbent assay (ELISA) was performed to measure the concentrations of TNFα and HSV-TK from each group by taking three samples. All tests followed the instructions of ELISA test kit. At the wavelength of 450 nm, the OD value was measured and based on the standard curve, and thereby the expression levels of TNFα and HSV-TK in the sample were calculated.
Western blot
The medium was removed, and the plates were washed twice with ice-cold PBS. The treated OCM-1 cells were lysed with sample buffer that contained 60 mM Tris, pH 6.8, 2% (w/v) sodium dodecyl sulfate (SDS), 100 mM 2-mercaptoethanol, and 0.01% bromophenol blue. The lysate was then incubated on ice for 30 minutes. The lysate was scraped by a cell scraper and harvested by a pipettor, and then centrifuged at 4°C for 30 minutes. The supernatant was collected and boiled for 5 minutes and stored at −20°C. Cellular extracts from the treated OCM-1 cells were processed for Western blot analysis. Fifty micrograms of protein per well was loaded on a 10% SDS-polyacrylamide gel electrophoresis gel. The protein was electrotransferred to polyvinylidene difluoride membranes for 1 hour and 45 minutes at 100 V, then blocked with Tris-buffered saline (TBS) containing 5% fat-free milk and 0.1% Tween-20 (TBST) for 1 hour and incubated with human anti-TNFα (TNF-α [N-19]: sc-1350; Santa Cruz Biotechnology, Dallas, TX, USA) overnight. After three washes with TBST, the membranes were incubated with secondary antibody for 1–2 hours at room temperature and washed again with TBST. Localization of the antibody was detected by chemiluminescence using an ECL kit (CoWin Biosciences, Inc., Menlo Park, CA, USA) following the manufacturer’s instructions.
Electron microscopy observation
For observation of transfected and exposed OCM-1 cells under electron microscopy, the nontransfected OCM-1 cells and OCM-1 cells in culture media with only dendrimeric nanoparticles were used as the negative control. At 24 hours after a combination of 0 Gy and 2 Gy 125I radiation exposure and GCV (50 μg/mL, pH 6.8–7.4; Life Technologies) for treatment, the cells were cultured to a logarithmic growth state. From each group, 1 × 107 cells were respectively obtained the medium removed and the plate washed twice with 4°C PBS. A cell scraper was used to scrap the OCM-1 cells slide by slide, and these were collected in a 1.5 mL Eppendorf tube. The solution was centrifuged at 1000 rpm for 3 minutes before its supernatant was discarded and 2.5% glutaraldehyde was added for 2 hours of fixation at 4°C. Samples were washed three times with PBS (pH 7.2), each time lasted 10 minutes with pipets prohibition, then stored at 4°C. When all samples were collected they would be sent to the electron microscope laboratory at the Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University. Samples were first observed under regular-light microscopy before being prepared on slides for electron microscopy for further investigation.
The cell-growth rate reflected the impact of transfection on OCM-1 cell proliferation
Respectively, TNF-TK, TNF-α, and TK were transfected into OCM-1 cells under a logarithmic growth pattern. The non-transfected OCM-1 cells and OCM-1 cells in culture media with only dendrimeric nanoparticles were used as the negative control. After a combination of 0 Gy and 2 Gy 125I radiation exposure and GCV (50 μg/mL, pH 6.8–7.4; Life Technologies) for treatment, the cells were cultured to a logarithmic growth state and prepared in a single-cell suspension. At approximately 2 × 104 cells per well, they were inoculated in six-well culture plates. On days 2, 4, 6, and 8, cells from three wells for each group were extracted and digested by 0.25% trypsin-EDTA into single cell suspension for cell count and plotting of the cell-growth curve.
Measuring the effect of transfection on promoting in vitro cell apoptosis with flow cytometry
Annexin V-FITC/PI
The nontransfected OCM-1 cells and OCM-1 cells in culture media with only dendrimeric nanoparticles were used as the negative control. After a combination of 0 Gy and 2Gy 125I radiation exposure and GCV (50 μg/mL, pH 6.8–7.4; Life Technologies) for treatment, the cells were cultured to a logarithmic growth state and prepared into single-cell suspension. At 2 × 106 cells per well, the cells were inoculated in six-well culture plates, with each group occupying six wells. These were incubated at 37°C, 5% CO2, and saturated humidity. By 48 and 72 hours after exposure, they were prepared into single-cell suspension. Approximately 1 × 106 cells were extracted and washed with 4°C PBS, before 100 μL annexin-binding buffer was added to resuspend the solution. Five microliters of annexin V fluorescein isothiocyanate (FITC) and 1 μL propidium iodide (PI) solution were added for another 15 minutes of incubation in the dark before 400 μL annexin-binding buffer was added. The solution was gently beaten while it was chilled on ice. Flow cytometry was then used within 1 hour to measure the level of apoptosis at a wavelength of 488 nm.
Caspase-3 fluorescent stain test
Quantitative analysis of apoptosis due to coexpression via the activated caspase-3 was undertaken. Each group of cells was prepared according to the aforementioned procedure. At 48 hours after exposure, 300 μL was extracted into a test tube from 1 × 106/mL concentration solution. After 1 μL FITC-DEVD-FMK (fluorescein isothiocyanate-Asp-Glu-Val-Asp-fluoromethyl ketone) was added it was placed into incubation for 1 hour at 37°C, 5% CO2, and saturated humidity, before it was centrifuged at 3,000 rpm for 5 minutes. The supernatant was discarded and the cells resuspended by adding 500 μL wash buffer. Five minutes of centrifugation at 3,000 rpm was performed twice. These steps were repeated by adding 300 μL wash buffer for resuspension. The sample was tested by flow cytometry via the FL-1 channel.
Statistical methods
All experiments were carried out in triplicate. Results are represented as means ± standard deviation. Statistical significance was tested using one-way analysis of variance. P < 0.05 was considered statistically significant.
Results
Construction of recombinant plasmid pEgr-TNFα-TK, plasmid pegr-TNFα, and plasmid pEgr-HSV-TK
According to the results of the gene-sequence analysis, we can synthesize the designed gene single-stranded oligo. The recombinant plasmids were ligased and transformed into the E. coli DH5α strain. Restricted digestion and sequencing carried out on the transformed DH5α proved that the genes was inserted to the vector correctly, indicating that the recombinant plasmids pEgr-TNFα-TK, hereafter referred to as TNF-TK, pEgr-TNFα, hereafter referred to as TNF, and pEgr-HSV-TK, hereafter referred to as TK were constructed successfully. The obtained target-gene fragments were sequenced and sequence aligned on NCBI. Agarose gel electrophoresis analysis is shown in Figure 1.
Figure 1.
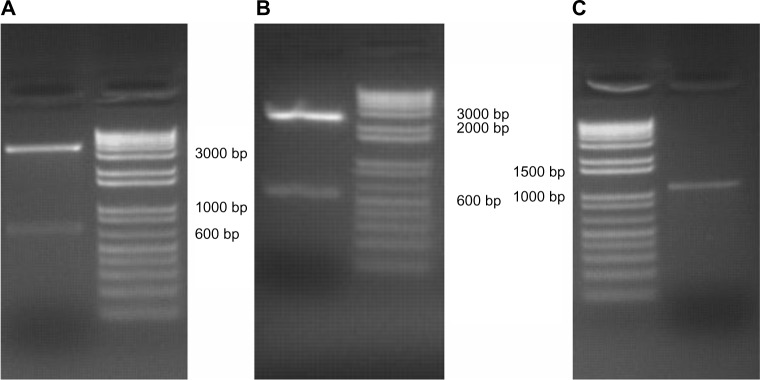
(A–C) Egr-1 promoter gene, TNF-α gene, HSV1-TK gene agarose gel electrophoresis. (A) Egr-1 promoter connected into pMD-18T vector double-cut by AseI/NheI into two bands −2.7 K and 0.6 K. (B) TNF-α gene connected into the PCR-XL-TOPO vector double-cut by XhoI/EcoRI into two bands −3.5 K and 0.7 K. (C) Repaired TK gene produced by PCR, approximately 1,150 bp.
Abbreviations: Egr-1, early growth response-1; TNF-α, tumor necrosis factor-α; HSV1-TK, herpes simplex virus thymidine kinase; TK, thymidine kinase; PCR, polymerase chain reaction.
Characterization of dendriplexes
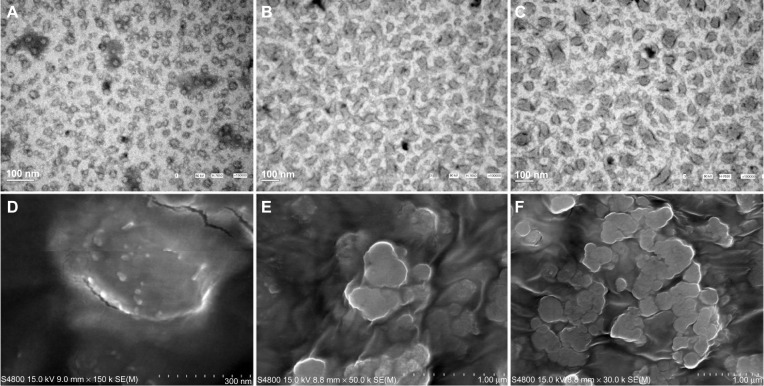
The dendriplexes of TNF-TK, TNF, and TK were verified with agarose gel electrophoresis, as shown in Figure 2A. Each group showed similar bands with the plasmid DNA, but the bands were larger due to agglomeration. DNase sensitivity examination showed that dendriplexes and plasmid DNA both had apparent bands in the original place, and a little trailing and extra bands showed as DNA degradation in 30 minutes. In 6 hours, dendriplexes could still be observed with bands in the original place, but not the plasmid DNA (Figure 2B, C and D). The electron microscopy sizing data of dendrimer nanoparticles and dendriplexes demonstrated particle sizes of about 20 nm and 100–200 nm, and the dendriplex agglomeration was about 500 nm (Figure 3). The zeta potentials of TNF-TK, TNF, and TK were 6.49 ± 0.83 mV, 6.71 ± 0.77 mV, and 7.91 ± 1.60 mV, respectively. There was no significantly statistical difference between them.
Figure 2.
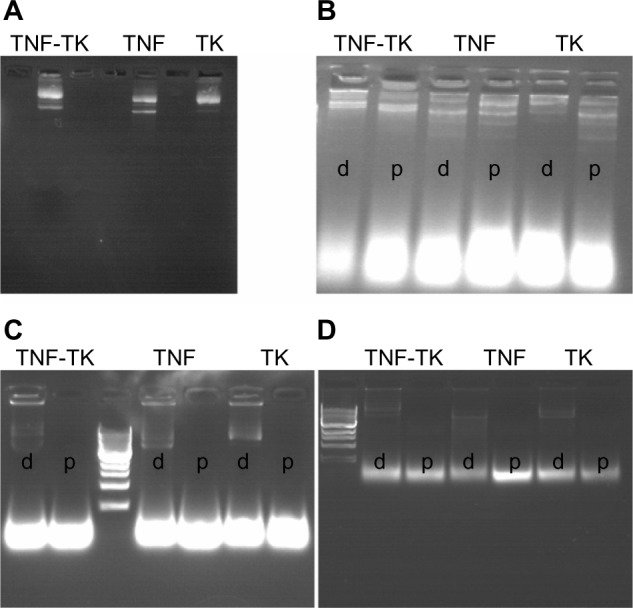
(A–D) Agarose gel electrophoresis of dendriplexes. (A) Dendripexes of TNF-TK, TNF, and TK were verified with gel electrophoresis. (B–D) DNase sensitivity examination results in 30 minutes, 4 hours, and 6 hours, respectively. The “d”s and “p”s refer to dendriplexes and plasmid DNA, respectively. We can see there are still bands around the original place.
Abbreviations: TNF-TK, tumor necrosis factor-thymidine kinase; TNF, tumor necrosis factor; TK, thymidine kinase.
Figure 3.
(A–F) Images of dendrimer nanoparticles and dendriplexes under electron microscope. TEM image of dendrimer nanoparticles (A) and dendriplexes (B and C) showed particle sizes of about 20 nm and 100–200 nm, respectively. SEM images of dendrimer nanoparticles (D) and dendriplexes (E and F) were matched with TEM and showed dendripolex agglomeration was approximately 500 nm.
Abbreviations: SEM, scanning electron microscopy; TEM, transmission electron microscopy.
Expression of human tumor necrosis factor-α protein
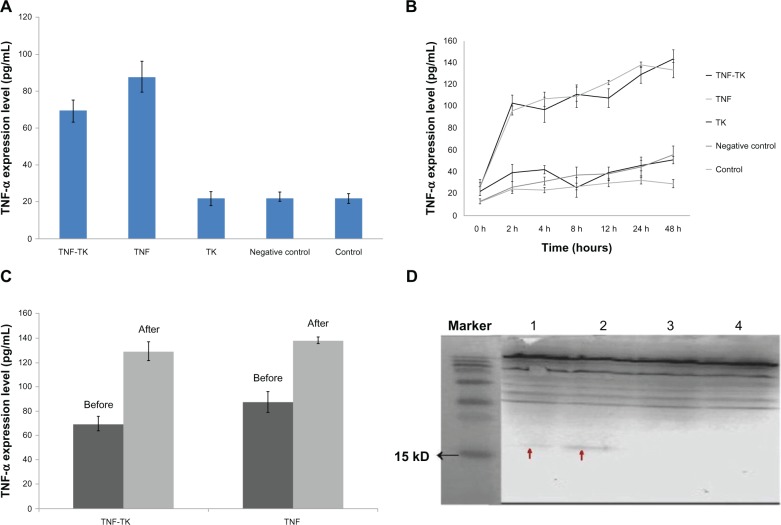
Without 125I radiation, the expression of TNFα was higher in TNF-TK and TNF group than the other group (Figure 4A). After 2 Gy 125I radiation, the expression of TNFα was higher in TNF-TK and TNF group and gradually increased with time; the highest expressions were shown at 24 hours and 48 hours (Figure 4B).
Figure 4.
(A–D) The expression of human tumor necrosis factor-α protein. (A) Without 125I radiation. (B) 2 Gy 125I irradiation, TNFα expression levels of each group change over time curve. (C) TNF-α expression at 24 hours. (D) TNFα Western blot strip: TNF-TK-radiation group, about 17 Kd (1); TNF-radiation group, about 17 Kd (2); TNF-TK and TNF groups, no obvious strip tape (3 and 4).
Note: The red arrows show the strip’s position.
Abbreviations: TNFα, tumor necrosis factor α; TNF-TK, tumor necrosis factor-thymidine kinase; TNF, tumor necrosis factor; 125I, iodine-125; TK, thymidine kinase.
After irradiation, the expression of TNFα in 2 hours to 48 hours versus 0 hours showed a significantly statistical difference (P < 0.01), but there was no significant difference (P = 0.95) between the TNF-TK and TNF groups at any time point (Figure 4C and D). The TNF-TK-radiation group versus the TNF-TK group showed a statistically significant difference (P < 0.01) at 24 hours. The TNF-radiation group versus the TNF group showed a statistically significant difference (P < 0.01) at 24 hours.
Expression of human herpes simplex virus thymidine kinase
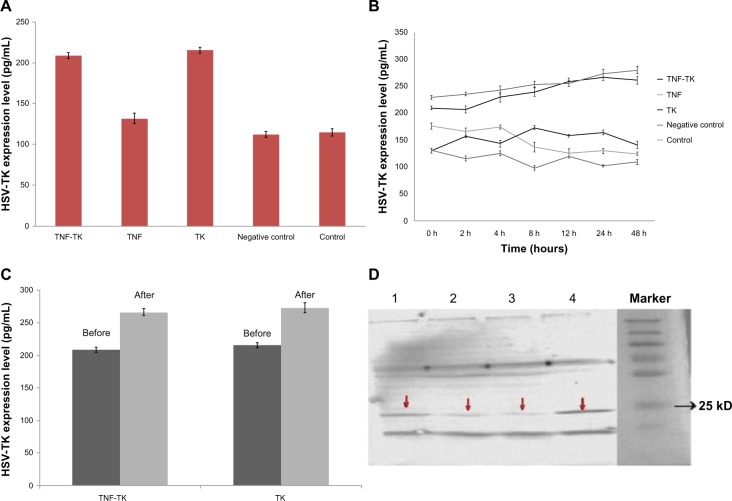
Without 125I radiation, the expression of HSV-TK was higher in the TNF-TK and TK groups than other groups (Figure 5A). After 2Gy 125I radiation, the expression of HSV-TK was higher in the TNF-TK and TK groups and gradually increased with time, the highest expressions were shown at 24 hours and 48 hours (Figure 5B). After irradiation, the expression of HSV-TK in 4 hours versus 0 hours showed a significantly statistical difference (P < 0.01), but there was no significant difference (P = 0.95) between the TNF-TK and TK groups at any time point. TNF-TK-radiation group versus the TNF-TK group showed statistically significant difference (P < 0.01) at 24 hours. The TK-radiation group versus the TK group showed a statistically significant difference (P < 0.01) at 24 hours. (Figure 5C and D).
Figure 5.
(A–D) The expression of human herpes simplex virus thymidine kinase. (A) Without 125I radiation. (B) 2 Gy 125I irradiation, HSV-TK expression levels of each group change over time curve. (C) HSV-TK expression at 24 hours. (D) HSV-TK Western blot strip: TNF-TK-radiation group, about 24 Kd (1); TNF-TK group, strip is pale and is about 24 Kd (2); TK group, about 24 Kd (3) the TK-radiation group had the most obvious strip, about 24 Kd (4).
Note: The red arrows show the strips position.
Abbreviations:125I, iodine-125; HSV-TK, herpes simplex virus-thymidine kinase; TNF-TK, tumor necrosis factor-thymidine kinase; TK, thymidine kinase.
Under electron microscopy
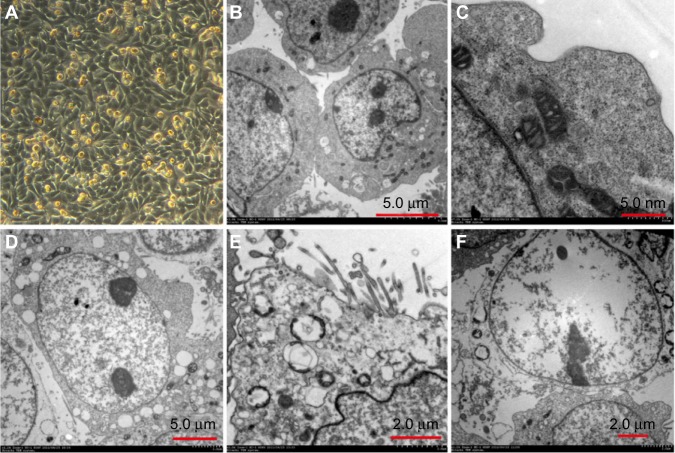
The sample in the TNF-TK group with exposure to 2 Gy 125I under regular light microscopy showed more cells in a necrotic state than other groups. There were large areas of vacuolar change, and some cells became smaller and oval in shape. When observed under electron microscopy, significant vacuoles were seen in the cytoplasm and there was expansion in nuclear space (Figure 6).
Figure 6.
(A–F) OCM-1 cell morphology. (A) human uveal melanoma OCM-1 (×100) was adherent cell in spindle shape. (B) Nontransfected control group of human uveal melanoma OCM-1 (×1.0 k) showed microvilli on cell surface, inverse ratio of nucleus to cytoplasm, and multiple nucleoli in nucleus, all suggesting a greater degree of malignancy. (C) Non-transfected control group of human uveal melanoma OCM-1 (×7.0 k): rough endoplasmic reticulum and mitochondrial structures could be observed. (D) TNF-TK group after using 2 Gy radio-applicator and cultured for 24 hours (×1.2 k): poorly defined cell membrane, formation of vacuole in cytoplasm, and expansion in nuclear space. (E) TNF-TK group after using 2 Gy radio-applicator and cultured for 24 hours (×2.0 k): swollen mitochondria, expanded rough endoplasmic reticulum, and vacuole formation in Golgi body. (F) TNF-TK group after using 2 Gy radio-applicator and cultured for 24 hours (×1.2 k): empty cell with poorly defined cell membrane and highly expanded organelles.
Abbreviations: OCM-1, human choroidal melanoma; TNF-TK, tumor necrosis factor-thymidine kinase.
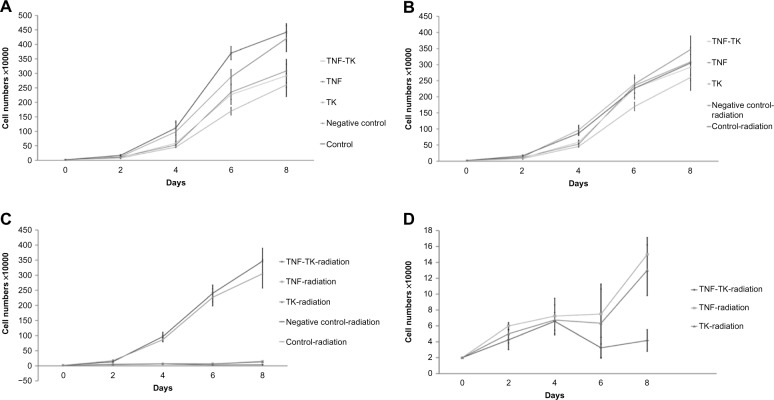
The cell-growth rate reflected the impact of transfection on OCM-1 cell proliferation
The cell numbers of each group were examined every 2 days in vitro. As shown in Figure 7A, irradiation only and transfection can suppress OCM-1 cell growth compared with the negative-control group and blank-control group, respectively. Growth conditions of the transfected group without irradiation were similar to the negative-control radiation and OCM-1 radiation groups (Figure 7B). The TNF-TK-radiation, TNF-radiation, and TK-radiation groups were significantly inhibited compared with the transfected groups and the irradiation groups (P < 0.05) (Figure 7C and D).
Figure 7.
(A) Cell-growth curves of non-125I radiation OCM-1 cells, the negative control group, with no statistically significant difference from the control group. (B) The transfected groups without irradiation were similar to the negative control radiation and OCM-1-radiation groups. (C and D) cell-growth curve of 125I radiation OCM-1 cells, the negative control group, with no statistically significant difference from the control group.
Abbreviations:125I, iodine-125; OCM-1, human choroidal melanoma; TNF-TK, tumor necrosis factor-thymidine kinase; TNF, tumor necrosis factor; TK, thymidine kinase.
Effect of recombinant plasmid pEgr-TNFα-TK on OCM-1 cell apoptosis
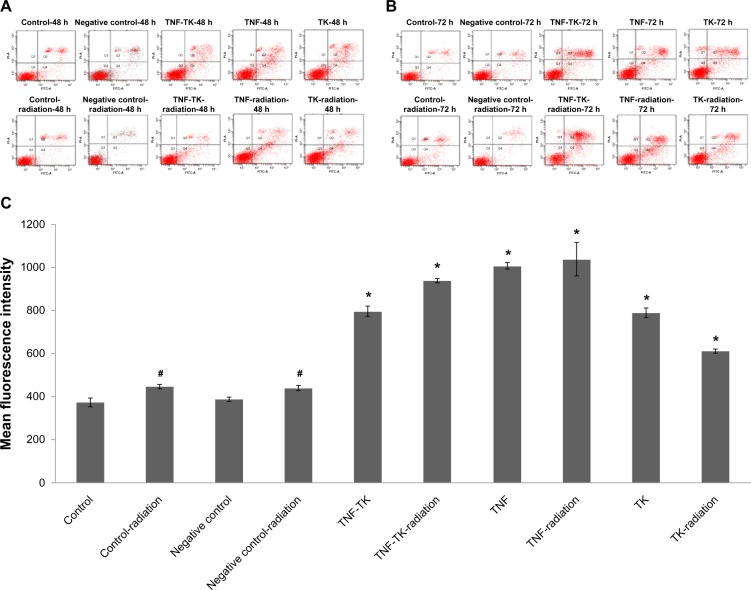
By using flow cytometry, it was possible to measure quantitatively and analyze the effect of coexpression plasmid on promoting apoptosis. In the transfected groups, which were either exposed or not exposed to 2 Gy 125I, results for signs of early cellular apoptosis between the two groups (annexin V-FITC+/PI cells, early apoptotic cells) showed pEgr-TNFα-TK treatment, irradiation, and combined treatments could evoke OCM-1 cell apoptosis (Figure 8A) when compared with the control group and the negative control group. However, 2 Gy 125I treatment did not induce OCM-1 cell apoptosis in 48 hours (P = 0.057); a statistically significant difference was revealed in 72 hours (P = 0.00). The apoptosis rate of the transfected groups with irradiation was higher than the unexposed transfection groups in 48 hours, while there was statistical significance in the TNF-radiation and TK-radiation groups in comparison with the unexposed transfection group (P < 0.05). In 72 hours (Figure 8B), the apoptosis rate of the transfected groups with irradiation was significantly higher than the unexposed transfection groups, and the difference in early apoptotic rate for cells in the TNF-radiation group was more significant when compared to others groups (P < 0.01).
Figure 8.
(A–C) Apoptosis analysis of OCM-1 cell lines after treatment for 48 and 72 hours by flow cytometry. (A) Apoptosis analysis using annexin V-FITC/PI double-staining after 48 hours. The negative control group compared with the control group showed no statistical significance (P > 0.05). (B) Apoptosis analysis using Annexin V-FITC/PI double-staining after 72 hours. The apoptosis ratios in pEgr-TNFα-TK treatment, irradiation, and combined groups were 7.86% ± 0.15%, 5.9% ± 0.17%, and 13.77% ± 0.76%, respectively; n = 3 replicates per condition. (C) Caspase-3 fluorescent stain after 48 hours. pEgr-TNFα-TK treatment, irradiation, and combined treatments significantly induced caspase-3 arrest in OCM-1 cells. The negative control group compared with the control group showed no statistical significance (P = 0.531).
Notes: *P < 0.005; #P < 0.01 compared with the negative control group and the control group by one-way analysis of variance; n = 3 replicates per condition.
Abbreviations: OCM-1, human choroidal melanoma; FITC, fluorescein isothiocyanate; PI, propidium iodide; pEgr-TNFα-TK, plasmid early growth response-1 tumor necrosis factor α thymidine kinase.
The result of activated caspase-3 fluorescent stain showed that in the unexposed group and the group exposed to 2 Gy 125I radiation, the average fluorescent intensity of caspase-3 stain between the two groups was more intense in the experiment group than the control (P < 0.01), and it was strongest in the TNF-radiation group (Figure 8C). There was statistical significance between the transfection and the nontransfection groups (P < 0.01).
Discussion
In this study, we use dendrimer nanoparticles as a vector to produce coexpression gene therapy of TNFα and HSV-TK, in order to find an efficient way to accomplish an improvement of human uveal primary tumor radiosensitivity.
Dendrimers have been shown to be nontoxic, and they are highly efficient carriers for the delivery of nucleic acids and short oligodeoxynucleotides.17–19 Ferenc et al proved that phosphorus dendrimers have the potential to become efficient carriers of small interfering RNA in anti-HIV therapy.20 Such complexes have been called dendriplexes by analogy with polyplexes (polymer/nucleic acid complexes) and lipoplexes (liposome/nucleic acid complexes).21 The results of our study show that these dendrimer nanoparticles are about 20 nm, and dendriplexes that formed with those plasmids that were well constructed could achieve 100–200 nm with neutral zeta potential.22,23 The properties of the dendriplexes suggest that they can perform the transfection appropriately and affect tumor-cell proliferation and apoptosis.
Nanomaterials are similar in scale to biologic molecules, and systems can yet be engineered to have various functions; therefore, nanotechnology is potentially useful for medical applications.24 The influence of nanotechnology on the field of medicine has given rise to a new field known as “nanomedicine,” which encompasses the utilization of nanoscale structures and devices for medical treatment and diagnosis.25 Numerous studies have reported that nanotechnology accelerates various regenerative therapies, such as those for the bone, vascular, heart, cartilage, bladder, and brain tissue.26–28 Nanomedicines having at least one dimension in the nanoscale include nanoparticles, micelles, nanotubes, and dendrimers, with and without targeting ligands, and are making a significant impact in the fields of ocular drug delivery and gene delivery.29 Nanoparticles have better cellular uptake than larger particles. This uptake process is most likely by endocytosis, and has been utilized for gene delivery investigating the uptake of different-sized nanoparticles (20, 100, 500, 1,000, and 2,000 nm) into retinal pigment epithelial cells.29,30
Dendrimer nanoparticles form a tree-like, globular, nanostructured polymer that is a new type of vector for genetic transfection.31 Some dendrimers possess antimicrobial properties, and can be used as surface-coating agents and drug carriers.32 The polymer has more accurate nanostructure, higher solubility, low viscosity, and composability by using the positive charge on its surface to bind with the glycoprotein and phospholipid on the cell membrane.33 Therefore nanoparticle systems diffuse rapidly and are well internalized in ocular tissues.34 Dendrimeric nanoparticles have received significant attention due to their well-defined size, tailorable structure, narrow polydispersity, and potentially favorable ocular biodistribution.35 Because of the unique physiological structure of an eye, it is better to use lower-toxicity and higher-efficacy transfection vectors to localize drug administration.36 As a result, using dendrimeric nanoparticles as the vector will be the most valuable treatment to treat intraocular tumor.
The involvement of gene therapy for tumor and transfection of genetic vectors consists of gene information for both direct or indirect antitumor effect, which will be expressed at the tumor-growth site to inhibit or even kill the tumor.37,38 In this study, we successfully combined human TNFα and HSV-TK genes into one single vector with Egr-1 promoter. Due to the eye’s unique anatomical structure, a radio applicator for the iris has always been deemed to be the best choice for localization as well as short-distance radiotherapy. More recently, 125I brachytherapy has gained acceptance as an effective treatment alternative for small and medium-sized melanomas.39 However, simple radiotherapy can still injure the surrounding tissues, and some tumors even develop resistance to the radiation, resulting in reduction of therapeutic effect and its application. These are the primary factors to cause the failure of radiotherapy.40–42 As a result, we hypothesize the combination of gene therapy and radiotherapy will have a better antitumor coeffect.
Egr-1 played an important role in combining gene therapy and radiotherapy effectively. Murugesan et al used the recombinant technology with adenovirus as vector to embed human TNFα, chemo- or radiosensitive fragment, and Egr-1 into a compound called TNFerade (AdEgr.TNF.11D).43 Weichselbaum and Kufe successfully proceeded to use TNFerade in phase III human trials for treating pancreatic carcinoma.44 The use of recombinant technology could assist in finding the most appropriate vector, which could in turn increase the precision of transfection of TNFα into tumor cells, for it to express and synthesize target enzymes while HSV-TK would also be used, and both could together act to kill tumor cells.
In this study, we not only constructed plasmid pEgr1-TNFα-TK with dendrimeric nanoparticles as the vector precisely but also combined 2 Gy 125I radiation for human uveal melanoma cells in vitro. DNase examination confirmed that dendrimer complexes can protect plasmid DNA from DNase I more effectively than naked plasmid DNA (Figure 2B–D).
Protein expression of the genes was tested by ELISA and Western blot, and both showed significant expression for each gene to protein. The expression level is related to 125I radiation exposure, and also showed a specific time–effect relationship. We also confirmed that the transfection with dendrimer nanoparticles was successful. Based on the expression levels of TNFα and HSV-TK in those transfected groups and the combination groups, the bar graphs in Figures 4C and 5C show increases in expression of these two proteins after exposure. Statistical analysis suggested a significant variation in expression before and after radiation. The study also proved that the radiation could activate the transcription of Egr-1 and induce downstream upregulation. However, the peak time and dosage for these two proteins in cells were partially different, probably due to the type of protein expression and the effect of transfection.
Under TEM, the sample in the TNF-TK group with exposure to 2 Gy 125I radiation showed more cells in a necrotic state than other groups. Cell growth-rate results suggested that the TNF-TK group combined with radiation presented the best effect to inhibit the proliferation rate of OCM-1 cells. On the contrary, the sample in the negative-control group showed no damage, just like the control group. This observation confirmed that it is safe to use dendrimer nanoparticles as vectors.
Also, our study proved that radiation can increase the efficiency of gene transfer to ameliorate the effect of gene therapy, and gene therapy can increase radiosensitivity, reduce radiation injury to normal tissue, and change oxygenation of tumor cells. The results of flow cytometry and caspase-3 fluorescent staining showed that there were no significant differences in the early apoptotic rate among the transfection groups that were not exposed to 2 Gy 125I, but there were significant differences between the transfection groups after irradiation. The TNF-radiation groups showed the highest early apoptotic rate compared with the other radiation groups. There was no statistical difference between the negative and control groups.
In vitro experimentation showed that dendrimer nanoparticles delivered the genes into OCM-1 lines successfully. The recombinant plasmid pEgr-1-TNFα-TK could significantly affect OCM-1 lines’ proliferation rate and cause apoptosis and necrosis. The effects were strong after 2 Gy 125I irradiation. However, the necrosis effect was more obvious, and the apoptosis effect was not as profound as the cells with transfection of pEgr-1-TNFα with irradiation. A possible reason could be the combinatory effect of TK/GCV, which would lead more cells to a necrotic state. We also confirmed that the dendrimer nanoparticles can deliver genes into the target cells effectively and safely.
In our previous study, we used New Zealand rabbits to build choroidal melanoma animal models and prove that domestic 125I plaque irradiation is effective for the treatment of choroidal melanoma.45 Therefore, we will further investigate the inhibitory effect and the antitumor mechanisms of coexpression plasmid pEgr-1-TNFα-TK on uveal melanoma in animal experiments. Experimental animal models under cyclosporine would have reduced immunity. The use of a gene-transfection system with a highly toxic and unstable viral carrier would make it difficult to maintain animal survival rates during experiments. Therefore, in this study, we confirmed the efficiency of dendrimer nanoparticle transfection and expression of cytotoxicity to provide reliable experimental data for further experiments to investigate a new approach for treating human uveal melanoma.
Conclusion
The present study indicated that the pEgr-1-TNFα-TK group had the best effect of destroying tumor cells after irradiation, and that dendrimer nanoparticles provided an efficient and safe avenue to deliver genes into target cells.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant 81272981), the Natural Sciences Foundation of Beijing, People’s Republic of China (grant 7112031), and the Advanced Health-Care Professional Development Project of the Beijing Municipal Health Bureau, People’s Republic of China (Grant 2009-3-32).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Singh AD, Topham A. Incidence of uveal melanoma in the United States: 1973–1997. Ophthalmology. 2003;110(5):956–961. doi: 10.1016/S0161-6420(03)00078-2. [DOI] [PubMed] [Google Scholar]
- 2.Virgili G, Gatta G, Ciccolallo L, et al. Incidence of uveal melanoma in Europe. Ophthalmology. 2007;114(12):2309–2315. doi: 10.1016/j.ophtha.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 3.Singh AD, Bergman L, Seregard S. Uveal melanoma: epidemiologic aspects. Ophthalmol Clin North Am. 2005;18(1):75–84. doi: 10.1016/j.ohc.2004.07.002. viii. [DOI] [PubMed] [Google Scholar]
- 4.Kujala E, Mäkitie T, Kivelä T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003;44(11):4651–4659. doi: 10.1167/iovs.03-0538. [DOI] [PubMed] [Google Scholar]
- 5.Kath R, Hayungs J, Bornfeld N, Sauerwein W, Hoffken K, Seeber S. Prognosis and treatment of disseminated uveal melanoma. Cancer. 1993;72(7):2219–2223. doi: 10.1002/1097-0142(19931001)72:7<2219::aid-cncr2820720725>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 6.Maatta AM, Samaranayake H, Pikkarainen J, Wirth T, Yla-Herttuala S. Adenovirus mediated herpes simplex virus-thymidine kinase/ganciclovir gene therapy for resectable malignant glioma. Curr Gene Ther. 2009;9(5):356–367. doi: 10.2174/156652309789753365. [DOI] [PubMed] [Google Scholar]
- 7.Moolten FL. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer Res. 1986;46(10):5276–5281. [PubMed] [Google Scholar]
- 8.Ha Thi HT, Lim HS, Kim J, Kim YM, Kim HY, Hong S. Transcriptional and post-translational regulation of Bim is essential for TGF-beta and TNF-alpha-induced apoptosis of gastric cancer cell. Biochim Biophysica Acta. 2013;1830(6):3584–3592. doi: 10.1016/j.bbagen.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Kearney CJ, Sheridan C, Cullen SP, et al. Inhibitor of apoptosis proteins (IAPs) and their antagonists regulate spontaneous and tumor necrosis factor (TNF)-induced proinflammatory cytokine and chemokine production. J Biol Chem. 2013;288(7):4878–4890. doi: 10.1074/jbc.M112.422410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurosaki T, Yamashita Y, Aki K, et al. Secure and effective gene vector of polyamidoamine dendrimer pharmaceutically modified with anionic polymer. J Pharm Sci. 2011;100(11):4855–4863. doi: 10.1002/jps.22701. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Kong W, Song Y, et al. Polyamidoamine dendrimers with a modified pentaerythritol core having high efficiency and low cytotoxicity as gene carriers. Biomacromolecules. 2009;10(3):617–622. doi: 10.1021/bm801333s. [DOI] [PubMed] [Google Scholar]
- 12.Weichselbaum RR, Hallahan DE, Beckett MA, et al. Gene therapy targeted by radiation preferentially radiosensitizes tumor cells. Cancer Res. 1994;54(16):4266–4269. [PubMed] [Google Scholar]
- 13.Meyer RG, Kupper JH, Kandolf R, Rodemann HP. Early growth response-1 gene (Egr-1) promoter induction by ionizing radiation in U87 malignant glioma cells in vitro. Eur J Biochem. 2002;269(1):337–346. doi: 10.1046/j.0014-2956.2001.02658.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Y, Song X, Jia R, et al. Radiation-inducible human tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene therapy: a novel treatment for radioresistant uveal melanoma. Pigment Cell Melanoma Res. 2010;23(5):661–674. doi: 10.1111/j.1755-148X.2010.00729.x. [DOI] [PubMed] [Google Scholar]
- 15.Xu XJ, Ding LH, Wang LX, et al. Construction of human Egr-1 promoter and its response to ionizing radiation in tumor cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2009;25(11):973–975. Chinese. [PubMed] [Google Scholar]
- 16.Zagurovskaya M, Shareef MM, Das A, et al. EGR-1 forms a complex with YAP-1 and upregulates Bax expression in irradiated prostate carcinoma cells. Oncogene. 2009;28(8):1121–1131. doi: 10.1038/onc.2008.461. [DOI] [PubMed] [Google Scholar]
- 17.Dufes C, Uchegbu IF, Schatzlein AG. Dendrimers in gene delivery. Adv Drug Deliv Rev. 2005;57(15):2177–2202. doi: 10.1016/j.addr.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Klajnert B, Bryszewska M. Dendrimers in Medicine. Hauppauge (NY): Nova Science; 2007. [Google Scholar]
- 19.Kim TI, Baek JU, Zhe Bai C, Park JS. Arginine-conjugated polypropylenimine dendrimer as a non-toxic and efficient gene delivery carrier. Biomaterials. 2007;28(11):2061–2067. doi: 10.1016/j.biomaterials.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 20.Ferenc M, Pedziwiatr-Werbicka E, Nowak KE, Klajnert B, Majoral JP, Bryszewska M. Phosphorus dendrimers as carriers of siRNA – characterisation of dendriplexes. Molecules. 2013;18(4):4451–4466. doi: 10.3390/molecules18044451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eliyahu H, Barenholz Y, Domb AJ. Polymers for DNA delivery. Molecules. 2005;10(1):34–64. doi: 10.3390/10010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clogston JD, Patri AK. Zeta potential measurement. Methods Mol Biol. 2011;697:63–70. doi: 10.1007/978-1-60327-198-1_6. [DOI] [PubMed] [Google Scholar]
- 23.Ramaswamy C, Sakthivel T, Wilderspin AF, Florence AT. Dendriplexes and their characterisation. Int J Pharm. 2003;254(1):17–21. doi: 10.1016/s0378-5173(02)00670-1. [DOI] [PubMed] [Google Scholar]
- 24.Kim BY, Rutka JT, Chan WC. Nanomedicine. N Engl J Med. 2010;363(25):2434–2443. doi: 10.1056/NEJMra0912273. [DOI] [PubMed] [Google Scholar]
- 25.Campolongo MJ, Tan SJ, Xu J, Luo D. DNA nanomedicine: engineering DNA as a polymer for therapeutic and diagnostic applications. Adv Drug Deliv Rev. 2010;62(6):606–616. doi: 10.1016/j.addr.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang L, Webster TJ. Nanotechnology controlled drug delivery for treating bone diseases. Expert Opinion Drug Deliv. 2009;6(8):851–864. doi: 10.1517/17425240903044935. [DOI] [PubMed] [Google Scholar]
- 27.Yao C, Webster TJ, Hedrick M. Decreased bacteria density on nanostructured polyurethane. J Biomed Mater Res A. June2013 doi: 10.1002/jbm.a.34856. Epub. [DOI] [PubMed] [Google Scholar]
- 28.Khang D, Carpenter J, Chun YW, Pareta R, Webster TJ. Nanotechnology for regenerative medicine. Biomed Microdevices. 2010;12(4):575–587. doi: 10.1007/s10544-008-9264-6. [DOI] [PubMed] [Google Scholar]
- 29.Kompella UB, Amrite AC, Ravi RP, Durazo SA. Nanomedicines for back of the eye drug delivery, gene delivery, and imaging. Prog Reti Eye Res. 2013 Apr 17; doi: 10.1016/j.preteyeres.2013.04.001. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahay G, Alakhova DY, Kabanov AV. Endocytosis of nanomedicines. J Control Release. 2010;145(3):182–195. doi: 10.1016/j.jconrel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kambhampati SP, Kannan RM. Dendrimer nanoparticles for ocular drug delivery. J Ocul Pharmacol Ther. 2013;29(2):151–165. doi: 10.1089/jop.2012.0232. [DOI] [PubMed] [Google Scholar]
- 32.Mintzer MA, Dane EL, O’Toole GA, Grinstaff MW. Exploiting dendrimer multivalency to combat emerging and re-emerging infectious diseases. Mol Pharm. 2012;9(3):342–354. doi: 10.1021/mp2005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stiriba SE, Frey H, Haag R. Dendritic polymers in biomedical applications: from potential to clinical use in diagnostics and therapy. Angew Chem Int Ed Engl. 2002;41(8):1329–1334. doi: 10.1002/1521-3773(20020415)41:8<1329::aid-anie1329>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 34.Honda M, Asai T, Oku N, Araki Y, Tanaka M, Ebihara N. Liposomes and nanotechnology in drug development: focus on ocular targets. Int J Nanomedicine. 2013;8:495–503. doi: 10.2147/IJN.S30725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Q, Kambhampati SP, Kannan RM. Nanotechnology approaches for ocular drug delivery. Middle East Afr J Ophthalmol. 2013;20(1):26–37. doi: 10.4103/0974-9233.106384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diebold Y, Calonge M. Applications of nanoparticles in ophthalmology. Prog Retin Eye Res. 2010;29(6):596–609. doi: 10.1016/j.preteyeres.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Hurtado A, Tseng JC, Meruelo D. Gene therapy that safely targets and kills tumor cells throughout the body. Rejuvenation Res. 2006;9(1):36–44. doi: 10.1089/rej.2006.9.36. [DOI] [PubMed] [Google Scholar]
- 38.Ogura M, Shibata T, Harada H, Hiraoka M. Tumor-specific gene therapy strategy for renal cell carcinomas. Nihon Rinsho. 2006;64(Suppl 2):672–675. Japanese. [PubMed] [Google Scholar]
- 39.Murray TG, Markoe AM, Gold AS, et al. Long-term followup comparing two treatment dosing strategies of (125) I plaque radiotherapy in the management of small/medium posterior uveal melanoma. J Ophthalmol. 2013;2013:517032. doi: 10.1155/2013/517032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Korkolis DP, Plataniotis GD, Gondikakis E, et al. Short-term preoperative radiotherapy is a safe approach for treatment of locally advanced rectal cancer. Int J Colorectal Dis. 2006;21(1):1–6. doi: 10.1007/s00384-005-0740-7. [DOI] [PubMed] [Google Scholar]
- 41.Palled SR, Doddihal HM, Mahantshetty UM. Choledochal cyst carcinoma treated with stereotactic radiotherapy. Clin Oncol. 2006;18(2):153–154. doi: 10.1016/j.clon.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 42.Franzone P, Berretta L, Barra S. Review of the role of radiotherapy in craniopharyngiomas: how does patient age influence management decisions? J Pediatr Endocrinol Metab. 2006;19(Suppl 1):395–397. [PubMed] [Google Scholar]
- 43.Murugesan SR, King CR, Osborn R, et al. Combination of human tumor necrosis factor-alpha (hTNF-alpha) gene delivery with gemcitabine is effective in models of pancreatic cancer. Cancer Gene Ther. 2009;16(11):841–847. doi: 10.1038/cgt.2009.32. [DOI] [PubMed] [Google Scholar]
- 44.Weichselbaum RR, Kufe D. Translation of the radio- and chemo-inducible TNFerade vector to the treatment of human cancers. Cancer Gene Ther. 2009;16(8):609–619. doi: 10.1038/cgt.2009.37. [DOI] [PubMed] [Google Scholar]
- 45.Zhou JQ, Wei WB, Li B, et al. Clinical effectiveness and safety of domestic 125I plaque irradiation for experimental choroidal melanoma. Chin J Exp Ophthalmol. 2012;30(8):692–698. [Google Scholar]