Abstract
There is increasing experimental evidence of the nongenomic action of thyroid hormones mediated by receptors located in the plasma membrane or inside cells. The aim of this work was to characterize the reverse T3 (rT3) action on calcium uptake and its involvement in immature rat Sertoli cell secretion. The results presented herein show that very low concentrations of rT3 are able to increase calcium uptake after 1 min of exposure. The implication of T-type voltage-dependent calcium channels and chloride channels in the effect of rT3 was evidenced using flunarizine and 9-anthracene, respectively. Also, the rT3-induced calcium uptake was blocked in the presence of the RGD peptide (an inhibitor of integrin-ligand interactions). Therefore, our findings suggest that calcium uptake stimulated by rT3 may be mediated by integrin αvβ3. In addition, it was demonstrated that calcium uptake stimulated by rT3 is PKC and ERK-dependent. Furthermore, the outcomes indicate that rT3 also stimulates cellular secretion since the cells manifested a loss of fluorescence after 4 min incubation, indicating an exocytic quinacrine release that seems to be mediated by the integrin receptor. These findings indicate that rT3 modulates the calcium entry and cellular secretion, which might play a role in the regulation of a plethora of intracellular processes involved in male reproductive physiology.
Introduction
Thyroid hormones (THs) are iodinated compounds known to influence gene expression in virtually every vertebrate cell. THs action is critically important for development, tissue differentiation, and maintenance of metabolic balance in mammals. Thyroxine (3,5,3’,5’-L-tetraiodothyronine; T4) is known to be the main secretory product of the thyroid gland in all vertebrates, and can be activated to triiodothyronine (3,5,3’-triiodothyronine; T3) in a stage- and tissue-specific manner by phenolic ring deiodination (outer ring deiodination) catalyzed by two iodothyronine deiodinases, D1 and D2. A third deiodinase, D3, promotes deiodination at the tyrosyl ring producing reverse T3 (3,3’,5’-triiodothyronine; rT3) and T2 (3,3’-diiodothyronine) [1,2]. All three deiodinases, D1, D2, and D3, are expressed in testis at different levels from weanling to adult life, however, D3 activity predominates in the developmental period and then declines in adult life [3].
THs are important modulators of spermatogenesis and steroidogenesis in the testis. The presence of specific nuclear thyroid hormone receptors (TRs), described in prepubertal Sertoli cells, implies the existence of an early and critical influence of thyroid hormones on testis development [4]. Accordingly, alterations in thyroid activity are frequently associated with changes in male reproductive functions, since hypothyroidism is associated with a marked delay in sexual maturation and development [5].
The classical mechanism of THs has been established as a genomic action, including binding to intracellular hormone receptors that share the characteristics of nuclear transcription and protein synthesis [6,7]. These events are characterized by a considerable latency with response times ranging from hours to days [7,8]. In general, THs modulate a large number of metabolic processes but not all of these actions are due to effects on nuclear transcription.
Actions of THs that are independent of ligand binding to nuclear thyroid receptors are called rapid or nongenomic actions. This mechanism is independent of active protein synthesis, initiating in the plasma membrane [9]. It typically has a time-course of seconds or minutes and is frequently associated with secondary messenger and kinase signaling pathways [10].
Previous studies in our laboratory demonstrated some nongenomic effects in testes cells, including, amino acid accumulation [11-14], ion fluxes across plasma membrane [14,15], hyperpolarization of Sertoli cells [12,13], calcium influx [14,16-18], modulation of extracellular nucleotide levels [19] and alteration in the intermediate filament cytoskeleton dynamics [15]. In the other tissues, THs promote, through nongenomic actions, insertion of Na+,K+-ATPase into the plasma membrane, as well as the modulatory activity of this enzyme [20-22], intracellular shuttling of TRs resident in cytoplasm to the nucleus [23,24], and regulation of the state of the actin cytoskeleton [25] such as regulation of specific gene expression.
Secretory activities of Sertoli cells are critical to spermatogenesis [26]. Sertoli cells express a variety of ion channels involved in cellular secretory functions [27,28], and an increase in the intracellular calcium concentration ([Ca2+]i) is a key signal triggering exocytosis in these cells [29]. The role of cytosolic Ca2+ is directly involved in the fusion of the secretory vesicles with the plasma membrane (for review [30]), and in the several distinct maturation steps of these secretory vesicles prior to fusion [31]. Sertoli cells contribute to spermatogenesis since they supply the seminiferous epithelium with a rich ionic fluid and synthesize specific proteins, such as transferrin and androgen-binding protein, in combination with a series of other important factors that maintain ongoing and normal germ cell development [32,33].
Until recently, rT3 was regarded as an inactive hormone, however, studies in our group has demonstrated that this T4 metabolite stimulates amino acid accumulation (a specific plasma membrane transport system) in immature rat testis [34]. Furthermore, rT3 regulates actin polymerization [25] and the mobility of brain cells during brain development through nongenomic signaling [25,35]. In fact, the rT3 assay has been used in order to clarify the specificity of TH and is currently used in our laboratory [34]. We have previously showed that T4 effect was 106 times more potent than T3 on amino acid accumulation [13]. Latter, we also demonstrated that T4 and T3 have particular specificity of action on calcium influx in cerebral cortex [56]. Also, in the testis, we showed a very fast and specific effect of T4 on calcium influx [14] that was not observed for T3. In addition, recently we find that rT3 and T4 have similar potency on amino acid accumulation, although rT3 to be significantly most efficient than T4, in immature rat testis to mediate plasma membrane rapid responses [34]. So, since these results clearly show that T4, T3 and rT3 specificity for rapid responses in testis or Sertoli cells are quite different, in this study we investigated the involvement of integrin on calcium uptake and exocytosis triggered by rT3 in immature rat Sertoli cells.
Materials and Methods
Materials
3,3’,5’-triiodothyronine (reverse T3, rT3; purity ≥ 97 %), Arg-Gly-Asp (RGD), 1,2-bis(2-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid tetrakis (acetoxymethyl ester) (BAPTA-AM), 9-anthracene carboxylic acid (9-AC), flunarizine, 2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran-4-one (PD 98059), stearoylcarnitine, quinacrine, Dulbecco’s modified Eagle’s medium (DMEM), Ham’s F12 medium, penicillin, streptomycin, kanamycin and amphotericin B, Serum Replacement 3, bovine pancreas deoxyribonuclease (DNase type I), hyaluronidase (type I-S), trypsin, soybean trypsin inhibitor, sodium pyruvate, D-glucose, Hepes, and sodium bicarbonate were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Collagenase-Dispase and bovine serum albumin (BSA) were acquired from Roche Diagnostics (Indianapolis, IN, USA). [45Ca]CaCl2 (sp. act. 321 KBq/mg Ca2+) and Optiphase Hisafe III biodegradable liquid scintillation were purchased from PerkinElmer (Boston, MA, USA). All other chemicals were of analytical grade.
Animals
Male wistar rats (Rattus norvegicus) weighing ± 20 g from the Central Animal House-UFSC were bred in our animal house and maintained in an air-conditioned room (21 °C) with controlled lighting (12 h/12 h light/dark cycle). The suckling rats were kept with their mothers until sacrifice by decapitation. Pelleted food (Nuvital, Nuvilab CR1, Curitiba, PR, Brazil) and tap water were available ad libitum. All the animals were carefully monitored and maintained in accordance with ethical recommendations of The Brazilian Veterinary Medicine Council and the Brazilian College of Animal Experimentation. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Federal University of Santa Catarina (Permit Number: CEUA/PP00418).
Primary Culture of Sertoli Cells and Calcium Uptake
Sertoli cells were obtained from 11-day-old Wistar rats. Rats were killed by decapitation, and testes were removed and decapsulated. Sertoli cells were obtained by sequential enzymatic digestion as previously described by Dorrington et al. [36]. Sertoli cells were seeded at a concentration of 200,000 cells/cm2 in 24-well culture plates (Falcon, Deutscher, Brummath, France) and cultured for 72 h in Ham’s F12/DMEM (1:1) medium supplemented with Serum Replacement 3, 2.2 g/L sodium bicarbonate, antibiotics (50,000 IU/L penicillin, 50 mg/L streptomycin, and 50 mg/L kanamycin), and a fungicide (0.25 mg/L amphotericin B), in a humidified atmosphere of 5% CO2 and 95% air at 34 °C. Three days after being plated, residual germ cells were removed by a hypotonic treatment using 20 mM Tris-HCl (pH 7.2) for 150 s. [37]. Cells were washed with PBS, and fresh Ham’s F12/DMEM (1:1) medium was added. Five days after being plated, cells were preincubated in Krebs Ringer-bicarbonate buffer (KRb) (122 mM NaCl, 3 mM KCl, 1.2 mM MgSO4, 1.3 mM CaCl2, 0.4 mM KH2PO4, 25 mM NaHCO3 and glucose 5 mM) for 15 min in a Dubnoff metabolic incubator at 34 °C (pH 7.4) and gassed with an O2/ CO2 mixture (95:5, v/v). The medium was then replaced with fresh KRb containing 0.1 μCi/mL 45Ca2+ and left for 60 min. For calcium uptake measurements, cells were incubated for a further 30 s, 1 min or 5 min, in the absence (control) or presence of rT3 (from 10-19 to 10-7 M). The rT3 was dissolved in 0.01 M NaOH-saline (stock solution) to be further diluted to the final concentrations in KRb buffer. In some experiments, channel blockers or kinase inhibitors were added during the last 15 min before the hormone was added and maintained during the incubation period (see figure legends). The following drugs were used: BAPTA-AM (50 μM) (intracellular calcium chelator; [17]), 9-AC (1 μM) (blocker for calcium-dependent Cl- channels; [18]), flunarizine (1 μM) (T-type voltage-dependent Ca2+ channel blocker; [18]), PD 98059 (30 μM) (MEK inhibitor; [18]), RGD peptide (500 nM) (TH binding on αvβ3 receptor blocker; [38]) and stearoylcarnitine (1 μM) (PKC inhibitor; [39]).
Extracellular 45Ca2+ from primary Sertoli cells culture was thoroughly washed off in 127.5 mM NaCl, 4.6 mM KCl, 1.2 mM MgSO4, 10 mM HEPES, 11 mM glucose, and 10 mM LaCl3, at pH 7.4 (30 min in washing solution). The presence of La3+ during the washing stage was found to be essential to prevent release of the intracellular 45Ca2+ [40]. After La3+ tissue washing, cells were homogenized with 0.5 M NaOH solution; 100 μL aliquots were placed in scintillation fluid for counting in a Beckman coulter beta liquid scintillation spectrometer (model LS 6500; Fullerton, California, USA), and 50 µL aliquots were used for total protein quantification by the Lowry method [41]. The results were expressed as pmol 45Ca2+/μg of protein [16].
Secretory activity of Sertoli cells
Sertoli cells were obtained from 11-day-old Wistar rats. On day 5 after plating, the cells were washed in Hank’s Buffered Salt Solution (HBSS) (136.9 mM NaCl, 16.7 mM NaHCO3, 1.3 mM CaCl2, 5.4 mM KCl, 0.65 mM MgSO4, 0.27 mM Na2HPO4, 0.44 mM KH2PO4, 6.1 mM glucose). The medium was then replaced with fresh HBSS containing 3 µM quinacrine and cells were incubated for 30 min at 34 °C. The time-course of rT3 (10-17 M) was carried out at 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10 min based on a similar approach previously used by our group [42]. When RGD and flunarizine were used, Sertoli cells were treated for 10 min with the drugs prior to incubation with quinacrine.
Exocytosis imaging in primary culture of Sertoli cells
Microscopy imaging was performed on quinacrine-loaded live Sertoli cells as described by Menegaz et al. [42]. Briefly, cells were washed with HBSS and loaded with 3 µM quinacrine dissolved in HBSS for 30 min at 34 °C. Sertoli cells were viewed under an Olympus BX41fluorescence microscope using a FITC filter. Exocytosis was identified as the rapid loss of quinacrine fluorescence when released into the medium, indicating fusion of secretory vesicles with the plasma membrane with/without the hormone stimulus. Images were obtained with a QColor 3C digital camera (Q-imaging) at a scanning rate of 1 image/60 s and processed with Q-capture Pro 5.1 software program (Q-imaging).
Statistical analysis
The results are means ± S.E.M. When multiple comparisons were performed, evaluation was carried out using one-way ANOVA followed by Bonferroni multiple comparison test or unpaired Student’s t-test was used to determine the significance of differences between groups. Differences were considered to be significant when p < 0.05.
Results
Rapid response of reverse T3 (rT3) in calcium uptake by Sertoli cells
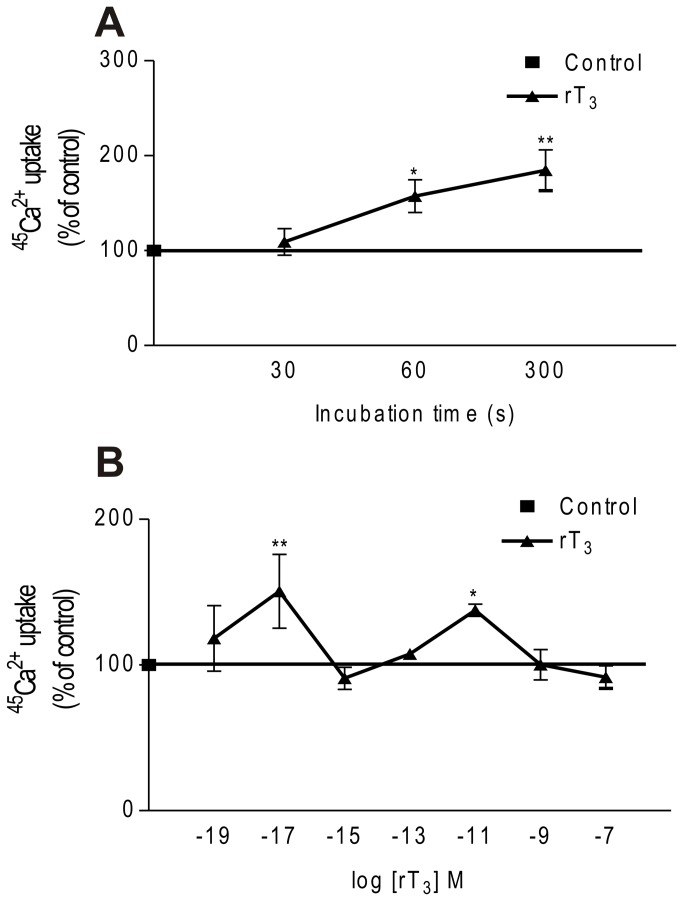
In this study we investigated the rapid action of rT3 in 11-day-old rat Sertoli cells using the radioisotope 45Ca2+, an accurate approach to measuring rapid effects on the plasma membrane. In the presence of 10-17 M rT3, the calcium uptake increased from 30 s until the maximum period studied (5 min). At 60 and 300 s a significant stimulatory effect of the hormone on calcium uptake was observed compared to the control group at 30 s (Figure 1A). As can be observed, addition of 10-17 and 10-11 M rT3 to the cultures for 60 s caused a significant increase (50% and 37%, respectively) in calcium uptake by these cells compared with the control group. On the other hand, 10-19, 10-15, 10-13, 10-9 and 10-7 M rT3 did not caused any significant calcium uptake (Figure 1B). Since the aim of this study was to evaluate the rapid response of rT3 we applied 60 s and 10-17 M in subsequent experiments.
Figure 1. Time-course and dose-response curve of rT3 on Ca2+ uptake in Sertoli cells.
(A) Time-course effect of rT3. Pre-incubation: 15 min in KRb, additional pre-incubation: 60 min with 0.1 µCi/mL of 45Ca2+ and incubation time: 30, 60 and 300 s with 0.1 µCi/mL of 45Ca2+ in the presence or absence of rT3 (10-17 M). Means ± S.E.M. n= 4 for all groups. **P < 0.01 and *p < 0.05 compared with control group. (B) Dose-response curve for rT3 in relation to Ca2+ uptake in Sertoli cells. Pre-incubation: 15 min in KRb, additional pre-incubation: 60 min with 0.1 µCi/mL of 45Ca2+ and incubation time: 60 s with 0.1 µCi/mL of 45Ca2+ in the presence or absence of rT3. Means ± S.E.M. For control and rT3 (10-19, 10-17, 10-15, 10-13, 10-11, 10-9 and 10-7 M), n=4 for each group. **P < 0.01 and *p < 0.05 compared with control group.
Evidence for plasma membrane receptor mediation of rT3 stimulation of calcium uptake
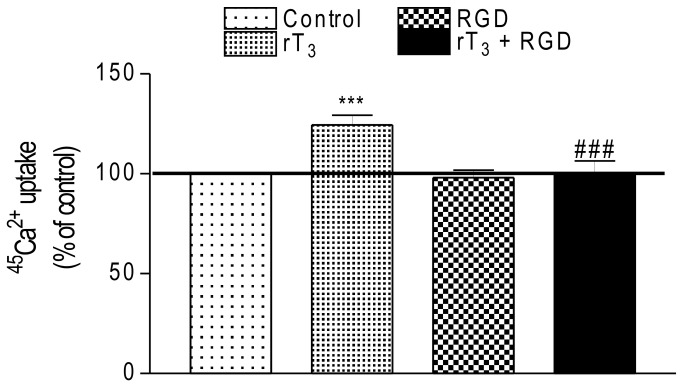
In order to evaluate the participation of αvβ3 integrin in the rT3 action on 45Ca2+ uptake, Sertoli cells were exposed to 10-17 M of the T4 metabolite in the presence or absence of RGD (a peptide that inhibits thyroid hormone binding to integrins) and the 45Ca2+ uptake was investigated. Figure 2 shows that RGD did not affect the basal calcium uptake. However, the rapid stimulatory effect of rT3 on calcium uptake was completely inhibited by the RGD peptide.
Figure 2. Influence of RGD peptide on stimulatory effect of rT3 on 45Ca2+ uptake in Sertoli cells.
Pre-incubation: 15 min in KRb, additional pre-incubation: 60 min with 0.1 µCi/mL of 45Ca2+ and incubation time: 60 s with 0.1 µCi/mL of 45Ca2+ in the presence or absence of RGD peptide (5 x 10-7 M) with/without rT3 (10-17 M). Means ± S.E.M. For control, rT3, RGD and rT3 + RGD, n=10 for each group. ***p < 0.001 compared with control group; ###p < 0.001 compared with rT3 group.
Involvement of voltage-dependent calcium and chloride channels on rT3 response in Sertoli cells
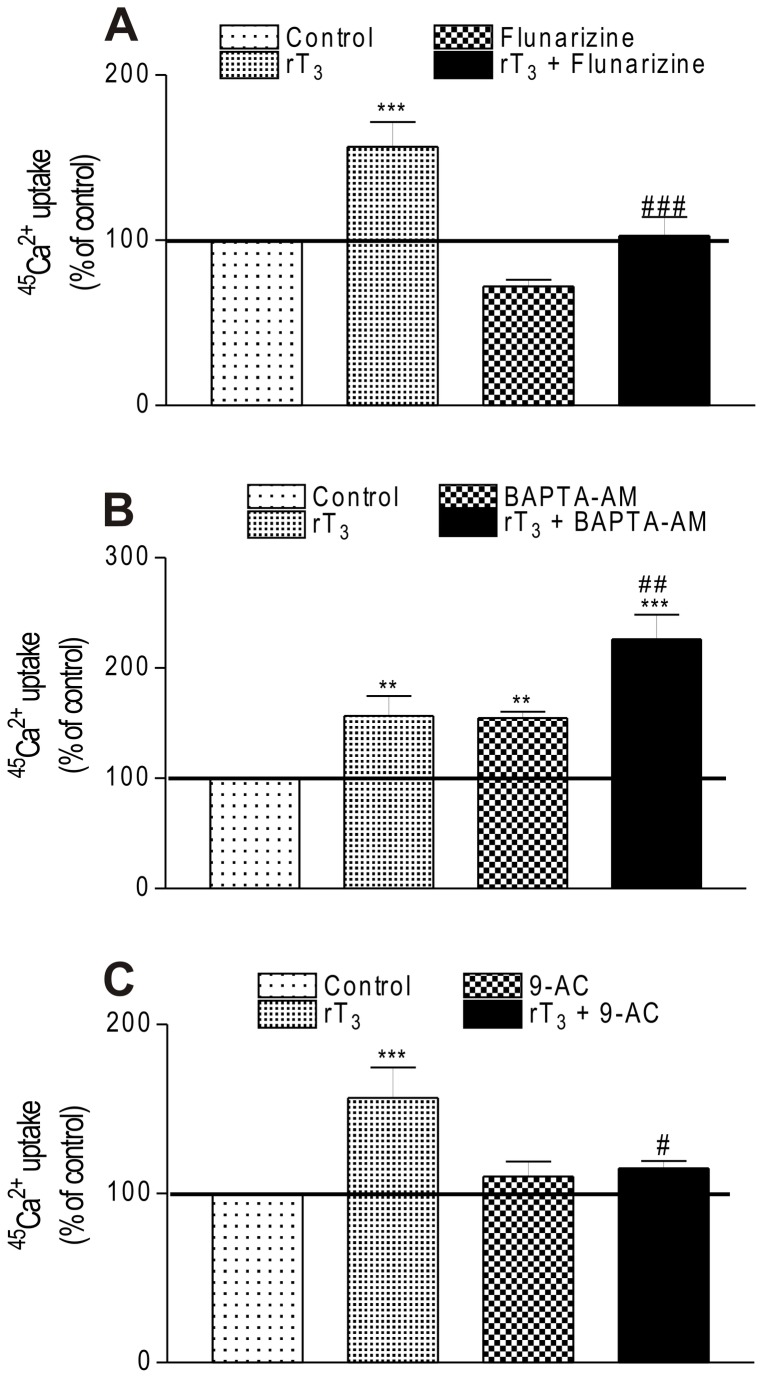
We also investigated whether T-type voltage-dependent calcium channels (T-VDCC) could be involved in the rT3 stimulatory action on 45Ca2+ uptake. To this aim, Sertoli cells were incubated in the presence of rT3 with/without flunarizine (1 µM) which blocks T-VDCCs [18]. In Figure 3A it can be observed that flunarizine nullified the rT3 stimulatory effect indicating the involvement of T-type VDCC in the calcium uptake in Sertoli cells.
Figure 3. Involvement of ionic channels and intracellular calcium on stimulatory effect of rT3 on 45Ca2+ uptake.
(A) Influence of flunarizine, (B) BAPTA-AM and (C) 9-AC on stimulatory effect of rT3 on 45Ca2+ uptake in Sertoli cells. Pre-incubation: 15 min in KRb, additional pre-incubation: 60 min with 0.1 µCi/mL of 45Ca2+ and incubation time: 60 s with 0.1 µCi/mL of 45Ca2+ in the presence or absence of flunarizine (1 µM), BAPTA-AM (50 µM) and 9-AC (1 µM) with/without rT3 (10-17 M). Means ± S.E.M. For control, n=10; rT3, n=7; flunarizine, n=8; rT3 + flunarizine, n=8; BAPTA-AM, n=8; rT3 + BAPTA-AM, n=6; 9-AC, n=6; rT3 + 9-AC, n=6. ***P < 0.001 and **p < 0.01 compared with control group; ###p < 0.001; ##p < 0.01 and #p < 0.05 compared with rT3 group.
Once the participation of T-VDCC on 45Ca2+ uptake in Sertoli cells had been established, we also sought to determine whether intracellular calcium levels could play a role in regulating the VDCC activity by using BAPTA-AM. Our findings demonstrated that when intracellular calcium was chelated by BAPTA-AM the 45Ca2+ uptake significantly increased and when BAPTA-AM was co-incubated with rT3 the stimulatory effect of rT3 was potentiated (Figure 3B).
Since VDCCs can open in response to changes in the resting plasma membrane potential, we investigated whether chloride influx might lead to 45Ca2+ uptake through VDCCs. The use of a specific blocker for calcium-dependent chloride channels (9-AC) demonstrated that it prevented the rT3-induced 45Ca2+ uptake (Figure 3C). These data allowed us to establish the implication of T-VDCC, intracellular calcium and chloride channels in mediating signal transduction of rT3 in immature Sertoli cells.
rT3 effect on 45Ca2+ uptake is mediated by PKC and MEK
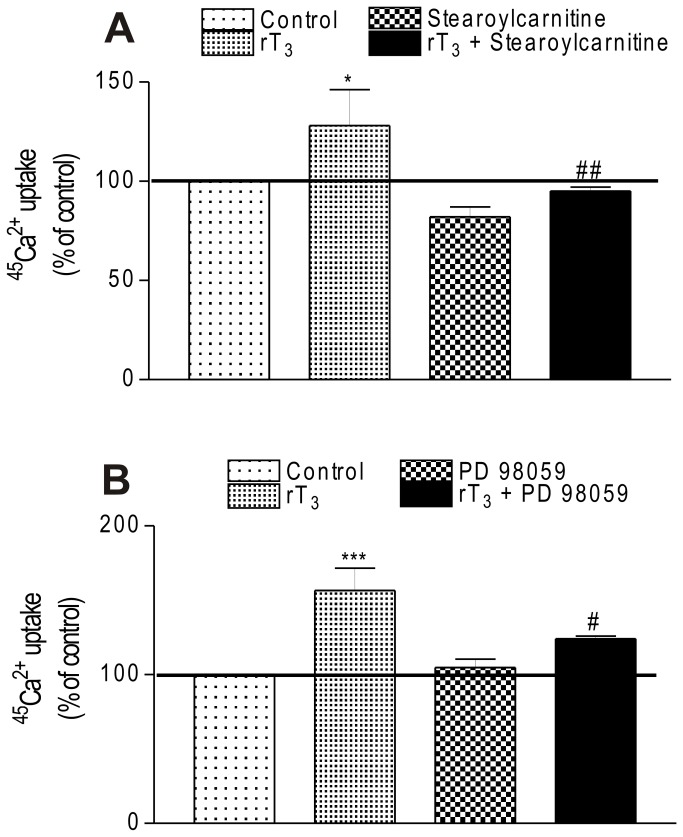
The contribution of different protein kinases known to target the calcium channels proteins [39] was investigated. To this aim, stearoyl carnitine and PD 98059 were used as PKC and MEK inhibitors, respectively. As shown in Figure 4, exposure to rT3 at 10-17 M for 60 s was able to increase the 45Ca2+ uptake but when the cells were previously preincubated with the kinase inhibitors the stimulatory effect of the hormone was totally prevented.
Figure 4. Involvement of kinases proteins on stimulatory effect of rT3 on 45Ca2+ uptake in Sertoli cells.
(A) Influence of stearoylcarnitine and (B) PD 98059. Pre-incubation: 15 min in KRb, additional pre-incubation: 60 min with 0.1 µCi/mL of 45Ca2+ and incubation time: 60 s with 0.1 µCi/mL of 45Ca2+ in the presence or absence of stearoylcarnitine (1 µM) and PD 98059 (30 µM) with/without rT3 (10-17 M). Means ± S.E.M. For control, n=9; rT3, n=6; stearoylcarnitine, n=8; rT3 + stearoylcarnitine, n=9; PD 98059, n=8; rT3 + PD 98059, n=8. ***p < 0.001 and *p < 0.05 compared with control group; ##p < 0.01 and #p < 0.05 compared with rT3 group.
rT3 and Sertoli cell secretion
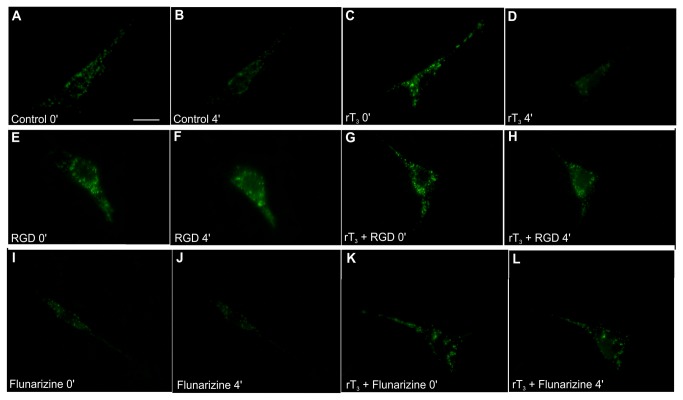
To demonstrate the potential of rT3 to induce cellular secretion, Sertoli cells were labeled with quinacrine. The panel in Figure 5 shows quinacrine loading in Sertoli cells monitored by fluorescence microscopy through changes in the fluorescence intensity or fluorescence lifetime. The images revealed non-uniform quinacrine distribution within the cells with punctate staining, which results from vesicular accumulation of the dye. In Figure 5A the granular staining is evident in the cytoplasm, but it was particularly abundant in the perinuclear region. Figure 5B represents a single quinacrine-stained cell imaged after 4 min in the basal condition without significant changes in fluorescence intensity. To study the exocytosis of quinacrine-stained vesicles induced by rT3, fluorescence changes of individual cells were analyzed and compared with basal conditions. Comparing the image in Figure 5D with the control (Figure 5C) demonstrates that after 4 min of exposure to rT3 the cells manifested a loss of fluorescence indicating an exocytic quinacrine release.
Figure 5. Fluorescence images of Sertoli cells stained with quinacrine.
Quinacrine stains individual secretory vesicles in the cell cytoplasm. Sertoli cells in culture were incubated with 3 µM quinacrine for 30 min, washed and photographed under fluorescence illumination immediately (A and C) and at 1 min intervals for 10 min of incubation in the absence or presence of rT3, respectively (B and D). Incubation of cells with 10-17 M rT3 caused fusion of quinacrine-loaded vesicles to the plasma membrane and release of the fluorescent content into the surrounding medium, as seen by the loss of fluorescence from most vesicles located at the cell periphery. This effect was observed after 4 min incubation with rT3. Also, Sertoli cells were incubated for 10 min with 500 nM of RGD peptide or 1 µM of flunarizine prior to incubation with quinacrine, washed and incubated with 3 µM quinacrine for 30 min. Quinacrine-loaded Sertoli cell cultures, pre-treated with RGD or flunarizine for 10 min, were incubated in the absence or presence of 10-17 M rT3 and photographed under fluorescence illumination immediately (E, G, I and K) and at 1-min intervals for 10 min of incubation in the absence or presence of rT3 (F, H, J and L). Incubation of cells in the presence of 500 nM RGD peptide or 1 µM flunarizine prevented the fusion of quinacrine-loaded vesicles to the plasma membrane and release of the fluorescent content. (A) Control, 0 min. (B) Control, 4 min. (C) rT3, 0 min. (D) rT3, 4 min. (E) RGD, 0 min. (F) RGD, 4 min. (G) rT3 + RGD, 0 min. (H) rT3 + RGD, 4 min. (I) Flunarizine, 0 min. (F) Flunarizine, 4 min. (G) rT3 + Flunarizine, 0 min. (H) rT3 + Flunarizine, 4 min. Experiments were performed 3 times with similar results. Bar = 10 µm.
Since the results for the calcium uptake indicated rapid response and plasma membrane-associated rT3 actions, we investigated the participation of αvβ3 integrin and T-type VDCCs in the mechanism of action of rT3 in Sertoli cell secretion. The findings demonstrated that RGD peptide and flunarizine did not produce alterations in cellular secretion (Figure 5F and 5J) when compared with respective control cells (Figure 5E and 5I). In addition these blockers prevented the exocytosis induced by rT3 (Figure 5H and 5L).
Discussion
Thyroid hormones T3 and T4 give rise to a wide range of effects on metabolism, growth and development [43]. T4 is the major form of TH secreted by the thyroid gland, whereas T3 is produced mainly in target tissues by deiodination of T4 [44]. While it is clear that many of the thyroid hormone actions are mediated by T3-dependent regulation of gene expression, in recent years the nongenomic action of thyroid hormones has also been reported (for review see [45]). Particularly in the male reproductive system, thyroid hormones play an important role where they regulate a diverse set of functions through rapid and genomic mechanisms (for review see [46]).
In this study, we obtained novel evidence that rT3, a T4 metabolite until recently regarded as inactive, is also involved in the regulation of 11 day-old Sertoli cell functions. It was demonstrated that rT3 stimulates calcium uptake in these cells within a very short time (60 s) and with a very low concentration (10-17 M) compared to thyroxine. A similar effect was previously reported by our group for T4 in whole testis [14]. However, the minimum concentration of T4 required to induce calcium influx in the testis was 10-9 M, highlighting the greater potency of rT3 when compared to T4.
Although the enzymes deiodinase 1 (D1) and deiodinase 3 (D3), which inactivate T4 and T3 by converting them to their reverse T3 (rT3) and 3,3′-T2 forms, respectively, exist in prepubertal and pubertal rat testis [3,47], there are no reports concerning the effect of rT3 in the testis or in Sertoli cells. Thus, as far as we are aware, this is the first demonstration of the rapid response of rat Sertoli cells in relation to calcium uptake by rT3. Based on this finding, the very potent effect of rT3 observed herein may represent a cell-specific modulatory event independent of high amounts of TH metabolites produced by the liver [48,49].
Nevertheless, several questions remain unanswered concerning the TH mechanism of action in the male reproductive system, especially related to rapid and nongenomic effects. For many years, TH action was viewed as dependent on the presence of nuclear receptors (TRs) and their major ligand, T3. Identification of a cell surface receptor for TH provides a molecular basis for certain nongenomic effects. Plasma membrane integrin αvβ3 is a cell surface receptor described for TH in the central nervous system, where nongenomic actions are initiated. It has been shown that integrin αvβ3 contains a binding domain for iodothyronines [50]. This domain contains an Arg-Gly-Asp (RGD) recognition site that is important for the binding of a variety of extracellular proteins and growth factors [51,52]. A family of adhesion proteins known as integrins has been described in relation to the reproductive system [53]. In addition, α6β1 integrin is expressed in Sertoli cells involved in cell-cell junctions [54]. In this regard, the RGD peptide was used to determine whether rT3-induced calcium uptake is mediated by integrin and, as expected, the results confirmed the participation of integrin in rT3 action on Sertoli cells.
Calcium helps regulate a variety of cellular functions in different cells, including germ cells and somatic cells in the testis in response to hormones and local regulators [55]. Considering the relevance of calcium overload on the modulation of a variety of Sertoli cell functions, especially cell secretion, different channel blockers and kinase inhibitors were used to determine the role and the mechanism of action of rT3 in calcium uptake. The rapid and/or sustained calcium uptake through VDCC seems to be required for physiological responses in Sertoli cells [14,16]. Therefore, in order to clarify its involvement in rT3 action, the T-VDCCs were previously inhibited with the use of a known calcium channel blocker. Our results showed that flunarizine totally prevented the rT3 effect on calcium uptake as has been reported for other hormones, such as T4, T3 and 1,25(OH)2 vitamin D3 [18,56]. Zamoner et al. [56] have demonstrated that the effects of T4 and T3 on the cerebral cortex of young rats are mediated by both L- and T-type VDCCs. Likewise, Rosso et al. [18] recently showed the involvement of T-type VDCC in calcium uptake induced by 1,25(OH)2 vitamin D3 in 10-day-old rat testis. These findings demonstrate that rT3-induced calcium uptake was directly and mostly related to VDCC.
The entrance of calcium into Sertoli cells can be triggered by depolarization, channel protein phosphorylation or depletion of intracellular calcium stores which requires functioning VDCC [33]. Electrophysiological studies demonstrated that T-type calcium channels of excitable cells are located in the plasma membrane of immature Sertoli cells [27]. Our findings indicated that calcium uptake induced by rT3 can result from T-VDCC opening but not from intracellular calcium depletion, since co-incubation of BAPTA-AM and rT3 produced a significant increase in calcium influx compared with that produced by rT3 alone. In order to evaluate the mechanisms that could lead to calcium uptake through T-VDCC in Sertoli cells, we investigated the participation of ionic channels and protein kinases by using pharmacological tools which allowed us to determine that effect of rT3 on calcium uptake is dependent on the chloride channel as well as PKC and MEK. In this context, the calcium influx through T-VDCCs could be modulated by complex mechanisms involving the activities of these protein kinases [46,57] or by changes in the plasma membrane potential generated by the opening of ionic channels [58].
Several hormones which regulate T-VDCCs have the ability to conduct calcium across the cellular membrane at potentials close to the resting potential [57]. In the testis, modulation of the voltage-dependent calcium conductance by changing the chloride concentration has been described [28]. Also, we recently showed the nongenomic effect of 1,25(OH)2 vitamin D3-induced calcium uptake in Sertoli cells through L- and T-VDCC modulation by Ca2+-dependent chloride channels [17,18] as well as in the cerebral cortex of young rats [59]. Also, T-channel activity, like that of most ion channels, can be modulated by hormones acting through signaling pathways such as protein kinases A and C [42].
It has been reported that PKC can modulate T-VDCC in a variety of cell systems [57]. Herein, we reveal the involvement of PKC in the calcium influx via T-VDCC in Sertoli cells. Besides the stimulatory effect of 1,25(OH)2-vitamin D3 on calcium uptake in the testis or Sertoli cells mediated by PKC and PKA, Costa et al. [60] also reported that the luteinizing hormone (LH) modulates T-type calcium currents in Leydig cells through PKA and PKC.
It has been reported that both conventional and novel PKCs can activate the MAPK signaling pathway [61] and, therefore, we also investigated the involvement of MEK in rT3-induced calcium uptake. The participation of MEK in calcium influx demonstrated in this study is in agreement with previous reports by our group for the effect of 1,25(OH)2-vitamin D3 on testis [17] and Sertoli cells [18].
There is an increasing body of evidence that T-type calcium channels can trigger fast and low-threshold exocytosis in neurons [62] as well as in chromaffin cells [63] and in retinal glial cells [64] controlling the release of neurotransmitters. In addition, these authors have reported that these channels are equally distributed near the docked secretory vesicles [63].
In this context, this study adds important evidence demonstrating that exocytosis in immature rat Sertoli cells is modulated by rT3. Similar granular quinacrine staining has been reported in the mouse Sertoli cell line (TM4) treated with 1,25(OH)2-vitamin D3, which was related to chloride channel activation [42]. Herein, the results reported suggest the involvement of calcium channels in cellular secretion induced by rT3. Moreover, the data obtained indicate that exocytosis is mediated by integrin and T-type VDCCs, since the preincubation of the cells with RGD and flunarizine abrogated the fusion of fluorescent vesicles with the plasma membrane leading to the disappearance of fluorescence.
Collectively, our findings reveal a new active metabolite of thyroid hormone in immature Sertoli cell. Our results strongly suggest that rT3 increases the calcium influx and that T-type VDCCs activation is implicated in Sertoli cell secretion. The activity of T-type VDCCs could be regulated by rT3 through integrin binding and consequent PKC, MEK and chloride channel activation. The modulation of calcium entry into Sertoli cells by rT3 might participate in the regulation of intracellular processes, such as cell secretion, reinforcing the role of rT3 in the male reproductive system physiology. Future studies are necessary to analyze further the physiological relevance of rT3 as well as to characterize the specific types of integrin that preferentially bind to the hormone in Sertoli cells. Ultimately, such knowledge could lead to the identification of novel means to regulate these possible physiological actions for therapeutic purposes.
Acknowledgments
APZ is registered on the Pharmacy Postgraduate Program of UFSC. RG is registered on the Biochemistry Postgraduate Program of UFSC. We are grateful to the department of BEG-CCB/UFSC for fluorescence microscopy facilities (FluorBEG) and the biologist Chirle Ferreira for technical assistance.
Funding Statement
This work was supported by grants from: MCT and CNPq (nº 471594/2010-5)- www.cnpq.br, CAPES/COFECUB nº 554/07 - www.capes.gov.br, FAPESC-SC (nº FCTP1518/000) - www.fapesc.sc.gov.br and CAPES/PPG-Pharmacy - www.pgfar.ufsc.br. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gereben B, Zeöld A, Dentice M, Salvatore D, Bianco AC (2008) Activation and inactivation of thyroid hormone by deiodinases: local action with general consequences. Endocrinol Rev 65: 570-590. PubMed: 17989921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dentice M, Salvatore D (2011) Deiodinases: the balance of thyroid hormone – Local impact of thyroid hormone inactivation. J Endocrinol 209: 273-282. doi: 10.1530/JOE-11-0002. PubMed: 21398344. [DOI] [PubMed] [Google Scholar]
- 3. Bates JM, St Germain DL, Galton VA (1999) Expression profiles of the three iodothyronine deiodinases, D1, D2, and D3, in the developing rat. Endocrinology 140: 844-851. doi: 10.1210/en.140.2.844. PubMed: 9927314. [DOI] [PubMed] [Google Scholar]
- 4. Jannini EA, Carosa E, Rucci N, Screponi E, D'Armiento M (1999) Ontogeny and regulation of variant thyroid hormone receptor isoforms in developing rat testis. J Endocrinol Invest 22: 843-848. PubMed: 10710271. [DOI] [PubMed] [Google Scholar]
- 5. Holsberger DR, Cooke PS (2005) Understanding the role of thyroid hormone in Sertoli cell development: a mechanistic hypothesis. Cell Tissue Res 322: 133-140. doi: 10.1007/s00441-005-1082-z. PubMed: 15856309. [DOI] [PubMed] [Google Scholar]
- 6. Lazar MA (1993) Thyroid hormone receptors: multiple forms, multiple possibilities. Endocrinol Rev 14: 184-193. doi: 10.1210/er.14.2.184. PubMed: 8325251. [DOI] [PubMed] [Google Scholar]
- 7. Kavok NS, Krasilnikova OA, Babenko NA (2001) Thyroxine signal transduction in liver cells involves phospholipase C and phospholipase D activation: genomic independent action of thyroid hormone. BMC Cell Biol 2: 5. doi: 10.1186/1471-2121-2-5. PubMed: 11312999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aranda A, Pascual A (2001) Nuclear hormone receptors and gene expression. Physiol Rev 81: 1269-1304. PubMed: 11427696. [DOI] [PubMed] [Google Scholar]
- 9. Davis PJ, Davis FB, Cody V (2005) Membrane receptors mediating thyroid hormone action. Trends Endocrinol Metab 16: 429-435. doi: 10.1016/j.tem.2005.09.007. PubMed: 16214361. [DOI] [PubMed] [Google Scholar]
- 10. Scapin S, Leoni S, Spagnuolo S, Gnocchi D, De Vito P et al. (2010) Short-term effects of thyroid hormones during development: Focus on signal transduction. Steroids 75: 576-584. doi: 10.1016/j.steroids.2009.10.013. PubMed: 19900468. [DOI] [PubMed] [Google Scholar]
- 11. Silva FR, Leite LD, Barreto KP, D’Agostini C, Zamoner A (2001) Effect of 3,5,3′-triiodo-L-thyronine on amino acid accumulation and membrane potential in Sertoli cells on the rat testis. Life Sci 69: 977-986. doi: 10.1016/S0024-3205(01)01186-9. PubMed: 11488410. [DOI] [PubMed] [Google Scholar]
- 12. Volpato KC, Menegaz D, Leite LD, Barreto KP, Garcia EV et al. (2004) Involvement of K+ channels and calcium-dependent pathways in the action of T3 on amino acid accumulation and membrane potential in Sertoli cells of immature rat testis. Life Sci 74: 1277-1288. doi: 10.1016/j.lfs.2003.08.005. PubMed: 14697410. [DOI] [PubMed] [Google Scholar]
- 13. Menegaz D, Zamoner A, Royer C, Leite LD, Bortolotto ZA et al. (2006) Rapid responses to thyroxine in the testis: active protein synthesis-independent pathway. Mol Cell Endocrinol 246: 128-134. doi: 10.1016/j.mce.2005.11.019. PubMed: 16387420. [DOI] [PubMed] [Google Scholar]
- 14. Menegaz D, Royer C, Rosso A, De Souza AZP, Dos Santos ARS et al. (2010) Rapid stimulatory effect of thyroxine on plasma membrane transport systems: Calcium uptake and neutral amino acid accumulation in immature rat testis. Int J Biochem Cell Biol 42: 1046-1051. doi: 10.1016/j.biocel.2010.03.015. PubMed: 20348014. [DOI] [PubMed] [Google Scholar]
- 15. Zamoner A, Corbelini PF, Funchal C, Menegaz D, Silva FR et al. (2005) Involvement of calcium-dependent mechanisms in T3-induced phosphorylation of vimentin of immature rat testis. Life Sci 77: 3321-3335. doi: 10.1016/j.lfs.2005.05.042. PubMed: 15985269. [DOI] [PubMed] [Google Scholar]
- 16. Zanatta L, Zamoner A, Gonçalves R, Zanatta AP, Bouraïma-Lelong H et al. (2011) 1α,25-Dihydroxyvitamin D(3) signaling pathways on calcium uptake in 30-day-old rat Sertoli cells. Biochemistry 50: 10284-10292. doi: 10.1021/bi201113n. PubMed: 22035182. [DOI] [PubMed] [Google Scholar]
- 17. Zanatta L, Zamoner A, Gonçalves R, Zanatta AP, Bouraïma-Lelong H et al. (2011) Effect of 1α,25-dihydroxyvitamin D3 in plasma membrane targets in immature rat testis: ionic channels and gamma-glutamyl transpeptidase activity. Arch Biochem Biophys 515: 46-53. doi: 10.1016/j.abb.2011.09.001. PubMed: 21933661. [DOI] [PubMed] [Google Scholar]
- 18. Rosso A, Pansera M, Zamoner A, Zanatta L, Bouraïma-Lelong H et al. (2012) 1α,25(OH)2-Vitamin D3 stimulates rapid plasma membrane calcium influx via MAPK activation in immature rat Sertoli cells. Biochimie 94: 146-154. doi: 10.1016/j.biochi.2011.10.001. PubMed: 22015633. [DOI] [PubMed] [Google Scholar]
- 19. Zamoner A, Bruno AN, Casali EA, Corbelini PF, Diniz GP et al. (2006) Genomic-independent action of thyroid hormones on NTPDase activities in Sertoli cell cultures congenital hypothyroid rats. Life Sci 80: 51-58. doi: 10.1016/j.lfs.2006.08.020. PubMed: 16978660. [DOI] [PubMed] [Google Scholar]
- 20. Lei J, Nowbar S, Mariash CN, Ingbar DH (2003) Thyroid hormone stimulates Na, K-ATPase activity and its plasma membrane insertion in rat alveolar epithelial cells. Am J Physiol 285: L762-L772. [DOI] [PubMed] [Google Scholar]
- 21. Lei J, Mariash CN, Bhargava M, Wattenberg EV, Ingbar DH (2008) T3 increases Na, K-ATPase activity via MAPK/ERK1/2-dependent pathway in rat adult alveolar epithelial cells. Am J Physiol 294: L749-L754. [DOI] [PubMed] [Google Scholar]
- 22. Scapin S, Leoni S, Spagnuolo S, Fiore AM, Incerpi S (2009) Short-term effects of thyroid hormone on the Na, K-ATPase activity of chick embryo hepatocytes during development: focus on signal transduction. Am J Physiol 296: C4-C12. [DOI] [PubMed] [Google Scholar]
- 23. Zhu XG, Hanover JA, Hager GL, Cheng SY (1998) Hormone-induced translocation of thyroid hormone receptors in living cells visualized using a receptor green fluorescent protein chimera. J Biol Chem 273: 27058-27063. doi: 10.1074/jbc.273.42.27058. PubMed: 9765220. [DOI] [PubMed] [Google Scholar]
- 24. Bunn CF, Neidig JA, Freidinger KE, Stankiewicz TA, Weaver BS et al. (2001) Nucleocytoplasmic shuttling of the thyroid hormone receptor α. Mol Endocrinol 15: 512-533. doi: 10.1210/me.15.4.512. PubMed: 11266504. [DOI] [PubMed] [Google Scholar]
- 25. Farwell AP, Dubord-Tomasetti SA, Pietrzykowski AZ, Leonard JL (2006) Dynamic nongenomic actions of thyroid hormone in the developing rat brain. Endocrinology 147: 2567-2574. doi: 10.1210/en.2005-1272. PubMed: 16469804. [DOI] [PubMed] [Google Scholar]
- 26. Russell LD, Griswold MD (1993) The Sertoli Cell, third ed.. Clearwater, FL: Cache River Press. [Google Scholar]
- 27. Lalevée N, Pluciennik F, Joffre M (1997) Voltage-dependent calcium current with properties of T-type current in Sertoli cells from immature rat testis in primary cultures. Biol Reprod 56: 680-687. doi: 10.1095/biolreprod56.3.680. PubMed: 9047014. [DOI] [PubMed] [Google Scholar]
- 28. Lalevée N, Joffre M (1999) Inhibition by cAMP of calcium-activated chloride currents in cultured Sertoli cells from immature testis. J Membr Biol 169: 167-174. doi: 10.1007/s002329900528. PubMed: 10354463. [DOI] [PubMed] [Google Scholar]
- 29. Taranta A, Morena AR, Barbacci E, D’Agostino A (1997) ω-Conotoxin-sensitive Ca2+ voltage-gated channels modulate protein secretion in cultured rat Sertoli cells. Mol Cell Endocrinol 126: 117–123. doi: 10.1016/S0303-7207(96)03973-1. PubMed: 9089649. [DOI] [PubMed] [Google Scholar]
- 30. Berridge MJ, Bootman MD, Roderick HL (2003) Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4: 517-529. doi: 10.1038/nrm1155. PubMed: 12838335. [DOI] [PubMed] [Google Scholar]
- 31. Voets T (2000) Dissection of three Ca2+-dependent steps leading to secretion in chromaffin cells from mouse adrenal slices. Neuron 28: 537-545. doi: 10.1016/S0896-6273(00)00131-8. PubMed: 11144362. [DOI] [PubMed] [Google Scholar]
- 32. Fujisawa M (2001) Cell-to-cell cross-talk in the testis. Urol Res 29: 144-151. doi: 10.1007/s002400100179. PubMed: 11482437. [DOI] [PubMed] [Google Scholar]
- 33. Gorczyńska-Fjälling E (2004) The role of calcium in signal transduction processes in Sertoli cells. Reprod Biol 4: 219-241. PubMed: 15592583. [PubMed] [Google Scholar]
- 34. Zanatta AP, Zanatta L, Gonçalves R, Zamoner A, Silva FRMB (2013) Integrin participates in the effect of thyroxine on plasma membrane in immature rat testis. Biochim Biophys Acta Gen Subj 1830: 2629-2637. doi: 10.1016/j.bbagen.2012.10.022. PubMed: 23137442. [DOI] [PubMed] [Google Scholar]
- 35. Farwell AP, Leonard JL (2005) Nongenomic actions of thyroid hormone during fetal brain development. Curr Opin. Endocrinol Metab 12: 17-22. [Google Scholar]
- 36. Dorrington JH, Roller NF, Fritz IB (1975) Effects of follicle-stimulating hormone on cultures of Sertoli cell preparations. Mol Cell Endocrinol 3: 57-70. doi: 10.1016/0303-7207(75)90031-3. PubMed: 168104. [DOI] [PubMed] [Google Scholar]
- 37. Galdieri M, Ziparo E, Palombi F, Russo M, Stefanini M (1981) Pure Sertoli cell cultures: a new model for the study of somatic–germ cell interactions. J Androl 5: 249-254. [Google Scholar]
- 38. Lin HY, Sun M, Tang HY, Lin C, Luidens MK et al. (2009) L-thyroxine vs. 3,5,3’-triiodo-l-thyronine and cell proliferation: activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Am J Physiol Cell Physiol 296: C980-C991. doi: 10.1152/ajpcell.00305.2008. PubMed: 19158403. [DOI] [PubMed] [Google Scholar]
- 39. Zamoner A, Pierozan P, Vidal LF, Lacerda BA, Dos Santos NG et al. (2008) Vimentin phosphorylation as a target of cell signaling mechanisms induced by 1alpha,25-dihydroxyvitamin D3 in immature rat testes. Steroids 73: 1400-1408. doi: 10.1016/j.steroids.2008.07.002. PubMed: 18687349. [DOI] [PubMed] [Google Scholar]
- 40. Batra S, Sjögren C (1983) Effect of estrogen treatment on calcium uptake by the rat uterine smooth muscle. Life Sci 32: 315-319. doi: 10.1016/0024-3205(83)90076-0. PubMed: 6827896. [DOI] [PubMed] [Google Scholar]
- 41. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193: 265-267. PubMed: 14907713. [PubMed] [Google Scholar]
- 42. Menegaz D, Barrientos-Duran A, Kline A, Silva FRMB, Norman AW et al. (2010) 1α,25(OH)2-Vitamin D3 stimulation of secretion via chloride channel activation in Sertoli cells. J Steroid Biochem Mol Biol 74: 264-269. [DOI] [PubMed] [Google Scholar]
- 43. Yen M (2001) Physiological and molecular basis of thyroid hormone action. Physiol Rev 81: 1097-1142. PubMed: 11427693. [DOI] [PubMed] [Google Scholar]
- 44. St Germain DL, Galton VA, Hernandez A (2009) Defining the role of the iodothyronine deiodinases: current concepts and challenges. Endocrinology 150: 1097-1107. PubMed: 19179439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cheng SY, Leonard JL, Davis PJ (2010) Molecular aspects of thyroid hormone actions. Endocrinol Rev 31: 139-170. doi: 10.1210/er.2009-0007. PubMed: 20051527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zamoner A, Pessoa-Pureur R, Silva FR (2011) Membrane-initiated actions of thyroid hormones on the male reproductive system. Life Sci 89: 507-514. doi: 10.1016/j.lfs.2011.04.006. PubMed: 21557952. [DOI] [PubMed] [Google Scholar]
- 47. Anguiano B, Aranda N, Delgado G, Aceves C (2008) Epididymis expresses the highest 5-deiodinase activity in the male reproductive system: kinetic characterization, distribution, and hormonal regulation. Endocrinology 149: 4209-4217. doi: 10.1210/en.2007-1679. PubMed: 18467445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Köhrle J (1999) Local activation and inactivation of thyroid hormones: the deiodinase family. Mol Cell Endocrinol 151: 103-119. doi: 10.1016/S0303-7207(99)00040-4. PubMed: 10411325. [DOI] [PubMed] [Google Scholar]
- 49. Van der Geyten S, Van den Eynde I, Segers IB, Kühn ER, Darras VM (2002) Diferential expression of iodothyronine deiodinases in chicken tissues during the last week of embryonic development. Gen Comp Endocrinol 128: 65-73. doi: 10.1016/S0016-6480(02)00065-5. PubMed: 12270789. [DOI] [PubMed] [Google Scholar]
- 50. Bergh JJ, Lin HY, Lansing L, Mohamed SN, Davis FB et al. (2005) Integrin αvβ3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology 146: 2864-2871. doi: 10.1210/en.2005-0102. PubMed: 15802494. [DOI] [PubMed] [Google Scholar]
- 51. Plow EF, Haas TA, Zhang L, Loftus J, Smith JW (2000) Ligand binding to integrins. J Biol Chem 275: 21785-21788. doi: 10.1074/jbc.R000003200. PubMed: 10801897. [DOI] [PubMed] [Google Scholar]
- 52. Tsou R, Isik FF (2001) Integrin activation is required for VEGF and FGF receptor protein presence on human microvascular endothelial cells. Mol Cell Biochem 224: 81-89. doi: 10.1023/A:1011947301849. PubMed: 11693202. [DOI] [PubMed] [Google Scholar]
- 53. Vinatier D (1995) Integrins and reproduction. Eur J Obstet Gynecol Reprod Biol 59: 71-81. doi: 10.1016/0028-2243(94)01987-I. PubMed: 7781865. [DOI] [PubMed] [Google Scholar]
- 54. Siu MK, Cheng CY (2004) Interactions of proteases, protease inhibitors, and the b1 integrin/laminin protein complex in the regulation of ectoplasmic specialization dynamics in the testis. Biol Reprod 70: 945–964. PubMed: 14645107. [DOI] [PubMed] [Google Scholar]
- 55. Lee JH, Kim JU, Kim C, Min CK (2010) Inhibitory actions of mibefradil on steroidogenesis in mouse Leydig cells: involvement of Ca2+ entry via the T-type Ca2+ channel. Asian J Androl 12: 807-813. doi: 10.1038/aja.2010.51. PubMed: 20694017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zamoner A, Royer C, Barreto KP, Pessoa-Pureur R, Silva FR (2007) Ionic involvement and kinase activity on the mechanism of nongenomic action of thyroid hormones on 45 Ca2+ uptake in cerebral cortex from young rats. Neurosci Res 57: 98-103. doi: 10.1016/j.neures.2006.09.012. PubMed: 17067709. [DOI] [PubMed] [Google Scholar]
- 57. Chemin J, Traboulsie A, Lory P (2006) Molecular pathways underlying the modulation of T-type calcium channels by neurotransmitters and hormones. Cell Calcium 40: 121-134. doi: 10.1016/j.ceca.2006.04.015. PubMed: 16797700. [DOI] [PubMed] [Google Scholar]
- 58. Iftinca MC (2011) Neuronal T-type calcium channels: what's new? J Med Life 4: 126-138. PubMed: 21776294. [PMC free article] [PubMed] [Google Scholar]
- 59. Zanatta L, Goulart PB, Gonçalves R, Pierozan P, Winkelmann-Duarte EC et al. (2012) 1α,25-Dihydroxyvitamin D(3) mechanism of action: Modulation of L-type calcium channels leading to calcium uptake and intermediate filament phosphorylation in cerebral cortex of young rats. Biochim Biophys Acta 1823: 1708-1719. doi: 10.1016/j.bbamcr.2012.06.023. PubMed: 22743040. [DOI] [PubMed] [Google Scholar]
- 60. Costa RR, dos Reis RI, Aguiar JF, Varanda WA (2011) Luteinizing hormone (LH) acts through PKA and PKC to modulate T-type calcium currents and intracellular calcium transients in mice Leydig cells. Cell Calcium 49: 191-199. doi: 10.1016/j.ceca.2011.02.003. PubMed: 21367452. [DOI] [PubMed] [Google Scholar]
- 61. Schönwasser DC, Marais RM, Marshall CJ, Parker PJ (1998) Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol 18: 790-798. PubMed: 9447975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Weiss N, Hameed S, Fernández-Fernández JM, Fablet K, Karmazinova M et al. (2012) Ca(v)3.2/syntaxin-1A signaling complex controls T-type channel activity and low-threshold exocytosis. J Biol Chem 287: 2810-2818. doi: 10.1074/jbc.M111.290882. PubMed: 22130660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mahapatra S, Calorio C, Vandael DH, Marcantoni A, Carabelli V et al. (2012) Calcium channel types contributing to chromaffin cell excitability, exocytosis and endocytosis. Cell Calcium 51: 321-330. doi: 10.1016/j.ceca.2012.01.005. PubMed: 22317919. [DOI] [PubMed] [Google Scholar]
- 64. Linnertz R, Wurm A, Pannicke T, Krügel K, Hollborn M et al. (2011) Activation of voltage-gated Na+ and Ca2+ channels is required for glutamate release from retinal glial cells implicated in cell volume regulation. Neuroscience 188: 23-34. doi: 10.1016/j.neuroscience.2011.04.058. PubMed: 21575684. [DOI] [PubMed] [Google Scholar]