Abstract
Background
Severe malaria risk varies between individuals, and most of this variation remains unexplained. Here, we examined the hypothesis that cytokine profiles at birth reflect inter-individual differences that persist and influence malaria parasite density and disease severity throughout early childhood.
Methods and Findings
Cytokine levels (TNF-α, IFN-γ, IL-1β, IL-4, IL-5, IL-6 and IL-10) were measured at birth (cord blood; N=783) and during subsequent routine follow-up visits (peripheral blood) for children enrolled between 2002 and 2006 into a birth cohort in Muheza, Tanzania. Children underwent blood smear and clinical assessments every 2-4 weeks, and at the time of any illness. Cord blood levels of all cytokines were positively correlated with each other (Spearman’s rank correlation). Cord levels of IL-1β and TNF-α (but not other cytokines) correlated with levels of the same cytokine measured at routine visits during early life (P < 0.05). Higher cord levels of IL-1β but not TNF-α were associated with lower parasite densities during infancy (P=0.003; Generalized Estimating Equation (GEE) method), with an average ~40% reduction versus children with low cord IL-1β levels, and with decreased risk of severe malaria during follow-up (Cox regression): adjusted hazard ratio (95% CI) 0.60 (0.39-0.92), P = 0.02.
Conclusion
IL-1β levels at birth are related to future IL-1β levels as well as the risk of severe malaria in early life. The effect on severe malaria risk may be due in part to the effect of inflammatory cytokines to control parasite density.
Introduction
Despite renewed efforts at control and elimination, malaria remains a major cause of morbidity and mortality in Africa, where 174 million clinical cases occur annually, resulting in an estimated 596,000 deaths [1]. Although factors such as sickle cell trait are known to influence malaria severity [2], most of the variation in risk between individuals remains unexplained [3]. Human genetics [4], parasite virulence [5], environmental factors [6] and acquired immunity [7] can all contribute to variations in risk.
During infection, cytokines play a dual role by controlling parasite growth on the one hand while exacerbating pathology on the other. These opposing effects have been attributed to the timing of cytokine expression [8] as well as the balance between inflammatory and anti-inflammatory cytokines [9]. For example, the inflammatory cytokines TNF-α and IFN-γ can mediate parasite inhibition and killing [10,11], but high levels of TNF-α have also been associated with severe malaria syndromes such as cerebral malaria [12,13]. Meanwhile, high levels of the anti-inflammatory cytokine IL-10, or high IL-10/TNF-α ratios, reduce the risk of severe malarial anemia [14,15], despite being associated with reduced parasite clearance in children with uncomplicated malaria [16].
Cytokine levels at birth might reflect inter-individual differences that persist and influence malaria outcomes during childhood. In malaria endemic areas, fetal sensitization to malaria antigens is common: cord blood lymphocytes often respond to stimulation with malaria antigens by proliferating [17,18] and producing type 1 and/or type 2 cytokine responses [19]. Some newborns of infected mothers display a “tolerant” phenotype (i.e. PBMC non-responsive to malaria antigens), and have an increased risk of infection and lower hemoglobin levels during early life [20].
To test the hypothesis that in utero immune profiles will persist during early childhood and influence malaria outcomes, we measured plasma cytokine levels at birth and analysed their relationship with cytokine levels and the risk of severe P. falciparum malaria during early life. We report for the first time that high levels of IL-1β in cord blood persist, and are associated with both improved control of parasite density and with decreased risk of severe malaria during infancy.
Materials and Methods
Study population and clinical procedures
Mothers and newborns were enrolled in a birth cohort study known locally as the Mother-Offspring Malaria Studies (MOMS) Project at Muheza Designated District Hospital, Muheza, Tanzania. Clinical procedures for the MOMS Project have been previously described [21]. Children whose data are reported in this study were enrolled between September 2002 and May 2006, and were followed for up to 4 years. Twins, stillbirths, early neonatal deaths, infants with any evidence of HIV infection (mother seropositive on voluntary testing, infant presented with suggestive signs or symptoms or suffered HIV/AIDS-related death during follow-up) or sickle-cell disease were excluded. Of the 882 children who remained after exclusions, 783 had cord blood cytokines measured and were thus included in this analysis.
Children were examined and blood smears obtained by finger or heel prick every 2 weeks during infancy and every 4 weeks post-infancy. Peripheral blood was collected into CPD anticoagulant during routine visits at roughly 3 months of age and then at ~6 month age intervals thereafter. Whenever children developed symptoms, they were examined by a study clinician. Children were classified as having severe malaria according to WHO criteria [22].
Ethics
Written informed consent was obtained from mothers prior to enrollment. Protocols for procedures used in this study were approved by the International Clinical Studies Review Committee of the Division of Microbiology and Infectious Diseases at the US National Institutes of Health, and ethical clearance was obtained from the Institutional Review Boards of Seattle BioMed and the National Medical Research Coordinating Committee in Tanzania.
Laboratory procedures
Cord blood samples were obtained by clamping the cord and cannulating umbilical vessels immediately after delivery. After removal of the umbilical cord and fetal membranes, placental blood samples were obtained by manual compression of the placental tissue in a grinder. Placental and cord blood samples were anticoagulated with EDTA, and stored on ice until processing the same day.
Malaria parasitemia diagnosis: Parasitemia was defined as identification of any parasites in a Giemsa-stained blood smear by microscopy, after counting at least 200 white blood cells at a magnification of 100x.
Determination of red blood cell disorders: Hemoglobin type (HbAA, HbAS and HbSS) was determined by cellulose acetate paper electrophoresis according to the manufacturer’s instructions (Helena Laboratories, Beaumont, Texas, USA). Genotyping for α-thalassemia was done according to the protocol described by Chong et al [23].
Cytokine assays: Plasma was obtained by centrifugation at 3,000 * g for 3 minutes and was stored frozen at -70°C until it was thawed on the day that cytokine assays were performed. Each plasma sample was analysed using a multiplex, bead-based platform (BioPlex; Bio-Rad, Irvine, CA) and custom-made assay kits as previously described [24]. For each plasma sample, all analytes were assayed in a single day, thus eliminating freeze-thaw cycles. All pipetting and sample identification were performed with a bar code-enabled, high-speed pipetting robot (Megaflex; Tecan, Research Triangle Park, NC). The detection limits for the different analytes were as follows: TNF-α, 0.10 pg/ml; IFN-γ, 0.04 pg/ml; IL-1β, 0.01 pg/ml; IL-4, 0.3 pg/ml; IL-5, 0.02 pg/ml; IL-6, 1.45 pg/ml; IL-10, 0.02 pg/ml. Cytokine levels were adjusted to account for dilution in anticoagulant at the time of sample collection.
Statistical analysis
Non-parametric tests (Mann-Whitney and Kruskal Wallis tests) were used to compare cord blood cytokine levels by baseline variables (gender, parity, placental malaria status, birth season, sickle cell and alpha-thalassemia genotypes). Malaria transmission season was defined as high between May and October, based on the peak incidence of parasitemia observed in our cohort. Correlations between cytokines at birth and at routine uninfected healthy visits were evaluated by Spearman’s rank correlation coefficient. Bonferroni correction was used in the pairwise correlations between cytokines at birth.
Cox regression models were fitted to evaluate the relationship between cord blood cytokine levels and the time to first episode of severe malaria. Schoenfeld residuals were used to test the proportional hazards assumption. Kaplan-Meier curves were used to display severe malaria rates in children with high versus low levels of cord cytokines IL-1β and TNF-α. To account for the correlation between visits of the same child, the association between cord blood cytokines and parasite density (log-transformed) was assessed by Generalized Estimating Equation (GEE) method. In this analysis, each positive blood smear was included as an observation. Exchangeable correlation structure and identity link function were used; and robust standard errors were estimated. Cytokine levels were included in Cox and GEE models as binary variables, determined by the median: values above the median were considered high and values below the median, low. Median values (in pg/ml) for cord blood cytokines were: TNF-α, 120.8; IL-1β, 6.0; IL-5, 2.6; IL-6, 7.0; IL-10, 3.5. Models were adjusted for sickle cell trait status, alpha-thalassemia, transmission season, bed net use, and village of residence. Since parity and placental malaria interact to influence malaria infection and clinical malaria risk [21,25], these variables were included with interaction term.
Average parasite densities during infections in infants, children aged 1 year or less, were estimated for children with high and low cord IL-1β levels: geometric mean parasite density during visits with infection was calculated for each child; the geometric mean of these values was then estimated for groups with different IL-1β levels at birth (Figure 1).
Figure 1. Average parasite densities in children with high and low cord IL-1β levels.
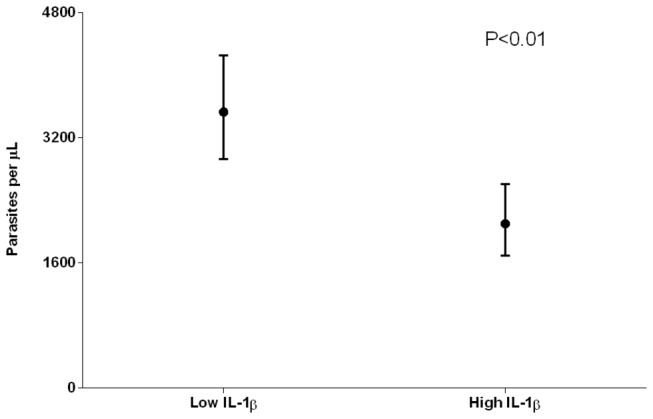
Only infections occurring in the first year of life were included in this analysis, since the association between levels of this cytokine at birth and subsequent parasite levels was only present during infancy (GEE model). (N=504, children with at least one infection during infancy) Concentrations of parasites per μL were estimated by assuming 8000 leukocytes/μL of blood. Children were defined as having high cord IL-1β levels if their IL-1β levels at birth were higher than the median in the study population (6 pg/ml); if IL-1β levels at birth were lower than the median value, these levels were considered low.
Data analyses were conducted using STATA version 11.1 (Stata Corporation, College Station, Texas, United States)
Results
Description of the study cohort
105 (13.4%) newborns were delivered by mothers with placental malaria (PM+), and this was most common in first and second time mothers (19.3%, 18.5% and 7.6% in primiparae, secundiparae and multiparae, respectively; P < 0.001) (Table 1 ). Mean birth weight was lower in offspring of PM+ versus PM- women in all parity groups (P < 0.05 for all groups) [26]. 61.5% of children used bed nets. Sickle cell trait was frequent in this population (16.6%).
Table 1. Demographic characteristics of the cohort.
| Number (%) | |
|---|---|
| Parity | |
| Primiparae | 218 (27.8) |
| Secundiparae | 184 (23.5) |
| Multiparae | 381 (48.7) |
| Gender | |
| Female | 379 (48.4) |
| Male | 404 (51.6) |
| Placental Malaria (PM) status | |
| PM+ | 105 (13.4) |
| PM- | 678 (86.6) |
| Birth season | |
| High | 367 (46.9) |
| Low | 416 (53.1) |
| Residence | |
| Bwembwera | 132 (16.9) |
| Magilla | 130 (16.6) |
| Mkanyageni | 169 (21.6) |
| Muheza township | 352 (45.0) |
| Median (Q1-Q3) | |
| Follow-up duration (in years) | 2.15 (1.22-2.98) |
| Mean (SD) | |
| Number of visits per child | 44.6 (19.6) |
Cord blood cytokine levels vary in relation to in utero factors
Cord blood levels of some cytokines differed according to malaria transmission season, placental malaria status and maternal parity (Table 2 ). Cord blood IFN-γ was more often detected during high versus low malaria transmission season among primi- (P=0.06) and multiparae (P<0.001), but not secundiparae (P=0.58). Placental malaria and birth during high transmission season were associated with higher cord blood levels of IL-10 (P=0.04 and P=0.05, respectively), a cytokine that is also elevated in maternal samples during episodes of inflammatory placental malaria [27,28]. Cord blood levels of IL-10 were also significantly higher in primiparae than other groups (P=0.003). Cord IL-4 was more frequently detected in children born during high transmission season (P=0.002). Sickle cell trait and alpha-thalassemia in the children did not influence levels of cord blood cytokines.
Table 2. Cord cytokine levels stratified by parity, transmission season and placental malaria status (Median [Q1-Q3]).
|
Parity
|
Transmission Season
|
Placental Malaria
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primiparae | Secundiparae | Multiparae | p-value | High | Low | p-value | PM + | PM - | p-value | |||
| TNF-α | 118.7 (70.5 - 180.9) | 132.7 (72.2 - 201.9) | 116.5 (68.1 - 173.8) | 0.15 | 122.8 (72.1–192.7) | 116.9 (64.5 - 173.5) | 0.31 | 128.9 (79.7 - 191) | 119.6 (68.2 - 180.7) | 0.29 | ||
| IL-1β | 5.8 (2.9 - 10.7) | 6.4 (3.0 - 11.8) | 5.9 (3 - 11.9) | 0.8 | 5.8 (3 - 11.1) | 6.3 (2.9 - 11.9) | 0.84 | 5.8 (3.1 - 9.9) | 6.1 (3 - 11.9) | 0.5 | ||
| IL-4 * | 12.4 | 9.8 | 10.5 | 0.67 | 14.4 | 7.7 | 0.002 | 10.5 | 10.9 | 0.89 | ||
| IL-5 | 2.4 (0.7 - 4.9) | 2.7 (1 - 5.2) | 2.7 (1.1 - 5.3) | 0.35 | 2.9 (0.7 - 5.4) | 2.4 (1 - 5.2) | 0.45 | 2.5 (0.4 - 6) | 2.6 (1 - 5.2) | 0.39 | ||
| IL-6 | 7.3 (2.3 - 17.5) | 5.6 (1.3 - 15.3) | 7.7 (2.4 -20.9) | 0.13 | 6.9 (2.3 -22.3) | 7 (1.9 - 16.2) | 0.44 | 5.6 (1.2 - 12.8) | 7.1 ( 2.3 - 19.1) | 0.14 | ||
| IL-10 | 4 (2.1 - 6.9) | 2.9 (1.3 - 5.5) | 3.3 (1.3 - 5.8) | 0.003 | 3.6 (1.6 - 6.5) | 3.2 (1.5 - 5.3) | 0.05 | 3.9 (1.9 - 6.9) | 3.3 (1.5 - 5.9) | 0.04 | ||
| IFN-γ * | 22.9 | 21.7 | 18.6 | 0.41 | 25.6 | 16.1 | 0.001 | 20.5 | 20.9 | 0.91 | ||
Cytokine levels are presented as pg/ml.
Percentage with detectable cytokine
Pro- and anti-inflammatory cytokines in cord blood are positively correlated
Pro-inflammatory and anti-inflammatory cytokines can be counter-regulatory. We analysed pairwise relationships between and within these two groups of cytokines at birth (Table 3 ). The pro-inflammatory cytokines TNF-α and IL-1β were highly correlated and both were correlated to IL-4 and IL-10 levels, which suggests that cytokines in cord blood were most likely influenced by a common process (eg, inflammation) rather than factors that alter expression of specific cytokines (eg, single nucleotide polymorphisms).
Table 3. Correlation between cord cytokine levels (Spearman’s rank correlation).
| TNF-α | IL-1β | IL-4 | IL-5 | IL-6 | IL-10 | IFN-γ | |
|---|---|---|---|---|---|---|---|
| TNF-α | |||||||
| IL-1β | 0.64 | ||||||
| IL-4 | 0.22 | 0.23 | |||||
| IL-5 | 0.4 | 0.34 | 0.17 | ||||
| IL-6 | 0.14 | 0.37 | 0.13 | 0.15 | |||
| IL-10 | 0.32 | 0.34 | 0.23 | 0.27 | 0.45 | ||
| IFN-γ | 0.35 | 0.3 | 0.34 | 0.25 | 0.16 | 0.34 |
All correlations had Bonferroni-adjusted p-values<0.001, except correlations between IL-6 and TNF-α (P=0.002) and between IL-6 and IL-4 (P=0.003)
IL-1β and TNF-α levels at birth correlate with levels during early childhood
We examined the relationship between cord levels of cytokines at birth, and levels measured in peripheral blood of these children during follow-up (Table 4 ). We included only measurements made at visits when the child was healthy and aparasitemic (N = 1,359 visits). IL-4 and IFN-γ levels were often undetectable, and therefore we used logistic regression to analyse these cytokines as binary variables (detectable vs. undetectable). Cord levels of TNF-α and IL-1β correlated significantly to levels measured later in life: TNF-α at birth correlated to levels measured throughout childhood, while IL-1β correlated to levels measured during the first year of life but not thereafter. Cord levels of other cytokines measured in this study did not correlate with peripheral blood levels throughout infancy or early childhood.
Table 4. Correlation between birth levels and early childhood levels of cytokines.
| Age (in weeks) | TNF-α | IL-1β | IL-5 | Il-6 | IL-10 | Number of Children |
|---|---|---|---|---|---|---|
| < 12 | 0.27* | 0.16* | 0.1 | 0.01 | 0 | 162 |
| 12-24 | 0.18* | 0.14* | 0.06 | 0.07 | 0.04 | 287 |
| 24-48 | 0.30* | 0.21* | 0.05 | -0.01 | 0.04 | 229 |
| 48-76 | 0.14* | 0.07 | 0.05 | 0.05 | 0.05 | 225 |
| 76-100 | 0.18* | -0.04 | -0.09 | 0.07 | 0.08 | 182 |
| 100-124 | 0.23* | 0.07 | 0.1 | -0.07 | -0.01 | 146 |
| 124-148 | 0.28* | 0.02 | 0.01 | 0.05 | -0.02 | 88 |
Correlation coefficients (Spearman’s rank correlation) at different age intervals are presented. IL-4 or IFN-γ were only detected in a minority of samples, and were therefore analyzed by logistic regression to assess whether cytokine positivity at birth predicted cytokine positivity during childhood. Detectable cord levels of IL-4 were associated with detectable IL-4 in samples collected before 12 weeks of age (odds ratio 2.46 95%CI [0.93 - 6.52], P=0.07). Detectable IFN-γ levels at birth were associated with IFN- γ detection in the first 12 weeks of life (odds ratio 2.26 95%CI [1.08 - 4.72], P=0.03), and with IFN- γ negativity between weeks 124 and 148 (odds ratio 0.20 95%CI [0.06 - 0.68], P=0.01).
P<0.05
High IL-1β levels at birth predict reduced parasite densities and severe malaria risk in infancy
During a malaria infection, pro-inflammatory cytokines are rapidly released from innate and adaptive immune cells, and may contribute to control of parasite density [29]. We analysed the relationship between cord levels of the cytokines IL-1β and TNF-α, and parasite densities during subsequent infections. High levels of IL-1β in cord blood were related to lower parasite levels during infections in infants (P=0.003, GEE model [Table 5]), equal to ~40% reduction in average parasite density (geometric mean parasite density in children with high cord IL-1β 2100 95% CI [1692 - 2607] versus children with low cord IL-1β 3528 95%CI [2927 - 4253] parasites per μL; Figure 1). Cord TNF-α levels did not predict parasite densities during subsequent infections.
Table 5. Cox model on time to first severe malaria episode and GEE model on parasite density.
| Severe Malaria | Parasite density | |
|---|---|---|
| Cytokine | Cox Model* | GEE model (< 1st year)¤ |
| IL-1β | 0.60 (0.39-0.92) P=0.02 | -0.16 (-0.26 -0.05) P=0.003 |
| TNF-α | 0.68 (0.45-1.03) P=0.07 | -0.09 (-0.20 - 0.01) P=0.09 |
For Cox regression results, hazard ratios adjusted for factors that might influence severe malaria risk (sickle cell trait status, alpha-thalassemia, transmission season, parity vs. placental malaria, bed net use and village of residence) are shown. Regression coefficients are presented for GEE models that assess the influence of IL-1β or TNF-α on parasite density
Hazard ratio
Regression coefficients
Finally, we analysed the influence of cord blood TNF-α and IL-1β levels on severe malaria risk. Kaplan-Meier curves (Figure 2) indicate that children with high cord levels of IL-1β (but not TNF-α) are protected against severe malaria. In Cox regression analyses, high levels of IL-1β at birth decreased the risk of first severe malaria episode (hazard ratio (95% CI, P-value) of 0.60 (0.39 - 0.92, P = 0.02)) (Table 5). In a multivariate Cox model that included all cord blood cytokines as well as other baseline covariates, only IL-1β levels at birth had a significant effect on the time to first severe malaria event, reducing the risk of severe malaria episode by 42% (hazard ratio (95% CI, P-value) 0.58 (0.35 - 0.97 , P=0.04)).
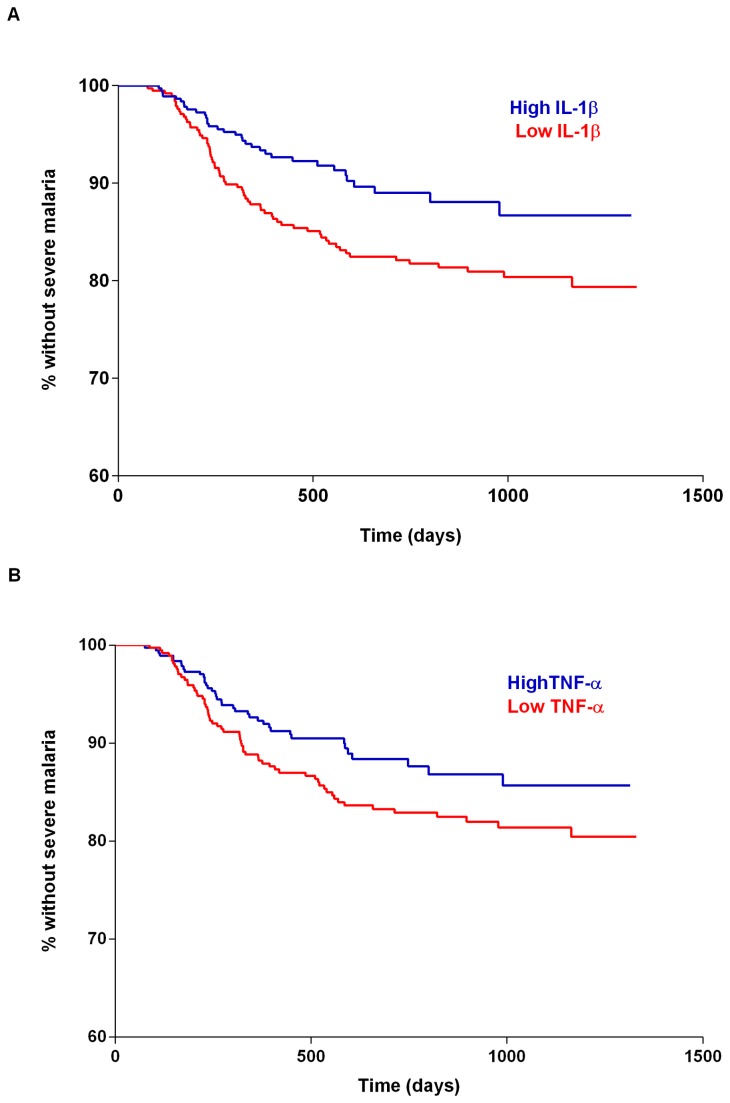
Figure 2. Kaplan-Meier curves for the risk of severe malaria.
(a) High cord levels of IL-1β were associated with longer time to first severe malaria episode (P=0.008, log-rank test); (b) High levels of TNF-α at birth were marginally associated with a longer time to first severe malaria episode (P=0.08, log-rank test). High levels of cord IL-1β and TNF-α were defined based on median values (TNF-α 120.8 pg/ml; IL-1β 6 pg/ml).
Discussion
In this study, we investigated whether cord blood cytokines of children living in a malaria endemic area are related to cytokine levels during early life and whether they predict severe malaria risk. Our results show for the first time that higher levels of the pro-inflammatory cytokine IL-1β at birth are significantly associated with improved control of parasite density and with decreased risk of severe malaria during early life. These findings support the idea that in utero sensitization or constitutive expression levels of cytokines, reflected by cord blood cytokine levels, contributes to the risk of severe malaria during childhood.
Previous studies have shown that cord blood cytokine responses might influence the risk of common childhood diseases. In the US, greater IFN-γ secretion by cord blood mononuclear cells (CBMC) reduces the risk of acute lower respiratory illness in the first year of life [30]. In a prospective birth cohort study in Kenya, children of malaria-infected women whose CBMC did not produce cytokines (IFN-γ, IL-2, IL-13, and/or IL-5) in response to blood stage malaria antigens were at increased risk of infection and had lower hemoglobin levels during childhood [20]. The effect of specific cytokines or immune responses on malaria severity has not previously been assessed.
In our birth cohort, infants with high levels of TNF-α and IL-1β at birth persist in this pattern during infancy, and these intrinsically higher levels might act early during an infection to control parasite density. TNF-α is a major effector cytokine and is implicated in both protection and pathogenicity during malaria infection. Several studies have observed that plasma levels of TNF–α are significantly higher in infected humans presenting with severe malaria [12,13]. In our study, high cord levels of TNF-α were associated with decreased risk of severe malaria, although this association was only marginally significant. The protective role of TNF-α is suggested by animal studies in which malaria-resistant C57BL/6 mice had higher levels of TNF-α mRNA in the spleen and liver during the early phase of infection, which enhanced clearance of infection [31]. In humans, TNF-α production during the acute phase of malaria similarly predicts a more rapid clinical and parasitological cure [32,33]. In a prospective study from Papua New Guinea, children with increased TNF-α levels during a P. falciparum infection were at lower risk of subsequent P. falciparum clinical episodes [34], which is consistent with more rapid clearance of parasites. In Gabon, children with a history of severe malaria had fewer T cells producing TNF-α in response to parasite antigen than children with a history of only mild malaria [35].
IL-1 acts synergistically with TNF-α to enhance NO and IFN-γ production in murine models of malaria [36]. PBMC IFN-γ production in response to malaria antigens has been associated with protection against reinfection in children with mild malaria [37], and NO has a direct parasite killing effect [38]. IL-1 also inhibits the intra-hepatocytic development of the rodent malaria parasite P. yoelii, an effect partly mediated by IL-6 secretion [39], and controls blood stage parasitemia in mice infected with P. berghei [40]. Our finding that high levels of cord blood IL-1β reduce the risk of severe malaria is also consistent with a study showing that IL-1β promoter haplotype -31C/-511A is associated with decreased production of IL-1 and increased risk of severe malarial anaemia in Kenyan children [41]. In our cohort, the protection associated with cord IL-1β against both parasite density and SM during infancy (reductions of ~40% and 40%, respectively) was similar in degree to the independent effects of HbAS throughout early childhood (reductions of 42% and 43%). Future studies should assess the contribution of polymorphisms affecting the inflammasome, a multi-protein complex responsible for processing and secretion of IL-1β: mutations that enhance inflammasome activity might result in increased levels of IL-1β at birth and afterwards.
Malaria infection during pregnancy modifies the risk of malaria infection and disease for the offspring [25,42]. In Tanzania, we previously observed that offspring of infected multiparae but not primiparae have increased risk of malaria infection [21], and more recently that these offspring have an increased risk of severe malaria (manuscript submitted). In Gabon, Schwarz et al found that the risk of clinical malaria is higher in children born to multigravidae with placental malaria [25]. We speculated that the effect of pregnancy malaria on malaria outcomes in their offspring might be mediated by altered cytokine responses in offspring that would be evident at birth. However our analyses do not identify a relationship of placental malaria at delivery to cord levels of IL-1β. Further research is warranted to identify the mechanisms by which maternal malaria modifies the risk of childhood malaria in offspring.
Future studies should also determine the mechanisms that control or modify cord blood cytokine profiles. Other maternal infections endemic in the study area such as lymphatic filariasis could be modifying cord blood cytokine production, but were not studied in this cohort. Alternatively, children may have intrinsic differences in cytokine expression, owing to, for example, genetic polymorphisms or in utero imprinting, and these differences could influence parasite density and severe malaria risk during early life. We have found that all cytokines tested are positively correlated at birth, suggesting that cytokine levels in cord blood might have been influenced by a common mechanism (such as inflammation) more so than by individual genetic polymorphisms.
Severe malaria represents a heterogeneous group of clinical presentations, including severe anemia, cerebral malaria, and respiratory distress, among others. These different syndromes might be associated with specific immune responses: for example, children with severe anemia have lower IL-10 levels compared to children with cerebral malaria [14,43]. Future studies with sufficient sample sizes should assess whether elevated IL-1β levels at birth reduce risk of all severe malaria symptoms equally or only of specific syndromes, and also whether a threshold level of IL-1β is required for the protective effect.
In conclusion, IL-1β levels in cord blood predict IL-1β levels, parasite density, and severe malaria risk throughout infancy. Therefore, factors that influence the cytokine profile or pro-inflammatory bias of the unborn child may have a significant impact on the outcome of malaria infections throughout early life. Placental malaria also increases parasite density and severe malaria risk in offspring, but it does not influence cord cytokine levels and therefore acts independently of the cord cytokine effect. Further study is needed to identify the factors that influence the fetal cytokine profile, as well as interventions that might target these factors to improve malaria outcomes during early childhood.
Acknowledgments
We thank the mothers and their children in Muheza District for participation in the studies. Theonest Mutabingwa led the clinical team that managed the care and data collection for study participants.
Funding Statement
This work was supported by the Division of Intramural Research (DIR) and the extramural program (grant R01AI52059) of NIAID, NIH; the Bill & Melinda Gates Foundation (grant 29202); the Foundation for the National Institutes of Health through the Grand Challenges in Global Health Initiative (grant 1364); the US National Institutes of Health Fogarty International Center (FIC) (grant D43 TW005509). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.(2012) World Malaria Report. Geneva: World Health Organization. [Google Scholar]
- 2. Williams TN, Mwangi TW, Wambua S, Alexander ND, Kortok M et al. (2005) Sickle cell trait and the risk of Plasmodium falciparum malaria and other childhood diseases. J Infect Dis 192: 178-186. doi: 10.1086/430744. PubMed: 15942909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mackinnon MJ, Mwangi TW, Snow RW, Marsh K, Williams TN (2005) Heritability of malaria in Africa. PLOS Med 2: e340. doi: 10.1371/journal.pmed.0020340. PubMed: 16259530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fortin A, Stevenson MM, Gros P (2002) Susceptibility to malaria as a complex trait: big pressure from a tiny creature. Hum Mol Genet 11: 2469-2478. doi: 10.1093/hmg/11.20.2469. PubMed: 12351583. [DOI] [PubMed] [Google Scholar]
- 5. Gupta S, Hill AV, Kwiatkowski D, Greenwood AM, Greenwood BM et al. (1994) Parasite virulence and disease patterns in Plasmodium falciparum malaria. Proc Natl Acad Sci U S A 91: 3715-3719. doi: 10.1073/pnas.91.9.3715. PubMed: 8170975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Al-Taiar A, Assabri A, Al-Habori M, Azazy A, Algabri A et al. (2009) Socioeconomic and environmental factors important for acquiring non-severe malaria in children in Yemen: a case-control study. Trans R Soc Trop Med Hyg 103: 72-78. doi: 10.1016/j.trstmh.2008.09.010. PubMed: 18950826. [DOI] [PubMed] [Google Scholar]
- 7. Doolan DL, Dobaño C, Baird JK (2009) Acquired immunity to malaria. Clin Microbiol Rev 22: 13-36. doi: 10.1128/CMR.00025-08. PubMed: 19136431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Angulo I, Fresno M (2002) Cytokines in the pathogenesis of and protection against malaria. Clin Diagn Lab Immunol 9: 1145-1152. PubMed: 12414742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dodoo D, Omer FM, Todd J, Akanmori BD, Koram KA et al. (2002) Absolute levels and ratios of proinflammatory and anti-inflammatory cytokine production in vitro predict clinical immunity to Plasmodium falciparum malaria. J Infect Dis 185: 971-979. doi: 10.1086/339408. PubMed: 11920322. [DOI] [PubMed] [Google Scholar]
- 10. Rockett KA, Awburn MM, Aggarwal BB, Cowden WB, Clark IA (1992) In vivo induction of nitrite and nitrate by tumor necrosis factor, lymphotoxin, and interleukin-1: possible roles in malaria. Infect Immun 60: 3725-3730. PubMed: 1500182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bouharoun-Tayoun H, Oeuvray C, Lunel F, Druilhe P (1995) Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J Exp Med 182: 409-418. doi: 10.1084/jem.182.2.409. PubMed: 7629503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grau GE, Taylor TE, Molyneux ME, Wirima JJ, Vassalli P et al. (1989) Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med 320: 1586-1591. doi: 10.1056/NEJM198906153202404. PubMed: 2657427. [DOI] [PubMed] [Google Scholar]
- 13. Kwiatkowski D, Hill AV, Sambou I, Twumasi P, Castracane J et al. (1990) TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet 336: 1201-1204. doi: 10.1016/0140-6736(90)92827-5. PubMed: 1978068. [DOI] [PubMed] [Google Scholar]
- 14. Kurtzhals JA, Adabayeri V, Goka BQ, Akanmori BD, Oliver-Commey JO et al. (1998) Low plasma concentrations of interleukin 10 in severe malarial anaemia compared with cerebral and uncomplicated malaria. Lancet 351: 1768-1772. doi: 10.1016/S0140-6736(97)09439-7. PubMed: 9635949. [DOI] [PubMed] [Google Scholar]
- 15. Othoro C, Lal AA, Nahlen B, Koech D, Orago AS et al. (1999) A low interleukin-10 tumor necrosis factor-alpha ratio is associated with malaria anemia in children residing in a holoendemic malaria region in western Kenya. J Infect Dis 179: 279-282. doi: 10.1086/314548. PubMed: 9841855. [DOI] [PubMed] [Google Scholar]
- 16. Hugosson E, Montgomery SM, Premji Z, Troye-Blomberg M, Björkman A (2004) Higher IL-10 levels are associated with less effective clearance of Plasmodium falciparum parasites. Parasite Immunol 26: 111-117. doi: 10.1111/j.0141-9838.2004.00678.x. PubMed: 15279621. [DOI] [PubMed] [Google Scholar]
- 17. Fievet N, Ringwald P, Bickii J, Dubois B, Maubert B et al. (1996) Malaria cellular immune responses in neonates from Cameroon. Parasite Immunol 18: 483-490. doi: 10.1046/j.1365-3024.1996.d01-19.x. PubMed: 9226685. [DOI] [PubMed] [Google Scholar]
- 18. King CL, Malhotra I, Wamachi A, Kioko J, Mungai P et al. (2002) Acquired immune responses to Plasmodium falciparum merozoite surface protein-1 in the human fetus. J Immunol 168: 356-364. PubMed: 11751981. [DOI] [PubMed] [Google Scholar]
- 19. Malhotra I, Mungai P, Muchiri E, Ouma J, Sharma S et al. (2005) Distinct Th1- and Th2-Type prenatal cytokine responses to Plasmodium falciparum erythrocyte invasion ligands. Infect Immun 73: 3462-3470. doi: 10.1128/IAI.73.6.3462-3470.2005. PubMed: 15908375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Malhotra I, Dent A, Mungai P, Wamachi A, Ouma JH et al. (2009) Can prenatal malaria exposure produce an immune tolerant phenotype? A prospective birth cohort study in Kenya. PLOS Med 6: e1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mutabingwa TK, Bolla MC, Li JL, Domingo GJ, Li X et al. (2005) Maternal malaria and gravidity interact to modify infant susceptibility to malaria. PLOS Med 2: e407. doi: 10.1371/journal.pmed.0020407. PubMed: 16259531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.(2000) Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Trans R Soc Trop Med Hyg 94 Suppl 1: S1-90. doi: 10.1016/S0035-9203(00)90413-9. PubMed: 11103309. [DOI] [PubMed] [Google Scholar]
- 23. Chong SS, Boehm CD, Higgs DR, Cutting GR (2000) Single-tube multiplex-PCR screen for common deletional determinants of alpha-thalassemia. Blood 95: 360-362. PubMed: 10607725. [PubMed] [Google Scholar]
- 24. Coutinho HM, McGarvey ST, Acosta LP, Manalo DL, Langdon GC et al. (2005) Nutritional status and serum cytokine profiles in children, adolescents, and young adults with Schistosoma japonicum-associated hepatic fibrosis, in Leyte, Philippines. J Infect Dis 192: 528-536. [DOI] [PubMed] [Google Scholar]
- 25. Schwarz NG, Adegnika AA, Breitling LP, Gabor J, Agnandji ST et al. (2008) Placental malaria increases malaria risk in the first 30 months of life. Clin Infect Dis 47: 1017-1025. doi: 10.1086/591968. PubMed: 18781874. [DOI] [PubMed] [Google Scholar]
- 26. Kabyemela ER, Fried M, Kurtis JD, Mutabingwa TK, Duffy PE (2008) Fetal responses during placental malaria modify the risk of low birth weight. Infect Immun 76: 1527-1534. doi: 10.1128/IAI.00964-07. PubMed: 18212078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kabyemela ER, Muehlenbachs A, Fried M, Kurtis JD, Mutabingwa TK et al. (2008) Maternal peripheral blood level of IL-10 as a marker for inflammatory placental malaria. Malar J 7: 26. doi: 10.1186/1475-2875-7-26. PubMed: 18230163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wenisch C, Parschalk B, Narzt E, Looareesuwan S, Graninger W (1995) Elevated serum levels of IL-10 and IFN-gamma in patients with acute Plasmodium falciparum malaria. Clin Immunol Immunopathol 74: 115-117. doi: 10.1006/clin.1995.1017. PubMed: 7994921. [DOI] [PubMed] [Google Scholar]
- 29. Hensmann M, Kwiatkowski D (2001) Cellular basis of early cytokine response to Plasmodium falciparum. Infect Immun 69: 2364-2371. doi: 10.1128/IAI.69.4.2364-2371.2001. PubMed: 11254594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ly NP, Rifas-Shiman SL, Litonjua AA, Tzianabos AO, Schaub B et al. (2007) Cord blood cytokines and acute lower respiratory illnesses in the first year of life. Pediatrics 119: e171-e178. doi: 10.1542/peds.2006-3134. PubMed: 17145902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jacobs P, Radzioch D, Stevenson MM (1996) In vivo regulation of nitric oxide production by tumor necrosis factor alpha and gamma interferon, but not by interleukin-4, during blood stage malaria in mice. Infect Immun 64: 44-49. PubMed: 8557372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kremsner PG, Winkler S, Brandts C, Wildling E, Jenne L et al. (1995) Prediction of accelerated cure in Plasmodium falciparum malaria by the elevated capacity of tumor necrosis factor production. Am J Trop Med Hyg 53: 532-538. PubMed: 7485713. [DOI] [PubMed] [Google Scholar]
- 33. Mordmüller BG, Metzger WG, Juillard P, Brinkman BM, Verweij CL et al. (1997) Tumor necrosis factor in Plasmodium falciparum malaria: high plasma level is associated with fever, but high production capacity is associated with rapid fever clearance. Eur Cytokine Netw 8: 29-35. PubMed: 9110145. [PubMed] [Google Scholar]
- 34. Robinson LJ, D'Ombrain MC, Stanisic DI, Taraika J, Bernard N et al. (2009) Cellular tumor necrosis factor, gamma interferon, and interleukin-6 responses as correlates of immunity and risk of clinical Plasmodium falciparum malaria in children from Papua New Guinea. Infect Immun 77: 3033-3043. doi: 10.1128/IAI.00211-09. PubMed: 19380468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ramharter M, Kremsner PG, Willheim M, Winkler H, Graninger W et al. (2004) Plasmodium falciparum-specific interleukin-2 and tumor necrosis factor-alpha expressing-T cells are associated with resistance to reinfection and severe malaria in healthy African children. Eur Cytokine Netw 15: 189-196. PubMed: 15542442. [PubMed] [Google Scholar]
- 36. Rockett KA, Awburn MM, Rockett EJ, Clark IA (1994) Tumor necrosis factor and interleukin-1 synergy in the context of malaria pathology. Am J Trop Med Hyg 50: 735-742. PubMed: 8024067. [DOI] [PubMed] [Google Scholar]
- 37. Luty AJ, Lell B, Schmidt-Ott R, Lehman LG, Luckner D et al. (1999) Interferon-gamma responses are associated with resistance to reinfection with Plasmodium falciparum in young African children. J Infect Dis 179: 980-988. doi: 10.1086/314689. PubMed: 10068595. [DOI] [PubMed] [Google Scholar]
- 38. Rockett KA, Awburn MM, Cowden WB, Clark IA (1991) Killing of Plasmodium falciparum in vitro by nitric oxide derivatives. Infect Immun 59: 3280-3283. PubMed: 1879941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pied S, Civas A, Berlot-Picard F, Renia L, Miltgen F et al. (1992) IL-6 induced by IL-1 inhibits malaria pre-erythrocytic stages but its secretion is down-regulated by the parasite. J Immunol 148: 197-201. PubMed: 1727866. [PubMed] [Google Scholar]
- 40. Curfs JH, van der Meer JW, Sauerwein RW, Eling WM (1990) Low dosages of interleukin 1 protect mice against lethal cerebral malaria. J Exp Med 172: 1287-1291. doi: 10.1084/jem.172.5.1287. PubMed: 2230643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ouma C, Davenport GC, Awandare GA, Keller CC, Were T et al. (2008) Polymorphic variability in the interleukin (IL)-1beta promoter conditions susceptibility to severe malarial anemia and functional changes in IL-1beta production. J Infect Dis 198: 1219-1226. doi: 10.1086/592055. PubMed: 18781863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Le Hesran JY, Cot M, Personne P, Fievet N, Dubois B et al. (1997) Maternal placental infection with Plasmodium falciparum and malaria morbidity during the first 2 years of life. Am J Epidemiol 146: 826-831. doi: 10.1093/oxfordjournals.aje.a009200. PubMed: 9384203. [DOI] [PubMed] [Google Scholar]
- 43. Thuma PE, van Dijk J, Bucala R, Debebe Z, Nekhai S et al. (2011) Distinct clinical and immunologic profiles in severe malarial anemia and cerebral malaria in Zambia. J Infect Dis 203: 211-219. doi: 10.1093/infdis/jiq041. PubMed: 21288821. [DOI] [PMC free article] [PubMed] [Google Scholar]