Abstract
A cholera epidemic has claimed the lives of more than 8,000 Haitians and sickened 650,000 since the outbreak began in October 2010. Early intervention in the epidemic focused on case-finding, treatment, and water and sanitation interventions for prevention of transmission. Use of oral cholera vaccine (OCV) as part of a complementary set of control activities was considered but initially rejected by policymakers. In December 2011, the Minister of Health of Haiti called for a demonstration of the acceptability and feasibility of the use of OCV in urban and rural Haiti. This paper describes the collaborative activity that offered OCV to one region of the Artibonite Department of rural Haiti in addition to other ongoing treatment and control measures. Despite logistics and cold chain challenges, 45,417 persons were successfully vaccinated with OCV in the region, and 90.8% of these persons completed their second dose.
Background
Haiti is fighting the world's largest cholera epidemic in 50 years. Since Vibrio cholerae was first detected in October 2010, 651,339 cases and 8,053 deaths have been reported by the Haitian Ministry of Health (MOH).1 According to the Pan-American Health Organization, some 1,500 new cases have been reported each week in 2013.2 The extensive initial local and international response to the cholera epidemic in Haiti largely accorded with World Health Organization (WHO) protocol: case-finding and rapid treatment by rehydration, clean water and sanitation efforts, public health messaging, and national surveillance.3 Oral cholera vaccine (OCV), however, remained unavailable in Haiti until 2012. In the first months of the epidemic, influential external policy advisors, a high profile international non-governmental organization, and certain academics voiced concerns that introducing vaccine might trigger further social instability at a time when election-year social frictions were already high and vaccine availability was limited.4,5 Others worried that vaccination would pull resources from other disease-control priorities.6 Still, some argued that vaccination could be integrated with other prevention and treatment activities and therefore strengthen the overall response.7,8
Two types of OCV are currently available: WC-rBS (marketed as Dukoral®; Crucell, Leiden, The Netherlands) and BivWC (marketed as Shanchol®; Shantha Biotechnics, Hyderabad, India). Neither vaccine is a panacea, but they have proven safe and effective in diverse settings, largely in endemic cholera regions.9 Both vaccines require use of cold chain and must be given in two doses, 1–6 weeks apart. In recent years, re-analysis of data from clinical trials demonstrated that both vaccines confer considerable herd immunity and interest in their use has increased.10 In 2010, WHO revised guidelines on the use of OCV and included a recommendation that in epidemic settings, reactive vaccination be considered as an additional control measure in conjunction with other prevention and control strategies.11
In December 2011, the MOH of Haiti requested that demonstration projects be conducted in rural and urban Haiti to establish the acceptability and feasibility of using OCV as part of a comprehensive cholera control strategy for the country. Partners In Health (PIH) and Le Groupe Haïtien d'Etude du Sarcome de Kaposi et des Infections Opportunistes (GHESKIO), both non-governmental healthcare organizations with long-time experience in Haiti, were early proponents of the inclusion of OCV in control efforts and were thus well positioned to respond rapidly to the Minister's request for technical and implementing partners. By that time, 523,904 Haitians had been sickened by cholera and 7,018 had died.12 Some 300 cases were being seen each week in the Artibonite and Central Departments where PIH supports 11 public health facilities, including cholera treatment and prevention activities.13
This report describes the use of OCV in a rural vaccination campaign undertaken by PIH as an implementing partner of the MOH. Our objective was to use available vaccine to provide protection to target recipients in rural Haiti before the onset of the annual rainy season three months later. In doing so, we aimed to demonstrate the acceptability and feasibility of such a campaign, despite the challenges of delivering healthcare in rural Haiti that were further heightened by the ongoing epidemic.
Procedures
Timeline.
Long lead-time items for the project included procurement and shipment of the vaccine and cold chain inputs. In part because months of lack of consensus among policymakers had resulted in public uncertainty, and in part because the vaccine had only recently been pre-qualified by WHO, concern arose from a national bioethics committee that the vaccination project was experimental and the start date was delayed while the appropriate ethics review took place. Once the committee had reviewed revised documents and understood that the project was a public health endeavor, they approved the project to advance without concern. This resulted in a six-week delay that impacted the strategy that was used (see below) and also meant that the campaign began at the start of the rainy season.
Project area.
The campaign aimed to provide vaccination access to a rural community in the Artibonite Department of Haiti. The project team chose to target an area called Bocozel (Figure 1). After a census was performed, the number of inhabitants of Bocozel was fewer than originally anticipated, so a neighboring community (Grand Saline) was included in the campaign to reach the objective of vaccinating 50,000 persons. Although a number of alternate targeting strategies might have been invoked, we elected to conduct the campaign in this region of Haiti for a number of reasons: its rural isolated nature with poor road infrastructure making access to health services difficult; the close interaction of its inhabitants with the Artibonite River (the source of the cholera epidemic); its low-lying topography resulting in salination of water boreholes and frequent flooding; and its lack of access to potable water and the lack of a clear plan by the regional water authority for investment in improved water access over the next five years. In short, these were the characteristics of precisely those places hardest-hit by epidemic cholera in Haiti.
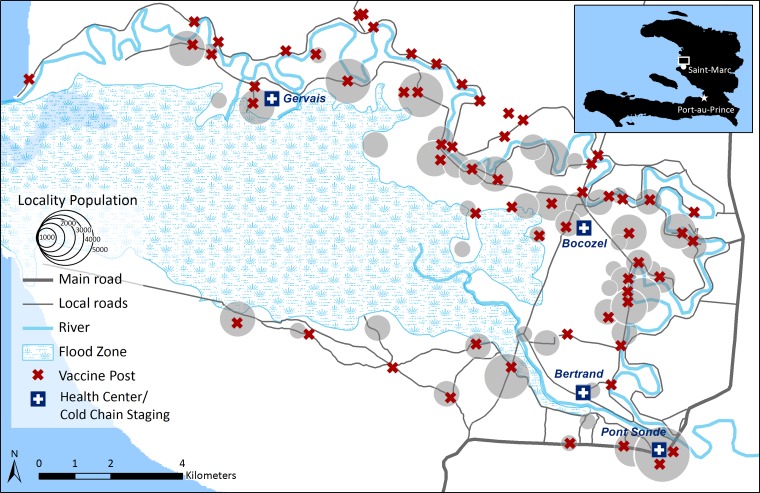
Figure 1.
Fixed vaccination posts and cold chain staging points in an oral cholera vaccination campaign in the communes of Bocozel and Grand Saline, Haiti, 2012.
Bocozel and Grand Saline are rural regions of Haiti near the town of St. Marc and home to the largest rice-growing region of Haiti. Vast plains of rice are irrigated by branches of the Artibonite River and those areas not receiving such irrigation are dry and desert-like. The inhabitants' economic situation is precarious; they live in shanty homes and have poor dietary diversity, few potable water sources, and poor access to sanitation. On most economic measures, their situation is worse than the nationwide average; 78% of persons earning less than $2 a day.14 Small health centers provide some primary care to people in the region but any complex health problems must be addressed in the town of St. Marc, a minimum of two hours away on foot. This region of Haiti had been affected early by the cholera epidemic and an attack rate two years into the epidemic was estimated at 5.1–7.5%.15
Stakeholder engagement and communications.
Leading up to the vaccination campaign, we undertook a series of key stakeholder meetings and focus groups to better understand how cholera vaccination might fit into the community context and to support the generation of communications materials. Stakeholder interviews were conducted with local public health practitioners, the district health office, and leaders of non-governmental organizations with activity in target areas. We also held eight community meetings with local authorities, religious leaders, school directors and teachers, traditional healers, and birth attendants and an additional five focus groups with 55 community residents. These meetings demonstrated that although there was initially a lack of knowledge about OCV, most stakeholders and potential beneficiaries were eager to have access to this tool to prevent cholera, in addition to improvements in clean water and sanitation access. We also gathered information from these meetings that was useful in the development of a communications campaign. Ten key messages around cholera vaccination and cholera prevention were generated and shared during the campaign via radio shows, sound trucks (local vehicles with megaphones), T-shirts, posters, and local television. At the start of the campaign, the director general of the MOH spoke on the radio about OCV.
Staff structure.
The project team was comprised of staff from PIH, as well as MOH representatives from the National Vaccine Program and the local health coordination office. Forty teams of four persons each were engaged in the delivery of vaccine doses to the target population. Each team had a medical records registrar, two vaccinators, and a crier. The crier was from the locality being vaccinated that day, guiding teams and alerting communities to the day's activities usually by using a megaphone. Twenty supervisors led these 40 teams. No existing training manuals were (to our knowledge) available on the use of OCV, so our PIH training department in Haiti created a community health worker training module and staff was trained over the course of two and a half days.16
Logistics and cold chain.
We chose between the two available vaccine brands based on cost and ease of use in the field. On December 9, 2011, PIH executed an order for all 200,000 available doses of BivWC at a pre-negotiated price of $1.85 per dose. On March 1, 2012, 168 shippers carrying 200,000 doses of oral cholera vaccine arrived to Port-au-Prince by air from India at 8:00 am.
To ensure cold chain during the five-day transit between India and Haiti, ice packs and 10-day electronic temperature monitoring devices (Q-tag®; Berlinger, Ganterschwil, Switzerland) were included in each shipper. The shipment was cleared through Haitian customs the same morning and awaiting trucks transported half of the doses directly to St. Marc for use in the rural component of the project, arriving by 2:00 pm the same day. The remaining 100,000 doses were trucked to a warehouse in Port-au-Prince for use in the urban campaign.
Because capacity for cold chain is limited in the rural isolated part of Haiti in which we work, we devised a series of steps to reinforce this capacity (Figure 1). In St. Marc, vaccine was transferred from shippers to a 20-foot refrigerated container where pre-arranged plastic shelving was used to store the boxes, each containing 10 vials, for a total volume of 6.31m3. From that time until the end of the campaign, the temperature of the cold container was monitored twice a day. Boxes remained wrapped until the day of use. Individual vials were only handled when vaccinators administered the vaccine.
Every evening, 4,000–7,000 doses were transferred from the cold container to refrigerated storage in a dispatching warehouse for use the next day. Each morning, supervisors loaded vaccines, ice packs, and a thermometer into cold boxes for further transport to one of four health centers in Bocozel that served as final staging areas. Here, vaccination teams met supervisors, filled vacuum flask carriers with vaccines and cold packs, and dispersed to their posts. A random selection of flasks was equipped with thermometers, and teams recorded temperatures throughout the vaccination day. Cold chain was thus maintained up to the time of vaccination. At the end of each day, unused vaccine was returned to refrigerated storage at the dispatch warehouse and prioritized for use the next day. Empty, used vials were stored in plastic bags with no other waste and incinerated under the supervision of the warehouse manager per the manufacturer's recommendation.
Use of handheld technology.
Data were collected by using Samsung Galaxy version 7.0 Plus tablets running on an Android operating system (Samsung, Seoul, South Korea) and stored on a web-hosted database. The data collection software used was Majella Insight® (Majella Global Technologies, Portland, ME). Because of cost concerns, data collected in the field were initially stored offline on the device and were later uploaded to our cloud database at the project office via local internet connection every evening. Because literacy levels are low in the target area and past experience had shown that personal identification cards are often unavailable, we required a robust method to identify vaccine recipients so as to document receipt of their second vaccine dose. We pre-printed vaccine cards with unique numeric barcodes, thus assigning each card a unique identification number. Using the tablet computer's built-in camera function, we scanned barcodes at the time of vaccination, registered the vaccinee in the database at which point the date, dose, and batch number were recorded. If barcodes did not successfully scan, or if vaccination cards were lost or missing, data were entered manually into the tablet database at the point of vaccination.
Census.
A census was performed in Bocozel before vaccination began. During the census, field teams moved from door-to-door across localities and collected information about the household from the household head or another adult. Each house was assigned a unique number. If no adult was present, the house was noted and the team returned on a subsequent day. Households were informed about cholera vaccination and general cholera prevention techniques were shared including water, sanitation, and hygiene messages. Residents who were eligible and expressed interest in vaccination were pre-registered and received a vaccine card with a unique barcode. Household members that were not present could be pre-registered by the responding adult and in this case vaccine cards were left with the household representative. Every tenth household was invited to participate in a short survey of knowledge and attitudes regarding cholera and also practices relevant to water-borne disease. When persons participated in the survey, it was conducted before team members shared information about the vaccine or other cholera prevention measures.
Vaccination strategy.
Vaccination occurred in two distinct phases that targeted children less than 10 years of age separately from other recipients. This phase was a necessary adjustment to project plans because the six-week delay in the timeline resulted in the OCV campaign schedule overlapping with a national oral polio vaccination (OPV) campaign. We received a manufacturer's letter advising that OPV should be avoided within 14 days of OCV administration. The first phase therefore occurred in Bocozel during April 15–May 24, 2012 and excluded children less than 10 years of age. The second phase targeted children less than 10 years of age, as well as any remaining eligible persons of all ages and occurred during May 27–June 19, 2012. Because the total census population in Bocozel was smaller than first expected, the campaign was expanded geographically in phase 2 to the neighboring community of Grand Saline.
Initial vaccinations were performed at fixed or rally posts in locations pre-determined by the communities and project team. These posts were usually the same locations that MOH officials use for childhood vaccination campaigns. Each evening, a daily report was generated from data collected in the field. It included information about vaccinations performed that day grouped by team and by locality, as well as the cumulative total number of vaccinations. A combination of these data, pre-registration data and field team feedback were used to inform the next day's field strategy. When this nightly review of the data suggested that attendance at fixed posts had slowed, teams moved to mobile posts. Mobile posts were comprised of the same vaccination team but the location was flexible, targeted to areas with low turn out to date. Teams sometimes completed multiple mobile posts in one day, moving on to the next position when attendance slowed. A final phase of vaccination occurred by using a door-to-door approach. We generated lists of persons who had pre-registered for the vaccine but who had not yet been vaccinated, along with their addresses. Teams moved door-to-door to offer vaccination to those houses. This whole approach was repeated after 14 days for the administration of the second vaccine dose.
At all times during the campaign, vaccination remained voluntary. Children less than one year of age and pregnant women were not eligible for vaccination. Pregnant women were asked to self-identify for exclusion at each stage of the campaign. If potential recipients presented requesting a first dose of vaccination out of cycle with the rest of the cohort, we did not offer vaccination unless the timing ensured that a second dose could be received in the project timeframe. In addition to vaccine-specific information, teams shared water and sanitation and hygiene messages during their interactions with community members because the objective of the project was to be part of a comprehensive control effort rather than to supplant one component with another.
Monitoring.
Persons were observed for side effects at the vaccination post for 30 minutes after receipt of the vaccine, and community health workers performed passive follow-up in the communities for side effects that might have occurred later. Trained community nurses made home visits to review persons reporting side effects. These side effects were designated as mild (no interference with daily activities), moderate (some interference with daily activities), or severe (significant, prevented daily activity).
In September 2012, three months after the vaccination campaign, a follow-up survey was conducted in Bocozel as part of routine program evaluation. Six hundred households were randomly selected from the census data and information was collected from respondents on vaccine coverage, as well as on knowledge and attitudes about cholera. No personally identifiable information was gathered during this survey. Institutional review board approval was obtained from Partners Institutional Review Board (Boston, MA) for secondary analysis of the data collected during this survey.
Results
Uptake.
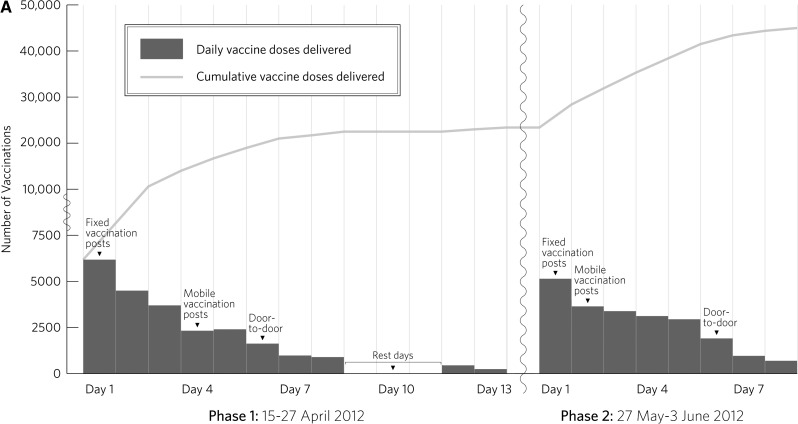
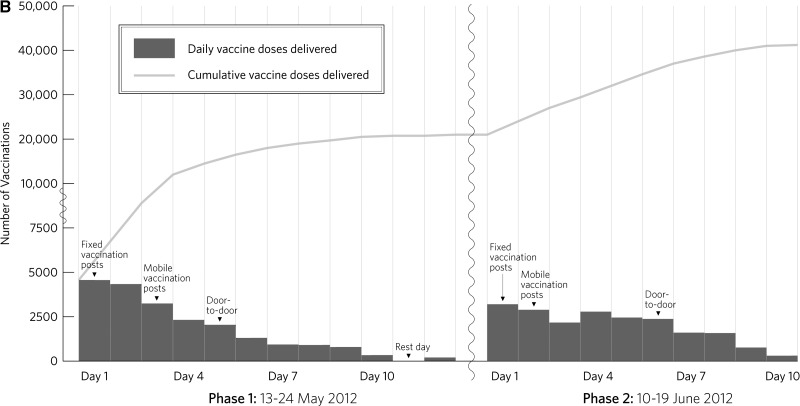
A total of 45,417 persons received at least one dose of OCV. Of these persons, 90.8% were confirmed to have received a second dose. Daily and cumulative vaccination counts across all phases and doses and the strategy used during each component are shown in Figure 2 . Uptake of vaccine and completion of second dose by location, sex, and age group are shown in Table 1.
Figure 2.
A, Daily and cumulative first doses delivered in an oral cholera vaccination campaign in rural Haiti, 2012. B, Daily and cumulative second doses delivered in an oral cholera vaccination campaign in rural Haiti, 2012.
Table 1.
Vaccination by location, age group and gender in an oral cholera vaccination campaign in rural Haiti, 2012
| Population | Bocozel, no. (%) | Grand Saline, no. (%) | Total, no. (%) |
|---|---|---|---|
| Community coverage | 76.7–92.7%* | 62.5%† | |
| Received at least 1 dose | 32,210 (70.9) | 13,207 (29.1) | 45,417 |
| Sex | |||
| F | 15,799 (49.0) | 6,630 (50.2) | 22,429 (49.4) |
| M | 16,383 (50.9) | 6,555 (49.6) | 22,938 (50.5) |
| Age, years | |||
| 1–4 | 3,630 (11.3) | 1,268 (9.6) | 4,898 (10.8) |
| 5–9 | 3,887 (12.1) | 1,792 (13.6) | 5,679 (12.5) |
| 10–17 | 5,744 (17.8) | 2,505 (19.0) | 8,249 (18.2) |
| ≥ 18 | 18,921 (58.7) | 7,620 (57.7) | 26,541 (58.4) |
| Completion rate | |||
| Received 2 doses | 29,204 (90.7) | 12,038 (91.1) | 41,242 (90.8) |
| Sex | |||
| F | 14,426 (91.3) | 6,147 (92.7) | 20,573 (91.7) |
| M | 14,752 (90.0) | 5,869 (89.5) | 20,621 (89.9) |
| Age, years | |||
| 1–4 | 3,400 (93.7) | 1,188 (93.7) | 4,588 (93.7) |
| 5–9 | 3,665 (94.3) | 1,697 (94.7) | 5,362 (94.4) |
| 10–17 | 5,217 (90.8) | 2,268 (90.5) | 7,485 (90.7) |
| ≥ 18 | 16,895 (89.3) | 6,863 (90.1) | 23,758 (89.5) |
Lower limit was calculated by census data, and upper limit was calculated by post-campaign coverage survey.
Calculated using government demographic data.
Coverage.
We measured community coverage in Bocozel in two ways. The first estimate used census data as the denominator and included in the numerator only those recipients of OCV that were confirmed to have been registered in that community (i.e., their domicile was registered by the census). Using these data, we found that community coverage was 76.7%. On vaccination days, some persons identified their domicile to be in a certain community, but they could not be found in the database. At the vaccine post, we had no way to prove or disprove a person's domicile. Thus, we also calculated coverage rates, including these self-reporting persons, whereby they were added to the numerator and denominator. Using this more inclusive method, we found that community coverage in Bocozel was 79.2%.
We further measured community coverage by asking about receipt of OCV during the post-campaign household survey. A total of 480 (92.7%) of 518 respondents reported that they had been vaccinated against cholera, of which 419 (87.3%) of 480 produced a vaccination card as confirmation. Among the 37 respondents reporting that they were not vaccinated, 18 (3.5%) reported not being present at the time of vaccination, 14 (2.7%) reported being ineligible at the time of vaccination, 3 (< 1%) did not want the vaccine, 2 (< 0.5%) reported other reasons; 1 person declined to respond. Because we did not perform a census or pre-registration phase in Grand Saline, we estimated community coverage, determined by using 2009 government demographic information as the denominator, to be 62.5%.
Waste and side effects.
Vaccine wastage was monitored by supervisors and project staff based on inventory of stock. Wastage was documented to be 495 doses (< 0.5%).
No severe side effects were reported during or after the campaign—19 vaccine recipients reported minor side effects. Gastrointestinal disturbance was the most common side effect, followed by dizziness, vomiting, or abdominal pain.
Discussion
Despite the fact that newer oral cholera vaccines have been demonstrated to be safe and effective in diverse settings, they have not been deployed extensively in the setting of epidemic cholera. Initial skepticism about the efficacy of such a strategy was reduced by the understanding that herd immunity plays an important role in cholera vaccination such that increasing levels of vaccination coverage in a community contribute to improved protection of not just the unvaccinated, but also further protection of the vaccinated population.10,17,18 In part, continued concern about cholera vaccination during epidemics has been about logistics and a fear of scarcity. Using a cold chain-requiring product for large, rapid, and reactive vaccination campaigns may seem less feasible during the chaos of epidemic disease. This finding is particularly notable given that such epidemics most often occur in regions with fragile health systems.19 Concerns about scarcity of resources may result in an attempt to pit one public health approach against another rather than prompt an attempt to integrate complementary approaches that might prevent disease, reduce mortality, and strengthen the health system.
We aimed to demonstrate the acceptability and feasibility of a reactive oral cholera vaccination campaign in the context of the ongoing Haitian epidemic in synergy with continued support of the other important components of cholera control, such as case-finding, treatment, access to potable water, and sanitation and hygiene education. We deemed the project successful by virtue of the uptake and coverage rates described.
Some key aspects of our program contributed to success including the staffing model and the engagement of community leaders. The partnership between PIH and the MOH occurred at all levels; MOH were key participants in strategic decision-making and in supervision of the field activities. Including a vaccination team member from the area being vaccinated each day provided important local context to the project and contributed to community trust in the activity. Engaging community leaders early in the process was important because once their questions and concerns were addressed, they naturally disseminated the information to their constituents. Their support ensured that the campaign was trusted and well understood. There had been some concern cited before the campaign that the targeted approach would lead to protests from communities not receiving vaccination, but this did not occur. We believe this was due in part to careful communications efforts.
Manufacturers of the OCV recommend that cold chain be used to the point of vaccination. Cold chain storage capacity in public or private health facilities in the region is limited, largely relying on outdated propane back-up refrigerators and lacking in cold boxes for community health workers. As part of the project plan, under guidance from the National Vaccine Program, we had purchased 10 solar powered refrigerators to store the vaccine, but delays in their procurement and arrival to Haiti meant that we had to rely on another source of storage. As such, we were obliged to use a cold storage container located inside a United Nations military base. Its location meant that frequent entry and exit of the entire project vaccination team was neither desirable nor logistically feasible, resulting in our need to use the dispatching warehouse for smaller batches of vaccine for daily use. This necessity added an additional transportation requirement and staffing that could potentially have been avoided. If cold chain requirements were needed up until the day of vaccination only, a significant savings in terms of equipment and logistics planning could also have been made.
The six-week delay in starting the OCV campaign resulted from a concern that the campaign was experimental and that a research activity was being planned without proper ethical consideration. In fact, using a pre-qualified vaccine with documented efficacy and side effects in a demonstration project was considered a public health service, not usually subject to ethics review. Those with the closest proximity to the risk of being sickened by cholera had expressed no ethical concerns. Thanks to swift communication between the ethics committee, the project team and the MOH, the issue was soon resolved, but the delay resulted in some important issues including loss of trained staff to other commitments, requiring another round of training for new staff; the onset of the rainy season, causing some transportation difficulties in this rural, flood-prone region; and the overlap with the OPV campaign. This overlap required us to split our campaign into two phases such that we ultimately returned to each area four times, two doses for each phase. This split had financial and logistics implications for the project. Despite our concerns that it would be difficult to explain the separation of children less than 10 years of age from others in the campaign, this message was received without too much complication by communities, as shown by high rates of follow-up both children and adults.
We had initially anticipated vaccination only in the community of Bocozel, but late in project planning realized that the target population there had been overestimated. We therefore added the second community of Grand Saline in the second phase of vaccination. Despite the shorter lead time to introduce the campaign, there was still excellent uptake and completion of second doses, leading us to believe that the messages about the campaign were already being shared from one community to the next by word of mouth. Because we had not initially planned to intervene in this area, no census or follow-up survey occurred in that region. The coverage rate of 62.4% may be an underestimate given that the denominator used for the calculation was from demographic data over three years old. Regardless of the method used for calculation, the overall coverage rates for both communities are within the range that would be expected to provide herd immunity.
Because the project was intended specifically to inform future practice on the use of OCV, we undertook data collection at a level more detailed than usually occurs during our community vaccination campaigns and chose to capture data electronically using handheld devices. We explored a number of technology platforms before choosing one. Important considerations were ease of use, durability, cost, and the limitations of our catchment area, including the rural environment, and lack of power and other infrastructure in the field. Scanning barcodes pre-printed on vaccine cards (rather than relying on manual entry) afforded our team swiftness and accuracy throughout pre-registration during the census, as well as during the vaccination phase. The technology platform used was accessible and user-friendly. High-school educated staff were able to quickly learn and familiarize themselves with the tablet and data collection software. Nightly review of the data also facilitated day-to-day changes in vaccination strategy that helped the efficiency of the vaccine teams and likely contributed to the excellent follow up rates.
There were no major side effects reported with the use of the vaccine, and this finding is consistent with previous experience with this vaccine.20 The project highlighted the fact that there is a weak formal Adverse Effects Following Immunization system in Haiti and this should be reinforced for all existing and future planned vaccination activities.
This was a public health intervention and research was not a primary objective. There remain a number of operational questions that would benefit from further study to facilitate the use of OCV in future scenarios. These questions include clarification on the need for maintaining cold chain until the point of administration versus until the day of vaccination, and whether the interaction with OPV is a real or just theoretical concern. A recent study of WC-rBS was reassuring regarding the safety of that vaccine during pregnancy; however, further data are needed to clarify recommendation on the use of OCVs in pregnant women.21 It is also important to consider the role that dissensus played in the failure to rapidly deploy an effective technology during the epidemic in Haiti. OCV has now been studied retrospectively and prospectively in epidemic cholera and this report adds to evidence of the feasibility of OCV in such circumstances.10,22–25
In conclusion, vaccination with OCV was acceptable and feasible in rural Haiti during an epidemic of cholera. There were high rates of community coverage in an initial area with intense pre-campaign communications and in a second community with a shorter pre-campaign communications. All communities had excellent rates of completion of second dose of the vaccine. Community engagement in the OCV campaign was critical to success.
ACKNOWLEDGMENTS
This oral cholera vaccination campaign was the result of a collaborative effort of many persons and organizations. We thank PIH and Zanmi Lasante staff and volunteers for their tireless dedication to the project; Amanda Schiff, Omowunmi Aibana, Wilfredo Matias, Matthew Peckarsky, Molly Franke, Nicole Armstrong, Zibiao Zhang, and Dana Thompson for their contributions; and community members and leaders in Bocozel and Grand Saline. This paper is dedicated to them, as well as to M.H.H.
Footnotes
Financial support: The vaccination campaign was supported by the American Red Cross and other private donors. Louise C. Ivers is supported by the National Institute of Allergy and Infectious Diseases (R01AI099243-01) and the National Institute of Child Health and Human Development (R01HD057627). Louise C. Ivers and Jessica E. Teng are supported by a subcontract from the Delivering Oral Vaccine Effectively (DOVE) program at Johns Hopkins University, which is funded by the Bill and Melinda Gates Foundation.
Authors' addresses: Louise C. Ivers and Jessica E. Teng, Division of Global Health Equity, Brigham and Women's Hospital, Boston, MA, E-mails: livers@pih.org and jteng@pih.org. Jonathan Lascher, Partners In Health, Haiti Programs Department, Boston, MA, E-mail: jlascher@pih.org. Max Raymond, Programs Department, Zanmi Lasante, St. Marc, Haiti, E-mail: maxraymondjr@gmail.com. Jonathan Weigel and Paul E. Farmer, Department of Global Health and Social Medicine, Harvard Medical School, Boston, MA, E-mails: jonweigel@gmail.com and paul_farmer@hms.harvard.edu. Nadia Victor, Programs Department, Zanmi Lasante, St. Marc, Haiti, E-mail: dianevic14@hotmail.com. J. Gregory Jerome and Isabelle J. Hilaire, Monitoring and Evaluation, Zanmi Lasante, St. Marc, Haiti, E-mails: gjerome@pih.org and isabelle.j.hilaire@gmail.com. Charles P. Almazor, Clinical Services, Zanmi Lasante, St. Marc, Haiti, E-mail: cpalmazor@pih.org. Ralph Ternier, Community Health Programs, Zanmi Lasante, St. Marc, Haiti, E-mail: ralphternier@hotmail.com. Jean Cadet and Jeannot Francois, Expanded Immunization Program, Ministry of Health and Population, Port-au-Prince, Haiti, E-mails: cadethaiti@yahoo.fr and francoisjeannot@ yahoo.fr. Florence D. Guillaume, Ministry of Health and Population, Port-au-Prince, Haiti, E-mail: minister@mspp.gouv.ht.
References
- 1.Ministère de la Santé et de la Population Rapport de Cas 24 Mars 2013. 2013. http://www.mspp.gouv.ht/site/index.php?option=com_content&view=article&id=120&Itemid=1 Available at. Accessed March 30, 2013.
- 2.Pan-American Health Organization PAHO/WHO Calls for International Funding of New Haiti Cholera Plan. 2013. http://reliefweb.int/report/haiti/pahowho-calls-international-funding-new-haiti-cholera-plan Available at. Accessed March 30, 2013.
- 3.World Health Organization Prevention and Control of Cholera Outbreaks: WHO Policy and Recommendations. 2013. http://www.who.int/cholera/technical/prevention/control/en/index.html Available at. Accessed March 30, 2013.
- 4.Date KA, Vicari A, Hyde TB, Mintz E, Danovaro-Holliday MC, Henry A, Tappero JW, Roels TH, Abrams J, Burkholder BT, Ruiz-Matus C, Andrus J, Dietz V. Considerations for oral cholera vaccine use during outbreak after earthquake in Haiti, 2010–2011. Emerg Infect Dis. 2011;17:2105–2112. doi: 10.3201/eid1711.110822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daniel T. New Cholera Campaign Faces Tough Questions. Associated Press: November 9, 2011. 2011. http://www.usatoday.com/news/health/story/health/story/2011-11-09/New-Haiti-cholera-campaign-faces-tough-questions/51136304/1 Available at. Accessed March 30, 2013.
- 6.Knox R. Cholera Vaccine Isn't the Answer for Haiti. NPR: October 28, 2010. 2010. http://www.npr.org/blogs/health/2010/10/28/130884642/why-the-cholera-vaccine-isn-t-the-answer-for-haiti Available at. Accessed March 30, 2013.
- 7.Ivers LC, Farmer P, Almazor CP, Léandre F. Five complementary interventions to slow cholera. Lancet. 2010;376:2048–2051. doi: 10.1016/S0140-6736(10)62243-X. [DOI] [PubMed] [Google Scholar]
- 8.Farmer P, Almazor CP, Bahnsen ET, Barry D, Bazile J, Bloom BR, Bose N, Brewer T, Calderwood SB, Clemens JD, Cravioto A. Meeting cholera's challenge to Haiti and the world: a joint statement on cholera prevention and care. PLoS Negl Trop Dis. 2011;5:e1145. doi: 10.1371/journal.pntd.0001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization Cholera vaccines: WHO position paper. Wkly Epidemiol Rec. 2010;85:117–128. [PubMed] [Google Scholar]
- 10.Ali M, Emch M, Seidlein L, Yunus M, Sack D, Rao M, Homgren J, Clemens JD. Herd immunity conferred by killed oral cholera vaccines: a reanalysis. Lancet. 2005;366:44–49. doi: 10.1016/S0140-6736(05)66550-6. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization Cholera vaccines: WHO position paper. Wkly Epidemiol Rec. 2010;85:117–128. [PubMed] [Google Scholar]
- 12.Ministère de la Santé et de la Population Rapport de Cas 31 Décembre 2011. 2013. http://www.mspp.gouv.ht/site/index.php?option=com_content&view=article&id=120&Itemid=1 Available at. Accessed March 30, 2013.
- 13.Ministère de la Santé et de la Population Rapport de Cas Par Semaine Épidémiologique. 2013. http://www.mspp.gouv.ht/site/index.php?option=com_content&view=article&id=119&Itemid=1 Available at. Accessed March 30, 2013.
- 14.World Bank Haiti Fact Sheet. 2013. http://www.worldbank.org/en/country/haiti/overview Available at. Accessed March 30, 2013.
- 15.Barzilay EJ, Schaad N, Magloire R, Mung KD, Boncy J, Dahourou GA, Mintz ED, Steenland MW, Vertefeuille J, Tappero JW. Cholera surveillance during the Haiti epidemic: the first 2 years. N Engl J Med. 2013;368:599–609. doi: 10.1056/NEJMoa1204927. [DOI] [PubMed] [Google Scholar]
- 16.Partners In Health Facilitator Manual, Participant Handbook. 2013. http://www.pih.org/publications/entry/community-health-worker-cholera-training-manuals Available at. Accessed August 19, 2013.
- 17.Emch M, Ali M, Jin-Kyung P, Yunus M, Sack DA, Clemens JD. Relationship between neighborhood-level killed oral cholera vaccine coverage and protective efficacy: evidence for herd immunity. Int J Epidemiol. 2006;35:1044–1050. doi: 10.1093/ije/dyl100. [DOI] [PubMed] [Google Scholar]
- 18.Jeuland M, Cook J, Poulos C, Clemens J, Whittington D. Cost-effectiveness of new-generation oral cholera vaccines: a multisite analysis. ISPOR. 2009;12:899–908. doi: 10.1111/j.1524-4733.2009.00562.x. [DOI] [PubMed] [Google Scholar]
- 19.Griffith DC, Kelly-Hope LA, Miller MA. Review of reported cholera outbreaks worldwide, 1995–2005. Am J Trop Med Hyg. 2006;75:973–977. [PubMed] [Google Scholar]
- 20.Saha A, Chowdhury MI, Khanam F, Bhuiyan S, Chowdhury F, Khan AI, Khan IA, Clemens J, Ali M, Cravioto A, Qadri F. Safety and immunogenicity study of a killed bivalent (O1 and O139) whole-cell oral cholera vaccine Shanchol, in Bangladeshi adults and children as young as 1 year of age. Vaccine. 2011;29:8285–8292. doi: 10.1016/j.vaccine.2011.08.108. [DOI] [PubMed] [Google Scholar]
- 21.Hashim R, Khatib AM, Enwere G, Park JK, Reyburn R, Ali M, Chang NY, Kim DR, Ley B, Thriemer K, Lopez AL, Clemens JD, Deen DJ, Shin S, Schaetti C, Hutubessv R, Aguado MT, Kieny MP, Sack D, Obaro S, Shaame AJ, Ali SM, Saleh AA, von Seidlein L, Jiddawi MS. Safety of the recombinant cholera toxin B subunit, killed whole-cell (rBS-WC) oral cholera vaccine in pregnancy. PLoS Negl Trop Dis. 2012;6:e1743. doi: 10.1371/journal.pntd.0001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chao DL, Halloran ME, Longini IM. Vaccination strategies for epidemic cholera in Haiti with implications for the developing world. Proc Natl Acad Sci USA. 2011;108:7081–7085. doi: 10.1073/pnas.1102149108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukandavire Z, Liao S, Wang J, Gaff H, Smith DL, Morris JG. Estimating the reproductive numbers for the 2008–2009 cholera outbreaks in Zimbabwe. Proc Natl Acad Sci USA. 2011;108:8767–8772. doi: 10.1073/pnas.1019712108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reyburn R, Deen JL, Grais RF, Bhattacharya SK, Sur D, Lopez AL, Jiddawi MS, Clemens JD, von Seidlein L. The case for reactive mass oral cholera vaccinations. PLoS Negl Trop Dis. 2011;5:e952. doi: 10.1371/journal.pntd.0000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anh DD, Lopez AL, Thiem VD, Grahek SL, Duong TN, Park JK, Kwon HJ, Favorov M, Hien NT, Clemens JD. Use of oral cholera vaccines in an outbreak in Vietnam: a case control study. PLoS Negl Trop Dis. 2011;5:e1006. doi: 10.1371/journal.pntd.0001006. [DOI] [PMC free article] [PubMed] [Google Scholar]