Abstract
Cholera, previously unrecognized in Haiti, spread through the country in the fall of 2010. An analysis was performed to understand the epidemiological characteristics, clinical management, and risk factors for disease severity in a population seen at the GHESKIO Cholera Treatment Center in Port-au-Prince. A comprehensive review of the medical records of patients admitted during the period of October 28, 2010–July 10, 2011 was conducted. Disease severity on admission was directly correlated with older age, more prolonged length of stay, and presentation during the two epidemic waves seen in the observation period. Although there was a high seroprevalence of human immunodeficiency virus (HIV), severity of cholera was not greater with HIV infection. This study documents the correlation of cholera waves with rainfall and its reduction in settings with improved sanitary conditions and potable water when newly introduced cholera affects all ages equally so that interventions must be directed throughout the population.
Introduction
A marker for inadequate water and sanitation infrastructure, cholera remains a significant cause of morbidity and mortality in developing countries. According to the World Health Organization (WHO), in 2011 there was an 85% increase in the number of cholera cases globally compared with 2010 with 58 countries reporting a cumulative total of 589,854 cases of cholera leading to 7, 816 deaths for an overall case fatality rate (CFR) of 1.3%.1 The Haitian Ministry of Health (MSPP) reported the first laboratory-confirmed case of cholera in early October 2010. It was later shown to be related to strains found in Southeast Asia.2 Since then, the epidemic has taken on such proportions that by 2011, Haiti and the Dominican Republic reported 41% of the global deaths caused by cholera. The introduction of cholera to Haiti led to tremendous demands for resources from the MSPP, nongovernmental organizations and the global public health community.3,4 After the onset of the outbreak, relief efforts were focused on developing a comprehensive package for effective cholera control.5,6 These implementations have led to a significant decrease in the fatality rate (CRF) from 3.5%, at the onset of the cholera epidemic, to less than the WHO should less than the WHO's benchmark of 1% for successful cholera intervention. Mitigation of spread of disease as addressed through the establishment of Cholera Treatment Centers (CTC), with an optimal capacity of 100 beds or more and/or smaller Cholera Treatment Units (CTUs), and community-based oral rehydration points (ORPs) for 1) administration of oral rehydration and clinical monitoring, 2) increased community recognition of the severity of cholera, 3) appropriate antibiotic use, and 4) development of a nationwide training program to build the capacity to respond to the epidemic.
Vibrio cholerae produces a clinical spectrum that ranges from asymptomatic colonization to life threatening disease. Approximately 2–5%, develop cholera gravis, the most severe and often fatal form of the disease.11 The morbidity and mortality caused by Vibrio cholerae may be under-reported given the rapid onset of dehydration before health care is sought. This may be particularly true in a country where other diarrheal illnesses are frequent and cholera has not previously occurred. In this paper, we will describe the demographics, clinical characteristics, as well as identify some predictors for acquisition of severe cases suspected cholera seen at the GHESKIO's CTC early in the epidemic (between October 28, 2010 and July 10, 2011).
Materials and Methods
Study area and population.
GHESKIO centers (The Haitian Group for the Study of Kaposi's Sarcoma and Opportunistic Infections) is an established Haitian institution that has provided care to patients infected with human immunodeficiency virus (HIV) and/or tuberculosis circa 1982. Soon after the earthquake of January 2010, GHESKIO assumed responsibility for the overall care of patients residing in the crowded slums surrounding it. Once cholera hit Port-au-Prince, GHESKIO, in a close working collaboration with the MSPP and international and non-governmental organizations, (NGO's) adapted quickly to the new pathogenic challenge facing its patient population by supplying clean water, basic sanitation, and medical care. The GHESKIO CTC was built in urban Port-au-Prince in the middle of a low-lying seaside area that is prone to flooding (Figure 1A in accompanying article by Rouzier and others1). The capacity of the CTC is currently to admit 40 patients per day with staff and support personnel to manage admitted patients around the clock. As of January, 2013, GHESKIO treated over 10,000 cases of acute diarrhea at that site. The majority of patients admitted to the CTC reside either in the surrounding communities, that were severely damaged by the earthquake or live in various camps for internally displaced persons (IDP) including one built near the GHESKIO grounds.
Most patients treated at the CTC live in crowded conditions with limited access to sanitation where drinking water sources included purchased water bags, bottled or filtered water, piped water and water delivered by tanker trucks.12 The GHESKIO camp for IDP established after the earthquake had a population of ∼5,000 during the time of this observation. Residents of the camp were provided with chlorinated water and portable toilets before the onset of and during the cholera outbreak. During most of the study period, the adjacent slum Village de Dieu, with a population of ∼15,000, remained an earthquake damaged slum with little organized access to sanitation or clean water.
Case definition.
In response to the earthquake, the MSPP setup two syndrome-based surveillance systems: the National Sentinel Surveillance System (NCSS) and the Internally Displaced Persons Surveillance System (IDPSS).14,15 The NCSS's case definition of cholera was persons presenting with acute watery diarrhea with or without vomiting aged 5 years or older, residing in an area withat least one case of laboratory-confirmed cholera. During the course of this study, as patients presented for care, treatment was guided by the clinical degree of dehydration at the time of presentation, based on the WHO guidelines used for treatment of acute diarrhea.16
With regards to treatment, the patients admitted with mild dehydration were placed under direct observation and given oral rehydration therapy, whereas most patients with moderate and all patients with severe dehydration were given fluids by the parenteral route. Furthermore, the majority of patients were given adjunctive antibiotics. According to WHO's Global Task Force on Cholera Control, once an outbreak is confirmed, a clinical diagnosis using the WHO standard case definition is sufficient, accompanied by sporadic testing at regular intervals.17 Therefore, most patients presenting to the CTC with acute diarrhea were presumed to have cholera and were subsequently treated empirically.
Data collection.
Records from October 28, 2010 to July 10, 2011 were reviewed to create a database of patients admitted to the CTC with acute diarrhea during the early stage of the cholera epidemic. As patients registered for admission, they were assigned an identifying number and entered into GHESKIO's electronic medical record to capture all admitted patients.
Paper charts were simultaneously created for documentation of hospital course and kept in a secure location. Charts were verified for completeness and accuracy before extracting the data points that were eventually entered into an Excel spreadsheet. The following details were available for analysis: age, gender, degree of dehydration on admission (classified as mild, moderate, or severe based on WHO guidelines),6 duration of hospitalization (defined as either < 24 hrs, 24–48 hrs, or > 48 hrs), referral to other hospitals/health centers, street address, death, and HIV status. The records were remarkably complete considering the setting in whereby provision of care was challenging. However, weights, volume of intravenous fluid, antibiotic administration, and full address were not routinely recorded.
Epidemic period definitions.
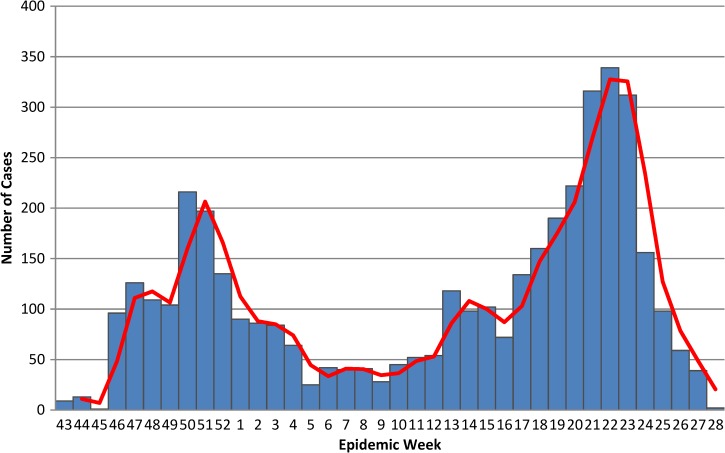
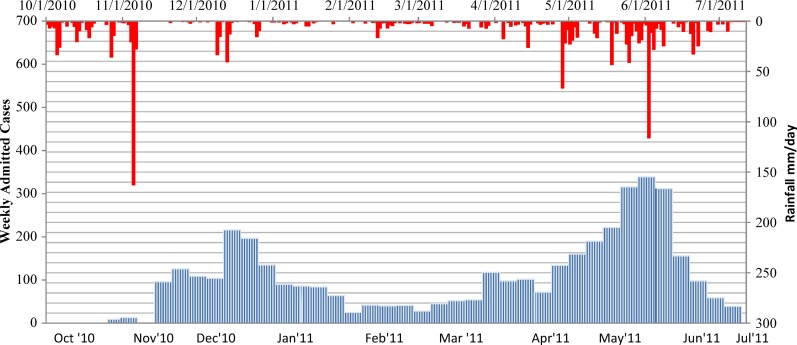
To assess the correlation between clinical characteristics of admitted patients with epidemic periods, we identified three time frames based on the varying densities of admitted patients noted during the study period (Figure 1). We termed the earlier epidemic period as the “first epidemic wave” and defined as the number of cases admitted between epidemic week (EW) 43 of 2010 to EW 5 of 2011. The second epidemic wave is defined by cases admitted between EWs 13–28 of 2011. The inter-epidemic period was the interval between these two peaks. EWs 6–12 of 2011.
Figure 1.
Epidemic curve of admitted suspected cholera cases at GHESKIO CTC October 28, 2010–July 10, 2011.
Confirmation of cholera and other laboratory assays.
Limited numbers of stool specimens were collected for culture and for testing in a Luminex assay designed to detect a battery of enteric pathogens. Stool specimens were tested for Vibrio cholerae and other enteric pathogens by the xTAG GPP (Luminex® Austin, TX) assay and were cultured in parallel for V. cholerae and Salmonella18; from April 1, 2011, admitted patients were systematically screened for HIV, whereas before that date, patients were tested primarily if there was clinical suspicion of an immunocompromised state.
Rainfall data.
Because cholera is a waterborne disease, real-time environmental monitoring for rainfall can play a significant role in understanding cholera dynamics19; hence, the number of cases of acute diarrhea admitted weekly to the CTC during the study period was correlated with daily precipitation estimates. Daily precipitation obtained from the National Aeronautics and Space Administration (NASA) and Tropical Rainfall Measuring Mission (TRMM), provided remote sensing data in Port-au-Prince at a resolution of 0.25 × 0.25 degrees from October 1, 2010 to August 31, 2011 (see http://trmm.gsfc.nasa.gov/ for details).
Statistical analyses.
We performed a descriptive statistical analysis of patients admitted with acute diarrhea during the ongoing cholera epidemic in Haiti for a logistic regression analysis of demographic factors on the disease severity composite. All analyses were conducted in SAS 9.2 (SAS Institute, Cary, NC).
Univariate and multivariate logistic regression analysis was performed to assess correlation of clinical characteristics (age, gender, and time of presentation in relation to the epidemic period) to assess risk factors for severe disease measured outcomes including: death, degree of dehydration (mild versus severe dehydration), and length of hospitalization. Odds ratios (ORs) with 95% confidence intervals (CIs), and all reported P values are two-tailed. For purpose of the analysis the population was divided into the following age groups 0–5, 5–19, 20–39, 40–59, and ≥ 60 years of age.
Results
Description of cohort and epidemic curve.
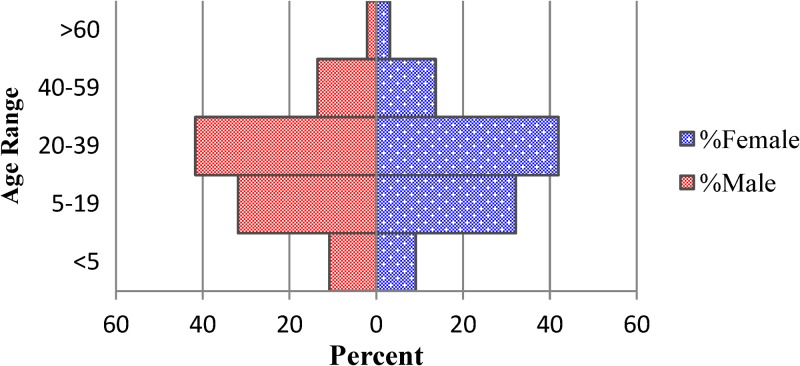
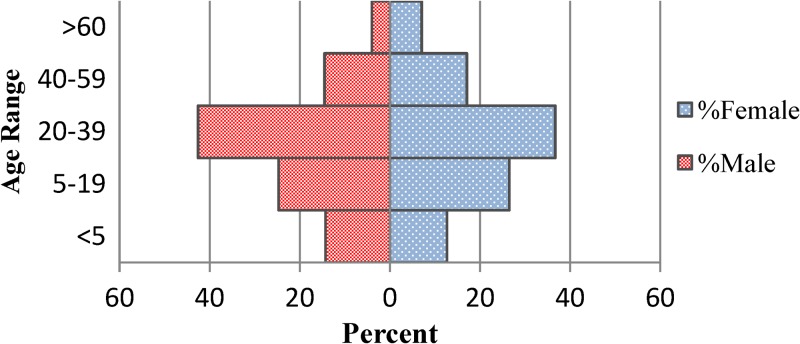
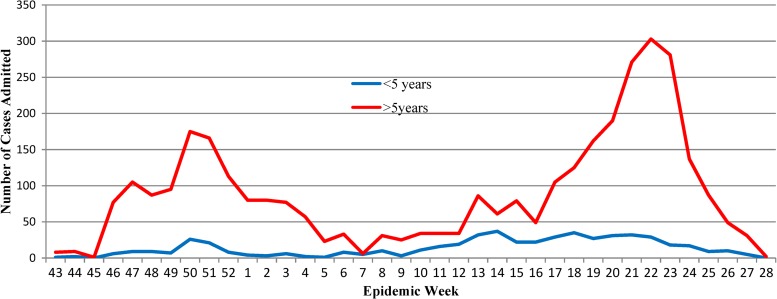
A total of 4,070 suspected cholera cases reported to GHESKIO's downtown campus for treatment between October 28, 2010 and July 10, 2011. The demographics of population are shown in Table 1, with data divided into epidemic periods, as defined previously, with the inter-epidemic period being notable for having < 50 admissions per week. There are two distinct peaks noted in the epidemic curve during epidemic weeks (EW) 43–4 and 12–28 (Figure 1). Notably, the sharp increase of the number of admissions around EW 22 corresponds with the beginning of the rainy season where 339 cases were admitted at the height of the second peak and was considerably higher than the first peak (216 cases). The age distribution of the study cohort almost exactly mirrored that of the population of the surrounding communities, including Village de Dieu and the GHESKIO IDP camp (Figures 2 and 3). The leading age group is adults 20–59 years of age, comprising a little over half (55.5%) of the admitted patients, whereas children < 5 years of age account for 13.5% of cases admitted to GHESKIO's CTC; this age group also showed the least increase in cases during the epidemic, implying that a higher percentage of acute diarrhea in this age group is likely not caused by cholera (Figure 4). Specifically, the shoulder at the beginning of the second wave, EW 12–16 of 2011, had a high percentage of admitted children under five perhaps because of rotavirus or other childhood enteric virus. In a study of 214 GHESKIO CTC patients cholera could be microbiologically confirmed in 100 patients (47%).18
Table 1.
Demographics of 4,070 patients admitted to GHESKIO CTC October 28, 2010–July 10, 2011
| Variables | n (%) | 1st epidemic wave | Interepidemic Period | 2nd epidemic wave |
|---|---|---|---|---|
| (%) | (%) | (%) | ||
| Age (years) | ||||
| < 5 | 531 (14) | 95 (7) | 24 (8) | 310 (12) |
| 5–19 | 1,002 (26) | 385 (28) | 50 (16) | 566 (22) |
| 20–39 | 1,564 (40) | 499 (37) | 78 (25) | 987 (39) |
| 40–59 | 616 (16) | 200 (15) | 22 (7) | 393 (16) |
| > 60 | 212 (5) | 68 (5) | 8 (3) | 136 (5) |
| Unknown | 249 (6) | 107 (8) | 13 (4) | 129 (5) |
| Total | 4,070 | 1,354 | 195 | 2,521 |
| Gender | ||||
| Male | 2,182 (54) | 713 | 117 | 1,352 |
| Female | 1,888 (46) | 641 | 78 | 1,169 |
Figure 2.
Population pyramid-patients admitted to GHESKIO CTC October 28, 2010–July 10, 2011.
Figure 3.
Population pyramid-surrounding community population.
Figure 4.
Number of cases admitted to GHESKIO by age group.
Predictors of disease severity and outcomes.
We conducted univariate analysis comparing the degree of dehydration of patients admitted during the first and second epidemic in periods. We found that the degree of dehydration was evenly distributed when comparing the proportion of patients admitted during the first and second periods (Table 2). However, when the inter-epidemic period was included in the analysis, we found that there was a statistically significant association (P < 0.0001) between degree of dehydration and epidemic period. The outcomes measured were death, likelihood of being referred to outside facilities for treatment of other co-morbidities in complex cases, and duration of hospitalization. There were a total of 15 deaths, with 9 deaths occurring within < 24 hours of presentation with overall mortality of 0.37% for our cohort. Causes of death were often a result of cardiovascular shock and were associated with more severe dehydration on admission Table 3A. A logistic model was applied to analyze likelihood of transfer to outside facilities for care as a predictor of disease severity (Table 3B). A total of 117 patients were recorded to have been transferred to other healthcare centers and 107 had a documented degree of dehydration. Causes for transfer were primarily for treatment of co-mordibities complicating their admission such as hypertensive crisis, diabetic emergencies, and respiratory infections. Our analysis indicated that patients with severe dehydration were more likely to be transferred out to other facilities for more advanced care (OR 2.88, 95% CI 1.73–4.77, P value < 0.0001). Using a logistic regression model we also measured the association between length of stay and degree of dehydration Table 4, to assess disease severity on admission as a predictor of duration of hospitalization.
Table 2.
Univariate analysis of degree of dehydration by epidemic period
| Mild (%) | Moderate (%) | Severe (%) | P value | |
|---|---|---|---|---|
| 1st epidemic wave | 416 (44.4) | 316 (33.8) | 204 (21.8) | < 0.0001 |
| Interepidemic period | 69 (35.4) | 101 (51.8) | 25 (12.8) | |
| 2nd epidemic wave | 1078 (42.8) | 971 (38.6) | 470 (18.7) |
Table 3A.
Univariate analysis of degree of dehydration and death
| Degree of dehydration | Deaths | Death rate | P value* |
|---|---|---|---|
| Mild | 0 | 0 | < 0.0001 |
| Moderate | 4 | 0.09 | |
| Severe | 11 | 0.27 | |
| Total | 15 | 0.037 |
Observation with unknown degree of hydration was excluded from χ2 test.
Table 3B.
Logistic regression of likelihood of referral to an outside facility*
| Referred | Not referred | Odds ratio (95% CI) | P value | |
|---|---|---|---|---|
| Dehydration | ||||
| Mild | 26 | 1097 | reference | NA |
| Moderate | 36 | 988 | 1.52 (0.90, 2.56) | 0.12 |
| Severe | 45 | 586 | 2.88 (1.73, 4.77) | < 0.0001 |
Observations with incomplete covariates were not included in this analysis.
Table 4.
Multivariate analysis/length of stay and degree of dehydration according to age and gender
| Length of stay | |||||
|---|---|---|---|---|---|
| < 24 hours | 24–48 hours | > 48 hours | |||
| Dehydration | Odds ratio (95% CI) | P value | |||
| Mild | 1131 (81.2) | 159 (11.4) | 103 (7.4) | Reference | NA |
| Moderate | 257 (20.5) | 624 (49.7) | 374 (29.8) | 12.91 (10.81, 15.41) | < 0.0001 |
| Severe | 86 (13.7) | 198 (31.4) | 346 (54.9) | 30.41 (24.44, 37.85) | < 0.0001 |
| Age | |||||
| < 5 yrs | 195 (51.9) | 100 (26.6) | 81 (21.5) | Reference | NA |
| 5–19 yrs | 392 (47.1) | 240 (28.8) | 201 (24.1) | 0.96 (0.74, 1.24) | 0.74 |
| 20–39 yrs | 610 (45.5) | 409 (30.5) | 322 (24.0) | 0.93 (0.72, 1.18) | 0.54 |
| 40–59 yrs | 211 (38.8) | 170 (31.3) | 163 (30.0) | 1.11 (0.84, 1.46) | 0.48 |
| > 60 yrs | 66 (4.5) | 62 (33.7) | 56 (30.4) | 0.90 (0.62, 1.30) | 0.57 |
| Gender | |||||
| Women | 680 (45.1) | 419 (27.8) | 408 (27.1) | Reference | NA |
| Men | 794 (44.8) | 562 (31.7) | 415 (23.4) | 0.85 (0.74, 0.98) | 0.03 |
These results were calculated taking into consideration that 19% of the cohort had undocumented either length of stay and/or degree of dehydration. Overall, our findings suggest that patients with more severe dehydration had a greater chance to have longer admission (P values were < 0.0001 for moderate and severe dehydration separately). Age did not influence length of hospitalization; however, men had shorter hospitalizations than women (OR 0.85; 95% CI 0.74–0.98, P value 0.03) (Table 4).
Association of degree of dehydration with host characteristics and epidemic period.
Based on the three ordinal categories of degree of dehydration (mild, moderate, and severe), a proportion of the odds model was applied to study the association between age, gender, HIV status, time of presentation during study period and the trend toward more severe dehydration Table 5. There was a statistically significant association between age and degree of dehydration, where compared with children < 5 years of age, patients 5 years of age or older had a progressive trend toward more severe dehydration with age. Notably, for people > 60 years ago, the chance was more than tripled (OR 3.5 95% CI 2.52–4.85 P < 0.0001) when compared with younger patients. We did not find a significant association between gender and degree of dehydration. Additionally, when adjusting for age and gender, we found no significant penchant toward more severe dehydration across epidemic periods. However, severe illness was more frequently seen during the two epidemic peaks, 19–22%, compared with the interepidemic period, 13%. Seven hundred and thirty-four patients (18% of cohort) had known HIV status, of including 83 HIV positive patients (11.3%) (Table 5). Of note, 22 patients were previously known to be HIV positive either through prior screening at GHESKIO or known history of HIV. The HIV seropositivity rate remained high when testing was routinely performed during the second epidemic arguing against a selection bias in choosing those to be tested. The HIV rate of patients admitted to GHESKIO during the cholera epidemic is higher than the reported seroprevalence in the community, 1.9%20 and the HIV rate of 10% noted in HIV at-risk individuals presenting to GHESKIO for voluntary counseling and testing. Despite the high rate of seropositivity, there was no significant correlation between HIV status and the degree of dehydration.
Table 5.
Severity of illness in patients with known human immunodeficiency virus (HIV) status
| Known HIV status | Total | Mild | Moderate | Severe | P value* |
|---|---|---|---|---|---|
| Positive | 83(11.3) | 15 (18.8) | 42 (52.5) | 23 (28.8) | 0.45 |
| Negative | 649 (88.4) | 168 (26.2) | 293 (45.6) | 181 (28.2) | |
| Indeterminate | 2 (0.3) | 1 | 1 | 0 | |
| Total | 734 | 184 | 336 | 204 |
Dynamics of cholera with rainfall.
The daily rainfall in Port-au-Prince was plotted against weekly GHESKIO CTC admissions (Figure 5); an association in the first epidemic with rainfall is hard to discern as cholera was spreading rapidly in a naive population. Tropical storm Thomas in early November of 2010 was accompanied by an increase in rainfall accumulation peaking at 162 mm/day. However, this preceded an increase in the number of admissions by several weeks. The second epidemic wave provided an opportunity to look more closely at the relationship of rainfall to an increase in cholera cases. There were two separate occurrences of increased rainfall accumulation on April 28, 2011 and on June 2, 2011. The peak in rainfall accumulation in April (66.3 mm/day) correlates with a steady increase in number of admissions at the beginning of the shoulder of the second epidemic wave. The most rain (116 mm/day) preceded the height of cases admitted during the second epidemic wave (339 cases) by only one day. Perhaps more striking is that cholera subsided when the rate of rainfall decreased.
Figure 5.
Time plot of weekly reported cases and daily rainfall.
Effect of sanitary interventions.
We attempted to crudely measure the impact of basic public health infrastructure in two adjacent populations whereby a census was conducted shortly following the study period. As previously described, the GHESKIO IDP had access to clean water and to basic sanitation before the cholera outbreak, whereas the neighboring shanty town of Village de Dieu did not have access to these measures until months after the onset of the epidemic. The incidence of admission of patients from the GHESKIO IDP was 30% lower than that of Village de Dieu (Table 5). However, the distribution of severity of illness in the two populations did not differ significantly (Table 6).
Table 6.
Incidence rates and severity of patients from GHESKIO internally displaced persons (IDP) and adjacent slum
| Camp | Pts admitted to CTC | Population | Incidence Rate (%) | |
|---|---|---|---|---|
| Cite de Dieu | 410 | 15,000 | 2.73 | |
| GHESKIO IDP camp | 95 | 5,000 | 1.9 | |
| Degree of dehydration | ||||
| Mild | Moderate | Severe | ||
| Cite de Dieu | 190 (50.4) | 124 (32.9) | 63 (16.7) | P value |
| GHESKIO IDP camp | 32 (36.8) | 43 (49.4) | 12 (13.8) | 0.014 |
Discussion
Because clinical cholera has not been reported in Haiti before October of 2010, our study describes the clinical implications of the disease in a naive population. This is in contrast to most descriptions of cholera that are in endemic areas. Another unique aspect of this study is that it was conducted during earlier parts of the epidemic—a time before extensive public health measures were in place. One purpose of this review was to identify at risk populations on the basis of age, gender, or HIV status. Although previous sero-epidemiologic studies have identified children as a group susceptible to contracting cholera,18,19 the results of this analysis indicate that the great majority of patients admitted were adults. Our study also shows that adults have a longer hospitalization, more severe dehydration on presentation, and a higher likelihood of referral to outside facilities for higher level care. During the two epidemic peaks, case counts increased among both children < 5 years of age and those 5 years and older; however, the increase in number of cases of diarrhea was less evident in those < 5 years of age (Figure 4), suggesting that other causes of diarrhea, e.g., rotavirus or norovirus are major players in the etiology of undercurrent diarrheal childhood illness. We certainly recognize that not all the illness presenting to the CTC is cholera, although the majority had the acute rice water diarrhea that typifies this disease. As anticipated, longer hospital stay, death, and likelihood of referral to outside facilities were all predictors of having more severe disease. However, HIV disease did not prove to be a risk factor for a trend toward more severe dehydration. One study suggested that HIV-infected patients were more susceptible to disease based on lower vibriocidal responses to cholera vaccine when compared with non-HIV infected recipients.23 In our cohort, HIV-status did not influence the severity of disease, but the HIV seroprevalence was high. This observation is the subject of an ongoing study at GHESKIO. Although the difference is striking, the higher prevalence of HIV patients could represent a selection bias because a considerable number of patients receive long-term care at GHESKIO for infectious diseases.
A sero-survey conducted in central Haiti within the first 6 months of the epidemic showed that children < 5 years of age had the highest prevalence of elevated antibody titers against cholera toxin.20 Conversely, previous studies performed in cholera-endemic countries show that vibriocidal antibodies increase proportionally with age.21 This would suggest that although cholera was recently introduced in Haiti, it is affecting all ages and that public health interventions are going to have to be universal to effectively curb the epidemic. In a previous study, we attempted to validate the use of oral mucosal fluid to measure cholera antibody response in 13 patients with culture confirmed cholera admitted to the study location CTC22; we hoped to have a simple, non-invasive tool to determine the penetrance of cholera in cholera-naïve populations and predict the added benefits of vaccine induced immunity. However, antibodies detected in oral fluid had 20-fold lower concentrations than serum and even at a 1:10 dilution were often but not uniformly positive in these culture proven cases. At this point without a firm population estimate of the catchment area, we cannot estimate what percentage of the population served by the study area has been infected with V. cholerae. At the height of the epidemic, ∼197 health care facilities were accessible to residents of Port-au-Prince. Many cholera treatment structures were established by various non-governmental organizations (NGOs), including 28 CTCs, 58 CTUs, and 111 oral rehydration posts (ORPs).13 With over 10,000 patients treated at the study location, the demands of maintaining an active CTC required 5 physicians, 12 nurses, and 30 support staff to provide rotating 24-hour coverage. In this study, we noted a higher number of patients presenting with acute diarrhea later during the epidemic, when compared with national data. One presumption is because a considerable number of treatment centers began closing during the latter part of the study period, resulting in an overflow of patients seeking care at alternate sites such as our study site. To replace the 40-bed tent facility used during the period described in this report a 100-bed permanent CTC has replaced the temporary structure on the GHESKIO grounds.
Our study has also depicted a known fact that increased rainfall is directly related to the number of suspected cholera cases admitted. This known fact will be useful in future plans aimed at cholera control measures. In addition to the impact of rainfall, we suspect that the number of admissions to the CTC was also affected by the sporadic opening and closing of other surrounding CTC's during the epidemic.
There were several limitations to this study relating to uniform data entry. The individuals retrieving data found that weight, volume of fluids administered, and precise addresses were often not available. The use of parenteral fluids and antibiotics were not strictly administered based on clinical assessment. Often, this was a result of a need for rapid resuscitation of large volume of patients and lack of medical staff to assure safe oral rehydration. Antibiotics were given to those moderately and severely ill but no assessment of their effectiveness can be derived from the data collected here. The difficulty in tracking the epidemic at a national level is even greater. A recent study by the Centers for Disease Control and Prevention (CDC) showed successful establishment of such a system that uses a monitoring and evaluation framework to implement cholera surveillance to aid interventions.7
Our study paints a picture of a severe diarrhea illness with rapid onset that appeared to increase in severity with advancing age. The mortality rate was below, better than, the acceptable rate for a cholera response. The burden placed on GHESKIO, which had functioned as an outpatient clinic to provide intensive care and fluid resuscitation in tents designed for 40 patients with as many as several hundred patients a day was considerable not only in staffing demands but in the use of pharmacy and coordinating activities. The Haitian cholera epidemic emphasizes the importance of developing sustainable methods of record keeping in settings of public health response to major epidemics. Additionally, there are a multitude of stakeholders involved in the health care in Haiti, that were magnified in the months after the earthquake. Historically, there have been issues with incomplete data, inaccurate data, lack of timely data collection, and parallel information systems among various partners working in health. In contrast, the Haitian response to the cholera epidemic as reflected in the experience reported here and replicated throughout the country is a strong reflection of the Haitian and international community's capacity to respond to a public health disaster. The difficult task ahead is to intervene to improve the underlying public health infrastructure and use all the tools available to make Haiti and the Western hemisphere again cholera free. The study, however, could roughly estimate the impact of public health interventions aimed at clean water and sanitation on cholera severity and case volume. The study location camp for IDP and Village de Dieu are two neighboring communities who had different access to clean water and sanitation during the study period. The study site IDP camp was established soon after the earthquake where residents direct access to sanitation, routine medical services and clean water, whereas these accommodations were not available for Village de Dieu residents. Our results show that the incidence admission to the GHESKIO CTC was 30% less in patients living in the GHESKIO IDP camp at 1.9% compared with patients living in the adjacent Village de Dieu community who had an incidence of 2.73%. Although results are crude, they suggest that public health interventions did diminish disease transmission but that sanitation alone might not eliminate cholera in the urban slums of Haiti. Despite the efforts underway to allay spread of cholera in the midst of a delicate lattice of political, economic and public health strife, it has now become endemic to Haiti.7 Our study cohort mostly resides in densely populated neighborhoods in flood-prone areas, with patients still residing in tents or in earthquake ravaged housing. In an endeavor to supplement ongoing public health implementations, this same population was the site of a successful demonstration project for the administration of two doses of oral cholera vaccine to 50,000 people, in the spring of 2012 (see accompanying paper by Rouzier and others)1. Because natural cholera infection elicits temporary immunity, acquired immune responses over time may result in progressive lengthening in the interval where low case volumes are reported at the population level therefore propagating transmission. This substantiates the need for further undertaking of measures to mitigate spread such as future vaccination programs.9,10
Cholera is now likely to remain endemic to Haiti as it has in most parts of Africa ever since 1971 and will prove a continued threat to its fragile public health system already overburdened by natural disasters and ongoing epidemics of AIDS and tuberculosis. Haiti's poor water poverty index ranking will be an ongoing challenge to curbing the epidemic.8,24 Studies looking at disease transmission patterns in Haiti have strongly suggested the benefits of cholera vaccination as a supplemental public health tool to avert mortality and morbidity.25,26 Recently, the MSPP in collaboration with CDC, Pan American Health Organization (PAHO), and the United Nations Children's Fund (UNICEF) launched a national plan to eradicate cholera in Haiti in 10 years. The MOH plan includes the use of oral cholera vaccines in addition to major interventions to provide potable water to 85% of the population, make latrines available, and improve sanitation.
ACKNOWLEDGMENTS
We thank the entire staff of Les Centres GHESKIO for their dedication and hard work.
Footnotes
Financial support: UNICEF cholera Grant « Renforcement et maintien des activités dans le cadre de la réponse pour le Cholera ». Centers for Disease Control: 5U2GGH000545-02 and The National Institutes of Health: 1K24 AI098627-02; 5 U2R TW006896-10; 5D43 TW 000018-25; 4UM1AI069421-07.
Authors' addresses: Claude-Lyne Valcin and Peter F. Wright, Dartmouth Medical School, Division of Infectious Disease and International Health, Hanover, NH, E-mails: cvalcin@gmail.com and peter.f.wright@dartmouth.edu. Karine Severe, Claudia T. Riche, Benedict S. Anglade, Colette Guiteau Moise, Macarthur Charles, and Patrice Joseph, Les Centres GHESKIO, Port-au-Prince, Haiti, E-mails: karinesevere@gheskio.org, claudiathriche@yahoo.ca, sanaglde@gheskio.org, colette0786@hotmail.com, mcharles@gheskio.org, and pjoseph@gheskio.org. Michael Woodworth, Duke University Health System, Internal Medicine, Durham, NC, E-mail: holmes.woodworth@gmail.com. Zhongze Li, Norris Cotton Cancer Center, Biostatistics Shared Resource, Lebanon, NH, E-mail: zhongze.li@dartmouth.edu. Jean W. Pape, Cornell University, Weill Medical College, New York, NY, E-mail: jwpape@gheskio.org.
References
- 1.World Health Organization Relevé épidémiologique hebdomadaire/Section d'hygiène du Secrétariat de la Société des Nations = Weekly epidemiological record/Health Section of the Secretariat of the League of Nations. 2012;87:289–304. [Google Scholar]
- 2.Chin C-S, Sorenson J, Harris JB, Robins WP, Charles RC, Jean-Charles RR, Bullard J, Webster DR, Kasarskis A, Peluso P, Paxinos EE, Yamaichi Y, Calderwood SB, Mekalanos JJ, Schadt EE, Waldor MK. The origin of the Haitian cholera outbreak strain. N Engl J Med. 2010;364:33–42. doi: 10.1056/NEJMoa1012928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Cure for Cholera—Improving Access to Safe Water and Sanitation—NEJM. http://www.nejm.org/doi/full/10.1056/NEJMp1214179 Available at. Accessed March 4, 2013. [DOI] [PubMed]
- 4.Ali M, Lopez AL, You YA, Kim YE, Sah B, Maskery B, Clemens J. The global burden of cholera. Bull World Health Organ. 2012;90:209A–218A. doi: 10.2471/BLT.11.093427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Periago MR, Frieden TR, Tappero JW, De Cock KM, Aasen B, Andrus JK. Elimination of cholera transmission in Haiti and the Dominican Republic. Lancet. 2012;379:e12–e13. doi: 10.1016/S0140-6736(12)60031-2. [DOI] [PubMed] [Google Scholar]
- 6.Ivers LC, Farmer P, Almazor CP, Léandre F. Five complementary interventions to slow cholera: Haiti. Lancet. 2010;376:2048–2051. doi: 10.1016/S0140-6736(10)62243-X. [DOI] [PubMed] [Google Scholar]
- 7.Barzilay EJ, Schaad N, Magloire R, Mung KS, Boncy J, Dahourou GA, Mintz ED, Steenland MW, Vertefeuille JF, Tappero JW. Cholera surveillance during the Haiti epidemic—the first 2 years. N Engl J Med. 2013;368:599–609. doi: 10.1056/NEJMoa1204927. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan CA, Meigh JR, Giacomello AM. The water poverty index: development and application at the community scale. Nat Resour Forum. 2003;27:189–199. [Google Scholar]
- 9.Desai SN, Clemens JD. An overview of cholera vaccines and their public health implications. Curr Opin Pediatr. 2012;24:85–91. doi: 10.1097/MOP.0b013e32834eb625. [DOI] [PubMed] [Google Scholar]
- 10.Tuite AR, Tien J, Eisenberg M, Earn DJ, Ma J, Fisman DN. Cholera epidemic in Haiti, 2010: using a transmission model to explain spatial spread of disease and identify optimal control interventions. Ann Intern Med. 2011;154:593–601. doi: 10.7326/0003-4819-154-9-201105030-00334. [DOI] [PubMed] [Google Scholar]
- 11.Sack DA, Sack RB, Chaignat C-L. Getting serious about cholera. N Engl J Med. 2006;355:649–651. doi: 10.1056/NEJMp068144. [DOI] [PubMed] [Google Scholar]
- 12.Center for Disease Control Epidemic cholera in a crowded urban environment, Port-au-Prince, Haiti. Emerg Infect Dis. 2011;17:2143–2145. doi: 10.3201/eid1711.110772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.International Federation of Red Cross and Red Crescent Societies . Geneva: International Federation of Red Cross and Red Crescent Societies; 2011. Haiti and the Dominican Republic—cholera outbreak: response and preparedness.http://reliefweb.int/rw/RWFiles2011.nsf/FilesByRWDocUnidFilename/MUMA-8DK2KCfull_report.pdf/$File/full_report.pdf Available at. Accessed February 13, 2013. [Google Scholar]
- 14.Center for Disease Control Launching a National Surveillance System after an earthquake—Haiti, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:933–938. [PubMed] [Google Scholar]
- 15.Center for Disease Control Rapid establishment of an internally displaced persons disease surveillance system after an earthquake—Haiti, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:939–945. [PubMed] [Google Scholar]
- 16.Duggan C, Santosham M, Glass RI. The management of acute diarrhea in children: oral rehydration, maintenance, and nutritional therapy. MMWR Morb Mortal Wkly Rep. 1992;41:1–20. [PubMed] [Google Scholar]
- 17.Swerdlow DL, Mintz ED, Rodriguez M, Tejada E, Ocampo C, Espejo L, Barrett TJ, Petzelt J, Bean NH, Petzelt J, Seminario L, Tauxe RV. Severe life-threatening cholera associated with blood group O in Peru: implications for the Latin American epidemic. J Infect Dis. 1994;170:468–472. doi: 10.1093/infdis/170.2.468. [DOI] [PubMed] [Google Scholar]
- 18.Charles M, Delva GG, Apollon A, Beaulieu ME, Pape JW. Etiology of acute diarrheal illness among patients visiting a cholera treatment center in Haiti using the xTAG gastrointestinal panel (GPP) assay. 2012. http://www.luminexcorp.com/prod/groups/public/documents/lmnxcorp/xtag-gpp-poster-charles-cvs.pdf Abstract. Available at. Accessed March 2, 2013.
- 19.Rinaldo A, Bertuzzo E, Lorenzo M, Lorenzo R, Blokesch MG, Casagrandi R, Murray M, Vesenbeckh SM, Rodrigurez-Iturbe I. Reassessment of the 2010–2011 Haiti cholera outbreak and rainfall-driven multiseason projections. Proceedings of the National Academy of Sciences. 2012 doi: 10.1073/pnas.1203333109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ministère de la Santé Publique et de la Population, Institut Haïtien de l'Enfance, Centres GHESKIO, Center for Disease Control and Prevention Etude de séro surveillance par method sentinelle de la prévalence du VIH, de la syphilis, de l'hépatite B et de l'hépatite C et chez les femmes. http://data.unaids.org/pub/Report/2007/haitihivserosurveill2007_fr.pdf Available at. Accessed March 2, 2013.
- 21.Harris JB, LaRocque RC, Chowdhury F, Khan AI, Logvinenko T, Faruque AS, Ryan ET, Qadri F, Calderwood SB. Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl Trop Dis. 2008;2:e221. doi: 10.1371/journal.pntd.0000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris J, Khan A, LaRocque R, Dorer D, Chowdhury F, Faruque A, Sack DA, Ryan ET, Quadri F, Calderwood SB. Blood group, immunity, and risk of infection with Vibrio cholerae in an area of endemicity. Infect Immun. 2009;73:7436–7441. doi: 10.1128/IAI.73.11.7422-7427.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson BR, Talkington D, Pruckler J, Gómez G, Fouché B, Lafosse E, Hooper C, Payne A, Paynel A, Nygren B, Archer RW, Dhourou G, Freeman N, Boncy J, Mintz E. Sero-epidemiologic survey of epidemic cholera in Haiti to assess spectrum of illness and risk factors for severe disease. 2012. Abstract–Annual meeting: American Society of Tropical Medicine and Hygiene: Abstract No. 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glass RI, Svennerholm AM, Khan MR, Huda S, Huq MI, Holmgren J. Seroepidemiological studies of El Tor cholera in Bangladesh: association of serum antibody levels with protection. J Infect Dis. 1985;151:236–242. doi: 10.1093/infdis/151.2.236. [DOI] [PubMed] [Google Scholar]
- 25.Valcin CL, Guiteau-Moise C, Joseph Taylor R, Son M, Housman M, Modern P, Wright PF, Pape JW. A Novel Method to Determine Immunity to Vibrio Cholerae in a previously Immunologically-Naïve Population. Boston, MA: 2008. Abstract–IDSA annual meeting. [Google Scholar]
- 26.Von Seidlein L, Wang XY, Macuamule A, Mondlane C, Puri M, Hendriksen I, Deen JL, Chaignat CL, Clemens JD, Ansaruzzaman M, Barreto A, Songane FF, Lucas M. Is HIV infection associated with an increased risk for cholera? Findings from a case-control study in Mozambique. Trop Med Intl Health. 2008;13:683–688. doi: 10.1111/j.1365-3156.2008.02051.x. [DOI] [PubMed] [Google Scholar]