Abstract
In 2010, toxigenic Vibrio cholerae was newly introduced to Haiti. Because resources are limited, decision-makers need to understand the effect of different preventive interventions. We built a static model to estimate the potential number of cholera cases averted through improvements in coverage in water, sanitation and hygiene (WASH) (i.e., latrines, point-of-use chlorination, and piped water), oral cholera vaccine (OCV), or a combination of both. We allowed indirect effects and non-linear relationships between effect and population coverage. Because there are limited incidence data for endemic cholera in Haiti, we estimated the incidence of cholera over 20 years in Haiti by using data from Malawi. Over the next two decades, scalable WASH interventions could avert 57,949–78,567 cholera cases, OCV could avert 38,569–77,636 cases, and interventions that combined WASH and OCV could avert 71,586–88,974 cases. Rate of implementation is the most influential variable, and combined approaches maximized the effect.
Introduction
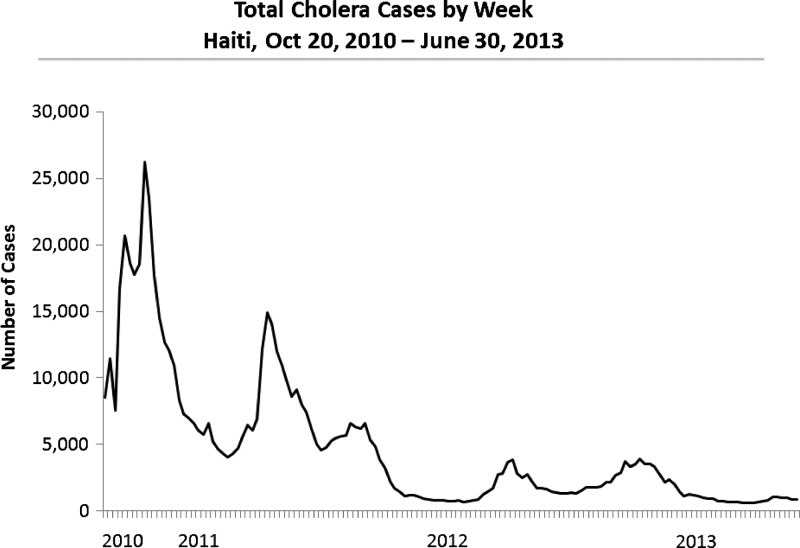
In October 2010, cholera was introduced to earthquake-stricken Haiti.1 Within days of its introduction, a National Cholera Surveillance System was implemented.1 Through June 30, 2013, Haiti had reported 663,134 cases of cholera (Figure 1)2; of these, 366,995 (55.3%) were hospitalized and 8160 (1.2%) died. As we approach the three-year mark, cholera will likely be considered endemic to Haiti.
Figure 1.
Total (n = 663,134) cholera cases by week, Haiti, October 20, 2010–June 30, 2013.
Water, sanitation, and hygiene (WASH) interventions, such as latrines, point-of-use chlorination and piped water, have long been recognized as effective prevention measures against cholera and other diarrheal diseases.3–12 In 2008, 63% of the Haitian population had access to improved water and 17% to improved sanitation.13 In 2010, after the earthquake, the Haitian Directorate for Potable Water and Sanitation reported that 26% of the rural population received improved water and 10% improved sanitation; in the Port-au-Prince metropolitan area, the coverage was 35% and 20%, respectively (Haitian Directorate for Potable Water and Sanitation five-year plan). Many public health scientists believe that sustained improvements in access to safe water and sanitation can eliminate transmission of cholera in Haiti, citing interventions used throughout South and Central America in the 1990s.14,15 The WASH interventions, including hand washing, have the additional benefit of reducing the incidence of other diarrheal and respiratory diseases.3,5,16,17 Although improving water and sanitation infrastructure is the ultimate goal of the Haitian Government and the international community, it will take considerable time.18
Oral cholera vaccine (OCV) has been proposed as an effective adjunct for cholera control in endemic and epidemic settings.19,20 Two whole-cell, killed, World Health Organization–prequalified OCVs are available: Dukoral® (Crucell, Stockholm, Sweden) and Shanchol™ (Shantha Biotechnics, Hyderabad, India). Both vaccines require two doses given two weeks apart, with protective immunity developing approximately one week after the second dose.21,22 The Haitian government sanctioned two pilot studies23 to assess the acceptability and feasibility of Shanchol™ vaccine, one in urban Haiti and one in rural Haiti.24 Based on these pilot study findings and findings from previous OCV studies, the Pan American Health Organization has recommended targeted or mass OCV campaigns that use Shanchol™ as an intermediate bridge to reduce cholera transmission in Haiti while improvements in water and sanitation infrastructure are implemented.24 We present results of a model that illustrates the potential impact of WASH and OCV interventions independently and in combination. These results can aid public health decision makers in allocating resources to prevent cholera transmission in Haiti.
Methods
We used Excel 2010 (Microsoft, Redmond, WA) to develop a spreadsheet-based, static mathematical model in which we allowed a degree of indirect protection (or herd immunity) for OCV and WASH interventions by including non-linear relationships between percentage of population covered and percentage of population effectively protected (i.e., for a given percentage vaccinated or who received WASH interventions, an additional percentage was also indirectly protected). For WASH interventions, we included latrines, point-of-use chlorination, and community piped water (standpipes). We divided Haiti's population into urban and rural elements. For both urban and rural populations, and for each intervention, we constructed three scenarios that illustrated potential rates-of-growth of coverage over 20 years. We also constructed scenarios in which we allowed a combination of WASH and OCV in rural and urban areas. In these combined scenarios, we conservatively assumed that persons who received OCV would not be covered by WASH interventions and vice versa. Thus, coverage for either WASH or for OCV interventions would never exceed 50%. We modeled 16 scenarios: six WASH, six OCV, and four that combined WASH and OCV interventions. For further details, see Online Supplemental Materials.
Demographics and expected annual incidence.
We used current population figures for Haiti stratified by rural and urban environments, and estimated population growth rate to project demographic growth over a 20-year period (Supplemental Table 1). Because toxigenic Vibrio cholerae was only recently introduced in Haiti, and cholera incidence has changed from an epidemic to an endemic pattern (Figure 1),2 there are no data describing the incidence of endemic cholera in Haiti over a 20-year period. Therefore, we estimated the 20-year annual incidence of endemic cholera in Haiti by using 1990–2010 annual incidence data from Malawi as reported to the World Health Organization.25 We chose Malawi because it faces similar socioeconomic challenges to those seen in Haiti (e.g., poor roads, relatively high infant mortality rate, large population without piped water, rates of literacy < 80%).26 We also performed sensitivity analyses by using annual incidence data for endemic cholera from Mozambique and India, as well as a set of hypothetical annual incidence data (Online Supplemental Material).
Intervention effectiveness.
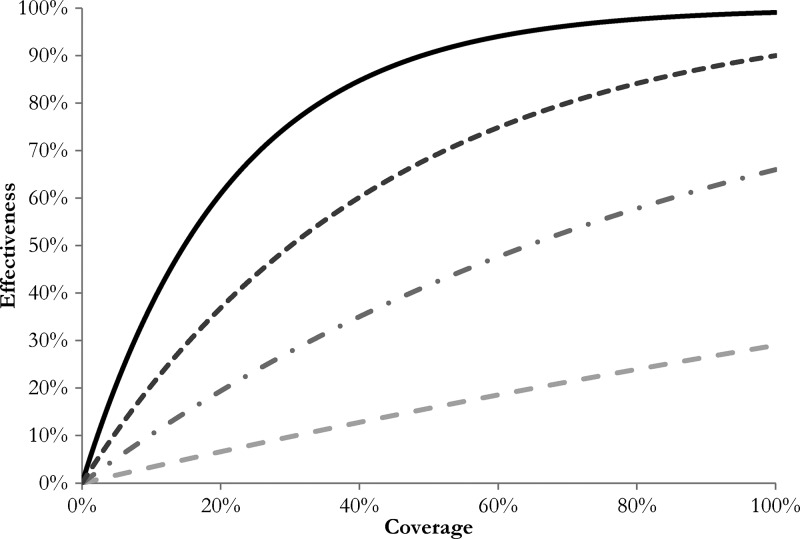
For each intervention, we included non-linear relationships between coverage and effectiveness that take into account indirect protective effects27 (Figure 2). For OCV (Shanchol™), we fitted an exponential curve to the OCV (Dukarol®) coverage-effectiveness modeling data from Longini and others.28 (Figure 2; Supplemental Table 2). The randomized control trial data for Shanchol™ administered as a two-dose regimen showed a direct efficacy of 67% after two years, which is nearly identical to that for Dukarol®21 and 66% after three years.29,30 Therefore, we assumed that our use of the Dukarol® coverage-effectiveness curve as a proxy for the Shanchol™ coverage-effectiveness curve was reasonable. We did not examine partial vaccination effect (i.e., receiving only one dose). The OCV coverage in our model implies effective coverage with two doses of Shanchol™ vaccine, and in addition, assumes that all two dose recipients will receive one booster dose every three years thereafter (Supplemental Table 4). For latrines and point-of-use chlorination, we estimated non-linear curves based on data from two reviews of interventions3,5 (Figure 2; Supplemental Table 2). For piped water, we assumed a non-linear curve with a protective effect of 90% at 100% coverage (Figure 2; Supplemental Table 2). Because there are little data on the synergistic effect of one or more WASH interventions, we used a conservative approach and assumed no additive effect across the various combinations of possible WASH interventions.7 Therefore, we used a stepwise introduction of WASH interventions over time, and the intervention with the stronger protective effect supplanted the other (i.e., piped water > chlorinated water > latrines). For further detail, see the Online Supplemental Material.
Figure 2.
Coverage-effectiveness curves for various interventions. Black line indicates oral cholera vaccine; dark gray dotted line indicates piped water; gray dotted-dashed line indicates point-of-use chlorination; light gray dashed line indicates latrines.
Intervention coverage over time.
For each urban (U) and rural (R) population, we modeled three rates of intervention implementation over 20 years for WASH (WASH/U 1, U 2, U 3 and WASH/R 1, R 2, R 3) and OCV (OCV/U 1, U 2, U 3 and OCV/R 1, R 2, R 3) interventions (Tables 1 and 2; Supplemental Figures 1, 3, and 4). We assumed that five persons shared one latrine and 50 persons shared one community piped water standpipe. Point-of use chlorination was assumed to occur at a household level. We also assumed that in the first five years of implementation, WASH resources would primarily be allocated towards point-of-use chlorination and that piped water would begin in year 6.
Table 1.
Water, sanitation, and hygiene (WASH) scenarios with percentage of urban (U) and rural (R) Haitian population covered at years 0, 5, and 20*
| Scenario | Intervention | Year 0 (%) | Year 5 (%) | Year 20 (%) |
|---|---|---|---|---|
| WASH/U1 | Latrines | 10 | 10 | 0 |
| Point-of-use chlorination + L | 20 | 80 | 25 | |
| Piped water + C + L | 10 | 10 | 75 | |
| Total | 40 | 100 | 100 | |
| WASH/U2 | Latrines | 10 | 10 | 0 |
| Point-of-use chlorination + L | 20 | 60 | 50 | |
| Piped water + C + L | 10 | 10 | 50 | |
| Total | 40 | 80 | 100 | |
| WASH/U3 | Latrines | 10 | 10 | 0 |
| Point-of-use chlorination + L | 20 | 40 | 80 | |
| Piped water + C + L | 10 | 10 | 20 | |
| Total | 40 | 60 | 100 | |
| WASH/R1 | Latrines | 10 | 30 | 0 |
| Point-of-use chlorination + L | 26 | 40 | 30 | |
| Piped water + C + L | 0 | 0 | 70 | |
| Total | 36 | 70 | 100 | |
| WASH/R2 | Latrines | 10 | 20 | 8 |
| Point-of-use chlorination + L | 26 | 30 | 42 | |
| Piped water + C + L | 0 | 0 | 50 | |
| Total | 36 | 50 | 100 | |
| WASH/R3 | Latrines | 10 | 10 | 10 |
| Point-of-use chlorination + L | 26 | 30 | 42 | |
| Piped water + C + L | 0 | 0 | 25 | |
| Total | 36 | 40 | 77 |
C = point-of-use chlorination; L = latrines.
Table 2.
Oral cholera vaccine (OCV) scenarios with percentage of Haitian urban (U) and rural (R) population covered at years 0, 5, and 20
| Scenarios | Year 0 (%) | Year 5 (%) | Year 20 (%) |
|---|---|---|---|
| OCV/U1 | 1 | 50 | 90 |
| OCV/U2 | 1 | 20 | 60 |
| OCV/U3 | 1 | 10 | 25 |
| OCV/R1 | 1 | 50 | 65 |
| OCV/R2 | 1 | 20 | 40 |
| OCV/R3 | 1 | 10 | 25 |
In addition, we generated two scenarios that combined WASH and OCV for each of the urban and rural settings (Table 3) . The four combined scenarios differ in coverage rate achieved by year 20 for each WASH and OCV intervention. For example, in the first urban and rural combined scenarios (Combined/U1, Combined/R1), we assumed that OCV reached peak coverage of 20% at year 5 and then decreased to 5% by year 20. For the second urban and rural combined scenarios (Combined/U2, Combined/R2), we assumed that OCV coverage peaked at 10% in year 5 and then decreased to 0% by year 20.
Table 3.
Combined oral cholera vaccine (OCV) and water, sanitation and hygiene (WASH) scenarios by percentage of Haitian urban (U) and rural (R) population covered, and at years 0, 5, and 20*
| Scenarios | Interventions | Year 0 (%) | Year 5 (%) | Year 20 (%) |
|---|---|---|---|---|
| Combined/U1 | OCV | 1 | 20 | 5 |
| WASH sub-total | 40 | 50 | 50 | |
| Latrines | 10 | 10 | 0 | |
| Point-of-use chlorination + L | 20 | 30 | 0 | |
| Piped water + C + L | 10 | 10 | 50 | |
| Combined/U2 | OCV | 1 | 10 | 0 |
| WASH sub-total | 40 | 50 | 50 | |
| Latrines | 10 | 10 | 0 | |
| Point-of-use chlorination + L | 20 | 30 | 25 | |
| Piped water + C + L | 10 | 10 | 25 | |
| Combined/R1 | OCV | 1 | 20 | 5 |
| WASH sub-total | 36 | 40 | 50 | |
| Latrines | 10 | 10 | 0 | |
| Point-of-use chlorination + L | 26 | 30 | 0 | |
| Piped water + C + L | 0 | 0 | 50 | |
| Combined/R2 | OCV | 1 | 10 | 0 |
| WASH sub-total | 36 | 40 | 50 | |
| Latrines | 10 | 10 | 0 | |
| Point-of-use chlorination + L | 26 | 30 | 25 | |
| Piped water + C + L | 0 | 0 | 25 |
C = point-of-use-chlorination; L = latrines.
Number of cholera cases averted.
Using endemic cholera incidence data from Malawi, we calculated potential cases averted for each scenario by multiplying the estimated incidence and the protective effect of the intervention(s). Cumulative cases averted were discounted by 3% per year.31
Uncertainty/sensitivity analyses.
To assess the robust nature of our model, we performed uncertainty/sensitivity analyses in three steps. First, we varied the baseline incidence rates to see if the change in input would change our results. We used endemic cholera incidence data from Mozambique (1990–2010) and India (1961–1981) to model countries with a higher and a lower mean incidence, respectively. We also created hypothetical scenarios with stable, growing, and decreasing cholera incidence to determine whether different secular trends in annual incidence would change our results. Second, we varied the coverage-effectiveness curves for latrines, point-of-use chlorination, and community piped water to enable uncertainty of the estimates of the protective effectiveness of these WASH interventions. The ranges for their protective effectiveness at 100% intervention coverage are latrines (95% confidence interval = 8–46%), point-of-use chlorination (95% CI = 32–83%), and piped water 90% (default value), and 100% (complete protection) (Supplemental Figure 2). Third, we varied the implementation rate of WASH, OCV, or a combination of both interventions to determine how the number of cumulative cholera cases averted would vary.
OCV uncertainty/sensitivity analyses.
We varied OCV coverage at year 20 from 1% to 100%. We assumed that effective OCV coverage increased linearly for 20 years.
WASH uncertainty/sensitivity analyses.
For latrines, we assumed that in urban Haiti, the percentage of persons with access to latrines only remained the same for the first five years and then was gradually replaced by point-of-use chlorination or piped water; in rural Haiti, the latrine coverage increased at a constant rate from 10% at year 0 to 30% at year 5 and continues to increase at the same rate thereafter until it is gradually replaced by point-of-use chlorination or piped water. Point-of-use chlorination coverage remained at baseline (20% in urban areas and 26% in rural areas) through year 20, or increased to various levels by year 5 (30%, 50%, 70%, or 90% in urban and rural areas, respectively), increasing thereafter through year 20 in the absence of piped water. Piped water coverage remained at baseline (10% in urban areas and 0% in rural areas) for the first five years, increasing thereafter through year 20.
Combined WASH and OCV uncertainty/sensitivity analyses.
In our combined scenarios, we assumed that the respective coverage of OCV and WASH does not exceed 50%. Those persons who would receive OCV would not receive any WASH interventions and vice versa. We assumed that latrine only coverage remained at the baseline (10%) until those persons also received point-of-use chlorination or piped water interventions. Point-of-use chlorination coverage increased from baseline (20% for urban areas and 26% for rural areas) to 30% at year 5, and continued to increase at a constant rate until piped water replaced it (sensitivity analysis scenario 1); or its coverage remained unchanged at the baseline from year 0 to year 5 and remained unchanged for subsequent years until piped water replaced it (sensitivity analysis scenario 2). Piped water coverage remained at baseline (10% in urban areas and 0% in rural areas) for the first 5 years, and increased at a constant rate thereafter to reach 10%, 20%, 30%, 40%, or 50%, respectively, by year 20. The OCV coverage increased at a constant rate from 1% (baseline) at year 0, peaked at year 5, and decreased thereafter at a constant rate to reach 5% at year 20. We varied the OCV coverage attained at year 5 from 1% (baseline) to 50%. Finally, we ran two sets of sensitivity analyses of the four combined interventions in which we first assumed that OCV coverage increased at a constant rate from 1% baseline at year 0 and reached 50% at year 5, and then either decreased at a constant rate to 5% at year 20 or remained at 50% through year 20 (i.e., no decrease) (see Online Supplemental Material).
Results
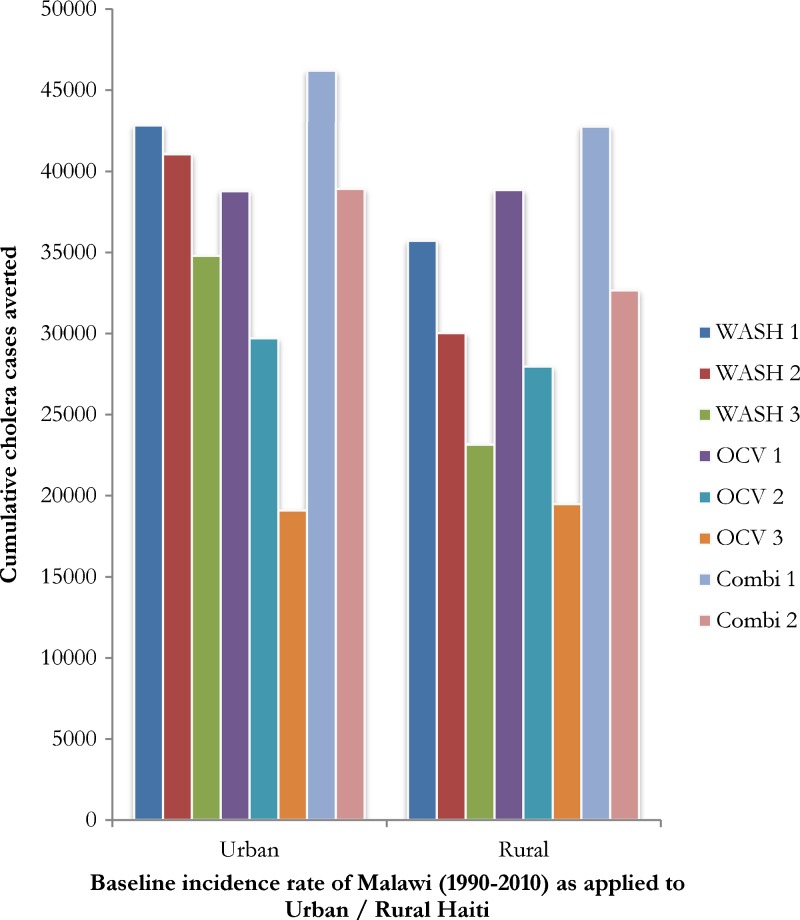
We developed eight urban scenarios (three WASH, three OCV, and two WASH/OCV combined) and eight rural scenarios (three WASH, three OCV, and two WASH/OCV combined). WASH scenario 1 (WASH/R1 + WASH/U1) averted 78,567 cases of cholera. WASH scenario 2 (WASH/R2 + WASH/U2) averted 71,106 cases of cholera. WASH scenario 3 (WASH/R3 + WASH/U3) averted 57,949 cases of cholera (Tables 1 and 4, Figure 3).
Table 4.
Comparisons of the cumulative number of cases of cholera averted by oral cholera vaccine (OCV) and water, sanitation and hygiene (WASH) scenarios applied to Haitian urban (U) and rural (R) populations by using Malawi, Mozambique, and India 20-year endemic cholera incidence data as baseline*
| Baseline incidence rate as applied to Haiti† | U/R | WASH 1 | WASH 2 | WASH 3 | OCV 1 | OCV 2 | OCV 3 | Combi 1 | Combi 2 |
|---|---|---|---|---|---|---|---|---|---|
| Malawi (1990–2010) | U | 42,828 | 41,072 | 34,794 | 38,793 | 29,704 | 19,093 | 46,213 | 38,913 |
| R | 35,739 | 30,034 | 23,155 | 38,843 | 27,964 | 19,476 | 42,761 | 32,673 | |
| Total | 78,567 | 71,106 | 57,949 | 77,636 | 57,668 | 38,569 | 88,974 | 71,586 | |
| Mozambique (1990–2010) | U | 61,879 | 59,313 | 49,427 | 59,223 | 45,931 | 29,529 | 65,384 | 54,827 |
| R | 52,541 | 43,865 | 33,452 | 59,229 | 43,085 | 30,121 | 62,704 | 47,076 | |
| Total | 114,420 | 103,178 | 82,879 | 118,452 | 89,016 | 59,650 | 128,088 | 101,903 | |
| India (1961–1981) | U | 3,711 | 3,530 | 3,010 | 3,421 | 2,508 | 1,588 | 4,124 | 3,473 |
| R | 2,911 | 2,454 | 1,956 | 3,437 | 2,387 | 1,620 | 3,753 | 2,856 | |
| Total | 6,622 | 5,984 | 4,966 | 6,858 | 4,895 | 3,208 | 7,877 | 6,329 |
Combi = combination of WASH and OCV.
Total cumulative cholera incidence (with a discounting rate of 3% per year): Malawi baseline incidence rate scenario: 106,994 cases; Mozambique baseline incidence rate scenario: 142,754 cases; India baseline incidence rate scenario: 9,635 cases.
Figure 3.
Cumulative cases of cholera averted by water, sanitation and hygiene (WASH) interventions, oral cholera vaccine interventions (OCV) or a combination of both (Combi) over a 20-year period in Haiti, and assuming a baseline national cholera incidence rate from Malawi (1990–2010) applied to urban and rural Haiti.
OCV scenario 1 (OCV/R1 + OCV/U1) averted 77,636 cases of cholera. OCV scenario 2 (OCV/R2 + OCV/U2) averted 57,668 cases of cholera. OCV scenario 3 (OCV/R3 + OCV/U3) averted 38,569 cases of cholera (Tables 2 and 4, Figure 3).
The rate of intervention coverage extension had the largest effect on cases of cholera averted (the difference between scenarios 1, 2 and 3 for either WASH or OCV).
Combined scenario 1 (Combined/R1 + Combined/U1) averted 88,974 cholera cases. Combined scenario 2 (Combined/R2 + Combined/U2) averted 71,586 cholera cases (Tables 3 and 4, Figure 3).
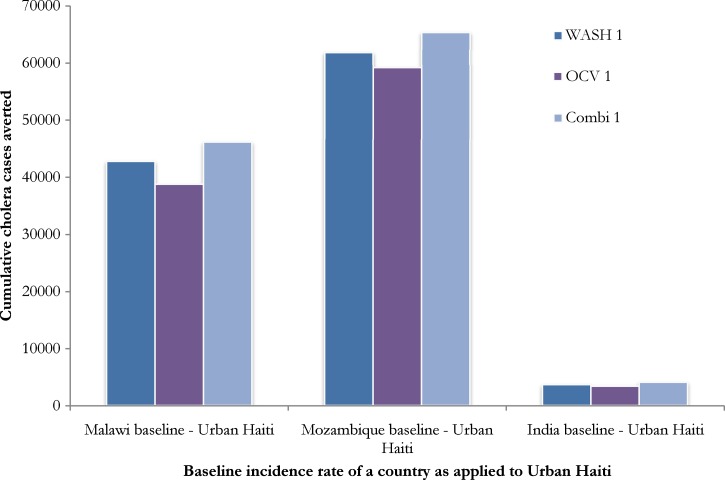
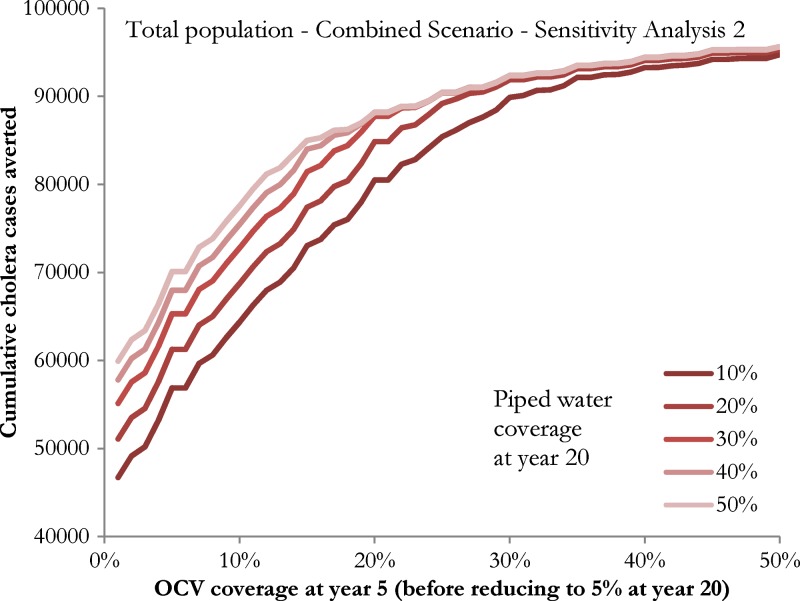
In our sensitivity analyses, we found that although the absolute number of cases of cholera averted is sensitive to the expected number of cholera cases given different baseline annual incidence of cholera, the relative effect of each intervention scenario is the same (Figure 4 ; Supplemental Figure 1). Our sensitivity analysis of combined interventions (Figure 5 ) demonstrated decreasing returns on investment (marginal increase of the number of cholera cases averted) when OCV coverage at year 5 and piped water coverage at year 20 are high. The OCV coverage of 30% at year 5 achieved similar outcomes with that of 50% coverage at year 5, regardless of piped water coverage of 10–50% at year 20 (Online Supplemental Material).
Figure 4.
Cumulative cases of urban (U) cholera cases averted by water, sanitation and hygiene (WASH/U1), oral cholera vaccine (OCV/U1) and a combination of WASH and OCV (Combined/U 1) scenarios when 20-year baseline annual incidence data from Malawi (1990–2010), Mozambique (1990–2010) and India (1961–1981) are applied to Haiti demographic data.
Figure 5.
Cumulative cholera cases averted over 20 years in Haiti by combined water, sanitation and hygiene (WASH) and oral cholera vaccine (OCV) intervention scenarios. The x-axis refers to OCV coverage at year 5, and the different lines refer to the proportion of total piped water coverage (10%, 20%, 30%, 40%, and 50%) at year 20. We assumed that 1) persons who received OCV would not be covered by WASH interventions and vice versa; 2) WASH and OCV interventions never exceed 50%, respectively; 3) point-of-use chlorination increases from 20% (urban), or 26% (rural) and will remain the same until piped water takes over (provided assumption 1 met); 4) latrine coverage remains 10% until it is taken over by point-of-use chlorination or piped water; 5) piped water baseline = 0% in rural areas and 10% in urban areas, and piped water coverage starts increasing at a constant rate from year 6 onwards; and 6) OCV coverage increases at a constant rate from 1% baseline at year 0, peaks at year 5 and decreases thereafter at a constant rate to reach 5% at year 20.
In our final sensitivity analysis, we explored scenarios to assess the impact on cases averted after a more rapid scale up of OCV coverage by year 5, as well as scenarios with sustained OCV coverage to year 20 (Table 5) . In our four combined scenarios described in Table 3, OCV coverage reached either 20% or 10% at year 5, and decreased to 5% or 0% by year 20, respectively (Combined/U1, Combined/R1, Combined/U2, Combined/R2). For Combined/U1 + R1, effective OCV coverage increased from 1% at year 0 at a constant rate, reached 20% at year 5, then decreased at a constant rate to 5% at year 20 (Table 3), thereby averting 88,974 cases over 20 years (Table 4). If in this scenario, effective OCV coverage was allowed to reach 50% at year 5, and then decrease at a constant rate to 5% at year 20, an additional 6,738 cases (95,712 cases) would be averted (Table 5). For Combined/U2 + R2, effective OCV coverage increased from 1% at year 0 at a constant rate, reached 10% at year 5, then decreased at a constant rate at 0% at year 20 (Table 3), thereby averting 71,586 cases over 20 years (Table 4). If in this scenario, effective OCV coverage were allowed to reach 50% at year 5, and then decrease at a constant rate to 5% at year 20, an additional 23,933 (95,519 cases) would be averted (Table 5). However, we estimated very small further increases in cases averted when we allowed for effective OCV coverage to reach 50% by year 5 and remain at that level to year 20 (Table 5). For example, in modified scenario Combined/U1 + R1, sustaining 50% coverage from year 5 through year 20 resulted in 95,777 cases averted (i.e., an additional 65 cases averted). Similar modest increases in case averted (95,703 cases averted; i.e., an additional 184 cases averted) were estimated for Combined/U2 + R2 (Table 5). For results of the other sensitivity/uncertainty analyses, see Online Supplemental Material.
Table 5.
Cumulative number of cases averted in sensitivity analyses of additional combined scenarios of WASH and OCV in urban (U) and rural (R) Haiti*
| Analysis | Combined/U1 | Combined/U2 | Combined/R1 | Combined/R2 | Combined/U1 + R1 | Combined/U2 + R2 |
|---|---|---|---|---|---|---|
| Main analysis in Table 3 | 46,213 | 38,913 | 42,761 | 32,673 | 88,974 | 71,586 |
| 50% OCV at year 5 decreasing to 5% at year 20† | 48,337 (+2,124) | 48,189 (+9,276) | 47,375 (+4,614) | 47,330 (+14,657) | 95,712 (+6,738) | 95,519 (+23,933) |
| Sustained 50% OCV from year 5 to 20† | 48,371 (+34) | 48,298 (+109) | 47,406 (+31) | 47,405 (+75) | 95,777 (+65) | 95,703 (+184) |
Incremental differences are indicated in parentheses.
All other assumptions are the same as the combination scenarios as described in Table 3. WASH = water, sanitation, and hygiene; OCV = oral cholera vaccine.
Discussion
Our results demonstrate that the rate of expanding coverage of WASH and OCV interventions affects the cumulative number of cases of cholera averted. The scenarios demonstrate that the modeled WASH and OCV interventions averted similar numbers of cholera cases. The assumptions of coverage for this model took into consideration the theoretical implementation of WASH and OCV interventions. Our goal was to demonstrate the scope of results given different rates of implementation and levels of coverage attained through a variety of scenarios, as well as with the sensitivity and uncertainty analyses. Scenarios that combined WASH and OCV interventions were most effective, which supports current efforts to implement both interventions when feasible.24
The WASH infrastructure provides a long-term, sustainable solution for prevention of cholera.12 Evidence from Europe and North America over the past two centuries, and more recently from Latin America, demonstrate that as water and sanitation coverage improves, the risk of epidemic or endemic cholera transmission is greatly reduced.12,14,15 WASH also prevents the transmission of many other diarrheal diseases, which in Haiti, as in many developing countries, is a leading killer of children less than five years of age.32,33 The overall benefit of expanding WASH coverage extends far beyond its effect on cholera alone.
The OCVs should help reduce the burden of cholera while WASH coverage is expanded, given the considerable amount of time required to improve WASH infrastructure (e.g., piped water and sewers). However, an OCV program should not be considered as a long-term alternative substitute for WASH. Implementation of OCVs will present its own challenges. Currently available OCVs are not 100% efficacious, induced immunity wanes over time thereby requiring periodic booster dosing, and today's globally available OCV supply is not sufficient to vaccinate the entire Haitian population with the required two-dose regimen. In addition, evidence from the routine childhood expanded program for immunizations and recent nationwide vaccine campaigns in Haiti has demonstrated varying ranges of coverage.34–37 Although rapid expansion of effective OCV coverage to 50% of Haitian population (10 million doses of administered vaccine or more) by year 5 may avert an additional 6,000–24,000 cases (Table 5), such rapid expansion is likely beyond the country's current capacity. Therefore, we highlight coverage scenarios (Table 3) in our model that we believe could be realistically achieved based on Haiti's recent experience with routine expanded program for immunizations and vaccine campaigns.
Our study has several limitations. First, we chose a static model while simultaneously incorporating an indirect effect by applying non-linear coverage-effective curves to WASH and OCV interventions. Thus, the model takes into account the current effect of an intervention (direct and indirect protection) and is an improvement over a classical static model. Unlike a model that simulates the transmission dynamics of cholera over time (e.g., ordinary differential equation models),38 a static model does not account for the future effect of the current intervention because the baseline incidence does not take into account the intervention applied in the previous year(s). However, our static model, like others,39,40 avoids having to estimate uncertain and unknown parameters required for dynamic models that explore the impact of multiple interventions introduced at various stages over time.41 More data will be needed to reduce the parameter uncertainty of existing dynamic models of cholera for Haiti.41,42 Second, although we accounted for population growth, we did not account for the likely migration of the Haitian population from rural to urban areas over the next 20 years. Third, we recognize that the baseline 20-year annual cholera incidence data from Malawi, Mozambique, and India that we used as illustrations for medium, high, and low incidence, respectively, may have been subject to under-reporting. However, our findings were robust across all three baseline country scenarios. However, it is clear that every country's experience with endemic cholera is unique. Only time will tell what Haiti's experience will be. Fourth, apart from modeling urban and rural Haiti separately, we did not study the impact of geographic variation on cholera incidence and intervention implementation (e.g., targeted immunization). Fifth, we acknowledge the uncertainty associated with the coverage-effectiveness curve used for each intervention. However, because data are sparse for OCV and WASH intervention coverage-effectiveness curves, we used modeling outputs of Longini and others28 to fit our exponential curves for OCV, and we also applied exponential curves to the WASH coverage-effectiveness relationship.27
Our study emphasizes that intervention coverage affects variation in estimated number of cumulative cholera cases averted over an extended period, and demonstrates the probable synergistic effects of WASH and OCV when used in combination. Our study should not be interpreted as an exact prediction for the number of cholera cases that could be averted in Haiti under the scenarios outlined, but it serves to demonstrate that WASH and OCV interventions can play an important role in decreasing the burden of cholera, and that maximizing intervention coverage is the central variable to their success. Transmission and intervention dynamics need to be understood so that informed decisions can be made about how to allocate limited resources. The Haitian Government recently released its National Plan for the Elimination of Cholera.18 This plan outlines a combination of public health interventions that include the use of OCV while expanding access to clean water and sanitation. Our study suggests that this combined strategy will be effective.
Supplementary Material
ACKNOWLEDGMENTS
We thank Manoj Gambhir, Richard Gelting, Tom Handzel, Eric Mintz, Daphne Moffett, Scott Santibanez, and Cathy Young for their valuable comments on earlier versions of this manuscript, and Cathy Young for editorial assistance.
Disclaimer: The findings and conclusions expressed in this report do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Authors' addresses: Isaac Chun-Hai Fung, Rebekah H. Borse, and Martin I. Meltzer, Health Economics and Modeling Unit, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: cfung@georgiasouthern.edu, rebekahheinzen@gmail.com, and qzm4@cdc.gov. David L. Fitter and Jordan W. Tappero, Center for Global Health, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: vid3@cdc.gov and jwt0@cdc.gov.
References
- 1.Barzilay EJ, Schaad N, Magloire R, Mung KS, Boncy J, Dahourou GA, Mintz ED, Steenland MW, Vertefeuille JF, Tappero JW. Cholera surveillance during the Haiti epidemic–the first 2 years. N Engl J Med. 2013;368:599–609. doi: 10.1056/NEJMoa1204927. [DOI] [PubMed] [Google Scholar]
- 2.Ministère de Santé Publique et de la Population Rapports Journaliers du MSPP sur l'Évolution du Cholera en Haiti. 2013. http://www.mspp.gouv.ht/site/downloads/Rapport%20journalier%20MSPP%20du%2030%20juin%202013.pdf Available at. Accessed July 26, 2013.
- 3.Clasen T, Roberts I, Rabie T, Schmidt W, Cairncross S. Interventions to improve water quality for preventing diarrhoea. Cochrane Database Syst Rev. 2006;3:CD004794. doi: 10.1002/14651858.CD004794.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Clasen T, Schmidt WP, Rabie T, Roberts I, Cairncross S. Interventions to improve water quality for preventing diarrhoea: systematic review and meta-analysis. BMJ. 2007;334:782. doi: 10.1136/bmj.39118.489931.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clasen TF, Bostoen K, Schmidt WP, Boisson S, Fung IC, Jenkins MW, Scott B, Sugden S, Cairncross S. Interventions to improve disposal of human excreta for preventing diarrhoea. Cochrane Database Syst Rev. 2010:CD007180. doi: 10.1002/14651858.CD007180.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waddington H, Snilstveit B. Effectiveness and sustainability of water, sanitation, and hygiene interventions in combating diarrhoea. J Development Effectiveness. 2009;1:295–335. [Google Scholar]
- 7.Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, Colford JM., Jr Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: a systematic review and meta-analysis. Lancet Infect Dis. 2005;5:42–52. doi: 10.1016/S1473-3099(04)01253-8. [DOI] [PubMed] [Google Scholar]
- 8.Cairncross S, Hunt C, Boisson S, Bostoen K, Curtis V, Fung IC, Schmidt WP. Water, sanitation and hygiene for the prevention of diarrhoea. Int J Epidemiol. 2010;39((Suppl 1)):i193–i205. doi: 10.1093/ije/dyq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnold BF, Colford JM., Jr Treating water with chlorine at point-of-use to improve water quality and reduce child diarrhea in developing countries: a systematic review and meta-analysis. Am J Trop Med Hyg. 2007;76:354–364. [PubMed] [Google Scholar]
- 10.Snow J. On the Mode of Communication of Cholera. London, England: John Churchill; 1855. [Google Scholar]
- 11.Snow J. On the communication of cholera by impure Thames water. Medical Times and Gazette. 1854;9:365–366. [Google Scholar]
- 12.Waldman RJ, Mintz ED, Papowitz HE. The cure for cholera: improving access to safe water and sanitation. N Engl J Med. 2013;368:592–594. doi: 10.1056/NEJMp1214179. [DOI] [PubMed] [Google Scholar]
- 13.WHO/UNICEF Joint Monitoring Programme for Water Supply and Sanitation . Progress on Sanitation and Drinking-Water: 2010 Update. Geneva: World Health Organization; 2010. [Google Scholar]
- 14.Sepulveda J, Valdespino JL, Garcia-Garcia L. Cholera in Mexico: the paradoxical benefits of the last pandemic. Int J Infect Dis. 2006;10:4–13. doi: 10.1016/j.ijid.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Roses Periago M, Frieden TR, Tappero JW, De Cock KM, Aasen B, Andrus JK. Elimination of cholera transmission in Haiti and the Dominican Republic. Lancet. 2012;379:e12–e13. doi: 10.1016/S0140-6736(12)60031-2. [DOI] [PubMed] [Google Scholar]
- 16.Cairncross S. Handwashing with soap: a new way to prevent ARIs? Trop Med Int Health. 2003;8:677–679. doi: 10.1046/j.1365-3156.2003.01096.x. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt WP, Cairncross S, Barreto ML, Clasen T, Genser B. Recent diarrhoeal illness and risk of lower respiratory infections in children under the age of 5 years. Int J Epidemiol. 2009;38:766–772. doi: 10.1093/ije/dyp159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Direction Nationale de L'Eau Potable et de L'Assainissement (Ministry of Public Health and Population of the Republic of Haiti) National Plan for the Elimination of Cholera in Haiti 2013–2022 (English Translation) 2013. http://new.paho.org/hq/index.php?option=com_docman&task=doc_download&gid=20326&Itemid=270&lang=en Available at. Accessed March 23, 2013.
- 19.Ivers LC, Farmer P, Almazor CP, Leandre F. Five complementary interventions to slow cholera: Haiti. Lancet. 2010;376:2048–2051. doi: 10.1016/S0140-6736(10)62243-X. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization Cholera vaccines: WHO position paper. Wkly Epidemiol Rec. 2010;85:117–128. [PubMed] [Google Scholar]
- 21.Date KA, Vicari A, Hyde TB, Mintz E, Danovaro-Holliday MC, Henry A, Tappero JW, Roels TH, Abrams J, Burkholder BT, Ruiz-Matus C, Andrus J, Dietz V. Considerations for oral cholera vaccine use during outbreak after earthquake in Haiti, 2010–2011. Emerg Infect Dis. 2011;17:2105–2112. doi: 10.3201/eid1711.110822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin S, Desai SN, Sah BK, Clemens JD. Oral vaccines against cholera. Clin Infect Dis. 2011;52:1343–1349. doi: 10.1093/cid/cir141. [DOI] [PubMed] [Google Scholar]
- 23.Ivers LC, Farmer PE, Pape WJ. Oral cholera vaccine and integrated cholera control in Haiti. Lancet. 2012;379:2026–2028. doi: 10.1016/S0140-6736(12)60832-0. [DOI] [PubMed] [Google Scholar]
- 24.Pan American Health Organization . Final Report of the XX Technical Advisory Group (TAG) Meeting on Vaccine-Preventable Diseases. Pan American Health Organization; Washington, DC: 2012. October 2012. [Google Scholar]
- 25.World Health Organization . Global Health Observatory. Number of Reported Cholera Cases. 2013. http://www.who.int/gho/epidemic_diseases/cholera/cases/en/index.html Available at. Accessed October 2, 2012. [Google Scholar]
- 26.Central Intelligence Agency The World Factbook. Malawi. 2013. https://www.cia.gov/library/publications/the-world-factbook/geos/mi.html Available at. Accessed March 22, 2013.
- 27.Fung IC-H, Fitter DL, Meltzer MI, Tappero JW, Borse RH. 2013. Coverage-effectiveness curve: evaluating the impact of water, sanitation and hygiene interventions. CDC/NCEZID/Division of Preparedness and Emerging Infections, white paper, Atlanta. [Google Scholar]
- 28.Longini IM, Jr, Nizam A, Ali M, Yunus M, Shenvi N, Clemens JD. Controlling endemic cholera with oral vaccines. PLoS Med. 2007;4:e336. doi: 10.1371/journal.pmed.0040336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sur D, Kanungo S, Sah B, Manna B, Ali M, Paisley AM, Niyogi SK, Park JK, Sarkar B, Puri MK, Kim DR, Deen JL, Holmgren J, Carbis R, Rao R, Nguyen TV, Han SH, Attridge S, Donner A, Ganguly NK, Bhattacharya SK, Nair GB, Clemens JD, Lopez AL. Efficacy of a low-cost, inactivated whole-cell oral cholera vaccine: results from 3 years of follow-up of a randomized, controlled trial. PLoS Negl Trop Dis. 2011;5:e1289. doi: 10.1371/journal.pntd.0001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sur D, Lopez AL, Kanungo S, Paisley A, Manna B, Ali M, Niyogi SK, Park JK, Sarkar B, Puri MK, Kim DR, Deen JL, Holmgren J, Carbis R, Rao R, Nguyen TV, Donner A, Ganguly NK, Nair GB, Bhattacharya SK, Clemens JD. Efficacy and safety of a modified killed-whole-cell oral cholera vaccine in India: an interim analysis of a cluster-randomised, double-blind, placebo-controlled trial. Lancet. 2009;374:1694–1702. doi: 10.1016/S0140-6736(09)61297-6. [DOI] [PubMed] [Google Scholar]
- 31.Corso PS, Haddix AC. Time Effects. Prevention Effectiveness: A Guide to Decision Analysis and Economic Evaluation. New York: Oxford University Press; 2003. [Google Scholar]
- 32.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basanez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabe E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fèvre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gonzalez-Medina D, Gosselin R, Grainger R, Grant B, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Laden F, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Levinson D, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mock C, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O'Connell M, O'Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA III, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leòn FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiebe N, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, AlMazroa MA, Memish ZA. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 33.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA III, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rainey JJ, Danovaro-Holliday MC, Magloire R, Kananda G, Lee CE, Chamouillet H, Lacapere F, Mung K, Luman ET. Haiti 2007–2008 national measles-rubella vaccination campaign: implications for rubella elimination. J Infect Dis. 2011;204((Suppl 2)):S616–S621. doi: 10.1093/infdis/jir488. [DOI] [PubMed] [Google Scholar]
- 35.Rainey JJ, Lacapere F, Danovaro-Holliday MC, Mung K, Magloire R, Kananda G, Cadet JR, Lee CE, Chamouillet H, Luman ET. Vaccination coverage in Haiti: results from the 2009 national survey. Vaccine. 2012;30:1746–1751. doi: 10.1016/j.vaccine.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 36.Lacapere F, Magloire R, Danovaro-Holliday MC, Flannery B, Chamoulliet H, Celestin EP. The use of rapid coverage monitoring in the national rubella vaccination campaign, Haiti 2007–2008. J Infect Dis. 2011;204((Suppl 2)):S698–S705. doi: 10.1093/infdis/jir480. [DOI] [PubMed] [Google Scholar]
- 37.Vertefeuille JF, Dowell SF, Domercant JW, Tappero JW. Cautious optimism on public health in post-earthquake Haiti. Lancet. 2013;381:517–519. doi: 10.1016/S0140-6736(13)60051-3. [DOI] [PubMed] [Google Scholar]
- 38.Chao DL, Longini IM, Jr, Morris JG., Jr Modeling cholera outbreaks. Curr Top Microbiol Immunol. 2013 doi: 10.1007/82_2013_307. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cook J, Jeuland M, Maskery B, Lauria D, Sur D, Clemens J, Whittington D. Using private demand studies to calculate socially optimal vaccine subsidies in developing countries. J Policy Anal Manage. 2009;28:6–28. doi: 10.1002/pam.20401. [DOI] [PubMed] [Google Scholar]
- 40.Reyburn R, Deen JL, Grais RF, Bhattacharya SK, Sur D, Lopez AL, Jiddawi MS, Clemens JD, von Seidlein L. The case for reactive mass oral cholera vaccinations. PLoS Negl Trop Dis. 2011;5:e952. doi: 10.1371/journal.pntd.0000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grad YH, Miller JC, Lipsitch M. Cholera modeling: challenges to quantitative analysis and predicting the impact of interventions. Epidemiology. 2012;23:523–530. doi: 10.1097/EDE.0b013e3182572581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fung IC-H. 2013. Cholera transmission dynamic models for public health practitioners. CDC/NCEZID/Division of Preparedness and Emerging Infections, white paper, Atlanta. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.