Abstract
Successful and sustained efforts have been made to curtail the major cholera epidemic that occurred in Haiti in 2010 with the promotion of hygiene and sanitation measures, training of health personnel and establishment of treatment centers nationwide. Oral cholera vaccine (OCV) was introduced by the Haitian Ministry of Health as a pilot project in urban and rural areas. This paper reports the successful OCV pilot project led by GHESKIO Centers in the urban slums of Port-au-Prince where 52,357 persons received dose 1 and 90.8% received dose 2; estimated coverage of the at-risk community was 75%. This pilot study demonstrated the effort, community mobilization, and organizational capacity necessary to achieve these results in a challenging setting. The OCV intervention paved the way for the recent launching of a national cholera vaccination program integrated in a long-term ambitious and comprehensive plan to address Haiti's critical need in water security and sanitation.
Introduction
Cholera is a toxin-mediated illness caused by ingestion of viable Vibrio cholerae that leads to a rapidly dehydrating diarrheal disease. The global disease burden is estimated to be 1.4–4.3 million cases and 28,000–142,000 deaths per year.1 Since the first reported outbreak in 1817, there have been seven pandemics and the disease remains endemic to Southeast Asia and Africa. The seventh pandemic began in 1961 when El Tor O1 strains replaced classical V. cholerae strains.2 In 1971, cholera was introduced in Africa where the disease became endemic with recurrent epidemic surges in many countries.3 In 1991, cholera arrived in Peru and South America, and it took approximately 10 years to control the disease.4
Vibrio cholerae strains have been rarely identified in Haiti. The first report of clinical case of cholera occurred on October 19, 2010. Three days later, the Haitian Ministry of Health and Population (MSPP) laboratory confirmed the first bacteriologically proven case of cholera ever recognized in Haiti.5 The strain of Vibrio cholerae in Haiti was identified as serogroup O1, serotype Ogawa, biotype El Tor and shown to be related to strains found in Southeast Asia.6 After its emergence in the Artibonite Valley in central Haiti, cholera spread rapidly around Haiti and as of March 13, 2013, it had caused an estimated 650,258 cases and 8,048 deaths in four major waves in what has been described as the worst national epidemic in recent memory.7,8 Cholera will remain a continued threat to Haiti's fragile public health system already overburdened by natural disasters and ongoing epidemics of acquired immunodeficiency syndrome (AIDS) and tuberculosis. Furthermore, the country is the most underserved in all of the Americas and has limited access to clean water (63% of the population has access to improved water) and a dearth of effective sanitation systems (17% access to improved sanitation).9 The sanitation, health care infrastructure, and economic conditions of Haiti are closer to those of countries in Africa plagued by endemic cholera rather than Latin America. Will cholera become endemic with intermittent epidemic waves or will it be controlled or even eradicated?
Facing these challenges, major efforts have been aimed at curbing the spread of cholera through establishing supplies of clean water, hand washing, and improving access to sanitation.10,11 Treatment has been addressed through 1) establishment of Cholera Treatment Centers (CTCs) for oral/IV rehydration and clinical monitoring, 2) setting up smaller community-based Oral Rehydration Points (ORP), 3) increasing community recognition of the severity of cholera, 4) use of antibiotics in treating severe disease,12 and 5) development of a nationwide training program to recognize the disease and build the capacity to respond to the epidemic.
From the earliest recognition of the epidemic in Haiti, there has been an effort to introduce oral cholera vaccine (OCV) as a component of the response.13–15 This vaccine has a proven track record in prevention of cholera in disease-endemic settings. It has been used less frequently to prevent the spread of an epidemic (preemptive vaccination) or to help control newly introduced disease (reactive vaccination). When approval was obtained to begin vaccination on April 9, 2012, Haiti was in the reactive stage because the disease had rapidly spread throughout the country with limited introductions to adjoining Dominican Republic and to the United States.16,17
This report documents a successful OCV demonstration project conducted by GHESKIO (The Haitian Group for the Study of Kaposi's Sarcoma and Opportunistic Infections) in collaboration with the MSPP in one of the most difficult settings in Haiti, urban slums. A parallel demonstration was conducted by Zanmi Lasante/Partners in Health (ZL/PIH) in the rural Artibonite Valley. GHESKIO undertook to deliver vaccine and define the safety, acceptability, and feasibility of giving the required two doses of oral cholera vaccine in the challenging urban slums of Port-au-Prince.
There is a strong history of safety and efficacy of OCV in numerous settings in adults and children as young as one year of age.18–21 This project was designed to demonstrate the acceptability and feasibility of vaccination at a scale that could be translated into national policy. The project was not designed to demonstrate efficacy, although the high vaccine coverage achieved in the targeted slum areas may ultimately provide an opportunity to measure the impact of reactive vaccination in decreasing the cholera outbreak in this setting.
Methods
Vaccine.
The vaccine used was an oral bivalent inactivated cholera vaccine (OCV) containing killed whole cells of V. cholerae O1 and V. cholerae O139 (Shanchol®; Shantha Biotechnics (Hyderabad, India) now a division of Sanofi-Aventis. It was prepared in India at a cost of less than $2 dollars a dose, which is compatible with use in developing countries. This vaccine has been licensed for use in Vietnam since 1994, and there have been multiple trials documenting its safety and immunogenicity.22 It received World Health Organization (WHO) pre-qualification in September 2011 (http:www.who.int/immunization_standards/vaccine_quality/PQ_vaccine_list_en/en/index.html). Two hundred thousand doses of the vaccine were purchased for use in Haiti through funds obtained from the American Red Cross through Partners in Health (Boston, MA).
Vaccine storage.
Vaccine was received in Haiti on February 19, 2012 and stored for GHESKIO at PROMESS, a Pan American Health Organization refrigerated vaccine storage warehouse in Port-au-Prince used for routine immunization vaccines by the MSPP until approval was obtained from the National Bioethics Committee to proceed with vaccination on April 9, 2012.
Site conducting the pilot operation.
Located in Port-au-Prince, Haiti, GHESKIO is the largest institution providing human immunodeficiency virus (HIV) infection and tuberculosis (TB) care in the Caribbean since the onset of the HIV epidemic in 1981. The GHESKIO integrated model of care for HIV infection, sexually transmitted infections, malaria, TB, and other opportunistic infections has been replicated at the national level in collaboration with the MSPP. GHESKIO currently supervises care at 23 public and private sites nationally. In 2013, 40% of all HIV patients in Haiti are receiving antiretroviral therapy through the GHESKIO–MSPP network.
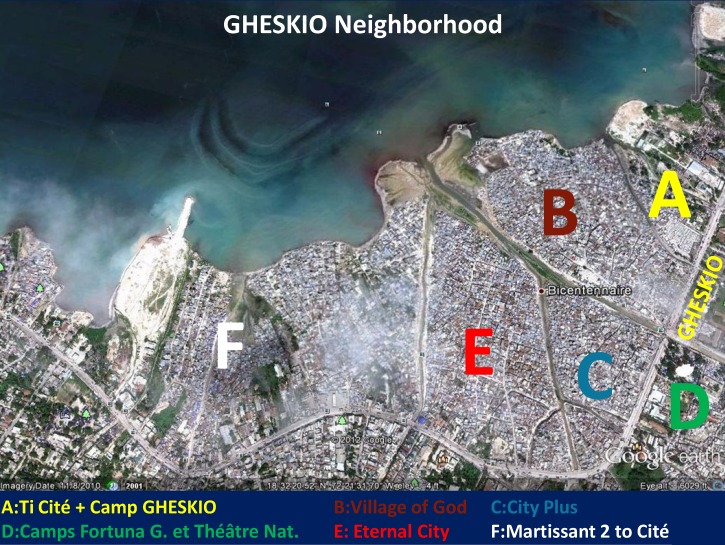
GHESKIO is located across the street from five densely populated slums that have an estimated population of 70,000 persons, where it is difficult to identify individual houses even with global positioning system (GPS) coordinates. This large slum area is located across from the GHESKIO downtown campus, extends all along Harry Truman Boulevard, and is composed of the GHESKIO tent City, the City of God, City of Eternity, City Plus, and Martissant (Figure 1). The population living in those urban slums has minimal access to basic hygiene and sanitation services and limited medical infrastructure. After the earthquake in January 2010, GHESKIO assumed responsibility for general health care of that disenfranchised population.23–25 These urban slums, infested with multiple gangs that defend their own territory, are built on fill and refuse. The houses are below sea level and latrines cannot be built making access to proper sanitation difficult. Water must be purchased from trucks from outside the community. Poverty is rampant and most slum inhabitants earn less than $1 U.S./day. Illiteracy and unemployment are high. The GHESKIO cholera vaccine demonstration targeted those disenfranchised populations because they are at high risk of contracting cholera. The earthquake and cholera epidemic added important new responsibilities at GHESKIO but also brought opportunities to work more closely with populations from tent cities and slums, all eventual potential beneficiaries of OCV.
Figure 1.
GHESKIO neighborhood, Haiti.
Two of the most important factors accounting for the successful completion of this pilot project in the urban setting are strong support and involvement of the MSPP and the fact that the institution in charge, GHESKIO, has the capacity to conduct operational research through an experienced community advisory board (CAB), well-trained Data Management and Pharmacy Units, and the capacity to offer training to medical and non–health-related personnel. GHESKIO has a 30-year history of capacity building by successive National Institutes of Health grants and longstanding collaborations with Cornell University and other major universities and institutions, such as Vanderbilt and Dartmouth, and the Mérieux Foundation in conducting clinically applied research.
The GHESKIO staffs are well trained in the conduct and rigor of National Institutes of Health trials, which was essential in developing and implementing the standard operating procedures needed at each level of this intervention. Research, training, and care are the three aspects of the GHESKIO mission. Since its inception GHESKIO has served as a national training center for medical and non-medical personnel in the prevention and treatment of sexually transmitted infections, HIV, TB, malaria, and most recently, cholera. The resilience of GHESKIO has been tested by the way it reacted after the major earthquake of January 2010 to provide acute care to the wounded and complete services to a large refugee population while continuing to deliver the usual HIV and TB services despite the loss of 43 key staff members and the destruction of 60% of its two campuses.23–25 Its history of working in a difficult environment with frequent social and political turmoil requiring the development of contingency plans that are reviewed and improved regularly also prepared it for operating in the difficult slum conditions for this pilot. All GHESKIO staff and CAB members were offered the OCV vaccine to demystify side effects associated with it. Sixty-eight percent were vaccinated, including all those who were perceived at risk (CTC workers, staff working at the treatment units at collaborating sites [CTUs], vaccinators, water testers, and workers involved in the sanitation effort in the slums), and 95% received a second dose.
GHESKIO also has maintained the trust of the community as a service provider and through the creation in 1997 of a 28 member CAB representing various key sectors of society: the three major religions, teachers, laboratory workers, AIDS patients, and human rights activists. When the cholera epidemic was confirmed, GHESKIO created a cholera-specific CAB, in addition to the old CAB, consisting of physicians, nurses, field workers, community, and religious leaders, health promoting agents, teachers, and school directors who were trained at the GHESKIO Training Unit. Their role was to multiply the transmission of information about the cholera epidemic to the community by going door-to-door, using megaphones, posters, pamphlets, and banderoles, and through the organization of workshops.
In response to the cholera epidemic, GHESKIO developed a comprehensive model of prevention and care in the GHESKIO tent city and neighboring slums that included 1) establishing two CTCs, one at each of its two main sites, eight cholera treatment units (CTUs), and 10 Oral Rehydration Points (ORPs) inside slums across its downtown center; 2) training medical and support personnel; 3) training, informing, and mobilizing the community; 4) improving water quality with water testing for chlorination at water vending points; 5) distributing oral rehydration salts, soap, Jerry cans to store water, and water purification tablets; 6) promoting hygiene with trained health promoting agents (agents promoteur de santé); 7) active screening of cholera cases in the community and referral to a CTC or ORP by the agents promoteur de santé; 8) cholera prevention campaigns in 12 market places and 16 tent camps; 9) trash removal and street cleaning in the slums; and 10) setting up a chlorine factory at GHESKIO. The GHESKIO CAB, a key player in prior HIV vaccine trials and who has headed all activities in the tent city after the earthquake, guided the cholera CAB to lead community sensitization and mobilization efforts. Particularly key to the successful cholera vaccine campaign were the trust developed with community leaders through the daily presence of GHESKIO personnel in the slums and the long history of provision by GHESKIO of free heath care to the slum's community.
Why we opted for cholera vaccination.
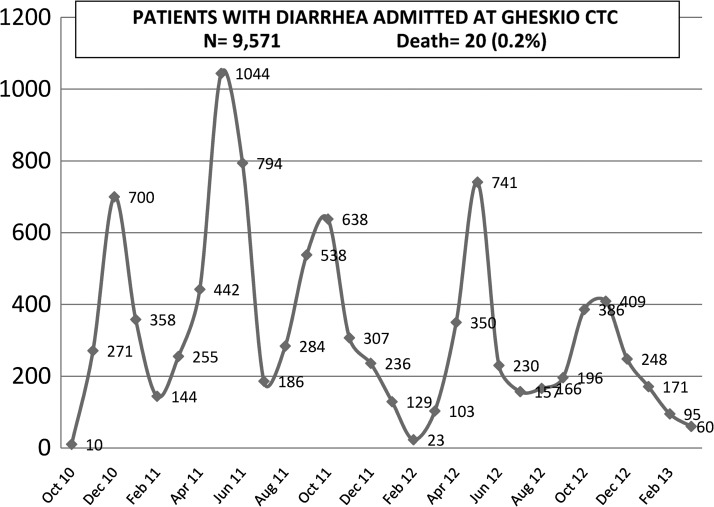
During the two-year period post-recognition of the cholera epidemic and initiation of the OCV pilot demonstration, GHESKIO had been extensively involved in all water, sanitation, and hygiene (WASH) activities. However, as the epidemic continued with ever increasing cases (Figure 2), it became evident that addition of another intervention known to be effective was essential to curtail the epidemic, particularly for the populations most at risk.
Figure 2.
Patients admitted with diarrhea at GHESKIO Cholera Treatment Center, Haiti, October 2010–February 2013.
Preparation for vaccination.
Although experienced in the conduct of experimental clinical trials, GHESKIO had not undertaken a vaccination project of this magnitude. The campaign was divided into three phases: pre-vaccination, vaccination, and post-vaccination. Once a national decision was made by the MSPP to proceed with vaccination, planning for the demonstration started on December 31, 2011.
Pre-vaccination phase.
The first four months in the pre-vaccination period were dedicated to setting up the various units that would be involved in the different phases: developing the logistics for vaccine receipt and cold chain, creating the necessary informatics tools to have a census of the targeted population, documenting their interest to receive OCV, managing information on all vaccine recipients, mobilizing the community and seeking volunteers via the CAB outreach, conducting a complete census of the targeted area for vaccination, and establishing a plan with the security unit to provide the necessary support to all staff and volunteers.
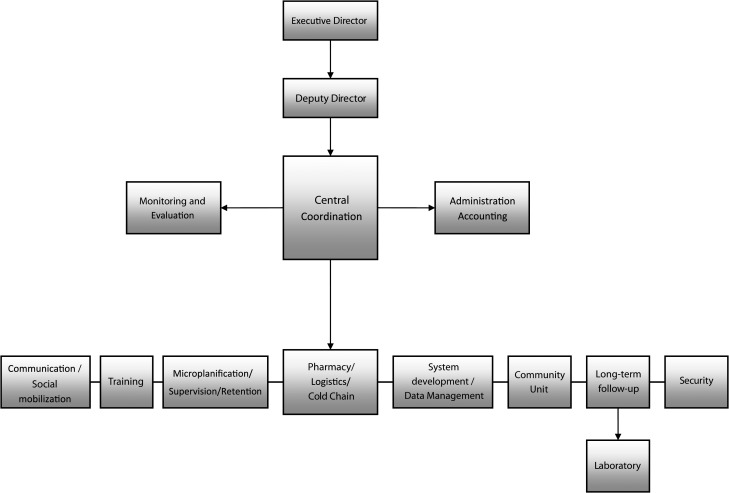
The vaccination campaign was no small task and it required the mobilization of 20% of the GHESKIO personnel during the pre-vaccination phase and virtually all GHESKIO personnel during the three-month vaccination period. Five percent of our staff remained involved in the post-vaccination phase. Personnel were relieved from clinical care, laboratory, pharmacy, research, training, and administrative duties and were engaged at all levels of this demonstration project. A dedicated organizational structure was created to manage the project with a central coordinating committee overseeing eight specialized subunits: community mobilization, training, pharmacy and cold chain, logistics, information technology (IT) and data management, monitoring and evaluation, post-vaccination follow-up and retention, and micro-planning (Figure 3). Specific places were reserved on the GHESKIO ground and training rooms for the various units involved in the vaccination project. Multiple plans were developed: a communication plan with the teams deployed in the slums using radio and phone, an evacuation plan in case a staff member or patient required rapid medical care, a security plan to anticipate and react to potential aggression or violence, a supervision plan of the various teams, and a logistics plan to replenish the teams operating in the slums with drinking water and additional vaccine.
Figure 3.
GHESKIO oral cholera vaccine pilot study organizational structure, Haiti.
The vaccination was intentionally conducted as a demonstration project in contradistinction to a vaccine trial to assess the feasibility of the use of the vaccine nationally because the OCV already had an extensive safety and efficacy record that had led to prequalification by WHO. It was determined after review by the National Ethical Committee that signed informed consent was not needed.
Fifty meetings were held with community and religious leaders (141 members) to present the project, review the impact of cholera disease, modes of transmission, treatment, and prevention methods and establish willingness of the population to receive OCV. After engaging community leaders, mass mobilization of the inhabitants was conducted with open-air activities in market places, schools, churches, setting up of posters and banners with information and with a door-to-door information campaign. The aim was to sensitize and pre-register 52,000 persons, determine the interest of the population to receive OCV with the aim to obtain at least 85% participation of those eligible more than one year of age, and greater than 90% acceptance of the second dose.
Data management.
The GHESKIO IT Unit played a key role in this effort. The EpiSurveyor software (DataDyne Group LLC, Washington, DC) was customized to enable digital documentation on the field of the census and vaccination of the targeted population on Nokia (Espoo, Finland) E5 smartphones equipped with GPS. One hundred and two smartphones were purchased for the pilot study. A daily deployment plan was prepared with the help of IT and logistics personnel and sent out the day before the planned vaccination to each team. The deployment plan was organized by slum, neighborhood, block, street and set of houses and indicated the names of all persons who been pre-registered in that area and agreed to receive OCV. This plan was important for the management of large number of vaccination teams on the field, ensuring systematic coverage of the five slums targeted for vaccination and facilitating real-time monitoring of the progress of the campaign.
Conduct of a census.
A door-to-door census of the five slums, all markets, and small workshops surrounding the downtown GHESKIO campus was undertaken to register all inhabitants and document the community's interest in receiving the vaccine after being presented with information on OCV. The entire area that includes the slums targeted for vaccination is called Kosovo because of the high crime rate. Habitants refused use of photo identification or fingerprints for fear that this could be used against them by the police or other authorities. The slums were divided into neighborhoods, blocks, and streets. Although GPS was used, it was not precise enough because houses were so closely located that they had to be numbered by the CAB staff. A questionnaire was administered to each household by the trained CAB volunteers documenting the number of persons living in each house, their sex, age, and pregnancy status. A pre-vaccination card was distributed to each person who agreed to vaccination. Over a three-month period, 51,814 persons were pre-registered for receipt of the cholera vaccine although not all of these persons could be found at the time of vaccination because of a high migration rate in and out of the slums and because pre-registered persons were not at their home at the time our teams came to provide the vaccine. Of those vaccinated, 65% were found during the first round. The rest required 1–3 more visits by the vaccination teams. This labor-intensive door-to-door strategy was chosen to maximize the probability that persons would received the required two doses of vaccine, but also because it served as a platform on which other global health interventions would be added for those communities.
Sensitization and social mobilization.
A communication plan was developed with a specific brochure that included information to be provided to the community about the risk factors for cholera transmission, methods of prevention, including WASH messages, the role of vaccines in general and that of OCV. Simple key messages were developed and disseminated during our campaign: vaccine was an additional weapon in the fight against cholera. It requires two doses and has minimal side effects. Vaccine protection is not 100% and hygiene precautions should always be maintained.
Training.
Training of the various teams involved in the three phases of pre-vaccination, vaccination, and post-vaccination was important to the success of this demonstration. From the start of the cholera epidemic to the launching of the OCV project, GHESKIO has offered training for medical personnel and support staff in the prevention and care of cholera. A total of 106 physicians, 291 nurses, 65 laboratory technicians, and 213 support staff were trained to work at CTCs and CTUs. The effort to inform non-medical personnel about the risk of cholera, modes of transmission, and prevention and treatment was commendable: community leaders (216), community health agents (80), water testers to evaluate the quality of the water (28), persons selling water from their cisterns (22), health promoting agents (1,003), merchants selling food and other goods at public markets (2,156), and the community at large of persons living in the slums across the downtown GHESKIO site (70,208) have been trained through the GHESKIO training unit. Training materials were developed and distributed. All personnel involved in all the phases of the project, community mobilization, vaccination, and follow up teams, received training regarding cholera transmission and prevention information. The 75 community agents identified to conduct the post-vaccination surveys were also trained to properly evaluate potential side effects of OCV and remind vaccine recipients to seek care at the closest ORP or the main GHESKIO CTC in case of side effects or apparition of diarrhea. These follow-up agents had to visit each home within less than 24 hours after each dose and record the following potential side effects: itching, rash, fever, nausea, vomiting, abdominal pain, diarrhea, and any other reported side effect.
Pharmacy.
Cold chain maintenance and equipment.
Two refrigerators, each with a capacity of 2.14 liters and equipped with integrated thermometers and a refrigerated 40-foot shipping container, were purchased for storage of vaccine at GHESKIO and were supplied with a priority electrical power line and backup generator. In collaboration with the MSPP, 250 cold boxes and 500 icepacks were acquired for use on the field by the vaccination teams. Freezers in the GHESKIO laboratory, as well as additional freezers purchased, were used to re-freeze the icepacks every night. Temperature control was evaluated twice a day by the pharmacy staff for the container, refrigerators, and freezers; temperatures were manually recorded on dedicated forms and icepacks returned at the end of the day of vaccination were visually inspected.
Vaccine distribution and logistics.
Vaccine receipt, storage, cold chain maintenance, preparation, distribution, and stock management were handled by the pharmacy team with 12 persons on a rotating schedule for seven days per week coverage during the entire three-month vaccination period. The container had a work area to prepare the individual vaccine vials into 25 or 50 dose batches for daily distribution to vaccination teams. Each day of vaccination, the pharmacy staff distributed a numbered thermos containing the ice packs and pre-prepared batches of vaccine vials to each vaccination team with a kit containing a biohazard bag and hand sanitizer. Additional batches were also prepared for replenishment on the field should a team need more vaccine.
Documentation and inventory.
Distribution of all doses was well recorded from receipt from the PROMESS storage warehouse to distribution to each team that signed for all vials received. Unused vials were registered and doses lost were documented and quarantined for rapid use the next day to minimize cold chain disruption. Daily reports were generated and cross-referenced with the paper vaccination team records, the pharmacy log sheets, and the IT database of persons vaccinated, which enabled close monitoring of the number of doses provided and progress of vaccination campaign.
Education and training of staff.
All vaccinators were GHESKIO employees. They received specific training at the Training Unit with a pharmacist on how to administer the vaccine and maintain cold chain requirements.
Waste management.
Empty vials were stored in biohazard bags, returned to the pharmacy to be registered, and isolated until destroyed by the MSPP.
Vaccination phase.
Vaccination teams were composed of GHESKIO employees (Figure 4) and community volunteers: recruited volunteer nurses and students from other schools, partnering institutions, and persons who lived in communities targeted for vaccination. A total of 75 vaccination teams of four members each were divided into three groups, each supervised by two leaders. Each team was composed of a vaccinator, a GHESKIO employee, who served as the team leader, a person for digital data entry into the smart phones, a person for paper documentation, and a person from the community for sensitization and mobilization. A daily deployment plan was prepared with the help of IT and logistics and sent out the day before the planned vaccination to each vaccination team and organized by slum, neighborhood, block, street, and set of houses for systematic coverage of the area. Cold chain was ensured and maintained for each vaccination team daily. Unused vials were registered and quarantined for rapid use the next day to minimize cold chain disruption and doses lost.
Figure 4.

GHESKIO employee working as vaccinator, Haiti.
Once vaccination began, two competing advertising companies known to work in the community and using media trucks were hired to go through the slums with promotional messages about cholera prevention methods including vaccination. GHESKIO CAB personnel went on the truck to ensure correct information was being disseminated. Before vaccination, detailed oral and visual non-verbal information was given to each recipient on cholera illness and the vaccine.
Data management.
All information regarding vaccination was recorded on Nokia E5 smartphones, downloaded at the end of each day into a secure database, and saved on a GHESKIO server. Paper documentation was also conducted for verification and backup purposes. Daily tallies were verified by vaccination team reports, pharmacy, and IT reports and crossed-checked with inventory stock counts monitoring campaign progress.
Post-vaccination phase.
Post-vaccination monitoring for adverse effects was ensured by a team of 75 trained volunteers who visited each vaccine recipient within 24–48 hours after each of the two doses to document any adverse reaction to the vaccine. The deployment plan of the vaccination teams used the day before was followed by the follow-up teams to trace back systematically all vaccine recipients by site, neighborhood, block and house number. This required constant communication and coordination between logistics and IT units to correctly manage the 375 volunteers on the field at the same time for vaccination and next day follow-up.
The follow-up teams reinforced WASH messages for the continued application of sanitation and clean water measures and reminded the community of the importance of coming to the GHESKIO CTCs or ORPs for any diarrhea in the year to come.
Results
The population targeted showed much interest in receiving OCV after being presented with information. Of 51,814 persons surveyed during the pre-vaccination phase, 96.7% indicated their interest in receiving the vaccine.
Vaccination was begun on April 12th, 2012 in adults and children more than 10 years of age. A concurrent national immunization campaign for measles and polio was being undertaken in children < 1–9 years of age and thus required us to modify the strategy and to postpone vaccination of younger children until two weeks after completion of the measles and polio campaign as concurrent OCV administration with other live attenuated vaccines had not been studied. Vaccination of these younger children with OCV was begun on May 23rd. The pre-registration information was essential in helping to rapidly locate and identify children to vaccinate. Administration of the 100,000 doses of OCV was completed on July 10th, three months later. A total of 52,357 persons received dose 1 and 47,520 received dose 2. Thus, 90.8% of persons received the recommended two doses of vaccine two weeks apart. At the end of the project, only 123 vials of vaccine were lost through breakage or manufacturing flaws, and 99,877 (99.9%) doses were administered.
We estimated the overall vaccine coverage at 74.8%; 52,357 of approximately 70,000 living in the target area received dose one. This finding would predict herd immunity.26 The distribution of vaccinees by age paralleled the age distribution in the targeted populations (Table 1). The population vaccinated was 47% male and 53% female. Acceptance of vaccine was high in all age groups and similar among men and women (Table 2). The lowest coverage rates were obtained in the Village of God and the tent camps surrounding GHESKIO because of the high migration seen there related to gang violence and transitional housing situations in the post-earthquake tent camps.
Table 1.
GHESKIO oral cholera vaccine coverage by age group, Haiti
| Age groups, years | Dose 1 | Dose 2 | % |
|---|---|---|---|
| 1–5 | 5,014 | 4,662 | 93.0 |
| 6–10 | 4,970 | 4,616 | 92.9 |
| 11–18 | 10,381 | 9,548 | 92.0 |
| > 18 | 31,992 | 28,694 | 89.7 |
| Total | 52,357 | 47,520 | 90.8 |
Table 2.
GHESKIO oral cholera vaccine campaign coverage by site and sex, Haiti
| Site | Population | Dose 1 | Dose 2 | % Completed | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | M | F | No. | M | F | % Covered | No. | M | F | % Covered | ||
| City of God | 11,747 | 4,867 | 6,880 | 7,247 | 3,003 | 4,244 | 62 | 6,260 | 2,594 | 3,666 | 53 | 86 |
| Mobile population around GHESKIO* | 8,333 | 3,958 | 4,475 | 6,584 | 3,134 | 3,450 | 154 | 5,699 | 692 | 1,076 | 48 | 82 |
| City Plus | 11,990 | 5,127 | 6,863 | 9,764 | 4,175 | 5,589 | 81 | 8,700 | 3,720 | 4,980 | 73 | 89 |
| City of Eternity | 15,789 | 6,723 | 9,066 | 11,314 | 4,818 | 6,496 | 72 | 10,431 | 4,442 | 5,989 | 66 | 92 |
| Martissant | 21,326 | 9,075 | 12,251 | 17,448 | 7,424 | 10,024 | 82 | 16,430 | 6,991 | 9,439 | 77 | 94 |
| Total population | 69,185 | 29,750 | 39,535 | 52,357 | 22,554 | 29,803 | 76 | 47,520 | 20,470 | 27,050 | 69 | 91 |
| % Coverage by sex | 43 | 57 | 76 | 75 | 69 | 68 | ||||||
Consists of persons living in four tent camps, marketplaces, and small workshops around GHESKIO.
Safety.
The vaccine was well tolerated; < 1.3% of patients reported minor side effects (Table 3) such as headache (25%), nausea (20%), and abdominal pain (11%). The reported side effect profile is consistent with published data. Because all recipients received vaccine, there was no control for complaints recorded. The high acceptance of a second dose (90.8%) suggests that there were no adverse effects that limited acceptance and uptake of OCV in this community.
Table 3.
Reported side effects during the GHESKIO oral cholera vaccine, Haiti*
| Reaction | Severity grade 1–2 | Severity grade 3–4 |
|---|---|---|
| Systemic | Grade 1–2 | Grade 3 |
| Headache | 325 | 8 |
| Dizziness | 100 | 0 |
| Fever | 73 | 12 |
| Gastrointestinal | ||
| Nausea | 256 | 6 |
| Vomiting | 107 | 5 |
| Abdominal pain | 145 | 12 |
| Diarrhea | 141 | 23 |
| Any | 1,309 (1.3%) | 78 (0.08%) |
Reported symptoms during 24–48 hours after cholera vaccination. n/N = 1,334/99,867 (data for two doses combined).
Post-vaccination follow-up is ongoing with passive surveillance of all cholera cases from the targeted communities that come to GHESKIO's downtown CTC. The populations followed-up in the vaccination area all seek care at the GHESKIO ORPs or CTC. A detailed intake questionnaire gathers demographic information and OCV vaccine history for each patient and household contacts. Stool specimens are collected for culture and Luminex PCR (Luminex Corporation, Austin, TX) to confirm cholera cases in patients presenting with acute watery diarrhea.
Discussion
Two years after its introduction in Haiti, cholera is still ongoing; four large waves paralleling the rainy seasons have caused more than 8,000 deaths and affected hundreds of thousands.7 Efforts to increase awareness, setting up treatment centers all over the country, and training of health personnel and educational campaign have had a favorable impact on the mortality associated with the cholera epidemic in Haiti but have not decreased ongoing transmission.7 Clearly, sanitation, clean water, hand washing, and hygienic food preparation are integral to the control of cholera. Temporary measures of chlorination, bottled or tank truck–supplied water are being put in place but are not sufficient. Long-term improvements that are needed for sanitation and clean water will be costly and take time. The Inter-American Development Bank estimates at $2.2 billion the needed investments in Haiti to improve access to water and sanitation by 2015, one of the targeted Millennium Development Goals. Offering effective cholera vaccination to the most at risk populations is thus an essential component in the fight against cholera, in addition to other preventive measures, to help save lives to a future time when sewage systems will exist at the national level. It should not be an either/or situation between vaccination and sanitation in the interim.15
The greatest challenge to the OCV demonstration project lay in reaching a consensus about the need and role for vaccination in the Haitian setting. Leading experts and donor agencies argued against the use of cholera vaccine in Haiti27,28 citing, among others, the paucity of available data on reactive vaccination campaigns, logistical challenges in a country with inadequate infrastructure and human resources further devastated by an earthquake 10 months before, cold chain requirements, and difficulties administering a two-dose vaccine to mobile populations.29 This demonstration pilot was successful despite tremendous challenges.
The obstacles were manifold. It was not a simple logistical task to vaccinate with two doses a highly mobile population living with permanent insecurity in gang-riddled slums, especially during the rainy season where the houses that are built on refuse below sea level were constantly flooded and mud-filled, rendering access difficult. We knew that this vaccination effort in the urban slums could not be conducted at vaccination posts, as usually done in Haiti. Fixed vaccination posts can have poor turnouts for the first dose, little uptake for a second dose, and limited opportunity to document side effects associated with the vaccine. A door-to-door strategy was thus chosen, despite being much more labor intensive, to reach the maximum number of people in their homes and increase their probability of receiving the second dose. This strategy was also essential for GHESKIO's global health mission to expand access to microcredit, conduct active surveillance for HIV and TB, promote reproductive health services, and identify high-risk pregnancies in those underserved communities. It was said that OCV could not be delivered in the urban slums of Port-au-Prince because it was too dangerous. GHESKIO personnel did have many reservations about delivering vaccines inside these dangerous neighborhoods. The GHESKIO leadership was essential in helping to overcome this fear and convincing the staff and the community that the GHESKIO institution and its entire leadership was committed to this effort and other global health interventions and accompanied the vaccination teams inside the slums. The staff was well received by the population, and there was no violent incident during the three-month effort. Furthermore, during the more than 30 year existence of GHESKIO, there has not been one violent attack against any member of the personnel or of the facilities, even during the worst periods of political turmoil. GHESKIO does not have armed security guards. This is a testimony of how well the institution is accepted and recognized in the community it serves.
The objections to OCV implementation were many. Some OCV opponents feared that use of vaccine in uneducated populations would give a false sense of protection and lead to a decrease of WASH practices, putting people at increased risk of acquiring not only cholera but all other waterborne pathogens. GHESKIO's long experience of caring for poor uneducated populations affected by diseases requiring complex long-term care such as AIDS and TB has shown that poor and uneducated patients can become responsive to key messages that are well explained. This finding is demonstrated by the high rate of adherence to antiretroviral therapy obtained in our population. Our message was loud and clear: the fight against cholera requires a package consisting of WASH plus OCV. The vaccination and follow-up teams reinforced the WASH messages on a house-to-house basis while giving the vaccine. A survey conducted in those communities one year after the beginning of the cholera outbreak and before OCV implementation showed that persons were familiar with the WASH concepts but had little means of implementing them because their living conditions were so dismal: no money to buy soap all the time, no latrines in greater than 95% of the houses, and scarce access to potable water. The OCV protection to that extremely vulnerable population then represents their greatest hope.
Another argument against use of OCV in countries with limited resources with an ongoing outbreak such as Haiti, especially after the earthquake that severely affected the healthcare infrastructure and led to an important brain drain of trained personnel, was that OCV implementation would compete with other WASH interventions and distract resources and health personnel from focusing on prevention and hygiene promotion. There was no evidence of competition for resources or staff between OCV and WASH during this demonstration. The staff of GHESKIO entered with enthusiasm and organization into this effort. The distribution of OCV, coupled with the census and supplying of other services, has changed the attitudes within and towards these areas. Finally, fear that riots by an angry population would ensue because of concerns of inequity that 100,000 doses were insufficient for a large urban city such as Port-au-Prince did not materialize. With proper information and communication and GHESKIO's continued commitment to those communities, the population clearly understood the nature of this pilot and chaos did not arise.
The logistical challenges to prepare, implement, and monitor the progress of an enterprise this size were taxing. Daily team deployments had to be altered to accommodate potential insecurity because of gang activity and flooding caused by rain; the targeted area for vaccine delivery had to be extended further to the south (Martissant) than originally planned (Figure 1) to account for higher than anticipated migration rate in and out of the slums. Because of the frequent raids by the police to arrest gang leaders and the indiscriminate practice of arresting for interrogation all those found, many adults, particularly those with less than perfect police records, periodically left the area to avoid getting arrested. This finding accounted in large part for the 9% of persons who did not receive the second dose of OCV. These obstacles and the need to delay childhood vaccination because of concurrent polio and measles campaigns in children ≤ 10 years of age prolonged the time to completion of the campaign.
The IT unit was instrumental in the success of this enterprise and showed great flexibility to constantly adjust its strategies to address the multiple constraints encountered. The pre-registration of all persons in the targeted for area for OCV greatly facilitated the modification required for children when we were informed of the concurrent Expanded Immunization Program with measles and polio vaccines. Daily deployment lists were modified to remove all children less than 10 years of age and later reprinted two weeks after the end of the Expanded Immunization Program. The flexibility to adapt and being used to developing contingency plans and working in difficult conditions were some important ingredients to the success of the intervention. Other challenges faced by the IT unit were limitation of the GPS accuracy to adequately locate the homes because of crowded living conditions in the slums; limited amount of time available to test the system before launching the vaccine operation; numerous unforeseen events, leading to continuous adjustment of the program, which slowed down the process of quality control of the collected data; issues of communication at times when rapid decisions had to be taken delaying the proper collection of data and occasionally errors in data collection; the necessity to register new persons on the day of vaccination making it necessary to provide a unique identification number in the field with the possibility of errors (having the same number being assigned to multiple vaccinees); and increasing delay in analyzing the follow-up data because of the need for a larger number of human resources than available.
This vaccination effort was successful because of the persistence of local institutions, such as GHESKIO and PIH, to convince national and international authorities of the need to add a new intervention that is known to be effective in the arsenal against cholera, particularly for the most at risk population. This approach to cholera has been advocated for by the Stop Cholera: Coalition for Cholera Prevention and Control30 and a recent WHO meeting was devoted to creating a stockpile of cholera vaccine, an essential step.31 It could also not have been feasible without the financial help of the American Red Cross that trusted PIH and GHESKIO in their capacity to conduct this pilot successfully. Further support and advocacy from multiple partners, including the Cholera Coalition, helped gather momentum to reverse opposition to the use of OCV during an outbreak. Finally, WHO pre-approval of the Shanchol® vaccine reduced barriers to the acquisition of the vaccine.
Despite innumerable challenges, the GHESKIO OCV pilot project was a success and demonstrated that 1) it is feasible to deliver two doses of OCV to 50,000 most at-risk persons living in difficult conditions in a dangerous urban slum; 2) that Shanchol® vaccine is safe and well-tolerated with minimal side effects; and 3) OCV vaccine is well received by the community that is well informed and mobilized.
The strong leadership and involvement of the directors of GHESKIO in all aspects of the intervention was crucial, leading by example and being present at the vaccination sites, meeting with all those involved to identify the problems and finding rapid solutions to them. Community support and participation were also essential. Although all agencies and embassies have deserted the downtown area, particularly after the earthquake, GHESKIO is the only health institution that has continued to offer uninterrupted services for more than 30 years to this population. Meeting with community leaders before and during the vaccination campaign was important to counter doubts voiced in some popular media on the efficacy and potential harmful effect of OCV. The leadership of the MSPP at the highest level in favor of providing OCV was also critical. The Minister of Health and Population, Dr. Florence Guillaume, had the courage to accept this challenge when many important international collaborating agencies were saying that it could not be conducted. She officially launched the OCV demonstration project and the Director General of the MSPP was present with the GHESKIO staff in the slums to give the first dose of OCV (Figure 5).
Figure 5.
GHESKIO oral cholera vaccine campaign, Haiti. Director General of the Ministry of Health and Population gives the first oral cholera vaccine dose.
Haiti offers a unique opportunity to evaluate the impact of mass vaccination at curtailing an ongoing outbreak and address the scientific question over the role of reactive vaccination. There is renewed interest in looking at different strategies aiming at better cholera control. Recent publications show OCV confers up to 67% protection at three years,32 and mathematical models have proposed that vaccinating high-exposure areas every three years could help significantly reduce disease burden.33 These findings have important programmatic implications for the epidemic in Haiti and other countries with high cholera rates. Another promising study on the way is looking at the effectiveness of a single dose of OCV in a large case-controlled trial in India. The Technical Advisory Group of the Pan American Health Organization has endorsed the use of cholera vaccine in attempts to eradicate cholera on the island of Hispaniola, noting that pre-emptive vaccination may prevent outbreaks and reactive vaccination may limit the spread of current outbreaks.34
The positive outcome of the two urban and rural OCV demonstration projects has led to the launching by the MSPP of a national campaign to scale up OCV targeting at first three at-risk communities in the North East, Center, and North Departments35 (Les Perches, Lascaobas, and Quartier Morin) as part of a 10-year plan to control cholera in Haiti. Preparations are under way for the imminent launch of the campaign. Furthermore, a national cholera prevention campaign in collaboration with the Centers for Disease Control and Prevention, the United Nations Children's Fund, and Pan American Health Organization aimed to bring fundamental changes in the availability of clean water and sanitation system on a country-wide basis is in discussion. However, the money for this multi-billion dollar program has not yet been secured. Reactive vaccination may now be regarded as an additional control measure, depending on local infrastructure, the epidemiologic situation, and identification of target areas.
This pilot study was conducted to demonstrate and detail the feasibility of implementing a cholera vaccination program in a difficult setting plagued with logistical challenges, insecurity, and a severely weakened health infrastructure in the aftermath of a major natural catastrophe. The approach and methods used in this effort were much more involved and labor intensive than would be required for a national program because we believed that it was important to clearly document the challenges and successful strategies adopted to overcome them. The lessons learned are being used to inform and guide the national extension of cholera vaccination in Haiti. GHESKIO is participating in the planning meetings with the MSPP and all training, social mobilization, and technical documents developed by GHESKIO for the pilot phase have been shared with the MSPP for use in the national extension. A less labor-intensive strategy using vaccination posts has been chosen to minimize personnel required, as well as limit time and cost of scaling up vaccination. Teams will be composed of three health staff: a vaccinator, a person for the documentation of vaccination in registries, and a person for community mobilization to seek out and encourage persons from the community to get vaccinated. Such vaccine posts can reach hundreds of persons per day. The door-to-door approach will be reserved for persons who do not come back for the second dose and for harder-to-reach subgroups.
The good safety profile of the Shanchol® vaccine was further confirmed in this pilot study in the Haitian population and showed only mild side effects registered in a thorough post-vaccination monitoring effort. A simpler vaccine pharmaco-vigilance strategy is being envisioned for the national extension of OCV with passive reporting of adverse effects at local healthcare structures and case-based surveillance in sentinel MSPP sites and laboratories. The tremendous success of the massive roll out campaigns for the new meningitis A conjugate vaccine in Africa where more than 100 million doses have been given in 13 countries in just two years speaks to the power of rallying public and private sectors, governments, and local partners to overcome public health threats.36,37 These successful mass vaccination campaigns teach us how community involvement is crucial to the success of national vaccination endeavors, and that well-organized campaigns should be supported at the highest political level.
Successful implementation of OCV programs can be successful, despite logistical challenges and should serve to capacitate national ministries by working with them and in collaboration with local institutions with long standing history of service in their communities, such as GHESKIO and PIH. Finally, OCV interventions can be integrated with other WASH activities without competing with them. GHESKIO's success is in great part because of its leadership and long-term commitment to global health that goes beyond simply offering cholera vaccination to most at-risk populations, but the institution continually seeking to offer integrated packages of care that make a notable impact in complex settings such as Haiti.
ACKNOWLEDGMENTS
We thank the staff of Les Centres GHESKIO for their dedication and hard work.
Footnotes
Financial support: This study was supported by the American Red Cross through Partners in Health; the United Nations Children's Fund (grant Renforcement et Maintien des Activités dans le Cadre de la Réponse pour le Cholera); the Centers for Disease Control and Prevention (5U2GGH000545-02); and the National Institutes of Health (1K24 AI098627-02, 5 U2R TW006896-10, 5D43 TW 000018-25, and 4UM1AI069421-07).
Authors' addresses: Vanessa Rouzier, Karine Severe, Marc Antoine Jean Juste, Mireille Peck, Christian Perodin, Patrice Severe, Marie Marcelle Deschamps, Rose Irene Verdier, and Sabine Prince, Les Centres GHESKIO, Port-au-Prince, Haiti, E-mails: vrouzier@gheskio.org, karinesevere@gheskio.org, mireillespeck@yahoo.com, cperodin@gheskio.org, patsevere@gheskio.org, mariehd@gheskio.org, riv@gheskio.org, and sprince@gheskio.org. Jeannot Francois, Jean Ronald Cadet, and Florence D. Guillaume, Ministry of Health and Population, Expanded Immunization Program, Port-au-Prince, Haiti, E-mails: francoisjeannot@yahoo.fr, cadethaiti@yahoo.fr, and minister@mspp.gouv.ht. Peter F. Wright, Department of Pediatrics, Dartmouth Medical School, Hanover, NH, E-mail: peter.f.wright@dartmouth.edu. Jean W. Pape, Division of Infectious Disease, Weill Cornell Medical College, New York, NY, E-mail: jwpape@gheskio.org.
References
- 1.Ali M, Lopez AL, You YA, Kim YE, Sah B, Maskery B, Clemens J. The global burden of cholera. Bull World Health Organ. 2012;90:209–218. doi: 10.2471/BLT.11.093427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Son MS, Megli CJ, Kovacikova G, Qadri F, Taylor RK. Characterization of Vibrio cholerae O1 El Tor biotype variant clinical isolates from Bangladesh and Haiti, including a molecular genetic analysis of virulence genes. J Clin Microbiol. 2011;49:3739–3749. doi: 10.1128/JCM.01286-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodgame RW, Greenough WB. III Cholera in Africa: a message for the West. Ann Intern Med. 1975;82:101–106. doi: 10.7326/0003-4819-82-1-101. [DOI] [PubMed] [Google Scholar]
- 4.Seas C, Miranda J, Gil AI, Leon-Barua R, Patz J, Huq A, Colwell RR, Sack RB. New insights on the emergence of cholera in Latin America during 1991: the Peruvian experience. Am J Trop Med Hyg. 2000;62:513–517. doi: 10.4269/ajtmh.2000.62.513. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) Cholera outbreak—Haiti, October 2010. MMWR Morb Mortal Wkly Rep. 2010;59:1411. [PubMed] [Google Scholar]
- 6.Chin CS, Sorenson J, Harris JB, Robins WP, Charles RC, Jean-Charles RR, Bullard J, Webster DR, Kasarskis A, Peluso P, Paxinos EE, Yamaichi Y, Calderwood SB, Mekalanos JJ, Schadt EE, Waldor MK. The origin of the Haitian cholera outbreak strain. N Engl J Med. 2011;364:33–42. doi: 10.1056/NEJMoa1012928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haiti Ministry of Public Health and Population. http://www.mspp.gouv.ht/site/index.php?option=com_content&view=article&id=120&Itemid=1 Available at. Accessed November 7, 2012.
- 8.Barzilay EJ, Schaad N, Magloire R, Mung KS, Boncy J, Dahourou GA, Mintz ED, Steenland MW, Vertefeuille JF, Tappero JW. Cholera surveillance during the Haiti epidemic: the first 2 years. N Engl J Med. 2013;368:599–609. doi: 10.1056/NEJMoa1204927. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan CA, Meigh JR, Giacomello AM. The Water Poverty Index: development and application at the community scale. Nat Resour Forum. 2003;27:189–199. [Google Scholar]
- 10.Waldman RJ, Mintz ED, Papowitz HE. The cure for cholera: improving access to safe water and sanitation. N Engl J Med. 2013;368:592–594. doi: 10.1056/NEJMp1214179. [DOI] [PubMed] [Google Scholar]
- 11.Periago MR, Frieden TR, Tappero JW, De Cock KM, Aasen B, Andrus JK. Elimination of cholera transmission in Haiti and the Dominican Republic. Lancet. 2012;379:e12–e13. doi: 10.1016/S0140-6736(12)60031-2. [DOI] [PubMed] [Google Scholar]
- 12.Nelson EJ, Nelson DS, Salam MA, Sack DA. Antibiotics for both moderate and severe cholera. N Engl J Med. 2011;364:5–7. doi: 10.1056/NEJMp1013771. [DOI] [PubMed] [Google Scholar]
- 13.Ivers LC, Farmer PE, Pape WJ. Oral cholera vaccine and integrated cholera control in Haiti. Lancet. 2012;379:2026–2028. doi: 10.1016/S0140-6736(12)60832-0. [DOI] [PubMed] [Google Scholar]
- 14.Ivers LC, Farmer P, Almazor CP, Leandre F. Five complimentary interventions to slow cholera. Haiti Lancet. 2010;376:2048–2051. doi: 10.1016/S0140-6736(10)62243-X. [DOI] [PubMed] [Google Scholar]
- 15.Farmer P, Almazor CP, Bahnsen ET, Barry D, Bazile J, Bloom BR, Bose N, Brewer T, Calderwood SB, Clemens JD, Cravioto A, Eustache E, Jérôme G, Gupta N, Harris JB, Hiatt HH, Holstein C, Hotez PJ, Ivers LC, Kerry VB, Koenig SP, Larocque RC, Léandre F, Lambert W, Lyon E, Mekalanos JJ, Mukherjee JS, Oswald C, Pape JW, Gretchko Prosper A, Rabinovich R, Raymonville M, Réjouit JR, Ronan LJ, Rosenberg ML, Ryan ET, Sachs JD, Sack DA, Surena C, Suri AA, Ternier R, Waldor MK, Walton D, Weigel JL. Meeting cholera's challenge to Haiti and the world: a joint statement on cholera prevention and care. PLoS Negl Trop Dis. 2011;5:e1145. doi: 10.1371/journal.pntd.0001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newton AE, Heiman KE, Schmitz A, Török T, Apostolou A, Hanson H, Gounder P, Bohm S, Kurkjian K, Parsons M, Talkington D, Stroika S, Madoff LC, Elson F, Sweat D, Cantu V, Akwari O, Mahon BE, Mintz ED. Cholera in United States associated with epidemic in Hispaniola. Emerg Infect Dis. 2011;17:2166–2168. doi: 10.3201/eid1711.110808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryan ET, Madoff LC, Ferraro MJ. Case records of the Massachusetts General Hospital. Case 20-2011. A 30-year-old man with diarrhea after a trip to the Dominican Republic. N Engl J Med. 2011;364:2536–2541. doi: 10.1056/NEJMcpc1100928. [DOI] [PubMed] [Google Scholar]
- 18.Shin S, Desai SN, Sah BK, Clemens JD. Oral vaccines against cholera. Clin Infect Dis. 2011;52:1343–1349. doi: 10.1093/cid/cir141. [DOI] [PubMed] [Google Scholar]
- 19.Sur D, Kanungo S, Sah B, Manna B, Ali M, Paisley AM, Niyogi SK, Park JK, Sarkar B, Puri MK, Kim DR, Deen JL, Holmgren J, Carbis R, Rao R, Nguyen TV, Han SH, Attridge S, Donner A, Ganguly NK, Bhattacharya SK, Nair GB, Clemens JD, Lopez AL. Efficacy of a low-cost, inactivated whole-cell oral cholera vaccine: results from 3 years of follow-up of a randomized, controlled trial. PLoS Negl Trop Dis. 2011;5:e1289. doi: 10.1371/journal.pntd.0001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinclair D, Abba K, Zaman K, Qadri F, Graves PM. Oral vaccines for preventing cholera. Cochrane Database Syst Rev Mar. 2011;16((3)):CD008603. doi: 10.1002/14651858.CD008603.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lucas ME, Deen JL, von Seidlein L, Wang XY, Ampuero J, Puri M, Ali M, Ansaruzzaman M, Amos J, Macuamule A, Cavailler P, Guerin PJ, Mahoudeau C, Kahozi-Sangwa P, Chaignat CL, Barreto A, Songane FF, Clemens JD. Effectiveness of a mass oral cholera vaccination in Beira, Mozambique. N Engl J Med. 2005;352:757–767. doi: 10.1056/NEJMoa043323. [DOI] [PubMed] [Google Scholar]
- 22.Clemens JD, Ke NT, Thuy HT, Son ND, Canh DG, Hang PV, Rao MR, Trach DD. Field trial of a locally produced, killed, oral cholera vaccine in Vietnam. Lancet. 1997;349:231–235. doi: 10.1016/s0140-6736(96)06107-7. [DOI] [PubMed] [Google Scholar]
- 23.Pape JW, Johnson WD, Jr, Fitzgerald DW. The earthquake in Haiti: dispatch from Port-au-Prince. N Engl J Med. 2010;362:575–577. doi: 10.1056/NEJMp1001015. [DOI] [PubMed] [Google Scholar]
- 24.Pape JW, Deschamps MM, Ford H, Joseph P, Johnson WD, Jr, Fitzgerald DW. The GHESKIO refugee camp after the earthquake in Haiti–dispatch 2 from Port-au-Prince. N Engl J Med. 2010;362:e27. doi: 10.1056/NEJMpv1001785. [DOI] [PubMed] [Google Scholar]
- 25.Pape JW, Rouzier V, Ford H, Joseph P, Johnson WD, Jr, Fitzgerald DW. The GHESKIO field hospital and clinics after the earthquake in Haiti: dispatch 3 from Port-au-Prince. N Engl J Med. 2010;362:e34. doi: 10.1056/NEJMpv1001787. [DOI] [PubMed] [Google Scholar]
- 26.Ali M, Sur D, You YA, Kanungo S, Sah B, Manna B, Puri M, Wierzba TF, Donner A, Nair GB, Bhattacharya SK, Dhingra MS, Deen JL, Lopez AL, Clemens J. Herd protection by a bivalent killed whole-cell oral cholera vaccine in the slums of Kolkata, India. Clin Infect Dis. 2013;56:1123–1131. doi: 10.1093/cid/cit009. [DOI] [PubMed] [Google Scholar]
- 27.PAHO Position on Cholera Vaccination in Haiti . Washington, DC: Pan American Health Organization; 2010. Version October 27, 2010. [Google Scholar]
- 28.World Health Organization Cholera vaccines: WHO position paper. Wkly Epidemiol Rec. 2010;85:117–128. [PubMed] [Google Scholar]
- 29.Date KA, Vicari A, Hyde TB, Mintz E, Danovaro-Holliday MC, Henry A, Tappero JW, Roels TH, Abrams J, Burkholder BT, Ruiz-Matus C, Andrus J, Dietz V. Considerations for oral cholera vaccine use during outbreak after earthquake in Haiti, 2010–2011. Emerg Infect Dis. 2011;17:2105–2112. doi: 10.3201/eid1711.110822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The Task Force for Global Health/Partners in Health . Stop Cholera: Coalition for Cholera Prevention and Control. Atlanta, GA: 2012. Summary conference proceedings, unpublished conference paper, March 15–16, 2012. [Google Scholar]
- 31.Waldor MK, Hotez PJ, Clemens JD. A national cholera vaccine stockpile: a new humanitarian and diplomatic resource. N Engl J Med. 2010;363:2279–2282. doi: 10.1056/NEJMp1012300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khatib AM, Ali M, von Seidlein L, Kim DR, Hashim R, Reyburn R, Ley B, Thriemer K, Enwere G, Hutubessy R, Aguado MT, Kieny MP, Lopez AL, Wierzba TF, Ali SM, Saleh AA, Mukhopadhyay AK, Clemens J, Jiddawi MS, Deen J. Effectiveness of an oral cholera vaccine in Zanzibar: findings from a mass vaccination campaign and observational cohort study. Lancet Infect Dis. 2012;12:837–844. doi: 10.1016/S1473-3099(12)70196-2. [DOI] [PubMed] [Google Scholar]
- 33.Longini IM, Jr, Nizam A, Ali M, Yunus M, Shenvi N, Clemens JD. Controlling endemic cholera with oral vaccines. PLoS Med. 2007;4:e336. doi: 10.1371/journal.pmed.0040336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Technical Advisory Group on Vaccine-Preventable Diseases . 2011. Final Report from the XIX TAG Meeting, July 6–8, 2011, Buenos Aires, Argentina [Google Scholar]
- 35.Bulletin MSPP Janvier–Mars 2013. http://issuu.com/jean-juniorjoseph/docs/bulletin_mspp_janvier-mars_2013_-_140513 Available at. Accessed August 4, 2013.
- 36.Djingarey MH, Barry R, Bonkoungou M, Tiendrebeogo S, Sebgo R, Kandolo D, Lingani C, Preziosi MP, Zuber PL, Perea W, Hugonnet S, Dellepiane de Rey Tolve N, Tevi-Benissan C, Clark TA, Mayer LW, Novak R, Messonier NE, Berlier M, Toboe D, Nshimirimana D, Mihigo R, Aguado T, Diomandé F, Kristiansen PA, Caugant DA, Laforce FM. Effectively introducing a new meningococcal A conjugate vaccine in Africa: the Burkina Faso experience. Vaccine. 2012;30((Suppl 2)):B40–B45. doi: 10.1016/j.vaccine.2011.12.073. [DOI] [PubMed] [Google Scholar]
- 37.Ouandaogo CR, Yaméogo TM, Diomandé FV, Sawadogo C, Ouédraogo B, Ouédraogo-Traoré R, Pezzoli L, Djingarey MH, Mbakuliyemo N, Zuber PL. Adverse events following immunization during mass vaccination campaigns at first introduction of a meningococcal A conjugate vaccine in Burkina Faso, 2010. Vaccine. 2012;30((Suppl 2)):B46–B51. doi: 10.1016/j.vaccine.2011.12.112. [DOI] [PubMed] [Google Scholar]