Abstract
Cost-effectiveness information on where malaria rapid diagnostic tests (RDTs) should be introduced is limited. We developed incremental cost-effectiveness analyses with data from rural health facilities in Ghana with and without microscopy. In the latter, where diagnosis had been presumptive, the introduction of RDTs increased the proportion of patients who were correctly treated in relation to treatment with antimalarials, from 42% to 65% at an incremental societal cost of Ghana cedis (GHS)12.2 (US$8.3) per additional correctly treated patients. In the “microscopy setting” there was no advantage to replacing microscopy by RDT as the cost and proportion of correctly treated patients were similar. Results were sensitive to a decrease in the cost of RDTs, which cost GHS1.72 (US$1.17) per test at the time of the study and to improvements in adherence to negative tests that was just above 50% for both RDTs and microscopy.
Introduction
Presumptive diagnosis and treatment of uncomplicated malaria continues to be common in many parts of Africa1–3 despite well-known problems with this strategy. Clinical symptoms of uncomplicated malaria are nonspecific and overlap with several other diseases4–6; as such, diagnosing malaria based on symptoms alone leads to substantial overdiagnosis of malaria.2,7–10 Massive overtreatment with antimalarials is likely to be unsustainable in an era where countries are using the relatively more expensive artemisinin-based combination therapy (ACT) as the first-line antimalarial.11
Current World Health Organization (WHO) guidelines on malaria case management recommend parasitological confirmation before treatment with an antimalarial.12 The gold standard has for many years been a blood slide examined under a light microscope. However, unless the laboratory technicians are well trained and the equipment well maintained, the accuracy of microscopy can be low under field conditions.13–16 Rapid diagnostic tests (RDTs) for malaria require limited training and have been found to perform well under field conditions17,18 and often better than routine microscopy.3,19,20
A major potential barrier to parasitological testing for malaria is the cost of the tests. However, improved targeting of antimalarial treatment to parasite-positive patients has been found to result in substantial cost savings of antimalarial drugs21–24 sometimes even enough to also cover the additional costs of parasitological testing.19,25,26
Previous cost-effectiveness analyses comparing malaria diagnostic approaches suggest that both RDTs and microscopy are more cost-effective than presumptive diagnosis.15,26–28 However, the relative cost-effectiveness of different diagnostic methods does appear to be highly dependent on a variety of factors. One important factor is the prescribers' degree of adherence to test results, meaning the likelihood that antimalarials are withheld from those patients who test negative. Although several economic studies assume high levels of adherence, multiple observational studies and trials have shown that this assumption is often incorrect with prescribers still prescribing an antimalarial despite a negative test result.8,10,29 Modeling studies confirm that any significant tendency to poor adherence to negative test results will decrease the cost-effectiveness of parasitological diagnosis relative to presumptive treatment.15,25,27,30 Similarly, high prevalence of malaria and therefore a high proportion of fever patients being parasite positive tends to improve the cost-effectiveness of presumptive treatment relative to parasitological testing, whereas the opposite is the case at moderate and low prevalence levels.25,27,31 Other important factors identified include the cost of RDT and microscopy, the accuracy of one test compared with another, cost of drug regimens for parasite-positive and parasite-negative patients and the degree to which non-malarial patients treated with an antimalarial seek additional care.9,26,32,33
The appropriateness of introducing RDTs into health facilities also depends on the presence and quality of existing microscopy services. A previously published clinical trial from Ghana showed that the impact of introducing RDTs on prescribing behavior was much greater in peripheral facilities without access to microscopy where malaria treatment had previously been presumptive, compared with a higher level facility with microscopy.10 Peripheral clinics with no microscopy make up the majority of formal care for patients with acute fevers in West Africa. However, the cost-effectiveness of the introduction of RDTs in these different settings is unknown. Whether, and where to introduce RDTs is currently a central question for policymakers, with cost-effectiveness a major consideration. To help inform decisions about the most appropriate diagnostic approach this study presents an incremental cost-effectiveness analysis of introducing RDTs in Ghana: in one central clinic where microscopy has been routinely available and another two peripheral clinics without microscopy where treatment has been presumptive.
Methods
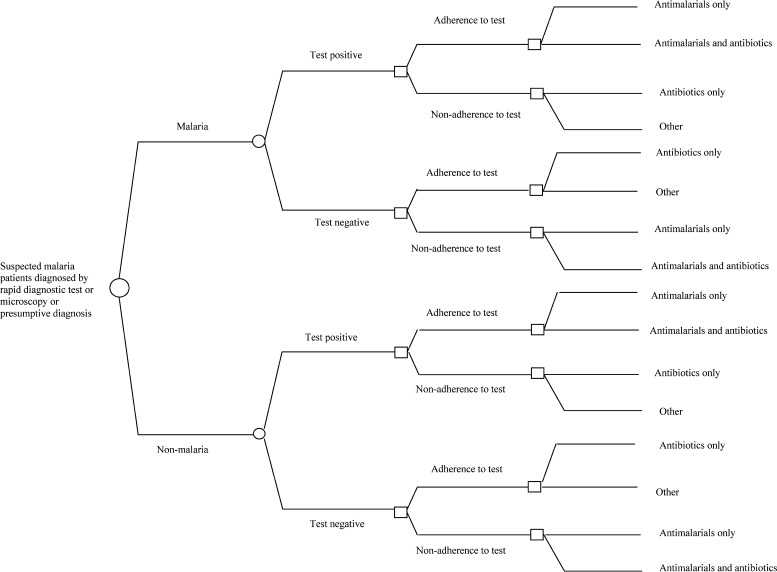
This study estimated the societal cost and effects of malaria treatment after one of three possible diagnostic methods: microscopy, presumptive diagnosis, or RDT. Incremental cost-effectiveness analyses were performed for displacing microscopy with RDT diagnosis and also for introducing RDT diagnosis instead of presumptive diagnosis. The incremental cost-effectiveness analyses were designed as a decision analysis where a reference population of 1,000 suspected malaria patients in each diagnostic option (RDT or current practice) in the two settings was exposed to the decision tree displayed in Figure 1. The decision models by diagnostic method and setting were populated using data collected in Dangme West District in rural Ghana. Data captured included the relevant probabilities (i.e., accuracy of tests), cost borne by the public health sector for diagnosis and treatment, household cost of health care seeking, and the effects of the health care received for patients.
Figure 1.
Decision tree.
Study setting.
Dangme West is a rural district with an estimated 2008 mid-year population of about 130,000. The people live mostly in scattered, small communities. The most densely populated area is Dodowa, the main administrative area of the district with 37,700 inhabitants and with the widest range of health providers. The district has 10 public health facilities of varying size and patient load, one publicly owned laboratory, and two privately owned laboratories located in other parts of the district. Private pharmacies and drug shops sell a range of medicines including antimalarials.
Three public health facilities were selected for this study. One was a large health facility with a high patient load staffed by medical doctors, medical assistants, and nurses, and equipped with a laboratory including microscopy, subsequently referred to as the “microscopy setting.” The remaining two health facilities situated in rural areas 15 and 29 km, respectively, from Dodowa were smaller, with lower patient loads and were staffed by medical assistants and nurses. These health facilities had no access to parasitological testing for malaria so diagnosis was mainly presumptive. These facilities will be referred to as the “presumptive diagnosis setting.” The nearest public sector laboratory was located in the large health facility in the administrative area, and there was a small private laboratory located < 5 km away from the furthest health facility.
Interventions.
At the time of the study, the national malaria control policy in Ghana was not yet recommending RDTs and they were therefore not routinely available in public health facilities. In the period from August 2007 to December 2008 a previously reported randomized, controlled, open label clinical trial was conducted in which diagnosis by malaria RDT was introduced in the study health facilities in both settings.10 Health workers from the study health facilities participated in a workshop focusing on how to perform an RDT and reviewing the clinical symptoms of malaria and other differential diagnoses of febrile illnesses in both adults and children. Patients visiting the health facility in the microscopy setting and suspected by a healthcare professional to have malaria were randomly allocated to have a blood sample taken for diagnosis either by RDT (RDT arm) or microscopy (microscopy arm). In both arms, the result of the test was given to healthcare professionals who then decided the treatment of the patient. In the same period, suspected malaria patients coming to the two health facilities in the presumptive diagnosis setting were randomly allocated to either have an RDT (RDT arm) or to be treated presumptively (presumptive diagnosis arm). There were 7,263 suspected malaria patients of whom 3,811 visited the health facility in the microscopy setting and 3,452 came to the health facilities in the presumptive diagnosis setting. A blood sample for a research slide was taken from all study patients (see trial description10 for details).
Measurement of effect.
The effect measure for the cost-effectiveness analysis was the proportion of patients correctly treated. This was taken from each of the study arms in the clinical trial described previously. Correct treatment was defined as a patient with a parasite-positive research slide who was prescribed an antimalarial, or a patient with a parasite-negative research slide who was not prescribed an antimalarial. In addition, other results from this trial such as the malaria slide positivity rate, accuracy of diagnostic testing methods, and adherence to test results (defined as the proportion of RDT or microscopy negative patients who did not receive an antimalarial) were applied to the reference populations in the decision models.
Measurement of costs.
Economic costs of resources were measured both from the perspective of the public health sector and the patients seeking care. Total cost by intervention depended on the specific pathways taken by suspected malaria patients through the decision tree (Figure 1). Direct costs to the public health facilities included the value of all resources needed to perform RDT or microscopy diagnosis and treatment in the outpatient department (OPD). Household costs of the initial visits to the study health facilities and any subsequent health-seeking visits incorporated both direct costs such as out-of-pocket expenditure for travel and the indirect costs in the form of value of time taken to travel and wait at the health facility and time unable to perform usual activities (income generation or housework). All cost figures were adjusted to the 2009 price level using the consumer price index34 and presented in Ghana cedis (GHS1 = US$0.68).
All three study health facilities were visited in 2009 to collect information on all resources used by an individual health facility during the financial year 2008, which included salaries for personnel and recurrent expenditure on drugs, disposables, RDTs, reagents, maintenance, utilities, transport, etc. Annual equivalents of the value of capital goods including buildings, equipment, and furniture were estimated using a discount rate of 5% and expected life spans of 30, 7, and 10 years, respectively, of the mentioned capital goods. Applying the standard step-down costing methodology,35,36 health facility-level cost was allocated in a stepwise fashion to the relevant cost centers of this study, which were the laboratory and the outpatient departments.
This was supplemented by bottom-up costing methods37,38 to separate the cost of malaria-specific activities from other activities. Interviews with laboratory staff captured self-reported time required to prepare and read a microscope slide and perform an RDT. Laboratory staff also listed the types and amounts of reagents and disposables required per individual test. A share of the annualized value of a microscope was allocated to malaria slide testing according to relative use. The average cost per RDT provided was estimated as the health system cost (personnel and other health facility cost) plus the price of the RDT used in the trial (Optimal, Diamed AG, Cressier, Switzerland) which was GHS1.72 (US$1.17) including freight.
Similarly, detailed bottom-up costing was used to capture malaria-related cost for treating malaria in the outpatient department. Personnel time was measured through actual observation. Details of all the drugs including their dosages prescribed to suspected malaria patients were captured from patient exit surveys in all three study health facilities (to be described below). Individual prescriptions to patients were valued using prices from Ghana Central Medical Stores for malaria drugs and the median supplier price from the International Drug Price Indicator Guide39 for all remaining drugs with an addition of 15% to allow for shipping cost as recommended by this guide. In both settings, an ACT in the form of fixed dose Artesunate-Amodiaquine was by far the most commonly used antimalarial. At the time of the study the reported cost of an adult course of nine tablets (50 mg/153 mg) was GHS1.26 (US$0.86). Drug prescriptions per visit were classified into four treatment categories: 1) antimalarials but no antibiotics, 2) antimalarials and antibiotics, 3) antibiotics but no antimalarials, and 4) drugs other than antimalarials and antibiotics. Average drug cost per visit was then calculated by these four treatment categories.
Cost data from the standard step-down costing methodology and the bottom-up costing were combined to form full unit costs per malaria microscope test, testing using an RDT, and outpatient treatments. Care was taken not to double count any resources.
Household costs of health care seeking and time lost were captured for a 2-week period starting from the first visit to a study health facility. Household cost data were obtained through a patient exit survey followed by a visit to the homes of the same patients 2 weeks later and all interviews took place in August–September 2009. All patients visiting the health facility and who met the inclusion criteria were eligible for enrollment in the study. Patients suspected of malaria by healthcare professionals at the health facilities were sent to research assistants for evaluation of eligibility and possible recruitment into the study. Informed consent was sought if the patient fulfilled the eligibility criteria. Allocations to either microscopy or RDT at the health center in the microscopy setting and to either presumptive diagnosis or RDT at the health facilities in the presumptive diagnosis setting were computer generated. Information on the allocations to diagnostic techniques were placed in sequentially numbered sealed opaque envelopes, which were previously labeled with unique study ID numbers and patients brought these envelopes to the consultation with the healthcare professional. The allocated test was carried out and a research slide taken at the same time. Eligible patients or their caregivers in the case of children < 15 years of age were approached on leaving a health facility by members of the study team who interviewed patients and caregivers on transport cost, transport time, and waiting time at the health facility on the day of visit. All drugs prescribed at this health facility were also captured from their patient folders and used for estimating drug cost during outpatient department visits as described earlier. The follow-up interview in the homes of patients investigated if the patient carried out a repeat visit at the same health facility or sought care from other health providers. Household cost related to additional health care was also captured. Finally, patients were asked if he/she had been unable to perform his/her usual activities because of illness and for how long.
Interviewers were high school leavers who were experienced data collectors and most of whom had participated in a number of previous similar studies. They had collected data for the main trial10 during which time they had received 12 days of training covering among others: the objectives of the study, community entry skills, and interviewing techniques.
The valuation of opportunity cost of time spent seeking treatment and time unable to perform usual activities was guided by the human capital approach40,41 but with deliberate departures from this theory. It was decided to assign an equal value of time lost for all patients regardless of their gender, age, and socio-economic background (type of work, actual income). The choice of an identical value of time rather than valuation based on actual income was made to avoid disadvantaging certain population groups. For instance, an intervention would tend to be relatively more cost-effective than another intervention simply if the former was more successful in treating individuals with salaried work rather than individuals outside the labor market such as housewives, children, and the elderly. Self-reported monthly salaries among patients 18 years of age and above in the household cost survey were used to estimate the opportunity cost of time. The distribution of patients across main work activity was similar by arm within settings (Table 1). The average self-reported monthly salaries were not significantly different by arm within settings and 81% of all patients 18 years of age and above reported a monthly salary above zero. Overall, the average monthly salary was GHS46.2 after removing the 5% highest salaries to diminish the influence of right skewness. Dividing by 21 work days in a month, this amounted to GHS2.2/day, which was what was used as the estimate of opportunity cost for all patients. The value of time of the care providers of the patients was not included.
Table 1.
Socio-economic background of patients and household cost in GHS per fever episode among participants in household cost survey in two settings in Dangme West District, 2009, Ghana, GHS1 = US$0.68
| Microscopy setting | Presumptive diagnosis setting | |||
|---|---|---|---|---|
| RDT arm | Microscopy arm | RDT arm | Presumptive diagnosis arm | |
| Number of patients | 101 | 101 | 102 | 102 |
| Gender | ||||
| Male | 37 | 49 | 40 | 41 |
| Female | 64 | 52 | 62 | 61 |
| Age | ||||
| 0–5 | 30 | 37 | 22 | 17 |
| 5–18 | 27 | 24 | 30 | 27 |
| 18–60 | 30 | 32 | 40 | 42 |
| 60+ | 14 | 8 | 10 | 16 |
| Main activity of patient (18 years and above) | ||||
| Farmer, fisherman | 3 | 3 | 5 | 8 |
| Small-scale trader | 9 | 15 | 16 | 22 |
| Other private sector | 7 | 8 | 10 | 8 |
| Public sector | 7 | 3 | 6 | 6 |
| Retired, housewife, unemployed | 18 | 11 | 13 | 14 |
| Monthly salary in GHS of patient (18 years and above)* | ||||
| Mean | 64.6 | 64.1 | 54.3 | 68.6 |
| Standard deviation | 97.7 | 92.4 | 60.8 | 108.4 |
| Household cost of health care seeking | ||||
| % of pts seeking additional care† | 17% | 17% | 11% | 11% |
| Hh cost per fever episode (GHS)‡ | 6.8 | 6.9 | 6.9 | 7.7 |
Self-reported monetary income.
Public health facility or chemical seller.
Household out-of pocket expenditure and opportunity cost of time lost (waiting at health provider, traveling, unable to perform usual activities).
The sample size for the household cost survey was calculated on the assumption that the household cost would be lower in the RDT arms as compared with current diagnosis in both settings. The average household cost for seeking malaria care was assumed to be GHS10 per episode in the current diagnosis arms; an order of magnitude similar to findings from previous empirical research.42,43 Assuming further that the standard deviation of the mean household cost was 80% of the mean, a power of 80%, a significance level of 5%, and an ability to detect a 30% decrease in the mean household cost between arms with current diagnostic technique and RDT resulted in 83 interviews per arm or 332 interviews in total.44 A total of 418 patients were interviewed during the exit survey and 406 patients could subsequently be identified for the follow-up interview in their homes 2 weeks later. The number of interviews was evenly distributed across the four study arms (Table 1).
Sensitivity analysis.
Univariate sensitivity analyses were used to assess the robustness of the baseline incremental cost-effectiveness ratios (ICERs) (Table 2). Parameters included in the sensitivity analyses were the sensitivities and specificities of the diagnostic tests and presumptive diagnosis, provider adherence to the test results, cost per outpatient department visit, cost per microscopy test, RDT price, and health facility cost of performing an RDT. Finally, a much lower ACT price of US$0.02–0.08 per course45 was incorporated into the sensitivity analysis to reflect the actual cost of ACTs to the public sector as a result of the Affordable Medicines Facility—malaria (AMFm) strategy, which provides a manufacturer level co-payment for ACTs in pilot countries including Ghana.45
Table 2.
Outcomes, total cost by arm, and incremental cost-effectiveness ratio of replacing current diagnostic methods by rapid diagnostic tests in two settings in Dangme West District, 2009*
| Outcomes, costs | Microscopy setting | Presumptive diagnosis setting | ||
|---|---|---|---|---|
| RDT arm | Microscopy arm | RDT arm | Presumptive diagnosis arm | |
| Suspected malaria patients, No. (%) | 1,000 (100%) | 1,000 (100%) | 1,000 (100%) | 1,000 (100%) |
| Of which received: | ||||
| Antimalarials, no antibiotics | 508 (51%) | 527 (53%) | 550 (55%) | 696 (70%) |
| Antimalarials and antibiotics | 116 (12%) | 116 (12%) | 150 (15%) | 231 (23%) |
| Antibiotics, no antimalarials | 168 (17%) | 159 (16%) | 158 (16%) | 38 (4%) |
| Other than antimal. and antibiot. | 207 (21%) | 198 (20%) | 141 (14%) | 35 (3%) |
| Correctly treated patients, No. (%)† | 601 (60%) | 569 (57%) | 651 (65%) | 420 (42%) |
| Total cost, GHS (%) | ||||
| Diagnostics | 2,824 (13%) | 2,028 (9%) | 3,919 (16%) | 0 (0%) |
| Drugs | 2,743 (12%) | 3,433 (16%) | 2,891 (12%) | 3,131 (15%) |
| Salaries, supplies, buildings | 9,849 (44%) | 9,743 (44%) | 10,451 (43%) | 10,564 (49%) |
| Total public health sector cost‡ | 15,416 (69%) | 15,204 (69%) | 17,260 (71%) | 13,695 (64%) |
| Out-of-pocket (travel, drugs) | 973 (4%) | 986 (4%) | 901 (4%) | 896 (4%) |
| Opportunity cost (travel, waiting) | 1,619 (7%) | 1,603 (7%) | 1,556 (6%) | 1,572 (7%) |
| Opportunity cost (work time lost) | 4,257 (19%) | 4,303 (19%) | 4,466 (18%) | 5,209 (24%) |
| Total patient cost§ | 6,849 (31%) | 6,892 (31%) | 6,924 (29%) | 7,677 (36%) |
| Total societal cost | 22,265 (100%) | 22,096 (100%) | 24,184 (100%) | 21,373 (100%) |
| Incremental analysis | Replace microscopy diagnosis by RDT diagnosis | Replace presumptive diagnosis by RDT diagnosis | ||
| Increase in no. of corr. treated pts | 32 | 231 | ||
| Incremental cost, health sector, GHS | 212 | 3,565 | ||
| Incremental cost, societal, GHS | 170 | 2,812 | ||
| ICER, health sector, GHS¶ | 6.7 | 15.4 | ||
| ICER, societal, GHS¶ | 5.3 | 12.2 | ||
Ghana, normalized to a population of 1,000 patients per arm. (GHS1 = US$0.68).
Patients with a parasite positive research slide who were prescribed an antimalarial or patients with a parasite negative research slide who were not prescribed an antimalarial.
Including initial health facility visits (patient recruitment) plus additional health facility visits within two weeks.
Including initial health facility visits (patient recruitment) plus additional health facility and chemical seller visits within 2 weeks.
ICER = Incremental Cost-Effectiveness Ratio.
Since the finalization of data collection for this study in 2009, more recent studies suggest a development toward improved provider adherence to RDT results24,46,47 and high patient acceptance of negative RDT results48 in many parts of Africa and a decline in the price of malaria RDTs. The impact of these trends was estimated by calculating the ICERs in both settings under a scenario where the provider adherences to negative RDT results were improved to 80% as opposed to around 50% found in this study (Table 3) and a low RDT price of US$0.65. The cost per malaria microscopy test and provider adherence to negative test results were assumed to be unchanged as were the price of a course of ACT.
Table 3.
Outcomes and cost in GHS per service in study health facilities in two settings in Dangme West District, 2009, Ghana, GHS1 = US$0.68
| Outcomes, costs | Microscopy setting | Presumptive diagnosis setting | ||
|---|---|---|---|---|
| RDT arm | Microscopy arm | RDT arm | Presumptive diagnosis arm | |
| Outcomes | ||||
| Malaria positivity rate* | 27% | 27% | 37% | 37% |
| Sensitivity of diagnostic test† | 87% | 61% | 93% | 97% |
| Specificity of diagnostic test† | 88% | 81% | 90% | 10% |
| Adherence to negative test | 54% | 51% | 51% | na |
| % of patients correctly treated‡ | 60% | 57% | 65% | 42% |
| Cost per service (GHS)§ | ||||
| Rapid diagnostic test | 2.6 | na | 3.7 | na |
| Microscopy | na | 1.9 | na | na |
| OPD visit¶ | 9.0 | 9.0 | 9.9 | 9.9 |
| Cost of drugs per OPD visit by prescription category (GHS)∥ | ||||
| Antimalarials, no antibiotics | ||||
| Mean cost | 2.1 | 2.9 | 2.6 | 2.8 |
| Median cost | 1.9 | 2.2 | 2.0 | 2.0 |
| Interquartile range | [1.3; 2.4] | [1.5; 3.5] | [1.5; 3.2] | [1.6; 4.1] |
| N | 46 | 59 | 58 | 70 |
| Antimalarials and antibiotics | ||||
| Mean cost | 4.8 | 6.2 | 4.2 | 3.6 |
| Median cost | 4.1 | 6.9 | 3.2 | 3.0 |
| Interquartile range | [2.8; 8.0] | [3.7; 8.1] | [2.9; 5.7] | [1.9; 5.3] |
| N | 12 | 17 | 12 | 8 |
| Antibiotics, no antimalarials | ||||
| Mean cost | 3.6 | 3.9 | 2.8 | 2.4 |
| Median cost | 3.0 | 4.1 | 2.6 | 1.7 |
| Interquartile range | [2.1; 4.5] | [2.3; 5.2] | [1.2; 4.7] | [1.5; 5.6] |
| N | 24 | 16 | 6 | 11 |
| Other than antimal. and antibiot. | ||||
| Mean cost | 1.4 | 1.6 | 1.5 | 2.0 |
| Median cost | 1.0 | 1.1 | 0.8 | 1.8 |
| Interquartile range | [0.2; 2.5] | [0.3; 2.3] | [0.3; 2.6] | [0.5; 3.6] |
| N | 23 | 13 | 28 | 15 |
According to the research slide.
Using the research slide as gold standard.
Patients with a parasite positive research slide who were prescribed an antimalarial or patients with a parasite negative research slide who were not prescribed an antimalarial.
In the presumptive diagnosis setting, the cost per service is a weighted average of two health facilities (weighted by outpatient department attendance).
Excluding cost of diagnostics and drug prescriptions.
Cost of all drugs prescribed at a visit.
Probabilistic sensitivity analyses (PSAs) were used to assess the influence of multivariate uncertainty on the incremental cost-effectiveness ratios.49,50 Parameters included in the PSA were the same as listed previously with assumed distributions as laid out in Table 4. Cost variables were all described by gamma distributions, which are defined for non-negative values only and skewed to the right and are therefore particularly relevant for cost data.50 For unit costs per service for an outpatient visit, a malaria microscopy test, and performance of an RDT, the costs were recalculated under different levels of overall attendance in the health facilities (between 20% below and above the observed) and these unit costs were used to estimate the necessary parameters for the gamma distributions.50 Parameters for the distributions for drug cost per prescription were calculated based on the data on drugs given as captured in this study (Table 3).
Table 4.
Input variables and distributions used for the sensitivity analyses, Dangme West District, 2009, Ghana, GHS1 = US$0.68
| Input variable | Microscopy setting | Presumptive diagnosis setting | ||
|---|---|---|---|---|
| RDT arm | Microscopy arm | RDT arm | Presumptive diagnosis arm | |
| Sensitivity of test | Triangular | Triangular | Triangular | Triangular |
| Min = 0.86 | Min = 0.60 | Min = 0.93 | Min = 0.96 | |
| Mode = 0.87 | Mode = 0.61 | Mode = 0.93 | Mode = 0.97 | |
| Max = 0.92 | Max = 0.66 | Max = 0.98 | Max = 1.00 | |
| Specificity of test | Triangular | Triangular | Triangular | Triangular |
| Min = 0.87 | Min = 0.80 | Min = 0.89 | Min = 0.09 | |
| Mode = 0.88 | Mode = 0.81 | Mode = 0.90 | Mode = 0.10 | |
| Max = 0.93 | Max = 0.86 | Max = 0.95 | Max = 0.15 | |
| Adherence to test if (false) negative | Triangular | Triangular | Triangular | |
| Min = 0.45 | Min = 0.26 | Min = 0.42 | ||
| Mode = 0.45 | Mode = 0.26 | Mode = 0.42 | ||
| Max = 0.70 | Max = 0.51 | Max = 0.67 | ||
| Adherence to test if (true) negative | Triangular | Triangular | Triangular | |
| Min = 0.55 | Min = 0.55 | Min = 0.51 | ||
| Mode = 0.55 | Mode = 0.55 | Mode = 0.51 | ||
| Max = 0.80 | Max = 0.80 | Max = 0.76 | ||
| Cost of drugs per OPD visit by prescription category | ||||
| Antimalarials, no antibiotics | Gamma | Gamma | Gamma | Gamma |
| α = 4.57 | α = 2.26 | α = 2.03 | α = 1.80 | |
| β = 0.45 | β = 1.26 | β = 1.29 | β = 1.55 | |
| Antimalarials and antibiotics | Gamma | Gamma | Gamma | Gamma |
| α = 2.70 | α = 6.14 | α = 2.23 | α = 2.39 | |
| β = 1.79 | β = 1.01 | β = 1.90 | β = 1.51 | |
| Antibiotics, no antimalarials | Gamma | Gamma | Gamma | Gamma |
| α = 2.31 | α = 4.93 | α = 3.10 | α = 2.24 | |
| β = 1.58 | β = 0.80 | β = 0.92 | β = 1.06 | |
| Other than antimal. and antibiot. | Gamma | Gamma | Gamma | Gamma |
| α = 1.17 | α = 0.68 | α = 1.05 | α = 1.23 | |
| β = 1.16 | β = 2.35 | β = 1.42 | β = 1.65 | |
| Unit cost per OPD visit (excl drugs) | Gamma | Gamma | Gamma | Gamma |
| α = 374 | α = 374 | α = 93 | α = 93 | |
| β = 0.02 | β = 0.02 | β = 0.11 | β = 0.11 | |
| Unit cost per malaria microscope test | Gamma | |||
| α = 140 | ||||
| β = 0.01 | ||||
| Unit cost per RDT procedure | Gamma | Gamma | ||
| α = 60 | α = 78 | |||
| β = 0.01 | β = 0.03 | |||
| Unit cost per RDT procedure | Uniform | Uniform | ||
| Min = 0.60 | Min = 0.60 | |||
| Max = 1.72 | Max = 1.72 | |||
RDT = rapid diagnostic test.
The PSAs were conducted as Monte Carlo simulations with 10,000 iterations using the @Risk software (Palisade Corporation, Ithaca, NY) and the distributions displayed in Table 4 to calculate costs and effects for a reference population of 1,000 individuals in each study arm as well as ICERs of introducing RDTs instead of the current diagnostic techniques.51 The degree of uncertainty surrounding the ICERs was summarized by scatter plots of joint incremental costs and incremental effects (change in the number of correctly treated fever patients) and cost-effectiveness acceptability curves.49,52–54
Ethical clearance.
All aspects of this study were approved by the Ethical Review Board of Ghana Health Service (Ethical Clearance ID no. GH-ERC 06/3/07) and the Ethics Committee of the London School of Hygiene and Tropical Medicine (application no. 5071).
Results
Table 3 presents the outcomes and cost per service in health facilities in the two study settings. In the microscopy setting, the proportion of patients with malaria, as confirmed by a double read research slide, was 27% with no significant differences between the two arms10; adherence to a negative RDT result was 54% and to a negative microscopy result was 51%. In this setting, the sensitivity of microscopy was 61% and specificity 81% compared with double read research slide, although these were 87% and 88%, respectively, for the RDT. In the presumptive diagnosis setting, the research slide positivity rate was 37% with no significant differences between the arms.10 Adherence to a negative RDT result was 51% and the sensitivity and specificity of RDT were 93% and 90% compared with a double read research slide, whereas these percentages were 97% and 10% for presumptive diagnosis.
In the microscopy setting the cost of diagnosis by RDT was higher than the cost of diagnosis by microscopy, GHS1.9 (US$1.3) per test compared with GHS2.6 (US$1.8), which is similar to the finding of other studies.30 Furthermore, the cost of diagnosis by RDT was higher in the presumptive diagnosis setting than in the microscopy setting reflecting a lower patient load in the former and therefore also higher fixed cost per test (cost of buildings and other overhead cost). Cost per outpatient visit was slightly lower in the microscopy setting health facility reflecting higher patient throughput, although the cost of personnel was higher because of staff with higher qualifications as compared with presumptive diagnosis setting health facilities. With respect to drug costs, it was found that patients randomized to microscopy were generally prescribed slightly more drugs or more expensive drugs than patients in the other arms.
With respect to seeking additional health care after the initial visit to a study health facility, 17% of patients in both arms of the microscopy setting went for a subsequent visit to a public health facility or a chemical seller (Table 1). In the presumptive diagnosis setting, 11% of patients in both arms sought additional care. Patient out-of-pocket expenditure and opportunity cost of lost time per fever episode were at similar levels across settings and arms except in the presumptive diagnosis arm where the patient cost was slightly higher.
Table 2 presents the cost and effects of a reference population of 1,000 suspected malaria patients in each study arm using the malaria positivity rate, behavior of healthcare professionals, cost per health sector services, and household costs related to health care seeking as presented in Table 3. In terms of overall costs, in the microscopy setting, the cost to the health sector in the two arms was very similar (Table 2). This is because although the cost of diagnosis was higher with RDTs than with microscopy, the cost of drugs was higher in the microscopy arm than in the RDT arm. Household costs were also almost identical because there were identical numbers of additional health care seeking visits and patients reported a similar average number of days being unable to perform usual activities. The societal cost was therefore also the same in the two arms with household costs forming 31% of total societal cost.
The number of correctly treated patients in the RDT arm was slightly higher than in the microscopy arm (601 versus 569) (Table 2). The incremental ICER of replacing microscopy diagnosis by RDT-based diagnosis was GHS6.7 (US$4.6) in terms of health sector costs and an ICER of GHS5.3 (US$3.6) in terms of societal costs. These low ICERs suggest that the cost of replacing microscopy diagnosis by RDT diagnosis may be low. However, it was also found that such a change in diagnostic technique would not lead to an increase in the number of correctly treated patient significantly different from zero.
In the presumptive diagnosis setting the addition of RDTs led to an overall increase in health sector costs of 26% with the cost of RDT diagnosis accounting for 23% of total health sector cost (Table 2). Although adherence to the negative results was 51%, this still led to a significant reduction in the prescription of antimalarials compared with the presumptive diagnosis arm. There was however a small increase in the prescription of antibiotics in the RDT arm and as a result, the total drug cost in the RDT arm was only 8% lower than in the presumptive diagnosis arm and the extra cost for performing RDTs was therefore recovered only to a limited extent. With respect to household cost, out-of-pocket spending was similar between the two arms. However, patients in the presumptive diagnosis arm reported being unable to perform their usual activities for a slightly longer period (average of 2.4 days versus 2.0 days) resulting in overall higher patient costs by 11%. Total societal cost was therefore higher by 13% in the RDT arm relative to the presumptive diagnosis arm with household costs forming 29% and 36% of societal cost respectively.
There was a much higher number of correctly treated patients in the RDT arm as compared with the presumptive diagnosis arm (651 versus 420). The ICER indicates that the additional cost of introducing RDT diagnosis instead of presumptive diagnosis was GHS15.4 (US$10.5) per additional patient correctly from a health sector perspective and GHS12.2 (US$8.3) per additional patient correctly treated from a societal perspective. In summary, replacing presumptive diagnosis by RDT diagnosis would lead to a significant increase in the number of correctly treated patients at a relatively low cost per additional correctly treated patient.
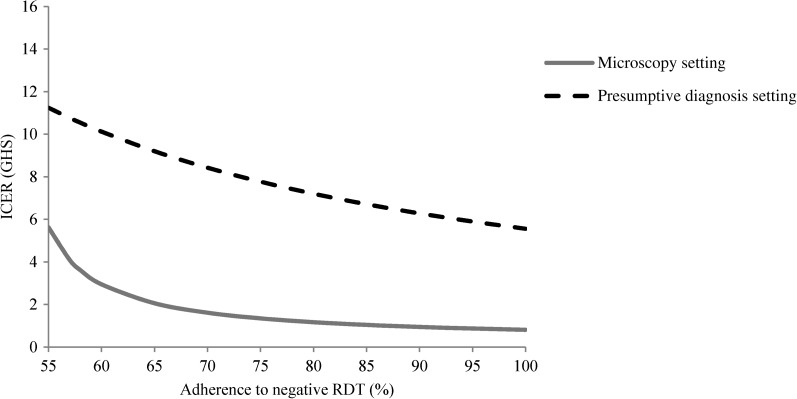
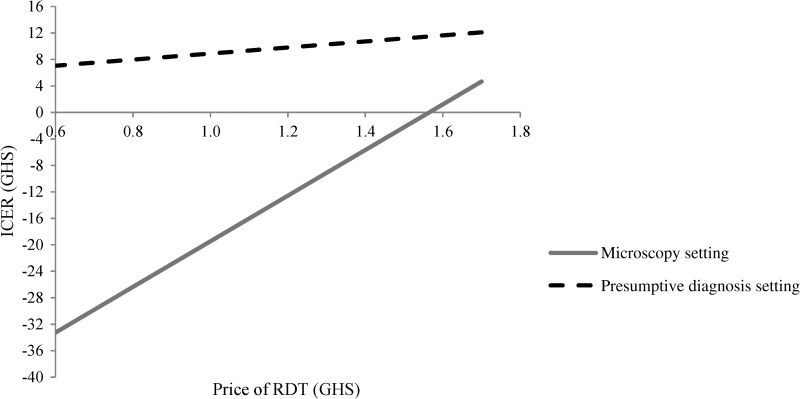
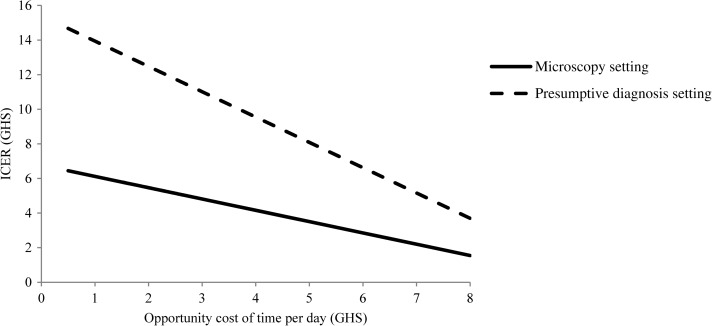
The one-way sensitivity analyses explored the influence of a range of factors on the ICERs from a societal perspective in the two settings. Improving the adherence of healthcare professionals to negative RDT test results from the observed level of just above 50% to higher adherence would significantly increase the desirability of RDTs relative to other diagnostic methods (Figure 2 ) as also found in other studies.25,27,30 For instance, an improvement from the observed level to an adherence of 85% resulted in a significant increase in the number of correctly treated patients and a reduction in the ICER from GHS5.3 (US$3.6) to GHS1.0 (US$0.7) in the microscopy setting and from GHS12.2 (US$8.3) to GHS6.7 (US$4.6) in the presumptive diagnosis setting. Similarly, improving the adherence to a negative microscopy test tended to improve the relative desirability of microscopy over RDT diagnosis. For instance, an increase in the adherence rate to microscopy from 51% to 85% led to a significant increase in the number of correctly treated patient while keeping the cost at similar levels in the two arms (results not shown). A two-way sensitivity analysis assuming a simultaneous improvement in the adherence to negative RDT and microscopy tests in the microscopy setting resulted in unchanged ICER (results not shown). A reduction in the RDT price strongly influenced the relative cost-effectiveness of RDT diagnosis compared with other diagnostic methods (Figure 3 ). If the RDT price was reduced from GHS1.72 (US$1.17) as used in this study to a price of GHS0.60 (US$0.41), this lowered the ICER by 42% in the presumptive diagnosis setting. In the microscopy setting, a reduction in RDT price lowered the cost in the RDT arm compared with the microscopy arm significantly, whereas the number of correctly treated patients remained identical in the two arms. The valuation of opportunity cost of time could potentially influence the ICER in the presumptive diagnosis setting (Figure 4) because a significant increase in the value of time from GHS2 to GHS6 per day would halve the ICER thus improving the cost-effectiveness of introducing RDTs in this setting. As socioeconomic developments occur in Ghana the trend will be in this direction.
Figure 2.
Sensitivity of the incremental societal cost-effectiveness ratio to improvements in adherence to rapid diagnostic tests with negative test results in Dangme West District, 2009, Ghana, GHS1 = US$0.68.
Figure 3.
Sensitivity of the incremental societal cost-effectiveness ratio to different price levels of rapid diagnostic tests in Dangme West District, 2009, Ghana, GHS1 = US$0.68.
Figure 4.
Sensitivity of the incremental societal cost-effectiveness ratio to changes in the value of opportunity cost of time in Dangme West District, 2009, Ghana, GHS1 = US$0.68.
Contrary to these factors, improvements in the sensitivities and specificities of diagnostic methods (from the observed levels of above 86% and 88% for the RDT and 61% and 81% for the microscopy) and changes in the cost per outpatient department visit did not give reason to change the observations made earlier on introducing RDTs into the two settings (results not shown). Finally, if the price of ACTs at the time of the study had been subsidized to the level proposed in the AMFm (US$0.02–0.08 in the public sector), the total drug cost would have been 21–43% lower in the study arms than displayed in Table 2 and the ICER of replacing the current diagnostic strategies with RDT diagnosis would increase in both settings; from GHS5.3 to GHS9.2 in the microscopy setting and from GHS12.2 to GHS14.8 in the presumptive diagnosis setting.
The scenario analysis simultaneously assuming a high provider adherence of 80% to negative RDT results and an RDT price of US$0.65, whereas holding all other parameters constant profoundly affected the ICERs (results not shown). In the microscopy setting, the number of correctly treated patients was significantly higher (75% versus 57%) and both total health sector and societal cost lower in the RDT arm as compared with the microscopy arm. In this scenario, it would therefore be cost-effective to replace microscopy by RDT testing. Similarly, in the presumptive diagnosis setting, the advantage in terms of correct treatment of RDT diagnosis over presumptive diagnosis improved even further (80% versus 42%), whereas the total health sector and societal cost in the RDT arm decreased. As a result the health sector incremental cost per correctly treated patient declined from GHS15.4 (US$10.5) to GHS7.0 (US$4.8) with societal incremental cost per correctly treated patient declining from GHS12.2 (US$8.3) to GHS5.1 (US$3.5) as compared with the baseline results (Table 2).
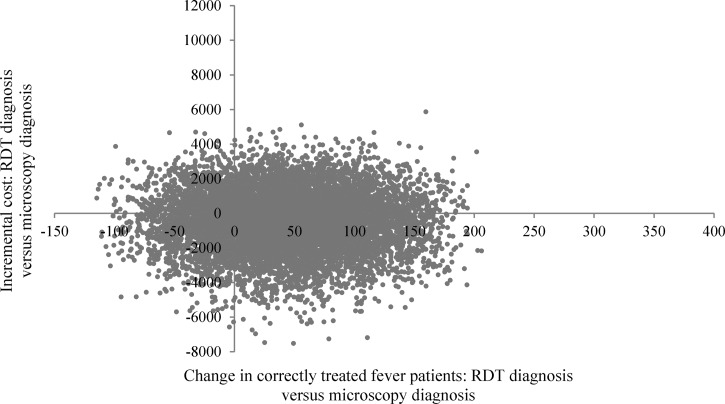
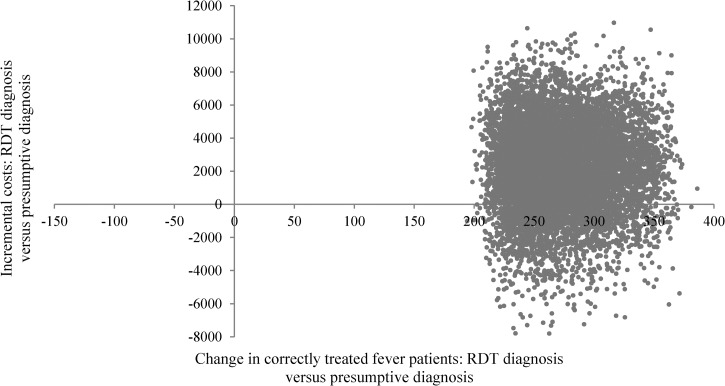
The results of the PSA assessing the impact of replacing microscopy by RDT diagnosis in the microscopy setting are displayed in Figure 5 showing pairs of incremental costs and incremental effects. The swarm of joint pairs of incremental costs and effects overlaps the origin suggesting that the effect in terms of increased number of correctly treated fever patients was not significantly different from zero and that the incremental cost of shifting from microscopy to RDT diagnosis did not significantly differ from zero. The PSA conducted for the presumptive diagnosis setting resulted in most of the joint pairs of incremental costs and incremental effects being situated in the upper right quadrant of the cost-effectiveness plane (Figure 6 ) suggesting that replacing presumptive diagnosis with RDT diagnosis resulted in a large and significant increase in the number of correctly treated fever patients but also in positive incremental costs in most cases.
Figure 5.
Scatter plot of incremental societal costs in GHS and incremental effects [change in correctly treated patients] resulting from replacing microscopy diagnosis by rapid diagnostic test (RDT) diagnosis in the microscopy setting in Dangme West District, 2009, Ghana, GHS1 = US$0.68.
Figure 6.
Scatter plot of incremental societal costs in GHS and incremental effects (change in correctly treated patients) resulting from replacing presumptive diagnosis by rapid diagnostic test (RDT) diagnosis in the presumptive diagnosis setting in Dangme West District, 2009, Ghana, GHS1 = US$0.68.
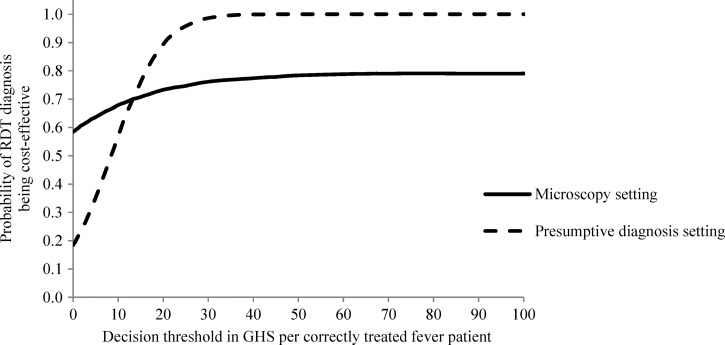
The ICER for each pair of incremental cost and effects is given by the slope of a line connecting a point and the origin in Figures 5 and 6. The ICERs generated from the PSA can be used to construct cost-effectiveness acceptability curves, which plot the share (probability) of ICERs being below specified thresholds that represent policymakers “willingness to pay”.53 The cost-effectiveness acceptability curves for this analysis are shown in Figure 7. If for instance health policymakers in Ghana are willing to pay GHS15 (US$10.2) per additional correctly treated fever patient, there is a 71% probability that introducing RDTs in the microscopy setting is cost-effective and this probability will only increase to 79% even at a much higher willingness-to-pay (WTP) such as GHS100 (US$68.0). Similarly for the presumptive diagnosis setting, at WTP threshold of GHS15 (US$10.2), the probability that it is cost-effective to replace presumptive diagnosis by RDT diagnosis is 76% and this increases to 99% if the WTP is doubled to GHS30 (US$20.4) (Figure 7). For most levels of WTP, it is more likely that introducing RDT diagnosis instead of presumptive diagnosis is a cost-effective intervention than it is to replace microscopy by RDT diagnosis.
Figure 7.
Cost-effectiveness acceptability curves for replacing microscopy diagnosis by rapid diagnostic test (RDT) diagnosis in the microscopy setting and for replacing presumptive diagnosis by RDT diagnosis in the presumptive diagnosis setting in Dangme West District, 2009, Ghana, GHS1 = US$0.68.
In summary, the range of sensitivity analyses performed did not give reason to alter the conclusions made previously based on Table 2, except that increased provider adherence to negative RDT results, lower RDT price, and higher valuation of opportunity cost of patient time would make RDT diagnosis relatively more cost-effective in both the microscopy and the presumptive diagnosis setting, whereas the AMFm would make RDT diagnosis slightly less cost-effective in both settings.
Discussion and Conclusions
This study randomized suspected malaria patients to different diagnostic approaches in two different settings of Dangme West District; one where microscopy already exists, and one where treatment of malaria was presumptive (clinical with no tests). This work sets out the economic analysis of these interventions.
The results of the cost-effectiveness analysis provided substantial support for the introduction of RDTs in the setting with no existing parasitological testing facilities. Although overall cost to the health sector were higher, patients benefitted from correct treatment more frequently when the diagnosis had been determined by an RDT compared with a presumptive diagnosis. The incremental cost to the health sector per additional correctly treated patient was GHS15.4 (US$10.5) and incremental societal cost was GHS12.2 (US$8.3). Although this ICER may be considered low, it will ultimately be up to health policy makers in Ghana to decide if this extra cost for improved treatment is good value for money compared with alternative uses of these resources in the health sector. In the likely event that the price of RDTs becomes lower or prescriber adherence to negative RDT results becomes higher than reported in this study then the relative desirability in cost-effectiveness terms of RDT diagnosis over presumptive diagnosis would improve. Recent studies have shown that significant improvements in adherence are possible with sustained training and supervision.55–57 Similarly, increasing the valuation of opportunity cost of patient time increases the cost-effectiveness of introducing RDTs; in common with many countries in Africa the rapidly growing economy makes this likely to be the direction in which opportunity cost of time will go.
This study found that it was less clear if replacing microscopy with RDT-based diagnostics should be recommended on cost-effectiveness grounds in Ghana given the low levels of adherence to both microscopy and RDTs observed. Both the health sector cost and the societal cost were at similar levels in the two arms, and there was only a minor advantage of RDTs over microscopy in terms of the proportion of correctly treated patients. Improvements in health worker adherence to negative RDT results would increase the number of correctly treated patients and a lower RDT price would lead to significant decreases in the total cost in the RDT arm thus improving the cost-effectiveness advantage of RDT diagnosis over microscopy. Conversely, improvements in health worker adherence to negative microscopy tests and higher accuracy of microscopy would improve the relative cost-effectiveness of microscopy over RDT diagnosis. The PSA found that the effect in terms of increased number of correctly treated fever patients was not significantly different from zero if microscopy would be replaced by RDT diagnosis. Similarly, the PSA suggested that the additional cost from introducing RDT diagnosis instead of microscopy was not significantly different from zero.
The low adherence to negative RDT and microscopy test results found in this study and other studies8,29 suggested a substantial potential for improving performance of parasitological testing leading to better health care. This would, in particular, be the case for RDTs because this diagnostic method was found to have considerably higher sensitivity and specificity than microscopy.10 If there had been perfect adherence, the proportion of correctly treated patients would increase to 88% and 91% in the RDT arms of the microscopy and presumptive diagnosis settings from the observed 60% and 65% and increase to 76% in the microscopy arm from the observed 57%. Improved provider adherence would also save costs from avoiding unnecessary prescriptions of antimalarials resulting in potentially lower total drug cost of 3% and 8% in the RDT arms and 9% in the microscopy arm as compared with the drug cost estimates in Table 2.
Household cost was found to be an important cost component constituting 29–36% of total societal cost in the study arms. For comparison, a cost-effectiveness study of three antimalarial drug combinations in Tanzania found that household cost comprised 39–54% of total societal cost.58 Our study assigned an opportunity cost of GHS2.2 (US$1.5) per day of lost time which may become an underestimate as Ghana develops, considering that the World Bank defines poverty as a situation where an individual has to live for US$1.25 or less per day59 and that the gross national income per capita in 201060 per working day was GHS5.2. Assuming an opportunity cost per day of GHS4.0 would increase the share of patient cost to 38–47% of total societal cost. Improved diagnosis may therefore not only result in higher health benefits to the population but also to significant decreases in lost patient resources. Despite this, cost-effectiveness studies of malaria diagnostics often do not include patient cost.15,21,27,31
A number of limitations of any cost-effectiveness study have to be taken into account. The patient survey conducted with the aim of estimating average drug cost by prescription category (Table 3) consisted of just over 400 interviews. This resulted in a low number of interviews for some of the groups classified by prescription type and study arm. For instance, there were only eight patient interviews to capture the average drug cost per outpatient department visit of “antimalarials and antibiotics” in the presumptive diagnosis arm of the presumptive diagnosis setting. These low numbers may have resulted in increased uncertainty of the cost-effectiveness ratios presented (Table 2).
The outcome measure chosen for this study was a “correctly treated” fever patient rather than a final health outcome or a utility measure such as disability-adjusted life-years averted. Although, a correctly treated patient may not necessarily lead to cure for a number of reasons, including poor patient adherence to the prescribed treatment, drug resistance, and poor quality of drugs, it can be argued that correct prescription is an important requisite. This study took place in only three health facilities in Ghana, at a time when RDTs had not been widely rolled out, and the AMFm pilot had not yet been launched. Generalizing from this study to other contexts should be done with caution. However, the examination of the included cost and parameter estimates and the results of the sensitivity analyses should facilitate applicability of the results found in this study to other settings.
Overall, this study provides support that introducing RDTs is cost-effective and clinically useful in settings where currently only presumptive treatment exists. If adherence to test results in negative cases improves, cost of RDTs falls, or a higher value is placed on the opportunity cost of time for patients the benefits will strengthen. In settings with availability of microscopy diagnosis, the economic case for substituting RDTs for microscopy is however balanced and dependent on key factors including adherence to negative results and accuracy of microscopy. If the observed trend of increasing provider adherence to negative RDT results and declining RDT price continues, this will likely lead to RDTs becoming more cost-effective than microscopy and to a significant decline in the cost per correctly treated patient when using RDT diagnosis as compared with presumptive diagnosis.
Footnotes
Financial support: This research was supported by the ACT Consortium, which is funded through a grant from the Bill & Melinda Gates Foundation to the London School of Hygiene and Tropical Medicine [grant no. 39640].
Authors' addresses: Evelyn Ansah and Michael Epokor, Research and Development Division, Ghana Health Service, Accra, Ghana, E-mails: ansahekdr@yahoo.co.uk and emkudjoe@yahoo.com. Christopher J. M. Whitty, Department of Clinical Research, London School of Hygiene and Tropical Medicine, London, UK, E-mail: Christopher.Whitty@lshtm.ac.uk. Shunmay Yeung and Kristian Schultz Hansen, Department of Global Health and Development, London School of Hygiene and Tropical Medicine, London, UK, E-mails: Shunmay.Yeung@lshtm.ac.uk and Kristian.Hansen@lshtm.ac.uk.
References
- 1.Amexo M, Tolhurst R, Barnish G, Bates I. Malaria misdiagnosis: effects on the poor and vulnerable. Lancet. 2004;364:1896–1898. doi: 10.1016/S0140-6736(04)17446-1. [DOI] [PubMed] [Google Scholar]
- 2.Reyburn H, Mbatia R, Drakeley C, Carneiro I, Mwakasungula E, Mwerinde O, Saganda K, Shao J, Kitua A, Olomi R, Greenwood BM, Whitty CJ. Overdiagnosis of malaria in patients with severe febrile illness in Tanzania: a prospective study. BMJ. 2004;329:1212. doi: 10.1136/bmj.38251.658229.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nankabirwa J, Zurovac D, Njogu JN, Rwakimari JB, Counihan H, Snow RW, Tibenderana JK. Malaria misdiagnosis in Uganda–implications for policy change. Malar J. 2009;8:66. doi: 10.1186/1475-2875-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandramohan D, Jaffar S, Greenwood B. Use of clinical algorithms for diagnosing malaria. Trop Med Int Health. 2002;7:45–52. doi: 10.1046/j.1365-3156.2002.00827.x. [DOI] [PubMed] [Google Scholar]
- 5.Kallander K, Nsungwa-Sabiiti J, Peterson S. Symptom overlap for malaria and pneumonia–policy implications for home management strategies. Acta Trop. 2004;90:211–214. doi: 10.1016/j.actatropica.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Rafael ME, Taylor T, Magill A, Lim YW, Girosi F, Allan R. Reducing the burden of childhood malaria in Africa: the role of improved diagnostics. Nature. 2006;444((Suppl 1)):39–48. doi: 10.1038/nature05445. [DOI] [PubMed] [Google Scholar]
- 7.Agnamey P, Brasseur P, Cisse M, Gaye O, Dumoulin J, Rigal J, Taylor WR, Olliaro P. Economic evaluation of a policy change from single-agent treatment for suspected malaria to artesunate-amodiaquine for microscopically confirmed uncomplicated falciparum malaria in the Oussouye District of south-western Senegal. Trop Med Int Health. 2005;10:926–933. doi: 10.1111/j.1365-3156.2005.01482.x. [DOI] [PubMed] [Google Scholar]
- 8.Hamer DH, Ndhlovu M, Zurovac D, Fox M, Yeboah-Antwi K, Chanda P, Sipilinyambe N, Simon JL, Snow RW. Improved diagnostic testing and malaria treatment practices in Zambia. JAMA. 2007;297:2227–2231. doi: 10.1001/jama.297.20.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hume JC, Barnish G, Mangal T, Armazio L, Streat E, Bates I. Household cost of malaria overdiagnosis in rural Mozambique. Malar J. 2008;7:33. doi: 10.1186/1475-2875-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ansah EK, Narh-Bana S, Epokor M, Akanpigbiam S, Quartey AA, Gyapong J, Whitty CJ. Rapid testing for malaria in settings where microscopy is available and peripheral clinics where only presumptive treatment is available: a randomized controlled trial in Ghana. BMJ. 2010;340:c930. doi: 10.1136/bmj.c930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization . World Malaria Report 2011. Geneva: World Health Organization; 2011. [Google Scholar]
- 12.World Health Organization . Guidelines for the Treatment of Malaria. Geneva: World Health Organization; 2010. [Google Scholar]
- 13.Barat L, Chipipa J, Kolczak M, Sukwa T. Does the availability of blood slide microscopy for malaria at health centers improve the management of persons with fever in Zambia? Am J Trop Med Hyg. 1999;60:1024–1030. doi: 10.4269/ajtmh.1999.60.1024. [DOI] [PubMed] [Google Scholar]
- 14.Reyburn H, Ruanda J, Mwerinde O, Drakeley C. The contribution of microscopy to targeting antimalarial treatment in a low transmission area of Tanzania. Malar J. 2006;5:4. doi: 10.1186/1475-2875-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chanda P, Castillo-Riquelme M, Masiye F. Cost-effectiveness analysis of the available strategies for diagnosing malaria in outpatient clinics in Zambia. Cost Eff Resour Alloc. 2009;7:5. doi: 10.1186/1478-7547-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Batwala V, Magnussen P, Nuwaha F. Are rapid diagnostic tests more accurate in diagnosis of Plasmodium falciparum malaria compared to microscopy at rural health centres? Malar J. 2010;9:349. doi: 10.1186/1475-2875-9-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abba K, Deeks JJ, Olliaro P, Naing CM, Jackson SM, Takwoingi Y, Donegan S, Garner P. Rapid diagnostic tests for diagnosing uncomplicated P. falciparum malaria in endemic countries. Cochrane Database Syst Rev. 2011:CD008122. doi: 10.1002/14651858.CD008122.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization . Malaria Rapid Diagnostic Test Performance: Results of WHO Product Testing of Malaria RDTs, Round 2. Geneva: World Health Organization; 2009. [Google Scholar]
- 19.Zurovac D, Larson BA, Skarbinski J, Slutsker L, Snow RW, Hamel MJ. Modeling the financial and clinical implications of malaria rapid diagnostic tests in the case-management of older children and adults in Kenya. Am J Trop Med Hyg. 2008;78:884–891. [PMC free article] [PubMed] [Google Scholar]
- 20.Wongsrichanalai C, Barcus MJ, Muth S, Sutamihardja A, Wernsdorfer WH. A review of malaria diagnostic tools: microscopy and rapid diagnostic test (RDT) Am J Trop Med Hyg. 2007;77:119–127. [PubMed] [Google Scholar]
- 21.Rolland E, Checchi F, Pinoges L, Balkan S, Guthmann JP, Guerin PJ. Operational response to malaria epidemics: are rapid diagnostic tests cost-effective? Trop Med Int Health. 2006;11:398–408. doi: 10.1111/j.1365-3156.2006.01580.x. [DOI] [PubMed] [Google Scholar]
- 22.Zikusooka CM, McIntyre D, Barnes KI. Should countries implementing an artemisinin-based combination malaria treatment policy also introduce rapid diagnostic tests? Malar J. 2008;7:176. doi: 10.1186/1475-2875-7-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yukich J, D'Acremont V, Kahama J, Swai N, Lengeler C. Cost savings with rapid diagnostic tests for malaria in low-transmission areas: evidence from Dar es Salaam, Tanzania. Am J Trop Med Hyg. 2010;83:61–68. doi: 10.4269/ajtmh.2010.09-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thiam S, Thior M, Faye B, Ndiop M, Diouf ML, Diouf MB, Diallo I, Fall FB, Ndiaye JL, Albertini A, Lee E, Jorgensen P, Gaye O, Bell D. Major reduction in anti-malarial drug consumption in Senegal after nation-wide introduction of malaria rapid diagnostic tests. PLoS ONE. 2011;6:e18419. doi: 10.1371/journal.pone.0018419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shillcutt S, Morel C, Goodman C, Coleman P, Bell D, Whitty CJ, Mills A. Cost-effectiveness of malaria diagnostic methods in sub-Saharan Africa in an era of combination therapy. Bull World Health Organ. 2008;86:101–110. doi: 10.2471/BLT.07.042259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uzochukwu BS, Obikeze EN, Onwujekwe OE, Onoka CA, Griffiths UK. Cost-effectiveness analysis of rapid diagnostic test, microscopy and syndromic approach in the diagnosis of malaria in Nigeria: implications for scaling-up deployment of ACT. Malar J. 2009;8:265. doi: 10.1186/1475-2875-8-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lubell Y, Reyburn H, Mbakilwa H, Mwangi R, Chonya S, Whitty CJ, Mills A. The impact of response to the results of diagnostic tests for malaria: cost-benefit analysis. BMJ. 2008;336:202–205. doi: 10.1136/bmj.39395.696065.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Batwala V, Magnussen P, Hansen KS, Nuwaha F. Cost-effectiveness of malaria microscopy and rapid diagnostic tests versus presumptive diagnosis: implications for malaria control in Uganda. Malar J. 2011;10:372. doi: 10.1186/1475-2875-10-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reyburn H, Mbakilwa H, Mwangi R, Mwerinde O, Olomi R, Drakeley C, Whitty CJ. Rapid diagnostic tests compared with malaria microscopy for guiding outpatient treatment of febrile illness in Tanzania: randomized trial. BMJ. 2007;334:403. doi: 10.1136/bmj.39073.496829.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lubell Y, Reyburn H, Mbakilwa H, Mwangi R, Chonya K, Whitty CJ, Mills A. The cost-effectiveness of parasitologic diagnosis for malaria-suspected patients in an era of combination therapy. Am J Trop Med Hyg. 2007;77:128–132. [PubMed] [Google Scholar]
- 31.Lemma H, San Sebastian M, Lofgren C, Barnabas G. Cost-effectiveness of three malaria treatment strategies in rural Tigray, Ethiopia where both Plasmodium falciparum and Plasmodium vivax co-dominate. Cost Eff Resour Alloc. 2011;9:2. doi: 10.1186/1478-7547-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zurovac D, Larson BA, Akhwale W, Snow RW. The financial and clinical implications of adult malaria diagnosis using microscopy in Kenya. Trop Med Int Health. 2006;11:1185–1194. doi: 10.1111/j.1365-3156.2006.01674.x. [DOI] [PubMed] [Google Scholar]
- 33.Lubell Y, Hopkins H, Whitty CJ, Staedke SG, Mills A. An interactive model for the assessment of the economic costs and benefits of different rapid diagnostic tests for malaria. Malar J. 2008;7:21. doi: 10.1186/1475-2875-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bank of Ghana Inflation. 2011. http://www.bog.gov.gh/index.php?option=com_wrapper&view=wrapper&Itemid=263 Available at. Accessed November 3, 2011.
- 35.Drummond MF, Sculpher MJ, Torrance GW, O'Brien B, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005. [Google Scholar]
- 36.Conteh L, Walker D. Cost and unit cost calculations using step-down accounting. Health Policy Plan. 2004;19:127–135. doi: 10.1093/heapol/czh015. [DOI] [PubMed] [Google Scholar]
- 37.Luce BR, Manning WG, Siegel JE, Lipscomp J. Estimating costs in cost-effectiveness analysis. In: Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. pp. 176–213. [Google Scholar]
- 38.Wordsworth S, Ludbrook A, Caskey F, Macleod A. Collecting unit cost data in multicentre studies. Creating comparable methods. Eur J Health Econ. 2005;6:38–44. doi: 10.1007/s10198-004-0259-9. [DOI] [PubMed] [Google Scholar]
- 39.Management Sciences for Health . International Drug Price Indicator Guide 2009. Cambridge, MA: Management Sciences for Health; 2010. [Google Scholar]
- 40.Sculpher M. The role and estimation of productivity costs in economic evaluation. In: Drummond M, McGuire A, editors. Economic Evaluation in Health Care. Oxford: Oxford University Press; 2001. pp. 94–112. [Google Scholar]
- 41.Liljas B. How to calculate indirect costs in economic evaluations. Pharmacoeconomics. 1998;13:1–7. doi: 10.2165/00019053-199813010-00001. [DOI] [PubMed] [Google Scholar]
- 42.Conteh L, Patouillard E, Kweku M, Legood R, Greenwood B, Chandramohan D. Cost effectiveness of seasonal intermittent preventive treatment using amodiaquine & artesunate or sulphadoxine-pyrimethamine in Ghanaian children. PLoS ONE. 2010;5:e12223. doi: 10.1371/journal.pone.0012223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asenso-Okyere WK, Dzator JA. Household cost of seeking malaria care. A retrospective study of two districts in Ghana. Soc Sci Med. 1997;45:659–667. doi: 10.1016/s0277-9536(96)00383-8. [DOI] [PubMed] [Google Scholar]
- 44.Armitage P, Berry G, Matthews JN. Statistical Methods in Medical Research. Malden, MA: Blackwell Science; 2002. [Google Scholar]
- 45.The Global Fund Affordable Medicines Facility—Malaria (AMFm): Summary Report on Co-paid ACTs. 2012. http://www.theglobalfund.org/en/amfm/firstlinebuyers/reports/. Available at. Accessed May 16, 2012.
- 46.Masaninga F, Sekeseke-Chinyama M, Malambo T, Moonga H, Babaniyi O, Counihan H, Bell D. Finding parasites and finding challenges: improved diagnostic access and trends in reported malaria and anti-malarial drug use in Livingstone district, Zambia. Malar J. 2012;11:341. doi: 10.1186/1475-2875-11-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ishengoma DS, Francis F, Mmbando BP, Lusingu JP, Magistrado P, Alifrangis M, Theander TG, Bygbjerg IC, Lemnge MM. Accuracy of malaria rapid diagnostic tests in community studies and their impact on treatment of malaria in an area with declining malaria burden in north-eastern Tanzania. Malar J. 2011;10:176. doi: 10.1186/1475-2875-10-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baiden F, Owusu-Agyei S, Okyere E, Tivura M, Adjei G, Chandramohan D, Webster J. Acceptability of rapid diagnostic test-based management of Malaria among caregivers of under-five children in rural Ghana. PLoS ONE. 2012;7:e45556. doi: 10.1371/journal.pone.0045556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Briggs A. Handling uncertainty in economic evaluation and presenting the results. In: Drummond M, McGuire A, editors. Economic Evaluation in Health Care. Oxford: Oxford University Press; 2001. pp. 172–214. [Google Scholar]
- 50.Briggs A, Claxton C, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006. [Google Scholar]
- 51.Doubilet P, Begg CB, Weinstein MC, Braun P, McNeil BJ. Probabilistic sensitivity analysis using Monte Carlo simulation. A practical approach. Med Decis Making. 1985;5:157–177. doi: 10.1177/0272989X8500500205. [DOI] [PubMed] [Google Scholar]
- 52.Briggs AH, O'Brien BJ. The death of cost-minimization analysis? Health Econ. 2001;10:179–184. doi: 10.1002/hec.584. [DOI] [PubMed] [Google Scholar]
- 53.O'Brien BJ, Briggs AH. Analysis of uncertainty in health care cost-effectiveness studies: an introduction to statistical issues and methods. Stat Methods Med Res. 2002;11:455–468. doi: 10.1191/0962280202sm304ra. [DOI] [PubMed] [Google Scholar]
- 54.Fenwick E, O'Brien BJ, Briggs A. Cost-effectiveness acceptability curves–facts, fallacies and frequently asked questions. Health Econ. 2004;13:405–415. doi: 10.1002/hec.903. [DOI] [PubMed] [Google Scholar]
- 55.D'Acremont V, Kahama-Maro J, Swai N, Mtasiwa D, Genton B, Lengeler C. Reduction of anti-malarial consumption after rapid diagnostic tests implementation in Dar es Salaam: a before-after and cluster randomized controlled study. Malar J. 2011;10:107. doi: 10.1186/1475-2875-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams HA, Causer L, Metta E, Malila A, O'Reilly T, Abdulla S, Kachur SP, Bloland PB. Dispensary level pilot implementation of rapid diagnostic tests: an evaluation of RDT acceptance and usage by providers and patients–Tanzania, 2005. Malar J. 2008;7:239. doi: 10.1186/1475-2875-7-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Msellem MI, Martensson A, Rotllant G, Bhattarai A, Stromberg J, Kahigwa E, Garcia M, Petzold M, Olumese P, Ali A, Bjorkman A. Influence of rapid malaria diagnostic tests on treatment and health outcome in fever patients, Zanzibar: a crossover validation study. PLoS Med. 2009;6:e1000070. doi: 10.1371/journal.pmed.1000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wiseman V, Kim M, Mutabingwa TK, Whitty CJ. Cost-effectiveness study of three antimalarial drug combinations in Tanzania. PLoS Med. 2006;3:e373. doi: 10.1371/journal.pmed.0030373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ravallion M, Chen S, Sangraula P. Dollar a Day Revisited. Washington, DC: World Bank; 2008. [Google Scholar]
- 60.Ghana Statistical Service National Income 2010. 2010. www.statsghana.gov.gh/docfiles/GDP/national_%20income_2010.pdf Available at. Accessed November 3, 2011.