Abstract
Sri Lanka reports significantly more cutaneous leishmaniasis (CL) cases than visceral leishmaniasis (VL) cases, both of which are caused by Leishmania donovani MON-37. A cross-sectional study conducted in an area with a high prevalence of CL prevalent included 954 participants of an estimated population of 61,674 to estimate the number of CL cases, ascertain whether there is a pool of asymptomatic VL cases, and identify risk factors for transmission. A total of 31 cases of CL were identified, of whom 21 were previously diagnosed and 10 were new cases. Using rK39 rapid diagnostic test to detect antibodies against Leishmania spp., we found that only one person was seropositive but did not have clinical symptoms of CL or VL, which indicated low transmission of VL in this area. χ2 test, independent sample t-test, and multivariate analysis of sociodemographic and spatial distribution of environmental risk factors showed that living near paddy fields is associated with increased risk for transmission of CL (P ≤ 0.01).
Introduction
Leishmaniasis is a neglected tropical disease that affects poor and marginalized communities.1 The disease is prevalent in 98 countries in the tropics and subtropics, of which 72 are developing countries.1–3 It is estimated that 350 million persons are at risk globally and up to 1.2 million cases of cutaneous leishmaniasis (CL) and 0.4 million cases of visceral leishmaniasis (VL) occur each year worldwide.3 Most (90%) VL patients reported globally are from India, Bangladesh, Nepal, Sudan, Ethiopia, and Brazil3 and 90% of CL patients are reported from Afghanistan, Algeria, Iran, Saudi Arabia, Syria, Brazil, Colombia, Peru, and Bolivia.3 Leishmaniasis is reported to be emerging in new foci in Africa4,5 and Asia.6–8
Sri Lanka is a tropical island in the Indian Ocean that is divided into 26 administrative districts and 9 provinces.9 Leishmaniasis is a relatively newly established disease in Sri Lanka, and is now considered an emerging public health problem in this country.10–12
Cutaneous leishmaniasis is the commonest form of leishmaniasis in Sri Lanka12 that is now reported throughout the country but mostly in the Anuradhapura and Polonnaruwa Districts of North Central province and the Hambantota and Matara Districts of Southern Province.9 Two cases of indigenous VL and one case of mucosal leishmaniasis (ML) have been reported from the Anuradhapura District.13–15
The first indigenous case of CL in Sri Lanka was reported in 1992 in the Hambantota District in Southern Province, and to date, more than 2,000 CL cases have been reported.12,16 The parasite strain causing CL and VL in Sri Lanka was identified as Leishmania donovani MON-376,17 a strain known to cause VL in India and Kenya.18–20 Leishmania donovani MON-37 strain differs from the L. donovani MON-2 strain, which is the most common strain causing VL in India, by multi-locus isoenzyme analysis and sequencing that identified a single nucleotide substitution at position 976 in the 6-phospogluconate dehydrogenase gene.6,17,21
Few studies have been conducted on leishmaniasis (i.e., parasitic aspects, disease surveillance, reservoir host and vectorial aspects) in Sri Lanka. The sand fly vector and the existence of a sylvatic cycle are yet to be identified, although there are two studies suggesting the dog as a potential reservoir host.22,23
Epidemiologic studies related to human leishmaniasis are limited for Sri Lanka. One study conducted in the Anuradhapura, Hambantota, and Moneragala Districts of Sri Lanka, which used active and passive case detection, estimated the prevalence of CL to range from 2.4% to 3.4%.24 The same study reported that in the northern region of the country, young 21–40-year-old men who spent greater than five hours/day outdoors were at higher risk for acquiring CL. In the southern region of Sri Lanka, transmission was considered as mainly peridomestic.
Leishmania donovani infections can remain asymptomatic, and most persons with these infections often show positive serodiagnostic results for rK39 antigen and may act as reservoirs for symptomatic infections.25–28 Poverty, poor housing with dampness, malnutrition, close proximity to animal reservoirs, overcrowding in the presence of anthropophilic sand flies, vegetation, presence of subsoil water, alluvial soil, and scrub jungles are some of the sociodemographic risk factors associated with transmission of leishmaniasis.1,4,29–31
The aim of this study was to identify potential sociodemographic and environmental risk factors of CL transmission in a disease-endemic focus in Sri Lanka and to examine seropositivity rates for antibodies against Leishmania spp. to determine if there is a potential reservoir for VL transmission in the country.
Materials and Methods
Study area.
The Thalawa Medical Officer of Health (MOH) area in the Anuradhapura District of North-Central Province, which has an area of 252 km2 and had an estimated population of 61,674 in 2011, was selected for the survey (Figure 1). The Thalawa MOH area reported the second highest CL case incidence in 2010 and 2011 (Regional Epidemiology Unit, Anuradhapura District, unpublished data) and is the area of residence of the first VL and ML cases reported in the country.13,15
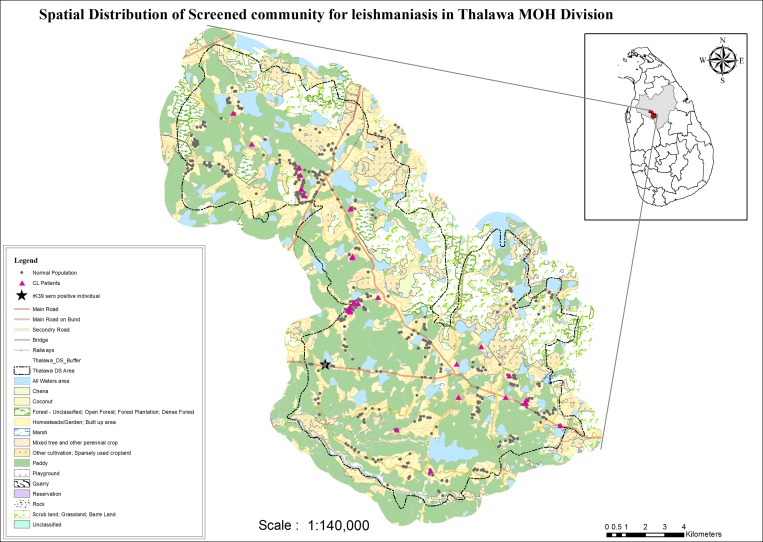
Figure 1.
Land use patterns of the study area in Sri Lanka from the Thalawa Medical Officer of Health (MOH) showing distribution of persons screened. Cutaneous leishmaniasis cases are indicated as pink triangles, rice fields as green areas, and persons with no cutaneous leishmaniasis or visceral leishmaniasis as black dots. The black star indicates the residence of the rK-39-seropositive person.
The Thalawa MOH area is a semi-dry and has an annual average rainfall of 1,205 mm. The highest rainfall in this area is the northeast monsoonal rains, which last from December through February. There are inter-monsoonal rains during October and November. This area has an average day and night relative humidity of 70% and 90%, respectively, and an average daily night and day temperature ranging between 24°C and 33°C (Department of Meteorology, Sri Lanka). The altitude of the area is 75–125 meters above mean sea level, and the area has numerous paddy fields where rice is grown, water bodies, scrub jungles, marshes, and forests (Figure 1).
We analyzed CL passive case detection incidence in North-Central Province during January 1, 2009–December 31, 2012 based on the data published by the Epidemiology Unit, Ministry of Health Sri Lanka32 to determine whether there is seasonal variation of CL incidence in this area.
Sampling method.
The Thalawa MOH area consists of 17 public health midwife (PHM) areas; 34 clusters with 25 persons in each cluster were selected randomly for the study. Two clusters from each PHM area were selected commencing from two random points; one resident living in every third house to the left of the previous house was selected for the study until the required sample size was obtained. Each participant in a selected house was enrolled randomly by the lottery method. Children less than two years of age, any person who had traveled overseas to a leishmaniasis-endemic country at any time, and any person who did not give written informed consent were excluded from the study. To detect a proportion of 0.05 seropositivity of antibodies against Leishmania spp. in the community with an acceptable difference of 0.0175, an alpha error of 0.05, and a design effect of 1.5, a minimum sample of 834 persons had to be screened. One thousand persons were informed of their selection a priori by the PHMs and community social workers living in the areas and requested to be present at predetermined community centers on selected days (November 18 and 19, 2011). The two centers were located in two locations within the study area and had easy access to selected participants. Participants were allocated to each center to prevent a possible effect on awareness based on information to be gathered in the survey in advance.
Rapid diagnostic test.
The rK39 rapid diagnostic test (RDT) (InBios Corporation, Seattle, WA), which was used for diagnosis of VL, is a rapid immunochromatographic strip assay used to qualitatively detect antibodies against L. donovani to the K39 antigen in human serum.1,25,33,34 The test kit is prepared with recombinant rK39 Leishmania sp. antigen and is used widely in VL-endemic countries in community surveys to determine the subclinical VL case incidence and the presence of antibodies against the K39 antigen of Leishmania spp. in the population.1,33,34
Blood (1.0 mL) was obtained by venipuncture from participants more than 12 years of age and 3–5 drops of blood was obtained by finger prick from persons 2–12 years of age by a doctor or a trained nurse under aseptic conditions using sterile equipment. Serum was separated by centrifugation. Twenty microliters of separated serum of each participant was placed on the RDT strip. Results were interpreted within 10 minutes according to the manufacturer's guidelines. The serum stored at –80°C during the acute phase of illness of the third VL-positive patient17 in Sri Lanka was used as a positive control.
Slit skin smear.
Slit skin smears were obtained by a doctor from participants who had suspected but previously undiagnosed CL lesions. Cutaneous leishmaniasis had the appearance of one or more lesions typically in an uncovered part of the body at the site of inoculation where a nodule appears, which may have also become an indolent ulcer with raised edges. The slides were stained with Giemsa and examined under a light microscope under oil immersion in the Department of Parasitology, Faculty of Medical Sciences, University of Sri Jayewardenepura. Amastigotes were identified as round to ovoid and characterized by a distinctive nucleus and adjacent kinetoplast.
The previously diagnosed CL patients who were selected randomly for the study had been diagnosed within the past five years and were either treated or undergoing treatment at the time of the survey. The definitive diagnosis had been made by performing a slit skin smear or punch biopsy and histologic analysis in a hospital by a histopathologist or a parasitologist. Case-patients already diagnosed had records with them.
Sociodemographic data collection.
Sociodemographic data were collected by trained PHMs by using pre-tested interviewer administered questionnaires. Information on age, sex, occupation, monthly income, housing, animal rearing practices, access to advanced medical facilities, awareness of leishmaniasis, and the vector sand fly were obtained. Occupations were categorized as indoor and outdoor. Children, housewives, and elderly persons who did not have outdoor occupations or professions, office workers, teachers, and any other skilled or unskilled indoor professionals were categorized as having indoor occupations. Persons engaged in farming, irrigation work, the armed forces, or any other outdoor skilled or unskilled professions were categorized as having outdoor occupations.
Animal rearing was defined as rearing an animal within 50 meters from houses. Rearing dogs, cattle, and chickens were documented to assess the existence of a putative reservoir host, zoopotentiation, or zooprophylaxis. Cattle and chickens are the two most common household farm animals reared in the Thalawa area (MOH Thalawa, unpublished data).
Monthly income was classified by categorizing persons into two groups: those who are social beneficiaries and those who are not social beneficiaries. A social benefit of Samurdhi is given to poor families identified by the Government of Sri Lanka.35 Houses were classified into two groups. Any house having a thatched roof, a clay wall, or a clay floor was considered a poor house (house with a high risk for transmission of leishmaniasis) because sand flies are known to breed in cracks and crevices of clay huts and can rest in dark places.1 A house that had brick walls, asbestos or tiled roofs, and cemented floors was considered a good house (house with a low risk for transmission of leishmaniasis). Daily use of bed nets, with and without impregnation, was also identified.
A medical history and clinical features of lesion(s) in persons given a diagnosis of CL or having a suspected lesion detected at the survey were obtained. Information on whether ayurvedic (traditional medicine) treatment was used and the delay between onset of a lesion and seeking advanced treatment was obtained.
Data collection for environmental risk factors.
Because environmental factors can influence leishmaniasis transmission, global positioning system coordinates were recorded for all households. The closest distances of participants’ residences to forest, marsh, paddy (rice) fields, scrub jungles, and water bodies were calculated based on the map prepared by the Geographic Information System Branch of the Department of Surveys, Sri Lanka, by using ArcGIS10 software (ESRI, Redlands, CA).
Social awareness program.
A social awareness program was conducted after the survey in community centers. Lectures were given by a local medical specialist on leishmaniasis and handouts containing information on clinical features of leishmaniasis that would help one to identify CL, ML or VL at an early stage of illness, available treatment measures, closest treatment centers, and measures on prevention of human–vector contact and control of sand flies were distributed among the participants.
Statistical analysis.
A chi-square test and independent sample t-test were used to test for associations between CL cases and sociodemographic and environmental risk factors. Binary logistic regression analysis was used to assess associations controlling for other variables. SPSS version 18 (IBM, Armonk, NY) was used for statistical analyses.
Ethics.
Ethical approval was obtained from the Ethical Review Committee, Faculty of Medical Sciences, University of Sri Jayewardenepura, Sri Lanka. Written informed consent was obtained from every adult participant and from either of the parents or the legal guardian of children less than 18 years of age. A thumb impression was obtained from persons unable to write after reading the form. This procedure ensured that participants understood the contents contained therein. All information collected was kept confidential. An approval to conduct the study, including use of the rK39 RDT as a screening tool for VL, was obtained from the Provincial Director of Health Services, North-Central Province, Sri Lanka.
Management and follow up of newly diagnosed leishmaniasis patients.
Any patient who had antibodies against Leishmania spp. was referred to the Teaching Hospital in Anuradhapura for further management. Persons who had smear-positive CL detected during the study were informed later via the MOH in Thalawa and referred to the Dermatology Clinic at the Teaching Hospital in Anuradhapura for treatment.
Results
Sociodemographic characteristics and clinical aspects of CL case-patients.
As shown in Table 1, 954 of 1,000 selected persons participated in the survey. The dropout rate was 4.6%. There were of 31 confirmed CL patients, of whom 21 were previously diagnosed and 10 were newly diagnosed during the survey. The slit skin smear was positive in the 10 previously undetected persons who had suspected lesions. These newly diagnosed CL patients who had Leishmania amastigotes in their slit skin smears were successfully treated with 1–3 mL of intralesional sodium stibogluconate injections (100 mg/mL) per session given twice a week until the lesion was completely healed with re-epithelialization and flattening of edges at the Dermatology Clinic at the Teaching Hospital in Anuradhapura. The treatment doses ranged from three to five sessions depending on size of the lesion and response to treatment.
Table 1.
Distribution of population by sociodemographic characteristics and results of rK-39 rapid diagnostic test for antibodies against Leishmania spp., Sri Lanka*
| Risk factor | CL positive† (n = 31) | CL negative (n = 923) | χ2 | df | P |
|---|---|---|---|---|---|
| Age category, years | |||||
| Pre-school (1–5) | 2 | 109 | |||
| Attending school (6–17) | 5 | 154 | |||
| Adult (18–65) | 24 | 644 | |||
| Elderly (> 65) | 1 | 15 | 1.378 | 3 | 0.711 |
| Sex | |||||
| M | 13 | 307 | |||
| F | 19 | 615 | 0.745 | 1 | 0.388 |
| Occupation | |||||
| Outdoor (farming, military, and other) | 17 | 468 | |||
| Indoor (housewives, children, and other) | 15 | 454 | 0.069 | 1 | 0.792 |
| Social beneficiary‡ | |||||
| Yes | 3 | 133 | |||
| No | 29 | 719 | 0.808 | 1 | 0.369 |
| House type | |||||
| Good | 27 | 790 | |||
| Poor | 5 | 132 | 0.043 | 1 | 0.863 |
| Rearing animals | |||||
| Yes (≥ 1) | 18 | 535 | |||
| No | 14 | 388 | 0.040 | 1 | 0.841 |
| Type of animal reared | |||||
| Dogs | |||||
| Yes | 16 | 421 | |||
| No | 16 | 501 | 0.234 | 1 | 0.628 |
| Cattle | |||||
| Yes | 0 | 83 | |||
| No | 32 | 839 | 3.155 | 1 | 0.076 |
| Chickens | |||||
| Yes | 3 | 83 | |||
| No | 29 | 839 | 0.005 | 1 | 0.942 |
| Other | |||||
| Yes | 2 | 80 | |||
| No | 30 | 842 | 0.232 | 1 | 0.630 |
| Use of bed nets daily | |||||
| Yes | 31 | 885 | |||
| No | 1 | 37 | 0.064 | 1 | 0.801 |
| Use of impregnated bed nets | |||||
| Yes | 1 | 44 | |||
| No | 31 | 841 | 0.166 | 1 | 0.684 |
| rK39 results | |||||
| Positive | 0 | 1 | |||
| Negative | 31 | 922 | 0.195 | 1 | 0.534 |
CL = cutaneous leishmaniasis; df = degrees of freedom.
K39-seropositive person was included with CL group (as leishmaniasis) during analysis of sociodemographic risk factors except in rK39 results section.
Seventy-one persons had not responded.
All lesions (current and healed) had first appeared in exposed areas of the body. Lesions were found in the shoulder area and mid-abdomen in some women who wore sarees, which exposed the shoulders and mid-abdominal areas (Table 2). Most (58.1%) lesions were nodules. Approximately 42% of CL patients had sought ayurvedic treatment at least once before seeking advanced medical treatment. The overall mean delay of seeking advanced treatment was 7.3 months. None of the CL patients had hepatosplenomegaly at examination. Three of the CL-positive patients complained of having low-grade fevers lasting for more than two weeks. All patients were afebrile and showed negative results in the rK39 RDT.
Table 2.
Clinical assessment of confirmed 31 confirmed cutaneous leishmaniasis patients, Sri Lanka
| Variable | No. (%) |
|---|---|
| Had ayurvedic treatment | |
| Yes | 13 (41.9) |
| No | 18 (58.1) |
| Delay between onset of lesion and seeking treatment | |
| < 6 months | 22 (71.0) |
| 6 months–1 year | 7 (22.6) |
| >1 year | 2 (6.4) |
| Mean delay in seeking treatment, months | 7.29 |
| Site of lesion | |
| Face and neck | 6 (19.4) |
| Ears | – |
| Upper limbs | 13 (41.9) |
| Lower limbs | 7 (22.6) |
| Upper body (umbilicus to neck) | 8 (25.8) |
| Lower body (umbilicus to symphysis pubis) | – |
| Type of lesion | |
| Ulcer | 11 (35.5) |
| Nodule | 18 (58.1) |
| Plaque | 2 (6.4) |
| No. lesions | |
| 1 | 29 (93.6) |
| > 1 | 2 (6.4) |
| Size of largest lesion, cm | |
| < 1 | 19 (61.3) |
| ≥ 1 | 12 (38.7) |
Of the CL-positive patients, 72% were 18–65 years of age. Women comprised two-thirds of the study population. Age, sex, outdoor or indoor occupation, housing conditions, being a beneficiary of social benefits with low income, daily use of bed nets, or animal rearing were not associated with increased of risk for disease (Table 1). Five personnel from the armed forces who were selected for the study did not have any signs of CL or VL (Table 1).
In this study, we identified a 37-year-old mother of three children who had a positive rK39 RDT result but did not have symptoms of VL. She was afebrile at examination, had no history of malaria, and did not have hepatosplenomegaly, pallor, or lymphadenopathy. Therefore, she was considered to have an symptomatic L. donovani infection. None of her family members showed positive results for the rK39 RDT.
Environmental risk factors.
As shown in Figure 1 and Table 3, most CL case-patients were lived near rice paddy fields. The distance from residences to paddy fields was significantly less for CL patients than for persons without CL. There was no difference in distances to forest, marsh, scrub jungles, and water bodies between residences of persons with and without CL. Logistic regression analysis showed that only distances to paddy fields was significantly associated with CL status (Table 4).
Table 3.
Environmental risk factors associated with CL, Sri Lanka
| Distances from household (meters)* | Cutaneous leishmaniasis patients (n = 30) + rK39-positive participant (n = 1) | Reference population (n = 812) | t-value (P) | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| To forest | 2,619.86 | 1,334.44 | 2,519.80 | 1,556.25 | 0.353 (0.724) |
| To marshy lands | 3,286.49 | 1,608.23 | 3,413.62 | 1,658.22 | −0.419 (0.675) |
| To paddy fields | 99.57 | 93.18 | 171.34 | 152.95 | −2.593 (0.010) |
| To scrub jungles | 520.86 | 228.07 | 534.25 | 368.51 | −0.312 (0.757) |
| To water bodies | 474.66 | 324.27 | 619.45 | 802.55 | −1.001 (0.317) |
Global positioning system coordinates could not be recorded for 110 persons.
Table 4.
Summary of logistic regression analysis using cutaneous leishmaniasis status as the dependent variable, Sri Lanka
| Variable | Regression coefficient (β) | Standard error of (β) | P | Odds ratio (95% confidence interval) |
|---|---|---|---|---|
| Constant | −2.448 | 0.286 | 0.000 | 0.086 |
| Distance to paddy | −0.006 | 0.002 | 0.009 | 0.994 (0.990–0.999) |
Social awareness.
Of the surveyed community, 37% had never heard of leishmaniasis and 24.4% knew a person who was affected (i.e., a family member, neighbor, friend, or co-worker). Nearly half (n = 485) of the community either did not know about the availability of treatment for leishmaniasis or did not have access to treatment, indicating poor social awareness, although almost all participants could reach the nearest hospital within two hours (Table 5).
Table 5.
Social awareness of leishmaniasis and the sand fly vector, Sri Lanka*
| Variable | No. (%) |
|---|---|
| Awareness of leishmaniasis | |
| Heard about leishmaniasis | |
| Yes | 602 (63.1) |
| No | 352 (36.9) |
| Mode of information | |
| Television | 215 (22.5) |
| Radio | 31 (3.2) |
| Printed media | 62 (6.5) |
| Friend | 82 (8.6) |
| Health care workers (PHMs) | 306 (32.2) |
| Any other known person affected | |
| Yes | 233 (24.4) |
| No | 721 (75.6) |
| Awareness of availability of advanced diagnostic and treatment facilities | |
| Yes | 469 (49.1) |
| No | 177 (18.6) |
| Non responders | 308 (32.3) |
| Time to reach nearest hospital, hours | |
| < 0.5 | 622 (65.1) |
| 1–2 | 331 (34.8) |
| > 2 | 1 (0.1) |
| Awareness of vector sandfly | |
| Biting nuisance | |
| Severe | 135 (14.2) |
| Moderately severe | 614 (64.4) |
| Mild | 45 (4.7) |
| None | 160 (16.8) |
| Peak biting time | |
| Dawn | 33 (3.5) |
| Day | 25 (2.6) |
| Dusk | 725 (76.1) |
| Night | 99 (10.4) |
| Indoor residual spraying for malaria | |
| Yes | 8 (0.8) |
| No | 946 (99.2) |
PHMs = public health midwives.
Most (64.4%) persons believed that sand fly biting was only a moderate nuisance. The peak biting time reported by most (76%) persons was dusk (Table 4).
Seasonal variation in CL incidence reported by the Ministry of Health, Sri Lanka.
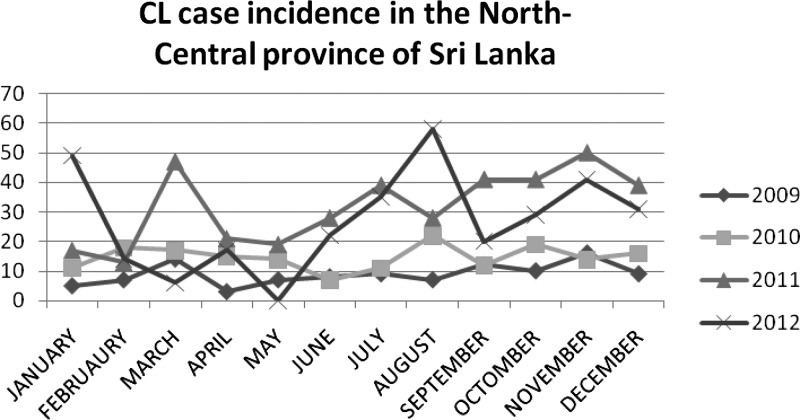
Transmission rates for each month were also examined for the past four years. As shown in Figure 2, there was overall more transmission during 2011–2012 than in the previous two years. In 2012, the highest transmission rates were observed during January, August, and November, although there was considerable variation from year to year.
Figure 2.
Cutaneous leishmaniasis (CL) case incidence data published by the Epidemiology Unit Ministry of Health, Sri Lanka.32
Discussion
Leishmaniasis has gradually spread to many parts of Sri Lanka and is now considered an endemic disease.10,11 A major observation of this study was the high prevalence (3.2%, 31 of 955) of CL among persons in the Thalawa MOH area. This finding is consistent with observations made in other highly endemic districts in Sri Lanka.24 Living at the edge of paddy fields was the only identified risk factor for development of leishmaniasis in this study.
There is some evidence suggesting that dogs could be a potential reservoir host. One study detected amastigotes in a slit skin smear and in blood of two dogs, and a second study detected an K39-positive dog.22,23 However, our present study did not identify any significant association with animal rearing. It is possible that other small mammals living near human habitats could be more significant reservoir hosts. Many small mammal species (i.e., Rattus spp.), which were identified as reservoir hosts for other Leishmania spp. (i.e., L. major) and Canis aureus, an identified reservoir host of L. infantum, are present in Sri Lanka and may play a role as a reservoir host.36 The fact that there was no association between occupation also favors other potential reservoir hosts in the peri-domestic environment.
Although it had been reported that outdoor occupation is a risk factor for CL in the northern area of Sri Lanka,24 our findings do not show such an association. The previous study reported an odds ratio of 6.5 to have CL among armed forces personnel who have significant exposure to forest areas compared with unemployed adults. This finding would further suggest the existence of a probable sylvatic cycle in Sri Lanka.
The high CL prevalence among armed forces personnel, males, younger age groups, and those engaged in the outdoor occupations may be caused by sampling of the population screened in the previous study.24 All of these factors seem interrelated. The population we screened in the Thalawa area was composed mainly of farmers, housewives, and children; there were only five armed forces personnel. None of the five armed forces personnel in our study had CL. Our findings suggest a peri-domestic pattern of leishmaniasis transmission in the Thalawa area. We also did not find any association between CL and house type or being a beneficiary of social benefits.
Spatial analysis showed that most persons in the study community live near paddy fields (mean ± SD distance = 168.5 ± 151.7 meters). In our study, living near paddy fields significantly increases the risk for leishmaniasis transmission (P = 0.010) (Tables 3 and 4). The ecology near paddy field comprises an aquatic environment, damp, moist, soil with organic matter, which has been shown to be conducive for breeding of Culex mosquito species that transmit Japanese enchphalitis.37,38 Whether this ecosystem aids sand fly breeding is not known and needs to be investigated. Wind speeds around paddy fields may also affect transmission of vector-borne diseases.
The most abundant putative vector in Sri Lanka is Phlebotomus argentipes.39,40 However, sand fly biology in Sri Lanka, although critically important for taking preventive measures, has not been studied in detail.
Leishmaniasis was declared a notifiable disease in Sri Lanka in September 2008.32 Data on CL passive case detection incidence related to seasonal variations over the past four years is variable32 (Figure 2). The higher CL incidence seen in 2011–2012 compared with lower CL incidence in 2009–2010 could be caused by improvement in case reporting in the health sector; increase spread of CL because sand fly control measures were not implemented, which resulted in the disease becoming established in the area; increased awareness in the community, which caused patients to seek more advanced treatment; or a combination of all of these factors. The higher CL incidence pattern seen in November in the past two years and in January 2012 correlates with monsoonal rainy seasons. The peaks seen in March 2011 could be attributed to delayed monsoons in 2011 (Department of Meteorology, Sri Lanka). The CL peak during July and August cannot be easily explained in relation to rainfall. The mean time between appearance of symptoms and seeking advanced medical care was 7.3 months. This delay may explain the peak that occurs in July–August (Figure 2).
One rK39-positive person was identified in this cohort. None of her family members were rK39 positive, suggesting that she probably was not a reservoir for transmission. Therefore, there did not appear to be a significant reservoir for rK39-positive persons in this CL-endemic region. Thus, risk for developing VL in this region can be considered low. Nevertheless, although the 1 case among 954 sampled persons is low, of a population of 20 million persons in Sri Lanka, the total number can be substantial and should not be ignored as a potential public health problem. Unavailability of another confirmatory serologic test to confirm the one seropositive person observed with the rK39 RDT was a limitation in our study. This limitation needs to be corrected in any future study.
Ten new cases of CL were detected, resulting in a prevalence of 1% for undetected CL in the community. This finding strongly indicates the need for better surveillance, case reporting, and early treatment, which are measures currently not used in Sri Lanka. Early detection and prompt treatment could minimize the human reservoir and subsequent transmission of the disease.
The clinical profile of CL lesions was similar to those reported in Sri Lanka.24 A high percentage (42%) of patients had sought ayurvedic treatment. There was poor social awareness of the disease and of the availability of treatment in this sampled population. Most of the community considered sand fly biting as a nuisance of moderate intensity and dusk as the peak biting time.
In conclusion, we recommended that more effective surveillance, case reporting, and control measures be implemented in high-risk areas. Further studies are needed to determine how CL (and VL) are transmitted, and these studies should include studies on sand fly biology, confirmation of the vector species, identifying reservoir hosts for transmission, and determining whether the same strain of L. donovani is responsible for CL and VL. Implementation of these measures are urgently needed before CL and possibly VL become major public health problems in Sri Lanka.
ACKNOWLEDGMENTS
We thank InBios International Corporation (Seattle, WA) for donating 500 Kalazar DetecTM rK39 Rapid Diagnostic tests. The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Footnotes
Financial support: Shalindra Ranasinghe and Renu Wickremasinghe were supported by National Research Council (grant no. 09-24) and the Ministry of Technology and Research of Sri Lanka. Greg Matlashewski was supported by the Canadian Institutes of Health Research.
Authors' addresses: Shalindra Ranasinghe, Ositha Silva, Hasini Wackwella, and Renu Wickremasinghe, Department of Parasitology, Faculty of Medical Sciences, University of Sri Jayewardenepura, Gangodawila, Nugegoda, Sri Lanka, E-mails: shalindraran12@gmail.com, ositha18@gmail.com, hasini_wackwella@yahoo.co.uk, and renuwickremasinghe@gmail.com. Rajitha Wickremasinghe, Department of Public Health, Faculty of Medicine, University of Kelaniya, Kelaniya, Sri Lanka, E-mail: arwicks@sltnet.lk. Asoka Munasinghe, Regional Epidemiology Unit, Regional Director of Health Services Office, Anuradhapura, Sri Lanka, E-mail: reanuradhapura@gmail.com. Sanjeeva Hulangamuwa, Dermatology Unit, Teaching Hospital, Anuradhapura, Sri Lanka, E-mail: chamilsanjeeva@yahoo.com. Sundaramoorthy Sivanantharajah, Geographic Information System Branch, Department of Surveys, Colombo, Sri Lanka, E-mail: ssgis@sltnet.lk. Kamal Seneviratne and Samantha Bandara, Office of the Medical Officer of Health, Anuradhapura Road, Thewala, Sri Lanka, E-mails: vbkamals@gmail.com and samanthaban99@gmail.com. Indira Athauda, Department of Parasitology, Faculty of Medical Sciences, University of Sri Jayewardenepura, Gangodawila, Nugegoda, Sri Lanka, E-mail: indiraathauda@yahoo.com. Chaturi Navaratne, Department of Parasitology, Faculty of Medical Sciences, University of Sri Jayewardenepura, Gangodawila, Nugegoda, Sri Lanka, E-mail: chaturi_navaratne@yahoo.com. Greg Matlashewski, Department of Microbiology and Immunology, McGill University, Montreal, Quebec, Canada, and Special Program for Research and Training in Tropical Diseases, World Health Organization, Geneva, Switzerland, E-mail: greg.matlashewski@mcgill.ca.
References
- 1.World Health Organization Control of the leishmaniases. World Health Organ Tech Rep Ser. 2010;949:1–186. [PubMed] [Google Scholar]
- 2.Dedet JP, Pratlong F. Leishmaniasis. In: Cook G, Zumla A, editors. Manson's Tropical Diseases. Twenty-first edition. London: Saunders Elsevier Sciences; 2003. pp. 1297–1302. [Google Scholar]
- 3.Alvar J, Velez I, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M, Leishmaniasis Control Team WHO. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson RN, Ngumbi PM, Gachihi GS, Mwanyumba JP, Mbugua J, Mosonik N, Were JB, Roberts CR. A new focus of kala-azar due to Leishmania donovani sensu lato in Kenya. Trans R Soc Trop Med Hyg. 1993;87:142–144. doi: 10.1016/0035-9203(93)90461-x. [DOI] [PubMed] [Google Scholar]
- 5.Marlet MV, Wuillaume F, Jacquet D, Quispe KW, Dujardin JC, Boelaert M. A neglected disease of humans: a new focus of visceral leishmaniasis in Bakool, Somalia. Trans R Soc Trop Med Hyg. 2003;97:667–671. doi: 10.1016/s0035-9203(03)80099-8. [DOI] [PubMed] [Google Scholar]
- 6.Karunaweera ND, Pratlong F, Siriwardane HV, Ihalamulla RL, Dedet JP. Sri Lankan cutaneous leishmaniasis is caused by Leishmania donovani zymodeme MON-37. Trans R Soc Trop Med Hyg. 2003;97:380–381. doi: 10.1016/s0035-9203(03)90061-7. [DOI] [PubMed] [Google Scholar]
- 7.Razmjou S, Hejazy H, Motazedian MH, Baghaei M, Emamy M, Kalantary M. A new focus of zoonotic cutaneous leishmaniasis in Shiraz, Iran. Trans R Soc Trop Med Hyg. 2009;103:727–730. doi: 10.1016/j.trstmh.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Fazaeli A, Fouladi B, Sharifi I. Emergence of cutaneous leishmaniasis in a border area at south-east of Iran: an epidemiological survey. J Vector Borne Dis. 2009;46:36–42. [PubMed] [Google Scholar]
- 9.United Nations Office for the Coordination of Humanitarian Affairs Sri Lanka . Sri Lanka Adminstrative Map 1:50. Map number: OCHA/LK/Colombo/Admin/001/02. 2007. http://www.humanitarianinfo.org/srilanka/catalogue/Files/Map%20Centre/Geographic%20Maps/Administrative%20Maps/LK00225_Admin_Sri%20Lanka_02July07.pdf Available at. Accessed February 20, 2012. [Google Scholar]
- 10.Karunaweera ND. Leishmania donovani causing cutaneous leishmaniasis in Sri Lanka: a wolf in sheep's clothing? Trends Parasitol. 2009;25:458–463. doi: 10.1016/j.pt.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Karunaweera ND, Rajapaksa US. Is leishmaniasis in Sri Lanka benign and be ignored? J Vector Borne Dis. 2009;46:13–17. [PubMed] [Google Scholar]
- 12.Minstry of Health Sri Lanka Colombo: Epidemiology Unit. Weekly Epidemiological Report. 2011;38:1–4. http://www.epid.gov.lk/web/attachments/article/188/Vol%2039%20NO%2002%20English.pdf Available at. Accessed February 28, 2012. [Google Scholar]
- 13.Abeygunasekara PH, Costa YJ, Seneviratne N, Ratnatunga N, Wijesundera Mde S. Locally acquired visceral leishmaniasis in Sri Lanka. Ceylon Med J. 2007;52:30–31. doi: 10.4038/cmj.v52i1.1047. [DOI] [PubMed] [Google Scholar]
- 14.Ranasinghe PH, Abeygunasekera PH, Athauda SB, Chandrasekharan NV, Mendis AV, Hulangamuwa CS, Wickremasinghe DR. First successful in vitro culture of the Leishmania strain causing visceral leishmaniasis in Sri Lanka. Ceylon Med J. 2011;56:179–180. doi: 10.4038/cmj.v56i4.3904. [DOI] [PubMed] [Google Scholar]
- 15.Rathnayake D, Ranawake RR, Sirimanna G, Siriwardhane Y, Karunaweera N, De Silva R. Co-infection of mucosal leishmaniasis and extra pulmonary tuberculosis in a patient with inherent immune deficiency. Int J Dermatol. 2010;49:549–551. doi: 10.1111/j.1365-4632.2010.04376.x. [DOI] [PubMed] [Google Scholar]
- 16.Athukorale DN, Seneviratne JK, Ihalamulla RL, Premaratne UN. Locally acquired cutaneous leishmaniasis in Sri Lanka. J Trop Med Hyg. 1992;95:432–433. [PubMed] [Google Scholar]
- 17.Ranasinghe S, Zhang WW, Wickremasinghe R, Abeygunasekera P, Chandrasekharan V, Athauda S, Mendis S, Hulangamuwa S, Matlashewski G, Pratlong F. Leishmania donovani zymodeme MON-37 isolated from an autochthonous visceral leishmaniasis patient in Sri Lanka. Pathog Glob Health. 2012;106:421–424. doi: 10.1179/2047773212Y.0000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreno G. Les Complexes Leishmania infantum. Implications Taxinomiques, Biogeographyques et Epidemiologiques. A Propos de l’Analysis Enzymatique de 548 Souches de l’Ancien et du Nouveau Monde. Montpelllier, France: University of Monpellier; 1989. [Google Scholar]
- 19.Kuhls K, Keilonat L, Ochsenreither S, Schaar M, Schweynoch C, Presber W, Schönian G. Multilocus microsatellite typing (MLMT) reveals genetically isolated populations between and within the main endemic regions of visceral leishmaniasis. Microbes Infect. 2007;9:334–343. doi: 10.1016/j.micinf.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Alam MZ, Haralambous C, Kuhls K, Gouzelou E, Sgouras D, Soteriadou K, Schnur L, Pratlong F, Schönian G. The paraphyletic composition of Leishmania donovani zymodeme MON-37 revealed by multilocus microsatellite typing. Microbes Infect. 2009;11:707–715. doi: 10.1016/j.micinf.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Siriwardana HV, Noyes HA, Beeching NJ, Chance ML, Karunaweera ND, Bates P. Leishmania donovani and cutaneous leishmaniasis, Sri Lanka. Emerg Infect Dis. 2007;13:476–478. doi: 10.3201/eid1303.060242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nawaratna SS, Weilgama DJ, Rajapaksha K. Cutaneous leishmaniasis in Sri Lanka: a study of possible animal reservoirs. Int J Infect Dis. 2009;13:513–517. doi: 10.1016/j.ijid.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 23.Rosypal AC, Tripp S, Kinlaw C, Hailemariam S, Tidwell RR, Lindsay DS, Rajapakse RP, Sreekumar C, Dubey JP. Surveillance for antibodies to Leishmania spp. in dogs from Sri Lanka. J Parasitol. 2010;96:230–231. doi: 10.1645/GE-2288. [DOI] [PubMed] [Google Scholar]
- 24.Siriwardana HV, Thalagala N, Karunaweera ND. Clinical and epidemiological studies on the cutaneous leishmaniasis caused by Leishmania (Leishmania) donovani in Sri Lanka. Ann Trop Med Parasitol. 2010;104:213–223. doi: 10.1179/136485910X12647085215615. [DOI] [PubMed] [Google Scholar]
- 25.Srivastava P, Dayama A, Mehrotra S, Sundar S. Diagnosis of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 2011;105:1–6. doi: 10.1016/j.trstmh.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Riera C, Fisa R, Udina M, Gallego M, Portus M. Detection of Leishmania infantum cryptic infection in asymptomatic blood donors living in an endemic area (Eivissa, Balearic Islands, Spain) by different diagnostic methods. Trans R Soc Trop Med Hyg. 2004;98:102–110. doi: 10.1016/s0035-9203(03)00015-4. [DOI] [PubMed] [Google Scholar]
- 28.Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet. 2005;366:1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 29.Singh SP, Hasker E, Picado A, Gidwani K, Malaviya P, Singh RP, Boelaert M, Sundar S. Risk factors for visceral leishmaniasis in India: further evidence on the role of domestic animals. Trop Med Int Health. 2010;15:29–35. doi: 10.1111/j.1365-3156.2010.02515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bern C, Hightower AW, Chowdhury R, Ali M, Amann J, Wagatsuma Y, Haque R, Kurkjian K, Vaz LE, Begum M, Akter T, Cetre-Sossah CB, Ahluwalia IB, Dotson E, Secor WE, Breiman RF, Maguire JH. Risk factors for kala-azar in Bangladesh. Emerg Infect Dis. 2005;11:655–662. doi: 10.3201/eid1105.040718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sudhakar S, Srinivas T, Palit A, Kar SK, Battacharya SK. Mapping of risk prone areas of kala-azar (visceral leishmaniasis) in parts of Bihar State, India: an RS and GIS approach. J Vector Borne Dis. 2006;43:115–122. [PubMed] [Google Scholar]
- 32.Minstry of Health SriLanka Colombo: Epidemiology Unit. Weekly Epidemiological Report. 2009–2012. http://www.epid.gov.lk/web/attachments/article/188/Vol%2039%20NO%2002%20English.pdf Available at. Accessed April 14, 2013.
- 33.El-Moamly AE-SM, Hafeez M. Performance of rK39 immunochromatography and freeze-dried direct agglutination tests in the diagnosis of imported visceral leishmaniasis. Parasitol Res. 2012;112:349–354. doi: 10.1007/s00436-011-2499-9. [DOI] [PubMed] [Google Scholar]
- 34.Sundar S, Pai K, Sahu M, Kumar V, Murray HW. Immunochromatographic strip-test detection of anti-K39 antibody in Indian visceral leishmaniasis. Ann Trop Med Parasitol. 2002;96:19–23. doi: 10.1179/000349802125000466. [DOI] [PubMed] [Google Scholar]
- 35.Department of the Commissioner General of Samurdhi The New Methodology of Family Categorization. Colombo: Department of the Commissioner General of Samurdhi. 2009. http://samurdhidept.gov.lk/new_methedology.html Available at. Accessed February 20, 2012.
- 36.Arudpragasam KD, Kotewala I, Kotagama SW. Checklist of Mammals of Sri Lanka. Colombo, Sri Lanka: National Science Council of Sri Lanka; 1982. [Google Scholar]
- 37.Yasuoka J, Levins R. Ecology of vector mosquitoes in Sri Lanka: suggestions for future mosquito control in rice ecosystems. Southeast Asian J Trop Med Public Health. 2007;38:646–657. [PubMed] [Google Scholar]
- 38.Sunish IP, Reuben R. Factors influencing the abundance of Japanese encephalitis vectors in ricefields in India. I. Abiotic. Med Vet Entomol. 2002;15:381–392. doi: 10.1046/j.0269-283x.2001.00324.x. [DOI] [PubMed] [Google Scholar]
- 39.Lane RP, Pile MM, Amerasinghe FP. Anthropophagy and aggregation behaviour of the sandfly Phlebotomus argentipes in Sri Lanka. Med Vet Entomol. 1990;14:79–88. doi: 10.1111/j.1365-2915.1990.tb00263.x. [DOI] [PubMed] [Google Scholar]
- 40.Ozbel Y, Sanjoba C, Alten B, Asada M, Depaquit J, Matsumoto Y, Matsumoto Y, Demir S, Siyambalagoda RR, Rajapakse RP, Matsumoto Y. Distribution and ecological aspects of sand fly (Diptera: Psychodidae) species in Sri Lanka. J Vector Ecol. 2011;36:S77. doi: 10.1111/j.1948-7134.2011.00115.x. [DOI] [PubMed] [Google Scholar]