Abstract
We describe the thirteenth reported case of human infection with Gongylonema spp. in the United States and the first to be confirmed as Gongylonema pulchrum. The parasite described was isolated from the oral cavity of a resident of Williamsburg, Virginia. The identity of the parasite was verified through morphological and genetic approaches, and provided the first genetic confirmation of a Gongylonema sp. in humans.
Nematodes of the genus Gongylonema have a cosmopolitan distribution and are frequently encountered in domestic livestock1 but are only rarely described in humans.2 Despite the apparent rarity of Gongylonema spp. in humans, healthcare providers should be aware of the possibility of human infection, even in the absence of any obvious risk factors for parasite acquisition. We report a case of human infection by the parasitic nematode Gongylonema pulchrum in one of the authors (JDA) that was genetically verified by polymerase chain reaction (PCR) analysis.
In December 2012, an otherwise healthy, 36-year-old man removed an intact, living, parasitic nematode from the mucosa of his cheek. The removal and subsequent analysis of the nematode occurred after approximately three months of symptoms. These symptoms involved the sensation of intermittent rough areas present in the gum mucosa, which could be felt by the tongue. These rough patches would appear and disappear on a daily basis, giving the patient the indirect sense that there was an organism moving within the oral cavity. Direct movement of the nematode could not be detected while feeling the rough patch with his tongue. There were no other symptoms reported during this period.
One day before removal of the parasite, the patient was able to visualize the rough patch in the mucosa of his lower lip after migration of the worm towards the opening of the mouth (Figure 1A). The patient, coincidentally trained as an invertebrate biologist, suspected the presence of a parasitic worm and immediately contacted his general practitioner, who then referred him to an oral surgeon for a formal medical evaluation. Upon presenting the oral surgeon with photographic evidence (Figure 1A and B) and a detailed description and preliminary diagnosis of gongylonemiasis, the surgeon disputed the patient's self-diagnosis, claiming this was simply normal discoloration of the affected area. However, at the time of the medical examination, the worm had moved toward the back of the oral cavity and was not in a position for easy removal or visual inspection.
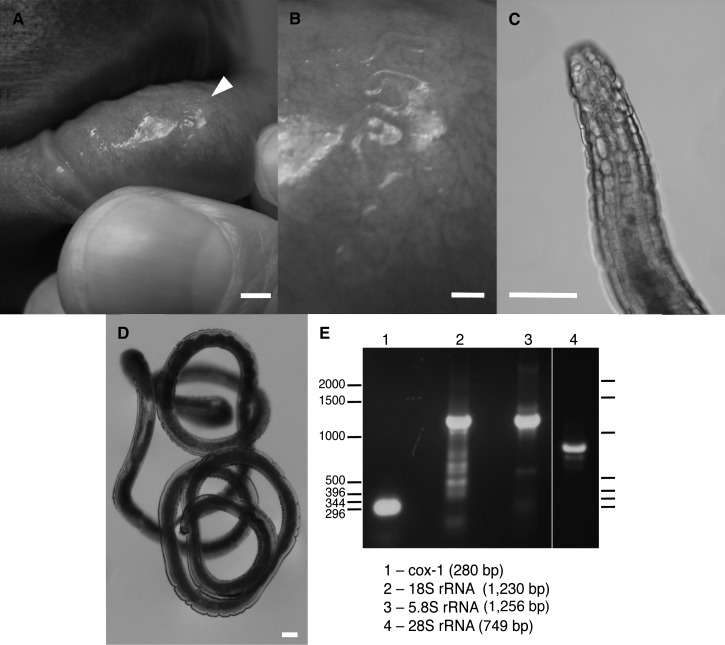
Figure 1.
A, Gongylonema pulchrum embedded in the lip mucosa of the patient. Arrowhead denotes location of living worm. B, Higher magnification of the same specimen, also in situ, in the lip mucosa. C, Anterior end of the specimen, showing rows of diagnostic bosses. D, Intact worm. E, Polymerase chain reaction fragments amplified from genomic DNA isolated from midbody of the worm shown in A–D for the mitochondrial cytochrome c oxidase subunit 1 (cox-1) gene and ribosomal RNA gene subunits 18S, 5.8S, and 28S (lanes 1–4) and subcloned and sequenced to genetically validate the worm as Gongylonema pulchrum. DNA ladder is indicated in basepairs (bp). Scale bar in A = 3 mm, scale bar in B = 1 mm, and scale bars in C–D = 0.1 mm.
The next morning, the patient awoke with the sensation that the worm was once again in the cheek mucosa near the front of the oral cavity. The patient used a pair of fine forceps (#5 super fine tip) to carefully remove the intact worm from the mucosa. The living and highly active parasite was transported to the patient's research laboratory at the College of William and Mary for identification, photomicrography, and subsequent analysis. The patient showed no additional symptoms after removal of this parasite and he was not administered treatment for nematode infection. The mucosa where the worm was removed recovered to its normal, healthy state within two days.
The parasite, upon collection, was analyzed and photographed while still alive by using a dissecting microscope. The long, transparent worm was readily identified as a nematode belonging to the genus Gongylonema through the presence of prominent bosses or scutes on the anterior end (Figure 1C). The specimen was an adult 20 mm in length and 0.12 mm in width (Figure 1D). We preserved the nematode in 70% ethanol approximately one hour after collection for subsequent analysis.
To genetically validate the morphological classification of the nematode as G. pulchrum, one of the authors (AEK) isolated genomic DNA from the parasite; amplified the mitochondrial cytochrome c oxidase subunit 1 (cox-1) gene and portions of the ribosomal RNA gene encoding the small and large subunits 18S, 5.8S, and 28S by PCR; and sequenced the subcloned fragments. Approximately 0.5 cm from the mid-section of the worm was removed and processed for genomic DNA isolation by lysing the tissue at 65°C for one hour in 40 μL of worm PCR lysis buffer (50 mM KCl, 10 mM Tris, pH 8.3, 2.5 mM MgCl2, 0.45% NP-40, 0.45% Tween-20, 0.01% gelatin, and 0.1 mg/mL of proteinase K).
Parasitic DNA (5 μL of lysate per reaction) was amplified by PCR using EconoTaq DNA polymerase (Lucigen Corp., Middleton, WI) and G. pulchrum-specific primers for cox-1 (coxFWD 5′-CCTGCATTTGGTATTATTAGGGAGTG-3′, coxREV 5′-TATGTCCAACATCACACAGGTTG-3′) and primers for ribosomal RNA subunits designed according to Halajian and others1) for18S (18FWD 5′-TCCAAGGAAGGCAGCAGGC-3′, 18REV 5′-CGACGGGCGGTGTGTACA-3′), 5.8S (5FWD 5′-AGGTGAACCTGCGGAAGGATCATT-3′, 5REV 5′-TTCACGCCCTCTTGAACTCT-3′), and 28S (28FWD 5′-GGGAAAGAAGACCCTGTTGAG-3′, 28REV 5′-TTCTGACTTAGAGGCGTTCAG-3′). Purified PCR fragments shown in Figure 1E were gel purified by electrophoresis (Gel/PCR DNA Fragment Extraction Kit; IBI Scientific, Peosta, IA) and cloned into the TOPO TA cloning vector (Invitrogen, Carlsbad, CA). Comparison of our genomic results with known sequences from G. pulchrum collected from wild and domestic animals by using the Basic Local Alignment Search Tool (National Center for Biotechnology Information, Bethesda, MD) showed 98–99% identities for all genes tested. These results verified the correct identification of this parasite as the same nematode species that infests animals such as cattle and other ungulates worldwide.1,3
This report represents only the thirteenth case of human infection by Gongylonema spp. in the United States and only the fifty-seventh case reported worldwide.4–8 It is also the first report to confirm the presence of G. pulchrum in humans through molecular methods. The small size and rarity of these specimens, along with the lack of diagnostic morphological characteristics for nematodes at the species level,9 means that it was not immediately clear whether this worm belonged to the same species as has been reported now from countries worldwide.4
In addition, there were no obvious risk factors for acquisition of this parasite. The patient had not traveled out of the country for many years and had not knowingly or intentionally consumed any insects (the known intermediate host for G. pulchrum). The patient had consumed water from a rural well in the months preceding detection, which is a possible source of infection, but perhaps no more likely a source than the insects and insect parts common in the food supply in the United States.2
The difficulty that the patient experienced in getting a medical expert to correctly identify this as a case of gongylonemiasis is not unusual. Previous case studies have reported the misdiagnosis of gongylonemiasis as oral candidiasis7 and even psychosis.6 These misdiagnoses occurred despite prior recommendations in the literature not to dismiss the symptoms associated with gongylonemiasis as delusional parasitosis.10 The sometimes transient nature of symptoms, combined with the mobility of the worms within the oral cavity, and the apparent rarity of infection, can challenge healthcare providers to make the correct diagnosis. Fecal samples may provide one way of detecting the ova of Gongylonema spp.,11 but this method is not always effective and assumes infection by a reproductive female.6,10
Our confirmation of gongylonemiasis through molecular techniques expands the diagnostic possibilities for this and other rare parasitic worms that can infect humans. If only partially intact worms are collected, as is sometimes the case,10 and the anterior end of the parasite is missing, then the identification will be more difficult because key morphological features such as the diagnostic bosses will be lost. In this case, molecular analysis may be required to complete the diagnosis. In the future, it would be interesting to test the extent of human infestation with G. pulchrum in the United States and worldwide by developing sensitive PCRs for use with fecal specimens, esophageal swabs, or previously acquired esophageal and/or oral mucosal biopsy specimens.
Although in this case and in most reported cases of gongylonemiasis the source of the infection is unknown, insects are considered to be the intermediate host for this and many other parasitic nematodes. Increasing interest in insects as a renewable food resource for humans12 suggests that human consumption of insects may increase in the future with a concomitant increase in gongylonemiasis. Awareness of the symptoms and diagnostic options for physicians is therefore critical, particularly in light of the skepticism with which many cases (including that reported here) are met.
ACKNOWLEDGMENTS
We thank M. Pizer for assistance in isolation of the specimen described and for critical comments on the manuscript.
Footnotes
Authors' addresses: Jonathan D. Allen, Department of Biology, College of William and Mary, Williamsburg, VA, E-mail: jdallen@wm.edu. Aurora Esquela-Kerscher, Department of Microbiology and Molecular Cell Biology, Leroy T. Canoles Jr. Cancer Research Center, Eastern Virginia Medical School, Norfolk, VA, E-mail: kerschae@evms.edu.
References
- 1.Halajian A, Eslami A, Salehi N, Ashrafi-Helan J, Sato H. Incidence and genetic characterization of Gongylonema pulchrum in cattle slaughtered in Mazandaran Province, Northern Iran. Iran J Parasitol. 2010;5:10–18. [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson ME, Lorente CA, Allen JE, Eberhard ML. Gongylonema infection of the mouth in a resident of Cambridge, Massachusetts. Clin Infect Dis. 2001;32:1378–1380. doi: 10.1086/319991. [DOI] [PubMed] [Google Scholar]
- 3.Makouloutou P, Setsuda A, Yokoyama M, Tsuji T, Saita E, Torii H, Kaneshiro Y, Sasaki M, Maeda K, Une Y, Hasegawa H, Sato H. Genetic variation of Gongylonema pulchrum from wild animals and cattle in Japan based on ribosomal RNA and mitochondrial cytochrome c oxidase subunit I genes. J Helminthol Sep. 2012;12:1–10. doi: 10.1017/S0022149X12000442. [DOI] [PubMed] [Google Scholar]
- 4.Haruki K, Furuya H, Saito S, Kamiya S, Kagei N. Gongylonema infection in man: a first case of gongylonemosis in Japan. Helminthologia. 2005;42:63–66. [Google Scholar]
- 5.Urch T, Albrecht BC, Buttner DW, Tannich E. Human infection with Gongylonema pulchrum. Dtsch Med Wochenschr. 2005;130:2566–2568. doi: 10.1055/s-2005-918604. [DOI] [PubMed] [Google Scholar]
- 6.Molavi GH, Massoud J, Gutierrez Y. Human Gongylonema infection in Iran. J Helminthol. 2006;80:425–428. doi: 10.1017/joh2006355. [DOI] [PubMed] [Google Scholar]
- 7.Ayala MA, Yencha MW. Gongylonema: a parasitic nematode of the oral cavity. Arch Otolaryngol Head Neck Surg. 2012;138:1082–1084. doi: 10.1001/2013.jamaoto.386. [DOI] [PubMed] [Google Scholar]
- 8.Pesson B, Hersant C, Biehler JF, Abou-Bacar A, Brunet J, Pfaff AW, Ferte H, Candolfi E. First case of human gongylonemosis in France. Parasite. 2013;20:1–4. doi: 10.1051/parasite/2013007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Floyd R, Abebe E, Papert A, Blaxter M. Molecular barcodes for soil nematode identification. Mol Ecol. 2002;11:839–850. doi: 10.1046/j.1365-294x.2002.01485.x. [DOI] [PubMed] [Google Scholar]
- 10.Eberhard ML, Busillo C. Human Gongylonema infection in a resident of New York City. Am J Trop Med Hyg. 1999;61:51–52. doi: 10.4269/ajtmh.1999.61.51. [DOI] [PubMed] [Google Scholar]
- 11.Wilde H, Suankratay C, Thongkan C, Chaiyabutr N. Human Gongylonema infection in Southeast Asia. J Travel Med. 2001;8:204–206. doi: 10.2310/7060.2001.24242. [DOI] [PubMed] [Google Scholar]
- 12.Belluco S, Losasso C, Maggioletti M, Alonzi CC, Paoletti MG, Ricci A. Edible insects in a food safety and nutritional perspective: a critical review. Com Rev Food Sci Food Safety. 2013;12:296–313. [Google Scholar]