Abstract
Mosquito management within households remains central to the control of dengue virus transmission. An important factor in these management decisions is the spatial clustering of Aedes aegypti. We measured spatial clustering of Ae. aegypti in the town of Borbón, Ecuador and assessed what characteristics of breeding containers influenced the clustering. We used logistic regression to assess the spatial extent of that clustering. We found strong evidence for juvenile mosquito clustering within 20 m and for adult mosquito clustering within 10 m, and stronger clustering associations for containers ≥ 40 L than those < 40 L. Aedes aegypti clusters persisted after adjusting for various container characteristics, suggesting that patterns are likely attributable to short dispersal distances rather than shared characteristics of containers in cluster areas. These findings have implications for targeting Ae. aegypti control efforts.
Introduction
Management of Aedes aegypti mosquitoes, the primary vector for dengue virus, remains central to control of dengue virus transmission. Effective long-term disease management, therefore, requires a solid understanding of the biology and population dynamics of this mosquito.1 Elements of the population dynamics of this species, however, are not well understood.2,3 Specifically, although studies have described the spatial patterns of Ae. aegypti populations, few have attempted to examine the specific factors that cause these spatial patterns.
Individuals residing in houses containing Ae. aegypti females have an increased risk of contracting dengue compared with those who reside in houses that do not contain Ae. aegypti females.4 This is for two reasons: first, female Ae. aegypti tend to live in close quarters with humans, feeding almost exclusively on human blood; and second, water-filled containers around households act as optimal habitat environments for immature Ae. aegypti to develop.4,5 Typical breeding containers include water tanks, metal drums, drinking water jugs, discarded plastic containers, and old tires that collect rainwater.6 Infested containers tend to contain immatures for short periods of time, creating a temporally dynamic spatial pattern of adult mosquitoes.3
Because Ae. aegypti breed in and around households, the participation of community members is necessary to minimize mosquito breeding.1 The first step, therefore, is to identify breeding site characteristics that affect the presence and abundance of Ae. aegypti at a household level; these data can in turn be used to develop control measures that limit proliferation at the community level to disseminate accurate information about dengue risk factors.1 Such detailed knowledge of how human environments affect proliferation and abundance of Ae. aegypti is critical to controlling virus transmission.7 At the household level, one important question is whether the presence of Ae. aegypti in a given household is influenced by environments in neighboring households. One goal of this study was to examine such spatial dependence.
Evidence for spatial dependence can be obtained cross sectionally through cluster analysis of larvae and pupae. In one notable analysis of Ae. aegypti clustering, Getis and others found, in Iquitos, Peru, that Ae. aegypti adults tended to cluster within a 30 m radius of a given house, with clustering occurring most heavily within a 10 m radius.3 However, their data did not show clustering of immature Ae. aegypti-positive containers among households, only that of pupae-positive containers within individual households. In contrast, a study in a lowland Thai village identified clustering of immature-positive containers in neighboring households up to 20 m away from one another.8 Similar to these previous studies, in this study we assess clustering in the coastal Ecuadorian town of Borbón; additionally, we extend the analysis to discern which factors are responsible for this observed clustering.
One mechanism that could explain the clusters and household dependencies observed in these studies involves adult females from one household ovipositing in neighboring households close to the location of their emergence. Numerous mark-release-recapture studies have shown that Ae. aegypti has a relatively short flight range, with adult females spending their lives in or near the house from which they emerged.7 The most important factor in the dispersal of Ae. aegypti females is likely to be the availability of suitable oviposition sites, i.e., sites that females will choose to oviposit in (principally because of the presence of adequate suitable water)9; however, other possible factors include mating and host-seeking behavior. Several mark-release-recapture studies have showed that dispersal distances of adult females increase as the availability of suitable oviposition sites decreases, and vice versa.10,11 Furthermore, different types of breeding containers have been shown to have differential success as rearing environments for Ae. aegypti, with some typically producing more pupae than others.12 Thus, clustering of larvae/pupae may occur if these suitable oviposition sites tend to be near each other, and if they have the conditions necessary for larvae and pupae to thrive in them. Evidence has shown that female Ae. aegypti engage in “skip oviposition,” laying small clutches of eggs in many sites during a single gonotrophic cycle.11 Therefore, human social factors related to water management that result in high numbers of suitable oviposition sites in neighboring houses may result in clusters of Ae. aegypti within those areas.
Accordingly, we systematically sampled immature Ae. aegypti from containers in and around houses in Borbón, Ecuador, and analyzed spatial patterns of mosquito abundance to address two primary questions: 1) Is there statistically significant clustering of sampled households or containers holding immature Ae. aegypti? 2) Do common environmental features of containers and households in clusters explain any patterns, or, conversely, are these patterns more likely to be attributable to the dispersal of adult mosquitoes? Although the first question has often been addressed, the second question has seldom if ever been considered. This second question is particularly important in planning effective community-level mosquito-control efforts.
Methods
Study population/time frame.
The city of Borbón, located on the northwestern coast of Ecuador in the province of Esmeraldas, and spanning a geographic area of 1.3 km2, has ∼5,000 residents living in 1,175 houses. Though once considered remote, Borbón is now connected by a paved road with the Atlantic coast to the west and the Andes Mountains to the east. The new road has encouraged in-migration, creating new settlements on the outer edges of the town.
We selected the 199 households enrolled in this study during May 2010 (shortly after the period usually with the greatest precipitation) from a cohort of 400 randomly selected households that were already participating in an ongoing study on water-associated diseases. Of these 400 households, the 199 were semi-randomly selected to be spatially representative of the original 400, and were located across the geographic extent of the town. All 400 households could not be surveyed because of time and personnel constraints. Surveying of households occurred from May 20 to May 27, 2010. Enrollment involved oral consent of household residents who were 18 years of age or older.
Institutional review boards at the University of Michigan and Universidad San Francisco de Quito in Ecuador approved our protocol.
Container surveillance and data collection.
All containers at each household and surrounding property, which held enough water at the time of sampling to potentially serve as a breeding site, were inspected for the presence of immature Ae. aegypti. Pupae in each container were individually counted and removed, and the presence or absence of larvae was recorded. To confirm species identification, all pupae and a representative sample of larvae were returned to a laboratory. Most larvae were examined under a stereoscopic microscope and identified based on morphological characteristics.13 For further verification, a subset of the larvae and all of the pupae were reared to adulthood before species identification, also under a stereoscopic microscope.
Containers were evaluated based on type Table 1, the presence of temephos (trade name Abate) larvicide tablets, the use of chlorine in the water, location indoors or outdoors, and whether they were rainwater-fed and capped or covered. Caps or tops for containers may include tight coverings, such as screw-on caps on jugs, or loose coverings, such as plywood boards on water tanks. Although the type of covering can affect Ae. aegypti oviposition,14 we did not differentiate between cover types during data collection. Most rainwater-fed containers would be purposefully placed to collect rain, although discarded containers often served as unintentional collectors of rainwater. The entire volume of each container was also estimated in liters (container volume, not necessarily the volume of the water inside the container).
Table 1.
Aedes aegypti-positive containers by type
| Container type | No. sampled | No. positive containers | % of total positive containers | No. positive containers topped/capped upon inspection |
|---|---|---|---|---|
| Tank | 211 | 24 | 41 | 7 |
| Gasoline jug | 299 | 10 | 17 | 1 |
| Tire | 50 | 6 | 10 | 0 |
| Bucket | 231 | 13 | 22 | 4 |
| Cistern (large cement) | 32 | 3 | 5 | 1 |
| Discarded container (non-tire) | 51 | 2 | 3 | 0 |
| Wash bin | 64 | 1 | 2 | 0 |
| Canoe/boat | 1 | 0 | 0 | n/a |
| Metal pot | 28 | 0 | 0 | n/a |
| Gallon jug (plastic) | 191 | 0 | 0 | n/a |
| Other | 24 | 0 | 0 | n/a |
| Total | 1178 | 59 | 100 | 13 |
Adult mosquitoes were collected with a Prokopak backpack aspirator,15 both inside and immediately outside of houses, focusing on important resting areas, such as walls, the underside of beds, and the underside of house floors. Aspiration was conducted in all rooms of houses. Total time of aspiration at each house was recorded; the median aspiration time at houses was 4 minutes, 53 seconds. Collected mosquitoes were killed in the field using ethyl acetate and were placed into storage containers for identification in the laboratory in Quito, Ecuador. Adult mosquitoes were stored at −20°C and identified as Ae. aegypti or other genera according to morphological characteristics.13
Data analysis.
Frequency of larval and pupal presence was evaluated by container type (e.g., tank, bucket, discarded plastic container, metal pot, large jug, gallon jug), size (estimated volume ≥ 40 L or < 40 L), location (indoors or outdoors), and covers (tops or caps at the time of inspection). Logistic regression analyses were conducted using SAS 9.3 (SAS Institute, Inc., Cary, NC) to determine which of the aforementioned variables (type, size, location, top/cap status) were associated with containers being positive for Ae. aegypti larvae and pupae.
Clustering was investigated at two scales: the household-level, which is ultimately the unit of most interventions, and the container level, to assess how different container characteristics affect spatial patterning. To determine clustering of positive households (at least one container with one or more immature mosquitoes), the number of positive households within a given radius interval [d-10, d] around a target household was calculated (ArcGIS 10). The “target household” was the household of interest in the clustering analysis. In this analysis we modeled the increase in odds that a target household will be positive for Ae. aegypti given that a neighboring household is positive for Ae. aegypti. Values for d ranged from 10 to 100 m. To count the number of positive neighbor households at each distance interval, we used the “Near” geospatial tool function in ArcGIS 10 to inventory the positive neighbors that each surveyed household had for each radius value d. We exported the resulting table into Microsoft Excel (Microsoft Corp., Redmond, WA), where we used the pivot table function to sum up the number of positive neighbors within a given radius interval for each house. We used logistic regression to determine whether presence of immature Ae. aegypti at neighboring households was associated with the odds of Ae. aegypti presence at each target household of interest. Because we were only concerned with the effect of neighboring houses containing Ae. aegypti, in this analysis we ignored negative neighboring houses and treated them as if they were unsampled or not present in the radius interval. Figure 1 provides a visual example of this first analysis, where positive and negative neighboring households surrounding a target households are shown within two radius intervals (d = 10 m and d = 20 m). Because sampling took place over the entire geographic extent of the town, and there are no houses beyond the town (the town is bordered by the junction of the Santiago and Cayapas rivers to the southeast and forest on other sides), no edge effect corrections were used in our clustering analysis.
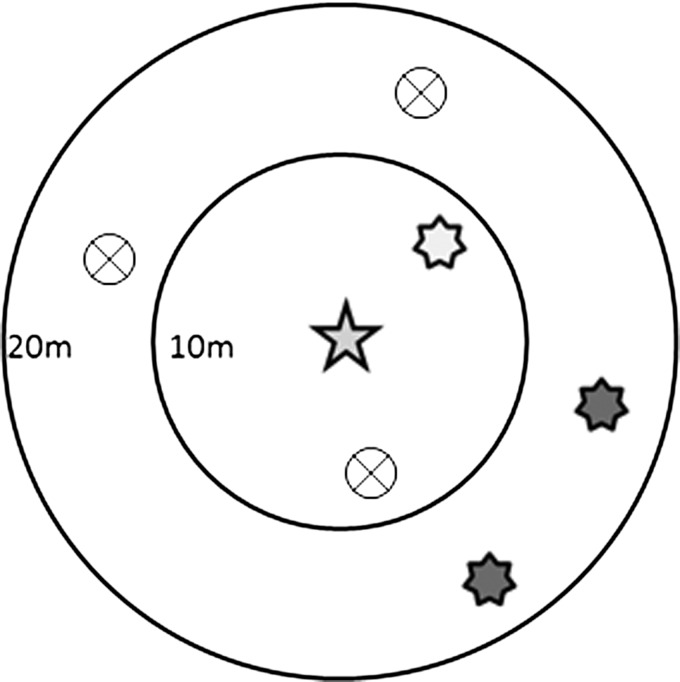
Figure 1.
Schematic diagram of framework for analysis presented in Table 3–6 where the outcome variable is defined as the vector status of a target household/container (depicted here as a 5-point star), and the exposure variable is defined as the vector status of a neighboring household/containers within a given radius interval (depicted here as 7-point stars). A positive household/container means one or more vectors were identified. For example, the light gray 7-point star inside a 10 m radius ring is a positive neighbor house/container in 0–10 m radius interval; whereas the dark gray stars between 10 and 20 m radius rings are positive houses/containers in 10–20 m radius interval. Circles with an X are negative neighboring houses/containers (no Aedes aegypti present), which are ignored in the analysis. The positive houses in the 0–10 m radius interval and the 10–20 m radius interval are analyzed separately, except in the case of Table 6, where appositive neighboring houses are aggregated into a single 0–40 m radius interval for the analysis. Every household/container is a target household/container and has the possibility of being a neighboring household/container for a given radius interval.
The data collected in this study are inherently spatial and thus likely to display spatial correlation. To determine whether there was a need to account for spatial correlation in our analysis, we inspected the residuals of the logistic regression model and constructed empirical semivariograms.16,17 The latter revealed that residual spatial correlation was still present even after adjusting for the effect of positive neighboring households within a given neighborhood buffer (see Supplemental Figures for semi-variograms). For most unadjusted models, the spatial correlation was estimated to decay to negligible values when the distance between households is greater than 2.1 km, a far greater distance than the spatial extents at which we attempt to observe clustering. To account for this residual spatial correlation, therefore, we introduced a spatial random effect using an exponential correlation function; i.e., using the statistical software package R, we fit the following model:
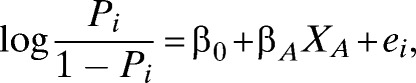 |
where pi is the probability that house i is positive for Ae. aegypti, β0 is an intercept, βA is the parameter associated with the presence of each positive neighbor household in a given radius interval, XA is the number of positive neighbor households in that radius interval, and ei is the spatial random effect for the location of house i.
This logistic regression approach provides analogous information to that produced by Ripley's K function, which has been used in other similar studies,3,8 but permits easier interpretation of binary variables. We compared our clustering results using the logistic regression approach to those using a Ripley's K function variant (ArcGIS 10 Multi-distance spatial cluster analysis tool, with no edge effect corrections), and obtained similar results (data not shown). We note that Ripley's K function is typically used for census data with complete or nearly complete sampling of a given area. The fact that the logistic regression and Ripley's K function provided similar results shows that our analysis is robust.
To further investigate clustering of Ae. aegypti at the container level, neighboring containers were grouped into two sizes (≥ 40 L and < 40 L), as container size has been proposed as an important factor in Ae. aegypti oviposition.10 Using a similar approach to the one used to calculate positive neighbor households in the household-level clustering analysis, we calculated (ArcGIS 10) the number of positive containers belonging to either size category within a radius interval [d-10, d] of each container. Because the goal of our clustering analysis was to assess the inter-household population dynamics of Ae. aegypti, containers belonging to the same household as the target container were excluded; only immature positive containers in neighbor households were considered. As with the household-level analysis, the target container in these logistic regression models was the container of interest in the clustering analysis, and the model estimated the odds that a target container was positive for Ae. aegypti given that containers in neighboring households were positive for Ae. aegypti. The association between target positive containers and the number of neighboring positive containers, stratified by size, was examined within given distance intervals of [d-10, d]. Because each household contains multiple containers, to account for possible correlation among containers belonging to the same household, we used a logistic regression model with a household-level random effect. We also investigated whether there was a need to account for spatial correlation in these models once random effects for households were introduced. Inspection of the empirical semi-variograms of the residuals of the logistic regression models with household-specific random effects revealed no residual spatial correlation (see Supplemental Figures 1–4). Hence, the logistic regression models for the container-level analysis included only household random effects to account for the repeated measures within each household. Estimates of the odds ratios (ORs) from both adjusted and unadjusted logistic regression models are reported in our results. Potential confounders that we adjusted for included: temephos insecticide use, chlorine use, presence/absence of a top or cap, number of household residents, water source (rainwater or not), container location (indoor/outdoor), the number of containers in the household, and volume (≥ 40 L or < 40 L). Through the adjusted models, we evaluated whether spatial clustering was attributable to spatial trends in these potential confounding variables, and if these spatial trends could fully explain the clustering observed. If the spatial trends did not explain the clustering, this would increase the likelihood that the clustering is attributable to the dispersal patterns of Ae. aegypti mosquitoes, as opposed to common environmental characteristics of houses in close proximity to one another. Using the same methods, we also assessed whether a container was more likely to be positive for Ae. aegypti (larvae and pupae) if there were other positive containers (also stratified by container volume) in the same household. The estimated ORs from these models, and models adjusting for the potential confounders, are reported.
We next investigated whether the presence of positive containers in neighboring households (< 40 m away) was associated with adult Ae. aegypti presence at target households. For this goal, we evaluated OR estimates from five logistic regression models. The 40 m cutoff point was chosen because it was the greatest distance at which clustering associations among Ae. aegypti-positive households were found. We used these models to analyze the associations between positive containers ≥ 40 L, positive containers < 40 L, and positive containers at the target household with adult Aedes aegypti presence at the target household. In three of these models, we assessed each variable independently. In the other two models, we adjusted the OR associated with positive containers in neighboring houses for the number of positive containers at the target house as well, because that variable could act as a confounder in the association between positive containers in neighboring houses and adult Ae. aegypti presence in target houses. Each of these five logistic regression models included spatial random effects, as was done in the household-level clustering analysis.
Results
Descriptive statistics.
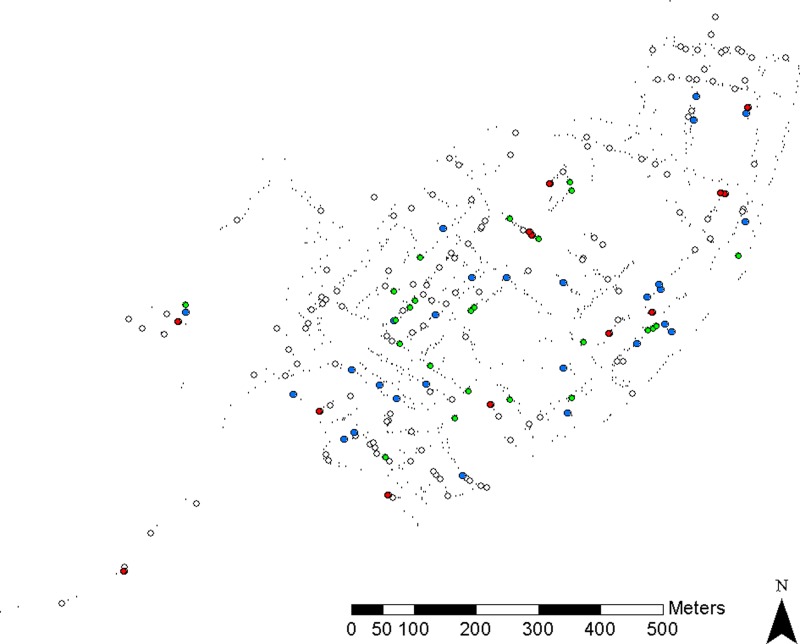
A total of 1,185 containers were found during surveillance, of which 58(4.9%) contained immature mosquitoes. Of these 58 positive containers, 27 (42%) harbored pupae. Most of the positive containers held water that lacked visible organic material (77%), were uncovered when inspected (80%), and were outdoors (61%). Tanks, including plastic water tanks and metal drums, accounted for 41% of the positive containers. “Pomas” (gasoline-jug style water containers) and buckets also were important breeding containers, comprising 15.6% and 20.3% of total positive containers, respectively. Notably, seven positive tanks and four positive buckets were covered upon inspection, indicating that covering of containers was either incomplete or not done consistently. A total of 59% of the positive containers had estimated volumes ≥ 40 L, as did 63% of the pupa-positive containers. Water tanks, buckets, and “pomas” were the most prevalent positive containers as well as the most prevalent container types (Table 1). The distribution of sampled households and Ae. aegypti-positive households is shown in Figure 2.
Figure 2.
Distribution of sampled households and Ae. aegypti-positive households. Green dots indicate houses positive for juveniles, blue dots indicate houses positive for adults, red dots indicate houses positive for juveniles and adults, white dots indicate sampled houses without Ae. aegypti, and small black dots indicate unsampled houses.
Univariate logistic regression analysis.
Containers that were ≥ 40 L (OR = 4.27, 95% confidence interval [CI]: 2.49,), uncovered (OR = 1.99, 95% CI: 1.06, 3.74), and with chlorine (OR = 1.72, 95% CI: 1.01, 2.94) all had significantly increased odds of harboring immature Ae. aegypti. A greater number of containers per household were associated with decreased odds for a container in that household to be immature-positive (OR = 0.95, 95% CI: 0.92, 0.99). That is, for any single container in the house, the odds of containing immature mosquitoes decreased as the total number of containers in the house increased. The visually observed presence of temephos larvicide tablets (OR = 0.56, 95% CI: 0.17, 1.83) and the number of residents in the household (OR = 1.04, 95% CI: 0.94, 1.16) had no significant association with the odds of containers being immature-positive Table 2.
Table 2.
Univariate logistic regression analyses
| Independent variable | OR (95% CI) |
|---|---|
| Container ≥ 40 L | 4.27 (2.49, 7.33) |
| Temephos usage | 0.56 (0.17, 1.83) |
| Chlorine usage | 1.72 (1.01, 2.94) |
| Uncovered container | 1.99 (1.06, 3.74) |
| No. of containers in house | 0.95 (0.92, 0.99) |
| No. of residents of house | 1.04 (0.94, 1.16) |
For the first four variables, odds ratios (ORs) indicate the increased odds of the container being juvenile-positive given the variables are true. For the last two variables ORs indicate decreased/increased odds of container being juvenile-positive for each additional container/resident in the house.
CI = confidence interval.
Clustering analysis.
Target houses located within 10 m of a single immature-positive household had nearly 11 times the odds of being immature-positive than those not near immature-positive neighbors (OR = 10.8, 95% CI: 3.8, 35.5). These households also had about 5 times the odds of being positive if an immature-positive neighboring household was 10–20 m away (OR = 5.3, 95% CI: 2.0, 13.9). Although a weaker association was found in the 30–40 m radius interval (OR = 2.9, 95% CI: 1.6, 5.4), no association was found at 20–30 m (OR = 1.1, 95% CI: 0.4, 2.3). Curiously, a negative association was found at 50–60 m (OR = 0.02, 95% CI: 0.03, 0.6) Table 3.
Table 3.
Cluster analysis of houses positive for Aedes aegypti juveniles and adults*
| Radius interval ([d-10],d) | No. of households within radius interval of all target households | No. of households positive for juveniles within radius interval of positive target households | No. of households positive for adults within radius interval of positive target households | Juveniles OR (95% CI) | Adults OR (95% CI) |
|---|---|---|---|---|---|
| 0–10 m | 16 | 10 | 8 | 10.8 (3.8, 35.5) | 7.6 (2.5, 23.0) |
| 10–20 m | 20 | 10 | 6 | 5.3 (2.0, 13.9) | 3.4 (1.1, 12.4) |
| 20–30 m | 33 | 6 | 7 | 1.1 (0.4 2.3) | 1.7 (0.8, 3.4) |
| 30–40 m | 46 | 18 | 6 | 2.9 (1.6, 5.4) | 0.8 (0.3, 1.9) |
| 40–50 m | 47 | 11 | 8 | 1.4 (0.7, 2.6) | 0.6 (0.2, 1.3) |
| 50–60 m | 45 | 2 | 20 | 0.2 (0.03, 0.6) | 3.0 (1.7, 5.5) |
| 60–70 m | 59 | 14 | 10 | 1.1 (0.6, 2.1) | 0.9 (0.4, 1.7) |
| 70–80 m | 76 | 12 | 12 | 0.9 (0.5, 1.6) | 0.6 (0.3, 1.6) |
| 80–90 m | 75 | 12 | 28 | 0.9 (0.5, 1.7) | 1.3 (0.8, 2.0) |
| 90–100 m | 92 | 24 | 14 | 1.6 (0.9, 2.5) | 0.6 (0.3, 1.3) |
Odds ratios (ORs) indicate increased odds of target house being positive associated with additional positive house within radius interval. Data collected in May 2010, with 199 houses sampled, 37 of which were positive for juveniles and 40 of which were positive for adults. Bold and italicized values are significant using Bonferroni correction: a = 0.005 (0.05/n tests = 0.05/10).
CI = confidence interval.
Adult mosquito-positive households showed a significant association with adult-positive target households in the radius intervals 0–10 m (OR = 7.6, 95% CI: 2.5, 23.0), 10–20 m (OR = 3.4, 95% CI: 1.1, 12.4), and 50–60 m (OR = 3.0, 95% CI: 1.7, 5.5) (Table 3).
Positive containers of ≥ 40 L in neighboring households were significantly associated with the presence of immature (larval or pupal) mosquitoes at target containers when they were in households that were located at distances of 0–10 m (OR = 7.6, 95% CI: 2.5, 23.0), 10–20 m (OR = 3.4, 95% CI: 1.1, 12.4), or 30–40 m (OR = 4.1, 95% CI: 1.7, 9.8. Positive neighboring containers < 40 L were significantly associated with the presence of immature mosquitoes at target containers only when they were located within a 0–10 m (OR = 4.3, 95% CI: 1.9, 10.0) radius interval. These associations persisted in adjusted logistic regression analyses Table 4. Within households, the number of positive containers of ≥ 40 L was significantly associated with the odds of the target container in that household being positive (OR = 1.6, 95% CI: 1.1, 2.6). This association was weaker in the adjusted logistic regression analysis (OR = 1.5, 95% CI: 0.9, 2.4). In contrast, the presence of other positive containers that were < 40 L had no significant association with the odds of the container of interest being positive in unadjusted analysis (OR = 1.20, 95% CI: 0.74, 1.95), but was significantly associated with the target container being positive in the adjusted analysis (OR = 2.0, 95% CI: 1.3, 3.1).
Table 4.
Clustering of containers positive for Aedes aegypti juveniles where neighboring containers are stratified by size*
| Radius interval ([d-10],d) | ≥ 40 L OR (95% CI) | ≥ 40 L adjusted* OR (95% CI) | < 40 L OR (95% CI) | < 40 L adjusted† OR (95% CI) |
|---|---|---|---|---|
| 0–10 m | 11.0 (3.0, 40.3) | 8.6 (2.6, 28.3) | 4.3 (1.9, 10.0) | 3.4 (1.6, 7.3) |
| 10–20 m | 7.0 (1.7, 28.7) | 5.7 (1.5, 21.2) | 2.0 (0.9, 4.8) | 1.6 (0.7, 3.4) |
| 20–30 m | 1.0 (0.2, 4.4) | 0.7 (0.2, 2.9) | 1.5 (0.4, 5.7) | 1.3 (0.4, 4.3) |
| 30–40 m | 4.1 (1.7, 9.8) | 3.4 (1.5, 7.8) | No observations | No observations |
Odds ratio (OR) indicates increased odds of target container being positive associated with additional juvenile-positive container within radius interval. Bold and italicized values are statistically significant at the α = 0.05 level.
Logistic regression models adjusted for: Temephos usage in the target container, chlorine usage in the target container, whether the target container has a top/cap, the number of individuals reported as living in the household with the target container, the number of containers at the household, whether the target container is rainwater-fed, whether the target container is indoors or outdoors, and whether the target container itself is ≥ 40 L or < 40 L. For a complete list of the co-variates and their estimates used in the adjusted models see Supplemental Tables S4a, S4b.
CI = confidence interval.
For pupae, immature-positive neighboring containers ≥ 40 L in the 0–10 m (OR = 7.7, 95% CI: 1.8, 33.5) and 10–20 m (OR = 5.1, 95% CI: 1.1, 24.9) radius intervals were significantly associated with the presence of pupae in target containers. Immature-positive neighboring containers < 40 L in the 0–10 radius interval were also significantly associated with the presence of pupae in target containers (OR = 3.0, 95% CI: 1.1, 8.2). These associations persisted in adjusted logistic regression analysis Table 5. Within households, the number of positive containers ≥ 40 L was significantly associated with the odds of the target container in that household being positive for pupae (OR = 2.6, 95% CI: 1.5, 3.4). This association persisted in the adjusted logistic regression analysis (OR = 2.1, 95% CI: 1.3, 3.3). The presence of other positive containers < 40 L had no significant association with the odds of the container of interest being positive for pupae in unadjusted analysis (OR = 1.6, 95% CI: 0.97, 2.8), but was significantly associated with the target container being positive in the adjusted analysis (OR = 2.1, 95% CI: 1.3, 3.5).
Table 5.
Clustering of containers positive for Aedes aegypti pupa(e) where neighboring containers are stratified by size*
| Radius interval ([d-10],d) | ≥ 40 L OR (95% CI) | ≥ 40 L adjusted* OR (95% CI) | < 40 L OR (95% CI) | < 40 L adjusted† OR (95% CI) |
|---|---|---|---|---|
| 0–10 m | 7.7 (1.8, 33.5) | 5.0 (1.9, 13.1) | 3.0 (1.1, 8.2) | 2.2 (1.0, 4.6) |
| 10–20 m | 5.1 (1.1, 24.9) | 3.5 (1.2, 10.2) | 2.4 (1.0, 5.9) | 1.7 (0.9, 3.2) |
| 20–30 m | 0.6 (0.02, 17.6) | 0.6 (0.1, 3.6) | 0.94 (0.03, 29.3) | 0.9 (0.1, 5.6) |
| 30–40 m | 2.5 (0.7, 8.9) | 2.0 (0.7, 5.4) | No observations | No observations |
Odds ratios (ORs) indicate increased odds of target container being positive associated with additional juvenile-positive container within radius interval. Data collected in May 2010, with 1,178 containers sampled, 37 of which were positive for pupae. Bold and italicized values are statistically significant at the α = 0.05 level.
Logistic regression models adjusted for: Temephos usage in the target container, chlorine usage in the target container, whether the target container has a top/cap, the number of individuals reported as living in the household with the target container, whether the target container is rainwater-fed, whether the target container is indoors or outdoors, the number of containers at the household, and whether the target container itself is ≥ 40 L or < 40 L. For a complete list of the co-variates and their estimates used in the adjusted models see Supplemental Tables S5a, S5b.
CI = confidence interval.
For adult mosquitoes, positive ≥ 40 L containers at neighboring households that were 0–40 m from target households were significantly associated with the presence of adult Ae. aegypti at target households (OR = 1.6, 95% CI: 1.1, 2.4). This association persisted after adjusting for positive containers within the target household. No significant association was found for positive < 40 L containers at neighbor households Table 6.
Table 6.
Logistic regression analysis with spatial random effects predicting presence of adult Aedes aegypti*
| Positive container ≥ 40 L in neighboring households up to 40 m OR (95% CI) | Positive container < 40 L in neighboring households up to 40 m OR (95% CI) | Positive container in target household OR (95% CI) | |
|---|---|---|---|
| Model 1 | 1.6 (1.1, 2.4) | ||
| Model 2 | 1.2 (0.8, 1.6) | ||
| Model 3 | 1.6 (1.0, 2.4) | ||
| Model 4 | 1.6 (1.1, 2.0) | 1.3 (0.9, 1.9) | |
| Model 5 | 1.1 (0.7, 1.6) | 1.5 (1.0, 2.4) |
Odds ratio (OR) for first two columns indicates increased odds of target household containing adult Ae. aegypti associated with an additional juvenile-positive container at neighboring households within 0–40 m of target household. Odds ratio for last column indicates increased odds of target household containing adult Ae. aegypti associated with additional positive container within target household.
CI = confidence interval.
Discussion
Spatial clustering of immature and adult Ae. aegypti was identified in this study. Common environmental factors related to community larvicide treatment and water management practices did not explain these patterns in our study, strengthening our hypothesis that the observed clustering is a result of the limited dispersal range of Ae. aegypti. Specifically, the observed clustering of Ae. aegypti immature-positive households within a 40 m radius persisted at the container level, even after adjusting for factors associated with common environmental characteristics (Tables 4 and 5). We also determined that larger containers played a major role in this clustering. When stratifying by container size, the association between the presence of Ae. aegypti in target and neighboring containers increased in strength if the neighboring containers were ≥ 40 L. This importance in container size remained when either larvae, pupae, or adults were used as the outcome variable for the target household. Intra-household clustering was not as strong as inter-household clustering. This may be attributable to the relatively small number (17) of households that had more than one positive container, making differences in associations based on container size harder to detect.
This study was conducted over a short time frame, and may contain temporal artifacts; however, our results are largely consistent with those of other studies of Ae. aegypti spatial patterning. Using a variant of a Ripley's K function, Getis and others3 found strong evidence of adult Ae. aegypti clustering within a 10 m radius in Iquitos, Peru, and weaker evidence of clustering within 30 m. Unlike our study, they reported no significant clustering of positive containers between houses. Our investigation also showed clustering of adult mosquitoes; but in contrast, we found strong evidence of clustering of immature-positive houses and individual immature-positive containers within 20 m, which is in agreement with a different study in Thailand that identified clustering of larvae and pupae up to 20 m.8 Furthermore, the stronger positive container clustering for containers ≥ 0 L than those < 40 L that we found is consistent with results of Maciel-de-Freitas and others,18 who, noted that large water-holding containers, especially tanks, tend to produce more adults than smaller containers. Additionally, in a mark-release-recapture experiment, Maciel-de-Freitas and others found that distribution of large size water containers was a highly important factor in the dispersal pattern of Ae. aegypti females.9 These results indicate that larger containers, which tend to be both more productive and attractive oviposition sites than smaller ones, may be the primary source of mosquitoes that are ovipositing in other nearby sites.
Although the Ripley's K function is a standard approach to identify clustering, our logistic regression method provides an opportunity to more closely examine potential causes for this clustering, by adjusting for common environmental factors related to water containers. We adjusted for chlorine use, temephos use, number of individuals living in the household, container size, and container coverage, as well as location and water source. Through this statistical adjustment, factors that did not affect the association between target and neighboring containers were identified. These factors did not appear to explain the observed clustering, again supporting the hypothesis that the observed clustering of Ae. aegypti was attributable to patterns of adult mosquito dispersal. We recognize however, there may be additional environmental factors that contribute to patterns of Ae. aegypti clustering, which were not measured in our study.
It is important to note that the ORs presented in Tables 3–6 correspond to the increase in odds for each additional positive neighbor. For example, in Table 3, a positive neighbor house in the 0–10 m radius interval would correspond to a 10.8 times greater odds of a target household being positive. If a target household had two positive neighbors in that interval, it would correspond to a 10.82 or 116.6 times greater odds of that household being positive. However, given that only one household in our sample had two positive neighbors at that interval, this estimate is not informed by the data and is a model extrapolation that is likely an overestimate (OR estimates from logistic regression coefficients are accurate only when the computed independent variable is close to the mean value). Accounting for multiple immature positive neighbors is more important at radius intervals farther from the target households or containers, which encompass greater areas, and are more likely to contain multiple positive neighbors than radius intervals at shorter distances. The alternative to this would be using a binary indicator for a target household or container having any number of positive neighbors. However, this would lead to an overestimation of the increase in odds associated with a target household having a single positive neighbor at a distant radius interval.
The distances of < 40 m, at which we found evidence of Ae. aegypti clustering, are consistent with previous literature on this mosquito's usual flight distance and spatial patterns. Most studies suggest that Ae. aegypti tend to disperse over short distances (tens of meters). For example, two Kenyan studies found that Ae. aegypti tended to disperse < 20 m19or < 50 m.20 Another study in Mexico noted a mean female flight distance of 30.5 m.21 Yet another study in three Thai villages reported somewhat longer mean dispersal distances that varied between 31 and 199 m, depending on the time and village; most mosquitoes, however, were recaptured in their house of release or an adjacent one.7 Thus, one inference from our findings is that dispersal distances may define spatial patterns of positive containers. The limited movement of mosquitoes lends support to the idea that human movement, as opposed to the dispersal of Ae. aegypti, is the primary driver of dengue transmission at fine spatial scales.22
One practical implication for vector management is that control needs to go beyond the individual household level; i.e., households need to be not only concerned about their own control efforts but also the control efforts of their neighbors. Communities, therefore, need to address control at a neighborhood level. Additionally, large container types, when infested, will produce mosquitoes that will oviposit in other nearby containers more often than small containers. Specifically targeting large containers at a neighborhood level,23 therefore, may be a more efficient strategy to reduce Ae. aegypti abundance aimed at reducing dengue transmission.
Supplementary Material
ACKNOWLEDGMENTS
We thank Renato Leon at the Universidad San Francisco de Quito for his help in developing the study; Katherine Connors for developing the original protocol; Jason Goldstick for his assistance in developing our statistical methodology; Reed Sorensen for his work in planning, data collection, and laboratory identification of mosquitoes; and the EcoDess staff members in Borbón for their assistance in our data collection efforts.
Footnotes
Financial support: This research was supported by grants from the National Institute of Allergy and Infectious Diseases (RO1AI050038), the University of Michigan Office of the Vice President for Research.
Authors' addresses: Nathaniel H. Schafrick, Mark L. Wilson, and Joseph N. S. Eisenberg, Department of Epidemiology, School of Public Health, The University of Michigan, Ann Arbor, MI, E-mails: natescha@umich.edu, wilsonml@umich.edu, and jnse@umich.edu. Meghan O. Milbrath and Veronica J. Berrocal, Department of Epidemiology and Department of Environmental Health Sciences, School of Public Health, University of Michigan, Ann Arbor, MI, E-mails: meghanom@umaich.edu, and berrocal@umich.edu.
References
- 1.Focks DA. A Review of Entomological Sampling Methods and Indicators for Dengue Vectors. Geneva: World Health Organization; 2003. [Google Scholar]
- 2.Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10:100–103. doi: 10.1016/s0966-842x(01)02288-0. [DOI] [PubMed] [Google Scholar]
- 3.Getis A, Morrison AC, Gray K, Scott TW. Characteristics of the spatial pattern of the dengue vector, Aedes aegypti, in Iquitos, Peru. Am J Trop Med Hyg. 2003;69:494–505. [PubMed] [Google Scholar]
- 4.Scott TW, Amerasinghe PH, Morrison AC, Lorenz LH, Clark GG, Strickman D, Kittayapong P, Edman JD. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: blood feeding frequency. J Med Entomol. 2000;37:89–101. doi: 10.1603/0022-2585-37.1.89. [DOI] [PubMed] [Google Scholar]
- 5.Scott TW, Morrison AC, Lorenz LH, Clark GG, Strickman D, Kittayapong P, Zhou H, Edman JD. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: population dynamics. J Med Entomol. 2000;37:77–88. doi: 10.1603/0022-2585-37.1.77. [DOI] [PubMed] [Google Scholar]
- 6.Barbazan P, Tuntaprasart W, Souris M, Demoraes F, Nitatpattana N, Boonyuan W, Gonzalez J. Assessment of a new strategy based on Aedes aegypti (L.) pupal productivity, for the surveillance and control of dengue transmission in Thailand. Ann Trop Med Parasitol. 2008;102:161–171. doi: 10.1179/136485908X252296. [DOI] [PubMed] [Google Scholar]
- 7.Harrington LC, Scott TW, Lerdthusnee K, Coleman RC, Costero A, Clark GG, Jones JJ, Kitthawee S, Kittayapong P, Sithiprasasna R, Edman JD. Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am J Trop Med Hyg. 2005;72:209–220. [PubMed] [Google Scholar]
- 8.Chansang C, Kittayapong P. Application of mosquito sampling count and geospatial methods to improve dengue vector surveillance. Am J Trop Med Hyg. 2007;77:897–902. [PubMed] [Google Scholar]
- 9.Maciel-de-Freitas R, Souza-Santos R, Codeço CT, Lourenço-de-Oliveira R. Influence of the spatial distribution of human hosts and large size containers on the dispersal of the mosquito Aedes aegypti within the first gonotrophic cycle. Med Vet Entomol. 2010;24:74–82. doi: 10.1111/j.1365-2915.2009.00851.x. [DOI] [PubMed] [Google Scholar]
- 10.Edman JD, Scott TW, Costero A, Morrison AC, Harrington LC, Clark GG. Aedes aegypti (Diptera: Culicidae) movement influenced by availability of oviposition sites. J Med Entomol. 1998;35:578–583. doi: 10.1093/jmedent/35.4.578. [DOI] [PubMed] [Google Scholar]
- 11.Reiter P. Oviposition, dispersal, and survival in Aedes aegypti: implications for the efficacy of control strategies. Vector Borne Zoonotic Dis. 2007;7:261–273. doi: 10.1089/vbz.2006.0630. [DOI] [PubMed] [Google Scholar]
- 12.Aldstadt J, Koenraadt CJ, Fansiri T, Kijchalao U, Richardson J, Jones JW, Scott TW. Ecological modeling of Aedes aegypti (L.) pupal production in rural Kamphaeng Phet, Thailand. PLoS Negl Trop Dis. 2011;5:e940. doi: 10.1371/journal.pntd.0000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darsie RF, Jr, Ward RA. Identification and Geographical Distribution of the Mosquitoes of North America, North of Mexico. Gainesville, FL: University Press of Florida; 2005. [Google Scholar]
- 14.Strickman D, Kittayapong P. Laboratory demonstration of oviposition by Aedes aegypti (Diptera: Culicidae) in covered water jars. J Med Entomol. 1993;30:937–949. doi: 10.1093/jmedent/30.5.947. [DOI] [PubMed] [Google Scholar]
- 15.Vazquez-Prokopec GM, Galvin WA, Kelly R, Kitron U. A new, cost-effective, battery-powered aspirator for adult mosquito collections. J Med Entomol. 2009;46:1256–1259. doi: 10.1603/033.046.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cressie NAC. Statistics for Spatial Data. Second edition. New York: Wiley; 1993. [Google Scholar]
- 17.Banerjee S, Carlin BP, Gelfand AE. Hierarchical Modeling and Analysis for Spatial Data. Boca Raton, FL: Chapman and Hall/CRC; 2004. [Google Scholar]
- 18.Maciel-De-Freitas R, Codeço CT, Lourenço-De-Oliveira R. Daily survival rates and dispersal of Aedes aegypti females in Rio de Janeiro, Brazil. Am J Trop Med Hyg. 2007;76:659–665. [PubMed] [Google Scholar]
- 19.McDonald PT. Population characteristics of domestic Aedes aegypti (Diptera: Culicidae) in villages on the Kenya coast. J Med Entomol. 1977;14:49–53. doi: 10.1093/jmedent/14.1.49. [DOI] [PubMed] [Google Scholar]
- 20.Trips M, Hausermann W. Dispersal and other population parameters of Aedes aegypti in an African village and their possible significance in epidemiology of vector-borne diseases. Am J Trop Med Hyg. 1986;35:1263–1279. doi: 10.4269/ajtmh.1986.35.1263. [DOI] [PubMed] [Google Scholar]
- 21.Ordóñez-Gonzalez JG, Mercado-Hernandez R, Flores-Suarez AE, Fernández-Salas I. The use of sticky ovitraps to estimate dispersal of Aedes aegypti in northeastern Mexico. J Am Mosq Control Assoc. 2001;17:93–97. [PubMed] [Google Scholar]
- 22.Stoddard ST, Forshey BM, Morrison AC, Paz-Soldan VA, Vazquez-Prokopec GM, Astete H, Reiner RC, Vilcarromero S, Elder JP, Halsey ES, Kochel TJ, Kitron U, Scott TW. House-to-house human movement drives dengue virus transmission. Proc Natl Acad Sci USA. 2013;110:994–999. doi: 10.1073/pnas.1213349110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maciel-de-Freitas R, Lourenço-de-Oliveira R. Does targeting key-containers effectively reduce Aedes aegypti population density? Trop Med Int Health. 2011;16:965–973. doi: 10.1111/j.1365-3156.2011.02797.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.