Abstract
Approximately 2–7% of the Omani population has chronic hepatitis B virus (HBV) infection. To decrease this burden, universal childhood hepatitis B vaccination was introduced in Oman in 1990. The hepatitis B vaccination strategy and reported coverage were reviewed. To assess the impact of the program on chronic HBV seroprevalence, a nationally representative seroprevalence study was conducted in Oman in 2005. Since 1991, hepatitis B vaccination in Oman has reached almost every eligible child, with reported coverage of ≥ 97% for the birth dose and ≥ 94% for three doses. Of 175 children born pre-vaccine introduction, 16 (9.1%) had evidence of HBV exposure, and 4 (2.3%) had evidence of chronic infection. Of 1,890 children born after vaccine introduction, 43 (2.3%) had evidence of HBV exposure, and 10 (0.5%) had evidence of chronic infection. Oman has a strong infant hepatitis B vaccination program, resulting in a dramatic decrease in chronic HBV seroprevalence.
Introduction
Worldwide, more than 2 billion people have been infected with hepatitis B virus (HBV), and ∼240 million have chronic HBV infection, which puts them at high risk for cirrhosis and hepatocellular carcinoma.1,2 An estimated 4.3 million people were infected with HBV yearly in the Eastern Mediterranean Region (EMR) of the World Health Organization (WHO) before routine childhood hepatitis B vaccination was introduced.3 Because of this burden, in 2010, the member countries of EMR established a hepatitis B control goal of achieving a seroprevalence of < 1% among children < 5 years of age by 2015.3
Limited data indicate a moderate burden of chronic HBV infection in Oman, with an estimated 2–7% of the population having chronic HBV infection before the introduction of vaccine4; however, other studies have found even higher prevalence of chronic infection. In a 1988–1989 study, 38.4% of pregnant Omani women who were not eligible for hepatitis B vaccine were positive for hepatitis B surface antibody (anti-HBs, a biomarker for having been infected with HBV in the past in a non-vaccinated person), and 8.9% were positive for hepatitis B surface antigen (HBsAg, a biomarker of acute or chronic HBV infection).5 A study in 2000 among pregnant women 25–46 years of age in nine health centers found a HBsAg seroprevalence of 7.1%.6
Because of the burden of chronic HBV infection, routine infant hepatitis B vaccination beginning at birth was introduced into the national vaccination program in August 1990. Surveillance data have shown a decrease in the burden of hepatitis B in the population; reported acute hepatitis B cases decreased 81% from 85 cases in 1999 to 16 in 2010.7,8 In addition, the prevalence of HBsAg among blood donors has decreased from 4% in 1990 to 1% in 2010.5,9,10 However, these data do not show the impact of the hepatitis B vaccination program among children, who are the target of the vaccination program and the most likely to acquire chronic HBV infection caused by perinatal and early childhood HBV transmission.11 In this report, we describe the progress of the routine hepatitis B immunization program in achieving high vaccine coverage and describe a seroprevalence evaluation of school children to document the program's impact in achieving the EMR hepatitis B control target.
Childhood hepatitis B vaccination strategy.
The Oman Ministry of Health (MOH) introduced a 3-dose schedule of hepatitis B vaccine into the routine infant immunization schedule in August 1990. During August 1990–June 2003, doses were scheduled at birth (within 24 hours), and at 6 weeks and 7 months of age. In July 2003, a new 4-dose schedule was introduced in the Expanded Program on Immunization (EPI) to accommodate use of a pentavalent vaccine (DTwP-HepB-Hib). Monovalent hepatitis B vaccine was administered at birth and pentavalent vaccine doses were given at 6 weeks, 3 months, and 5 months of age. In 2008, the schedule was changed to birth (monovalent), 2, 4, and 6 months (pentavalent).
In Oman, a birth dose of hepatitis B vaccine is recommended for all infants within 24 hours of birth to prevent perinatal HBV transmission10; pregnant women are not routinely screened for HBsAg during prenatal care, and there is no national policy for the administration of hepatitis B immunoglobulin to newborns born to HBsAg positive mothers at birth. An EPI staff nurse visits the post-natal ward twice daily to identify newborns and deliver the birth dose at every public and private hospital in the country. For ∼2% of children who are born at home, a skilled birth attendant (SBA) who attends the delivery administers the birth dose. Pregnant women choosing to deliver at home without an SBA are informed during antenatal visits to report to the hospital or their primary health clinic within 24 hours of delivery to register and have the birth dose administered to the baby. If a newborn is unable to come to a health facility, an EPI staff member or SBA conducts a home visit to vaccinate the child.
Every birth regardless of location of birth is registered (“MR2 register”), and data of vaccination are recorded in the EPI registry. Hepatitis B vaccination is also documented on a child health card, which is kept by the mother and updated with each subsequent vaccine dose given to the child. Once a child is registered, his or her information is easily accessible at other health centers, so despite internal migration, the vaccination status of each child can be verified. Children not vaccinated at the recommended age are followed up by telephone and/or a home visit to vaccinate the child.
In addition to vaccination during infancy, all children matriculating in school (> 98% of the school-age population) are required to show evidence of complete vaccination. Students whose vaccinations are not complete are directed to a primary health care institution for vaccination.
To supplement routine vaccination, school-based catch-up hepatitis B vaccination campaigns were conducted to protect older children and adolescents from infection. During 2000–2005, adolescents 11–18 years of age (1982–1990 birth cohorts) were vaccinated with a 3-dose series at 0, 1, and 6 months. Thus, as of 2012, all individuals aged 30 and below, 60% of the total population, were eligible to have received the hepatitis B vaccine series.
Methods
Vaccination coverage.
The number of children vaccinated with hepatitis B-containing vaccine each month is reported to the district health office by clinical healthcare facilities. Hepatitis B vaccination coverage is reported monthly for the birth dose (HepB-BD), 1st dose (HepB1), 2nd dose (HepB2), and 3rd dose (HepB3) by all district health offices to province health offices and then to the central headquarters of the MOH. Yearly administrative hepatitis B vaccination coverage is calculated by using the number of children vaccinated with a given dose as the numerator and the number of live births as the denominator for birth dose or the number of surviving infants as the denominator for doses 1–3 in a given year. In line with the WHO Reach Every District (RED) vaccination strategy, coverage is calculated at the district-level and nationally.12 Children who are vaccinated as part of the school-entry program or during catch-up campaigns are not included in coverage estimates.
Vaccination coverage during the school-based campaigns was calculated by dividing the number vaccinated in each round by the number of students enrolled in each grade for each province. This was repeated for each of the three rounds in a given grade.
Hepatitis B vaccination and seroprevalence study.
Because acute hepatitis B is rare in children, the WHO recommends monitoring the impact of infant hepatitis B vaccination programs by conducting a seroprevalence study.13 In 2005, in collaboration with the WHO, the Oman MOH conducted a two-stage cluster survey, stratified by province, among grade 1 (6–7 years of age) and grade 7 students (12–13 years of age), attending public school to assess hepatitis B vaccination and seroprevalence. Children enrolled in private schools were ineligible for this evaluation (∼10% of children). Both age cohorts were eligible for vaccination services at the time of birth, though some individuals in grade 7 could have been born before the start of the national hepatitis B vaccination policy (August 1990). Timely 3-dose vaccination was defined as having received a hepatitis B vaccine dose within 24 hours of birth plus two subsequent doses separated by at least 4-week intervals, administered before the first birthday.
Sampling was conducted so seroprevalence data could be analyzed by province and nationally; all provinces are similar in terms of access to health services, ethnicity distribution, and standard of living. The sample size was based on provincial primary school grade 1 class sizes, with an expectation that the HBsAg seroprevalence would be 1% ± 1% with 95% confidence. With a design effect of two and a 15% refusal rate, the minimum sample size was 875 grade 1 students. All public schools with grade 1 classes were eligible for inclusion. After stratification into nine strata based on provinces, a random sample of classes was selected in each stratum. All members of a selected class were enrolled. Based on these specifications and including an accounting for refusals and absentees, a combined total of 2,285 grade 1 and grade 7 students were chosen by probability proportional to size from 31 1st grade and 28 7th grade classrooms (provincial distribution of 2–7 classrooms/grade/province).
If families provided informed consent, vaccination data on each student was collected from vaccination cards; if no vaccination card was available, the vaccination status of the student was then considered unknown. Additionally, a 5 mL blood specimen was obtained from each student.
The HBsAg testing was conducted using Hepanostika HBsAg Ultra ELISA test kits (Biomeriuex, Marcy l'Etoile, France). Hepatitis B core antibody (Anti-HBc) testing was conducted using Hepanostika anti-HBc Uni-Form ELISA test kits (Biomeriuex). All serologic testing was conducted at the Central Public Health Laboratory in Oman. Serum was tested first for total anti-HBc; if anti-HBc was not detected, the sample was assumed to be negative for HBsAg. Anti-HBc positive serum samples were tested for HBsAg. If both HBsAg and anti-HBc were detected, the child was defined as having chronic HBV infection. If only anti-HBc was detected, the child was defined as having a resolved HBV infection.
All data were entered into an Excel 2003 (Microsoft Corp., Redmond, WA) database and analyzed using SAS v9.3 (Cary, NC). Proportions were calculated for HBsAg and anti-HBc by province and nationally among the three groups: grade 1 students born after the national vaccine policy, grade 7 students born after the national vaccine policy, and grade 7 students born before the national vaccine policy. Wilson 95% confidence intervals (CIs) are given for HBsAg proportions. Data presented does not take into account weights or cluster design because school identifiers for children were not recorded. To assess associations between participants and non-participants, chi-square (χ2) P-values were calculated for categorical variables and Wilcoxon rank-sum P-values were calculated to assess differences in continuous variables.
Approval for the study was provided by the Omani National Research Ethical Committee of the MOH. All students with chronic HBV infection were referred to a hospital for medical care. Additionally, all family contacts of HBsAg-positive students were offered screening for anti-HBc and HBsAg; those without evidence of immunity were offered hepatitis B vaccination and HBsAg-positive persons were referred for medical care. Counseling was provided free of charge to parents or guardians of all HBsAg-positive children and any HBsAg-positive contacts.
Results
Vaccination coverage.
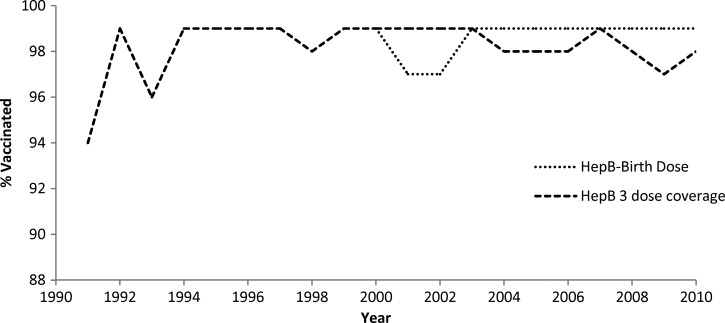
Since 1991, hepatitis B vaccination in Oman has reached almost every eligible child, with reported coverage of ≥ 97% for HepB-BD and ≥ 94% for HepB3 each year since inception (Figure 1).14 In 2010, ≥ 95% HepB-BD and HepB3 coverage were reported in all 61 districts.
Figure 1.
Hepatitis B vaccination reported administrative coverage among infants by year—Oman, 1990–2010. The national routine infant hepatitis B vaccination program started in 1991.
The school-based vaccination campaigns targeted ∼330,000 children born from 1982 to 1990. In each of the three rounds, ≥ 95% vaccination coverage was achieved in each of the 10 provinces in each of the birth cohorts targeted.15,16
Hepatitis B vaccination and seroprevalence study.
Of 2,285 children selected to participate in the 2005 hepatitis B serosurvey, 152 did not have consent to participate and 68 were absent or refused to provide assent for participation; thus, 2,065 children participated in the serosurvey. Non-participants and participants were similar with regards to sex (participants: 51% female versus nonparticipants: 47%, χ2 P-value = 0.3) and age (median age of participants and non-participants = 12 years, Wilcoxon P-value = 0.26).
Timely receipt of three doses of hepatitis B vaccine was 8% (14 of 175) among grade 7 students born before institution of the national hepatitis B vaccination policy, 78% (698 of 899) among grade 7 students born after institution of the national policy, and 95% (940 of 991) among grade 1 students (Table 1) . Provincial coverage of timely receipt of three doses of hepatitis B vaccine ranged from 0% to 29% among grade 7 students born before institution of the national policy, 54% to 90% among grade 7 students born after institution of the national policy, and 82% to 100% among grade 1 students (Table 2) .
Table 1.
Hepatitis B vaccination status among grade 1 and grade 7 serosurvey participants, Oman, 2005
| Timely 3-dose vaccination* n (%) | Partial vaccination (1–2 doses) n (%) | Unvaccinated (0 doses) n (%) | Unknown vaccination status n (%) | Total n | |
|---|---|---|---|---|---|
| Grade 7, born before national hepatitis B vaccination policy | 14 (8%) | 6 (3%) | 90 (51%) | 65 (37%) | 175 |
| Grade 7, born after national hepatitis B vaccination policy | 698 (78%) | 81 (9%) | 4 (0%) | 116 (13%) | 899 |
| Grade 1 | 940 (95%) | 38 (4%) | 1 (0%) | 12 (1%) | 991 |
Timely 3-dose vaccination was defined as having received a hepatitis B vaccine dose within 24 hours of birth plus two subsequent doses separated by at least 4-week intervals, administered before the first birthday.
Table 2.
Timely* 3-dose hepatitis B vaccination coverage among grade 1 and grade 7 serosurvey participants by province, Oman, 2005
| Grade 7, born before national hepatitis B vaccination policy | Grade 7, born after national hepatitis B vaccination policy | Grade 1, born after national hepatitis B vaccination policy | ||||
|---|---|---|---|---|---|---|
| Province | No. vaccinated with 3 doses/no. enrolled | % | No. vaccinated with 3 doses/no. enrolled | % | No. vaccinated with 3 doses/no. enrolled | % |
| Dakhilyah | 4/14 | 29 | 81/101 | 80 | 118/119 | 99 |
| Dhahira | 0/12 | 0 | 90/115 | 78 | 93/113 | 82 |
| Dhofar | 0/30 | 0 | 50/93 | 54 | 106/108 | 98 |
| Musandam | 1/4 | 25 | 35/51 | 69 | 46/48 | 96 |
| Muscat | 3/16 | 19 | 66/90 | 73 | 122/122 | 100 |
| N. Sharqiyah+Al Wustah | 3/36 | 8 | 84/101 | 83 | 119/124 | 96 |
| North Batinah | 0/22 | 0 | 90/102 | 88 | 120/123 | 98 |
| S. Batinah | 2/30 | 7 | 149/166 | 90 | 111/117 | 95 |
| S. Sharqiyah | 1/11 | 9 | 53/80 | 66 | 105/117 | 90 |
| Total | 14/175 | 8 | 698/899 | 78 | 940/991 | 95 |
Timely 3-dose vaccination was defined as having received a hepatitis B vaccine dose within 24 hours of birth plus two subsequent doses separated by at least 4-week intervals, administered before the first birthday.
Of 175 grade 7 students born before institution of the national policy with serum, 16 (9.4%) were positive for anti-HBc. Of 899 grade 7 students born after institution of the national policy, 26 (2.9%) were positive for anti-HBc. Of 991 grade 1 students born after institution of the national policy, 17 (1.7%) were positive for anti-HBc. HBsAg seroprevalence was 2.3% (4 of 175, 95% CI 0.9–5.7%) among grade 7 students born before institution of the national policy. The HBsAg seroprevalence was 0.7% (6 of 899, 95% CI 0.3–1.5%) among grade 7 students born after institution of the national policy; HBsAg seroprevalence was 0.4% (4 of 991, 95% CI 0.2–1.0%) among grade 1 students. There was no statistically significant difference between males and females for either anti-HBc or HBsAg seropositivity (data not shown).
In the nine provinces, anti-HBc seroprevalence ranged from 0% to 21.4% and HBsAg seroprevalence ranged from 0% to 7.1% among grade 7 students born before institution of the national vaccine strategy. For grade 7 students born after institution of the national policy, seroprevalence ranged from 0% to 5.9% for anti-HBc and 0% to 2.2% for HBsAg; for grade 1 students, seroprevalence ranged from 0% to 4.6% for anti-HBc and 0% to 2.1% for HBsAg (Table 3) .
Table 3.
Hepatitis B core antibody (anti-HBc) and surface antigen (HBsAg) seroprevalence among grade 1 and grade 7 serosurvey participants by province, Oman, 2005
| Grade 7, born before national hepatitis B vaccination policy | Grade 7, born after national hepatitis B vaccination policy | Grade 1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-HBc | HBsAg | Anti-HBc | HBsAg | Anti-HBc | HBsAg | |||||||
| Province | No. positive/no. tested | % | No. positive/no. tested | % | No. positive/no. tested | % | No. positive/no. tested | % | No. positive/no. tested | % | No. positive/no. tested | % |
| Dakhilyah | 3/14 | 21.4 | 1/14 | 7.1 | 6/101 | 5.9 | 1/101 | 1.0 | 3/119 | 2.5 | 1/119 | 0.8 |
| Dhahira | 0/12 | 0.0 | 0/12 | 0.0 | 3/115 | 2.6 | 0/115 | 0.0 | 0/113 | 0.0 | 0/113 | 0.0 |
| Dhofar | 2/30 | 6.7 | 1/30 | 3.3 | 4/93 | 4.3 | 2/93 | 2.2 | 5/108 | 4.6 | 1/108 | 0.9 |
| Musandam | 0/4 | 0.0 | 0/4 | 0.0 | 0/51 | 0.0 | 0/51 | 0.0 | 1/48 | 2.1 | 1/48 | 2.1 |
| Muscat | 0/16 | 0.0 | 0/16 | 0.0 | 0/90 | 0.0 | 0/90 | 0.0 | 1/122 | 0.8 | 1/122 | 0.8 |
| N. Sharqiyah+Al Wustah | 4/36 | 11.1 | 0/36 | 0.0 | 4/101 | 4.0 | 1/101 | 1.0 | 4/124 | 3.2 | 0/124 | 0.0 |
| North Batinah | 3/22 | 13.6 | 0/22 | 0.0 | 3/102 | 2.9 | 0/102 | 0.0 | 2/123 | 1.6 | 0/123 | 0.0 |
| S. Batinah | 4/30 | 13.3 | 2/30 | 6.7 | 4/166 | 2.4 | 2/166 | 1.2 | 0/117 | 0.0 | 0/117 | 0.0 |
| S. Sharqiyah | 0/11 | 0.0 | 0/11 | 0.0 | 2/80 | 2.5 | 0/80 | 0.0 | 1/117 | 0.9 | 0/117 | 0.0 |
| Total | 16/175 | 9.1 | 4/175 | 2.3 | 26/899 | 2.9 | 6/899 | 0.7 | 17/991 | 1.7 | 4/991 | 0.4 |
Discussion
Great progress has been made in Oman toward eliminating transmission of HBV among children by achieving and sustaining high hepatitis B birth dose coverage and high completion of the hepatitis B vaccine series. After introduction of the national policy for hepatitis B vaccination, 0.4% of grade 1 and 0.7% of grade 7 children had evidence of chronic HBV infection, compared with 2.3% of grade 7 students who were ineligible for infant vaccination. These findings support Oman's progress toward elimination of HBV transmission.
In Oman, routine hepatitis B vaccination coverage, and coverage with other vaccines, is very high, with a reported coverage of ≥ 97% for the birth dose and ≥ 94% for three doses of hepatitis B since 1991.14,17 The data are corroborated by a 1994 EPI coverage survey of 1,818 children 1 year of age, which showed 98% HepB3 coverage, compared with reported administrative HepB3 coverage of 99%.17 The coverage data in the serosurvey among grade 1 children also corroborates the fact that district-level coverage is high, though one must note that Dhahira has a timely 3-dose coverage of only 82%. This is most likely caused by the home births occurring in this area that might not have received a birth dose within 24 hours, and thus, by our conservative definition, have not received a timely 3-dose series. Coverage is high for a variety of reasons. First, all primary health care centers offer free hepatitis B vaccine daily helping to ensure adequate access. High-quality vaccine management has prevented shortages from occurring. The population also has a high awareness and acceptance of vaccinations. Finally, good child tracking systems allow the MOH to know the true number of children requiring vaccination and identification of missed children.
High hepatitis B birth dose coverage was achieved in Oman by addressing challenges to administering hepatitis B vaccine at the time of delivery. For example, EPI staff was placed in all health facilities with maternity units to ensure administration of the birth dose within 24 hours. In addition, a vaccine delivery strategy using birth attendants was developed for births not occurring in healthcare facilities, because although 98% of births in Oman are in facilities, nearly 100% of all births are attended by a birth attendant. High hepatitis B vaccine coverage also benefits from supporting elements that help reinforce the EPI program. All persons residing in Oman, including expatriates (25–30% of the population), have access to local health services including hepatitis B vaccination, which is provided free of charge in government health facilities. Furthermore, public acceptance of all vaccines is high.18
Many of the strategies used in Oman have been implemented in other resource-rich countries.19,20 However, Oman's resource-intense vaccination strategy might not be replicable in other countries; other strategies have been used with success to achieve high birth dose and 3-dose coverage. In China, a multi-faceted strategy has been aimed to increase hepatitis B vaccine coverage, including promoting facility deliveries, increasing awareness among both the public and health care providers, and increasing vaccine availability.21,22 The efforts in China have resulted in a drop in chronic HBV infection prevalence from 10% to < 1%.23 In Indonesia, the birth dose vaccine is stored at room temperature in the homes of community midwives to improve timeliness and administration of the birth dose; they also provide all birth dose by a single dose prefilled auto-disabled injection device (i.e., Uniject).24 Countries with low birth dose and/or 3-dose coverage should look to both Oman and elsewhere to learn about potential strategies for achieving higher coverage and should implement those that are most feasible within their current context.
The national seroprevalence study is subject to several limitations. Although the study was designed as a two-stage cluster survey, we were unable to analyze the data taking into account the study design. Because of an error in data collection, school information was unavailable and thus variance estimates do not account for children within clusters; however, because the number of HBsAg-positive children was so small, and the number of positives in any one region did not exceed two, the effect of the cluster sampling on the variance would be negligible. Second, the small sample size in the pre-vaccine cohort limits the precision of seroprevalence before vaccine introduction. It must also be noted that 98% of children in Oman attend school; therefore, children who are not in school and who might be less likely to be vaccinated and/or are at higher risk for hepatitis B are not included in this evaluation. Additionally, 10% of children attend private schools, which were not included in this evaluation; we suspect they have a vaccination rate comparable to the one found in this study, but there could be differences. Finally, this is a cross-sectional study showing how effective the program was in the 1990s (because it was done in 2005 among children mostly born in the 1990s).
In 2010, the regional committee of the EMR committed to a goal to reduce hepatitis B surface antigen seroprevalence among children < 5 years of age to < 1% by 2015. The results of this study indicate that the target of < 1% HBsAg seroprevalence has been achieved among children in Oman. Other countries aiming to achieve hepatitis B control goals should consider best practices that have been effective in Oman. Future steps to continue progress toward elimination of HBV transmission in Oman include maintaining high birth dose and HepB3 coverage, and ensuring continued political and financial support for the hepatitis B vaccination program.
ACKNOWLEDGMENTS
We acknowledge the support of H E Ali Jaffer M, Department of School Health, especially Sahar Abdo in the endeavor to have a successful immunization strategy for Oman. We sincerely thank the World Health Organization Headquarters and the Eastern Mediterranean Regional Office for providing technical and financial support. We especially thank Chris Nelson and all our colleagues at Central Public Health Laboratory, Darseit, Public Health Laboratory, and regional hospital laboratories. Finally, we acknowledge the efforts undertaken by the staff of the Department of Communicable Disease Surveillance and Control, regional epidemiologists and national and governorate supervisors of Communicable Disease Control, staff of regional and national EPI Sections, and regional school health focal points for the successful implementation of the national hepatitis B seroprevalence study.
Footnotes
Financial support: Funding for this seroprevalence study was provided by the World Health Organization and the Omani Ministry of Health.
Authors' addresses: Salah Thabit Al Awaidy, Office of H E of Health Affairs, Ministry of Health, Muscat Sultanate of Oman, E-mail: salah.awaidy@gmail.com. Shyam Pandurang Bawikar, Salim Al Mahrouqi, Said Al Baqlani, and Idris Al Obaidani, Department of Communicable Disease Surveillance and Control, Directorate General of Health Affairs, Ministry of Health, Muscat, Sultanate of Oman, E-mails: shyam.bawikar@gmail.com, salem.mahrooqi@gmail.com, saidalisaif@yahoo.co.uk, and dr.idris.oman@gmail.com. Suleiman Salim Al Busaidy, Central Public Health Laboratory, Directorate General of Health Affairs, Ministry of Health, Muscat, Sultanate of Oman, E-mail: mohdl@omantel.net.om. James Alexander and Minal K. Patel, Global Immunization Division, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: Axj1@cdc.gov and hgo9@cdc.gov.
References
- 1.WHO Hepatitis B vaccines. Wkly Epidemiol Rec. 2004;79:255–263. [PubMed] [Google Scholar]
- 2.Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212–2219. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 3.Regional Committee for the Eastern Mediterranean Resolution: the growing threats of hepatitis B and C in the Eastern Mediterranean Region: a call for action. EM/RC56/R. 2009:5. [Google Scholar]
- 4.CDC World Hepatitis Day. 2011. http://www.cdc.gov/Features/dsHepatitisAwareness/ Available at. Accessed August 2, 2011, 2011.
- 5.Ministry of Health-Oman Annual Health Report. 2009. http://www.moh.gov.om/en/stat/2009/index_eng.htm Available at. Accessed December 1, 2012.
- 6.Al Awaidy S, Abu-Elyazeed R, Al Hosani H, Al Mulla A, Al Busaiedy S, Al Amiry A, Farah Z, Al Marrie A, Bock HL, Al-Shaar I, Shah S. Sero-epidemiology of hepatitis B infection in pregnant women in Oman, Qatar and the United Arab Emirates. J Infect. 2006;52:202–206. doi: 10.1016/j.jinf.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Ministry of Health-Oman Annual Health Report. 2010. http://www.moh.gov.om/en/stat/2010/Chapters/CH08Y10.pdf Available at. Accessed December 1, 2012.
- 8.Ministry of Health-Oman Annual Health Report. 2006. http://www.moh.gov.om/en/stat/2006/Chapters/CH8Y06.pdf Available at. Accessed December 1, 2012.
- 9.Department of Family & Community Health Programmes, Department of Surveillance & Disease Control Viral hepatitis: current status in the Sultanate. Community Health & Disease Surveillance Newsletter H. 1993:1–4. [Google Scholar]
- 10.Ministry of Health-Oman Oman viral hepatitis survey: 2005. Community Health and Disease Surveillance Newsletter. 2005;14:1–3. 9–10. [Google Scholar]
- 11.Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol. 2005;34:1329–1339. doi: 10.1093/ije/dyi206. [DOI] [PubMed] [Google Scholar]
- 12.Vandelaer J, Bilous J, Nshimirimana D. Reaching Every District (RED) approach: a way to improve immunization performance. Bull World Health Organ. 2008;86:A–B. doi: 10.2471/BLT.07.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO . Documenting the Impact of Hepatitis B Immunization: Best Practices for Conducting a Serosurvey. Geneva: World Health Organization; 2011. [Google Scholar]
- 14.World Health Organization Immunization Profile - Oman. 2011. http://apps.who.int/immunization_monitoring/en/globalsummary/countryprofileselect.cfm Available at. Accessed January 1, 2012.
- 15.Ministry of Health-Oman Frequently asked questions (FAQ): hepatitis B. Community Health and Disease Surveillance Newsletter. 2002;11:8. [Google Scholar]
- 16.Ministry of Health-Oman Coverage of target groups (1985 & 1986 birth cohorts) by region/governorate: hepatitis B catch-up immunization campaign in schools: 2002–03. Community Health and Disease Surveillance Newsletter. 2003;12:7. [Google Scholar]
- 17.WHO/UNICEF . Review of Immunization Coverage-Oman: 1980–2008. Geneva: World Health Organization, UNICEF; 2009. [Google Scholar]
- 18.WHO-EMRO Country Profile: Oman. 2010. http://www.emro.who.int/emrinfo/index.aspx?Ctry=oma Available at. Accessed August 2, 2011, 2011.
- 19.Kao JH, Chen DS. Global control of hepatitis B virus infection. Lancet Infect Dis. 2002;2:395–403. doi: 10.1016/s1473-3099(02)00315-8. [DOI] [PubMed] [Google Scholar]
- 20.Mast EE, Weinbaum CM, Fiore AE, Alter MJ, Bell BP, Finelli L, Rodewald LE, Douglas JM, Jr, Janssen RS, Ward JW. Advisory Committee on Immunization Practices Centers for Disease C, Prevention A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep. 2006;55:1–33. quiz CE31–34. [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention Progress in hepatitis B prevention through universal infant vaccination–China, 1997–2006. MMWR Morb Mortal Wkly Rep. 2007;56:441–445. [PubMed] [Google Scholar]
- 22.Cui F, Li L, Hadler SC, Wang F, Zheng H, Chen Y, Gong X, Hutin YJ, Cairns KL, Liang X, Yang W. Factors associated with effectiveness of the first dose of hepatitis B vaccine in China: 1992–2005. Vaccine. 2010;28:5973–5978. doi: 10.1016/j.vaccine.2010.06.111. [DOI] [PubMed] [Google Scholar]
- 23.Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, Wang F, Zheng H, Guo J, Jia Z, Ma J, Wang H, Luo H, Li L, Jin S, Hadler SC, Wang Y. Evaluation of the impact of hepatitis B vaccination among children born during 1992–2005 in China. J Infect Dis. 2009;200:39–47. doi: 10.1086/599332. [DOI] [PubMed] [Google Scholar]
- 24.Creati M, Saleh A, Ruff TA, Stewart T, Otto B, Sutanto A, Clements CJ. Implementing the birth dose of hepatitis B vaccine in rural Indonesia. Vaccine. 2007;25:5985–5993. doi: 10.1016/j.vaccine.2007.05.055. [DOI] [PubMed] [Google Scholar]