Abstract
Recently, rearranged during transfection (RET) fusions have been identified in approximately 1% of non-small cell lung cancer (NSCLC). To know the prevalence of RET fusion genes in Korean NSCLCs, we examined the RET fusion genes in 156 surgically resected NSCLCs using a reverse transcriptase polymerase chain reaction. Two KIF5B-RET fusions and one CCDC6-RET fusion were identified. All three patients were females and never smokers with adenocarcinomas. RET fusion genes were mutually exclusive from EGFR, KRAS mutations and EML4-ALK fusion. RET fusion genes occur 1.9% (3 of 156) of surgically treated NSCLC patients in Koreans.
Keywords: RET Fusion; KIF5B; CCDC6; Carcinoma, Non-Small-Cell Lung; Korean
Lung cancer is the most common cause of cancer-related deaths worldwide. Surgical resection is the best treatment modality for early-stage non-small cell lung cancer (NSCLC). About two thirds of NSCLC is diagnosed with locally advanced or metastatic disease and treated with chemotherapy (1). Cytotoxic chemotherapy with platinum-based doublets has been the mainstay of treatment for advanced NSCLC (2). However, recent development of molecular technology has enabled targeted therapies for NSCLC. For example, epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, such as gefitinib, is used as 1st line chemotherapy for EGFR mutated adenocarcinoma (AC) (3). Crizotinib, an anaplastic lymphoma receptor tyrosine kinase (ALK) inhibitor, has been available after discovery of ALK fusion gene in NSCLC (4).
Most recently, novel fusions of rearranged during transfection (RET) gene have been identified in 1%-2% of NSCLC (5-9). RET proto-oncogene, located on chromosome 10q11.2, encodes a receptor tyrosine kinase expressed in tissues derived from neural crest (10). RET plays a crucial role in neural crest development. Oncogenic activation of RET is related with inherited cancer syndromes, such as multiple endocrine neoplasia type 2 (MEN 2), which includes medullary thyroid cancer (10). RET rearrangements is also associated with papillary thyroid cancer after exposure to external radiation (11). In lung cancer, genomic rearrangement of RET partnered with kinesin family member 5B (KIF5B) or coiled-coil domain containing 6 (CCDC6) have been reported (5-9). Similarly to echinoderm microtubule-associated protein-like 4 (EML4), which is common fusion partner to ALK gene, KIF5B and CCDC6 contain a coiled-coil domain (CCD). In RET fusion genes, a CCD of KIF5B or CCDC6 functions as a dimerization unit, which induces homodimerization and activates the oncogenic protein tyrosine kinase domain by autophophorylation (12). Ligand-independent activation of RET fusion gene may serve as the driving force for carcinogenesis.
To date, seven variants in KIF5B-RET fusion and only one type in CCDC6-RET fusion have been reported (13). Among 7 KIF5B-RET fusion variants, the fusion of KIF5B exon 15 and RET exon 12 (K15;R12) is the most common, and accounts for 60%-70% of KIF5B-RET fusion (5-7). The other variants, such as K16;R12, K23;R12, K22;R12 etc. were found infrequently.
Takeuchi et al. (5) found 13 (0.9%) RET fusion genes, which consist of 12 KIF5B-RET and 1 CCDC6-RET, in 1,482 Japanese NSCLCs. Wang et al. (7) discovered 13 patients (1.4%) harboring RET fusion genes among 936 Chinese NSCLCs. KIF5B-RET fusion gene was identified in 0.8% (1 of 121) NSCLCs in Caucasians (6). KIF5B-RET fusion gene was not found in 34 Norwegian and 5 African American (6, 9). In the present study, to know the prevalence of RET fusion genes in Korean NSCLCs, we investigated the RET fusion status of 156 surgically resected NSCLCs.
Tumor samples were provided by the National Biobank of Korea, Kyungpook National University Hospital, Daegu, Korea, which is supported by the Ministry of Health, Welfare, and Family Affairs. All materials derived from the National Biobank were obtained in accordance with the institutional review board approved protocol, Kyungpook National University Medical Center (Approval No., KNUHBIO_10_1016). This study included 156 NSCLC patients with available RNA who underwent curative surgical resection at the Kyungpook National University Hospital (Daegu, Korea) between January 2005 and July 2011. All the tissues were rapidly frozen in liquid nitrogen and stored at -80℃ until assayed. Written informed consent was obtained from all patients. All patients included were ethnic Koreans. The histologic types of lung cancers were as follows: 104 patients (66.7%) with AC and 52 patients (33.3%) with squamous cell carcinoma (Supplement Table 1). There were 105 males and 51 females, with the mean age of 63.8±8.7 yr. Patients consisted of 102 ever smokers and 54 never smokers. Of the 104 AC patients, 51 were never-smokers.
Total RNA was extracted from fresh frozen tissues using the RNeasy Mini kit (Qiagen, Valencia, CA, USA), and the RNA extract was incubated with RNase-free DNase I to remove contaminating DNA. Reverse transcription of total RNA was carried out using a Qiagen kit to generate complementary DNA (cDNA). Among 7 KIF5B-RET variants, we targeted the most common 5 KIF5B-RET fusion variants, K15;R12, K16;R12, K23;R12, K22;R12, and K24;R11. We conducted reverse transcriptase-polymerase chain reaction (RT-PCR) assays using sense primers 5'-ATTAGGTGGCAACTGTAGAACC-3' on exon 15 of KIF5B, to detect K15;R12 and K16;R12; 5'-AGCCACAGATCAGGAAAAGA-3' on exon 22 of KIF5B, to detect K22;R12, K23;R12 and K24;R11; and 5'-TGCAGCAAGAGAACAAGGTG-3' on exon 1 of CCDC6. Antisense primer was 5'-CAGGCCCCATACAATTTGAT-3' on exon 12 of RET. PCR reactions were performed in a total volume of 20 µL containing 200 ng of cDNA, 10 pM of each primer, 0.2 mM dNTPs, 1 unit of Taq polymerase (Genet Bio, Daejeon, Korea), 25 mM MgCl2, and 10×reaction buffer (10 mM Tris-HCl [pH8.3], 50 mM KCl, and 1.5 mM MgCl2). The PCR cycle conditions consisted of an initial denaturation step at 94℃ for 15 min, followed by 34 cycles of 30 sec at 94℃; 30 sec at 56℃; 1 min at 72℃; and a final elongation at 72℃ for 10 min. The PCR products were resolved on 1.2% agarose gels and stained with ethidium bromide for visualization under UV light. To identify the specific PCR product, the PCR products were digested 2 hr at 37℃ with the EcoRI restriction enzymes (New England BioLabs, Beverly, MA, USA). To confirm the RFLP results, selected PCR-amplified DNA samples were examined by DNA sequencing, and the results were also 100% concordant. We also analyzed mutations in the EGFR (exons 18-21), KRAS (exon 2) and EML4-ALK fusion using PCR and direct sequencing, as described in our previous study (14).
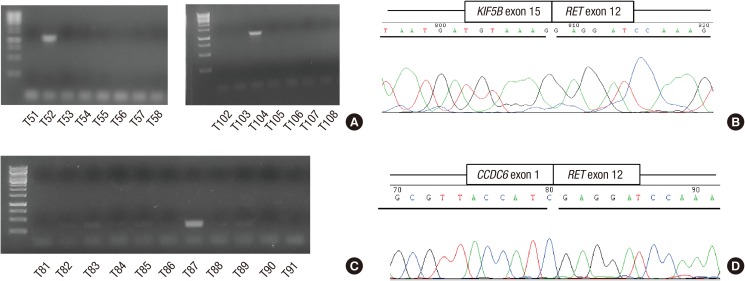
Using RT-PCR, RET fusion transcripts were detected in 3 (1.9%) of the 156 NSCLCs. RET fusion genes were found only in ACs. All the three patients were females and never smokers (Table 1). None of them had previous history of thyroid cancer or radiation therapy. Among three patients, two harbored KIF5B-RET fusion (KIF5B exon 15 fused with RET exon 12) and one harbored CCDC6-RET fusion (CCDC6 exon 1 fused with RET exon 12) (Fig. 1). Among 104 ACs, 26 cases (25.0%) had EGFR mutations, 7 cases (6.7%) had KRAS mutations, and 3 cases (2.9%) had EML4-ALK fusion. RET fusions were mutually exclusive with EGFR, KRAS mutations and EML4-ALK fusion.
Table 1.
Clinicopathological features of 3 patients with non-small cell lung cancer with RET fusions
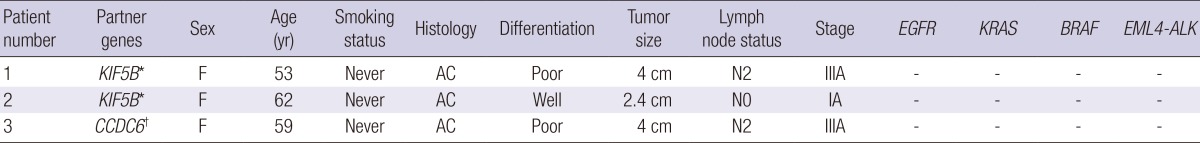
*Fusion between KIF5B exon 15 and RET exon 12; †Fusion between CCDC6 exon 1 and RET exon 12. F, female; AC, adenocarcinoma.
Fig. 1.
Detection of RET fusion genes by RT-PCR and sequencing. RT-PCR results of KIF5B-RET fusion genes (A). Nucleotide sequencing of the RCR product of KIF5B-RET (K15;R12) (B). RT-PCR result of CCDC6-RET fusion gene (C). Nucleotide sequencing of the RCR product of CCDC6-RET (D).
In this study, the frequency of RET fusion of NSCLCs was 1.9% (2.9% in ACs), which was comparable to the other studies, which reported that the frequency of RET fusion was 1%-2% in East Asian patients with NSCLCs (5-7, 9). However, this was different with the frequency reported in a previous Korean study, in which two cases (10%) of the KIF5B-RET fusion were identified in 20 primary lung ACs (15). This is mostly because the samples in the previous study were selected among triple-negative (EGFR, KRAS and EML4-ALK) or double-negative (EGFR and EML4-ALK) ACs.
There are some limitations in the present study. Among 7 variants of KIF5B-RET fusion gene, we examined only five variants. However, the other two variants, K24;R8 and K15;R11, are extremely rare and only one case for each fusion has been reported until now (6, 9). In addition, the results of RT-PCR analysis were not further examined by fluorescence in situ hybridization (FISH). Although FISH is more effective method for detection chromosome rearrangement, the high cost and need for technical expertise limits its wide usage. Furthermore, unlike PCR, FISH cannot distinguish between different RET fusion variants. Therefore, we employed RT-PCR and direct sequencing to evaluate the profile of RET fusion genes in Korean NSCLCs.
This study was supported by the National R&D Program for Cancer Control, Ministry of Health & Welfare (0720550-2) and the Converging Research Center Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012K001351).
The authors have no conflicts of interest to disclose.
Supplementary Material
Characteristics of study population and 3 patients with RET fusion gene
References
- 1.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goffin J, Lacchetti C, Ellis PM, Ung YC, Evans WK Lung Cancer Disease Site Group of Cancer Care Ontario's Program in Evidence-Based Care. First-line systemic chemotherapy in the treatment of advanced non-small cell lung cancer: a systematic review. J Thorac Oncol. 2010;5:260–274. doi: 10.1097/JTO.0b013e3181c6f035. [DOI] [PubMed] [Google Scholar]
- 3.Sequist LV, Martins RG, Spigel D, Grunberg SM, Spira A, Jänne PA, Joshi VA, McCollum D, Evans TL, Muzikansky A, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol. 2008;26:2442–2449. doi: 10.1200/JCO.2007.14.8494. [DOI] [PubMed] [Google Scholar]
- 4.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, Ou SH, Dezube BJ, Jänne PA, Costa DB, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeuchi K, Soda M, Togashi Y, Suzuki R, Sakata S, Hatano S, Asaka R, Hamanaka W, Ninomiya H, Uehara H, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18:378–381. doi: 10.1038/nm.2658. [DOI] [PubMed] [Google Scholar]
- 6.Lipson D, Capelletti M, Yelensky R, Otto G, Parker A, Jarosz M, Curran JA, Balasubramanian S, Bloom T, Brennan KW, et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med. 2012;18:382–384. doi: 10.1038/nm.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang R, Hu H, Pan Y, Li Y, Ye T, Li C, Luo X, Wang L, Li H, Zhang Y, et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J Clin Oncol. 2012;30:4352–4359. doi: 10.1200/JCO.2012.44.1477. [DOI] [PubMed] [Google Scholar]
- 8.Matsubara D, Kanai Y, Ishikawa S, Ohara S, Yoshimoto T, Sakatani T, Oguni S, Tamura T, Kataoka H, Endo S, et al. Identification of CCDC6-RET fusion in the human lung adenocarcinoma cell line, LC-2/ad. J Thorac Oncol. 2012;7:1872–1876. doi: 10.1097/JTO.0b013e3182721ed1. [DOI] [PubMed] [Google Scholar]
- 9.Kohno T, Ichikawa H, Totoki Y, Yasuda K, Hiramoto M, Nammo T, Sakamoto H, Tsuta K, Furuta K, Shimada Y, et al. KIF5B-RET fusions in lung adenocarcinoma. Nat Med. 2012;18:375–377. doi: 10.1038/nm.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eng C. RET proto-oncogene in the development of human cancer. J Clin Oncol. 1999;17:380–393. doi: 10.1200/JCO.1999.17.1.380. [DOI] [PubMed] [Google Scholar]
- 11.Bounacer A, Wicker R, Caillou B, Cailleux AF, Sarasin A, Schlumberger M, Suárez HG. High prevalence of activating ret proto-oncogene rearrangements, in thyroid tumors from patients who had received external radiation. Oncogene. 1997;15:1263–1273. doi: 10.1038/sj.onc.1200206. [DOI] [PubMed] [Google Scholar]
- 12.Chao BH, Briesewitz R, Villalona-Calero MA. RET fusion genes in non-small-cell lung cancer. J Clin Oncol. 2012;30:4439–4441. doi: 10.1200/JCO.2012.45.8240. [DOI] [PubMed] [Google Scholar]
- 13.Pao W, Hutchinson KE. Chipping away at the lung cancer genome. Nat Med. 2012;18:349–351. doi: 10.1038/nm.2697. [DOI] [PubMed] [Google Scholar]
- 14.Lee SY, Kim MJ, Jin G, Yoo SS, Park JY, Choi JE, Jeon HS, Cho S, Lee EB, Cha SI, et al. Somatic mutations in epidermal growth factor receptor signaling pathway genes in non-small cell lung cancers. J Thorac Oncol. 2010;5:1734–1740. doi: 10.1097/JTO.0b013e3181f0beca. [DOI] [PubMed] [Google Scholar]
- 15.Ju YS, Lee WC, Shin JY, Lee S, Bleazard T, Won JK, Kim YT, Kim JI, Kang JH, Seo JS. A transforming KIF5B and RET gene fusion in lung adenocarcinoma revealed from whole-genome and transcriptome sequencing. Genome Res. 2012;22:436–445. doi: 10.1101/gr.133645.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of study population and 3 patients with RET fusion gene