Abstract
Inconsistent findings on the relationship of sex partner concurrency to infection with HIV and other sexually transmitted diseases (STDs) may result from differences in how sex partner concurrency is conceptualized. We examine the relationship of reciprocal sex partner concurrency (RSPC) to diagnosed STDs among heterosexuals. Heterosexually active adults (N = 717) were recruited for a cross-sectional study using respondent-driven sampling (RDS) from high-HIV-risk areas in New York City (NYC, 2006–2007) and interviewed about their sexual risk behaviors, number of sex partners, last sex partners, and STD diagnoses (prior 12 months). RSPC was when both the participant and her/his last sex partner had sex with other people during their sexual relationship. Odds ratios (OR), adjusted odds ratios (aOR), and 95 % confidence intervals (95%CI) were estimated by logistic regression. The sample was 52.4 % female, 74.3 % Black; median age was 40 years. RSPC was reported by 40.7 % and any STD diagnoses by 23.4 %. Any STDs was reported by 31.5 % of those reporting RSPC vs. 17.9 % of those who did not (OR = 2.11, 95%CI = 1.49–3.0). Any STDs was independently associated with RSPC (aOR = 1.54, 95%CI = 1.02–2.32), female gender (aOR = 2.15, 95%CI = 1.43–3.23), having more than three sex partners (aOR = 1.72, 95%CI = 1.13–2.63), and unprotected anal sex (aOR = 1.65, 95%CI = 1.12–2.42). Heterosexuals in high-HIV-risk neighborhoods in sexual partnerships that involve RSPC are at greater risk of STDs and, potentially, HIV. RSPC, in addition to sexual risk behaviors and the number of sex partners, may facilitate the heterosexual spread of HIV through STD cofactors and linkage into larger STD/HIV sexual transmission networks.
Keywords: Heterosexuals, HIV, Sex partner concurrency, STDs, New York City
Introduction
A high proportion of HIV diagnoses in the United States are attributable to heterosexual transmission. In 2008, heterosexual transmission accounted for 32 % of new diagnoses in the 37 states with confidential name-based HIV infection reporting1 and 22 % in New York City (NYC).2 Understanding the risk factors for heterosexual transmission is essential for prevention. However, individual risk behaviors, such as condom use and the number of sex partners, and sexually transmitted disease (STD) cofactors do not by themselves explain the heterosexual spread of HIV.3,4 In addition to these risk factors, the characteristics of sexual networks among heterosexuals,5 specifically the pattern of sex partner concurrency (overlapping sex partnerships in the same time interval), may help to explain the heterosexual spread of HIV and other STDs in sub-Saharan Africa6–8 and in high-risk urban and rural areas in the United States.9–11
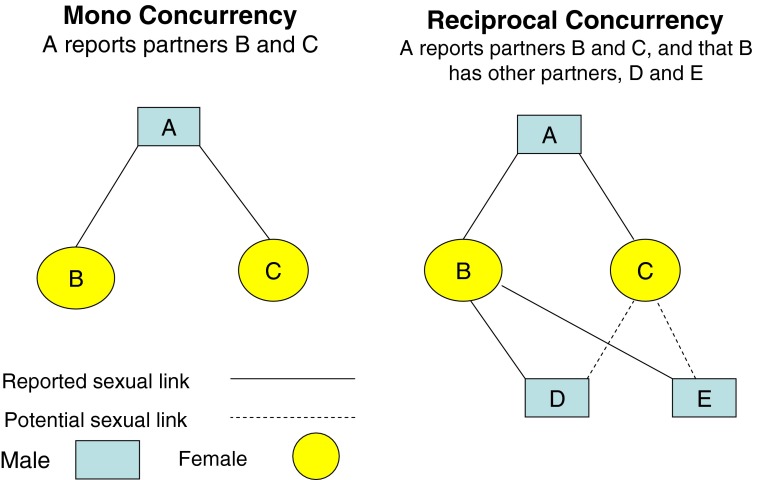
A high prevalence of sex partner concurrency in a population has been hypothesized to increase the number of direct or indirect sexual connections and thereby to influence the extent, rapidity, and persistence of HIV and other STD epidemics.12 However, the theory that sex partner concurrency is a driver of heterosexual HIV epidemics has been criticized for being based on limited evidence.13,14 Here, we propose that inconsistent findings on the relation of sex partner concurrency to HIV and STD epidemics may be a function of how the concept is specified and that sex partner concurrency may refer to different patterns of overlapping sex partnerships. In particular, we make a distinction between mono concurrency and reciprocal concurrency. This is described below and in Figure 1 for heterosexual partners.
Figure 1.
Mono and reciprocal concurrency in heterosexual partnerships.
Mono concurrency occurs when an index individual has two or more partners during the same time interval and these partners do not have other partners. In Figure 1, under mono concurrency, index A, a male, has two female partners, B and C, who do not have other partners. Reciprocal concurrency occurs when the index and one or more concurrent sex partners have other sex partners during the same time interval. In Figure 1, under reciprocal concurrency, B, a female partner of A, reports male partners D and E. In mono concurrency, if index A is uninfected, the risk of A becoming infected would arise if B or C was infected and A engaged in unprotected sex with B or C. In reciprocal concurrency, the risk to A is increased because B and/or C are linked to other partners and may be part of a larger sexual transmission network with more paths through which infectious pathogens can flow.
Reciprocal concurrency may be common among those with concurrent partners. In population-based studies of men and women in the United States, having concurrent sex partners was independently associated with having sex partners who were themselves nonmonogamous.15,16 Studies on network factors associated with the risk of infection with HIV and other STDs have shown that risk networks comprising direct and indirect ties among members (termed “sociocentric”6 or “sociometric”17 networks) influence the risk of infection, e.g., among injection drug users (IDU), men who have sex with men (MSM), sex workers, and at-risk rural and urban populations in sub-Saharan Africa.6,17–22
It is often difficult and not feasible to sample, recruit, and measure sociocentric networks.23 Another approach is to use egocentric network data (the direct relationships of an index individual [ego] with other individuals, e.g., sexual partners), whether directly measured or through self-report, to develop proxy measures of sociocentric network characteristics.24,25 In the following, we use self-reported egocentric data on recent sexual partnerships from a risk behavior survey of heterosexuals at high risk of HIV in NYC to examine the prevalence and correlates of reciprocal sex partner concurrency (RSPC) and its relationship to STDs. Although the relationship between STD infection and the sexual transmission of HIV is complex, STDs can be both biological cofactors and markers of greater sexual risk for HIV infection in vulnerable populations.26–29
Methods
Sampling, Eligibility, Data Collection
The data for this analysis are from the National HIV Behavioral Surveillance (NHBS) study in NYC. NHBS is a cross-sectional study conducted by the Centers for Disease Control and Prevention (CDC) and local partners on HIV prevalence and risk among MSM, IDU, and high-risk heterosexuals.30 The data are from the high-risk heterosexual cycle conducted in 2006–2007.
The NHBS methods for defining high-risk heterosexuals are explained in detail elsewhere.31 Using NYC HIV surveillance data and the United States Census data on household poverty, NYC zip codes were ranked by combined standardized rates of heterosexual HIV and poverty. The top 30 zip codes, which were clustered in Central Brooklyn, Harlem, and the South Bronx, were considered “high-risk areas” (HRAs).
Study participants had to reside in or have a social connection to an HRA. Participants were considered to have a social connection if they were recruited into the study by a previous participant who resided in an HRA. The sample was accrued using respondent-driven sampling (RDS), which is based on peer recruitment.32,33 Ethnographers selected initial recruits (n = 8), called seeds, through the assistance of key informants and community outreach. Seeds met eligibility criteria for the study, were socially gregarious within the local community, and had large social networks. Once the seeds completed the study, they were asked to recruit up to three peers (friends, relatives, or people they were close to, who were between 18 and 50 years of age, who lived in the project area, and who they had seen in the past 30 days). Peers who participated in the study were asked to recruit their peers and so on, until the target sample size was met. Participants who lived outside an HRA were not allowed to recruit others, in order to maintain the connection to HRAs.
Other eligibility criteria included opposite sex vaginal/anal sex in the past year, age between 18 and 50 years, NYC residence, and English/Spanish comprehension. Eligible participants were paid $20 for completing the questionnaire, $10 for taking the HIV test, and $10 for each eligible participant (up to three) they recruited.
After giving their informed consent, participants were administered a computer-assisted structured interview in private by trained interviewers. Interviews were conducted in English or Spanish in study offices located in HRAs in Central Brooklyn and Central Harlem. After the interview was completed, blood specimens were obtained through venipuncture by a trained phlebotomist from participants who consented to be tested. The blood specimens were tested for HIV-1 at the New York City Public Health Laboratory. All procedures involving human subjects were reviewed and approved by the Institutional Review Boards of the New York City Department of Health and Mental Hygiene, the National Development and Research Institutes, and the CDC.
Geographic Coverage and Demographic Characteristics of Seeds
The eight seeds resided in two HRA clusters: one was in Central Brooklyn (three seeds who resided in the same zip code) and the other in Central Harlem (five seeds who resided in the same zip code). In Central Harlem, the seeds included three men and two women who self-identified as being of Black race/ethnicity and ranged in age from 26 to 44 years. In Central Brooklyn, the three seeds were female, self-identified as Black, and ranged in age from 24 to 49 years. From these eight seeds, the study recruited participants who resided in all five boroughs (50.2 % in Brooklyn, 31.1 % in Manhattan, 17.2 % in the Bronx, 1.3 % in Queens, and 0.3 % in Staten Island) and in 74 zip codes.
Measures
In the interview, participants were asked about their sociodemographic characteristics, injection drug use history, noninjection drug use in the past 12 months, alcohol use in the past 30 days, sexual risk behaviors and number of sex partners in the past 12 months, the characteristics of their last sex partner in the past 12 months, e.g., whether the partner was HIV-positive, and recent (past 12 months) diagnoses with STDs.
The variable “any STD diagnoses” was based on the participant's self-report of being diagnosed by a doctor, nurse, or other health care provider with either syphilis, gonorrhea, chlamydia, herpes (HSV), genital warts (HPV), or other STDs. RSPC was measured for the last opposite gender sex partner during the previous 12 months who was not an exchange partner (an exchange partner was defined in the questionnaire as someone with whom the participant had sex in exchange for things like money or drugs). Participants were asked if they had sex with other people during their sexual relationship with this last sex partner. Participants were also asked if this last sex partner had sex with other people during their sexual relationship. The responses of “definitely did” and “probably did” were recoded as “yes”, and the responses “definitely did not” and “probably did not” were recoded as “no”. RSPC was defined as occurring when a participant reported that both the participant and the last sex partner had sex with other people during their sexual relationship in the past 12 months (coded as “yes/no”).
Other variables in the analysis included sociodemographic characteristics (gender, race/ethnicity, age, sexual identity, less than high school graduation or less than a General Educational Diploma [GED], marital status, and in the past 12 months, an income of less than $10,000, being homeless, and being arrested), ever (lifetime) injecting drugs, weekly use of noninjection drugs (crack, cocaine, heroin, and marijuana), binge alcohol use (for men, five or more drinks in one “sitting”; for women, four or more drinks in one “sitting”) in the last 30 days, sexual risk in the past 12 months (unprotected vaginal sex, unprotected anal sex, and more than three [the median] sex partners), characteristics of the last sex partner (five or more years older, ever injected drugs, ever used crack, ever incarcerated, HIV-infected or HIV status unknown, and duration of partnership was more than 2 years [the median]), the participant's HIV test result from being tested at the interview, and the size of the participant's personal network.31 Personal network size was determined by asking participants how many of their peers, as defined above, they had seen in the last 30 days.
Participants Analyzed
The eight seeds generated 1,015 recruits, of whom 850 were eligible. Of the 850 who were eligible, 133 were excluded from the analysis, including, in hierarchical order of elimination, 105 with last sex partners who were exchange partners, two with missing data for the questions on concurrency in the last sex partnership, four who reported that they were HIV-positive, and 22 men who had sex with men in the last 12 months. Those who self-reported as being HIV-positive were excluded because awareness of HIV status can influence risk behavior.34 The analysis was conducted on 717 participants.
Statistical Analysis
Bivariate analysis was conducted to test (Pearson's Chi-square) for variables that were significantly associated with RSPC. The association of any STD diagnoses with RSPC and the significant correlates of RSPC was determined using bivariate and multivariate logistic regression to estimate crude and adjusted odds ratios (OR and aOR, respectively) and 95 % confidence intervals (95%CI). Race/ethnicity, age, gender, the number of sex partners, the duration of the last sex partnership, and the reciprocal of the participant's reported personal social network size (to control for the probability of being selected for the sample) were forced into the multivariate model as control variables or covariates. A confirmatory multivariate analysis was conducted that included RSPC (the exposure variable), the variables forced into the original multivariate model, and variables (as listed in Table 1) that, when added separately to a model with only the exposure variable, changed the coefficient of the exposure variable by more than 10 %.35
Table 1.
Characteristics associated with reciprocal sex partner concurrency
| Characteristics | Total | RSPC | ||
|---|---|---|---|---|
| N | c% | r% | p | |
| Total | 717 | 100.0 | 40.7 | |
| Gender | 0.13 | |||
| Male | 341 | 47.6 | 37.8 | |
| Female | 376 | 52.4 | 43.4 | |
| Race/ethnicity | 0.81 | |||
| Black | 533 | 74.3 | 40.3 | |
| Hispanic | 130 | 18.1 | 43.1 | |
| White | 38 | 5.3 | 42.1 | |
| Other | 16 | 2.2 | 31.3 | |
| Age | 0.15 | |||
| 18–29 | 237 | 33.1 | 42.2 | |
| 30–39 | 116 | 16.2 | 47.4 | |
| 40–50 | 364 | 50.8 | 37.6 | |
| Other sociodemographics | ||||
| Nonheterosexual identity | 104 | 14.5 | 61.5 | <0.01 |
| Less than high school graduation/GED | 305 | 42.5 | 44.3 | <0.10 |
| Not married or not living as married | 635 | 88.6 | 41.9 | 0.08 |
| Income <10,000 (PY) | 476 | 66.4 | 43.3 | <0.06 |
| Homeless (PY) | 325 | 45.3 | 43.7 | <0.15 |
| Arrested (PY) | 198 | 27.6 | 41.4 | 0.82 |
| Substance use | ||||
| Drug injection (lifetime) | 138 | 19.3 | 44.9 | 0.27 |
| Weekly crack use (PY) | 140 | 19.5 | 50.0 | 0.01 |
| Weekly cocaine use (PY) | 66 | 9.2 | 57.6 | <0.01 |
| Weekly heroin use (PY) | 66 | 9.2 | 50.0 | 0.11 |
| Weekly marijuana use (PY) | 250 | 34.9 | 49.6 | <0.01 |
| Binge alcohol use (PM) | 301 | 42.0 | 49.8 | <0.01 |
| Past year sexual risks | ||||
| Unprotected vaginal sex | 658 | 91.8 | 42.9 | <0.01 |
| Unprotected anal sex | 240 | 33.5 | 53.3 | <0.01 |
| >3 total sex partners | 325 | 45.3 | 63.1 | <0.01 |
| Last sex partner characteristics | ||||
| ≥5 years older | 174 | 24.3 | 43.7 | 0.37 |
| IDU | 81 | 11.3 | 46.9 | 0.23 |
| Used crack | 242 | 33.8 | 45.9 | <0.05 |
| Ever incarcerated | 355 | 49.5 | 43.7 | <0.12 |
| HIV-positive or status unknown | 361 | 50.4 | 52.1 | <0.01 |
| Partnership >2 years | 343 | 47.8 | 37.0 | 0.06 |
| HIV test result | 0.78 | |||
| Negative | 644 | 92.5 | 41.0 | |
| Positive | 52 | 7.5 | 40.4 | |
| Not tested | 21 | 2.9 | 33.3 | |
c% column percent, r% row percent, PY past year (12 months), PM past month (30 days)
Multicollinearity between the explanatory variables included in the multivariate model was assessed.36 The diagnostic measures of collinearity utilized included Tolerance (TOL), the Variance Inflation Factor (VIF), and the weighted TOL and VIF.
Although RDS data are subject to recruitment biases related to network size and homophily (preferential in-group recruitment),33,37 we conducted unweighted analyses because RSPC is a network variable that is structured by network size and homophily.31 To assess whether a weighted analysis would influence the estimated effect of RSPC, we conducted a sensitivity analysis for the multivariate model using RDS weights for any STD diagnoses.38,39 Analyses were conducted using SAS© v9.1 (SAS Institute, Cary, NC, USA).
Results
Characteristics of the analysis sample (N = 717) are shown in Table 1 (c% represents the column percentages based on the total sample size denominator). The analysis sample was 52.4 % female and 47.6 % male. Almost three-quarters self-identified as being of Black race/ethnicity and close to a fifth as Hispanic, with few identifying as White or other race/ethnicity. The mean age was 36.0 years (standard deviation = 10.4 years), with a median age of 40 years (range 18 to 50 years). A minority (14.5 %) reported a nonheterosexual identity (0.8 % homosexual, gay or lesbian, and 13.7 % bisexual). Over 40 % did not graduate high school or receive a GED. A majority (88.6 %) were unmarried or not living as married. Two-thirds reported an annual household income of less than $10,000. In the past year, many (45.3 %) had ever been homeless and just over a quarter had been arrested. Almost a fifth had ever (lifetime) injected drugs. Noninjection drug use and binge alcohol use were common. Over 90 % engaged in unprotected vaginal sex, a third in unprotected anal sex, and 45.3 % had more than three sex partners. Many reported last sex partners who were potentially high risk, e.g., 50.4 % had a last sex partner with a positive or unknown HIV status. HIV infection prevalence (7.5 % of 696 tested at the interview) was high for this population. The median self-reported personal social network size was ten (range 0 to 1,000).
Variables associated with RSPC are shown in Table 1 (r% represents row percentages based on row sample size denominators). RSPC was reported by 40.7 % of participants, with 36.3 % reporting that one partner had other partners (25.9 % only participants and 10.4 % only sex partners), and 23.0 % that neither partner had other partners. In the bivariate analysis, variables significantly associated with RSPC included nonheterosexual identity (p < 0.01), weekly noninjection use of crack (p = 0.01), cocaine (p < 0.01), and marijuana (p < 0.01), binge alcohol use (p < 0.01), unprotected vaginal sex (p < 0.01), unprotected anal sex (p < 0.01), having more than three sexual partners (p < 0.01), having a last sex partner who used crack (p < 0.05), and a last sex partner whose HIV status was reported as positive or unknown (p < 0.01).
Overall, almost one in four (23.4 %) reported that they had been diagnosed with any STDs in the prior 12 months (Table 2). This included 4.3 % reporting syphilis, 8.1 % gonorrhea, 9.4 % chlamydia, 2.1 % herpes, 1.7 % genital warts, and 3.6 % other STDs (not mutually exclusive). In the bivariate analysis, those who reported RSPC compared with those who did not were twice as likely to report being diagnosed with any STDs (31.5 % vs. 17.9 %, OR = 2.11, 95%CI = 1.49–3.00, p < 0.01). In additional contrast analyses (data not shown in Table 2), those who reported RSPC were significantly more likely to report any STD diagnoses than were those who reported that neither the participant nor the last sex partner had other sex partners (17.6 %, OR = 2.16, 95%CI = 1.35–3.45, p < 0.002), only the participant had other sex partners (19.9 %, OR = 1.85, 95%CI = 1.20–2.87, p < 0.01), and only the last sex partner had other sex partners (13.5 %, OR = 2.94, 95%CI = 1.45–6.00, p < 0.003). Other significant variables included female gender, unprotected anal sex, and reporting more than three sex partners in the past year.
Table 2.
Bivariate and multiple logistic regression of STD diagnoses
| Characteristic | Total | STD | Crude | Adjusted | |||
|---|---|---|---|---|---|---|---|
| N | n | r% | OR | 95 % CI | OR | 95 % CI | |
| Total | 717 | 168 | 23.4 | – | – | – | – |
| Gender | |||||||
| Male | 341 | 59 | 17.3 | 1.00 | 1.00 | ||
| Female | 376 | 109 | 29.0 | 1.95 | 1.36–2.79 | 2.15 | 1.43–3.23 |
| Race/ethnicity | |||||||
| Black | 533 | 127 | 23.8 | 1.01 | 0.47–2.19 | 1.34 | 0.59–3.07 |
| Hispanic | 130 | 28 | 21.5 | 0.89 | 0.38–2.08 | 1.17 | 0.47–2.93 |
| White | 38 | 9 | 23.7 | 1.00 | 1.00 | ||
| Other | 16 | 4 | 25.0 | 1.07 | 0.28–4.17 | 1.57 | 0.37–6.60 |
| Age | |||||||
| 18–29 | 237 | 58 | 24.5 | 1.00 | 1.00 | ||
| 30–39 | 116 | 28 | 24.1 | 0.98 | 0.56–1.65 | 0.83 | 0.46–1.47 |
| 40–50 | 364 | 82 | 22.5 | 0.90 | 0.61–1.32 | 0.92 | 0.57–1.49 |
| Nonheterosexual identity | 104 | 31 | 29.8 | 1.48 | 0.93–2.34 | 0.84 | 0.49–1.45 |
| Substance use | |||||||
| Weekly crack use (PY) | 140 | 39 | 27.9 | 1.34 | 0.88–2.04 | 0.96 | 0.57–1.59 |
| Weekly cocaine use (PY) | 66 | 15 | 22.7 | 0.96 | 0.52–1.75 | 0.74 | 0.38–1.43 |
| Weekly marijuana use (PY) | 250 | 65 | 26.0 | 1.24 | 0.87–1.78 | 1.15 | 0.77–1.71 |
| Binge alcohol use (PM) | 301 | 74 | 24.6 | 1.12 | 0.79–1.58 | 0.93 | 0.63–1.37 |
| Past year sexual risks | |||||||
| Unprotected vaginal sex | 658 | 160 | 24.3 | 2.05 | 0.95–4.41 | 1.31 | 0.58–2.93 |
| Unprotected anal sex | 240 | 76 | 31.7 | 1.94 | 1.36–2.77 | 1.65 | 1.12–2.42 |
| >3 total sex partners | 325 | 99 | 30.5 | 2.05 | 1.44–2.91 | 1.72 | 1.13–2.63 |
| Last sex partner characteristics | |||||||
| Used crack | 242 | 66 | 27.3 | 1.37 | 0.96–1.96 | 1.30 | 0.83–2.03 |
| HIV-positive or status unknown | 361 | 93 | 25.8 | 1.30 | 0.92–1.84 | 1.07 | 0.73–1.56 |
| Partnership >2 years | 343 | 82 | 23.9 | 1.05 | 0.75–1.49 | 1.15 | 0.79–1.67 |
| Reciprocal sex partner concurrency | |||||||
| No | 425 | 76 | 17.9 | 1.00 | |||
| Yes | 292 | 92 | 31.5 | 2.11 | 1.49–3.00 | 1.54 | 1.02–2.32 |
r%, row percent, PY past year (12 months), PM past month (30 days)
Having a last sex partner who was an exchange sex partner (N = 93) may be a proxy variable for RSPC. “Any STD diagnoses” was reported by 29.0 % of those reporting exchange partners (data not shown in Table 2).
In the multivariate analysis, RSPC (yes vs. no) was independently associated with any STD diagnoses after accounting for the effects of sexual, drug use, and other risk variables (aOR = 1.54, 95%CI = 1.02–2.32, p < 0.04). Other significant variables included female gender (aOR = 2.15, 95%CI = 1.43–3.23, p < 0.01), engaging in unprotected anal sex (aOR = 1.65, 95%CI = 1.12–2.42, p < 0.02), and having more than three sex partners in the past year (aOR = 1.72, 95%CI = 1.13–2.63, p < 0.02). Personal social network size was not significant (aOR = 1.92, 95%CI = 0.74–5.00, p < 0.18). To account for high outliers in personal social network size, the natural log of personal social network size was included as a control variable. The final model was essentially unchanged when this variable was included.
In the confirmatory analysis, only having more than three sex partners in the past year changed the coefficient of RSPC by more than 10 %. The effect of RSPC was similar in this reduced multivariate model (aOR = 1.65, 95%CI = 1.12–2.44, p < 0.02).
None of the measures for assessing multicollinearity indicated that the explanatory variables in the multivariate model were collinear. The lowest TOL was 0.70 and the highest VIF, 1.43, which was also confirmed by the weighted assessment.
With the exception of personal social network size, all of the variables included in the original unweighted multivariate analysis were included in the RDS weighted multivariate analysis. The effect of RSPC on any STD diagnoses was similar in the weighted analysis, although statistical significance became marginal (aOR = 1.85, 95%CI = 0.98–3.49, p < 0.06).
Discussion
Among heterosexuals who lived in or were socially connected to high HIV-risk areas in NYC, the prevalence of RSPC was high and almost a quarter had been diagnosed with STDs in the past year. Any STD diagnoses was associated with RSPC but not with mono concurrency (for either the participant or the participant's last sex partner). Those who reported RSPC during their last sexual relationship were twice as likely to report being diagnosed with STDs as those who did not. When we accounted for potential confounders, RSPC remained independently associated with any STD diagnoses.
Although sex partner concurrency is a plausible theory to explain the extent, rapidity, and persistence of heterosexual STD and HIV epidemics, questions remain over whether it is empirically supported. A possible source for inconsistent findings is that sex partner concurrency is often defined and operationalized as an indicator of individual risk rather than of network or population risk.40 In our analysis, sex partner concurrency was specified to apply not only to participants but also to participants' sex partners. Inconsistent findings on the relationship of sex partner concurrency to infection with HIV and other STDs may result from undifferentiated measures of sex partner concurrency, since some with concurrent sex partners may not have sex partners who have other sex partners, who may be more likely to be transmitters of HIV and other STDs. In studies of STD transmission in the United States, Koumans et al.41 and Potterat et al.42 found that those with concurrent partners were more likely to be STD transmitters. Among STD clinic attendees in St. Petersburg, Russia partner concurrency but not individual concurrency was significantly associated with diagnosed STDs.43 Moreover, Adimora and et al.,10 in a study of heterosexually transmitted HIV among African Americans in North Carolina, found that the risk for infection with HIV among those who denied high-risk behaviors or partners was independently associated with having a sex partner who was not monogamous during the relationship with the participant. However, these studies did not specifically analyze the combined effect of both individual and partner concurrency (RSPC). In our study, we found that RSPC was associated with a greater risk of having diagnosed STDs not only compared with neither the participant nor the partner having other sex partners but also with only participant concurrency and only partner concurrency.
Other variables independently associated with any STD diagnoses included unprotected anal sex and female gender. In a previous analysis of these data, we found that unprotected anal sex was an independent predictor of any STD diagnoses among women.38 The association of unprotected anal sex with STD diagnoses persisted in this larger sample of men and women. There is increasing evidence that the prevalence of anal sex is considerable in some high-risk heterosexual populations44–46 and may place women engaging in this sexual practice at high-risk for infection with HIV and other STDs.38,47 The higher risk of STD diagnoses among women is consistent with other research which finds that women are more susceptible to becoming infected with some STDs.48,49 Women may also be more likely to be screened for STDs, which may increase their likelihood of being diagnosed.49
RSPC, independent of the number of sex partners and individual sexual risk behaviors, may generate heterosexual HIV epidemics in HRAs at both the individual and population levels by increasing the individual risk of acquiring or transmitting STD cofactors and HIV and, at the population level, by increasing the prevalence of STDs and HIV and their spread through larger and longer sexual transmission networks. Interventions that target high-risk urban areas, such as those in NYC, could help to reduce the incidence of HIV and other STDs by promoting the reduction of sex partner concurrency through direct messages emphasizing “one partner at one time”12 as well as by addressing individual risk factors, such as stimulant drug use and binge alcohol use, or structural risk factors, such as high rates of unemployment and incarceration,50 which may generate sex partner concurrency. Such interventions could create a multiplier effect in which the reduction of RSPC could add value at the population level to existing individual-level interventions and increase efficiency in the use of scarce prevention resources.
Limitations
Some participants may have been misclassified if they did not report RSPC at the last sex partnership but RSPC occurred in prior sex partnerships in the last 12 months. In addition, the identification of whether the last sex partner had other sex partners is based on self-report, which may be inaccurate if the last sex partner did not disclose or the participant did not suspect other sex partnerships.51 Since the measure of sex partner concurrency was a direct measure and not based on the specific timing of partnerships,52 some participants may have misreported closely spaced sequential partnerships as concurrent partnerships. Those with asymptomatic STDs, particularly gonorrhea and chlamydia, would have been less likely to report being diagnosed with STDs. Even with these sources of potential misclassification and conservative bias, RSPC was found to be associated with being diagnosed with STDs. The summary measure of diagnosed STDs used in the analysis may not reflect heterogeneity in how different STDs are distributed in sexual networks and geographically,53–55 although focusing RDS in HRAs may have increased the overlap between the sexual networks and geographic concentration of different STDs.56 The inference of a causal relationship between RSPC and STDs is limited by the cross-sectional design of the study and differences in the time frames used to measure RSPC and STD diagnoses. The sample was not randomly selected and caution is thereby necessary in generalizing the results to heterosexuals living in or socially connected to the HRAs in the sample. Additionally, because the HIV epidemic in the United States overall is concentrated while in some African countries it is generalized, caution is also necessary in transferring inferences between the two contexts. However, HIV prevalence in NYC HRAs and in similar urban areas in the United States may be generalized within these areas and is comparable to that found in some generalized heterosexual epidemics in Africa.9,57
Conclusions
In order to determine the effect of sex partner concurrency on the risk of acquiring or transmitting heterosexually transmitted HIV and other STDs, greater theoretical specificity is needed in measuring sex partner concurrency so that it reflects the dynamics of underlying sexual transmission networks. One such measure, RSPC, can be developed using egocentric sexual network data reported by participants in standard surveys, such as the NHBS survey.
Heterosexuals in high-HIV-risk neighborhoods in sexual partnerships that involve RSPC are at greater risk of STDs and, potentially, HIV. RSPC, in addition to sexual risk behaviors and the number of sex partners, may facilitate the heterosexual spread of HIV through STD cofactors and linkage into larger STD/HIV sexual transmission networks.
Acknowledgments
The authors would like to acknowledge James Hadler, MD; Colin Shepard, MD; and Monica Sweeney, MD of the New York City Department of Health and Mental Hygiene for their critical reading of earlier drafts of the paper. We would like to thank Elizabeth DiNenno, Amy Drake, Amy Lansky, and Isa Miles of the CDC for contributing to the NHBS study design locally and nationally, and the New York City NHBS field staff for their efforts in data collection as well as the study participants who consented to be in the study.
This work was funded by a cooperative agreement between the New York City Department of Health and Mental Hygiene and the Centers for Disease Control and Prevention (Grant #U62/CCU223595-03-1).
References
- 1.HIV/AIDS surveillance report, 2008. 20. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2010. [Google Scholar]
- 2.New York City Department of Health and Mental Hygiene. New York City HIV/AIDS Annual Surveillance Statistics 2008. 2009.
- 3.Hallfors DD, Iritani BJ, Miller WC, Bauer DJ. Sexual and drug behavior patterns and HIV and STD racial disparities: the need for new directions. Am J Public Health 2007;97(1). [DOI] [PMC free article] [PubMed]
- 4.Grosskurth H, Gray R, Hayes R, Mabey D, Wawer M. Control of sexually transmitted diseases for HIV-1 prevention: understanding the implications of the Mwanza and Rakai trials. Lancet. 2000;355(9219):1981–7. doi: 10.1016/S0140-6736(00)02336-9. [DOI] [PubMed] [Google Scholar]
- 5.Rothenberg R. HIV transmission networks. Curr Opin HIV AIDS. 2009;4(4). [DOI] [PMC free article] [PubMed]
- 6.Helleringer S, Kohler HP. Sexual network structure and the spread of HIV in Africa: evidence from Likoma Island, Malawi. AIDS. 2007;21(17). [DOI] [PubMed]
- 7.Helleringer S, Kohler HP, Kalilani-Phiri L. The association of HIV serodiscordance and partnership concurrency in Likoma Island (Malawi). AIDS. 2009;23(10). [DOI] [PMC free article] [PubMed]
- 8.Mah TL, Halperin DT. Concurrent sexual partnerships and the HIV epidemics in Africa: evidence to move forward. AIDS Behav. 2010;14(1):11–6. doi: 10.1007/s10461-008-9433-x. [DOI] [PubMed] [Google Scholar]
- 9.Magnus M, Kuo I, Shelley K et al. Risk factors driving the emergence of a generalized heterosexual HIV epidemic in Washington, District of Columbia networks at risk. AIDS. 2009;23(10). [DOI] [PubMed]
- 10.Adimora AA, Schoenbach VJ, Martinson FE, et al. Heterosexually transmitted HIV infection among African Americans in North Carolina. J Acquir Immune Defic Syndr. 2006;41(5):616–23. doi: 10.1097/01.qai.0000191382.62070.a5. [DOI] [PubMed] [Google Scholar]
- 11.Adimora AA, Schoenbach VJ, Martinson FE, Donaldson KH, Stancil TR, Fullilove RE. Concurrent partnerships among rural African Americans with recently reported heterosexually transmitted HIV infection. J Acquir Immune Defic Syndr. 2003;34(4):423–9. doi: 10.1097/00126334-200312010-00010. [DOI] [PubMed] [Google Scholar]
- 12.Morris M, Kretzschmar M. Concurrent partnerships and the spread of HIV. AIDS. 1997;11(5):641–8. doi: 10.1097/00002030-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Lurie MN, Rosenthal S. Concurrent partnerships as a driver of the HIV epidemic in sub-Saharan Africa? The evidence is limited. AIDS Behav. 2010;14(1):17–24. doi: 10.1007/s10461-009-9583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lurie MN, Rosenthal S. The concurrency hypothesis in sub-Saharan Africa: convincing empirical evidence is still lacking. Response to Mah and Halperin, Epstein, and Morris. AIDS Behav. 2010;14(1):34. doi: 10.1007/s10461-009-9640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adimora AA, Schoenbach VJ, Doherty IA. Concurrent sexual partnerships among men in the United States. Am J Public Health. 2007;97(12):2230–7. doi: 10.2105/AJPH.2006.099069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adimora AA, Schoenbach VJ, Taylor EM, Khan MR, Schwartz RJ. Concurrent partnerships, nonmonogamous partners, and substance use among women in the United States. Am J Public Health. 2011;101(1):128–36. doi: 10.2105/AJPH.2009.174292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman SR, Neaigus A, Jose B, et al. Sociometric risk networks and risk for HIV infection. Am J Public Health. 1997;87(8):1289–96. doi: 10.2105/AJPH.87.8.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klovdahl AS. Social networks and the spread of infectious diseases: the AIDS example. Soc Sci Med. 1985;21(11):1203–16. doi: 10.1016/0277-9536(85)90269-2. [DOI] [PubMed] [Google Scholar]
- 19.Rothenberg RB, Woodhouse DE, Potterat JJ, Muth SQ, Darrow WW, Klovdahl AS. Social networks in disease transmission: the Colorado Springs Study. NIDA Res Monogr. 1995;151:3–19. [PubMed] [Google Scholar]
- 20.Haraldsdottir S, Gupta S, Anderson RM. Preliminary studies of sexual networks in a male homosexual community in Iceland. J Acquir Immune Defic Syndr. 1992;5(4):374–81. [PubMed] [Google Scholar]
- 21.Friedman SR, Kottiri BJ, Neaigus A, Curtis R, Vermund SH, Des Jarlais DC. Network-related mechanisms may help explain long-term HIV-1-seroprevalence levels that remain high but do not approach population-group saturation. Am J Epidemiol. 2000;152(10):913–22. doi: 10.1093/aje/152.10.913. [DOI] [PubMed] [Google Scholar]
- 22.Epstein H. The mathematics of concurrent partnerships and HIV: a commentary on Lurie and Rosenthal, 2009. AIDS Behav. 2010;14(1):29–30. doi: 10.1007/s10461-009-9627-x. [DOI] [PubMed] [Google Scholar]
- 23.Ghani AC, Donnelly CA, Garnett GP. Sampling biases and missing data in explorations of sexual partner networks for the spread of sexually transmitted diseases. Stat Med. 1998;17(18). [DOI] [PubMed]
- 24.Morris M. Epidemiology and social networks. Sociol Methods Res. 1993;22(99-126).
- 25.Morris M. Overview of network survey designs. In: Morris M, editor. Network epidemiology: a handbook for survey data design and data collection. New York: Oxford; 2004. pp. 8–21. [Google Scholar]
- 26.Wasserheit JN. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis. 1992;19(2):61–77. [PubMed] [Google Scholar]
- 27.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75(1):3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rothenberg RB, Wasserheit JN, St. Louis ME, Douglas JM. The effect of treating sexually transmitted diseases on the transmission of HIV in dually infected persons: a clinic-based estimate. Ad Hoc STD/HIV Transmission Group. Sex Transm Dis. 2000;27(7). [DOI] [PubMed]
- 29.Hagan H, Jenness SM, Wendel T, Murrill CR, Neaigus A, Gelpi-Acosta C. Herpes simplex virus type 2 associated with HIV infection among New York heterosexuals living in high-risk areas. Int J STD AIDS. 2010;21(8):580–3. doi: 10.1258/ijsa.2010.010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lansky A, Sullivan PS, Gallagher KM, Fleming PL. HIV behavioral surveillance in the U.S.: a conceptual framework. Public Health Rep. 2007;122 Suppl 1. [DOI] [PMC free article] [PubMed]
- 31.Jenness SM, Neaigus A, Hagan H, Murrill CS, Wendel T. Heterosexual HIV and sexual partnerships between injection drug users and noninjection drug users. AIDS Patient Care STDS. 2010;24(3):175–81. doi: 10.1089/apc.2009.0227. [DOI] [PubMed] [Google Scholar]
- 32.Heckathorn DD. Respondent-driven sampling: a new approach to the study of hidden populations. Soc Probl. 1997;44(2):174–99. doi: 10.2307/3096941. [DOI] [Google Scholar]
- 33.Heckathorn DD. Respondent-driven sampling II: deriving valid population estimates from chain-referral samples of hidden populations. Soc Probl. 2002;49(1):11–34. doi: 10.1525/sp.2002.49.1.11. [DOI] [Google Scholar]
- 34.Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005;39(4). [DOI] [PubMed]
- 35.In: Hosmer DW, Lemeshow S, eds. Applied logistic regression. New York: Wiley; 2000. p. 116
- 36.Allison PD. Logistic regression using the SAS system: theory and application. Cary, NC, USA: SAS Institute Inc.; 1999. [Google Scholar]
- 37.Heckathorn D. Extensions of respondent-driven sampling: analyzing continuous variables and controlling for differential recruitment. Sociol Methodol. 2007;37(1):151–207. doi: 10.1111/j.1467-9531.2007.00188.x. [DOI] [Google Scholar]
- 38.Jenness SM, Begier EM, Neaigus A, Murrill CS, Wendel T, Hagan H. Unprotected anal intercourse and sexually transmitted diseases in high-risk heterosexual women. Am J Public Health. 2010 June 17. [DOI] [PMC free article] [PubMed]
- 39.Johnston L, O'Bra H, Chopra M, et al. The associations of voluntary counseling and testing acceptance and the perceived likelihood of being HIV-infected among men with multiple sex partners in a South African township. AIDS Behav. 2010;14(4):922–31. doi: 10.1007/s10461-008-9362-8. [DOI] [PubMed] [Google Scholar]
- 40.Morris M. Barking up the wrong evidence tree. Comment on Lurie & Rosenthal, “Concurrent partnerships as a driver of the HIV epidemic in sub-Saharan Africa? The evidence is limited”. AIDS Behav. 2010;14(1):31–3. doi: 10.1007/s10461-009-9639-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koumans EH, Farley TA, Gibson JJ et al. Characteristics of persons with syphilis in areas of persisting syphilis in the United States: sustained transmission associated with concurrent partnerships. Sex Transm Dis. 2001;28(9). [DOI] [PubMed]
- 42.Potterat JJ, Zimmerman-Rogers H, Muth SQ et al. Chlamydia transmission: concurrency, reproduction number, and the epidemic trajectory. Am J Epidemiol. 1999;150(12). [DOI] [PubMed]
- 43.Zhan W, Krasnoselskikh TV, Niccolai LM, Golovanov S, Kozlov AP, Abdala N. Concurrent sexual partnerships and sexually transmitted diseases in Russia. Sex Transm Dis. 2011 January 20. [DOI] [PMC free article] [PubMed]
- 44.Friedman SR, Flom PL, Kottiri BJ, et al. Prevalence and correlates of anal sex with men among young adult women in an inner city minority neighborhood. AIDS. 2001;15(15):2057–60. doi: 10.1097/00002030-200110190-00025. [DOI] [PubMed] [Google Scholar]
- 45.Risser JM, Padgett P, Wolverton M, Risser WL. Relationship between heterosexual anal sex, injection drug use and HIV infection among black men and women. Int J STD AIDS. 2009;20(5). [DOI] [PubMed]
- 46.Neaigus A, Gyarmathy VA, Zhao M, Miller M, Friedman SR, Des J. Sexual and other noninjection risks for HBV and HCV seroconversions among noninjecting heroin users. J Infect Dis. 2007;195(7):1052–61. doi: 10.1086/512081. [DOI] [PubMed] [Google Scholar]
- 47.Cohen MS, Miller WC. Sexually transmitted diseases and human immunodeficiency virus infection: cause, effect, or both? Int J Infect Dis. 1998;3(1). [DOI] [PubMed]
- 48.Buvé A, Gourbin C, Laga M. Gender and sexually transmitted diseases. In: KK Holmes et al., editor. Sexually transmitted diseases. 4th Edition ed. New York: McGraw-Hill; 2008. p. 151-64.
- 49.Sexually transmitted disease surveillance, 2009. Atlanta, GA: U.S. Department of Health and Human Services; 2010. [Google Scholar]
- 50.Adimora AA, Schoenbach VJ. Social context, sexual networks, and racial disparities in rates of sexually transmitted infections. J Infect Dis. 2005;191(Suppl 1):S115–S122. doi: 10.1086/425280. [DOI] [PubMed] [Google Scholar]
- 51.Helleringer S, Kohler HP, Kalilani-Phiri L, Mkandawire J, Armbruster B. The reliability of sexual partnership histories: implications for the measurement of partnership concurrency during surveys. AIDS. 2011;25(4):503–11. doi: 10.1097/QAD.0b013e3283434485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manhart LE, Aral SO, Holmes KK, Foxman B. Sex partner concurrency: measurement, prevalence, and correlates among urban 18-39-year-olds. Sex Transm Dis. 2002;29(3):133–43. doi: 10.1097/00007435-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 53.Zimmerman HL, Potterat JJ, Dukes RL, et al. Epidemiologic differences between chlamydia and gonorrhea. Am J Public Health. 1990;80(11):1338–42. doi: 10.2105/AJPH.80.11.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stoner BP, Whittington WL, Hughes JP, Aral SO, Holmes KK. Comparative epidemiology of heterosexual gonococcal and chlamydial networks: implications for transmission patterns. Sex Transm Dis. 2000;27(4):215–23. doi: 10.1097/00007435-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Potterat JJ. Socio-geographic space and sexually transmissible diseases in the 1990. Today's Life Sci. 1992;4(12):16–22. [Google Scholar]
- 56.Rothenberg R, Muth SQ, Malone S, Potterat JJ, Woodhouse DE. Social and geographic distance in HIV risk. Sex Transm Dis. 2005;32(8):506–12. doi: 10.1097/01.olq.0000161191.12026.ca. [DOI] [PubMed] [Google Scholar]
- 57.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2010. 2010.