Background: The epithelial Ca2+ channel TRPV5 facilitates Ca2+ reabsorption in the kidney and is regulated by sialidase and the hormone klotho.
Results: Sialidase stimulates TRPV5 plasma membrane stabilization in a lipid raft-dependent manner, while klotho increased cell surface expression of the channel via its N-glycan.
Conclusion: Klotho and sialidase regulate TRPV5 membrane stabilization in a different manner.
Significance: Understanding the regulation of TRPV5 is crucial to treat patients with Ca2+-related disorders.
Keywords: Calcium, Calcium Channels, Calcium Transport, Glycosylation, Membrane Trafficking, trpv5
Abstract
The transient receptor potential vanilloid type 5 (TRPV5) Ca2+ channel facilitates transcellular Ca2+ transport in the distal convoluted tubule (DCT) of the kidney. The channel is glycosylated with a complex type N-glycan and it has been postulated that hydrolysis of the terminal sialic acid(s) stimulate TRPV5 activity. The present study delineates the role of the N-glycan in TRPV5 activity using biochemical assays in Human Embryonic Kidney 293 cells expressing TRPV5, isoelectric focusing and total internal reflection fluorescent microscopy. The anti-aging hormone klotho and other glycosidases stimulate TRPV5-dependent Ca2+ uptake. Klotho was found to increase the plasma membrane stability of TRPV5, via the TRPV5 N-glycan. Sialidase mimicked this stimulatory action. However, this effect was independent of the N-glycosylation state of TRPV5, since the N-glycosylation mutant (TRPV5N358Q) was activated to the same extent. We showed that the increased TRPV5 activity after sialidase treatment is caused by inhibition of lipid raft-mediated internalization. In addition, sialidase modified the N-glycan of transferrin, a model glycoprotein, differently from klotho. Previous studies showed that after klotho treatment, galectin-1 binds the TRPV5 N-glycan and thereby increases TRPV5 activity. However, galectin-3, but not galectin-1, was expressed in the DCT. Furthermore, an increase in TRPV5-mediated Ca2+ uptake was detected after galectin-3 treatment. In conclusion, two distinct TRPV5 stimulatory mechanisms were demonstrated; a klotho-mediated effect that is dependent on the N-glycan of TRPV5 and a sialidase-mediated stimulation that is lipid raft-dependent and independent of the N-glycan of TRPV5.
Introduction
Calcium (Ca2+) plays a crucial role in multiple physiological processes such as muscle contraction, neuronal excitability, enzymatic activity, bone formation, cell membrane formation, and exocytosis. It is of physiological importance to tightly regulate intra- and extracellular Ca2+ levels. To this end, the Ca2+ balance is maintained by the bone, kidney, duodenum and parathyroid glands (1). Secretion of parathyroid hormone (PTH)2 by the parathyroid glands results in increased Ca2+ re(ab)sorption from both bone and kidney. The rate-limiting step in renal active Ca2+ reabsorption is the uptake by the transient receptor potential vanilloid type 5 (TRPV5) channel, which is apically expressed in the distal convoluted tubule (DCT) of the kidney (2–5). The channel is responsible for 8% of the total Ca2+ reabsorption (6). TRPV5 is a tetrameric membrane protein containing 6 transmembrane segments, with a cytosolic amino and carboxyl terminus and an extracellular N-glycan at asparagine 358. The N-glycan is an important post-translational regulatory site, which can affect the plasma membrane retention of the channel (7).
Previous studies have shown that intracellular N-glycans contribute to signaling processes, dimer formation, membrane trafficking and polarized sorting of glycoproteins to the apical surface of epithelial cells (8, 9). Extracellular N-glycans of plasma membrane glycoproteins are involved in signaling processes, apoptosis and cell-cell contacts. The composition of these glycans and the properties of the glycoproteins can be influenced by the activity of extracellular glycosidases (10–12). For example, previous studies demonstrated that sialidase, a glycosidase, affects caveolar-mediated endocytosis by interacting with glycolipids in the plasma membrane (13). Trafficking of TRPV5 is crucial for controlling its abundance at the plasma membrane and therefore, the Ca2+ reabsorption.
A protein that modifies the extracellular N-glycan of TRPV5 is the type-1 glycoprotein, klotho (7, 14, 15). Klotho is a glycosidase that participates in the degradation of glycans and thereby affects different glycoproteins (7, 15–17). Other glycosidases, such as β-glucuronidase and sialidase, mimic the plasma membrane stabilization effect of klotho on TRPV5 by modifying its N-glycan. It was hypothesized that klotho has β-glucuronidase activity, because this enzyme has homology with β-glucuronidase and is able to hydrolyze glucuronic acids (16). Treatment of Human Embryonic Kidney 293 cells (HEK293) expressing TRPV5 with β-glucuronidase also increased the Ca2+ uptake through TRPV5 (7). However, Tohyama et al. found that the β-glucuronidase activity of klotho is ∼26 times lower than that of β-glucuronidase (16). In addition, two highly conserved glutamate residues, essential for the enzymatic activity of β-glucuronidase, are not conserved in klotho. Furthermore, glucuronic acids are uncommon moieties in N-glycans of membrane proteins such as TRPV5. Cha et al. proposed that klotho operates instead as an exo-sialidase, specifically breaking the α-2,6-linked bonds of the negatively charged sialic acids (14). They postulated that in Chinese Hamster Ovary (CHO) cells, cleavage of sialic acids by klotho exposes the underlying disaccharide galactose-β-(1–4)-N-acetylglucosamine, followed by binding of galectins, a group of carbohydrate-binding proteins with a high affinity for the exposed galactose-N-acetylglucosamine disaccharides.
One of the galectin family members is galectin-1, a polyvalent protein expressed in various tissues and with a wide range of biological activities. It was suggested that galectin-1 forms a stable complex with TRPV5, resulting in a stimulatory effect on Ca2+ reabsorption by TRPV5 retention at the cell surface (14). Galectin-1 is present in both extra- and intracellular compartments, where it binds to carbohydrate and non-carbohydrate molecules, respectively (18). Galectin-1 is found in human tubular epithelial cells and in extensive amounts in the porcine kidney (19).
The aim of the present study was to investigate the underlying molecular mechanism of the klotho-mediated TRPV5 regulation and the involvement of galectins. To this end, we delineated the action of different glycosidases, viz. klotho and sialidase. Total Internal Reflection Fluorescent Microscopy (TIRF-M) combined with a photo-switchable fluorescent protein enabled us to study the dynamics of TRPV5 proteins on the plasma membrane in HEK293 cells. Finally, isoelectric focusing (IEF), allowed the detection of klotho-mediated sialic acid hydrolysis on a model glycoprotein, transferrin.
EXPERIMENTAL PROCEDURES
DNA Constructs
The pCINeo/IRES-GFP plasmid encoding TRPV5 was generated as described previously (20). TRPV5N358Q was obtained by in vitro mutagenesis of TRPV5-pCINeo/IRES-GFP cDNA according to the manufacturer's instructions (Stratagene, La Jolla, CA). The pcDendra-2 (Evrogen, Moscow, Russia) was amplified by PCR and ligated into the pCINeo/IRES-HA TRPV5 construct using the NheI and EcoNI (New England Biolabs, Ipswich, MA) restriction sites. All constructs were verified by DNA sequence analysis.
Immunoblotting and Protein Concentration Determination
TRPV5 protein expression was determined by 8% (w/v) SDS-PAGE and Western blotting using anti-TRPV5 (1:4.000, (21)) with peroxidase-labeled goat anti-mouse IgG (1:10.000, Sigma-Aldrich) antibodies. Protein concentration was measured using the BCA protein assay kit (Thermo Scientific, Rockford, IL), according to the manufacturer's manual.
Electrophysiology
Whole-cell currents were measured with an EPC-10 (HEKA electronic) amplifier using Patchmaster V2.20 software. The borosilicate glass electrode resistance was between 2.5 and 4 mΩ. The ramp protocol for measuring the current-voltage (I/V) relationship of Na+ consisted of linear voltage ramps from −100 to +100 mV within 450 ms repeated every 5 s. The step protocol for measuring the Ca2+ current consisted of a 10 s long voltage-step applied from +70 to −100 mV. Current traces were sampled at 0.5 ms for the ramp and 2 ms for step protocol. Reported current densities were calculated from the current at −80 mV during the ramp protocol. The standard extracellular solution contained 150 mm NaCl, 6 mm CsCl, 10 mm HEPES (pH 7.4 adjusted with NaOH), 50 μm EDTA, and 10 mm glucose. For Ca2+ current measurements NaCl was replaced with equimolar NMDG-Cl and 10 mm CaCl2 was added. Osmotic differences were adjusted by adding a respective amount of mannitol. The internal (pipette) solution contained 20 mm CsCl, 100 mm Cs-aspartate, 1 mm MgCl2, 10 mm BAPTA, 4 mm Na2ATP, and 10 mm HEPES (pH 7.2 adjusted with CsOH). Data were analyzed using Igor-pro software (WaveMetrics, Oswego, OR).
45Ca2+ Uptake Assay
HEK293 cells were transiently transfected with TRPV5 in pCINeo/IRES-GFP, TRPV5N358Q in pCINeo/IRES-GFP or the empty pCINeo/IRES-GFP vector (mock). One day after transfection, cells were reseeded on poly-l-lysine-coated (0.1 mg/ml) culture dishes and incubated with recombinant klotho (2 μg/ml, R&D systems, Minneapolis, MN), sialidase from Vibrio cholerae (27 mU/ml, Sigma-Aldrich), β-glucuronidase from bovine liver (310 units/ml, Sigma-Aldrich), endoF (1 kU/ml, New England), a combination of glycosidases, or human galectin-3 (0.67 μg/ml, Prospec, Ness Ziona, Israel) for 16 h at 37 °C. Radioactive 45Ca2+-uptake in TRPV5-transfected HEK293 cells was determined as described previously (22).
Plasma Membrane Biotinylation and TRPV5 Cell Surface Turnover
HEK293 cells were transiently transfected with TRPV5 in pCINeo/IRES-GFP, TRPV5N358Q in pCINeo/IRES-GFP or the empty pCINeo/IRES-GFP vector (mock). One day after transfection, cells were reseeded on poly-l-lysine-coated (0.1 mg/ml) culture dishes and incubated with recombinant klotho (2 μg/ml, R&D systems), sialidase from V. cholerae (27 mU/ml, Sigma-Aldrich), β-glucuronidase from bovine liver (310 units/ml, Sigma-Aldrich), or endoF (1 kU/ml, New England Biolabs) for 16 h at 37 °C. Subsequently, cells were biotinylated as described previously (7). For time point 0 h cells were collected from the plates and disrupted in 1 ml of lysis buffer [1% (v/v) Nonidet P-40, 150 mm NaCl, 5 mm EDTA, 50 mm Tris (pH 7.5 adjusted with HCl), 1 mm PMSF, 10 μg/ml leupeptin, 10 μg/ml pepstatin, 5 μg/ml proteinase A] immediately after biotinylation. For the other investigated time points, cells were cultured in the absence of glycosidases for an additional 1 or 3 h at 37 °C, subsequently washed with ice-cold PBS and homogenized in 1 ml of lysis buffer. TRPV5 protein expression at the cell surface and in total cell lysate was investigated as described previously (7).
Total Internal Reflection Microscopy (TIRF-M)
HEK293 cells were transiently transfected with pcDNA3-Dendra2-HA-TRPV5 and pcDNA3-Dendra2-HA-TRPV5N358Q 24 h before the experiment. TIRF-M cells were harvested in 15 ml tubes and resuspended in conditioned medium containing recombinant klotho (2 μg/ml, R&D systems) or sialidase from V. cholerae (27 mU/ml, Sigma-Aldrich). The tubes were rotated for 1.5 h at 37 °C to increase the effective reactive surface of the cells. Cells were reseeded on small Petri dishes (WilCo, Amsterdam, The Netherlands) coated with 50 μg/ml fibronectin and cultured for 2–7 h before subjected to TIRF-M. Dishes were washed and placed in a custom-made chamber with Krebs buffer (135 mm NaCl, 5 mm KCl, 1.5 mm MgCl2, 1.5 mm CaCl2, 20 mm HEPES (pH 7.4 with NaOH), 10 mm d-glucose,) and imaged at room temperature. The Olympus Fluoview FV1000-IX81 confocal microscope, with fully integrated TIRF module, and equipped with a PLAPON60xO/TIRFM-SP-PlanApochromatic objective 60x oil, NA 1.45 and WD = 0,10 mm lens was used to measure fluorescence. Photoconversion of Dendra2 was carried out using a 405 nm laser for 2 s. TRPV5 membrane trafficking was followed for about 36 min and every 3 min images were acquired of unswitched (488 nm laser, 20 frames/5 s) and switched (559 nm laser, 20 frames/5 s) dendra-TRPV5 proteins. All imaging acquisition was performed with Cell̂M (Tokyo, Japan).
Biotinylation of TRPV5
HEK293 cells transiently transfected with TRPV5 in pCINeo/IRES-GFP, TRPV5N358Q in pCINeo/IRES-GFP, or the empty pCINeo/IRES-GFP vector (mock) were reseeded on poly-l-lysine-coated (0.1 mg/ml) culture dishes 1 day after transfection and incubated with sialidase from V. cholerae (27 mU/ml, Sigma-Aldrich) or recombinant klotho (2 μg/ml, R&D systems) for 16 h at 37 °C. Subsequently, cells were biotinylated, lysed, and the biotinylated proteins were precipitated from the cell lysate with neutravidin beads (Pierce, Etten-leur, The Netherlands) as described previously (7). For the biotinylation after filipin treatment, the same protocol was used, but the cells were treated with filipin (5 μg/ml, Sigma-Aldrich) for 45 min prior to lysis.
Transferrin Isoelectric Focusing (tIEF)
The IEF assay was carried out as previously described, with the following differences (23). Briefly, plasma samples were incubated for 30 min, with a solution of 6.7 mm ferric citrate and 0.17 m sodium hydrogen carbonate, in a ratio of 1:1 (plasma to solution), to saturate the transferrin with iron. The iron-saturated plasma was treated with 2 units/ml sialidase (Roche Applied Science, Mannheim, Germany), 150 μg/ml recombinant klotho (R&D systems) or left untreated for each of the investigated time points (1, 2, 4, 7 h or overnight). Subsequently, the samples were applied to a hydrated immobiline gel (pH 5–7) on a PhastSystem (GE-Healthcare Life Sciences, Piscataway). Transferrin isoforms were detected after immunofixation with rabbit anti-human transferrin antibody (Dako, Glostrup, Denmark) and Coomassie brilliant blue staining.
COPAS Sorting and PCR
Transgenic mice expressing eGFP under the control of the TRPV5 promoter have been described previously (24). Fluorescently labeled DCT/CNT tubules were isolated from transgenic animals, using a Complex Object Parametric Analyzer and Sorter (COPAS) sorter (Union Biometrica, Somerville, MA) as previously described (25, 26). The animal ethics board of the Radboud University Nijmegen approved all of the experimental procedures. Tubular, HEK293 and CHO cell RNA was extracted using TRIzol Total RNA Isolation Reagent (Invitrogen BRL, The Netherlands) and processed into cDNA. The cDNA was mixed with Power SYBR green PCR Mastermix (Applied Biosystems, Foster City, CA) and exon-overlapping primers against TRPV5 and galectin-1 and −3.
Statistical Analysis
In all experiments, the data are expressed as mean ± S.E. Statistical significance (p < 0.05) was determined by analysis of variance and a Bonferroni post-hoc test.
RESULTS
Stimulation of TRPV5 Plasma Membrane Retention by Glycosidases
To study the molecular mechanism of the klotho-dependent TRPV5 stimulation, we investigated the effect of various glycosidases on the activity of the channel. HEK293 cells transiently expressing TRPV5 were treated with recombinant mouse klotho (2 μg/ml), sialidase (27 mU/ml), β-glucuronidase (310 units/ml) or endoglycosidase-F (endoF) (1 kU/ml) for 16 h and subjected to patch clamp analysis. HEK293 cells transfected with TRPV5 all showed typical representative inwardly-rectifying I/V curves. Fig. 1A represents cells transiently transfected with TRPV5, untreated or treated with klotho or endoF. The tested glycosidases significantly increased the TRPV5-mediated Na+ current density (Fig. 1B), while the current density of mock-transfected cells was not affected (data not shown). The stimulation of TRPV5 channel activity was confirmed by measuring 45Ca2+ uptake in cells after glycosidase treatment (Fig. 1C). EndoF showed the highest stimulation of TRPV5 (205 ± 6%), but also β-glucuronidase, sialidase, and klotho increased significantly the TRPV5-dependent Ca2+ uptake (166 ± 4%, 147 ± 8%, 155 ± 4%, respectively, p < 0.05).
FIGURE 1.
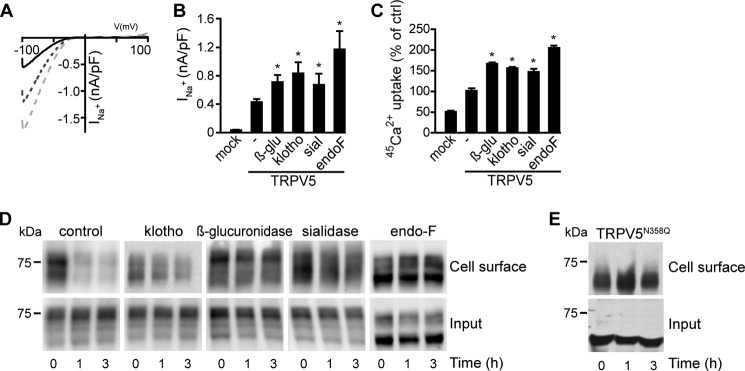
Different glycosidases increase the TRPV5-mediated Ca2+ uptake via plasma membrane stabilization of the channel. A, I/V relationships of untreated, klotho- or endoF-treated HEK293 cells expressing TRPV5 are presented. The solid line represents untreated cells, dashed line represents klotho-treated cells (light gray), and the dotted line represents endoF-treated cells (dark gray). B, TRPV5-mediated Na+ current in HEK293 cells was measured. Treatment with β-glucuronidase, klotho, sialidase, or endoF all resulted in a significantly increased current (n = 17–42). C, 45Ca2+ uptake assays of HEK293 cells transfected with TRPV5 or the empty vector (mock). These cells were untreated or treated with glycosidase (β-glucuronidase, klotho, sialidase, or endoF); D, time-chase assay of cells transfected with TRPV5. The membrane proteins were biotinylated at 0 h. After 3 h incubation without glycosidase, the membrane fraction of TRPV5 was decreased. E, time-chase assay of cells transfected with TRPV5N358Q. All data are presented as mean ± S.E.; n = 3; *, p < 0.05, statistically significant.
Subsequently, we investigated the plasma membrane retention via time-chase analysis. HEK293 cells expressing TRPV5 were treated for 16 h with different glycosidases and subjected to cell-surface biotinylation. To follow the fate of the biotinylated proteins, the cells were incubated for 0, 1, or 3 h without glycosidases. In untreated cells, the amount of biotinylated TRPV5 decreased significantly after 1 and 3 h compared with the initial time point (0 h) (Fig. 1D, upper panels). For klotho-, sialidase-, and endoF-treated cells, only a small decrease of biotinylated TRPV5 channels was detected after 1 and 3 h (Fig. 1D, upper panels). The total expression of TRPV5 was not significantly altered by the glycosidase treatment (Fig. 1D, lower panels).
To further elucidate the mechanism responsible for the increase in TRPV5 activity after glycosidase treatment, the N-glycosylation-deficient mutant TRPV5N358Q, was subjected to time-chase analysis. Compared with wild-type TRPV5 the plasma membrane abundance of the mutant TRPV5N358Q channel was markedly stable, even after 3 h of incubation (Fig. 1E).
Glycosidase Treatment Retains TRPV5 at the Plasma Membrane
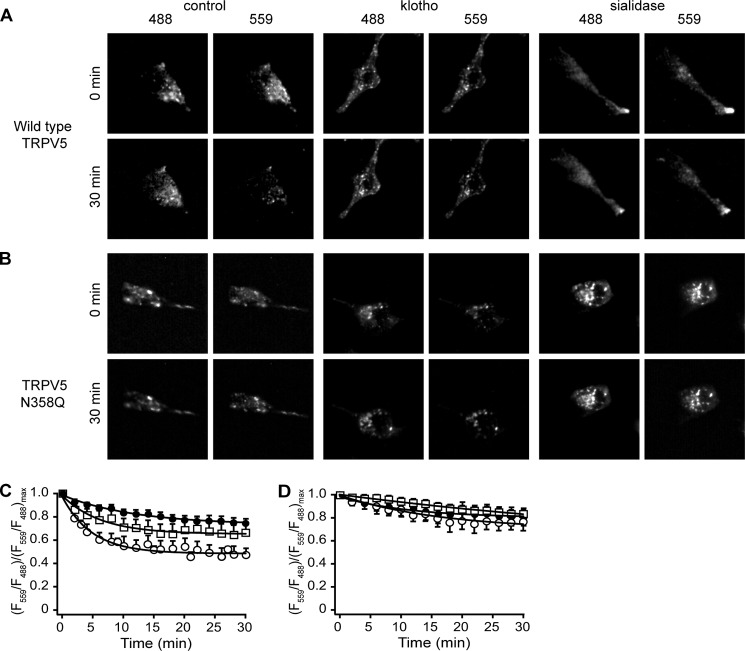
Traditional time-chase experiments are not able to distinguish between delayed recycling of proteins from the plasma membrane or reduced degradation. To overcome this limitation, we established a novel approach by combining live-cell TIRF-M with a photo-convertible (green to red) fluorescent protein, Dendra-2, that was fused to the N terminus of the TRPV5 channel (27). Via this technique an evanescent field at the glass-cell membrane interface permitted specific detection and excitation of the channels close to the plasma membrane (∼70–100 nm). Proteins in close vicinity to the plasma membrane were photo-switched in TIRF mode using evanescent UV light (λ = 405 nm). TIRF-M images are shown of the control and treated conditions for Dendra-2-TRPV5 and Dendra-2-TRPV5N358Q, captured at the initial time point after photo-switching and after 30 min (Fig. 2, A and B). The ratio of fluorescent intensity at an initial time point was calculated by (Fi − Fo)/Fo, where Fo is fluorescent intensity at an initial time point and Fi = fluorescent intensity at a new time point. To follow the presence of the protein at the plasma membrane in time, the ratio between the switched red fraction (F559) and unswitched green fraction (F488) was calculated ((F559/F488)/(F559/F488)max). After treatment with sialidase (70 ± 3%) or klotho (75 ± 4%) the ratio at 30 min was significantly increased compared with control (47 ± 8%) indicating that TRPV5 is more stable at the plasma membrane (Fig. 2C). To investigate whether there is a glycosidase-dependent effect on the N-glycosylation-deficient mutant, Dendra-2-TRPV5N358Q was subjected to klotho and sialidase treatment. After glycosidase treatment, no significant decrease in the fluorescent ratio of Dendra-2-TRPV5N358Q was found (Fig. 2D).
FIGURE 2.
Klotho and sialidase stabilize TRPV5 on the plasma membrane. A and B, TIRF-M images of HEK293 cells expressing Dendra-2-TRPV5 or Dendra-2-TRPV5N358Q in both red and green channels, after 0 and 30 min. C, ratio of switched red/unswitched green fraction of HEK293 cells expressing Dendra-2-TRPV5. These cells were subjected to live-cell TIRF-M. At 0 min Dendra-2 was subjected to UV light (λ = 405 nm), resulting in a switch from green to red. D, ratio of switched red/unswitched green fraction of HEK293 cells expressing Dendra-2-TRPV5N358Q. These cells showed no increase in plasma membrane retention after glycosidase treatment. ○, control; □, klotho-treated; ●, sialidase-treated. All data are expressed as mean ± S.E.; n = 3.
Klotho and Sialidase Regulate TRPV5 Plasma Membrane Retention Differently
TIRF-M showed that the cells expressing TRPV5N358Q appeared brighter after sialidase treatment, but not after klotho treatment. The difference between sialidase-treated and klotho-treated HEK293 cells expressing TRPV5N358Q was further investigated. Treatment with klotho of the cells expressing TRPV5N358Q did not alter the plasma membrane availability of this mutant as detected by cell surface biotinylation (104 ± 6%, p > 0.2) (Fig. 3, A and B). For the cells transfected with wild-type TRPV5, the TRPV5 plasma membrane fraction significantly increased after klotho treatment (140 ± 9%, p < 0.05). In contrast, sialidase treatment enhanced the plasma membrane fraction of both TRPV5 and TRPV5N358Q (151 ± 8% and 153 ± 7%, respectively, p < 0.05) (Fig. 3, C and D).
FIGURE 3.
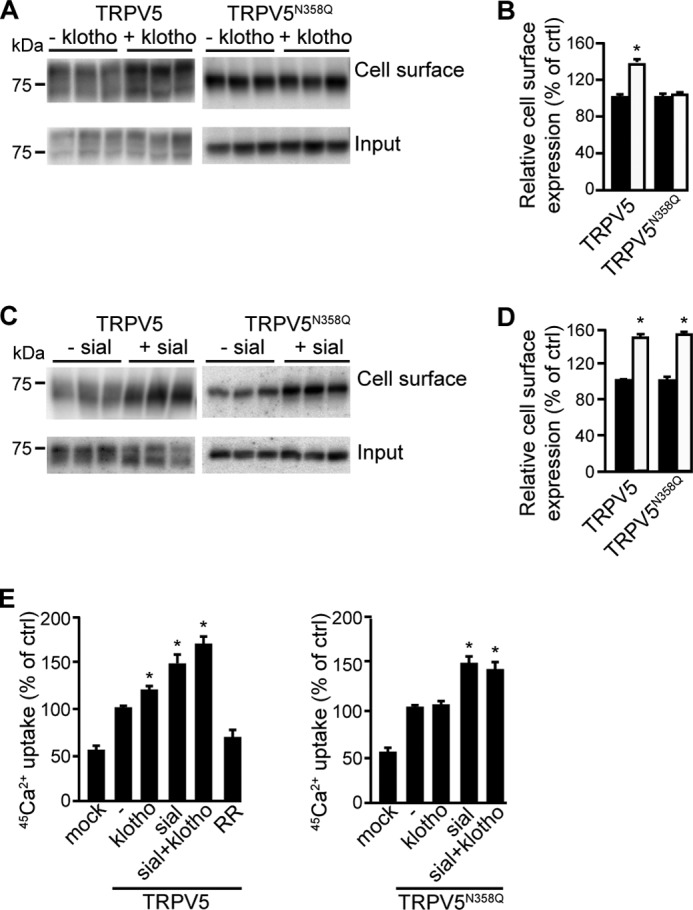
TRPV5 stabilization via klotho is N-glycan-dependent. A, biotinylation of HEK293 cells expressing TRPV5 or TRPV5N358Q after klotho treatment, a representative blot is depicted; B, relative TRPV5 cell surface expression with or without klotho treatment (white and black bar, respectively); C, biotinylation of HEK293 cells expressing TRPV5 or TRPV5N358Q after sialidase treatment, a representative blot is depicted; D, relative TRPV5 cell surface expression with or without sialidase treatment (white and black bar, respectively); E, 45Ca2+ uptake assay of cells transfected with TRPV5 or TRPV5N358Q after treatment with klotho, sialidase, a combination of these glycosidases, ruthenium red or left untreated. All data are presented as mean ± S.E.; n = 3; *, p < 0.05, statistically significant.
The distinct actions of klotho and sialidase were further studied by 45Ca2+ uptake assays. In line with our biochemical observations, klotho augmented the 45Ca2+ influx in HEK293 cells transfected with TRPV5 (120 ± 2%), but not in cells expressing TRPV5N358Q (103 ± 5%) (Fig. 3E). In contrast, sialidase increased TRPV5-mediated 45Ca2+ uptake in TRPV5-expressing cells(150 ± 11%) as well as in cells expressing TRPV5N358Q (149 ± 10%) (Fig. 3E). Co-treatment with sialidase and klotho resulted in an additive increase in TRPV5-mediated Ca2+ influx (170 ± 8%). In contrast, no additional 45Ca2+ influx was observed after sialidase and klotho treatment of HEK293 cells over-expressing TRPV5N358Q (149 ± 10% and 144 ± 9%, sialidase versus sialidase and klotho, respectively, p > 0.2). The TRPV5 blocker ruthenium red was applied to determine the magnitude of the TRPV5-mediated 45Ca2+ influx (28).
Disruption of Lipid Rafts Increases TRPV5 Plasma Membrane Abundance
Sialidase is known to affect protein trafficking by interacting with glycolipids in lipid rafts (13). To determine whether TRPV5 is present in lipid rafts, lipid rafts in HEK293 cells expressing either TRPV5 or TRPV5N358Q were disrupted using the cholesterol-chelating drug filipin. After filipin treatment, cell surface biotinylation showed a significant increase in TRPV5 and TRPV5N358Q abundance at the plasma membrane (Fig. 4, A–C). Likewise, 45Ca2+ uptake assays revealed a proportional increase in the functional activity of TRPV5 (119 ± 3%) and TRPV5N358Q (122 ± 4%), after filipin treatment versus control (p < 0.05) (Fig. 4D). A combination of filipin and klotho significantly stimulated TRPV5-mediated 45Ca2+ uptake (100 ± 6% and 143 ± 10%, control versus filipin and klotho, respectively p < 0.05). No additional 45Ca2+ uptake was observed after combining sialidase with filipin (100 ± 6% and 113 ± 6%, control versus filipin and sialidase, respectively p > 0.2) (Fig. 4E).
FIGURE 4.
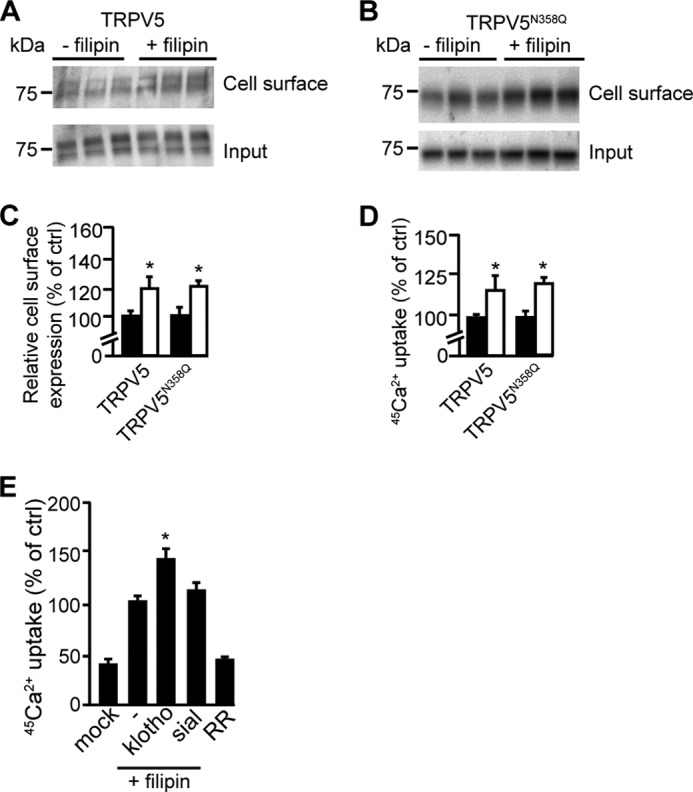
TPRV5 plasma membrane retention is regulated via lipid rafts. A, biotinylation of HEK293 cells expressing TRPV5 after filipin treatment, a representative blot is depicted. B, biotinylation of cells expressing TRPV5N358Q after filipin treatment, a representative blot is depicted. C, relative cell surface expression, with or without filipin treatment (white and black bar, respectively) of TRPV5 (n = 6) or TRPV5N358Q (n = 3) overexpressing cells; D, 45Ca2+ uptake assay in the presence of filipin (n = 3). Filipin significantly increases Ca2+ uptake independent on the N-glycan of TRPV5 (white bar). E, 45Ca2+ uptake assay in the presence of filipin and klotho or sialidase (n = 3). Klotho significantly increased TRPV5-dependent 45Ca2+-uptake in the presence of flilipin, while sialidase treatment gave no additional effect. All data are presented as mean ± S.E.; *, p < 0.05, statistically significant.
Sialidase and Klotho Modify the N-glycan of Transferrin in a Different Manner
The difference between klotho and sialidase was further confirmed by isoelectric focusing (IEF), using human blood transferrin as a model glycoprotein. Via this technique, proteins are separated based on their isoelectric focus point. Since sialic acids are negatively charged, a change in the isoelectric focusing pattern can be detected after sialidase treatment. Plasma transferrin was used as model glycoprotein, since the isoelectric focusing point after removal of the sialic acids has been characterized. The main isoform of human plasma transferrin contains two complex biantennary N-glycans, each terminated by two α-2,6-linked sialic acids (23). The protein was treated with klotho (150 μg/ml) or sialidase (2 units/ml) for different time intervals. Sialidase treatment resulted in a clear shift in the IEF pattern of transferrin, indicating the loss of sialic acids (Fig. 5A). Before treatment, a main isoform with four sialic acids was detected and after 2 h of sialidase treatment all sialic acids were cleaved. Treatment with klotho did not significantly alter the IEF pattern of transferrin (Fig. 5B).
FIGURE 5.
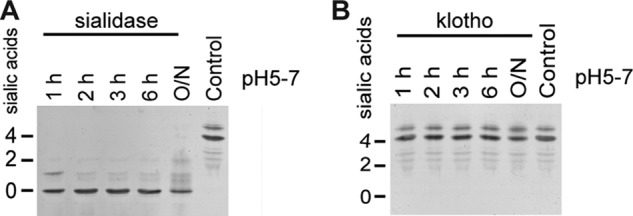
The isoelectric focusing pattern of transferrin is not modified by klotho. A, tIEF after sialidase treatment. Hydrolysis of sialic acids by sialidase treatment results in a shift of the IEF pattern. B, isoelectric focusing of transferrin after klotho treatment. Klotho has no influence on the amount of sialic acids.
Galectin-3 Partially Mediates Klotho-dependent TRPV5 Activity
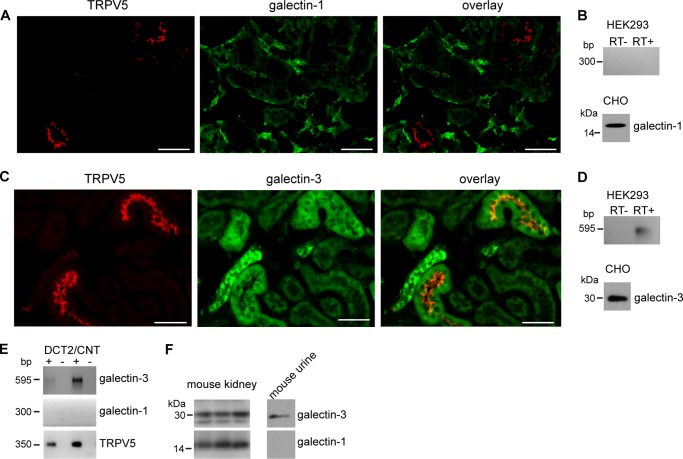
The premise that klotho might not be a specific sialidase, would add new mechanistic data to the hypothesis proposed by Cha et al., who suggested that galectin-1 stabilizes TRPV5 on the plasma membrane after N-glycan modification by klotho-mediated sialidase activity. In CHO cells they showed that the stimulatory klotho effect on TRPV5 was absent after the knock down of galectin-1 (14). However, immunohistochemistry of mouse kidney sections failed to show co-localization between TRPV5 and galectin-1 (Fig. 6A). In addition, galectin-1 was expressed in CHO cells, but not in HEK293 cells, that were used for this study (Fig. 6B). Since the potential ligands for galectin-1 and galectin-3 are similar (29), the expression of galectin-3 in mice sections, HEK293 cells and in Complex Object Parametric Analyzer and Sorter (COPAS)-sorted distal convoluted and connecting tubules (DCT/CNT) was checked. Galectin-3 showed a clear co-localization with TRPV5 in mice kidney sections. Some tubuli were positive for galectin-3, but not for TRPV5, indicating that galectin-3 activity is not restricted to TRPV5-expressing tubuli (Fig. 6C). Galectin-3 was also expressed in HEK293 cells, as was shown by RT-PCR (Fig. 6D). The expression of galectins in the DCT/CNT was further studied after collecting the nephron segments from mouse kidney, by the COPAS large particle flow cytometry (25). In isolated DCT/CNT segments only galectin-3 mRNA was detected by RT-PCR, but not galectin-1 (Fig. 6E). In addition, also in mouse urine, only galectin-3 was found, which implies that it has access to the glycan of TRPV5 (Fig. 6F).
FIGURE 6.
Galectin-3 is a candidate mediator of the klotho effect on TRPV5. A, immunohistochemistry on DCT/CNT for TRPV5 and galectin-1 shows no co-localization. B, immunoblot of CHO cell lysate demonstrated that galectin-1 was present. After RT-PCR on the cDNA of HEK293 cells, no galectin-1 was detected. C, immunohistochemistry on DCT/CNT for TRPV5 and galectin-3 demonstrate a co-localization; D, immunoblot of CHO cell lysate demonstrated that galectin-3 was expressed. After RT-PCR on the cDNA of HEK293 cells, galectin-3 was detected. E, cDNA of the DCT/CNT cells was analyzed by RT-PCR and only galectin-3 was detected. F, total kidney lysate of mouse contains both galectin-1 and galectin-3, while mouse urine only contains galectin-3.
The involvement of galectin-3 in the regulation of TRPV5 was further investigated. HEK293 cells, transiently overexpressing TRPV5 were treated with 0.67 μg/ml recombinant human galectin-3. 45Ca2+ uptake assays revealed an increase in TRPV5 channel activity after galectin-3 treatment (100 ± 2% and 131 ± 6%, control versus galectin-3, respectively, p < 0.05) (Fig. 7A), while the activity of the TRPV5N358Q mutant was not affected by galectin-3 treatment (100 ± 2% and 104 ± 4%, control versus galectin-3, respectively, p > 0.2) (Fig. 7A). Combining galectin-3 and klotho treatment, or galectin-3 and sialidase treatment, did not result in additional stimulation of channel activity (Fig. 7B).
FIGURE 7.
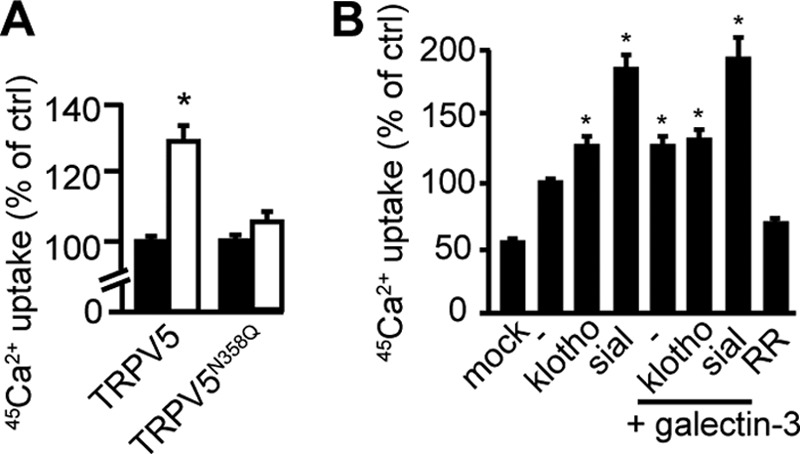
TRPV5 channel activity is regulated by extracellular galectin-3. A, 45Ca2+-uptake assay in cells overexpressing TRPV5 (n = 5) or TRPV5N358Q (n = 3) in the presence and absence of human galectin-3. Galectin-3 significantly increased wild type TRPV5 (white bar) but not TRPV5N358Q channel activity (white bar). All data are presented as mean ± S.E.; *, p < 0.05, statistically significant. B, 45Ca2+-uptake assay of cells overexpressing TRPV5 treated with klotho, sialidase, galectin-3, or a combination of these enzymes. No additional stimulation was detected after treatment with klotho and galectin-3. All data are presented as mean ± S.E.; n = 5; *, p < 0.05, statistically significant.
DISCUSSION
Our study demonstrates that glycosidases augment the plasma membrane retention of TRPV5, where klotho and sialidase increase the TRPV5 plasma membrane fraction via distinct mechanisms. The effect of sialidase, in contrast to klotho, is independent of the glycosylation status of TRPV5. Moreover, galectin-3 is a potential mediator for klotho-dependent TRPV5 stabilization. Our conclusions are based on the following observations: (i) biochemical assays showed that klotho, sialidase, β-glucuronidase and endoF increase the TRPV5 channel activity and membrane retention; (ii) the plasma membrane stability of TRPV5N358Q was altered by sialidase, but not by klotho; (iii) IEF demonstrated no modification of the N-glycan sialic acids after klotho treatment, in transferrin; (iv) only galectin-3, but not galectin-1, co-localizes with TRPV5 in DCT/CNT of the kidney; (v) galectin-3 increased the TRPV5-mediated Ca2+ influx in a N-glycan-dependent manner.
It was demonstrated that glycosidase treatment increases the TRPV5 channel activity. This effect was detected by treatment with different types of glycosidase (endoF, sialidase, and β-glucuronidase). TRPV5 channel activity was measured via time-chase experiments in combination with functional analyses (7, 14). A general mechanism or pathway for all the applied glycosidases remains unknown, since their enzymatic effects are distinct: endoF cleaves the entire N-glycan (30), sialidase hydrolyzes end-standing sialic acids, while β-glucuronidase cleaves glucuronic acids (31). One hypothesis is that any modification of the N-glycan improves the plasma membrane stabilization of TRPV5. Alternatively, since all glycans on the plasma membrane were hydrolyzed, the detected stabilizing effect could be independent of the N-glycan of TRPV5. This would mean that the plasma membrane stabilization is due to an indirect effect on TRPV5. However, the stimulatory effect of klotho is dependent on the N-glycan of TRPV5.
Plasma membrane retention can occur via two distinct routes; a delayed retrieval from the plasma membrane and subsequent degradation of the channel, or via an accelerated/increased insertion at the plasma membrane. It is difficult to distinguish between both routes based on time-chase experiments. To pinpoint the fate of glycosidase-treated TRPV5, we developed a novel assay, combining the live-cell imaging technique, TIRF-M, with photoactivatable fluorophores. Important advantages of TIRF-M over confocal microscopy are the higher Z resolution, high signal to noise ratio, increased contrast in fluorescence and reduced bleaching. The ease in combining kinetic studies with local information in living samples results in fast data acquisition (32). We showed that TRPV5 has a reduced mobile fraction after sialidase or klotho treatment, indicating that these glycosidases enable TRPV5 to reside longer on the plasma membrane. Our control, TRPV5N358Q lacks N-glycosylation and is devoid of any glycosylation-dependent regulation. This mutant demonstrated no decrease in the mobile fraction after glycosidase treatment. Overall this mutant had a smaller mobile fraction compared with TRPV5.
While conducting the TIRF-M based live-cell imaging it was observed that after sialidase treatment, the cells expressing TRPV5N358Q appeared brighter. This effect was not detected after klotho treatment. This prompted us to investigate the effect of sialidase on TRPV5N358Q. An increased plasma membrane abundance of TRPV5N358Q was detected via biotinylation, after sialidase treatment. It was noticed that biotinylated TRPV5 often runs as a smear band on SDS-PAGE. The reason for this effect remains unclear, however, it could indicate that plasma membrane TRPV5 is more heterogeneous compared with intracellular TRPV5.
The effect of sialidase treatment on the TRPV5N358Q mutant was confirmed using 45Ca2+ uptake experiments. TRPV5-mediated 45Ca2+ uptake was increased after treatment with both sialidase and klotho in an additive manner, indicating that klotho and sialidase act via different pathways. Interestingly, Cha et al. postulated that sialidase did not increase the plasma membrane abundance of TRPV5N358Q (14). However, in their study CHO cells were used as a model system, while in the present study HEK293 cells were employed.
We proved that filipin, an inhibitor of microdomain clustering, increases the cell surface expression of TRPV5/TRPV5N358Q and 45Ca2+ uptake through TRPV5 and TRPV5N358Q. This finding indicated that TRPV5 and TRPV5N358Q channels are present and internalized in clustered microdomains. Recently, it has been suggested that sialidase prevents microdomain clustering and lipid raft formation by hydrolyzing sialic acids from glycosphingolipids (13). Microdomain clustering is known to stimulate caveolae-mediated endocytosis and since sialidase treatment inhibits this microdomain clustering, the endocytosis is reduced (13). Because TRPV5 is present in these microdomains, the channel is most likely stabilized on the plasma membrane by sialidase via reduced lipid raft formation and thereby a decreased caveolae-mediated endocytosis. In agreement with this hypothesis, sialidase treatment did not result in additional stimulation of 45Ca2+ uptake after disrupting the lipid rafts by filipin. This finding, together with the sialidase-mediated stimulation of Ca2+ uptake via TRPV5N358Q, and increased wild type TRPV5 activation after klotho treatment in the presence of filipin, suggested that klotho and sialidase act via two different pathways to modulate channel activity. Sialidase, in contrast to klotho, stimulates TRPV5 via a mechanism that is independent of the N-glycan of TRPV5.
To further confirm this hypothesis IEF was employed using the well-characterized transferrin as a model protein. Sialidase altered the IEF pattern of transferrin, a substrate exposing terminal sialic acids, including α2,6-linked sialic acids (33, 34). Klotho treatment did not result in any modification, suggesting a lack of sialidase activity of klotho. Alternatively, it is possible that sialidase activity of klotho is tightly structure-related, cleaving only one sialic acid that is presented in a specific sugar signature, which may be absent in transferrin. The difference between sialidase and klotho prompted us to investigate the following step in the proposed mechanism. Cha et al. suggested that galectin-1 is a key player in the regulation of TRPV5. Interestingly, immunohistochemistry on renal mouse cortex sections showed no co-localization of galectin-1 with TRPV5. Galectin-1 is not expressed in HEK293 cells and therefore the in vitro measured effect of klotho is likely to be galectin-1 independent. Since the potential ligands for galectin-1 and galectin-3 are highly similar, we investigated the role of galectin-3 in this process. Galectin-3 is a carbohydrate-binding protein that is highly abundant in type A intercalated cells of the cortical collecting duct and the distal tubules (35, 36). An important difference between galectin-1 and galectin-3 is that the dimer galectin-1 requires a terminal galactose for binding, while the oligomer galectin-3 binds to repeating [-3Gal-β-(1–4)-GlcNAc-β-1-]n or poly-N-acetyllactosamine sequences that not necessarily contain a terminal β-galactoside residue (37). Galectin-3 co-localized with TRPV5, although the presence of galectin-3 is not restricted to TRPV5-expressing tubuli. In addition, COPAS sorted DCT/CNT cells were enriched with galectin-3 mRNA, while galectin-1 mRNA was not detected, indicating that galectin-3 may be involved in TRPV5 regulation. In contrast to the TRPV5N358Q mutant, wild type TRPV5 channel activity was increased upon treatment with galectin-3. This suggests that galectin-3-mediated channel activity is dependent on the N-glycan of TRPV5. Interestingly, co-treatment with galectin-3 and klotho did not additively stimulate TRPV5 activity implying that klotho and galectin-3 stimulate TRPV5 activity in a similar manner. An interesting observation, as detected with TIRF-M, biotinylation, 45Ca2+ uptake experiments and electrophysiology was the stable expression of TRPV5N358Q mutant on the plasma membrane without any treatment. It was proposed that galectin stabilizes TRPV5 on the plasma membrane. However, due to the absence of an N-glycan on the TRPV5N358Q mutant, it is striking that this mutant retains on the plasma membrane. Since the TRPV5N358Q mutant was transiently overexpressed, one could hypothesize that some glycosylation-deficient channels are forced to the plasma membrane. Since the entire N-glycan is absent, the protein cannot be appropriately regulated by the cell and will remain more stable on the plasma membrane.
Likewise, Kohno et al. demonstrated a significant difference between the internalization rates of the wild type endothelial differentiation gene-1 product (Edg-1, a G-coupled receptor) and the nonglycosylated N30D-Edg-1 mutant (39). The wild type G-coupled receptor was internalized at higher rates compared with the nonglycosylated N30D-Edg-1 receptor. Another explanation could be that the channel is destabilized due to steric hindrance in the presence of the N-glycan, resulting in a reduced amount of TRPV5 on the cell surface. Consequently, any reduction of the N-glycan of TRPV5 results in an increase of plasma membrane stability and, therefore, channel activity.
Acknowledgments
We thank Dr. S. Boros (Dept. of Physiology, Nijmegen) for help with the performed experiments, and F. van Zeeland and E. Lenssen for their assistance and support with the COPAS experiments.
This work was supported by grants from the Nijmegen Centre for Molecular Life Sciences (NCMLS) (to J. G. H.), the Dutch Kidney Foundation (C06.2170, CP10.11), the Netherlands Organization for Scientific Research (NWO-CW 700.55.302), and EURenOmics funding from the European Union Seventh Framework Programme (FP7/2007-2013, agreement n° 305608).
- PTH
- parathyroid hormone
- CNT
- connecting tubule
- COPAS
- complex object parametric analyzer and sorter
- DCT
- distal convoluted tubule
- EndoF
- endoglycosidase-F
- IEF
- isoelectric focusing
- tIEF
- isoelectric focusing of transferrin
- TIRF-M
- total internal reflection fluorescent microscopy
- TRPV5
- transient receptor potential vanilloid type 5.
REFERENCES
- 1. Huang C. L., Moe O. W. (2011) Klotho: a novel regulator of calcium and phosphorus homeostasis. Pflug. Arch. Eur. J. Physiol. 462, 185–193 [DOI] [PubMed] [Google Scholar]
- 2. Venkatachalam K., Montell C. (2007) TRP channels. Annu. Rev. Biochem. 76, 387–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van de Graaf S. F., Bindels R. J., Hoenderop J. G. (2007) Physiology of epithelial Ca2+ and Mg2+ transport. Rev. Physiol. Biochem. Pharmacol. 158, 77–160 [DOI] [PubMed] [Google Scholar]
- 4. Suzuki Y., Landowski C. P., Hediger M. A. (2008) Mechanisms and regulation of epithelial Ca2+ absorption in health and disease. Annu. Rev. Physiol. 70, 257–271 [DOI] [PubMed] [Google Scholar]
- 5. Hoenderop J. G., Bindels R. J. (2008) Calciotropic and magnesiotropic TRP channels. Physiology 23, 32–40 [DOI] [PubMed] [Google Scholar]
- 6. Hoenderop J. G. J., Willems P. H. G. M., Bindels R. J. M. (2000) Toward a comprehensive molecular model of active calcium reabsorption. Am. J. Physiol. 278, F352–F360 [DOI] [PubMed] [Google Scholar]
- 7. Chang Q., Hoefs S., van der Kemp A. W., Topala C. N., Bindels R. J., Hoenderop J. G. (2005) The β-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science 310, 490–493 [DOI] [PubMed] [Google Scholar]
- 8. Takahashi M., Yokoe S., Asahi M., Lee S. H., Li W., Osumi D., Miyoshi E., Taniguchi N. (2008) N-glycan of ErbB family plays a crucial role in dimer formation and tumor promotion. Biochem. Biophys. Acta 1780, 520–524 [DOI] [PubMed] [Google Scholar]
- 9. Moen A., Hafte T. T., Tveit H., Egge-Jacobsen W., Prydz K. (2011) N-Glycan synthesis in the apical and basolateral secretory pathway of epithelial MDCK cells and the influence of a glycosaminoglycan domain. Glycobiology 21, 1416–1425 [DOI] [PubMed] [Google Scholar]
- 10. Zhuo Y., Bellis S. L. (2011) Emerging Role of alpha 2,6-Sialic Acid as a Negative Regulator of Galectin Binding and Function. J. Biol. Chem. 286, 5935–5941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kawamura S., Sato I., Wada T., Yamaguchi K., Li Y., Li D., Zhao X., Ueno S., Aoki H., Tochigi T., Kuwahara M., Kitamura T., Takahashi K., Moriya S., Miyagi T. (2012) Plasma membrane-associated sialidase (NEU3) regulates progression of prostate cancer to androgen-independent growth through modulation of androgen receptor signaling. Cell Death Differ. 19, 170–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miyagi T., Yamaguchi K. (2012) Mammalian sialidases: physiological and pathological roles in cellular functions. Glycobiology 22, 880–896 [DOI] [PubMed] [Google Scholar]
- 13. Singh R. D., Marks D. L., Holicky E. L., Wheatley C. L., Kaptzan T., Sato S. B., Kobayashi T., Ling K., Pagano R. E. (2010) Gangliosides and beta1-integrin are required for caveolae and membrane domains. Traffic 11, 348–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cha S. K., Ortega B., Kurosu H., Rosenblatt K. P., Kuro-O. M., Huang C. L. (2008) Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc. Natl. Acad. Sci. U.S.A. 105, 9805–9810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu P., Boros S., Chang Q., Bindels R. J., Hoenderop J. G. (2008) The β-glucuronidase klotho exclusively activates the epithelial Ca(2+) channels TRPV5 and TRPV6. Nephrol. Dial. Transpl. 23, 3397–3402 [DOI] [PubMed] [Google Scholar]
- 16. Tohyama O., Imura A., Iwano A., Freund J. N., Henrissat B., Fujimori T., Nabeshima Y. (2004) Klotho is a novel β-glucuronidase capable of hydrolyzing steroid β-glucuronides. J. Biol. Chem. 279, 9777–9784 [DOI] [PubMed] [Google Scholar]
- 17. Cohen D. M. (2006) Regulation of TRP channels by N-linked glycosylation. Semin Cell Dev. Biol. 17, 630–637 [DOI] [PubMed] [Google Scholar]
- 18. Cho M. J., Cummings R. D. (1995) Galectin-1, a β-Galactoside-Binding Lectin in Chinese-Hamster Ovary Cells. 1. Physical and Chemical Characterization. J. Biol. Chem. 270, 5198–5206 [DOI] [PubMed] [Google Scholar]
- 19. Bürger A., Filsinger S., Cooper D. N. W., Hänsch G. M. (1996) Expression of the 14 kDa galactose-binding protein, galectin-1, on human tubular epithelial cells. Kidney Int. 50, 754–759 [DOI] [PubMed] [Google Scholar]
- 20. van de Graaf S. F., Hoenderop J. G., Gkika D., Lamers D., Prenen J., Rescher U., Gerke V., Staub O., Nilius B., Bindels R. J. (2003) Functional expression of the epithelial Ca(2+) channels (TRPV5 and TRPV6) requires association of the S100A10-annexin 2 complex. EMBO J. 22, 1478–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoenderop J. G., van der Kemp A. W., Hartog A., van de Graaf S. F., van Os C. H., Willems P. H., Bindels R. J. (1999) Molecular identification of the apical Ca2+ channel in 1, 25-dihydroxyvitamin D3-responsive epithelia. J. Biol. Chem. 274, 8375–8378 [DOI] [PubMed] [Google Scholar]
- 22. de Groot T., Lee K., Langeslag M., Xi Q., Jalink K., Bindels R. J., Hoenderop J. G. (2009) Parathyroid hormone activates TRPV5 via PKA-dependent phosphorylation. J. Am. Soc. Nephrol. 20, 1693–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Eijk H. G., van Noort W. L. (1992) The analysis of human serum transferrins with the PhastSystem: quantitation of microheterogeneity. Electrophoresis 13, 354–358 [DOI] [PubMed] [Google Scholar]
- 24. Meyer A. H., Katona I., Blatow M., Rozov A., Monyer H. (2002) In vivo labeling of parvalbumin-positive interneurons and analysis of electrical coupling in identified neurons. J. Neurosci. 22, 7055–7064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dimke H., San-Cristobal P., de Graaf M., Lenders J. W., Deinum J., Hoenderop J. G., Bindels R. J. (2011) γ-Adducin stimulates the thiazide-sensitive NaCl cotransporter. J. Am. Soc. Nephrol. 22, 508–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miller R. L., Zhang P., Chen T., Rohrwasser A., Nelson R. D. (2006) Automated method for the isolation of collecting ducts. Am. J. Physiol. 291, F236–F245 [DOI] [PubMed] [Google Scholar]
- 27. Gurskaya N. G., Verkhusha V. V., Shcheglov A. S., Staroverov D. B., Chepurnykh T. V., Fradkov A. F., Lukyanov S., Lukyanov K. A. (2006) Engineering of a monomeric green-to-red photoactivatable fluorescent protein induced by blue light. Nat. Biotechnol. 24, 461–465 [DOI] [PubMed] [Google Scholar]
- 28. Hoenderop J. G., Vennekens R., Müller D., Prenen J., Droogmans G., Bindels R. J., Nilius B. (2001) Function and expression of the epithelial Ca(2+) channel family: comparison of mammalian ECaC1 and 2. J. Physiol. 537, 747–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Varki A. (2009) Essentials of Glycobiology, 2nd Ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: [PubMed] [Google Scholar]
- 30. Suzuki T., Park H., Lennarz W. J. (2002) Cytoplasmic peptide:N-glycanase (PNGase) in eukaryotic cells: occurrence, primary structure, and potential functions. Faseb J. 16, 635–641 [DOI] [PubMed] [Google Scholar]
- 31. Chen X., Varki A. (2010) Advances in the biology and chemistry of sialic acids. ACS Chem. Biol. 5, 163–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Toomre D., Bewersdorf J. (2010) A New Wave of Cellular Imaging. Annu. Rev. Cell Dev. Biol. 26, 285–314 [DOI] [PubMed] [Google Scholar]
- 33. Coddeville B., Regoeczi E., Strecker G., Plancke Y., Spik G. (2000) Structural analysis of trisialylated biantennary glycans isolated from mouse serum transferrin. Characterization of the sequence Neu5Gc(α2–3)Gal(β1–3)[Neu5Gc(α2–6)]GlcNAc(β1–2)Man. Biochim. Biophys. Acta 1475, 321–328 [DOI] [PubMed] [Google Scholar]
- 34. Spik G., Debruyne V., Montreuil J., van Halbeek H., Vliegenthart J. F. (1985) Primary structure of two sialylated triantennary glycans from human serotransferrin. FEBS Lett. 183, 65–69 [DOI] [PubMed] [Google Scholar]
- 35. Winyard P. J., Bao Q., Hughes R. C., Woolf A. S. (1997) Epithelial galectin-3 during human nephrogenesis and childhood cystic diseases. J. Am. Soc. Nephrol. 8, 1647–1657 [DOI] [PubMed] [Google Scholar]
- 36. Kikuchi Y., Kobayashi S., Hemmi N., Ikee R., Hyodo N., Saigusa T., Namikoshi T., Yamada M., Suzuki S., Miura S. (2004) Galectin-3-positive cell infiltration in human diabetic nephropathy. Nephrol. Dial. Transpl. 19, 602–607 [DOI] [PubMed] [Google Scholar]
- 37. Patnaik S. K., Potvin B., Carlsson S., Sturm D., Leffler H., Stanley P. (2006) Complex N-glycans are the major ligands for galectin-1, -3, and -8 on Chinese hamster ovary cells. Glycobiology 16, 305–317 [DOI] [PubMed] [Google Scholar]
- 38.Deleted in proof
- 39. Kohno T., Wada A., Igarashi Y. (2002) N-Glycans of sphingosine 1-phosphate receptor Edg-1 regulate ligand-induced receptor internalization. Faseb J. 16, 983–992 [DOI] [PubMed] [Google Scholar]