Background: Cypher/ZASP plays an essential structural role in cardiac muscle.
Results: Cypher/ZASP specifically interacted with PKARIIα and calcineurin.
Conclusion: Cypher/ZASP is a novel AKAP acting as a sarcomeric signaling center for potential phosphorylation regulation the function of channels and myofilament proteins.
Significance: Cypher/ZASP-PKA-calcineurin complex expands our understanding the role of Cypher/ZASP in the heart.
Keywords: Akap, Calcineurin, Calcium Channels, Phosphorylation, Protein Kinase A (PKA), Cypher/ZASP
Abstract
PKA signaling is important for the post-translational modification of proteins, especially those in cardiomyocytes involved in cardiac excitation-contraction coupling. PKA activity is spatially and temporally regulated through compartmentalization by protein kinase A anchoring proteins. Cypher/ZASP, a member of PDZ-LIM domain protein family, is a cytoskeletal protein that forms multiprotein complexes at sarcomeric Z-lines. It has been demonstrated that Cypher/ZASP plays a pivotal structural role in the structural integrity of sarcomeres, and several of its mutations are associated with myopathies including dilated cardiomyopathy. Here we show that Cypher/ZASP, interacting specifically with the type II regulatory subunit RIIα of PKA, acted as a typical protein kinase A anchoring protein in cardiomyocytes. In addition, we show that Cypher/ZASP itself was phosphorylated at Ser265 and Ser296 by PKA. Furthermore, the PDZ domain of Cypher/ZASP interacted with the L-type calcium channel through its C-terminal PDZ binding motif. Expression of Cypher/ZASP facilitated PKA-mediated phosphorylation of the L-type calcium channel in vitro. Additionally, the phosphorylation of the L-type calcium channel at Ser1928 induced by isoproterenol was impaired in neonatal Cypher/ZASP-null cardiomyocytes. Moreover, Cypher/ZASP interacted with the Ser/Thr phosphatase calcineurin, which is a phosphatase for the L-type calcium channel. Taken together, our data strongly suggest that Cypher/ZASP not only plays a structural role for the sarcomeric integrity, but is also an important sarcomeric signaling scaffold in regulating the phosphorylation of channels or contractile proteins.
Introduction
In cardiomyocytes, the cAMP/PKA signaling initiated by β-adrenergic receptors (β-ARs)3 is of central importance for all functions, including excitation-contraction coupling, metabolism, hypertrophy, and survival (1, 2). The PKA holoenzyme is a heterotetramer containing two catalytic (C) and two regulatory (R) subunits (3). Four genes encode PKA regulatory subunits (RIα, RIβ, RIIα, and RIIβ) in mammals. Binding of the second-messenger cAMP to the regulatory subunits releases the catalytic subunits of PKA, which catalyze its substrate to be phosphorylated at either the serine or the threonine within the consensus sequence motif (RRX(S/T)X, X is variable) (4). The regulatory subunits also regulate the cellular localization of PKA by binding to a special group of proteins: A-kinase anchoring proteins (AKAPs).
AKAPs spatially and temporally restrict or compartmentalize the activity of PKA. To date, ≥70 AKAP genes have been identified, among which ∼20 are expressed in the heart (5). Three types of AKAPs have been classified. Type II AKAPs specifically bind to PKA RII, whereas type I AKAPs bind to RI. A few AKAPs with dual specificity bind to both RII and RI.
PKA-mediated phosphorylation of sarcomeric proteins induced by β-AR stimulation, including cardiac troponin I (cTnI) (6), myosin-binding protein C (MyBP-C) (7), titin (8), and myosin light chain (9), is also important for cardiac contraction and left ventricular torsion. The phosphorylation of cTnI and cardiac MyBP-C leads to decreased calcium responsiveness, thus increasing the myofibril relaxation rate. Some cardiac AKAPs have been shown to localize at sarcomeres, such as synemin (10), cardiac troponin T (11), myospryn (12), and myomegalin (13). Synemin and myospryn co-localize with PKARII at the Z-line or the Z-line/costamere in striated muscle. Myospryn also interacts with calcineurin (CaN) (14). Myomegalin might be an AKAP for the sacomeric proteins MyBP-C and cTnI. Cardiac troponin T is a dual specificity AKAP regulating cTnI phosphorylation through the troponin complex.
Initially, AKAPs were regarded as recruiters of PKA and phosphatases to form a signaling complex for each of its unique substrates. Recently, AKAP complexes have also been reported to regulate gene transcriptional expression. A direct role of AKAP79/150 has been suggested through its organized signal complexes cAMP/CREB (cAMP-response element-binding protein) or CaN/NFAT (15, 16).
Cypher/ZASP is a striated Z-line protein, which plays an important structural role in cardiac muscle in maintaining the integrity of sarcomeres under the stress of contraction force (17–20). Here, we report that the Z-line protein Cypher/ZASP is also a typical type II AKAP that specifically interacts with the RIIα regulatory subunit of PKA and the Ser/Thr phosphatase CaN, making Cypher/ZASP-PKA-CaN a signaling center for sarcomeric proteins or channels such as the L-type calcium channel (LTCC).
EXPERIMENTAL PROCEDURES
Antibodies and Mice
FLAG epitope, α-actinin, and plakoglobin antibodies were from Sigma-Aldrich. GST and Myc epitope antibodies were from Abcam. Antibodies against PKA-c, p-Erk1/2, Erk1/2, CaN, and PKA substrate were from Cell Signaling. LTCC phospho-Ser1928 antibody was from Badrilla Ltd. LTCC and GAPDH antibodies were from Santa Cruz Biotechnology. PKA RII antibody was either from Millipore or from Abcam. Calmodulin antibody was from Assay Biotech. The rabbit polyclonal Cypher antibody was generated by us.
Generation of Cypher knock-out mice has been described previously (19). Mice were maintained in a pathogen-free vivarium, and all procedures involving mice were approved by the Institutional Animal Care and Use Committee of Zhejiang University.
Plasmids
Plasmids containing the coding sequences for RIα, RIβ, RIIα, and RIIβ were gifts from Dr. Susan S. Taylor (University of California San Diego, La Jolla, CA). All tagged expression vectors (GST-, FLAG-, Myc-) were constructed in the pXJ40 vector as described previously (21). KOD polymerase was used for amplification, and DNA sequences were confirmed by DNA sequencing.
Protein-Protein Interactions in Vitro
Protein-protein interactions were studied in vitro using overexpression of tagged proteins in HEK293 cells. Plasmids were co-transfected using Lipofectamine 2000 (Invitrogen). 30 h later, cells were harvested and resuspended in radioimmune precipitation buffer assay (50 mm Tris, pH 7.4, 150 mm NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS) with protease inhibitors (Roche Applied Science) and phosphatase inhibitors (Roche Applied Science). To analyze LTCC phosphorylation, transient transfected cells were incubated with 100 μm forskolin (Sigma), 250 μm isobutylmethylxanthine for 30 min before harvesting the cells. Co-immunoprecipitation assays were performed using anti-protein tag antibodies and protein A-agarose beads (Roche Applied Science). Binding proteins were further analyzed by SDS-PAGE and immunoblot.
Immunostaining
3-month-old male mice were injected with isoproterenol (15 mg/kg, intraperitoneal). Saline was injected as control. 30 min later, mice were anesthetized, and hearts were dissected, immersed in iso-pentane, and snap-frozen for cryosectioning (Leica CM3050S).
Immunostaining of frozen sections (12 μm) was done as described (22). The samples were fixed in ice-cold acetone for 5 min, rehydrated in PBS, and incubated with primary antibody in gold buffer (155 mm NaCl, 2 mm EGTA, 2 mm MgCl2, 20 mm Tris-HCl, pH 7.5) overnight at 4 °C. After washing out primary antibody by PBS, the samples were incubated with secondary antibodies and DAPI at room temperature for 1 h, washed with PBS, and embedded in fluorescent embedding medium (DAKO). Images were taken using a Leica SP5 confocal microscope equipped with a 63× glycerol immersion objective in sequential scanning mode.
Neonatal Rat Cardiomyocyte Isolation
Neonatal rat cardiomyocytes were isolated from 1-day-old rats as described previously (23). Lentivirus-mediated gene transfer was performed 12 h later followed by 24 h in culture before harvesting the cells for protein analysis.
Structure Modeling
The PKARII-Cypher AA202–215 structure was modeled using Xtalview (24) based on the crystal structure of the RII-D/D-AKAP-IS complex (Protein Data Bank (PDB) accession code: 2izx) (25). The modeled structure was further refined using REFMA (26). All structural illustrations were prepared with PYMOL (52). The model showed that Thr203 from the Cypher peptide formed van der Waals contacts with Ile3, Ile5, and Ala21 from RII dimerization/docking domain (D/D). The substitution of Thr203 to Ile203 enhanced the van der Waals interactions.
CaN Enzymatic Activity Measurement
The CaN activity assay was performed as described (27). The CaN enzymatic activity was measured by using a colorimetric assay kit according to the manufacturer's instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) (28). The free phosphate released from synthetic RII phosphopeptide substrate by calcineurin was measured using malachite green assay. Cyclosporin A, a specific inhibitor for calcineurin, was used. The calcineurin specific activity was calculated from subtracting the value measured in the presence of cyclosporin A from total activity measured without cyclosporin A.
To measure activated calcineurin, calcineurin/calmodulin co-immunoprecipitations were performed as described (27, 29). Mouse hearts were lysed in co-immunoprecipitation buffer (20 mmol/liter NaPO4, 150 mmol/liter NaCl, 2 mmol/liter MgCl2, 0.1% Nonidet P-40, 10% glycerol, 1 mmol/liter DTT) with protease inhibitor (Roche Applied Science) and phosphatase inhibitor (Roche Applied Science). The lysate was incubated with calcineurin or calmodulin antibody followed by the addition of protein A-agarose beads. The washed beads were subjected to SDS-PAGE and immunoblot.
Real-time PCR
Total RNA was isolated from 1-day-old mouse hearts using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. Real-time PCR was performed as described previously (30). The oligonucleotide primers for MCIP1.4 were: forward: 5′-TCCAGCTTGGGCTTGACTGAG-3′, and reverse, 5′-ACTGGAAGGTGGTGTCCTTGTC-3′.
Statistics
Data were expressed as mean ± S.E. We performed statistical evaluation using Student's unpaired t test. p < 0.05 was considered to be statistically significant.
RESULTS
Cypher/ZASP Interacted with the Regulatory Subunit RIIα of PKA
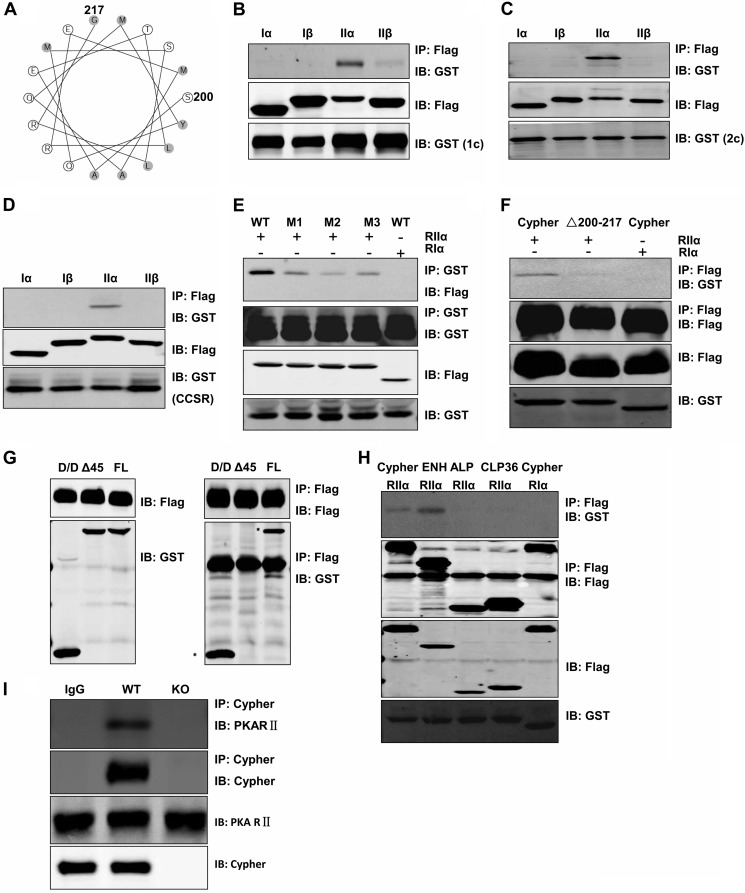
Cypher/ZASP contains a PDZ domain at its N terminus and three LIM domains at its C terminus with a short ZASP motif (ZM, AA186–211) in the middle. By analyzing the secondary structure of the Cypher1c amino acid sequence (AF114378_1), we found that the fragment AA200–217 could potentially form an amphipathic helix (Fig. 1A), a feature of AKAPs. The AA200–217 sequence partly overlaps with the ZM region (AA186–211). To determine whether Cypher indeed interacts with PKA regulatory subunits through its ZM domain, GST-tagged Cypher was co-expressed with each of the four FLAG-tagged PKA regulatory subunits (RIα, RIβ, RIIα and RIIβ) in HEK293 cells. Cypher protein was specifically immunoprecipitated with RIIα (Fig. 1B). Similarly, both Cypher2c (2c), without the LIM domains, and the Cypher cardiac-specific region (CCSR, AA108–227), being encoded by the exon 4, interacted with the PKA RIIα (Fig. 1, C and D). To further determine whether the α helix was essential for the interaction, we substituted proline at each of the following amino acid residue positions, Thr203, Leu204, and Leu215, to disrupt the helix. All three variants (T203P, L204P, and L215P) significantly decreased the binding of Cypher to PKARIIα (Fig. 1E), whereas the mutant Cypher with an AA200–217 deletion had no interaction (Fig. 1F). Therefore, the CCSR of Cypher specifically interacted with PKA RIIα.
FIGURE 1.
Cypher interacted directly with RIIα regulatory subunit of PKA. A, an amphipathic helical wheel plot for amino acid residues AA200–217 of Cypher1c (AF114378_1) reveals a hydrophobic surface on one side (shaded). B–D, GST-tagged Cypher1c (B), Cypher2c (C), or Cypher cardiac-specific region (CCSR, AA108–227) (D) and FLAG-tagged PKA regulatory subunits (RIα, RIβ, RIIα, RIIβ) were co-expressed in HEK293 cells. FLAG-tagged proteins were immunoprecipitated with anti-FLAG antibody, and interacting proteins were verified with anti-GST antibody in immunoblot (IB). IP, immunoprecipitation. E, GST-tagged Cypher CCSR and its variants with an introduced proline (M1, T203P; M2, L204P; M3, L215P) were co-transfected with FLAG-tagged RIIα (RIα as a negative control) in HEK293 cells. Cell lysates were used for immunoprecipitation to verify the interaction. F, FLAG-tagged Cypher or its AA200–217 deletion mutant (ΔAA200–217) was co-expressed with GST-tagged RIIα. Cell lysates were used for immunoprecipitation to verify the interaction. G, FLAG-tagged Cypher was co-expressed with GST-tagged RIIα full-length (FL), its D/D (AA1–45), or its D/D deletion mutant (Δ45) in HEK293 cells. Cell lysates were used for immunoprecipitation to verify the interaction. H, FLAG-tagged Cypher, ENH, ALP, and CLP36 were co-expressed with GST-tagged RIIα (RIα as a negative control) in HEK293 cells. Protein interactions were determined by immunoprecipitation. I, Cypher protein was immunoprecipitated from neonatal mouse heart lysate by anti-Cypher antibody, and the interaction with PKA RII protein was verified by anti-PKARII antibody. Rabbit IgG and Cypher-null heart lysate were used as controls.
The D/D of RIIα is involved in the interactions of typical AKAPs. To further study whether the D/D domain was involved in the interaction with Cypher, FLAG-tagged Cypher1c was co-expressed with either the GST-tagged full-length, D/D domain (1–45) or the D/D deletion (ΔD/D) of RIIα in HEK293 cells. Both full-length and D/D domain interacted with Cypher, whereas ΔD/D did not (Fig. 1G). Therefore, Cypher interacted with the D/D region of RIIα, displaying the characteristics of a genuine AKAP.
Cypher belongs to the ALP/Enigma subfamily of the PDZ-LIM domain protein family. We further investigated the interactions between PKA RIIα and other members of the ALP/Enigma subfamily. We found that, like Cypher, enigma homolog (ENH) bound to PKA RIIα, whereas ALP and CLP36 did not (Fig. 1H).
To confirm the interaction in vivo, an anti-Cypher antibody was used for a co-immunoprecipitation assay. RII protein was pulled down together with Cypher (Fig. 1I) in wild-type but not Cypher-null mouse hearts. Rabbit IgG failed to pull down either Cypher or PKARII.
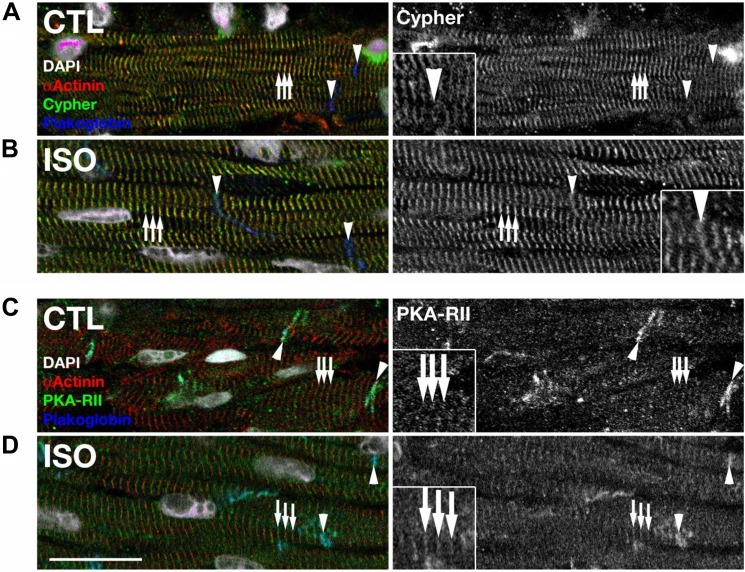
To further verify the interaction between Cypher and PKARII in cardiomyocytes, immunostaining was performed (Fig. 2). As shown previously (17), Cypher localized predominantly at Z-lines together with sarcomeric α-actinin (Fig. 2A). Isoproterenol treatment did not disturb the Z-line localization of Cypher (Fig. 2B); however, it lead to more pronounced localization of Cypher at intercalated disks (Fig. 2B, inset). PKARII localized at intercalated disks but did not show significant striated staining pattern in control hearts (Fig. 2C). However, hearts treated with isoproterenol showed increasing localization of PKARII at Z-lines, as judged by colocalization with α-actinin (Fig. 2D). Thus, endogenously expressed Cypher and PKARII may also form a protein complex at Z-lines of mouse cardiomyocytes.
FIGURE 2.
Subcellular localization of Cypher (A and B) and PKARII (C and D) in heart tissues of adult mice. A and B, Cypher localized at Z lines (arrows) in mouse cardiomyocytes as judged by α-actinin co-staining. Z line localization of Cypher was not perturbed by treatment of mice with isoproterenol; however, Cypher showed additionally slight intercalated disk localization (arrowheads) in treated hearts when compared with controls (compare inset in A and B). C and D, PKARII was found to localize at intercalated discs (arrowheads) in control hearts, as judged by plakoglobin counterstaining. Upon isoproterenol treatment, PKARII was found to localize in a more pronounced way to Z lines (arrows), in addition to its intercalated disk localization (compare inset in C and D). Sarcomeric α-actinin was used to mark Z-lines (arrows); plakoglobin was use to mark cardiac intercalated disks (arrowheads). Scale bars: 20 μm.
The Cardiomyopathy-associated Mutation T206I (T203I in Mouse) Enhanced the Interaction between ZASP and PKARII
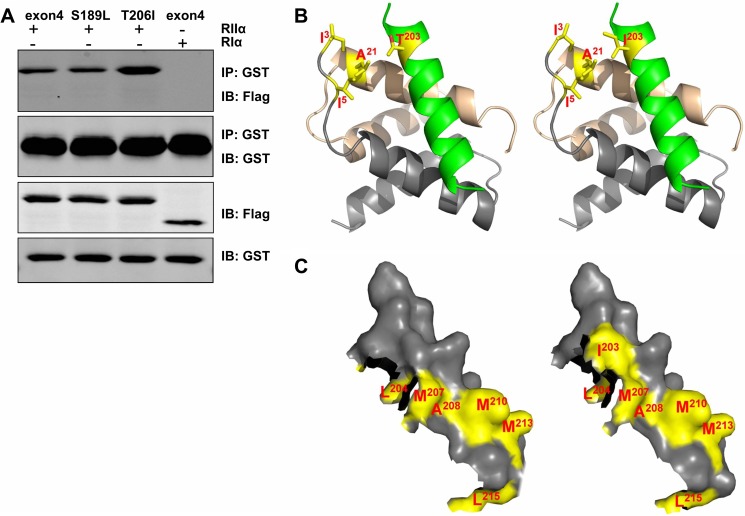
Several ZASP mutations have been identified in human patients with myopathies including dilated cardiomyopathy. Some of the mutations are localized to the sequence encoded by exon 4. Similar to Cypher CCSR, the peptide encoded by the ZASP exon 4 interacted with PKA RIIα (Fig. 3A). Mutation S189L did not change the interaction, whereas mutation T206I significantly enhanced it (Fig. 3A). For further investigation of the interaction, the RIIα-AKAP-IS crystal structure was adapted, and the AKAP-IS sequence was superimposed over the Cypher sequence (AA202–215). As shown in the model, the Thr203 residue forms van der Waals contacts with the Ala21, Ile3, and Ile5 residues of RII D/D, whereas substitution of Thr203 to Ile203 enhances the van der Waals interactions (Fig. 3B). Surface representations of the Cypher helix (AA202–215) showed that the substitution of Thr203 to Ile203 also increased the hydrophobic surface involving the PKA RII D/D interaction (Fig. 3C).
FIGURE 3.
Cardiomyopathy-associated Cypher/ZASP mutation enhanced the PKA-Cypher interaction. A, GST-tagged human ZASP exon 4 encoded fragment and two mutants (S189L and T206I) were co-transfected with FLAG-tagged PKA RIIα (RIα as a control) in HEK293 cells. Protein interactions were assayed by immunoprecipitation (IP). IB, immunoblotting. B, stick models of RIIα D/D-Cypher Glu202–Leu215 (WT, left; T203I mutant, right) complex (RII D/D dimer at the back in light brown and gray, Cypher Glu202–Leu215 at the front in green) superimposed onto the RIIα D/D-AKAP-IS structure. C, surface representation of the Cypher helix (Glu202–Leu215) (WT, left; T203I mutant, right) with the highlighted hydrophobic residues (yellow) involved in PKA RIIα binding.
Cypher/ZASP Facilitated Phosphorylation of the LTCC by PKA
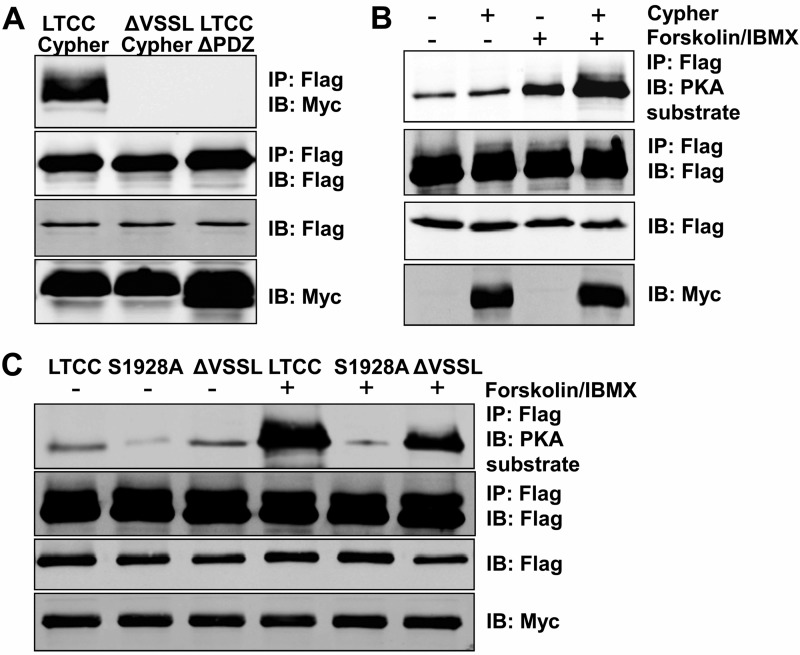
It has been reported that the LTCC contains a PDZ-binding motif at its cytosolic tail and interacts with the PDZ domain of ENH (31), which together with Cypher and Enigma, belongs to the Enigma subfamily of the PDZ-LIM domain protein family. To verify the interaction between Cypher and LTCC, the FLAG-tagged Cypher1c full-length or its PDZ (AA1–82) deletion mutant was co-expressed with the Myc-tagged LTCC cytosolic region (the last 662 amino acid residues) or a deletion mutant missing the last four residues (ΔVSSL) in HEK293 cells (Fig. 4A). Cypher interacted with the Myc-tagged LTCC cytosolic region, whereas either the deletion mutant of the LTCC or the PDZ deletion of Cypher completely obliterated the interaction (Fig. 4A). Subsequently, the phosphorylation of LTCC cytosolic region was studied in HEK293 cells in vitro. The addition of a PKA agonist forskolin dramatically increased the phosphorylation of the LTCC (Fig. 4B). Without stimulation, the expression of Cypher slightly increased the phosphorylation of the LTCC, whereas with forskolin stimulation, the phosphorylation of the LTCC was increased further (Fig. 4B). Furthermore, the S1928A mutation completely blocked the phosphorylation of LTCC mediated by PKA (Fig. 4C). Therefore, for the cytosolic region of LTCC, Ser1928 was the dominant PKA catalytic site. Disrupting the interaction between LTCC and Cypher by either the ΔVSSL deletion of the LTCC or the deletion of the Cypher PDZ domain significantly impaired the PKA-mediated phosphorylation. Our results showed that Cypher interacted with LTCC and facilitated PKA-mediated phosphorylation of the LTCC at Ser1928.
FIGURE 4.
Cypher/ZASP tethered PKA to phosphorylate the LTCC cytosolic region at Ser1928in vitro. A, the Cypher PDZ domain interacted with the LTCC C-terminal PDZ binding motif (VSSL). Myc-tagged Cypher or its PDZ domain deletion mutant (ΔPDZ) was co-expressed with FLAG-tagged LTCC cytosolic region (AA1510–2172) or its deletion mutant (ΔVSSL) in HEK293 cells. FLAG-tagged proteins were enriched by anti-FLAG antibody, and interacting proteins were verified by blotting with an anti-Myc antibody. IP, immunoprecipitation; IB, immunoblotting. B, FLAG-tagged LTCC cytosolic region with or without Myc-tagged Cypher was co-expressed in HEK293 cells. Forskolin (100 μm, 30 min) was used to activate PKA. LTCC protein was purified by anti-FLAG antibody, and the phosphorylation was detected by a phospho-(Ser/Thr) PKA substrate antibody. IBMX, 3-isobutyl-1-methylxanthine. C, LTCC and its mutants S1928A and C-terminal VSSL deletion (ΔVSSL) were expressed in HEK293 cells with Cypher. LTCC phosphorylation with or without the PKA agonist forskolin treatment was assessed.
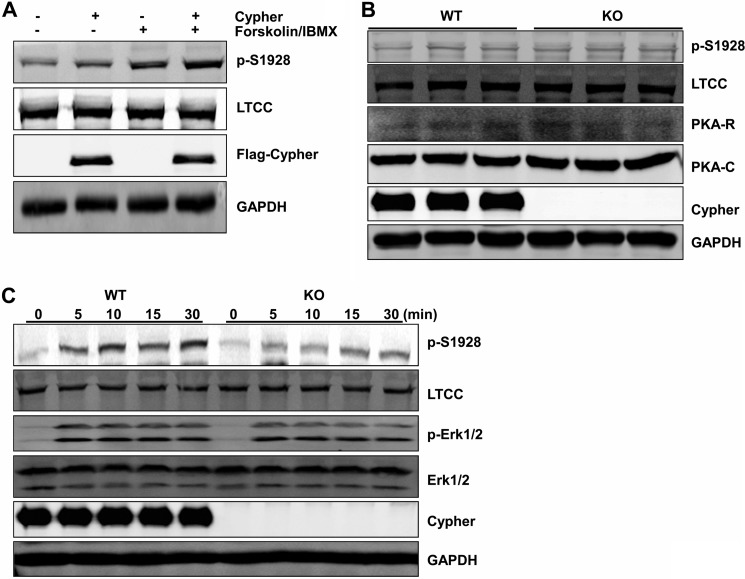
We further confirmed the role of Cypher in cultured cardiomyocytes utilizing virus-mediated overexpression of Cypher protein in neonatal rat cardiomyocytes. With Cypher overexpression and forskolin stimulation, phosphorylation of the LTCC was dramatically increased (Fig. 5A). We then evaluated the expression levels of the LTCC and PKA in Cypher-null mouse hearts. Cypher deletion did not change the protein levels of the catalytic subunit of PKA, the RII regulatory subunit of PKA, LTCC, nor the phosphorylated LTCC protein (Fig. 5B). Last, isoproterenol injection dramatically increased the phosphorylation of LTCC at Ser1928 in WT mouse hearts, and this was blunted in Cypher-null hearts (Fig. 5C). Similar to our in vitro data, PKA was tethered by Cypher to LTCC phosphorylating the LTCC at Ser1928 in cardiomyocytes.
FIGURE 5.
Cypher facilitated the phosphorylation of LTCC by PKA in vivo. A, neonatal rat cardiomyocytes were transfected with Lenti-FLAG-Cypher viruses (or empty vector viruses) and treated with forskolin (100 μm, 30 min). Cell lysates were analyzed for total and phosphorylated LTCC (phospho-Ser1928-specific antibody (p-S1928)). IBMX, 3-isobutyl-1-methylxanthine. B, heart lysates from 1-day-old wild-type (WT) or Cypher-null mice (KO) were analyzed for LTCC, phosphorylated LTCC, PKA RII subunit, PKA catalytic subunit, and Cypher. C, neonatal mice were given isoproterenol (15 mg/kg in saline, 20 μl) at different time points before the hearts were dissected, and lysates were analyzed for the LTCC and its phosphorylation; the phosphorylation of Erk1/2 was used to show that the PKA signal was activated by isoproterenol. GAPDH was used to show equal protein loading.
Cypher Interacted with Ser/Thr Phosphatase CaN, Blocking Its Activity
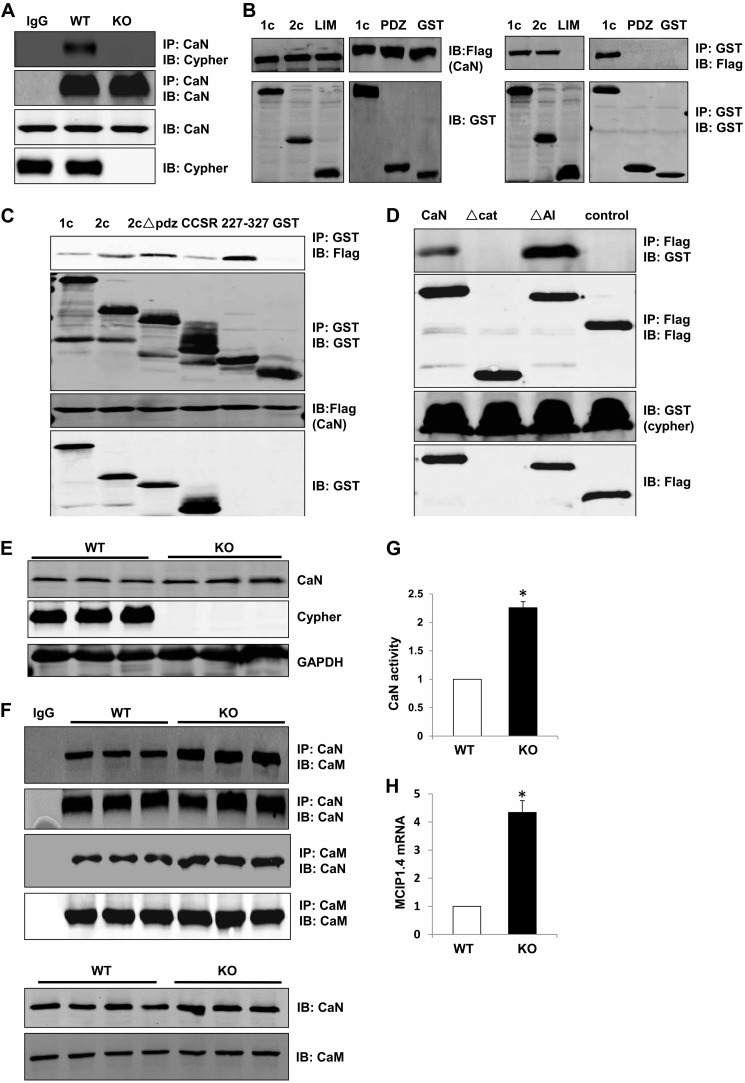
It is a hallmark of AKAPs to bind to not only PKA, but also phosphatases or phosphodiesterases. Hence we investigated the potential interaction between Cypher and the Ser/Thr phosphatase CaN. In the mouse hearts, CaN and Cypher were in the same protein complex, as shown by co-immunoprecipitation (Fig. 6A). To confirm this interaction, tagged Cypher1c and 2c, along with the PDZ and LIM domains, were co-expressed with tagged CaN in HEK293 cells. Cypher1c and 2c interacted with CaN, whereas the N-terminal PDZ domain and C-terminal LIM domains did not (Fig. 6B). However, we discovered that different regions of Cypher2c (ΔPDZ, CCSR, and AA227–327) interacted with CaN (Fig. 6C). Deletion of the catalytic domain (AA45–345, Δcat) of CaN impaired the interaction with Cypher, whereas deletion of the AI region (AA455–477) enhanced it (Fig. 6D). Thus, Cypher interacted with CaN at the catalytic domain competing with the AI region of CaN to bind to the catalytic domain. Further, deletion of Cypher in mouse hearts did not change the protein level of CaN (Fig. 6E); however, we did detect more CaM (or CaN) protein co-immunoprecipitating with CaN (or CaM) (Fig. 6F) and increased CaN enzymatic activity (Fig. 6G). This was further evidenced by an increase in the mRNA level of modulatory calcineurin-interacting protein 1, exon 4 isoform (MCIP1.4) (32), a downstream target of calcineurin signaling, in Cypher-null hearts when compared with WT controls (Fig. 6H). Therefore, the enzymatic activity of CaN was increased in Cypher-null hearts, although the protein expression of CaN was unchanged (Fig. 6, E–H).
FIGURE 6.
Cypher directly interacted with CaN and blocked its activity. A, CaN protein was precipitated from adult mouse heart lysates, and the interaction with Cypher was verified with an anti-Cypher antibody. Rabbit IgG was used for control. IP, immunoprecipitation; IB, immunoblotting. B, GST alone or GST-tagged Cypher1c, 2c, PDZ domain (AA1–80), and LIM domains (AA547–723) were co-transfected with FLAG-tagged CaN in HEK293 cells. Interacting proteins were verified by immunoprecipitation. C, GST alone or GST-tagged Cypher1c, 2c, 2c with PDZ domain deletion (2cΔPDZ), CCSR, and 2c227–327 was co-transfected with FLAG-tagged CaN in HEK293 cells. Interactions were verified by immunoprecipitation. D, FLAG-tagged CaN and its mutants (Δcat: Δ45–345, ΔAI: Δ455–477) were co-transfected with GST-tagged Cypher1c in HEK293 cells. Protein interactions were verified by immunoprecipitation. E, WT and Cypher-null neonatal heart lysates were probed with an anti-CaN antibody. F, CaN proteins (or CaM) were precipitated from neonatal WT, and Cypher-null hearts and interacting CaM (or CaN) were determined by probing with an anti-CaM antibody (or CaN antibody). G, CaN enzymatic activity from WT and Cypher-null neonatal heart lysates was measured (n = 4). The values were normalized to the average WT value. H, the mRNA levels of modulatory calcineurin-interacting protein 1, the exon4 isoform, MCIP1.4, in 1-day-old WT and Cypher KO mouse hearts (n = 3) measured by real-time PCR. The data are presented as the ratios of MCIP1.4 to 18 S RNA, an internal standard, and were normalized to the WT values. *, p < 0.05. Error bars indicate mean ± S.E.
Cypher/ZASP Was Phosphorylated at Ser265 and Ser296 by PKA
Cypher/ZASP interacted with the RIIα regulatory subunit of PKA. Next we further analyzed whether Cypher could be phosphorylated by PKA. We searched the Cypher1c sequence for the PKA substrate consensus sequence ((K/R)(K/R)X(S/T)X, X is variable), and two putative PKA substrate phosphorylation sites, Ser265 and Ser296, were identified (Fig. 7A). Ser265 is conserved between mouse and human, whereas Ser296 is unique to mouse. To begin to address the question, we first observed enhanced phosphorylation of the Cypher protein following isoproterenol treatment in the WT mouse hearts (Fig. 7B). We then analyzed whether mutants of S265A and S296A altered the phosphorylation of Cypher. Although both single mutants significantly decreased the phosphorylation of Cypher following forskolin stimulation in HEK293 cells, the double mutation S265A/S296A completely eliminated the Cypher phosphorylation (Fig. 7C).
FIGURE 7.
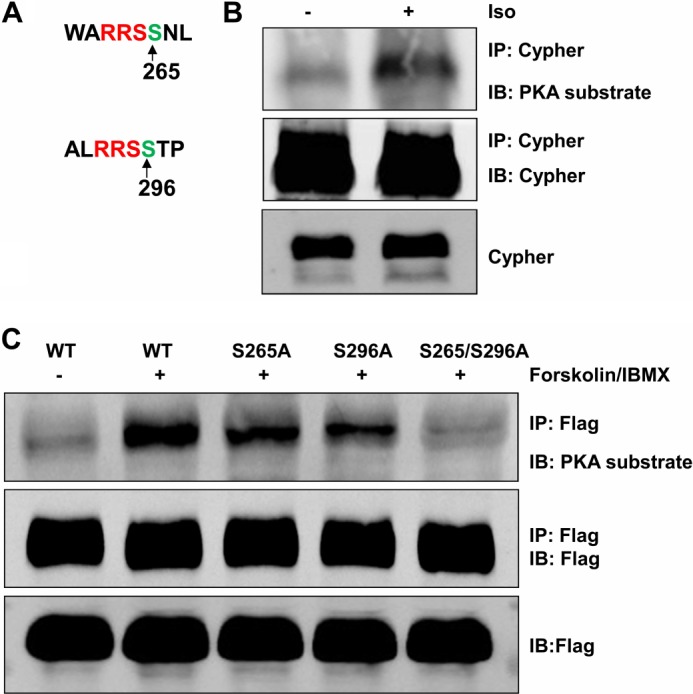
Cypher was phosphorylated at Ser265 and Ser296 by PKA. A, Cypher Ser265 and Ser296 were potential PKA phosphorylation sites. B, heart lysates from adult mice treated with isoproterenol (Iso, 15 mg/kg, 20 μl) were immunoprecipitated for Cypher, and Cypher phosphorylation was detected by phospho-(Ser/Thr) PKA substrate antibody. C, FLAG-tagged Cypher and its variants Cypher S265A, S296A, and the double mutation S265A/S296A (indicated by S265/S296A) were expressed in HEK293 cells, and purified proteins were assayed for phosphorylation by phospho-(Ser/Thr) PKA substrate antibody.
DISCUSSION
Cypher/ZASP, containing a PDZ domain at its N terminus and three LIM domains at its C terminus, belongs to the PDZ-LIM domain protein family (17, 33). Cypher/ZASP is a sarcomeric protein interacting with the Z-line proteins α-actinin-2, myotilin, and calsarcin and the signaling molecule protein kinase Cs (17, 18, 34). We have demonstrated that Cypher plays essential roles in the maintenance of the sarcomere integrity especially under contraction force stress (18, 19, 30). Cypher global-null mice display postnatal death with cardiac and skeletal muscle defects. Conditional or postnatal inducible deletion of Cypher in mouse cardiomyocytes causes premature death and severe dilated cardiomyopathy. The identification of ZASP variants associated with myopathies such as dilated cardiomyopathy, left ventricular noncompaction cardiomyopathy, and myofibrillar myopathy (zaspopathy) further demonstrates the requirement of Cypher/ZASP for the normal function of striated muscle (35–37). It is a feature that the LIM domains of Enigma subfamily proteins interact with protein kinase C (38). For Cypher/ZASP, the increased affinity to PKC caused by the substitution Asp626 to Asn is associated with dilated cardiomyopathy (35). Moreover, deletion of Cypher or its splice isoform in mouse hearts leads to abnormalities of certain signaling pathways (18, 30). However, no direct evidence has linked Cypher/ZASP to any signal transduction pathways. Currently, our study shows that Cypher/ZASP is an AKAP. Thus, Cypher/ZASP plays not only an important structural role in sarcomeric Z-lines, but also forms a signaling center to regulate the phosphorylation of some proteins, including the LTCC and Cypher/ZASP itself. These findings are in line with the concept that cardiac Z-lines are signaling centers as well as important structural components of sarcomeres (39).
Here we provided new evidence that Cypher/ZASP is a typical type II AKAP, which tethers PKA and Ser/Thr phosphatase CaN to their specific substrates, such as LTCC, to control its function by modulating post-translational phosphorylation. The AA200–217 fragment of Cypher, forming an amphipathic helix, specifically docks to the D/D domain of PKA RIIα. Deletion of either the Cypher AA200–217 region or the RII D/D domain completely abolished the interaction (Fig. 1, F and G). The amino acid residues AA200–217 partially overlap with the ZM motif (AA186–211), which has been assumed to be involved in the interaction with the rod domain of α-actinin (40). Interestingly, other members of the Enigma/ALP subfamily, such as ALP, which has a ZM motif (20), did not bind to PKA RIIα; however, ENH, without ZM, did bind to PKA RIIα (Fig. 1H).
Similarly to ENH, the PDZ domain of Cypher/ZASP binds to the LTCC at its C-terminal PDZ binding motif (31). We have demonstrated that, as an AKAP, Cypher/ZASP tethers PKA to the LTCC and phosphorylates Ser1928 both in vitro and in vivo. CaN, which has been reported as a phosphatase for the LTCC (41), also interacts with Cypher/ZASP. All these findings point to an additional role for Cypher/ZASP as a signaling center for regulating the Ser1928 phosphorylation of LTCC in cardiomyocytes. A few membrane-associated AKAPs, such as AKAP18α and AKAP79, have been reported to restrict PKA kinase activation to the LTCC (16, 42, 43). Cypher/ZASP is a dominant sarcomeric Z-line protein. The T-tubules and sarcomeric Z-lines are in close proximity within cardiomyocytes, facilitating the interaction between the sarcomeric protein Cypher/ZASP and the membrane protein LTCC, aiding in PKA regulation of LTCC channel function (44).
Our data showed that Cypher/ZASP facilitated PKA-mediated phosphorylation of LTCC at its Ser1928. The S1928A substitution abolished phosphorylation of LTCC, even with forskolin treatment, which is consistent with a previous study (45). Cypher-null hearts had impaired LTCC phosphorylation at Ser1928 induced by isoproterenol. However, the role of Ser1928 phosphorylation in LTCC pump function is under debate. Ser1928 is a well characterized PKA/PKC/PKG phosphorylation site (46). Our data show that Ser1928 is the unique PKA substrate site in the cytosolic region of the LTCC (Fig. 4C). A number of in vitro studies have shown that the calcium pump function of the LTCC is regulated by modification of the phosphorylation at Ser1928 mediated by PKA, which is triggered by β-AR stimulation (2). However, ablation of LTCC Ser1928 phosphorylation in a knock-in transgenic mouse model does not affect the calcium current induced by the β-AR-cAMP-PKA signaling in mouse cardiomyocytes (45). Therefore, the actual in vivo function of Cypher/ZASP as an AKAP requires further study, including identification of the central target in the Cypher/ZASP-PKA-CaN multi-molecule signaling complex.
CaN is a Ca2+/CaM dependent Ser/Thr phosphatase contributing to hypertrophic signaling in cardiomyocytes (47). Our previous work showed that deletion of the Cypher long isoforms leads to late onset dilated cardiomyopathy with overactivated CaN signaling that includes NFATc4 dephosphorylation and nuclear translocation followed by increased transcription of the NFAT downstream gene RCAN1.4 (30). The catalytic domain of CaN interacted with the middle region of Cypher/ZASP (Fig. 6, C and D). Interestingly, the deletion of the autoinhibitory region of CaN enhanced the interaction. Moreover, deletion of Cypher increased CaN-bound CaM, and therefore, the CaN enzymatic activity was increased. Several proteins have been reported to inhibit CaN activity such as calsarcin (48), RCAN1.4 (49), AKAP79 (50), and FHL2 (51). It is also worthy of note that Cypher/ZASP might be a new physiological inhibitor of CaN.
Acknowledgments
We thank Iain C. Bruce for a critical reading and language revision of the manuscript. We acknowledge the help of the University of California San Diego (UCSD) Microscopy Core Facility (National Institutes of Health Grant P30 NS047101) and Jennifer Santini.
This work was supported by National Natural Science Foundation of China Grant 81170117 (to H. C.), Zhejiang Provincial Natural Science Foundation of China Grant LY12H02006 (to X. G.), Major Research Program from the state Ministry of Science and Technology of China Grant 2010CB912004 (to Y. K.), and by National Institutes of Health K99/R00 Grant HL107744 (to S. L.).
- β-AR
- β-adrenergic receptor
- AKAP
- A-kinase anchoring proteins
- PKARII
- PKA regulatory subunit II
- MyBP-C
- myosin-binding protein C
- cTnI
- cardiac troponin I
- CaN
- calcineurin
- CaM
- calmodulin
- NFAT
- nuclear factor of activated T-cells
- LTCC
- L-type calcium channel
- D/D
- dimerization/docking domain
- CCSR
- Cypher cardiac-specific region
- 2c
- Cypher2c
- ENH
- enigma homolog
- ZM
- ZASP motif
- ZASP
- Z-band alternatively spliced PDZ motif protein
- ALP
- actinin-associated LIM protein
- MCIP
- modulatory calcineurin-interacting protein.
REFERENCES
- 1. Woo A. Y., Xiao R. P. (2012) β-Adrenergic receptor subtype signaling in heart: from bench to bedside. Acta Pharmacol. Sin. 33, 335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harvey R. D., Hell J. W. (2013) CaV1.2 signaling complexes in the heart. J. Mol. Cell. Cardiol. 58, 143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taylor S. S., Kim C., Vigil D., Haste N. M., Yang J., Wu J., Anand G. S. (2005) Dynamics of signaling by PKA. Biochim. Biophys. Acta 1754, 25–37 [DOI] [PubMed] [Google Scholar]
- 4. Zhang J., Ma Y., Taylor S. S., Tsien R. Y. (2001) Genetically encoded reporters of protein kinase A activity reveal impact of substrate tethering. Proc. Natl. Acad. Sci. U.S.A. 98, 14997–15002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blant A., Czubryt M. P. (2012) Promotion and inhibition of cardiac hypertrophy by A-kinase anchor proteins. Can J. Physiol. Pharmacol. 90, 1161–1170 [DOI] [PubMed] [Google Scholar]
- 6. Dong X., Sumandea C. A., Chen Y. C., Garcia-Cazarin M. L., Zhang J., Balke C. W., Sumandea M. P., Ge Y. (2012) Augmented phosphorylation of cardiac troponin I in hypertensive heart failure. J. Biol. Chem. 287, 848–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang Q., Hewett T. E., Klevitsky R., Sanbe A., Wang X., Robbins J. (2001) PKA-dependent phosphorylation of cardiac myosin binding protein C in transgenic mice. Cardiovasc Res. 51, 80–88 [DOI] [PubMed] [Google Scholar]
- 8. Yamasaki R., Wu Y., McNabb M., Greaser M., Labeit S., Granzier H. (2002) Protein kinase A phosphorylates titin's cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes. Circ. Res. 90, 1181–1188 [DOI] [PubMed] [Google Scholar]
- 9. Sheikh F., Ouyang K., Campbell S. G., Lyon R. C., Chuang J., Fitzsimons D., Tangney J., Hidalgo C. G., Chung C. S., Cheng H., Dalton N. D., Gu Y., Kasahara H., Ghassemian M., Omens J. H., Peterson K. L., Granzier H. L., Moss R. L., McCulloch A. D., Chen J. (2012) Mouse and computational models link Mlc2v dephosphorylation to altered myosin kinetics in early cardiac disease. J. Clin. Invest. 122, 1209–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Russell M. A., Lund L. M., Haber R., McKeegan K., Cianciola N., Bond M. (2006) The intermediate filament protein, synemin, is an AKAP in the heart. Arch. Biochem. Biophys. 456, 204–215 [DOI] [PubMed] [Google Scholar]
- 11. Sumandea C. A., Garcia-Cazarin M. L., Bozio C. H., Sievert G. A., Balke C. W., Sumandea M. P. (2011) Cardiac troponin T, a sarcomeric AKAP, tethers protein kinase A at the myofilaments. J. Biol. Chem. 286, 530–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reynolds J. G., McCalmon S. A., Tomczyk T., Naya F. J. (2007) Identification and mapping of protein kinase A binding sites in the costameric protein myospryn. Biochim. Biophys. Acta 1773, 891–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Uys G. M., Ramburan A., Loos B., Kinnear C. J., Korkie L. J., Mouton J., Riedemann J., Moolman-Smook J. C. (2011) Myomegalin is a novel A-kinase anchoring protein involved in the phosphorylation of cardiac myosin binding protein C. BMC Cell Biol. 12, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kielbasa O. M., Reynolds J. G., Wu C. L., Snyder C. M., Cho M. Y., Weiler H., Kandarian S., Naya F. J. (2011) Myospryn is a calcineurin-interacting protein that negatively modulates slow-fiber-type transformation and skeletal muscle regeneration. FASEB J 25, 2276–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang J., Shapiro M. S. (2012) Activity-dependent transcriptional regulation of M-Type (Kv7) K+ channels by AKAP79/150-mediated NFAT actions. Neuron 76, 1133–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oliveria S. F., Dell'Acqua M. L., Sather W. A. (2007) AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron 55, 261–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou Q., Ruiz-Lozano P., Martone M. E., Chen J. (1999) Cypher, a striated muscle-restricted PDZ and LIM domain-containing protein, binds to α-actinin-2 and protein kinase C. J. Biol. Chem. 274, 19807–19813 [DOI] [PubMed] [Google Scholar]
- 18. Zheng M., Cheng H., Li X., Zhang J., Cui L., Ouyang K., Han L., Zhao T., Gu Y., Dalton N. D., Bang M. L., Peterson K. L., Chen J. (2009) Cardiac-specific ablation of Cypher leads to a severe form of dilated cardiomyopathy with premature death. Hum. Mol. Genet. 18, 701–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou Q., Chu P. H., Huang C., Cheng C. F., Martone M. E., Knoll G., Shelton G. D., Evans S., Chen J. (2001) Ablation of Cypher, a PDZ-LIM domain Z-line protein, causes a severe form of congenital myopathy. J. Cell Biol. 155, 605–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zheng M., Cheng H., Banerjee I., Chen J. (2010) ALP/Enigma PDZ-LIM domain proteins in the heart. J Mol. Cell. Biol. 2, 96–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cheng H., Kimura K., Peter A. K., Cui L., Ouyang K., Shen T., Liu Y., Gu Y., Dalton N. D., Evans S. M., Knowlton K. U., Peterson K. L., Chen J. (2010) Loss of enigma homolog protein results in dilated cardiomyopathy. Circ. Res. 107, 348–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lange S., Ouyang K., Meyer G., Cui L., Cheng H., Lieber R. L., Chen J. (2009) Obscurin determines the architecture of the longitudinal sarcoplasmic reticulum. J. Cell Sci. 122, 2640–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shen T., Zheng M., Cao C., Chen C., Tang J., Zhang W., Cheng H., Chen K. H., Xiao R. P. (2007) Mitofusin-2 is a major determinant of oxidative stress-mediated heart muscle cell apoptosis. J. Biol. Chem. 282, 23354–23361 [DOI] [PubMed] [Google Scholar]
- 24. McRee D. E. (1999) XtalView/Xfit—A versatile program for manipulating atomic coordinates and electron density. J. Struct. Biol. 125, 156–165 [DOI] [PubMed] [Google Scholar]
- 25. Gold M. G., Lygren B., Dokurno P., Hoshi N., McConnachie G., Taskén K., Carlson C. R., Scott J. D., Barford D. (2006) Molecular basis of AKAP specificity for PKA regulatory subunits. Mol. Cell 24, 383–395 [DOI] [PubMed] [Google Scholar]
- 26. Collaborative Computational Project, Number 4 (1994) The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol. Crystallogr 50, 760–763 [DOI] [PubMed] [Google Scholar]
- 27. Frey N., Barrientos T., Shelton J. M., Frank D., Rütten H., Gehring D., Kuhn C., Lutz M., Rothermel B., Bassel-Duby R., Richardson J. A., Katus H. A., Hill J. A., Olson E. N. (2004) Mice lacking calsarcin-1 are sensitized to calcineurin signaling and show accelerated cardiomyopathy in response to pathological biomechanical stress. Nat. Med. 10, 1336–1343 [DOI] [PubMed] [Google Scholar]
- 28. Pang X., Sun N. L. (2009) Calcineurin-NFAT signaling is involved in phenylephrine-induced vascular smooth muscle cell proliferation. Acta Pharmacol. Sin. 30, 537–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lim H. W., De Windt L. J., Steinberg L., Taigen T., Witt S. A., Kimball T. R., Molkentin J. D. (2000) Calcineurin expression, activation, and function in cardiac pressure-overload hypertrophy. Circulation 101, 2431–2437 [DOI] [PubMed] [Google Scholar]
- 30. Cheng H., Zheng M., Peter A. K., Kimura K., Li X., Ouyang K., Shen T., Cui L., Frank D., Dalton N. D., Gu Y., Frey N., Peterson K. L., Evans S. M., Knowlton K. U., Sheikh F., Chen J. (2011) Selective deletion of long but not short Cypher isoforms leads to late-onset dilated cardiomyopathy. Hum. Mol. Genet. 20, 1751–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maturana A. D., Wälchli S., Iwata M., Ryser S., Van Lint J., Hoshijima M., Schlegel W., Ikeda Y., Tanizawa K., Kuroda S. (2008) Enigma homolog 1 scaffolds protein kinase D1 to regulate the activity of the cardiac L-type voltage-gated calcium channel. Cardiovasc Res. 78, 458–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang J., Shelton J. M., Richardson J. A., Kamm K. E., Stull J. T. (2008) Myosin regulatory light chain phosphorylation attenuates cardiac hypertrophy. J. Biol. Chem. 283, 19748–19756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. te Velthuis A. J., Bagowski C. P. (2007) PDZ and LIM domain-encoding genes: molecular interactions and their role in development. Scientific World Journal 7, 1470–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. von Nandelstadh P., Ismail M., Gardin C., Suila H., Zara I., Belgrano A., Valle G., Carpen O., Faulkner G. (2009) A class III PDZ binding motif in the myotilin and FATZ families binds enigma family proteins: a common link for Z-disc myopathies. Mol. Cell. Biol. 29, 822–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arimura T., Hayashi T., Terada H., Lee S. Y., Zhou Q., Takahashi M., Ueda K., Nouchi T., Hohda S., Shibutani M., Hirose M., Chen J., Park J. E., Yasunami M., Hayashi H., Kimura A. (2004) A Cypher/ZASP mutation associated with dilated cardiomyopathy alters the binding affinity to protein kinase C. J. Biol. Chem. 279, 6746–6752 [DOI] [PubMed] [Google Scholar]
- 36. Vatta M., Mohapatra B., Jimenez S., Sanchez X., Faulkner G., Perles Z., Sinagra G., Lin J. H., Vu T. M., Zhou Q., Bowles K. R., Di Lenarda A., Schimmenti L., Fox M., Chrisco M. A., Murphy R. T., McKenna W., Elliott P., Bowles N. E., Chen J., Valle G., Towbin J. A. (2003) Mutations in Cypher/ZASP in patients with dilated cardiomyopathy and left ventricular non-compaction. J. Am. Coll Cardiol 42, 2014–2027 [DOI] [PubMed] [Google Scholar]
- 37. Selcen D., Engel A. G. (2005) Mutations in ZASP define a novel form of muscular dystrophy in humans. Ann Neurol. 57, 269–276 [DOI] [PubMed] [Google Scholar]
- 38. Kuroda S., Tokunaga C., Kiyohara Y., Higuchi O., Konishi H., Mizuno K., Gill G. N., Kikkawa U. (1996) Protein-protein interaction of zinc finger LIM domains with protein kinase C. J. Biol. Chem. 271, 31029–31032 [DOI] [PubMed] [Google Scholar]
- 39. Frank D., Frey N. (2011) Cardiac Z-disc signaling network. J. Biol. Chem. 286, 9897–9904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Klaavuniemi T., Kelloniemi A., Ylänne J. (2004) The ZASP-like motif in actinin-associated LIM protein is required for interaction with the α-actinin rod and for targeting to the muscle Z-line. J. Biol. Chem. 279, 26402–26410 [DOI] [PubMed] [Google Scholar]
- 41. Tandan S., Wang Y., Wang T. T., Jiang N., Hall D. D., Hell J. W., Luo X., Rothermel B. A., Hill J. A. (2009) Physical and functional interaction between calcineurin and the cardiac L-type Ca2+ channel. Circ. Res. 105, 51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Scott J. D., Dessauer C. W., Taskén K. (2013) Creating order from chaos: cellular regulation by kinase anchoring. Annu. Rev. Pharmacol. Toxicol. 53, 187–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hulme J. T., Ahn M., Hauschka S. D., Scheuer T., Catterall W. A. (2002) A novel leucine zipper targets AKAP15 and cyclic AMP-dependent protein kinase to the C terminus of the skeletal muscle Ca2+ channel and modulates its function. J. Biol. Chem. 277, 4079–4087 [DOI] [PubMed] [Google Scholar]
- 44. Zhang R., Yang J., Zhu J., Xu X. (2009) Depletion of zebrafish Tcap leads to muscular dystrophy via disrupting sarcomere-membrane interaction, not sarcomere assembly. Hum. Mol. Genet 18, 4130–4140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lemke T., Welling A., Christel C. J., Blaich A., Bernhard D., Lenhardt P., Hofmann F., Moosmang S. (2008) Unchanged β-adrenergic stimulation of cardiac L-type calcium channels in Cav1.2 phosphorylation site S1928A mutant mice. J. Biol. Chem. 283, 34738–34744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kamp T. J., Hell J. W. (2000) Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ. Res. 87, 1095–1102 [DOI] [PubMed] [Google Scholar]
- 47. Heineke J., Ritter O. (2012) Cardiomyocyte calcineurin signaling in subcellular domains: from the sarcolemma to the nucleus and beyond. J. Mol. Cell Cardiol 52, 62–73 [DOI] [PubMed] [Google Scholar]
- 48. Frey N., Richardson J. A., Olson E. N. (2000) Calsarcins, a novel family of sarcomeric calcineurin-binding proteins. Proc. Natl. Acad. Sci. U.S.A. 97, 14632–14637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yang J., Rothermel B., Vega R. B., Frey N., McKinsey T. A., Olson E. N., Bassel-Duby R., Williams R. S. (2000) Independent signals control expression of the calcineurin inhibitory proteins MCIP1 and MCIP2 in striated muscles. Circ. Res. 87, E61-E68 [DOI] [PubMed] [Google Scholar]
- 50. Coghlan V. M., Perrino B. A., Howard M., Langeberg L. K., Hicks J. B., Gallatin W. M., Scott J. D. (1995) Association of protein kinase A and protein phosphatase 2B with a common anchoring protein. Science 267, 108–111 [DOI] [PubMed] [Google Scholar]
- 51. Hojayev B., Rothermel B. A., Gillette T. G., Hill J. A. (2012) FHL2 binds calcineurin and represses pathological cardiac growth. Mol. Cell. Biol. 32, 4025–4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. DeLano W. L. (2010) The PyMOL Molecular Graphics System, version 1.3r1, Schrödinger, LLC, New York [Google Scholar]