Background: VLDLR is a receptor for Reelin in brain neurons, but its mode of regulation is unclear.
Results: BDNF increases VLDLR gene expression in hippocampal neurons, whereas Reelin decreases the receptor post-transcriptionally.
Conclusion: Reelin down-regulates VLDLR via the E3 ubiquitin ligase Mylip/Idol.
Significance: The dynamic regulation of VLDLR by BDNF and Reelin may be important for neuronal migration and brain development.
Keywords: Brain-derived Neurotrophic Factor (BDNF), Neurodevelopment, Neurons, Receptor Regulation, Ubiquitin Ligase
Abstract
BDNF positively influences various aspects of neuronal migration, maturation, and survival in the developing brain. Reelin in turn mediates inhibitory signals to migrating neuroblasts, which is crucial for brain development. The interplay between BDNF and Reelin signaling in neurodevelopment is not fully understood. We show here that BDNF increased the levels of the Reelin receptor (VLDL receptor (VLDLR)) in hippocampal neurons by increasing gene expression. In contrast, Reelin decreased VLDLRs, which was accompanied by an increase in the levels of the E3 ligase Mylip/Idol in neurons. Down-regulation of Mylip/Idol using shRNAs abrogated the decrease in VLDLRs induced by Reelin. These results show that VLDLRs are tightly regulated in hippocampal neurons by both transcriptional and post-transcriptional mechanisms. The regulation of VLDLR by BDNF and Reelin may affect the migration of neurons and contribute to neurodevelopmental disorders in the nervous system.
Introduction
Reelin is a large secreted glycoprotein that binds to the VLDL receptor (VLDLR)2 and to ApoER2 (apolipoprotein E receptor 2) (1–3). Mutation in Reelin leads to severe developmental defects in the buildup of the laminated structures of the brain as exemplified by the disorganized cortex in the Reelin mutant mouse (4, 5). In humans, a lack of proper Reelin function affects neuronal migration and leads to neurodevelopmental disorders such as lissencephaly (6). Disturbed Reelin signaling has also been associated with neurological diseases such as Alzheimer disease, epilepsy, and schizophrenia (7–10). Interaction of Reelin with VLDLRs at the cell surface induces signaling and tyrosine phosphorylation of the protein Dab1 (Disabled-1) via the Src family of kinases (11–13). Phosphorylated Dab1 then transmits the Reelin signal into the cell and influences the cytoskeleton and the expression of specific genes (9, 13, 14). The precise mechanisms by which Reelin influences neuronal migration and neurodevelopment are, however, not fully understood.
BDNF is a neurotrophic factor of the neurotrophin family that is widely expressed in the brain (15, 16). BDNF is important in neurodevelopment and contributes to the regulation of neurogenesis, neuroblast migration, and neuronal survival and maturation and to the function of neuronal networks and plasticity in adults (17–21). BDNF acts by binding to the high affinity TrkB receptors, leading to receptor phosphorylation and activation of downstream signaling pathways (22). As the receptors TrkB and VLDLR can be expressed by the same neuron, we reasoned that the signals induced by BDNF and Reelin may functionally interact. To study this, we cultured hippocampal neurons from developing rat brain and analyzed the regulation of VLDLR. The results show that both BDNF and Reelin can affect VLDLR levels in hippocampal neurons, but the mechanisms are different. BDNF increased VLDLR gene expression, whereas Reelin down-regulated VLDLR levels via the E3 ubiquitin ligase Mylip (myosin regulatory light chain-interacting protein)/Idol (inducible degrader of the LDL receptor) (23, 24). The dynamic regulation of VLDLR levels by contrasting BDNF and Reelin signals is likely to affect neurodevelopment and may have consequences for neuronal functions also in the mature brain.
EXPERIMENTAL PROCEDURES
Reagents and Plasmids Constructs
Expression plasmids and shRNA constructs for Mylip/Idol constructs were reported previously (23–27). Adenoviral vector expressing wild-type Mylip/Idol was as described (24). The cloning and characterization of the VLDLR promoter construct (28) and the mouse Mylip/Idol promoter (29) have been described previously.
Cell Culture and Transfections
Hippocampal neurons were prepared from embryonic day 17 Wistar rats (Harlan Laboratories) and plated onto polyornithine (Sigma)-coated 6-well plates at a density of 2 × 106 cells. Cells were cultured in Neurobasal medium containing 2% B27 supplement as described previously (30, 31). Cells were cultured for 5–7 days and stimulated with 50 μg/ml BDNF (PeproTech, London, United Kingdom) or with 0.1–2 μg/ml recombinant Reelin (R&D Systems) alone or together for various periods of times. The synthetic liver X receptor (LXR) ligand GW3965 was used to induce Mylip/Idol (27, 29), actinomycin D (Sigma) was used to inhibit gene transcription, and bafilomycin A1 (Sigma) was used to block lysosomes. To down-regulate Mylip/Idol, freshly prepared neurons were transfected with Mylip shRNA constructs using the Nucleofector rat neuron system (Amaxa GmbH) as described previously (32, 33). To modulate Mylip/Idol activity, cultured neurons were infected with adenoviruses encoding wild-type Mylip/Idol (24) for 3 days following stimulation with Reelin for 24 h.
Immunoblotting and Immunoprecipitation
Neurons were lysed, and immunoblots were made essentially as described previously (31–33). Protein concentrations were determined using the Bradford assay, and an equal amount of protein per sample was subjected to SDS-PAGE and blotted onto nitrocellulose filters (Amersham Biosciences). The filters were first incubated for 1 h in 50 mm Tris-HCl (pH 7.5), 150 mm NaCl, 0.1% Tween 20, and 5% skim milk and then overnight at 4 °C with anti-VLDLR (1:250; Santa Cruz Biotechnology clone 6A6), anti-ApoER2 (1:10,000; kind gift of Dr. Noam Zelcer), anti-Mylip/Idol (1:1000; Abcam), rabbit anti-MIR (23), anti-Cnpy2 (Canopy-2)/Msap (MIR-interacting saposin-like protein) (1:2000; Proteintech), anti-phospho-Dab1 (1:1000; Abcam), anti-Dab1 (1:200; Santa Cruz Biotechnology), or anti-β-actin (1:5000; Sigma) primary antibody. After washing, the filter was incubated with horseradish peroxidase-conjugated secondary antibodies (1:2500; Jackson ImmunoResearch Laboratories), followed by detection using enhanced chemiluminescence (Pierce). Quantification was performed using Gel Doc (Bio-Rad).
To study ubiquitination of endogenous VLDLRs, lysates were made from control and 24-h Reelin-stimulated neurons. Lysates from precleared cell supernatants were incubated overnight at 4 °C on a rotary shaker using 2 μg/ml anti-VLDLR antibodies. Immunocomplexes were bound to Sepharose A for 2 h at 4 °C and recovered by centrifugation (29). Beads were washed three times, and the samples were resuspended in SDS-PAGE buffer and subjected to immunoblotting using anti-VLDLR and anti-ubiquitin antibodies. Blots were quantified by densitometry.
Promoter Assays
Hippocampal neurons in 6-well plates were transfected for 24 h with 0.5 μg of VLDLR promoter-luciferase reporter (28). To control for transfection efficiency, 0.02 μg of pRL-TK Renilla luciferase was used. Cells were treated with 50 ng/ml BDNF for 24 h and harvested by using Passive lysis buffer, and the Renilla and firefly luciferase activities were measured using a luminometer (Promega) (29, 31). The Mylip/Idol promoter assay was performed similarly, but cells were stimulated with 1 μg of Reelin. The results of gene activities are shown as -fold increase in firefly luciferase activity normalized to Renilla activity. The control empty vector pGL was included as an additional control and showed no change in activity.
RNA Isolation and Quantitative PCR
Total RNA was extracted using the RNeasy tissue kit (Qiagen), followed by cDNA synthesis essentially as described (29, 34). LightCycler® 480 SYBR Green I Master (Roche Applied Science) real-time quantitative PCR (qPCR) assays were performed on a LightCycler 480 (Roche Applied Science) with a 96-well block. Each 20-μl qPCR contained 2 μl of the cDNA product (1:50 dilution) and 1 μl of 10 μm each of the forward and reverse primers. The reaction was run for 15 min at 95 °C for initial activation of the enzyme, followed by 40 cycles of 15 s at 95 °C for denaturation, 20 s at 60 °C for annealing, and 10 s at 72 °C for extension. After completion of the reaction, the PCR products were subjected to a melting curve analysis spanning the temperature range from 60 to 95 °C. The specificity of the amplification was further confirmed by electrophoresis on 2% agarose gels stained with SYBR Safe (Invitrogen). The results show the averages of three replicate experiments normalized to GAPDH and actin. The following primer sequences were used for qPCR: VLDLR, 5′-AGCAATCTCAGTTGTAAGC-3′ (forward) and 5′-TAGGGTGTTATGGGTGTAG-3′ (reverse); Mylip/Idol, 5′-AGGCATCTCAATTTGTAAAG-3′ (forward) and 5′-GTAGACATTCTTTCCTGACT-3′ (reverse); β-actin, 5′-TGGGTATGGAATCCTGTG-3′ (forward) and 5′-GGTCTTTACGGATGTCAAC-3′ (reverse); and GAPDH, 5′-GCCAAGTATGATGACATCAAG-3′ (forward) and 5′-AAGGTGGAAGAATGGGAG-3′ (reverse).
Quantification
Statistical comparisons were performed using one-way analysis of variance (ANOVA), followed by a Bonferroni post hoc test (more than three groups). Student's t test was used in experiments with two groups with GraphPad Prism version 5.0 (GraphPad Software). Values are expressed as means ± S.E. p ≤ 0.05 was considered significant.
RESULTS
BDNF Increases VLDLR Levels in Hippocampal Neurons via TrkB Receptors
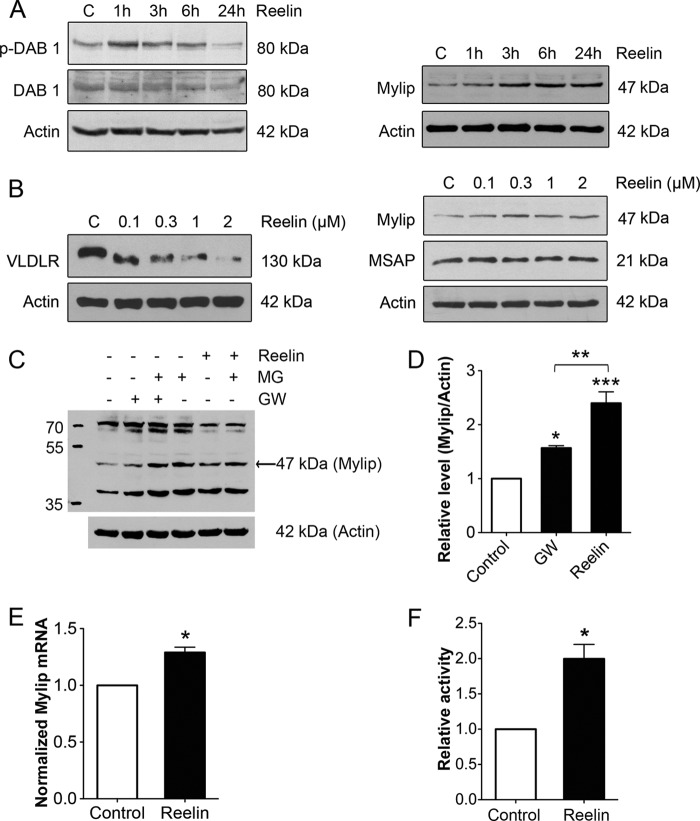
Hippocampal neurons prepared from embryonic rat brain express TrkB receptors and respond to treatments with BDNF (17, 18). We observed that stimulation of cultured hippocampal neurons with 50 ng/ml BDNF increased the VLDLR levels in a time-dependent manner (Fig. 1, A and C). The increase in VLDLRs by BDNF involved the TrkB receptors as shown by using the inhibitor K252a (Fig. 1D). In contrast to VLDLR, BDNF did not significantly elevate ApoER2 in hippocampal neurons (Fig. 1B). As shown previously, stimulation of cells with the LXR agonist GW3965 leads to the degradation of lipoprotein receptors following ubiquitination that is induced by the E3 ligase Mylip/Idol (23, 27). In hippocampal neurons, the addition of GW3965 down-regulated VLDLR, and this effect was counteracted by BDNF (Fig. 1E). In contrast to GW3965, BDNF did not significantly influence Mylip/Idol levels in hippocampal neurons (data not shown).
FIGURE 1.
BDNF increases VLDLR levels in hippocampal neurons. Hippocampal neurons were prepared from embryonic day 17 rats and cultured for 7 days as described under “Experimental Procedures.” Cells were stimulated with 50 ng/ml BDNF for various times, and immunoblotting was performed using specific antibodies against VLDLR and ApoER2. β-Actin was used as a control. Left panels, immunoblots; right panels, quantifications done using ImageJ. A and B, BDNF added for 24 h significantly increased VLDLR (A) but not ApoER2 (B) levels in hippocampal neurons. Values are means ± S.D. (n = 3). **, p < 0.01 for BDNF versus the control (C). C, time course for the increase in VLDLR by BDNF. A typical immunoblot is shown, and the experiments were repeated three times. D, cells were stimulated with BDNF in the presence of 500 nm K252a to inhibit TrkB receptors. Values are means ± S.D. (n = 3; ANOVA p value, 0.0016; F, 19.92; Tukey's test). **, p < 0.01 for BDNF versus the control. E, the addition of 1 μm GW3965 (GW), which activates LXRs, decreased VLDLR levels in hippocampal neurons, and this was counteracted by co-treatment with BDNF. Values are means ± S.D. (n = 3; ANOVA p value, 0.0001; F, 259; Tukey's test). ***, p < 0.001 for BDNF versus the control and for BDNF + GW3965 versus GW3965; *, p < 0.05 for GW3965 versus the control.
BDNF Increases VLDLR Gene Transcription
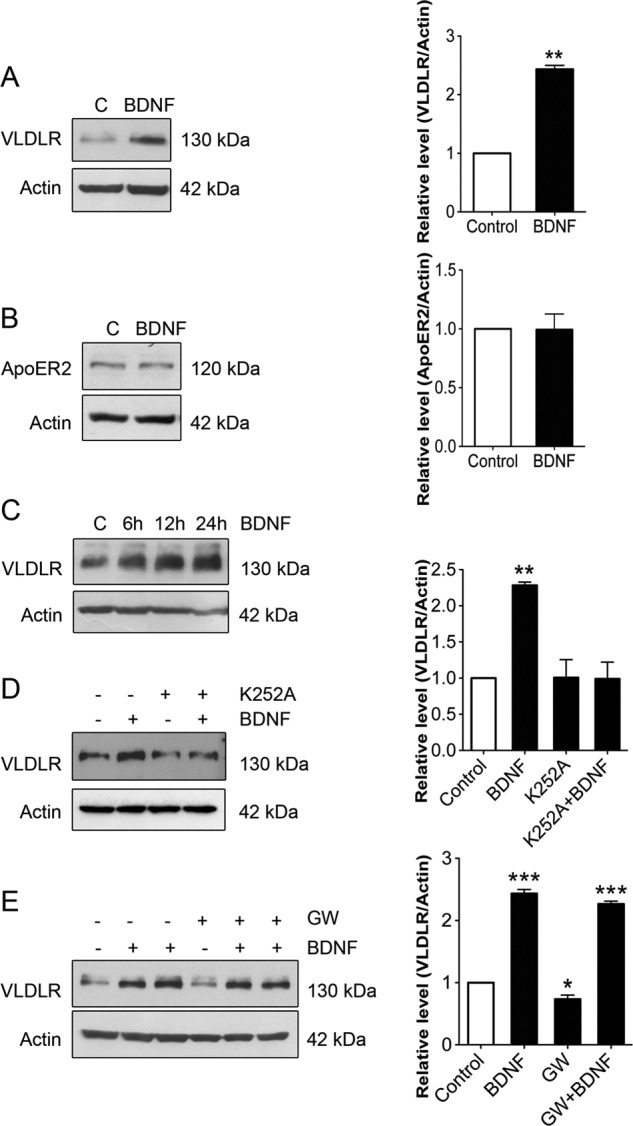
Experiments using actinomycin D revealed that BDNF increased VLDLR at the RNA level (Fig. 2A). We also observed an increase in VLDLR mRNA by BDNF in hippocampal neurons using qPCR (Fig. 2B), suggesting an effect on VLDLR expression. To study this further, we employed the 5′-upstream sequence of the VLDLR gene linked to the luciferase reporter construct. Previously, the VLDLR gene promoter was shown to be active in different human cell lines, including human hepatocyte cells and trophoblasts (28). We observed that the VLDLR promoter is active in hippocampal neurons also, and stimulation with BDNF significantly increased this activity, showing a direct effect of BDNF on VLDLR gene transcription (Fig. 2C). Together, these results show that BDNF increases VLDLR gene transcription in hippocampal neurons, leading to an increase in VLDLR levels.
FIGURE 2.
BDNF increases VLDLR gene expression in hippocampal neurons. A, hippocampal neurons were stimulated with 50 ng/ml BDNF for 12 h in the absence or presence of 30 ng/ml actinomycin D to block gene transcription. β-Actin was used as a control. The increase in VLDLR was inhibited by actinomycin D. A typical immunoblot is shown, and the experiment was repeated three times. B, RNA was prepared from control and 24-h BDNF-stimulated neurons, and qPCR was performed as described under “Experimental Procedures.” Note the increase in VLDLR mRNA by BDNF. Values are means ±S.D. (n = 3). ***, p < 0.001 for BDNF versus the control. C, hippocampal neurons were transfected with the VLDLR promoter-luciferase reporter construct as described under “Experimental Procedures” and further stimulated for 24 h with 50 ng/ml BDNF. Luciferase activity was measured, and values were corrected against Renilla activity. VLDLR gene activity was increased by BDNF. The basic promoter showed no activity and is not included here. Values are means ± S.D. (n = 3). *, p < 0.05 for BDNF versus the control.
Reelin Down-regulates VLDLRs in Hippocampal Neurons by Increasing Receptor Degradation
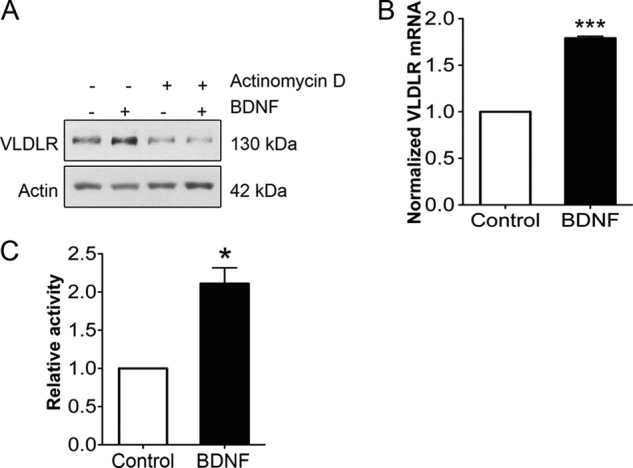
BDNF levels in the brain and hippocampus are known to increase during early development (35), whereas VLDLR levels are low in the mature brain. We therefore reasoned that there must be other mechanisms in addition to BDNF for regulation of VLDLRs in the hippocampus. VLDLR and ApoER2 are thought to undergo internalization and endocytosis upon binding Reelin at the cell surface (13, 14, 36). However, the precise mechanisms regulating trafficking of these receptors are not fully understood, but lysosomes play a role in receptor degradation. We observed that stimulation of hippocampal neurons with Reelin for 24 h drastically reduced VLDLRs in these cells (Fig. 3A). The decrease in VLDLR levels by Reelin was blocked by bafilomycin A1 (Fig. 3B), which inhibits lysosomal degradation. Reelin also decreased VLDLR levels induced by BDNF for 24 h (Fig. 3C). To compare Reelin with the effect of LXR signaling, neurons were stimulated with Reelin alone or together with GW3965, and the VLDLR levels were determined. The data show that both Reelin and GW3965 reduced VLDLR levels in hippocampal neurons, and the effect was additive (Fig. 3D). Moreover, Reelin was relatively stronger in decreasing the VLDLR levels compared with GW3965 (Fig. 3D). We studied also whether Reelin can induce ubiquitination of VLDLRs in neurons. The results obtained by immunoprecipitation show that VLDLR was more heavily ubiquitinated in hippocampal neurons after stimulation with Reelin compared with controls. These results suggest that Reelin down-regulates VLDLRs in hippocampal neurons via lysosomal degradation of the receptor involving protein ubiquitination.
FIGURE 3.
Reelin down-regulates VLDLR levels and induces receptor ubiquitination in hippocampal neurons. Hippocampal neurons were stimulated with 1 μg/ml Reelin under various conditions as indicated below. Immunoblotting was performed using specific antibodies against VLDLR and β-actin (used as a control). Left panels, immunoblots; right panels, quantification. A, cells were stimulated for 24 h. Values are means ± S.D. (n = 3). *, p < 0.05 for Reelin versus the control (C). B, the addition of 0.3 μm bafilomycin A1 (Baf), which inhibits lysosomes, blocked the degradation of VLDLR induced by Reelin. Values are means ± S.D. (n = 3). ***, p < 0.001 for bafilomycin A1 + Reelin versus Reelin; **, p < 0.01 for bafilomycin A1 versus the control; *, p < 0.05 for Reelin versus the control and bafilomycin A1 + Reelin versus the control. C, Reelin down-regulates VLDLRs that were increased by 50 ng/ml BDNF. Values are means ± S.D. (n = 3; ANOVA p value, 0.0023; F, 39.36; Tukey's test). **, p < 0.01 for BDNF versus the control. D, cells were stimulated in the absence or presence of 1 μm GW3965 (GW). Note the decrease in VLDLR levels by GW3965 and Reelin. Values are means ± S.D. (n = 3; ANOVA p value, 0.0001; F, 50.93; Tukey's test). ***, p < 0.001 for Reelin versus the control and for GW3965 + Reelin versus the control; **, p < 0.05 for GW3965 versus the control and for GW3965 + Reelin versus GW3965. E, VLDLR ubiquitination. Lysates were prepared from control hippocampal neurons and from cells stimulated for 24 h with Reelin. Immunoprecipitations (IP) were done employing 2 μg of anti-VLDLR monoclonal antibody, followed by immunoblotting as described under “Experimental Procedures.” Left panel, ubiquitin species bound to VLDLR in the immunoprecipitate; right panel, total amount of VLDLRs in lysates. Note the increase in the degree of ubiquitination of VLDLR in Reelin-treated cells. A typical experiment is shown and was repeated three times.
Reelin Increases Mylip/Idol in Hippocampal Neurons Essential for VLDLR Down-regulation
Binding of Reelin to VLDLR is known to activate intracellular signaling pathways in target neurons. Dab1 is a signaling protein for VLDLRs (4, 5) and was phosphorylated by Reelin at 1 h of stimulation (Fig. 4A). In addition, Reelin significantly increased the level of Mylip/Idol from 3 h onwards (Fig. 4A). Reelin decreased the VLDLR levels in a concentration-dependent manner, whereas the Mylip/Idol levels were increased (Fig. 4B). In contrast, Cnpy2/Msap, which is known to modulate LDL receptor (LDLR) levels in hepatocytes (29), was not changed in neurons after stimulation with Reelin (Fig. 4B).
FIGURE 4.
Reelin activates VLDLR signaling and increases Mylip/Idol expression in hippocampal neurons. Hippocampal neurons were stimulated with Reelin under various conditions as indicated below. Immunoblotting was performed using specific antibodies, and β-actin was used as a control. A, time course. Cells were stimulated with 1 μg/ml Reelin for different times. Note that phosphorylation of Dab1 (p-Dab1) was increased at 1 h of stimulation. The levels of Mylip/Idol increased at 3 h onwards. Typical immunoblots are shown and were repeated. C, control. B, concentration curve. Cells were stimulated with different concentrations of Reelin for 24 h. Note the decrease in VLDLR and the increase in Mylip/Idol levels by Reelin with no apparent change in Cnpy2/Msap. Typical immunoblots are shown and were repeated three times. C, immunoblot with antibody testing. Hippocampal neurons were stimulated 1 μg/ml Reelin or 1 μm GW3965 (GW) for 24 h alone or together with the proteasomal inhibitor MG321 (MG; 4 μg/ml), which was added for 4 h. Lysates were made, and immunoblotting was performed using anti-Mylip antibodies. β-Actin was used as a control. Note the increase in endogenous Mylip/Idol levels (47-kDa band) by MG321 and GW3965 and the additive effect using both compounds. Reelin also increased Mylip/Idol. For sake of clarity, the whole blot is shown here. Of note, there are also unspecific (high and low molecular mass) bands using the anti-Mylip antibody that have been observed before, the nature of which is unclear. D, summary of data on Mylip/Idol. Reelin was relatively more effective than GW3965 in increasing Mylip/Idol in neurons. Values are means ± S.D. (n = 6; ANOVA p value, 0.0006; F, 32.86; Tukey's test). ***, p < 0.001 for Reelin versus the control; **, p < 0.01 for Reelin versus GW3965; *, p < 0.05 for GW3965 versus the control. E, RNA was prepared from control and 3-h Reelin-stimulated neurons, and qPCR was performed as described under “Experimental Procedures.” Note the increase in Mylip/Idol mRNA by Reelin. Values are means ±S.D. (n = 3). *, p < 0.05 for Reelin versus the control. F, hippocampal neurons were transfected with the Mylip/Idol promoter-reporter construct as described under “Experimental Procedures” and further stimulated with Reelin for 24 h. Luciferase activity was measured as described under “Experimental Procedures,” and values were corrected against those for Renilla. The activity of the Mylip/Idol promoter was increased by Reelin. The basic promoter showed no activity and is not included here. Values are means ± S.D. (n = 3). *, p < 0.05 for Reelin versus the control.
Mylip/Idol is a labile protein that has been hard to detect on immunoblots (24, 27, 37). In lysates from rat cultured neurons, we detected endogenous levels of Mylip/Idol that were further increased by blocking proteasomes using the compound MG321 and by stimulation of LXRs with GW3965 (Fig. 4C). The precise band of Mylip/Idol using the antibody was ∼47 kDa (Fig. 4C). This is in agreement with recent data obtained using an anti-Idol monoclonal antibody and lysates from mouse peritoneal macrophages (37). Statistical analyses of the effects revealed that Reelin more strongly increased Mylip/Idol levels in these neurons compared with GW3965 (Fig. 4D). This is in accordance with data showing that the down-regulation of VLDLR observed with Reelin was greater than that with GW3965 (Fig. 3D).
Using qPCR, we observed that the addition of Reelin increased the Mylip/Idol mRNA levels in neurons (Fig. 4E). Reelin also increased the activity of the Mylip/Idol promoter in these cells, demonstrating an effect of Reelin on gene transcription (Fig. 4F).
To study the dynamics of Mylip/Idol in neurons in more detail, we infected the cells with adenovirus expressing wild-type Mylip/Idol (24). The results show that expression of wild-type Mylip/Idol decreased VLDLR levels in neurons (Fig. 5A). This indicates that these receptors are efficiently degraded in neurons due to the presence of active Mylip/Idol.
FIGURE 5.
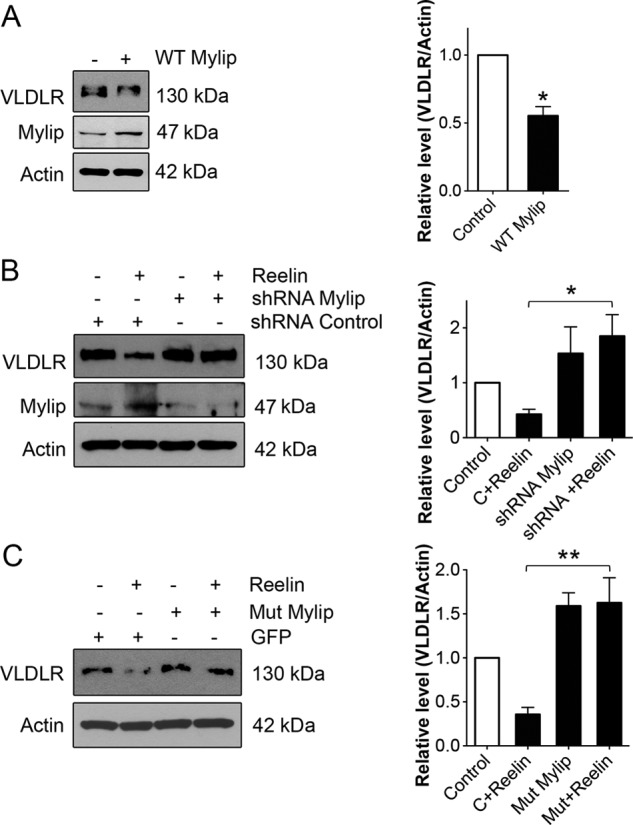
Involvement of Mylip/Idol in the regulation of VLDLR levels by Reelin. Hippocampal neurons were treated as indicated below and further stimulated with 1 μg/ml Reelin for 24 h. VLDLR and Mylip/Idol levels were determined by immunoblotting. β-Actin was used as a control. Left panels, immunoblots; right panels, quantification. A, neurons were infected with adenovirus expressing wild-type Mylip/Idol as described under “Experimental Procedures.” VLDLR levels decreased in the Mylip/Idol-overexpressing cells. Values are means ± S.D. (n = 3). *, p < 0.05 for Reelin versus the control. B, neurons were transfected for 3 days with control (C) scrambled or Mylip/Idol shRNA plasmids for 3 days and further stimulated with 1 μg/ml Reelin for 24 h. Reelin reduced VLDLRs in control shRNA- but not Mylip/Idol shRNA-treated cells. Mylip/Idol levels were down-regulated by >70%. Values are means ± S.D. (n = 3). *, p < 0.05 for the control + Reelin versus shRNA + Reelin. C, neurons were transfected with control EGFP and mutant Mylip/Idol plasmids. Reelin reduced VLDLRs in control but not mutant Mylip/Idol-expressing cells. Mutant Mylip/Idol also increased the basal levels of Mylip/Idol. Values are means ± S.D. (n = 3; ANOVA p value, 0.0023; F, 17.34; Tukey's test). **, p < 0.01 for the mutant + Reelin versus the control + Reelin.
To clarify the role of Mylip/Idol in Reelin-mediated VLDLR regulation, we down-regulated Mylip/Idol using shRNA constructs for the rat protein (27). The data show that down-regulation of Mylip/Idol using RNA silencing largely counteracted the decrease in VLDLRs brought about by Reelin, with no effect observed with control shRNA (Fig. 5B). Expression of mutant Mylip/Idol lacking the active E3 ligase (25, 27) also reversed the Reelin-mediated decrease in VLDLRs (Fig. 5C). Interestingly, there was an increase in the basal levels of VLDLRs after expression of Mylip/Idol in neurons (Fig. 5C). This suggests that inactive mutant Mylip/Idol may act in a dominant-negative manner with regard to endogenous Mylip/Idol, thereby increasing VLDLR levels.
DISCUSSION
In this work, we have shown that VLDLR levels in developing hippocampal neurons are regulated by BDNF and Reelin in a reciprocal manner. The dynamic regulation of VLDLRs by these factors is likely to be important for the development of various brain structures. Reelin plays a role in neuronal migration and in the positioning of cells in the brain. In the hippocampus, Reelin has been shown also to specifically influence dendritic maturation and synaptogenesis (38, 39). Reelin also enhances cognitive performance and increases synaptic plasticity in adult mouse brain (40). Mutation of Reelin or its signaling pathways can lead to developmental defects with an altered connectivity of neurons that is characteristic of many neurological disorders.
Previous studies have shown that VLDLR and ApoER2 are receptors for Reelin (1–3). VLDLR and ApoER2 have a similar expression pattern in the brain, but they do not completely overlap. VLDLR is expressed in different parts of the developing brain, including the hippocampus, cortex, and cerebellum, with the highest levels found in the cerebellum (4, 5). In the hippocampus, VLDLR is expressed preferentially by neurons, although some glial cells also express this receptor (see GENSAT).
BDNF is an important neurotrophic factor in the brain that influences various aspects of neuronal development. In the hippocampus, the level of BDNF increases with postnatal development concomitant with neuronal differentiation and maturation (35, 41). The precise downstream genes and mechanisms by which BDNF influences hippocampal neuron development are not fully understood. Here, we have shown that BDNF elevates VLDLRs in developing neurons, with smaller effects on ApoER2. The increase in VLDLRs by BDNF was observed at both the RNA and protein levels. The data obtained using the upstream promoter region of VLDLR linked to a luciferase reporter revealed that BDNF increased the gene activity of VLDLR. These data show that VLDLR is a BDNF-responsive gene, adding to the complexity of genes that are induced by BDNF in developing neurons.
Previous transfection studies have shown that the 4-kb large upstream region of the VLDLR gene is an active gene promoter in different cell types, including human hepatoma cells (28). However, there have been no studies so far on VLDLR gene activity in neuronal cells. The VLDLR promoter contains several regulatory elements for binding of transcription factors such as the C/EBP (CCAAT/enhancer-binding protein), CREB (cAMP response element-binding protein), and GATA-1 factors (28). It is also known that BDNF via TrkB is able to increase CREB levels in neurons (22). As shown previously in non-neuronal cells, the VLDLR promoter is regulated in a cell-specific manner by a combination of transcription factors acting in a positive or negative manner (28). The precise mechanisms by which BDNF activates VLDLR gene expression and the transcription factors involved in this in neuronal cells warrant further detailed study.
In contrast to BDNF, we observed that Reelin down-regulated VLDLR levels largely via a post-transcriptional mechanism. The VLDLR and LDLR levels have been previously shown to be regulated by receptor ubiquitination that is induced by the E3 ligase Mylip/Idol (24, 27). Mylip/Idol itself is increased by activation of nuclear LXRs via oxysterols or by using synthetic ligands such as GW3965 (24, 27, 29). Furthermore, the LDLR levels in hepatocytes are influenced by expression of Cnpy2/Msap, which antagonizes the biological effect of Mylip/Idol (29). In comparison with LDLRs, little is known about the regulation of VLDLRs particularly in neuronal cells. We have shown here that VLDLRs are expressed by hippocampal neurons in culture and that the cells respond to Reelin by rapid activation of VLDLR signaling as shown by Dab1 phosphorylation. Reelin reduced VLDLR levels in neurons at 24 h, showing that Reelin stimulation causes receptor down-regulation. The addition of bafilomycin A1, which inhibits lysosomes, blocked the decrease in VLDLR induced by Reelin, indicating that VLDLR is degraded in lysosomes following Reelin stimulation. To study this in more detail, we determined the levels of ubiquitin species bound to VLDLRs by immunoprecipitation. The data show that the endogenous VLDLRs were more heavily ubiquitinated in Reelin-treated hippocampal neurons compared with controls. Collectively, these results show that Reelin is able to down-regulate its own receptor by promoting VLDLR ubiquitination and lysosomal degradation. In the future, it will be important to study which lysine residues in VLDLR become ubiquitinated and whether the receptor is preferentially mono- and/or polyubiquitinated following Reelin treatment. As such, the regulation of receptors by their own ligands in target cells is not surprising and has been shown previously for different growth factors and their tyrosine kinase receptors (42).
To clarify the mechanisms by which Reelin influences VLDLR levels, we focused on Mylip/Idol, which is known to ubiquitinate and down-regulate this receptor. The data show that Reelin elevated Mylip/Idol in hippocampal neurons mainly via increased gene expression. Interestingly, the increase in Mylip/Idol by Reelin was even stronger than that observed with the LXR ligand GW3965 (Fig. 4D). This may contribute to the larger effect of Reelin in down-regulation of VLDLR in these neurons compared with GW3965 (Fig. 3D; compare Figs. 1E and 3C). The precise mechanisms by which Reelin stimulates gene expression of Mylip/Idol are currently under investigation.
To study the role of Mylip/Idol in VLDLR regulation in neurons, we down-regulated Mylip/Idol using shRNA, which virtually abolished the decrease in VLDLR induced by Reelin. This result demonstrates that Mylip/Idol is essentially involved in the regulation of VLDLR by Reelin. A similar finding was observed in experiments using a functionally inactive form of Mylip/Idol that lacks the important cysteine in the RING domain (C387A mutant) (25, 27). We observed that expression of mutant Mylip/Idol was able to counteract the decrease in VLDLRs induced by Reelin in hippocampal neurons. The basal levels of VLDLRs were also increased in these neurons, suggesting that mutant Mylip/Idol may have a dominant-negative effect compared with the wild-type protein. However, more experiments in the future are needed to clarify the biochemical bases for this effect of mutant Mylip/Idol particularly in neuronal cells. Further evidence supporting the view that the VLDLRs are tightly regulated in neurons was provided by the dose-response curve for Reelin showing that there is an inverse linear relationship between the VLDLR levels and the amount of Mylip/Idol present (Fig. 4B). Moreover, expression of wild-type Mylip/Idol using adenoviruses decreased VLDLRs in neurons (Fig. 5C).
Physiologically, this study shows a reciprocal regulation of VLDLR levels by BDNF and Reelin in developing neurons, which may increase our understanding about VLDLRs in neurodevelopment. Reelin was originally thought to induce a stop signal in migrating neuroblasts, which is crucial for the right lamination of different brain structures during development (4, 5). This view has been modified by recent experiments showing that Reelin may rather act as a “detach and go” signal in neuronal migration (13, 14, 43). The results of this study could be in favor of such a model by showing that VLDLRs are down-regulated by Reelin and that the block in cell migration may be transient in nature possibly and followed by a re-entry of cell movements. The increase in VLDLRs by BDNF may in turn allow for alternative waves of VLDLR expression, which is important for cell migration and proper establishment of brain structures during development. Previous studies have shown that expression of BDNF in the developing brain cortex of transgenic animals results in reduced Reelin levels (44). BDNF has also been shown to influence Cajal-Retzius cells (45), which express Reelin during hippocampal development (46). BDNF is further regulated in an activity-dependent manner in the hippocampus and brain cortex (16, 47), suggesting that VLDLR expression could be similarly controlled. This attractive hypothesis is currently under investigation.
This study has shown that VLDLR is regulated in hippocampal neurons by both transcriptional and post-transcriptional mechanisms. BDNF increased gene expression of VLDLR, whereas Reelin decreased VLDLR levels largely via induction of the E3 ligase Mylip/Idol. The results for VLDLRs show an important functional connection between BDNF and Reelin signaling pathways during brain development. Cell signaling disturbed by BDNF and Reelin is associated with neurological disorders with impaired neuronal function and connectivity. In particular, altered Reelin signaling is thought to be involved in diseases such as Alzheimer disease, schizophrenia, and epilepsy (7–10). It remains to be studied whether the reciprocal regulation of VLDLR in neurons as shown here by Reelin and BDNF plays a functional role in these or other human neurological diseases.
Acknowledgments
We thank Noam Zelcer for Mylip/Idol constructs and anti-ApoER2 antibody, Anne K. Soutar for the VLDLR promoter, Tom Curran for Reelin-expressing cells, Tho Huu Ho for help with qPCR, and Kristina Söderholm and Riikka Kosonen for skillful technical assistance.
This work was supported by Finska Läkaresällskapet, the Sigrid Juselius Foundation, Liv och Hälsa, Magnus Ehrnrooth, the Minerva Foundation, and the Academy of Finland.
- VLDLR
- VLDL receptor
- LXR
- liver X receptor
- qPCR
- quantitative PCR
- ANOVA
- analysis of variance
- LDLR
- LDL receptor.
REFERENCES
- 1. D'Arcangelo G., Homayouni R., Keshvara L., Rice D. S., Sheldon M., Curran T. (1999) Reelin is a ligand for lipoprotein receptors. Neuron 24, 471–479 [DOI] [PubMed] [Google Scholar]
- 2. Hiesberger T., Trommsdorff M., Howell B. W., Goffinet A., Mumby M. C., Cooper J. A., Herz J. (1999) Direct binding of Reelin to VLDL receptor and apoE receptor 2 induces tyrosine phosphorylation of Disabled-1 and modulates tau phosphorylation. Neuron 24, 481–489 [DOI] [PubMed] [Google Scholar]
- 3. Trommsdorff M., Gotthardt M., Hiesberger T., Shelton J., Stockinger W., Nimpf J., Hammer R. E., Richardson J. A., Herz J. (1999) Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell 97, 689–701 [DOI] [PubMed] [Google Scholar]
- 4. Rice D. S., Curran T. (2001) Role of the Reelin signaling pathway in central nervous system development. Annu. Rev. Neurosci. 24, 1005–1039 [DOI] [PubMed] [Google Scholar]
- 5. Tissir F., Goffinet A. M. (2003) Reelin and brain development. Nat. Rev. Neurosci. 4, 496–505 [DOI] [PubMed] [Google Scholar]
- 6. Hong S. E., Shugart Y. Y., Huang D. T., Shahwan S. A., Grant P. E., Hourihane J. O., Martin N. D., Walsh C. A. (2000) Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat. Genet. 26, 93–96 [DOI] [PubMed] [Google Scholar]
- 7. Impagnatiello F., Guidotti A. R., Pesold C., Dwivedi Y., Caruncho H., Pisu M. G., Uzunov D. P., Smalheiser N. R., Davis J. M., Pandey G. N., Pappas G. D., Tueting P., Sharma R. P., Costa E. (1998) A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 95, 15718–15723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fatemi S. H., Snow A. V., Stary J. M., Araghi-Niknam M., Reutiman T. J., Lee S., Brooks A. I., Pearce D. A. (2005) Reelin signaling is impaired in autism. Biol. Psychiatry 57, 777–787 [DOI] [PubMed] [Google Scholar]
- 9. Herz J., Chen Y. (2006) Reelin, lipoprotein receptors and synaptic plasticity. Nat. Rev. Neurosci. 7, 850–859 [DOI] [PubMed] [Google Scholar]
- 10. Durakoglugil M. S., Chen Y., White C. L., Kavalali E. T., Herz J. (2009) Reelin signaling antagonizes β-amyloid at the synapse. Proc. Natl. Acad. Sci. U.S.A. 106, 15938-15943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Howell B. W., Gertler F. B., Cooper J. A. (1997) Mouse disabled (mDab1): a Src binding protein implicated in neuronal development. EMBO J. 16, 121–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bock H. H., Herz J. (2003) Reelin activates SRC family tyrosine kinases in neurons. Curr. Biol. 13, 18–26 [DOI] [PubMed] [Google Scholar]
- 13. Gao Z., Godbout R. (2013) Reelin-Disabled-1 signaling in neuronal migration: splicing takes the stage. Cell Mol. Life Sci. 70, 2319–2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Förster E., Bock H. H., Herz J., Chai X., Frotscher M., Zhao S. (2010) Emerging topics in Reelin function. Eur. J. Neurosci. 31, 1511–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leibrock J., Lottspeich F., Hohn A., Hofer M., Hengerer B., Masiakowski P., Thoenen H., Barde Y. A. (1989) Molecular cloning and expression of brain-derived neurotrophic factor. Nature 341, 149–152 [DOI] [PubMed] [Google Scholar]
- 16. Zafra F., Hengerer B., Leibrock J., Thoenen H., Lindholm D. (1990) Activity dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors. EMBO J. 9, 3545–3550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Korte M., Carroll P., Wolf E., Brem G., Thoenen H., Bonhoeffer T. (1995) Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc. Natl. Acad. Sci. U.S.A. 92, 8856–8860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lindholm D., Carroll P., Tzimagiogis G., Thoenen H. (1996) Autocrine-paracrine regulation of hippocampal neuron survival by IGF-1 and the neurotrophins BDNF, NT-3 and NT-4. Eur. J. Neurosci. 8, 1452–1460 [DOI] [PubMed] [Google Scholar]
- 19. Sairanen M., Lucas G., Ernfors P., Castrén M., Castrén E. (2005) Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J. Neurosci. 25, 1089–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Egan M. F., Kojima M., Callicott J. H., Goldberg T. E., Kolachana B. S., Bertolino A., Zaitsev E., Gold B., Goldman D., Dean M., Lu B., Weinberger D. R. (2003) The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112, 257–269 [DOI] [PubMed] [Google Scholar]
- 21. Autry A. E., Monteggia L. M. (2012) Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol. Rev. 64, 238–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang E. J., Reichardt L. F. (2003) Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 72, 609–642 [DOI] [PubMed] [Google Scholar]
- 23. Olsson P.-A., Korhonen L., Mercer E. A., Lindholm D. (1999) MIR is a novel ERM-like protein that interacts with myosin regulatory light chain and inhibits neurite outgrowth. J. Biol. Chem. 274, 36288–36292 [DOI] [PubMed] [Google Scholar]
- 24. Zelcer N., Hong C., Boyadjian R., Tontonoz P. (2009) LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science 325, 100–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bornhauser B. C., Johansson C., Lindholm D. (2003) Functional activities and cellular localization of the ezrin, radixin, moesin (ERM) and RING zinc finger domains in MIR. FEBS Lett. 553, 195–199 [DOI] [PubMed] [Google Scholar]
- 26. Bornhauser B. C., Olsson P.-A., Lindholm D. (2003) MSAP is a novel MIR-interacting protein that enhances neurite outgrowth and increases myosin regulatory light chain. J. Biol. Chem. 278, 35412–35420 [DOI] [PubMed] [Google Scholar]
- 27. Hong C., Duit S., Jalonen P., Out R., Scheer L., Sorrentino V., Boyadjian R., Rodenburg K. W., Foley E., Korhonen L., Lindholm D., Nimpf J., van Berkel T. J., Tontonoz P., Zelcer N. (2010) The E3 ubiquitin ligase IDOL induces the degradation of the low density lipoprotein receptor family members VLDLR and ApoER2. J. Biol. Chem. 285, 19720–19726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kreuter R., Soutar A. K., Wade D. P. (1999) Transcription factors CCAAT/enhancer-binding protein β and nuclear factor-Y bind to discrete regulatory elements in the very low density lipoprotein receptor promoter. J. Lipid Res. 40, 376–386 [PubMed] [Google Scholar]
- 29. Do H. T., Tselykh T. V., Mäkelä J., Ho T. H., Olkkonen V. M., Bornhauser B. C., Korhonen L., Zelcer N., Lindholm D. (2012) Fibroblast growth factor-21 (FGF21) regulates low-density lipoprotein receptor (LDLR) levels in cells via the E3-ubiquitin ligase Mylip/Idol and the Canopy2 (Cnpy2)/Mylip-interacting saposin-like protein (Msap). J. Biol. Chem. 287, 12602–12611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Korhonen L., Belluardo N., Lindholm D. (2001) Regulation of X-chromosome-linked inhibitor of apoptosis protein in kainic acid-induced neuronal death in the rat hippocampus. Mol. Cell. Neurosci. 17, 364–372 [DOI] [PubMed] [Google Scholar]
- 31. Sokka A. L., Putkonen N., Mudo G., Pryazhnikov E., Reijonen S., Khiroug L., Belluardo N., Lindholm D., Korhonen L. (2007) Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J. Neurosci. 27, 901–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kairisalo M., Korhonen L., Sepp M., Pruunsild P., Kukkonen J. P., Kivinen J., Timmusk T., Blomgren K., Lindholm D. (2009) NF-κB-dependent regulation of brain-derived neurotrophic factor in hippocampal neurons by X-linked inhibitor of apoptosis protein. Eur. J. Neurosci. 30, 958–966 [DOI] [PubMed] [Google Scholar]
- 33. Putkonen N., Kukkonen J. P., Mudo G., Putula J., Belluardo N., Lindholm D., Korhonen L. (2011) Involvement of cyclin-dependent kinase-5 in the kainic acid-mediated degeneration of glutamatergic synapses in the rat hippocampus. Eur. J. Neurosci. 34, 1212–1221 [DOI] [PubMed] [Google Scholar]
- 34. Hyrskyluoto A., Pulli I., Törnqvist K., Ho T. H., Korhonen L., Lindholm D. (2013) Sigma-1 receptor agonist PRE084 is protective against mutant huntingtin-induced cell degeneration: involvement of calpastatin and the NF-κB pathway. Cell Death Dis. 4, e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. da Penha Berzaghi M., Cooper J., Castrén E., Zafra F., Sofroniew M., Thoenen H., Lindholm D. (1993) Cholinergic regulation of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) but not neurotrophin-3 (NT-3) mRNA levels in the developing rat hippocampus. J. Neurosci. 13, 3818–3826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Duit S., Mayer H., Blake S. M., Schneider W. J., Nimpf J. (2010) Differential functions of ApoER2 and very low density lipoprotein receptor in Reelin signaling depend on differential sorting of the receptors. J. Biol. Chem. 285, 4896–4908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Scotti E., Calamai M., Goulbourne C. N., Zhang L., Hong C., Lin R. R., Choi J., Pilch P. F., Fong L. G., Zou P., Ting A. Y., Pavone F. S., Young S. G., Tontonoz P. (2013) IDOL stimulates clathrin-independent endocytosis and multivesicular body-mediated lysosomal degradation of the low-density lipoprotein receptor. Mol. Cell. Biol. 33, 1503–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Qiu S., Weeber E. J. (2007) Reelin signaling facilitates maturation of CA1 glutamatergic synapses. J. Neurophysiol. 97, 2312–2321 [DOI] [PubMed] [Google Scholar]
- 39. Niu S., Yabut O., D'Arcangelo G. (2008) The Reelin signaling pathway promotes dendritic spine development in hippocampal neurons. J. Neurosci. 28, 10339–10348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rogers J. T., Rusiana I., Trotter J., Zhao L., Donaldson E., Pak D. T., Babus L. W., Peters M., Banko J. L., Chavis P., Rebeck G. W., Hoe H. S., Weeber E. J. (2011) Reelin supplementation enhances cognitive ability, synaptic plasticity, and dendritic spine density. Learn. Mem. 18, 558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zafra F., Castrén E., Thoenen H., Lindholm D. (1991) Interplay between glutamate and γ-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proc. Natl. Acad. Sci. U.S.A. 88, 10037–10041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Haglund K., Sigismund S., Polo S., Szymkiewicz I., Di Fiore P. P., Dikic I. (2003) Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat. Cell Biol. 5, 461–466 [DOI] [PubMed] [Google Scholar]
- 43. Cooper J. A. (2008) A mechanism for inside-out lamination in the neocortex. Trends Neurosci. 31, 113–119 [DOI] [PubMed] [Google Scholar]
- 44. Ringstedt T., Linnarsson S., Wagner J., Lendahl U., Kokaia Z., Arenas E., Ernfors P., Ibáñez C. F. (1998) BDNF regulates reelin expression and Cajal-Retzius cell development in the cerebral cortex. Neuron 21, 305–315 [DOI] [PubMed] [Google Scholar]
- 45. Marty S., Carroll P., Cellerino A., Castrén E., Staiger V., Thoenen H., Lindholm D. (1996) Brain-derived neurotrophic factor promotes the differentiation of various hippocampal nonpyramidal neurons, including Cajal-Retzius cells, in organotypic slice cultures. J. Neurosci. 16, 675–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Del Río J. A., Heimrich B., Borrell V., Förster E., Drakew A., Alcántara S., Nakajima K., Miyata T., Ogawa M., Mikoshiba K., Derer P., Frotscher M., Soriano E. (1997) A role for Cajal-Retzius cells and reelin in the development of hippocampal connections. Nature 385, 70–74 [DOI] [PubMed] [Google Scholar]
- 47. Castrén E., Zafra F., Thoenen H., Lindholm D. (1992) Light regulates expression of brain-derived neurotrophic factor mRNA in rat visual cortex. Proc. Natl. Acad. Sci. U.S.A. 89, 9444–9448 [DOI] [PMC free article] [PubMed] [Google Scholar]