Background: Certain mRNAs are anchored to the endoplasmic reticulum (ER) by p180.
Results: The placental alkaline phosphatase mRNA requires its transmembrane domain coding region to be anchored by p180.
Conclusion: Translational-independent ER targeting of mRNA by p180 can be mediated by regions of the ORF that encode hydrophobic peptides.
Significance: Translational-independent targeting of mRNA to membranes may be mediated by a mechanism conserved between prokaryotes and eukaryotes.
Keywords: mRNA, Protein Secretion, Ribonuclear Protein (RNP), RNA Transport, Subcellular Organelles
Abstract
In both eukaryotic and prokaryotic cells, it has been recently established that mRNAs encoding secreted and membrane proteins can be localized to the surface of membranes via both translation-dependent and RNA element-mediated mechanisms. Previously, we showed that the placental alkaline phosphatase (ALPP) mRNA can be localized to the ER membrane independently of translation, and this localization is mediated by p180, an mRNA receptor present in the ER. In this article, we aimed to identify the cis-acting RNA element in ALPP. Using chimera constructs containing fragments of the ALPP mRNA, we demonstrate that the ER-localizing RNA element is present within the 3′ end of the open reading frame and codes for a transmembrane domain. In addition, we show that this region requires p180 for efficient ER anchoring. Taken together, we provide the first insight into the nature of cis-acting ER-localizing RNA elements responsible for localizing mRNAs on the ER in mammalian cells.
Introduction
In eukaryotes, mRNAs encoding secretory, membrane-bound, and most organellar proteins must be targeted to, and then maintained at, the surface of the endoplasmic reticulum (ER).2 In mammalian cells this localization is mediated by at least two general mechanisms. The first, which is universally conserved across all life, is the co-translational dependent mechanism. In this pathway, mRNAs encoding secreted and membrane proteins are initially translated in the cytoplasm by the free ribosomes. Once the signal sequence exits the ribosome, it recruits the signal recognition particle (SRP), which inhibits further translation and directs the nascent polypeptide chain-ribosome-mRNA complex, to the surface of the ER by binding to the SRP receptor (1, 2). At the ER, the signal sequence is inserted into the translocon, and translation resumes (3, 4). During this process, the mRNA remains anchored on the ER by direct interactions between the translocon and ribosomes (3).
In both prokaryotes and eukaryotes there are alternative mechanisms that act independently of translation, SRP, and ribosomes (5–7). In mammalian cells, this alternative localization pathway is mediated by at least one RNA receptor, p180 (7). This highly abundant membrane-bound protein resides on the ER surface and contains a large positively charged cytoplasmic domain that can directly interact with RNA in vitro, likely by forming nonspecific ionic interactions with the phosphate backbone (7). In addition, other mRNA receptors are probably present in the mammalian ER, as p180 depletion does not abolish translation-independent anchoring of bulk mRNA to the surface of this organelle (7).
Interestingly, it appears that only a subset of mRNAs is able to utilize this translation-independent pathway. Individual examples include the human GRP94 (6), and calreticulin (7) mRNAs, which encode chaperones that reside in the lumen of the ER, and the human placental alkaline phosphatase (ALPP) mRNA (7). In addition, mRNAs encoding certain cytosolic proteins also co-fractionate with the ER (8). Importantly, both the calreticulin gene and ALPP require p180 for their translation-independent maintenance at the ER (7). In contrast, certain engineered reporter mRNAs, such as t-ftz, and natural transcripts, such as the insulin-like 3 and cytochrome p450 8B1 (CYP8B1) genes, predominantly use the translation-dependent pathway for ER localization (7, 9). In light of this, p180 probably collaborates with supplementary proteins, which have motif-discriminating RNA-binding domains.
Although p180 appears to be metazoan-specific, a similar system is present in Escherichia coli (5, 10). In particular, it was found that the Bgl polycistronic transcript is anchored to the plasma membrane of E. coli independently of translation. The anchoring of this transcript required a portion of the BglF ORF, which encodes several transmembrane domains (TMDs).
To better understand this basic cellular process in mammalian cells, it is critical that we identify RNA elements that promote p180-dependent ER localization. Here we identify a region of the ALPP mRNA that promotes ER localization. Furthermore, we demonstrate that this RNA fragment uses the p180-dependent pathway.
EXPERIMENTAL PROCEDURES
DNA Plasmid, Construction of Fusion Genes
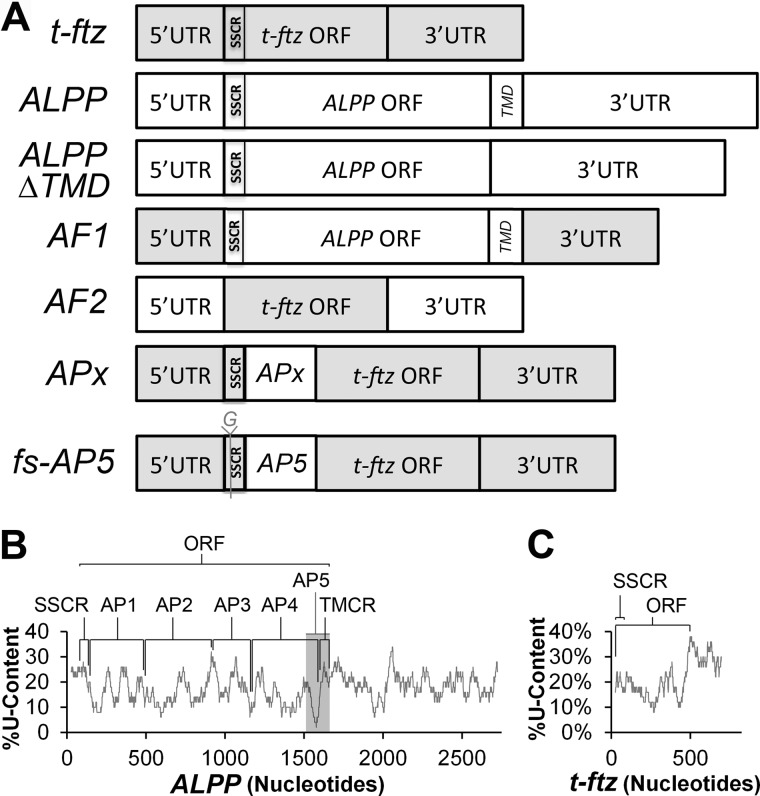
All chimeric constructs were constructed by restriction free subcloning (11) using full-length ALPP in pSPORT6 (7) and t-ftz (also known as MHC-ftz) in pcDNA3 (12). Briefly, inserts were amplified by PCR, followed by a second vector-based PCR step that either inserts the fragment or uses the fragment to replace a targeted sequence. AF1 was constructed by replacing the t-ftz ORF with the ALPP ORF, whereas the converse is true for AF2 (Fig. 1A). APx constructs (AP1, AP2, AP3, AP4, and AP5, Fig. 1A) were constructed by inserting various fragments of ALPP (as indicated in Fig. 1B) between the signal sequence coding region (SSCR) and ORF of t-ftz. In particular, AP1 contains nucleotides 134–480 of the full-length ALPP cDNA transcript, AP2 contains 481–917, AP3 contains 918–1172, AP4 contains 1173–1564, and AP5 contains 1472–1671. Note that AF1 and AP1–5 constructs are in pCDNA3, whereas AF2 is present in pSPORT6. Frameshift AP5 (fs-AP5) was constructed by site-directed mutagenesis using restriction-free subcloning to insert a guanine between the 3rd and 4th nucleotides of the ORF. ALPP-ΔTMD was made by removing nucleotides 1504–1594 from the ALPP construct using restriction-free cloning. H1B-GFP and M1-ftz were described previously (7, 13).
FIGURE 1.
Schematic diagrams of constructs and U content in ALPP and t-ftz. A, schematic of the chimeric constructs used in this study. All t-ftz sequences are depicted in gray and ALPP sequences in white. SSCRs and TMCRs are also indicated. B, percentage of U content in ALPP, as analyzed using a moving window of 50 nucleotides, plotted against the length of the construct. The SSCR, ORF, TMCR, and various fragments (AP1–5) are indicated. Note that the gray region represents AP5. C, percentage U content in t-ftz, as analyzed using a moving window of 50 nucleotides.
Computational Analysis of the Features of Encoded Proteins
The presence of signal sequences or TMDs was predicted using SignalP and TMHMM servers. A Kyte-Doolittle hydropathy plot was computed using ProtScale.
Cell Culture, Permeabilization, shRNA Treatment, Transfection, and Microinjection
Cell culture and transfection were carried out as described previously (7, 9). Depletion of p180 by lentivirus-mediated delivery of shRNA constructs was carried out in U2OS cells as described previously (7). 18–24 h after transfection cells were pretreated with DMEM containing DMSO, 200 μm puromycin (Sigma), or 5 μm homoharringtonine (HHT) (Tocris Bioscience) for 30 min, permeabilized with digitonin (Sigma), and then fixed as described previously (9). To determine the targeting of newly synthesized transcript, plasmids encoding ALPP, t-ftz, or AP5 were microinjected into U2OS or COS-7 cells pretreated with DMEM containing either DMSO or 5 μm HHT for 15 min as described previously (7, 14). After injection, cells were incubated with DMEM containing DMSO or HHT for 2 h and then permeabilized with digitonin and fixed as described previously (7, 9, 14).
Fluorescent in Situ Hybridization (FISH), Immunofluorescence, and Imaging
FISH, immunofluorescence, and imaging were performed, as described previously (7, 9, 14). For ALPP- and ftz-specific probes, see Ref. 7. For H1B-GFP, a DNA probe specific for EGFP was used (Alexa Fluor 546, 5′-CCG TCG CCG ATG GGG GTG TTC TGC TGG TAG TGG TCG GCG AGC TGC ACG CTG CC-3′, synthesized by IDT). For Trapα immunofluorescence, a polyclonal primary (15) and Alexa Fluor 647-conjugated secondary (Molecular Probes) were used as described previously (7). Images were analyzed, and subcellular localization was quantified using NIS-element software, as described previously (7, 9, 14).
Cell Fractionation, Western Blotting, and Northern Blotting
U2OS cells were transfected and allowed to express various mRNAs for 18–24 h and then separated into cytosolic and ER fractions as described previously (7). Briefly, cells were trypsinized, washed, and then permeabilized with low levels of digitonin. The nuclear ER fraction was separated from the cytosolic fraction by low speed centrifugation. The pellet was then treated with Triton X-100 detergent to lyse the ER, and the nuclear contents were removed by low speed centrifugation. Cell fractions were separated by SDS-PAGE and immunoprobed with antibodies against α-tubulin (mouse monoclonal DM1A, diluted 1:20,000; Sigma), Aly (rabbit polyclonal (16), 1:1000), Trapα (rabbit polyclonal (15), diluted 1:5000), or p180 (rabbit polyclonal, diluted 1:1000; Sigma). For Northern blots, RNA was isolated from fractions, separated on denaturing agarose gels, and probed using radiolabeled antisense ftz probes, as described previously (17).
RESULTS
Efficient Translation-independent Maintenance of ALPP mRNA at the ER Requires Its Open Reading Frame
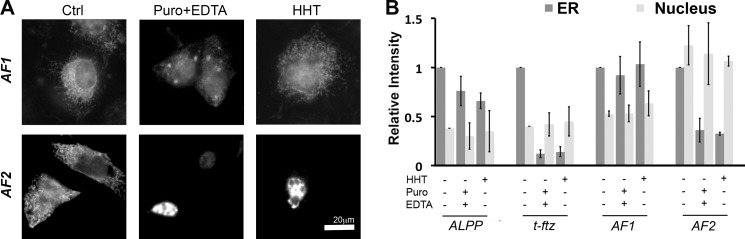
To identify putative ER-localizing RNA element(s), we created chimera constructs between ALPP, which can be targeted and then maintained on the ER in a translation-independent manner, and t-ftz, which strictly requires translation for its localization to the ER (7). First, we created two constructs that had UTRs from one gene and the ORF of the other. AF1 contains the ORF of ALPP and the UTRs of t-ftz, whereas AF2 contains the converse (Fig. 1A). These constructs were transfected into COS-7 cells, which were then allowed to express mRNA for 18–24 h. The cells were then treated with either control medium, puromycin, or HHT for 30 min. Puromycin promotes the ejection of nascent polypeptides and can help to disrupt ribosomes when used in combination with an extraction buffer that contains EDTA (7). In contrast, HHT, being an inhibitor of translation initiation, allows ribosomes to complete translation naturally while preventing the association of new ribosomes with the mRNA (18). Note that both drugs completely inhibit translation in <15 min (7). The cells were then treated with digitonin to permeabilize the plasma membrane and extract any cytoplasmic mRNAs that are not ER-bound, as described previously (7, 9). The cells were then fixed, and AF1 mRNA was detected by FISH using probes directed to the ALPP ORF, whereas AF2 was detected using probes that hybridize to the ftz ORF.
Surprisingly, the majority of the AF1 mRNA was maintained on the ER after either puromycin/EDTA or HHT treatments. In contrast, the ER association of AF2 mRNA was sensitive to these treatments (Fig. 2). As an internal control we also monitored the amount of nuclear mRNA, which is not affected by digitonin permeabilization (7), and as expected this remained relatively unchanged among different treatment groups (Fig. 2B). As published previously (7), ALPP remained associated with the ER under all conditions, whereas t-ftz required translation for ER association. These results indicated that the ER-localizing cis-element was present within the ORF of ALPP.
FIGURE 2.
Translation-independent ER localization is mediated by the ALPP ORF. COS-7 cells were transfected with plasmids containing either ALPP, t-ftz, or the AF1, AF2 fusion constructs and allowed to express mRNA for 18–24 h. The cells were then treated with DMSO (Ctrl), puromycin (Puro), or HHT for 30 min and then extracted with either digitonin alone, or for puromycin-treated cells, with 20 mm EDTA. The cells were then fixed, stained for mRNA using specific FISH probes (ALPP probe for AF1, ftz probe for AF2), and imaged. A, representative FISH images of cells expressing AF1 or AF2. B, quantification of the fluorescence intensities of mRNA in the ER and nucleus. All data were normalized to the ER staining intensity in the control treated group for each construct. Each bar represents the average ± S.E. (error bars) of three independent experiments (each experiment consisting of n>30 cells). Scale bar, 20 μm.
The TMD Coding Region of ALPP mRNA Promotes the Translation-independent Maintenance of mRNA at the ER
The ALPP transcript is translated into a protein product that is translocated into the ER and then anchored to the membrane by a carboxyl-terminal TMD. This TMD is then cleaved, and the processed protein becomes covalently linked to a phosphatidylinositol glycan moiety, which retains the protein at the membrane (19). Mature ALPP is then transported through the secretory pathway to the surface of cell.
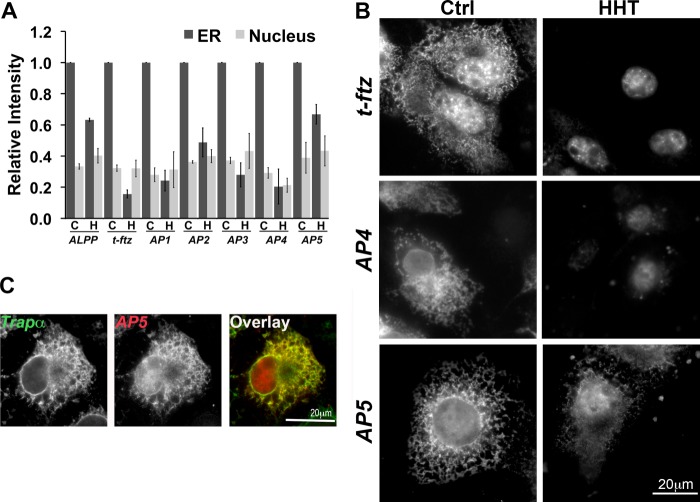
To determine where the ER-localizing RNA element is located, we inserted five segments of the ALPP ORF between the SSCR and ORF of t-ftz (AP1, AP2, AP3, AP4,and AP5) (Fig. 1, A and B). This allowed us to use the ftz FISH probe to detect each of these chimeric mRNAs. Moreover, the presence of the t-ftz SSCR ensured that these mRNAs would be properly exported from the nucleus to the cytoplasm (12). Plasmids containing each construct were transfected into COS-7 cells. After 18–24 h, translation-independent ER association was assessed. We found that the AP5 fusion mRNA was maintained on the ER independently of translation at a level similar to the full-length ALPP (Fig. 3 A and B). In HHT-treated cells, AP5 mRNA co-localized with Trapα, an ER marker, confirming that this localization is ER-specific (Fig. 3C). Of the other chimeras, only AP2 showed a modest increase in its localization on the ER in HHT treated cells compared with t-ftz, suggesting that this fragment may have some limited localization capability. Note that a region of AP5 is also found in AP4 (Fig. 1B); however, this later mRNA requires translation for efficient ER anchoring (Fig. 3, A and B).
FIGURE 3.
The element responsible for the translation-independent ER localization is present in AP5. COS-7 cells were transfected with plasmid containing either full-length ALPP, t-ftz, AP1, AP2, AP3, AP4, or AP5 and allowed to express mRNA for 18–24 h. The cells were then treated with DMSO (C or Ctrl) or HHT (H) for 30 min and then extracted with digitonin. Cells were then fixed, stained for mRNAs using specific FISH probes (ftz probe was used to detect AP1–5), and imaged. A, quantification of the fluorescence intensities of mRNA in the ER and nucleus. All data were normalized to the ER staining intensity in the control treated group for each construct. The results were normalized to the ER staining intensities in the control treated group for each construct. Each bar represents the average ± S.E. (error bars) of three independent experiments (each experiment consisting of n>30 cells). B, examples of COS-7 cells expressing either t-ftz, AP4, or AP5. C, example of a COS-7 cell expressing AP5 that has been treated with HHT for 30 min then extracted with digitonin. The cell was co-stained for AP5 using ftz FISH probes and Trapα, an ER marker, by immunofluorescence. Scale bars, 20 μm.
From these experiments we conclude that the AP5 region of ALPP contains an ER-localizing sequence. It is likely that the region responsible for this activity resides in the region that is unique to AP5 (i.e. not found in AP4), in particular the TMD-coding region (TMCR).
The Coding Potential of AP5 Is Not Required for ER Localization
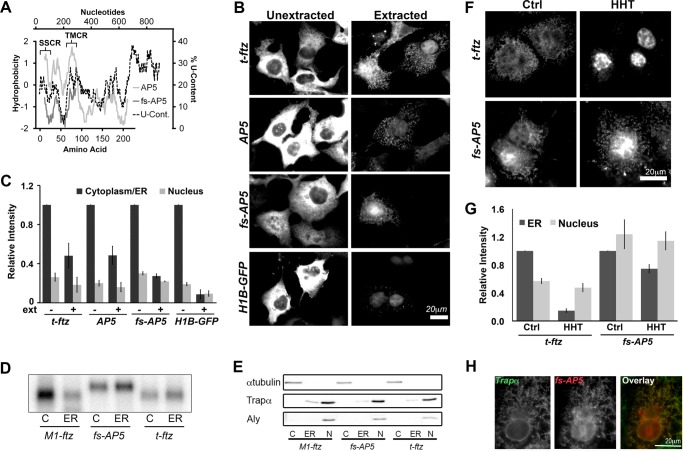
To ensure that ER localization of AP5 was not due to a low level of translation in HHT-treated cells, we inserted a guanine between the 3rd and 4th nucleotides of the ORF of this mRNA, creating frameshifted AP5 (fs-AP5, Fig. 1A). This mRNA encodes a polypeptide that does not contain a signal sequence or TMD, as predicted by either SignalP (20) or TMHMM (21) (data not shown). Furthermore, this peptide is mostly hydrophilic as measured by a Kyte-Doolittle hydropathy plot (22) (Fig. 4A), and is thus not a likely substrate for co-translational translocation into the ER.
FIGURE 4.
Frameshifted AP5 can still efficiently be maintained on the ER independently of ribosomes and translation. A, hydrophobicity (y axis, left) of the polypeptides encoded by AP5 and fs-AP5 plotted against the peptide length (x axis, bottom). The Kyte-Doolittle hydropathy plot was calculated using with a moving window size of 21 amino acids. Note that AP5 encodes the t-ftz signal sequence (residues 1–23 of the AP5 protein), followed by the C-terminal domain of ALPP (including the TMD of ALPP, residues 73–90 of the AP5 protein), and then ends with the hydrophilic ftz protein. In contrast, fs-AP5 does not encode any hydrophobic region. Also note that the frameshift mutation results in the creation of a premature stop codon and a smaller encoded protein. For comparison, the U content (x axis, right) along AP5 (y axis, top) was also plotted. B and C, COS-7 cells transfected with plasmid containing either t-ftz, AP5, fs-AP5, or H1B-GFP and allowed to express mRNA for 18–24 h. Cells were then fixed directly (Unextracted) or first digitonin-extracted (Extracted) and then fixed. Cells were then stained with specific FISH probes (ftz probes were used to for AP5 and fs-AP5) to visualize mRNA distribution. B, representative examples. C, quantification of fluorescence intensities of mRNA in the cytoplasm/ER and nucleus in unextracted (ext−) and extracted (ext+) cells. All data were normalized to the unextracted cytoplasmic/ER staining intensity for each construct. Each bar represents the average ± S.E. (error bars) of three independent experiments (each experiment consisting of n>40 cells). D and E, U2OS cells transfected with plasmid containing either t-ftz, fs-AP5, or M1-ftz. Cytoplasm (C), ER (ER), and nuclear (N) fractions were collected. D, total RNA purified from each fraction. The presence of mRNA was determined via Northern blotting using anti-ftz probes. E, denatured fractions, separated by SDS-PAGE and analyzed by immunoblotting using antibodies against either Trapα (a resident ER protein), α-tubulin (a cytoplasmic protein), and Aly (a nuclear protein). F and G, COS-7 cells transfected with plasmids containing the t-ftz or fs-AP5 constructs and allowed to express mRNA for 18–24 h. The cells were then treated with DMSO (Ctrl) or HHT for 30 min and then extracted with digitonin. Cells were fixed, stained for mRNA using a FISH probe against ftz, and imaged. F, representative images. G, quantification of the fluorescence intensities of mRNAs in the ER and nucleus. All data were normalized to the ER staining intensity in the control treated group for each construct. Each bar represents the average ± S.E. (error bars) of three independent experiments (each experiment consisting of n>30 cells). H, example of a COS-7 cell expressing fs-AP5 that has been treated with HHT for 30 min and then extracted with digitonin. The cell was co-stained for fs-AP5 using a ftz FISH probe and Trapα, an ER marker, by immunofluorescence. Scale bars, 20 μm.
Next, we expressed fs-AP5 in COS-7 cells and observed its distribution in normal and digitonin-permeabilized cells compared with AP5 and t-ftz. We also monitored H1B-GFP mRNA, which is not expected to be at the ER because it encodes a nuclear localized histone 1B-GFP fusion. In the majority of cells the distribution of t-ftz, AP5, fs-AP5, and H1B-GFP mRNA was mostly diffuse in the cytoplasm, indicating that a substantial fraction of these transcripts were not ER-bound (Fig. 4B). However, after digitonin permeabilization, significant levels of t-ftz, AP5, and fs-AP5, but not H1B-GFP mRNA, remained in the cytoplasm in a reticular pattern (Fig. 4B; for quantification see 4C), that co-localized with Trapα (data not shown). Indeed, we calculated that approximately one quarter of the cytoplasmic fs-AP5 mRNA is bound to the ER (Fig. 4C). In contrast, <10% of the H1B-GFP mRNA was ER-associated. We next confirmed this result by analyzing the distribution of various mRNAs in different cell fractions. Transfected U2OS cells were separated into cytoplasm ER and nuclear fractions as described previously (7, 17), and the presence of RNA was determined by Northern blotting. fs-AP5 and t-ftz were present at slightly higher levels in the ER than in the cytoplasmic fraction (Fig. 4D). In contrast, M1-ftz mRNA, which encodes a cytosolic protein and contains the mRNA nuclear export-promoting element M1 (13), was present predominantly in the cytoplasmic fraction (Fig. 4D). To ensure that the fractions were not cross-contaminated, we examined them for various markers. Note that the ER fraction was free of cytosolic proteins, such as α-tubulin, and nuclear factors, such as the RNA-binding protein Aly (Fig. 4E). It is likely that the discrepancy in the degree of ER localization for fs-AP5 between Fig. 4, B and E, was because COS-7 cells tend to have higher levels of expression than U2OS and thus flood all of the mRNA-binding sites on the ER.
We then assessed whether the localization of fs-AP5 mRNA required translation. In contrast to t-ftz, fs-AP5 remained ER-associated in COS-7 cells treated with HHT (Fig. 4, F and G). This ER localization in HHT-treated cells was confirmed by the co-localization of the mRNA with the ER marker Trapα (Fig. 4H). These results confirm that AP5 contains an RNA element that can anchor transcripts on the ER independently of translation.
The TMCD Is Required for the Translation-independent ER Localization of ALPP
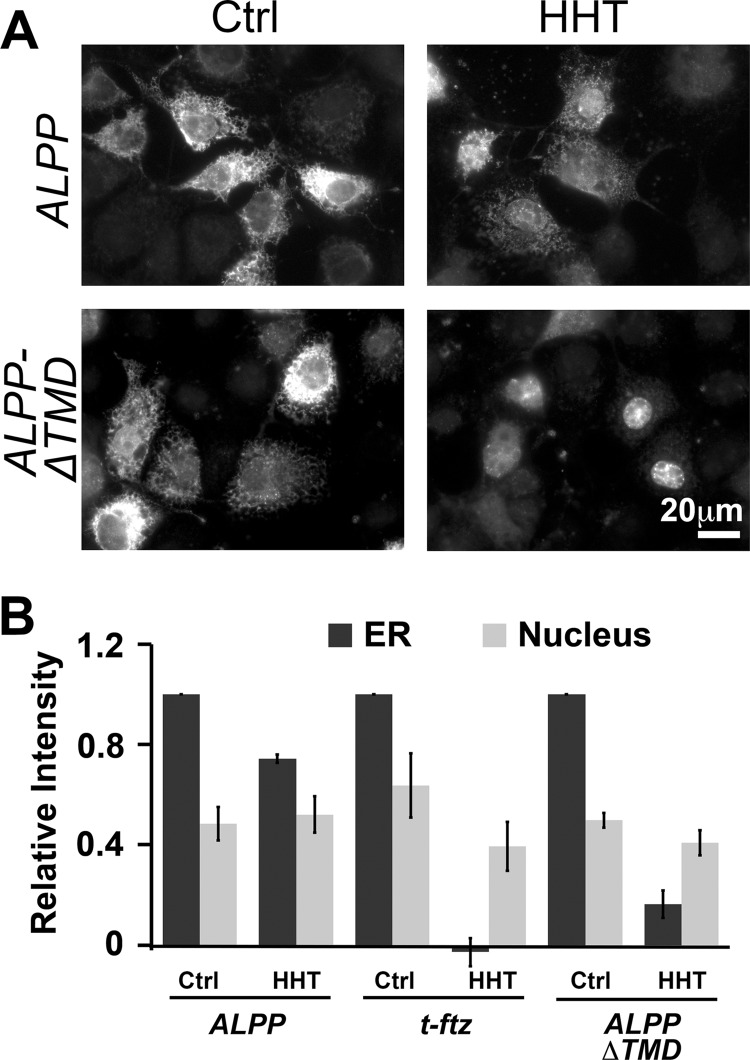
We next deleted the last 90 nucleotides of the ORF from the full-length ALPP. This construct, ALPP-ΔTMD, lacks the TMCR. Although this mRNA localized to the ER in COS-7 cells, we found that it was not retained there after HHT treatment, especially when compared with ALPP (Fig. 5). These observations indicate that the ER retention element likely resides in the TMCR.
FIGURE 5.
The TMCD is required for the translation-independent ER localization of ALPP. COS-7 cells were transfected with plasmid containing either ALPP or ALPP-ΔTMD and allowed to express mRNA for 18–24 h. The cells were treated with DMSO (Ctrl) or HHT for 30 min and then extracted with digitonin. The cells were fixed and stained for mRNA using a specific FISH probe against the ALPP ORF. A, representative examples. B, quantification of the fluorescence intensities of mRNAs in the ER and nucleus. All data were normalized to the ER staining intensity in the control treated group for each construct. Each bar represents the average ± S.E. (error bars) of three independent experiments (each experiment consisting of n>30 cells). Scale bar, 20 μm.
AP5 Promotes the Efficient Targeting of mRNA to the ER Independently of Translation
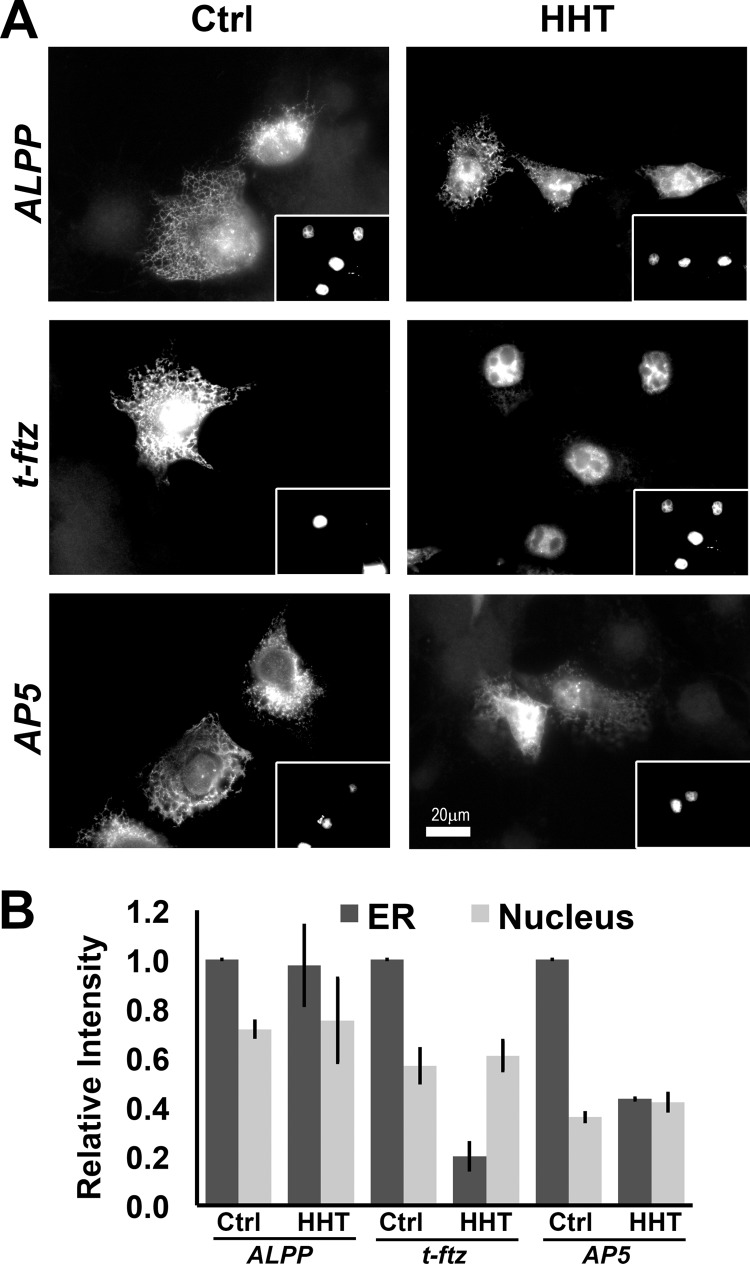
Thus far, our data indicate that once localized to the surface of the ER, AP5 is retained there even after ribosomes have dissociated from the transcript. It is however possible that the initial targeting of this mRNA to the ER is still dependent on translation, with the TMCR mediating its subsequent ER retention independently of translation. To test whether this region can promote the initial targeting to the ER independently of translation, we first treated COS-7 cells with HHT for 15 min to halt all translation, then microinjected plasmids and monitored the localization of the newly synthesized transcripts. In principle, an mRNA should be free of active ribosomes throughout its entire lifetime under these conditions. We found that newly synthesized AP5 was targeted to the ER in both control treated and in cells pretreated with HHT (Fig. 6). The targeting of AP5 was less efficient than the full-length ALPP, but enhanced compared with t-ftz (Fig. 6B). These results indicate that AP5 can both target to, and be retained on, the ER independently of translation.
FIGURE 6.
The initial ER targeting of AP5 can occur independently of translation and ribosomes. COS-7 cells were pretreated with DMSO (Ctrl) or HHT for 15 min, then microinjected with plasmids containing ALPP, t-ftz, or AP5. These plasmids were microinjected with Alexa Fluor 488-conjugated 70-kDa dextran, which marks injected cells and can be seen in the insets (A). Cells were allowed to express mRNAs for 2 h in the presence of DMSO or HHT, then extracted with digitonin, fixed, and stained with specific FISH probes, and imaged. A, representative examples. B, quantification of the fluorescence intensities of mRNAs in the ER and nucleus. All data were normalized to the ER staining intensity in the control treated group for each construct. Each bar represents the average ± S.E. (error bars) of three independent experiments (each experiment consisting of n>30 cells). Scale bar, 20 μm.
p180 Is Required for the Efficient Targeting of ALPP mRNA to the ER
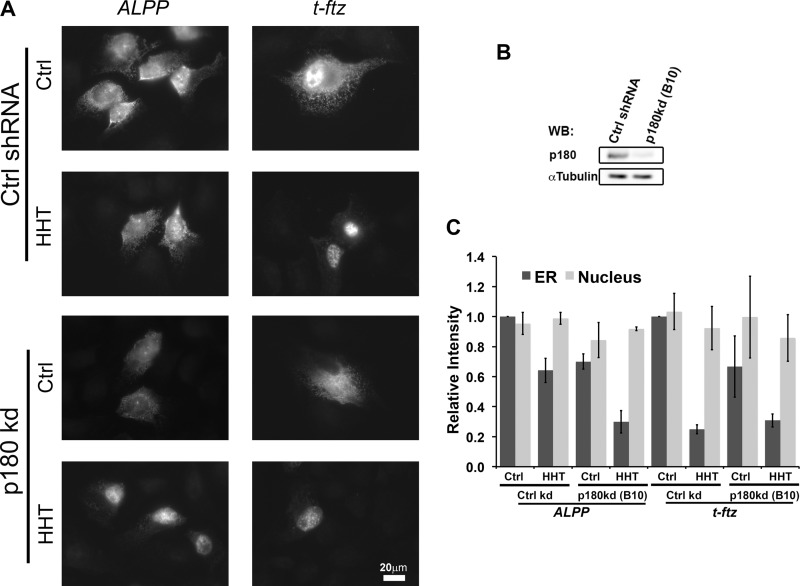
Previously, we showed that p180 acts as an mRNA receptor that anchors several different transcripts, such as ALPP, on the surface of the ER. Unfortunately, we never tested whether p180 was required for the initial ER targeting of this transcript independently of translation. To test this, we microinjected plasmids containing ALPP into p180-depleted U2OS cells (Fig. 7B). We observed that in the absence of p180, the ability of the newly synthesized ALPP mRNA to target to the ER decreased in both control and HHT-treated cells (Fig. 7, A and C). In contrast, the ER targeting of t-ftz was strictly dependent on active translation and was not affected by p180 depletion. These data indicate that p180 is required not only for ER anchoring, but also for the efficient ER targeting of a subset of mRNA, such as the ALPP transcript.
FIGURE 7.
p180 is required for the initial ER targeting of ALPP. U2OS cells were infected with lentivirus carrying control shRNA (Ctrl shRNA/Ctrl kd) or shRNA clone B10 against p180 (p180 kd). The control and p180-depleted cells were pretreated with DMSO (Ctrl) or HHT for 15 min, then microinjected with plasmids containing either the ALPP or t-ftz and allowed to express mRNAs for 2 h in the presence of DMSO or HHT. The cells were then extracted with digitonin, fixed, and stained with specific FISH probes and imaged. A, representative examples. B, cell lysate collected on the day of injection. The level of depletion was assessed by immunoblotting (WB) against p180 and α-tubulin. C, quantification of the fluorescence intensities of mRNAs in the ER and nucleus. All data were normalized to the ER staining intensity in the control treated/control shRNA group for each construct. Each bar represents the average ± S.E. (error bars) of three independent experiments (each experiment consisting of n>30 cells). Scale bar, 20 μm.
The fact that we can observe a p180 dependence in targeting even in the presence of active translation suggests that p180 also either works in parallel and/or directly enhances SRP-dependent processes. This first model is supported by the fact that p180 is required for enhanced ER targeting even when translation is inhibited. The second possibility is supported by observations that p180 may directly contact ribosomes (23) and/or the translocon (24). Nevertheless, our results strongly indicate that p180 is involved in both the initial targeting and also the anchoring of ALPP on the surface of the ER.
ER Maintenance of AP5 mRNA Requires p180
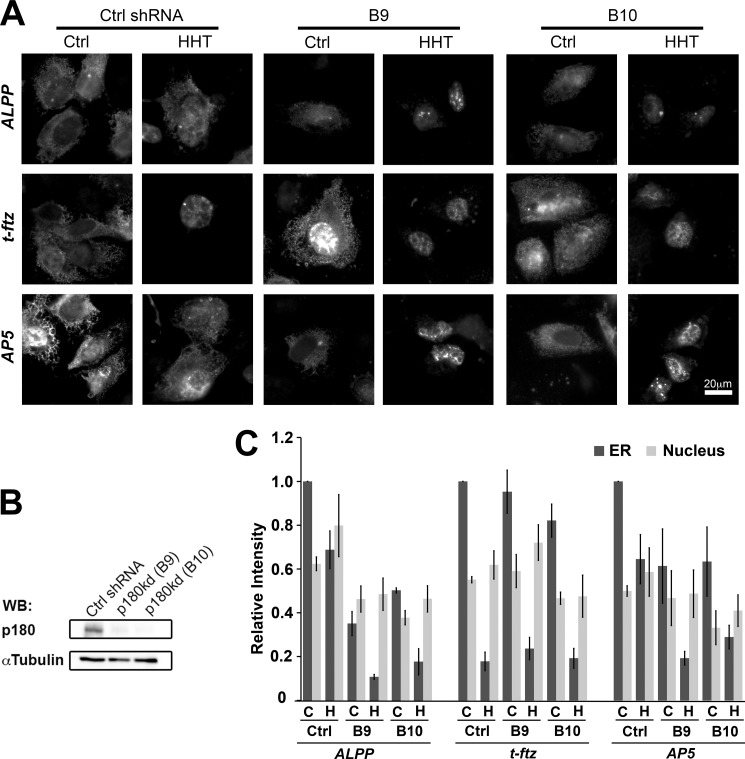
To examine whether TMCR-mediated ER localization is p180-dependent, we examined the distribution of AP5 in U2OS cells depleted of p180 (Fig. 8B). Indeed, similar to full-length ALPP mRNA, AP5 showed a significant decrease in its ability to associate with ER in p180-depleted cells (Fig. 8, A and C). This decrease was seen in both control and HHT-treated cells. Again, the ability of t-ftz to be maintained on the ER was strictly dependent on translation and was not affected by the depletion of p180. In conclusion, our results indicate that the AP5 region of ALPP, which includes a TMCR, contains an RNA element that anchors this mRNA to the surface of the ER in a p180-dependent manner.
FIGURE 8.
p180 is required for the ER association of AP5. U2OS cells were infected with lentivirus carrying control shRNA (Ctrl shRNA) or shRNAs (clones B9 or B10) against p180. The control and p180-depleted cells were transfected with plasmids containing the ALPP, t-ftz, or AP5 construct and allowed to express mRNAs for 18–24 h. The cells were then treated with either DMSO (C or Ctrl) or HHT for 30 min (H), digitonin-extracted, fixed, stained with specific FISH probes, and imaged. A, representative examples. B, cell lysate collected on the day of injection. The level of depletion was assessed by immunoblotting (WB) against p180 and α-tubulin. C, quantification of the fluorescence intensities of mRNAs in the ER and nucleus. All data were normalized to the ER staining intensity in the control treated/control shRNA group for each construct. Each bar represents the average ± S.E. (error bars) of three independent experiments (each experiment consisting of n>30 cells). Scale bar, 20 μm.
DISCUSSION
We have identified a region of ALPP that contains an RNA element that promotes ER localization. Furthermore, our data indicate that this element requires p180 for its activity. Interestingly, this element maps to a region of ALPP that encodes a TMD. This finding suggests that mammalian cells may have a propensity to recognize nucleotide features that are associated with certain protein-coding regions. These inherent biases would be maintained by natural selection primarily to constraints imposed by the encoded polypeptide; however, subsequently these features could be further exaggerated to enhance activities that act at the nucleotide level. For example, the amino acid composition of TMDs is enriched in hydrophobic residues such as Leu, Ile, Met, Val, and Phe. Interestingly, all of these amino acids are encoded by codons that have a uracil at their second position and are thus relatively U-rich (25). Indeed, it has been noted that uracil content is a good predictor of whether any given region within an ORF encodes a TMD or a signal sequence (26). As mentioned in the Introduction, it has also been shown that E. coli targets certain mRNAs to the plasma membrane by elements found within TMCRs that are U-rich (5). These results may indicate that the propensity for TMCR to promote the localization of mRNAs to sites of secretory protein production, independently of translation, maybe universally conserved.
This simplistic model, however, does not explain all of our observations. When the uracil content of the ALPP transcript was analyzed, the TMCR contains a relatively high level of uracil compared with other sections of the transcript; however, one can clearly find regions within the ORF and UTRs that have even higher levels (see Fig. 1B). Furthermore, regions of the t-ftz 3′-UTR (present in both t-ftz and AP5, see Figs. 1C and 4A) have levels of uracil that exceed what is found in the TMCR of ALPP, yet t-ftz does not exhibit much translation-independent ER targeting. We also recently reported that the CYP8B1 mRNA, which encodes a membrane-bound protein, also requires translation for efficient ER anchoring (9), suggesting that not all TMCRs have this activity. Finally, calreticulin mRNA, which does not encode any TMD, associates with the ER independently of translation in a p180-dependent manner (7). Thus, it is clear that although U-richness in TMCRs may help contribute to this activity, other features are also important. Work in budding yeast has identified other cis-elements that are responsible for ER targeting. One example is Pmp1, which contains several UG repeats in its 3′-UTR that can promote ER localization (27). Recently, other yeast mRNAs have been shown to localize to the ER independently of translation in manners that depend on either their ORFs and/or UTRs (28). Thus, it is likely that many different elements may target mRNAs to the ER.
Another element found within ORFs of metazoan mRNAs encoding secretory proteins is the SSCR. It promotes nuclear export (12) and translation (17) of mRNAs. Mutations within the SSCR redirect mRNAs from the ER to stress granules (12), large cytoplasmic aggregates of mRNAs with stalled translation initiation complexes (29). SSCRs also have unusual nucleotide compositions. They are depleted of adenine, enriched in guanine, cytosine, and uracil, and tend to contain CUG repeats (12, 13). SSCRs, however, do not appear to promote translation-independent ER targeting as exemplified by t-ftz and insulin-like 3 genes, which despite having SSCRs with high U content, absolutely require translation for efficient targeting and anchoring to the ER (Fig. 3A) (7). It is still possible that whereas the SSCR is not sufficient, it may still be required for translation-independent ER targeting.
We have attempted to analyze the degree of conservation in the 90 nucleotides at the end of the ALPP ORF; however, this analysis was hampered by the fact that mammals contain numerous ALPP paralogs, many of which lack TMCRs. Despite this, we found that these regions had features that are associated with SSCRs. In particular, they have CUG repeats, GC-rich regions and low adenine content. These observations raise the possibility that the number of SSCR-like segments present in a given mRNA impacts the efficiency of the p180-dependent pathway. We are currently identifying p180-associated mRNAs, and through this approach we hope to computationally determine which sequence features and motifs correlate with p180 dependence for ER anchoring.
It remains unclear how p180 promotes the specific anchoring of a subset of mRNAs to the ER. p180 likely binds the RNA backbone through ionic interactions (7) and therefore is not likely to act selectively. In light of this, other RNA-binding proteins, which have classic RNA recognition motifs, may act in conjunction with p180 to selectively retain certain mRNAs. Candidates were previously identified in a mass spectrometry screen of proteins that co-purify with ER-derived polysomes (7). Ultimately, the ALPP TMCR could be used to identify these accessory proteins.
Acknowledgments
We thank E. Lee, K. Mahadevan, A. Akef, and H. Zhang for helpful advice.
This work was supported by a graduate fellowship (to X. A. C.), summer student fellowships (to Y. Z. and S. J. H.), and a grant (to A. F. P.) all from the National Science and Engineering Research Council of Canada.
- ER
- endoplasmic reticulum
- ALPP
- placental alkaline phosphatase
- CYP8B1
- cytochrome P-450 8B1
- DMSO
- dimethyl sulfoxide
- fs
- frameshift
- HHT
- homoharringtonine
- SRP
- signal recognition particle
- SSCR
- signal sequence coding region
- TMCR
- transmembrane domain coding region
- TMD
- transmembrane domain.
REFERENCES
- 1. Walter P., Ibrahimi I., Blobel G. (1981) Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in vitro-assembled polysomes synthesizing secretory protein. J. Cell Biol. 91, 545–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gilmore R., Blobel G., Walter P. (1982) Protein translocation across the endoplasmic reticulum. I. Detection in the microsomal membrane of a receptor for the signal recognition particle. J. Cell Biol. 95, 463–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Görlich D., Prehn S., Hartmann E., Kalies K. U., Rapoport T. A. (1992) A mammalian homolog of SEC61p and SECYp is associated with ribosomes and nascent polypeptides during translocation. Cell 71, 489–503 [DOI] [PubMed] [Google Scholar]
- 4. Görlich D., Rapoport T. A. (1993) Protein translocation into proteoliposomes reconstituted from purified components of the endoplasmic reticulum membrane. Cell 75, 615–630 [DOI] [PubMed] [Google Scholar]
- 5. Nevo-Dinur K., Nussbaum-Shochat A., Ben-Yehuda S., Amster-Choder O. (2011) Translation-independent localization of mRNA in E. coli. Science 331, 1081–1084 [DOI] [PubMed] [Google Scholar]
- 6. Pyhtila B., Zheng T., Lager P. J., Keene J. D., Reedy M. C., Nicchitta C. V. (2008) Signal sequence- and translation-independent mRNA localization to the endoplasmic reticulum. RNA 14, 445–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cui X. A., Zhang H., Palazzo A. F. (2012) p180 promotes the ribosome-independent localization of a subset of mRNA to the endoplasmic reticulum. PLoS Biol. 10, e1001336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lerner R. S., Seiser R. M., Zheng T., Lager P. J., Reedy M. C., Keene J. D., Nicchitta C. V. (2003) Partitioning and translation of mRNAs encoding soluble proteins on membrane-bound ribosomes. RNA 9, 1123–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cui X. A., Palazzo A. F. (2012) Visualization of endoplasmic reticulum localized mRNAs in mammalian cells. J. Vis. Exp. 70, e50066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bibi E. (2011) Early targeting events during membrane protein biogenesis in Escherichia coli. Biochim. Biophys. Acta 1808, 841–850 [DOI] [PubMed] [Google Scholar]
- 11. van den Ent F., Löwe J. (2006) RF cloning: a restriction-free method for inserting target genes into plasmids. J. Biochem. Biophys. Methods 67, 67–74 [DOI] [PubMed] [Google Scholar]
- 12. Palazzo A. F., Springer M., Shibata Y., Lee C. S., Dias A. P., Rapoport T. A. (2007) The signal sequence coding region promotes nuclear export of mRNA. PLoS Biol. 5, e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cenik C., Chua H. N., Zhang H., Tarnawsky S. P., Akef A., Derti A., Tasan M., Moore M. J., Palazzo A. F., Roth F. P. (2011) Genome analysis reveals interplay between 5′-UTR introns and nuclear mRNA export for secretory and mitochondrial genes. PLoS Genetics 7, e1001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gueroussov S., Tarnawsky S. P., Cui X. A., Mahadevan K., Palazzo A. F. (2010) Analysis of mRNA nuclear export kinetics in mammalian cells by microinjection. J. Vis. Exp. 46, 2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Görlich D., Prehn S., Hartmann E., Herz J., Otto A., Kraft R., Wiedmann M., Knespel S., Dobberstein B., Rapoport T. A. (1990) The signal sequence receptor has a second subunit and is part of a translocation complex in the endoplasmic reticulum as probed by bifunctional reagents. J. Cell Biol. 111, 2283–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou Z., Luo M. J., Straesser K., Katahira J., Hurt E., Reed R. (2000) The protein Aly links pre-messenger-RNA splicing to nuclear export in metazoans. Nature 407, 401–405 [DOI] [PubMed] [Google Scholar]
- 17. Mahadevan K., Zhang H., Akef A., Cui X. A., Gueroussov S., Cenik C., Roth F. P., Palazzo A. F. (2013) RanBP2/Nup358 potentiates the translation of a subset of mRNAs encoding secretory proteins. PLoS Biol. 11, e1001545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fresno M., Jiménez A., Vázquez D. (1977) Inhibition of translation in eukaryotic systems by harringtonine. Eur. J. Biochem. 72, 323–330 [DOI] [PubMed] [Google Scholar]
- 19. Amthauer R., Kodukula K., Udenfriend S. (1992) Placental alkaline phosphatase: a model for studying COOH-terminal processing of phosphatidylinositol-glycan-anchored membrane proteins. Clin. Chem. 38, 2510–2516 [PubMed] [Google Scholar]
- 20. Petersen T. N., Brunak S., von Heijne G., Nielsen H. (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 8, 785–786 [DOI] [PubMed] [Google Scholar]
- 21. Krogh A., Larsson B., von Heijne G., Sonnhammer E. L. (2001) Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305, 567–580 [DOI] [PubMed] [Google Scholar]
- 22. Kyte J., Doolittle R. F. (1982) A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157, 105–132 [DOI] [PubMed] [Google Scholar]
- 23. Ueno T., Kaneko K., Sata T., Hattori S., Ogawa-Goto K. (2012) Regulation of polysome assembly on the endoplasmic reticulum by a coiled-coil protein, p180. Nucleic Acids Res. 40, 3006–3017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dejgaard K., Theberge J. F., Heath-Engel H., Chevet E., Tremblay M. L., Thomas D. Y. (2010) Organization of the Sec61 translocon, studied by high resolution native electrophoresis. J. Proteome Res. 9, 1763–1771 [DOI] [PubMed] [Google Scholar]
- 25. Palazzo A. F., Mahadevan K., Tarnawsky S. P. (2013) ALREX-elements and introns: two identity elements that promote mRNA nuclear export. WIREs RNA 4, 523–533 [DOI] [PubMed] [Google Scholar]
- 26. Prilusky J., Bibi E. (2009) Studying membrane proteins through the eyes of the genetic code revealed a strong uracil bias in their coding mRNAs. Proc. Natl. Acad. Sci. U.S.A. 106, 6662–6666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Loya A., Pnueli L., Yosefzon Y., Wexler Y., Ziv-Ukelson M., Arava Y. (2008) The 3′-UTR mediates the cellular localization of an mRNA encoding a short plasma membrane protein. RNA 14, 1352–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kraut-Cohen J., Afanasieva E., Haim-Vilmovsky L., Slobodin B., Yosef I., Bibi E., Gerst J. E. (August 7, 2013) Translation- and SRP-independent mRNA targeting to the endoplasmic reticulum in the yeast, Saccharomyces cerevisiae. Mol. Biol. Cell 10.1091/mbc.E13-01-0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anderson P., Kedersha N. (2006) RNA granules. J. Cell Biol. 172, 803–808 [DOI] [PMC free article] [PubMed] [Google Scholar]