Background: Channelrhodopsins are algal phototaxis receptors used in optogenetics.
Results: Channel activity and photochemistry of a new channelrhodopsin (PsChR) are characterized.
Conclusion: Blue-shifted PsChR has ∼3-fold greater unitary conductance, faster recovery from excitation, and higher sodium selectivity than channelrhodopsin 2 from Chlamydomonas.
Significance: These properties of PsChR facilitate further analysis of light-gated channel function and are potentially useful for optogenetics.
Keywords: Algae, Ion Channels, Optogenetics, Photobiology, Photoreceptors, Channelrhodopsin, Phototaxis
Abstract
Rhodopsin photosensors of phototactic algae act as light-gated cation channels when expressed in animal cells. These proteins (channelrhodopsins) are extensively used for millisecond scale photocontrol of cellular functions (optogenetics). We report characterization of PsChR, one of the phototaxis receptors in the alga Platymonas (Tetraselmis) subcordiformis. PsChR exhibited ∼3-fold higher unitary conductance and greater relative permeability for Na+ ions, as compared with the most frequently used channelrhodopsin-2 from Chlamydomonas reinhardtii (CrChR2). Photocurrents generated by PsChR in HEK293 cells showed lesser inactivation and faster peak recovery than those by CrChR2. Their maximal spectral sensitivity was at 445 nm, making PsChR the most blue-shifted channelrhodopsin so far identified. The λmax of detergent-purified PsChR was 437 nm at neutral pH and exhibited red shifts (pKa values at 6.6 and 3.8) upon acidification. The purified pigment undergoes a photocycle with a prominent red-shifted intermediate whose formation and decay kinetics match the kinetics of channel opening and closing. The rise and decay of an M-like intermediate prior to formation of this putative conductive state were faster than in CrChR2. PsChR mediated sufficient light-induced membrane depolarization in cultured hippocampal neurons to trigger reliable repetitive spiking at the upper threshold frequency of the neurons. At low frequencies spiking probability decreases less with PsChR than with CrChR2 because of the faster recovery of the former. Its blue-shifted absorption enables optogenetics at wavelengths even below 400 nm. A combination of characteristics makes PsChR important for further research on structure-function relationships in ChRs and potentially useful for optogenetics, especially for combinatorial applications when short wavelength excitation is required.
Introduction
Photomotility responses in green flagellate algae are mediated by retinylidene proteins with shared sequence homology to other microbial rhodopsins (for review see Refs. 1 and 2). Their photoexcitation leads to depolarization of the algal plasma membrane (3–5), which initiates a signaling cascade that eventually brings about correction of the cell swimming path according to the direction of light. An incisive method to probe rhodopsin-mediated light signaling in phototactic algae is measurement of photoinduced electrical signals in cell suspensions (6). With this assay, photoreceptor currents were detected in a variety of species, and their analysis revealed a dual receptor sensory system common for all tested algae with intrachloroplast eyespots (reviewed in Ref. 1).
Algal sensory rhodopsins demonstrate light-gated cation channel activity when heterologously expressed in animal cells and are therefore called “channelrhodopsins” (ChRs)2 (7, 8). They predominantly conduct protons but are also permeable for mono- and divalent metal cations. ChRs are widely used for control of neuronal firing, muscle contraction and other cellular processes by light (“optogenetics”; for review see Refs. 9–12). High efficiency and temporal precision are required of optogenetic tools, especially when low stimulus intensities or high stimulation frequencies are desirable, but the ChRs known so far are not ideal in these respects. Their performance has been somewhat increased by molecular engineering, e.g., by point mutations and/or construction of chimeras between different natural sequences (reviewed by Refs. 13 and 14). For example, the H134R and T159C mutants of ChR2 from Chlamydomonas reinhardtii generate ∼3- and ∼10-fold larger currents, respectively, as compared with the wild type, but this gain in amplitude is accompanied by a loss in rates of current rise and decay (15, 16). C1V1, a recently developed chimera of CrChR1 and VcChR1, produces 3-fold larger currents but retains extremely slow decay kinetics characteristic of parental VcChR1, which could be partially improved by introducing a combination of point mutations (17, 18).
A complementary strategy for optimization and extension of the optogenetic toolkit is searching for natural ChR variants in other algae. Previously we briefly reported cloning a new sequence, designated PsChR, from the phototactic alga Platymonas (Tetraselmis) subcordiformis (19). In this study, we show that PsChR is one of the two receptors for phototaxis in this alga. We also present a detailed analysis of its photoelectric activity in cultured animal cells and characterize optical properties of the purified pigment by spectroscopy and flash photolysis.
Here we report that currents generated by PsChR in HEK293 cells show higher amplitude, smaller inactivation, and faster peak recovery than those generated by CrChR2, the molecule of choice in most optogenetic studies, whereas their kinetics is similarly fast. The higher current amplitude of PsChR is due to its higher unitary conductance, as estimated by stationary noise analysis. PsChR shows greater relative permeability for Na+ ions over protons, as compared with CrChR2, which in optogenetic applications could minimize undesirable pH changes in the neuronal cytoplasm. We find that PsChR expressed in cultured hippocampal neurons evokes action potentials by short light pulses delivered at frequencies up to 50 Hz, which is the upper limit of functioning for this type of neuron (20). Light control of spiking on the time scale of seconds was more reliable with PsChR than with CrChR2, because of the faster recovery of PsChR from excitation. With its maximum sensitivity at 445 nm, PsChR is the most blue-shifted channelrhodopsin found so far, ideally suited for coupling with long wavelength absorbing rhodopsin pumps for light-regulated bidirectional control of the membrane potential (21–23) or with fluorescent voltage- or Ca2+-sensitive probes (24–27), which are becoming increasingly popular.
EXPERIMENTAL PROCEDURES
Photocurrents in Native Cells
P. (Tetraselmis) subcordiformis was obtained from the UTEX Culture Collection of Algae (strain 71) and grown in modified artificial seawater medium (28) under 16-h light/8-dark cycle (light: 2,000 lux). Photoelectric currents in the algal cells were measured with the population assay invented earlier (6). The method takes advantage of the directional sensitivity of the photoreceptor antenna complex in flagellates. Two platinum wires immersed in a cell suspension pick up an electrical current generated in response to a unilateral excitation flash from a Vibrant HE 355II Tunable Laser (OPOTEK Inc., Carlsbad, CA) set at desired wavelengths. To decrease the current noise, cells from a 2-week culture were harvested and resuspended in the measuring medium of a lower ionic strength (0.5 mm CaCl2 and 40 mm NaCl). The signal was amplified by a low noise current amplifier (model 428; Keithley Instruments, Cleveland, OH) and digitized by a Digidata 1322A supported by pClamp 10 software (both from Molecular Devices, Union City, CA).
Whole Cell Patch Clamp Measurements in HEK293 Cells
The mammalian expression vector that contained PsChR cDNA encoding the 7TM domain (amino acid residues 1–326) in frame with EYFP tag was generated as described previously (19). HEK293 (human embryonic kidney) cells were transfected using the TransPass COS/293 transfection reagent (New England Biolabs, Ipswich, MA). All-trans-retinal (Sigma) was added as a stock solution in ethanol at the final concentration of 5 μm. Measurements were performed 48–72 h after transfection with an Axopatch 200B amplifier (Molecular Devices) with a 2-kHz filter. The signals were digitized with a Digidata 1440A using the pClamp 10.2 software (both from Molecular Devices) at the sampling rate 50 μs/point for noise analysis and 200 μs/point for other experiments. Fabrication of patch pipettes and contents of pipette and bath solutions were as before (19). To test relative permeability for Na+ ions, Na+ in the bath solution was partially replaced with large monovalent cation N-methyl-d-glucamine that is not conducted by ChRs to a measurable degree (8). Light excitation was provided by a Polychrome IV light source (T.I.L.L. Photonics GMBH, Grafelfing, Germany) pulsed with a mechanical shutter (Uniblitz Model LS6; Vincent Associates, Rochester, NY; half-opening time, 0.5 ms). The light intensity was attenuated with the built-in Polychrome system or with neutral density filters. Maximal quantum density at the focal plane of the 40× objective lens was ∼2 × 1022 photons × m−2 × s−1.
Noise Analysis
An experimental procedure for stationary noise analysis of ChR-generated whole cell currents was modified from Ref. 29. Current traces were recorded at −60 mV at room temperature in the dark and during a 25-s light pulse of intensity, eliciting a half-maximal response. The plateau current was fit with a single exponential, and the fit signal was subtracted from the current trace (30). Power spectral densities were calculated from 5-s segments of the traces using pClamp software; 10 individual spectra for dark and light conditions were averaged for each trace. The difference (light minus dark spectrum) was fit between 2 and 1 kHz with a single Lorentzian function to determine the zero frequency asymptote and the corner frequency (31).
The theory of stationary noise analysis is based on the assumption that the channel stochastically alternates between a closed and an open state (31). The spectral density of resultant current fluctuations is described by a Lorentzian function,
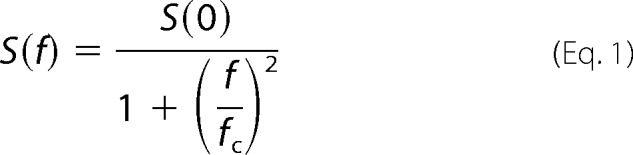 |
where S(f) is the spectral density, S(0) is the zero asymptote (spectral density at 0 Hz), f is the frequency, and fc is the corner frequency.
The unitary conductance (γ) is estimated from the parameters of this function using the amplitude of the whole cell channel current (I), the holding potential (Vh), and the reversal potential of the channel current (Vr).
 |
Electrical Measurements in Neurons
The 7TM domains of PsChR or CrChR2 in frame with an EYFP tag were inserted into pFUGW lentivirus vector backbone provided by Dr. Carlos Lois (Massachusetts Institute of Technology) between BamHI and EcoRI sites. The lentivirus was produced by triple transfection of HEK293FT cells (Invitrogen) with the envelope plasmid pCMV-VSVG, the packaging plasmid pΔ8.9 (both from Dr. Lois), and the pFUGW-PsChR/CrChR2-EYFP plasmids using Lipofectamine 2000 (Invitrogen), as described (32). The viral titer was determined by infection of HEK cells. Hippocampi of embryonic day 18 Sprague-Dawley rats were obtained as part of a kit from BrainBits (Springfield, IL), and primary neuronal cultures were prepared using the protocol provided by the company. Cells were cultured in NbActiv4 medium (70) on polylysine-coated coverslips and supplemented with 0.4 μm all-trans-retinal (final concentration, in addition to retinyl acetate present in the medium), unless otherwise indicated. Neurons were infected with the lentivirus 1 day after plating. Between 10 and 19 days after plating, the cells were used for patch clamp measurements with the same setup as described for HEK cells, except that neurons were bathed in Tyrode's solution (125 mm NaCl, 2 mm KCl, 3 mm CaCl2, 1 mm MgCl2, 25 mm HEPES, 30 mm glucose, pH 7.3), and the pipette solution contained 135 mm KCl, 6 mm NaCl, 0.35 mm EGTA, 4 mm Mg-ATP, 20 mm HEPES, pH 7.25. Spiking was measured in the current clamp mode to keep the membrane voltage at approximately −65 mV.
Expression and Purification of PsChR in Pichia pastoris
The 7TM domain of PsChR with a TEV protease site at the N terminus, and a nine-His tag at the C terminus was subcloned into the pPIC9K vector (Invitrogen) via its EcoRI and AvrII sites. P. pastoris strain SMD1168 (his4, pep4) (Invitrogen) was transformed by electroporation using the linearized resultant plasmid pPIC9K-PsChR-9His. A P. pastoris clone that grows on 4 mg/ml Geneticin was selected according to the manufacturer's instructions. Protein expression and purification was carried out as described earlier for Chlamydomonas augustae channelrhodopsin 1 (33). Cells were grown in buffered glycerol-complex medium to A600 2–6, transferred to buffered minimal methanol yeast medium supplemented with all-trans-retinal and induced with 0.5% methanol. After 24–30 h, the cells were harvested by low speed centrifugation and disrupted in a bead beater (BioSpec Products, Bartlesville, OK). The membranes were collected by ultracentrifugation and solubilized in 1.5% (w/v) dodecyl maltoside for 1 h at 4 °C. Nonsolubilized material was removed by ultracentrifugation, and protein was purified from the supernatant using a nickel-nitrilotriacetic acid column (Qiagen). The protein samples were concentrated in 100 mm NaCl, 0.02% dodecyl maltoside, 20 mm HEPES (pH 7.4) and used for measurements either directly or after reconstitution in nanodiscs with 1,2-dimyristoyl-sn-glycero-3-phosphocholine lipid from Avanti Polar Lipids (Alabaster, AL), as described for haloarchaeal sensory rhodopsin II in (34).
Absorption Spectroscopy and Flash Photolysis
Absorption spectra of partially purified PsChR in the UV-visible range were recorded on a Cary 4000 spectrophotometer (Varian, Palo Alto, CA). pH titration was carried out by the addition of small volumes of 1 m NaOH, 0.5 m Tris (pH 10), 0.5 m citric acid, or 1 m HCl. Light-induced absorption changes of Pichia-expressed pigment were measured with a laboratory-constructed cross-beam apparatus (35). Excitation flashes (532 nm, 6 ns, 40 mJ) were provided by a Surelite I Nd-YAG laser (Continuum, Santa Clara, CA). Measuring light was from a 250-W incandescent tungsten lamp combined with a McPherson monochromator (model 272, Acton, MA). A Hamamatsu Photonics (Bridgewater, NJ) photomultiplier tube (model R928) was protected from excitation laser flashes by a second monochromator of the same type and additionally with 12-nm bandwidth interference filters (Oriel Instruments, Stratford, CT). Signals were amplified by a low noise current amplifier (model SR445A; Stanford Research Systems, Sunnyvale, CA) and digitized by a Digidata 1320A (Molecular Devices) at the sampling rate 4 μs/point. The time interval between excitation flashes was 10 s, and 100 sweeps were averaged for each data point.
Retinal Extraction and Analysis
The protocol was adapted from Refs. 36 and 37. Protein samples were kept overnight in the dark and maintained in darkness or illuminated for 2 min using a tungsten halogen light beam from an FOI-150W Illuminator (Titan Tool Supply, Buffalo, NY) passed through a heat filter and a 432 ± 5-nm interference filter. Retinal was extracted by the addition of ice-cold methanol followed by ice-cold hexane and vortexing under dim red light. Phases were separated by centrifugation, and the top layer (hexane phase) was carefully withdrawn and dried under argon. The samples were dissolved in methanol and separated in 100% hexane on a Spherisorb S5 ODS2 analytical column using a Waters Delta 600 HPLC system (Waters Corporation, Milford, MA).
Data analysis was performed with pClamp 10.2 (Molecular Devices) and OriginPro 7 (OriginLab Corporation, Northampton, MA) software.
RESULTS
Photoreceptor Currents in the Algae
Unilateral light excitation of suspensions of P. subcordiformis cells elicited characteristic photoelectric responses (Fig. 1, inset) previously detected in many freshwater flagellates (6, 38–40). They are comprised of photoreceptor currents superimposed with the regenerative response triggered by depolarization of the plasma membrane. Gene silencing experiments in C. reinhardtii have demonstrated that photoreceptor currents are mediated by ChRs (4, 5, 41).
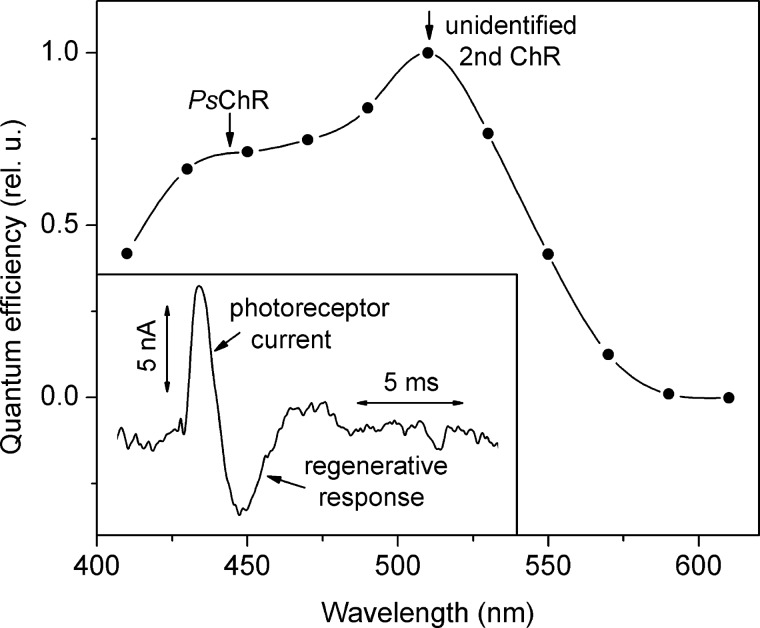
FIGURE 1.
Inset, a typical photoinduced electrical signal recorded from a cell suspension of the marine alga P. subcordiformis consisting of a photoreceptor current followed by a regenerative response. Main panel, the action spectrum of photoreceptor currents in P. subcordiformis, which shows a contribution of two photoreceptor pigments. rel. u., relative unit.
The action spectrum of photoreceptor currents in P. subcordiformis shows a main peak at 510 nm and a pronounced shoulder in the blue region (Fig. 1, main panel) that we attribute to the existence of two photoreceptor pigments, as demonstrated for the similar action spectrum in C. reinhardtii (4). Our data match closely the action spectrum of P. subcordiformis phototaxis determined by measuring accumulation of cells in an illuminated cuvette (42). Similar action spectra have also been measured in many other green flagellates, including the model organism C. reinhardtii, in which contribution of two ChRs to the phototaxis spectrum was directly proven by gene knockdown experiments (4). As we show below, the maximum of the spectral sensitivity of PsChR in HEK293 cells was at 445 nm, which strongly suggests it being one of the two receptors responsible for phototaxis in this organism. This hypothesis remains to be tested directly when methods for genetic modification of P. subcordiformis become available.
PsChR Primary Structure
The amino acid sequence of PsChR is comprised of 660 residues, and its N-terminal half forms the 7TM (rhodopsin) domain. Two more transmembrane helices are predicted in its C-terminal half, as in ChR1 from C. reinhardtii. Its N terminus (upstream of the 7TM domain) is relatively short and contains no predicted signal peptide, in contrast to CrChR1. Only Cys-73 (CrChR1 numbering) is conserved of three N-terminal Cys residues found to form disulfide bonds between protomers in the C1C2 dimer (43). P. subcordiformis is classified as a prasinophycean alga, but PsChR shows lower sequence homology with PgChR1 from another prasinophyte, Pyramimonas gelidicola (44), than with known ChRs from chlorophycean flagellates. It contains all five Glu residues and a Lys residue in helix B, conserved in ChRs from C. reinhardtii and Volvox carteri (supplemental Fig. S1). Glu and Asp residues are found, respectively, in the positions of the protonated Schiff base counterions Asp-85 and Asp-212 in bacteriorhodopsin (BR) and a His residue in the position of the proton donor Asp-96 in BR. Glu-87 of CrChR1, responsible for its pH-dependent color tuning and fast photocurrent inactivation (45), is also conserved in PsChR. The position of Tyr-226/Asn-187 (in CrChR1/CrChR2, respectively), identified as one of the molecular determinants of differences in spectra, desensitization, and current kinetics in response to a step-up and step-down of continuous light between CrChR1 and CrChR2 (46), in PsChR is occupied by a Ser residue.
Kinetics and Inactivation of PsChR Currents
The 7TM domain of PsChR expressed in HEK293 cells demonstrated light-gated channel activity typical of other high efficiency ChRs (Fig. 2). The mean peak current generated by PsChR at saturating light intensity was 4.6 ± 0.4 nA (n = 27 cells), compared with 2.5 ± 0.2 nA (n = 20 cells) for CrChR2, the channelrhodopsin most frequently used in optogenetic studies (Fig. 2C). The difference between the plateau currents for these two pigments was even greater: ∼3-fold (Fig. 2C). Under continuous illumination, the photocurrent decreased from a peak to a quasistationary level, which is also known for all other ChRs and is called light inactivation or desensitization. For PsChR the rate of this process was slower, and its extent was significantly less than that of CrChR2 (Fig. 2). In addition to the main phase of inactivation with the time constant (τ) ∼40 ms, which contributed 76% of the amplitude, a slow second phase with τ ∼7 s was observed (saturating light intensity, −60 mV, pH 7.4). Current inactivation after 1-s illumination, calculated as the difference between the peak and plateau current relative to the peak current, was ∼1.5-fold less for PsChR than for CrChR2 (Fig. 2D). After switching off the light, PsChR currents decayed biexponentially with τ of both phases ∼15% larger (τ1 = 9.8 ± 0.3 ms, τ2 = 102 ± 5.4 ms, n = 24 cells) than those for CrChR2 currents under the same conditions (τ1 = 8.3 ± 0.6 ms, τ2 = 84.9 ± 11.3 ms, n = 16 cells).
FIGURE 2.
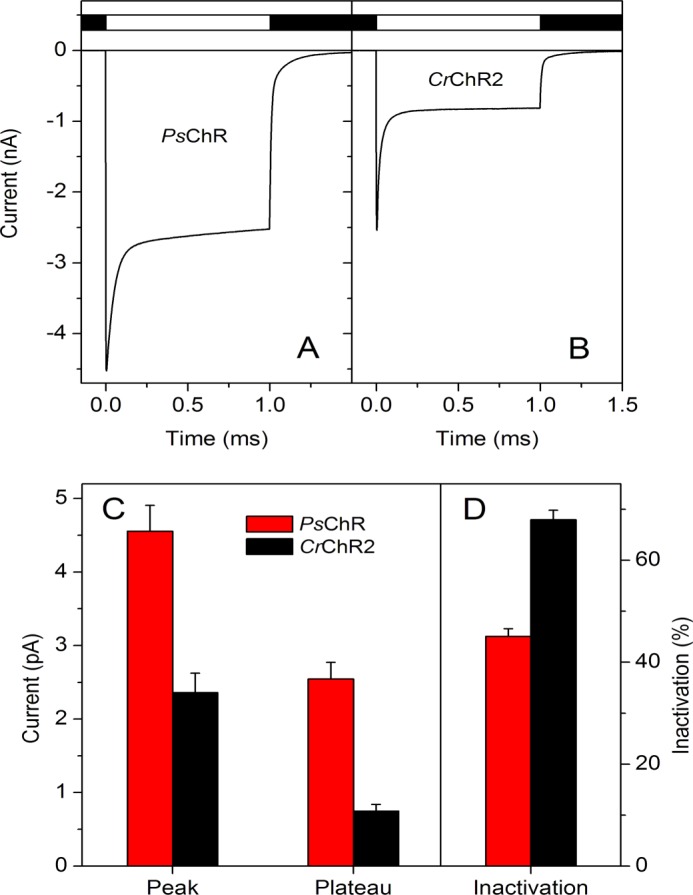
A and B, photocurrents generated by PsChR (A) and CrChR2 (B) expressed in HEK293 cells in response to a light pulse of saturating intensity (440 and 470 nm, respectively). The duration of the pulse is shown schematically on top. C and D, the mean amplitudes of peak and plateau currents (C) and the degree of current inactivation (D) for PsChR (n = 27 cells) and CrChR2 (n = 20 cells).
Current Amplitude and Unitary Conductance
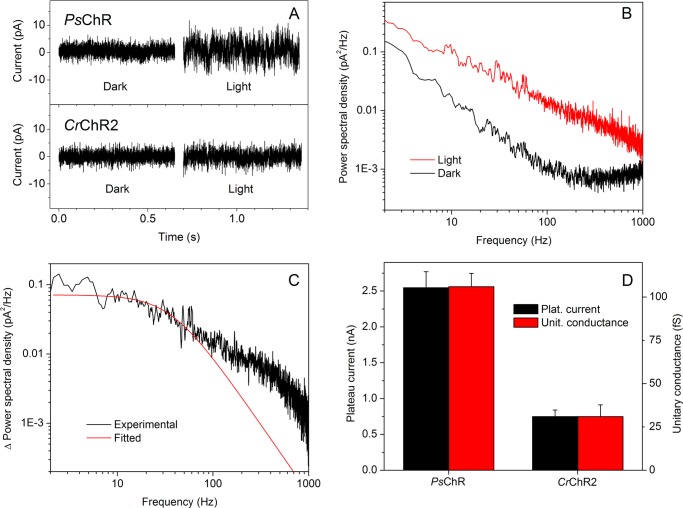
The amplitude of the whole cell current measured under continuous illumination depends on the number of photoactive molecules in the cell membrane, lifetime of the open channel, and its unitary conductance. Although single channel currents generated by CrChR2 are below the limit for direct recording, their parameters could be estimated by stationary noise analysis (29). We used this approach (for details see “Experimental Procedures”) to determine whether a greater plateau current of PsChR reflects an increased unitary conductance over that of CrChR2.
In PsChR-transfected cells, the noise amplitude became considerably larger under continuous illumination, as compared with the dark conditions (Fig. 3A, top traces). This increase in noise was much greater than observed in CrChR2-transfected cells under the same conditions (Fig. 3A, bottom traces). The noise of control untransfected cells did not change at all after switching on the light (data not shown). The power density spectra for both light and dark conditions contained a significant 1/f component, i.e., a component inversely proportional to the frequency (Fig. 3B). The origin of this noise component is unclear; it can arise simply from nonstationary processes such as base-line drift (30) or from the summation of a few Lorentzian components having appropriate amplitudes and corner frequencies (47). However, the light minus dark difference could be fit with a Lorentzian function between 2 and 1 kHz (Fig. 3C). The value of unitary conductance (γ) for PsChR obtained from this fit was ∼3-fold larger than for CrChR2. Our value for CrChR2 was close to that reported previously (29), taking into account the lower bath Na+ concentration in our experiments and the temperature dependence of γ. The close correlation between the whole cell plateau current amplitudes and the calculated unitary conductances (Fig. 3D) shows that the greater current amplitude of PsChR in HEK cells over that of CrChR2 is attributable to its greater unitary conductance. It should be noted that the absolute values of γ for both pigments determined by this method may be underestimated, because the plateau current generated by ChRs under prolonged illumination is largely determined by a third, inactivated state of the channel (8), not taken into account by the model used.
FIGURE 3.
A, whole cell current noise generated by PsChR (top) and CrChR2 (bottom) in the dark and under continuous illumination. B, noise power spectra calculated for the dark (black line) and light (red line) conditions of currents generated by PsChR. The spectra were smoothed by five-point adjacent averaging for presentation purposes. Other details are under “Experimental Procedures.” C, the difference spectrum (light minus dark) calculated from the spectra shown in B (black line) and its single Lorentzian fit (red line). D, plateau currents measured after 1-s illumination (left axis; same as in Fig. 2C) and unitary conductances (right axis) of PsChR and CrChR2. The data are the mean values calculated from 12 individual current traces for PsChR and 8 traces for CrChR2.
Current Peak Recovery
A general property of all ChRs tested so far is that a second light pulse delivered after a short dark interval elicits a response with a smaller transient peak than that invoked by the first pulse, although the plateau level is the same for both pulses (Fig. 4A). The time course of this process is multicomponential and depends on the membrane potential and extracellular pH (8). The recovery of the peak current generated by PsChR was faster than that of any ChR tested so far. In particular, 50% of the initial peak amplitude was recovered in a ∼30-fold shorter time than with CrChR2 measured under the same conditions (Fig. 4B).
FIGURE 4.
A, photocurrents generated by PsChR expressed in an HEK293 cell in response to a pair of 1-s light pulses (440 nm, saturating intensity) delivered with a 1-s dark interval. Current was normalized to the peak value of the first signal. The illumination protocol is shown schematically on top. B, the time course of the recovery of the transient component of the signal measured with PsChR (black squares) or CrChR2 (open circles). Recovery was calculated as the ratio of the differences between the peak and the plateau values measured in response to the second and the first pulse. Recovery percentage is plotted against the time interval between the pulses. The data are the mean values of measurements in three to five cells.
Ion Selectivity
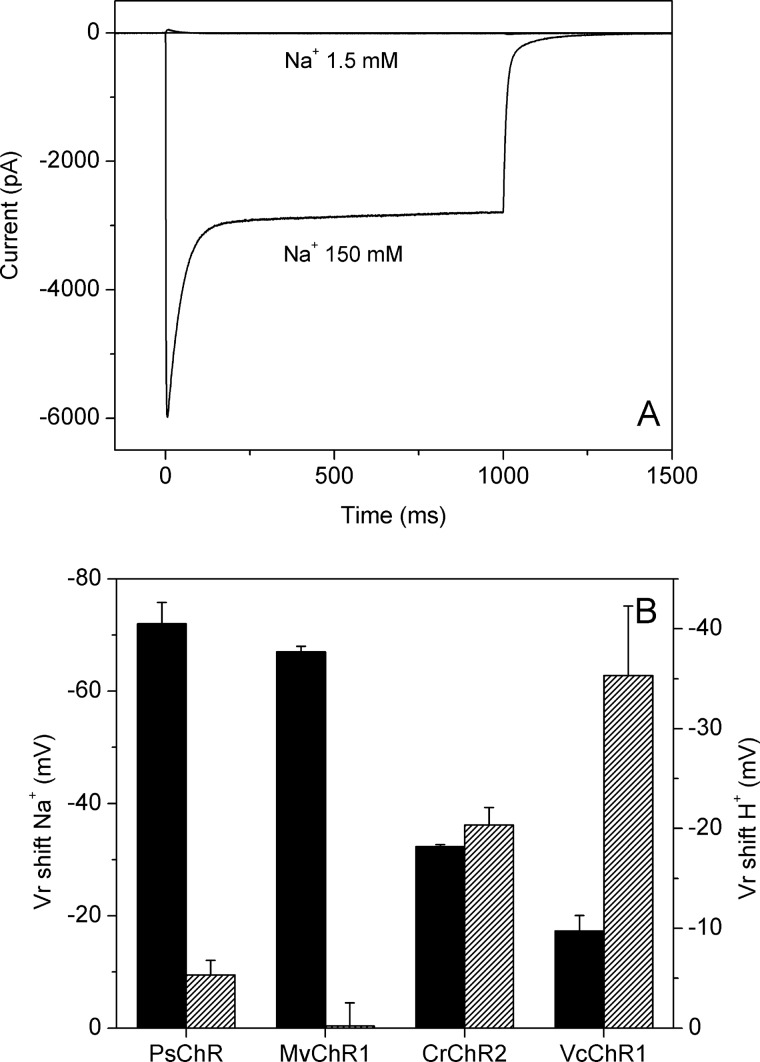
Inward photocurrents generated by PsChR under our standard conditions were almost entirely carried by Na+ ions, as revealed by their dramatic suppression after partial replacement of Na+ in the bath with nonpermeable organic N-methyl-d-glucamine (Fig. 5A). In comparison, only ∼80% of CrChR2 current was contributed by Na+, as estimated in a parallel experiment (data not shown; see Refs. 8 and 45). We measured the current-voltage relationships and calculated the shifts of the reversal voltage (Vr) upon a decrease of Na+ or H+ concentrations in the bath for PsChR and several other ChRs for comparison. The greatest negative shifts upon Na+ depletion were obtained with PsChR and MvChR1 from Mesostigma viride, which indicated their highest relative permeability to Na+ ions over protons of all tested ChRs (Fig. 5B). However, the Na+ conductance of MvChR1 was inhibited by protons; peak current amplitude decreased 24% when the bath pH was changed from 7.4 to 6.4, despite a ∼7-mV positive shift of the Vr, indicating that protons were also permeable through its channel. In contrast, no such inhibition was observed in PsChR: the current amplitude difference between pH 7.4 and 6.4 was <2%.
FIGURE 5.
A, photocurrents generated by PsChR in the same HEK293 cell at 150 and at 1.5 mm Na+ and 148.5 mm nonpermeable N-methyl-d-glucamine in the bath (1-s light stimulus, 440 nm). B, shifts of Vr measured for plateau currents after a decrease in the bath Na+ concentration from 150 to 1.5 mm (left axis, solid bars), or an increase in the bath pH from 7.4 to 9 (right axis, hatched bars). The data points are the mean values obtained from three cells.
Under our conditions, the Vr of CrChR2 current shifted to a less positive value during illumination (from 15.8 ± 1.0 mV for the initial current to 10.7 ± 1.2 mV for the plateau current; n = 6 cells). These observations confirmed the previously reported data interpreted as the presence of two open states with different ion selectivities in the photocycle of this pigment (48). In contrast, the corresponding Vr values calculated for PsChR current were approximately the same (13.1 ± 0.6 mV and 14.1 ± 0.6 mV, respectively; n = 7 cells). According to the above interpretation, PsChR appears to exhibit a single conductive state, in contrast to CrChR2.
Action Spectroscopy
The action spectra of PsChR-generated currents were measured using a 50-ms light pulse of low intensity, as described earlier for MvChR1 (40). Its maximum was at 445 nm (Fig. 6A, black line), which makes PsChR the shortest wavelength-absorbing ChR so far characterized.
FIGURE 6.
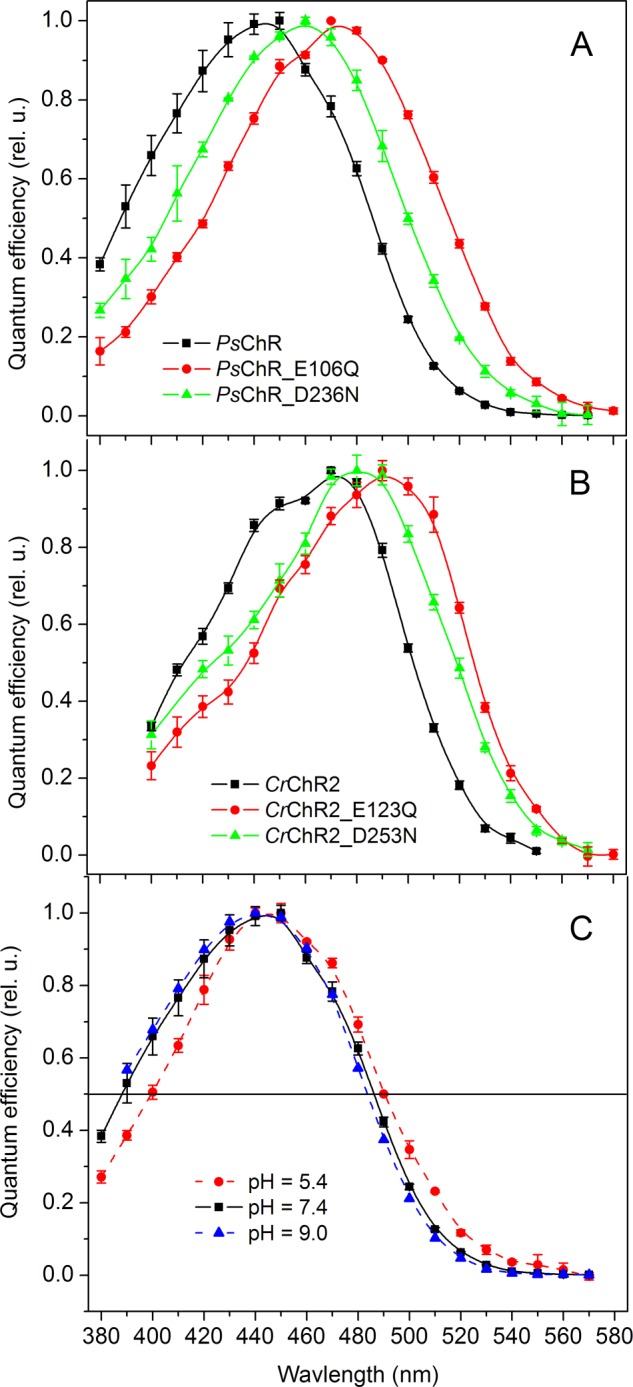
Action spectra of channel currents generated in HEK293 cells by wild-type PsChR and its mutants at the bath pH 7.4 (A), wild-type CrChR2 and its mutants at the bath pH 7.4 (B), and wild-type PsChR at the bath pH indicated in the legend (C). The data points are the mean values of three to five measurements. rel. u., relative unit.
Interaction of the protonated Schiff base and its counterion is one of the major mechanisms of spectral tuning in retinylidene proteins (49). Most ChRs (except DsChR1) contain two carboxylate residues homologous to Asp-85 and Asp-212 in BR (supplemental Fig. S1) that form hydrogen bonds with the Schiff base nitrogen, as revealed by the crystal structure of the C1C2 chimera (43). We generated the E106Q and D236N mutants to neutralize each of the corresponding carboxylate positions in PsChR and measured their spectral sensitivities. Both mutations caused a red shift of the spectrum (Fig. 6A), the magnitude of which was larger upon neutralization of the Asp-85 homolog (30 nm) than of the Asp-212 homolog (14 nm), as is seen in BR (50–52). Similar results (red shifts of 23 and 16 nm) were found in the corresponding CrChR2 mutants, E123Q and D253N (Fig. 6B), the first of which was previously tested in oocytes (53). This is in contrast to low efficiency C. augustae channelrhodopsin 1, in which the corresponding mutations caused a blue spectral shift,3 as did threonine substitution of the Asp-85 homolog in the C1V1 chimera (17). The opposite directions of the spectral shifts suggest different counterion arrangements in these ChRs.
Acidification of the medium also caused a red shift of the PsChR action spectrum, but its magnitude was relatively smaller compared with those observed in the counterion mutants: less than 3 nm when pH was shifted from 9.0 to 7.4 and 4 nm when pH was shifted from 7.4 to 5.4 (Fig. 6C). This observation may indicate low pKa values of the titrated carboxylates. However, taking into account that channel activity of the E106Q and D236N mutants was greatly reduced (∼44- and ∼100-fold, respectively, with respect to that of wild type), an alternative explanation could be smaller contributions of the protonated red-shifted forms of the pigment to the net photocurrents compared with that of the unprotonated forms.
Characterization of Purified PsChR
PsChR was expressed in P. pastoris and partially purified in detergent with yields of 0.2–0.3 mg/liter of yeast culture. The absorption spectrum of purified PsChR in dodecyl maltoside showed an absorption maximum at 437- and ∼90-nm half-bandwidth (Fig. 7A, solid line). The absorption spectrum shifted less than 1–2 nm to longer wavelengths when pigment molecules were incorporated into lipid nanodiscs (Fig. 7A, dashed line). Both spectra lacked obvious vibrational fine structure, characteristic of the other relatively blue-shifted microbial rhodopsins CrChR2 (54, 55) and haloarchaeal sensory rhodopsin II (56).
FIGURE 7.
A, the absorption spectrum of purified PsChR solubilized in detergent or reconstituted in nanodiscs. B, pH titration of the absorption maximum of detergent-purified PsChR. Experimental data were fit with a sum of three Henderson-Hasselbalch components (f(pH) = A × (1 − 10(pH−pKa))−1 + C). The pK values and amplitudes of the transitions denoted by A are derived from curve fitting. C, inset, the difference spectrum between dark- and light-adapted purified PsChR. Main panel, the time course of the spectral transition during dark adaptation determined as the dependence of the amplitude of the difference spectrum (as shown by the arrow in the inset) on time fit with an exponential function. rel. u., relative unit.
The absorption spectrum of purified PsChR also shifted to longer wavelengths upon acidification of the medium, as did the action spectrum of channel currents. Interestingly, the maximum at 445 nm (the peak of the action spectrum) was observed at the nonphysiological pH ∼4.3, which suggests different protein states in detergent and biological membranes (Fig. 7B). Titration of the peak revealed at least three titratable groups. The shape of the differential signal for the most alkaline transition (data not shown) indicated that it reflects deprotonation of the Schiff base; hence fitting of the titration data was performed with the base level parameter fixed at 360 nm. The pKa of this transition was ∼10.5, and those of the other two transitions were 3.8 and 6.6. It is probable that the most acidic transition reflects protonation of Glu-106, because neutralization of this residue caused a larger red shift of the action spectra of the two mutants tested (Fig. 6A, red line). The transition with pKa ∼6.6 and smaller amplitude may correspond to protonation of Asp-236, a neutral mutation of which caused a smaller red spectral shift (Fig. 6A, green line). This hypothesis needs to be tested with purified mutant proteins, which are, however, not yet available.
Retinal extraction experiments showed that the ratio of all-trans- to 13-cis-isomer in dark-adapted PsChR was ∼75:25, similar to that in CrChR2 (37). No significant changes in the isomer composition were observed upon light adaptation. In BR and Anabaena sensory rhodopsin, changes in the isomer composition (36, 57) upon dark adaptation give rise to characteristic difference spectra (36, 58). The direction of the observed changes are opposite in BR and Anabaena sensory rhodopsin. We carried out spectroscopic measurements to resolve possible small amplitude changes in PsChR that fall below the detection limit of retinal extraction and to follow their time course. Dark adaptation of PsChR led to a blue spectral shift with a typical light-dark adaptation shape of the difference spectrum (Fig. 7C, inset). As in BR, the amount of 13-cis-retinal increased in the dark. However, its magnitude in PsChR was dramatically smaller as judged from the difference spectrum (only 3% of the total absorbance). The time constant of dark adaptation in PsChR was ∼26 min (Fig. 7C, main panel).
Fig. 8A shows the spectra of laser flash-induced absorbance changes in detergent-purified PsChR. Although no positive signal was observed in the short wavelength region at room temperature, an increase in the absorption at 510 nm at the expense of a decrease at 380 nm at the early stages of the photocycle suggests contribution of an early M-like state, the absorption of which strongly overlaps with the absorption of the unphotolysed state. Therefore, kinetics of this intermediate was followed at 5 °C to improve the time resolution (Fig. 8B). Even at this low temperature, the rates of the M intermediate formation and decay were faster than those reported in CrChR2 (59). Consequently, maximal accumulation of the M intermediate of PsChR was observed at 50 μs, as compared with 200 μs in CrChR2. The main photoproduct (N/O-like) in the PsChR photocycle is red-shifted, similar to observations in CrChR2 (54), and biphasic in both its rise and decay. The rise and the fast component of the decay roughly correlated with the opening and closing of the channel measured in HEK cells (Fig. 8C).
FIGURE 8.

A, the spectra of absorbance changes induced by laser excitation (532 nm, 6 ns) in detergent-purified PsChR. B, the kinetics of the M-like intermediate in detergent-purified PsChR. The smooth line shows a computer fit to the data. C, superposition of the kinetics of the red-shifted intermediate in the photocycle of detergent-purified PsChR and the kinetics of the laser flash-induced photocurrent generated by PsChR in a HEK293 cell adapted from Ref. 19.
Function of PsChR in Neurons
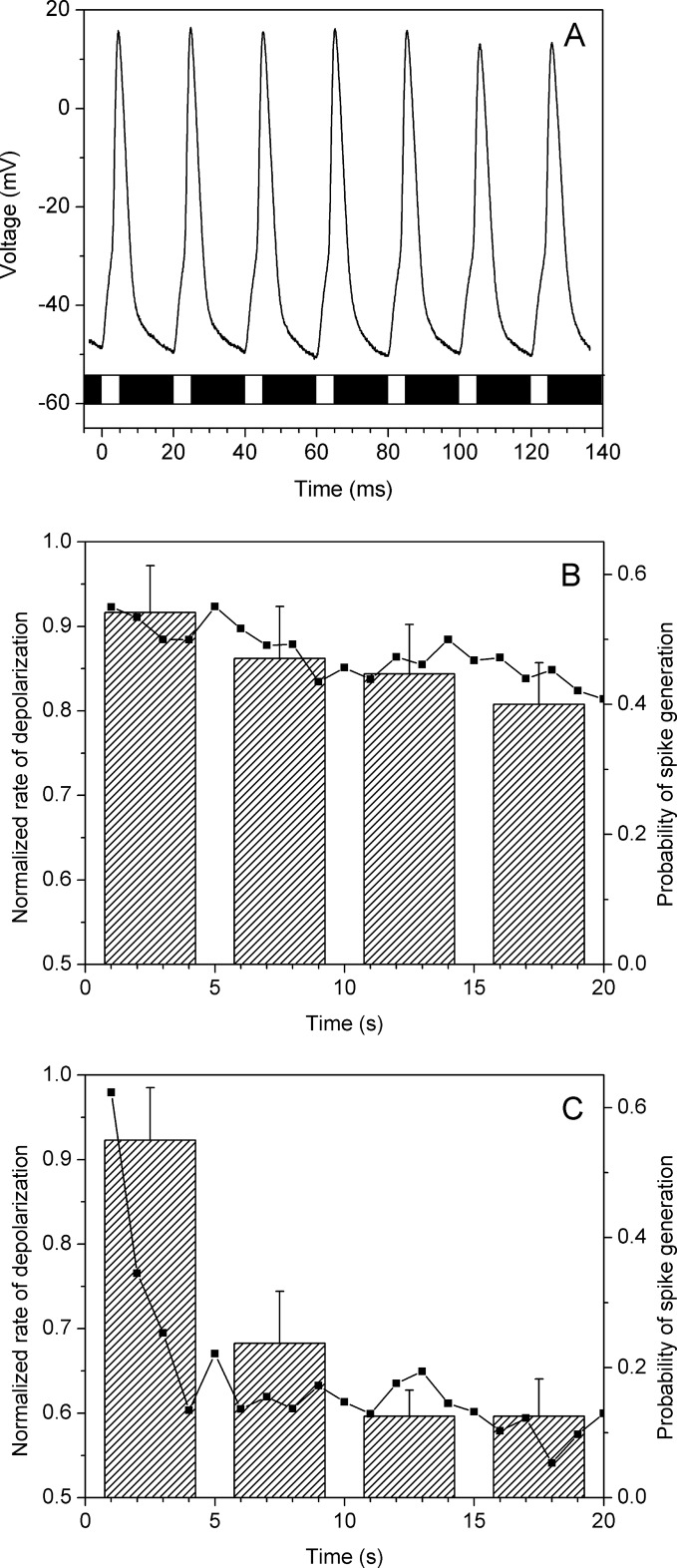
To test whether PsChR is relevant for optogenetic applications, we examined its performance in cultured hippocampal neurons. Neurons expressing PsChR were capable of firing action potentials upon light stimulation with brief light pulses at frequencies up to 50 Hz (Fig. 9A), which is the upper limit for pyramidal neurons (20). Expression of both PsChR and CrChR2, as judged by the tag fluorescence, was increased when the cultures were supplemented with all-trans-retinal (final concentration, 0.4 μm) in addition to 0.5 μm retinyl acetate present in the culture medium. Photoinduced channel currents generated in neurons by PsChR were increased ∼9-fold, and those generated by CrChR2 were increased ∼5-fold in cultures with additional retinal, which was similar to results obtained in HEK293 cells (∼12- and ∼2.4-fold increase for PsChR and CrChR2, respectively).
FIGURE 9.
A, typical voltage traces showing membrane depolarization and spikes recorded from a current-clamped PsChR-expressing pyramidal neuron in response to stimulation with brief light pulses (440 nm, 5 ms, 50 Hz). The times of light pulses are indicated by the bar at the bottom of the graph. B and C, the normalized rate of the light-induced membrane depolarization (squares, left axis) and the probability of spike generation (bars, right axis) in cultured hippocampal neurons that express PsChR (B) or CrChR2 (C). Trains of 20 light pulses (5-ms duration) were applied at a frequency of 1 Hz. The excitation wavelength was 440 nm for PsChR and 470 nm for CrChR2, and the light intensity was close to the threshold for spike generation (i.e., spikes were evoked by less than 100% of pulses). The spiking probability was calculated for each successive group of five pulses. The data are mean values recorded from 16 experiments.
Upon 1-Hz excitation, the rate of the membrane depolarization decreased significantly more slowly and to a lesser degree for PsChR than for CrChR2 (Fig. 9, B and C, left axes). Correspondingly, the probability of spike generation driven by this depolarization only slightly decreased during a 20-s period of excitation in PsChR-expressing neurons, whereas in CrChR2-expressing neurons it rapidly dropped to a much lower level (Fig. 9, B and C, bars, right axes).
DISCUSSION
Action spectroscopy and analysis of photoreceptor currents predict the existence of two spectrally distinct ChRs in all green algae tested so far (for review see Ref. 1), with a possible exception of M. viride (40). In C. reinhardtii, gene silencing experiments have directly confirmed the role of its ChRs as light receptors for phototaxis and the photophobic response (4, 5, 41). To date, two channelopsin genes per genome have been identified in C. reinhardtii (4, 7, 8, 60), V. carteri (61), and Pleudorina starrii (44). ChRs from C. reinhardtii and V. carteri have been heterologously overexpressed and purified, and their absorption spectra confirmed the results of action spectroscopy (41, 54, 61). In both organisms, the longer wavelength (green) absorbing ChR of the pair was designated as ChR1, and the shorter wavelength (blue) absorbing as ChR2.
Alignment of the 14 currently reported ChR sequences (supplemental Fig. S1) do not divide them in two distinct groups. Therefore, when only one ChR species of a presumed pair is known in a particular organism, it is difficult to assign it to ChR group 1 or 2 from the sequence information alone. (These numbers are not to be confused with type 1 and type 2 used to distinguish microbial rhodopsins from animal visual proteins (62).) However, matching the spectral properties of PsChR with the action spectrum of photoreceptor currents in P. subcordiformis clearly indicates that it is the blue-shifted member of the pair and consequently can be classified as PsChR2, despite it being the only ChR identified so far in this alga. PsChR absorption is the most blue-shifted of all known ChRs. Generally, for optogenetic tools long-wavelength absorption is preferable to minimize scattering of light by biological tissues and its absorption by hemoglobin. However, the blue-shifted spectrum of PsChR is ideal for combinatorial applications with long wavelength-absorbing rhodopsin pumps or fluorescent indicators.
All known ChRs are predominantly proton channels, so their use for optogenetics may lead to undesirable acidification of the cytoplasm. Indeed, a pH decrease of 0.3 units was detected in photoactivated HEK293 cells that express CrChR2 (15). The higher selectivity of PsChR for Na+ ions over protons may circumvent this side effect. Both PsChR and MvChR1 show high Na+ selectivity, but its mechanisms appear to be fundamentally different in these proteins. In MvChR1, which uniquely contains an alanine residue in place of His-134 (CrChR2 numbering), Na+ conductance is inhibited by protons, as it is in the H134(R/S) mutants of CrChR2 (63). On the other hand, no such inhibition was found in PsChR, in which His-134 is conserved (supplemental Fig. S1).
Also conserved in PsChR are Glu-90, Ser-63, and Asn-258 (supplemental Fig. S1), implicated in control of ion selectivity in CrChR2 (63–65), and Ser-136, which regulates the size of the channel pore (66). Of three positively charged residues that form a vestibule on the extracellular side of the channel pore in C1C2 (43), Lys-154 is substituted with Val in PsChR. However, neutral residues are also found in this position in other ChRs, including highly proton-selective DsChR1, which is inconsistent with a possible role for this residue in determination of Na+ selectivity. The structural determinants of the high Na+ selectivity in PsChR remain currently unknown.
We showed that PsChR is expressed in cultured hippocampal neurons and can be used to drive their spiking activity by light excitation. Moreover, a faster peak recovery of PsChR-generated currents provides an advantage in certain optogenetic applications. Recently it has been demonstrated that the level of expression and, correspondingly, the amplitude of channel currents generated by CrChR2 in animal cells dramatically increase upon the addition of exogenous retinal (67). We found that this increase was even larger for PsChR than for CrChR2. Retinal content of some heterologous systems, such as Caenorhabditis elegans or Drosophila, is so low that even CrChR2 cannot be used there without the addition of exogenous retinal (68, 69). On the other hand, the intact mammalian brain may have enough endogenous retinal to reconstitute fully functional PsChR.
Our characterization of PsChR augments understanding of functional mechanisms of ChRs and reveals its potentially useful properties as an optogenetic tool. Taking into account the great number and diversity of phototactic algal species in which the existence of ChRs is predicted, comparative analysis of different native ChR variants may be as beneficial as bioengineering of select model molecules both for basic research into their structure-function relationships and for optimization of their biotechnology applications.
Acknowledgment
We thank Dr. Carlos Lois (Massachusetts Institute of Technology) for pFUGW, pCMV-VSVG, and pΔ8.9 plasmids.
This work was supported, in whole or in part, by National Institutes of Health Grants R01GM027750 and R21MH098288. This work was also supported by the Hermann Eye Fund and Endowed Chair AU-0009 from the Robert A. Welch Foundation.

This article contains supplemental Fig. S1.
E. G. Govorunova, O. A. Sineshchekov, H. Li, and J. L. Spudich, manuscript in preparation.
- ChR
- channelrhodopsin
- PsChR
- P. subcordiformis channelrhodopsin
- CrChR
- C. reinhardtii channelrhodopsin
- MvChR1
- M. viride channelrhodopsin
- TM
- transmembrane
- BR
- bacteriorhodopsin.
REFERENCES
- 1. Sineshchekov O. A., Spudich J. L. (2005) Sensory rhodopsin signaling in green flagellate algae, in Handbook of Photosensory Receptors, pp. 25–42, Wiley-VCH, Weinheim [Google Scholar]
- 2. Hegemann P. (2008) Algal sensory photoreceptors. Annu. Rev. Plant. Biol. 59, 167–189 [DOI] [PubMed] [Google Scholar]
- 3. Litvin F. F., Sineshchekov O. A., Sineshchekov V. A. (1978) Photoreceptor electric potential in the phototaxis of the alga Haematococcus pluvialis. Nature 271, 476–478 [DOI] [PubMed] [Google Scholar]
- 4. Sineshchekov O. A., Jung K.-H., Spudich J. L. (2002) Two rhodopsins mediate phototaxis to low- and high-intensity light in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. U.S.A. 99, 8689–8694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Govorunova E. G., Jung K.-W., Sineshchekov O. A., Spudich J. L. (2004) Chlamydomonas sensory rhodopsins A and B. Cellular content and role in photophobic responses. Biophys. J. 86, 2342–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sineshchekov O. A., Govorunova E. G., Der A., Keszthelyi L., Nultsch W. (1992) Photoelectric responses in phototactic flagellated algae measured in cell suspension. J. Photochem. Photobiol. B Biol. 13, 119–134 [Google Scholar]
- 7. Nagel G., Ollig D., Fuhrmann M., Kateriya S., Musti A. M., Bamberg E., Hegemann P. (2002) Channelrhodopsin-1. A light-gated proton channel in green algae. Science 296, 2395–2398 [DOI] [PubMed] [Google Scholar]
- 8. Nagel G., Szellas T., Huhn W., Kateriya S., Adeishvili N., Berthold P., Ollig D., Hegemann P., Bamberg E. (2003) Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. U.S.A. 100, 13940–13945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deisseroth K. (2011) Optogenetics. Nat. Methods 8, 26–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mei Y., Zhang F. (2012) Molecular tools and approaches for optogenetics. Biol. Psychiatry 71, 1033–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hegemann P., Nagel G. (2013) From channelrhodopsins to optogenetics. EMBO Mol. Med. 5, 171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yawo H., Asano T., Sakai S., Ishizuka T. (2013) Optogenetic manipulation of neural and non-neural functions. Dev. Growth Differ. 55, 474–490 [DOI] [PubMed] [Google Scholar]
- 13. Hegemann P., Möglich A. (2011) Channelrhodopsin engineering and exploration of new optogenetic tools. Nat. Methods 8, 39–42 [DOI] [PubMed] [Google Scholar]
- 14. Mattis J., Tye K. M., Ferenczi E. A., Ramakrishnan C., O'Shea D. J., Prakash R., Gunaydin L. A., Hyun M., Fenno L. E., Gradinaru V., Yizhar O., Deisseroth K. (2012) Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat. Methods 9, 159–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin J. Y., Lin M. Z., Steinbach P., Tsien R. Y. (2009) Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys. J. 96, 1803–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berndt A., Schoenenberger P., Mattis J., Tye K. M., Deisseroth K., Hegemann P., Oertner T. G. (2011) High-efficiency channelrhodopsins for fast neuronal stimulation at low light levels. Proc. Natl. Acad. Sci. U.S.A. 108, 7595–7600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yizhar O., Fenno L. E., Prigge M., Schneider F., Davidson T. J., O'Shea D. J., Sohal V. S., Goshen I., Finkelstein J., Paz J. T., Stehfest K., Fudim R., Ramakrishnan C., Huguenard J. R., Hegemann P., Deisseroth K. (2011) Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477, 171–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prigge M., Schneider F., Tsunoda S. P., Shilyansky C., Wietek J., Deisseroth K., Hegemann P. (2012) Color-tuned channelrhodopsins for multiwavelength optogenetics. J. Biol. Chem. 287, 31804–31812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sineshchekov O. A., Govorunova E. G., Wang J., Li H., Spudich J. L. (2013) Intramolecular proton transfer in channelrhodopsins. Biophys. J. 104, 807–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang F., Prigge M., Beyrière F., Tsunoda S. P., Mattis J., Yizhar O., Hegemann P., Deisseroth K. (2008) Red-shifted optogenetic excitation. A tool for fast neural control derived from Volvox carteri. Nat. Neurosci. 11, 631–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Han X., Qian X., Stern P., Chuong A. S., Boyden E. S. (2009) Informational lesions. Optical perturbation of spike timing and neural synchrony via microbial opsin gene fusions. Front. Mol. Neurosci. 2, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tang W., Ehrlich I., Wolff S. B., Michalski A. M., Wölfl S., Hasan M. T., Lüthi A., Sprengel R. (2009) Faithful expression of multiple proteins via 2A-peptide self-processing. A versatile and reliable method for manipulating brain circuits. J. Neurosci. 29, 8621–8629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kleinlogel S., Terpitz U., Legrum B., Gökbuget D., Boyden E. S., Bamann C., Wood P. G., Bamberg E. (2011) A gene-fusion strategy for stoichiometric and co-localized expression of light-gated membrane proteins. Nat. Methods 8, 1083–1088 [DOI] [PubMed] [Google Scholar]
- 24. Tsuda S., Kee M. Z., Cunha C., Kim J., Yan P., Loew L. M., Augustine G. J. (2013) Probing the function of neuronal populations. Combining micromirror-based optogenetic photostimulation with voltage-sensitive dye imaging. Neurosci. Res. 75, 76–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li Y., Tsien R. W. (2012) pHTomato, a red, genetically encoded indicator that enables multiplex interrogation of synaptic activity. Nat. Neurosci. 15, 1047–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Collot M., Loukou C., Yakovlev A. V., Wilms C. D., Li D., Evrard A., Zamaleeva A., Bourdieu L., Léger J. F., Ropert N., Eilers J., Oheim M., Feltz A., Mallet J. M. (2012) Calcium rubies. A family of red-emitting functionalizable indicators for two-photon Ca2+ imaging. J. Am. Chem. Soc. 134, 14923–14931 [DOI] [PubMed] [Google Scholar]
- 27. Wu J., Liu L., Matsuda T., Zhao Y., Rebane A., Drobizhev M., Chang Y. F., Araki S., Arai Y., March K., Hughes T. E., Sagou K., Miyata T., Nagai T., Li W. H., Campbell R. E. (2013) Improved orange and red Ca2+ indicators and photophysical considerations for optogenetic applications. ACS Chem. Neurosci. 4, 963–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McLachlan J. (1960) The culture of Dunaliella tertiolecta Butcher. A euryhaline organism. Can. J. Microbiol. 6, 367–379 [Google Scholar]
- 29. Feldbauer K., Zimmermann D., Pintschovius V., Spitz J., Bamann C., Bamberg E. (2009) Channelrhodopsin-2 is a leaky proton pump. Proc. Natl. Acad. Sci. U.S.A. 106, 12317–12322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cherny V. V., Murphy R., Sokolov V., Levis R. A., DeCoursey T. E. (2003) Properties of single voltage-gated proton channels in human eosinophils estimated by noise analysis and by direct measurement. J. Gen. Physiol. 121, 615–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gray P. (1994) Analysis of whole cell currents to estimate the kinetics and amplitude of underlying unitary events. Relaxation and “noise” analysis, in Microelectrode Techniques: The Plymouth Workshop Handbook (Ogden D. C., ed) pp. 189–207, Company of Biologists, Cambridge, UK [Google Scholar]
- 32. Lois C., Hong E. J., Pease S., Brown E. J., Baltimore D. (2002) Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 295, 868–872 [DOI] [PubMed] [Google Scholar]
- 33. Hou S. Y., Govorunova E. G., Ntefidou M., Lane C. E., Spudich E. N., Sineshchekov O. A., Spudich J. L. (2012) Diversity of Chlamydomonas channelrhodopsins. Photochem. Photobiol. 88, 119–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang J., Sasaki J., Tsai A. L., Spudich J. L. (2012) HAMP domain signal relay mechanism in a sensory rhodopsin-transducer complex. J. Biol. Chem. 287, 21316–21325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang W.-W., Sineshchekov O. A., Spudich E. N., Spudich J. L. (2003) Spectroscopic and photochemical characterization of a deep ocean proteorhodopsin. J. Biol. Chem. 278, 33985–33991 [DOI] [PubMed] [Google Scholar]
- 36. Sineshchekov O. A., Trivedi V. D., Sasaki J., Spudich J. L. (2005) Photochromicity of Anabaena sensory rhodopsin, an atypical microbial receptor with a cis-retinal light-adapted form. J. Biol. Chem. 280, 14663–14668 [DOI] [PubMed] [Google Scholar]
- 37. Nack M., Radu I., Bamann C., Bamberg E., Heberle J. (2009) The retinal structure of channelrhodopsin-2 assessed by resonance Raman spectroscopy. FEBS Lett. 583, 3676–3680 [DOI] [PubMed] [Google Scholar]
- 38. Sineshchekov O. A., Govorunova E. G., Jung K.-H., Zauner S., Maier U.-G., Spudich J. L. (2005) Rhodopsin-mediated photoreception in cryptophyte flagellates. Biophys. J. 89, 4310–4319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kreimer G. (1994) Cell biology of phototaxis in flagellate algae. Int. Rev. Cytol. 148, 229–310 [Google Scholar]
- 40. Govorunova E. G., Spudich E. N., Lane C. E., Sineshchekov O. A., Spudich J. L. (2011) New channelrhodopsin with a red-shifted spectrum and rapid kinetics from Mesostigma viride. mBio 2, e00115–00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Berthold P., Tsunoda S. P., Ernst O. P., Mages W., Gradmann D., Hegemann P. (2008) Channelrhodopsin-1 initiates phototaxis and photophobic responses in Chlamydomonas by immediate light-induced depolarization. Plant Cell 20, 1665–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Halldal P. (1958) Action spectra of phototaxis and related problems in Volvocales, Ulva gametes and Dinophyceae. Physiol. Plantarum 11, 118–153 [Google Scholar]
- 43. Kato H. E., Zhang F., Yizhar O., Ramakrishnan C., Nishizawa T., Hirata K., Ito J., Aita Y., Tsukazaki T., Hayashi S., Hegemann P., Maturana A. D., Ishitani R., Deisseroth K., Nureki O. (2012) Crystal structure of the channelrhodopsin light-gated cation channel. Nature 482, 369–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang F., Vierock J., Yizhar O., Fenno L. E., Tsunoda S., Kianianmomeni A., Prigge M., Berndt A., Cushman J., Polle J., Magnuson J., Hegemann P., Deisseroth K. (2011) The microbial opsin family of optogenetic tools. Cell 147, 1446–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tsunoda S. P., Hegemann P. (2009) Glu 87 of channelrhodopsin-1 causes pH-dependent color tuning and fast photocurrent inactivation. Photochem. Photobiol. 85, 564–569 [DOI] [PubMed] [Google Scholar]
- 46. Wang H., Sugiyama Y., Hikima T., Sugano E., Tomita H., Takahashi T., Ishizuka T., Yawo H. (2009) Molecular determinants differentiating photocurrent properties of two channelrhodopsins from Chlamydomonas. J. Biol. Chem. 284, 5685–5696 [DOI] [PubMed] [Google Scholar]
- 47. Sauvé R., Szabo G. (1985) Interpretation of 1/f fluctuations in ion conducting membranes. J. Theor. Biol. 113, 501–516 [DOI] [PubMed] [Google Scholar]
- 48. Berndt A., Prigge M., Gradmann D., Hegemann P. (2010) Two open states with progressive proton selectivities in the branched channelrhodopsin-2 photocycle. Biophys. J. 98, 753–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Honig B., Greenberg A. D., Dinur U., Ebrey T. G. (1976) Visual-pigment spectra. Implications of the protonation of the retinal Schiff base. Biochemistry 15, 4593–4599 [DOI] [PubMed] [Google Scholar]
- 50. Subramaniam S., Marti T., Khorana H. G. (1990) Protonation state of Asp (Glu)-85 regulates the purple-to-blue transition in bacteriorhodopsin mutants Arg-82 → Ala and Asp-85 → Glu. The blue form is inactive in proton translocation. Proc. Natl. Acad. Sci. U.S.A. 87, 1013–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Marti T., Rösselet S. J., Otto H., Heyn M. P., Khorana H. G. (1991) The retinylidene Schiff base counterion in bacteriorhodopsin. J. Biol. Chem. 266, 18674–18683 [PubMed] [Google Scholar]
- 52. Gergely C., Ganea C., Száraz S., Váró G. (1995) Charge motions studied in the bacteriorhodopsin mutants D85N and D212N. J. Photochem. Photobiol. B Biol. 27, 27–32 [Google Scholar]
- 53. Gunaydin L. A., Yizhar O., Berndt A., Sohal V. S., Deisseroth K., Hegemann P. (2010) Ultrafast optogenetic control. Nat. Neurosci. 13, 387–392 [DOI] [PubMed] [Google Scholar]
- 54. Bamann C., Kirsch T., Nagel G., Bamberg E. (2008) Spectral characteristics of the photocycle of channelrhodopsin-2 and its implication for channel function. J. Mol. Biol. 375, 686–694 [DOI] [PubMed] [Google Scholar]
- 55. Ritter E., Stehfest K., Berndt A., Hegemann P., Bartl F. J. (2008) Monitoring light-induced structural changes of Channelrhodopsin-2 by UV-visible and Fourier transform infrared spectroscopy. J. Biol. Chem. 283, 35033–35041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Takahashi T., Yan B., Mazur P., Derguini F., Nakanishi K., Spudich J. L. (1990) Color regulation in the archaebacterial phototaxis receptor phoborhodopsin (sensory rhodopsin II). Biochemistry 29, 8467–8474 [DOI] [PubMed] [Google Scholar]
- 57. Pettei M. J., Yudd A. P., Nakanishi K., Henselman R., Stoeckenius W. (1977) Identification of retinal isomers isolated from bacteriorhodopsin. Biochemistry 16, 1955–1959 [DOI] [PubMed] [Google Scholar]
- 58. Ohno K., Takeuchi Y., Yoshida M. (1977) Effect of light-adaptation on the photoreaction of bacteriorhodopsin from Halobacterium halobium. Biochim. Biophys. Acta 462, 575–582 [DOI] [PubMed] [Google Scholar]
- 59. Verhoefen M. K., Bamann C., Blöcher R., Förster U., Bamberg E., Wachtveitl J. (2010) The photocycle of channelrhodopsin-2. Ultrafast reaction dynamics and subsequent reaction steps. Chemphyschem 11, 3113–3122 [DOI] [PubMed] [Google Scholar]
- 60. Suzuki T., Yamasaki K., Fujita S., Oda K., Iseki M., Yoshida K., Watanabe M., Daiyasu H., Toh H., Asamizu E., Tabata S., Miura K., Fukuzawa H., Nakamura S., Takahashi T. (2003) Archaeal-type rhodopsins in Chlamydomonas. Model structure and intracellular localization. Biochem. Biophys. Res. Commun. 301, 711–717 [DOI] [PubMed] [Google Scholar]
- 61. Kianianmomeni A., Stehfest K., Nematollahi G., Hegemann P., Hallmann A. (2009) Channelrhodopsins of Volvox carteri are photochromic proteins that are specifically expressed in somatic cells under control of light, temperature, and the sex inducer. Plant Physiol. 151, 347–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Spudich J. L., Yang C.-S., Jung K.-H., Spudich E. N. (2000) Retinylidene proteins. Structures and functions from archaea to humans. Annu. Rev. Cell Dev. Biol. 16, 365–392 [DOI] [PubMed] [Google Scholar]
- 63. Gradmann D., Berndt A., Schneider F., Hegemann P. (2011) Rectification of the channelrhodopsin early conductance. Biophys. J. 101, 1057–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ruffert K., Himmel B., Lall D., Bamann C., Bamberg E., Betz H., Eulenburg V. (2011) Glutamate residue 90 in the predicted transmembrane domain 2 is crucial for cation flux through channelrhodopsin 2. Biochem. Biophys. Res. Commun. 410, 737–743 [DOI] [PubMed] [Google Scholar]
- 65. Plazzo A. P., De Franceschi N., Da Broi F., Zonta F., Sanasi M. F., Filippini F., Mongillo M. (2012) Bioinformatic and mutational analysis of channelrhodopsin-2 cation conducting pathway. J. Biol. Chem. 287, 4818–4825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Richards R., Dempski R. E. (2012) Re-introduction of transmembrane serine residues reduce the minimum pore diameter of channelrhodopsin-2. PLoS One 7, e50018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ullrich S., Gueta R., Nagel G. (2013) Degradation of channelopsin-2 in the absence of retinal and degradation resistance in certain mutants. Biol. Chem. 394, 271–280 [DOI] [PubMed] [Google Scholar]
- 68. Nagel G., Brauner M., Liewald J. F., Adeishvili N., Bamberg E., Gottschalk A. (2005) Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr. Biol. 15, 2279–2284 [DOI] [PubMed] [Google Scholar]
- 69. Schroll C., Riemensperger T., Bucher D., Ehmer J., Völler T., Erbguth K., Gerber B., Hendel T., Nagel G., Buchner E., Fiala A. (2006) Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr. Biol. 16, 1741–1747 [DOI] [PubMed] [Google Scholar]
- 70. Brewer G. J., Boehler M. D., Jones T. T., Wheeler B. C. (2008) NbActiv4 medium improvement to Neurobasal/B27 increases neuron synapse densities and network spike rates on multielectrode arrays. J. Neurosci. Methods 170, 181–187 [DOI] [PMC free article] [PubMed] [Google Scholar]