Abstract
In traditional medicine, several medicinal plants or their extracts have been used to treat diabetes. Zingiber officinale Roscoe, known commonly as ginger, is consumed worldwide in cookeries as a spice and flavouring agent. It has been used as the spice and medicine for thousands of years. The present study was undertaken to investigate the potential protective effect of Zingiber officinale Rosc. in a model of oxidative damage to pancreatic β cells. The free radical scavenging activities and composition of the isolated n-hexane and ethanolic extracts were confronted with their protective, antioxidant and cytotoxic effects in INS-1E β cells. Unlike the n-hexane extract (exerting, paradoxically, stronger antiradical capacity), both low cytotoxicity and remarkable protective effects on β cell viability, followed by lowering oxidative stress markers were found for the ethanolic extract Zingiber officinale Rosc. The present study is the first pilot study to assess the protective potential of Zingiber officinale Rosc. in a model of cytotoxic conditions imposed by diabetes in β cells.
Keywords: Zingiber officinale Roscoe, oxidative stress, diabetes, pancreatic β cells
Introduction
Oxidative stress plays a critical role in diabetes type 1 and 2. With regard to their reduced antioxidant reserves, pancreatic insulin producing β cells represent one of its primary targets.
Zingiber officinale Roscoe (family, Zingiberaceae), known universally as ginger, is commonly used as a spice and food as well as medicinal agent in Indian, Asian and Arabic traditional medicine in the form of a fresh paste, dried powder, candy (crystallized ginger) or slices preserved in syrup (Ali et al., 2008; Ojewole, 2006). The ginger rhizomes have been used in herbal medicinal practice for the treatment of a range of diseases such as rheumatoid arthritis, hypercholesterolemia, neurological diseases, asthma, stroke, constipation, diabetes or cancer (Lantz et al., 2007).
The research confirmed multiple health benefit of Z. officinale rhizome extracts including analgetic and anti-inflammatory effects (Ojewole, 2006; Lantz et al., 2007). Moreover, previous reports point to the therapeutic usefulness of ginger extracts also with regard to diabetes and diabetic complications (Ojewole, 2006; Bhandari et al., 2005). Hyperglycaemia- as well as dyslipidaemia-lowering effects belong to the most known anti-diabetic benefits of ginger.
Hydroethanolic extract of the rhizome ginger is known for its strong free-radical reducing efficacy (Ali et al., 2008). It is mediated mostly by its phenolic constituents that may be divided into two groups: gingerol-, gingeron- and shogaol-related group and diarylheptanoids. A mixture of non-phenolics (sesquiterpene hydrocarbons, carbonyl compounds, monoterpene hydrocabons and esters) contributes to the antioxidative activity and is responsible for the strong aroma of ginger in food, beverages and dietary supplements (Ali et al., 2008).
The essential oil with volatile compounds (prepared by hydrodistillation) showed also a good antioxidant activity with correspondence to its phenolic content (El-Ghorab et al., 2010). Furthermore, antioxidant activity of both volatile and non-volatile fractions has been ascribed to the synergistic effects of phenolics (such as eugenol, shoagols, zingerone, gingerdiols, gingerols, etc.). As confirmed by GC-MS, the most abundant terpenes in ginger essential oil are zingiberene, ar-turmerone, curcumene, tumerone and curlone (Singh et al., 2008).
Considering frequent applications of ginger extracts in medicine or food industry, there is a need for better understanding of the commercial drugs composition, based on Z. officinale extracts also with regard to their biological efficacies.
In this study, the composition of two different Z. officinale extracts (prepared with solvents n-hexane and ethanol) was investigated. Furthermore, their intrinsic antiradical properties with their cytotoxicities and protective effects in a model of oxidative damage to pancreatic INS-1E β cells were compared.
Methods
Plant material
The dried powdered roots of ginger were purchased from the local vegetable and fruit market of Vido, Co., Ltd., Bratislava, Slovak Republic (September 2009).
Chemicals and INS-1E cell culture
DPPH (1,1′-diphenyl-2-picrylhydrazyl) radical was purchased from Sigma Co. (St.Louis, MO, USA) and Folin-Ciocalteu′s phenol reagent from Merck KGaA, Darmstadt, Germany).
INS-1E β cells (kindly provided by Prof. Claes Wollheim, University of Geneva (Merglen et al., 2004) were cultured in RPMI-1640 supplemented with 10% fetal calf serum, 100 U.ml–1 penicillin, 100 µg.l–1 streptomycin, 2 mmol.l–1 L-glutamine, 1 mmol.l–1 Na-pyruvate, 55 µmol.l–1, 2-mercaptoethanol, 10 mmol.l–1 HEPES, pH 7.0–7.4 (KRD molecular technologies, ltd, Slovakia). For assays, the cells were detached by 5–10-min incubation with 0.05% trypsin/EDTA (KRD molecular technologies, ltd., Bratislava, Slovakia).
Extracts preparation
Air-dried and powdered ginger (Z. officinale rhizomes, 2×150 g) was separately extracted with five volumes of n-hexane and 60% aqueous ethanol (v:v), for 48 h, subsequently the solid phase was removed by filtration and combined extracts were concentrated under reduced pressure at 40 °C, to give 10.2 g and 13.2 g crude extract, respectively. The prepared extracts were stored at 4 °C until further analyzed.
GC-MS analysis
The extracts were submitted to qualitative and quantitative analysis by GC-MS system (Agilent Technologies 7890, Palo Alto, USA) gas chromatograph equipped with an Agilent Technologies 5975C inert XL mass selective spectrometer. The mass range was scanned from m/z 29–420 Daltons.
The non-polar column Agilent 19091B-102 Ultra 2, 25 m × 0.20 mm, film thickness 0.33 µm was programmed from 40–320 °C.min-1. Injector temperature was 250 °C, injections 0.5 µl, and split 1:100. The carrier gas (helium) flow was maintained at 0.8 ml.min–1 by an electron control of pressure. Identification of the compounds was based on (i) comparison of substance mass spectra with the GC-MS system data bank (NIST 05 library), (ii) comparison of mass spectra with data in the literature (Jain et al., 2007; Jolad et al., 2004).
DPPH radical-scavenging assay
Free radical-scavenging ability of both extracts tested was determined as described by Wong et al. (2006). The capacity to scavenge the lipid soluble DPPH radical was monitored at 517 nm. DPPH methanolic solution (2.9 ml, 1 mmol.l–1 solution of DPPH radical solution in methanol) was mixed with the samples tested (0.1 ml) at different concentrations. After 30 min the absorbance was read at 517 nm (Thermo Electron Corporation Genesis 6 Spectrophotometer (UK)). The percentage of absorbance decrease, relative to non-reduced control DPPH solution was evaluated.
Total phenolic content
The total phenolic content was determined using Folin-Ciocalteu′s method (Lako et al., 2007). Briefly, a 0.5 ml aliquot of extract solution, dissolved in methanol was transferred into the test tube containing 8 ml of distilled water. Afterwards, 0.5 ml of the Folin Ciocalteu′s phenol reagent (1:10 dilution) was added to react completely with the oxidizable compounds or phenolates, and 1 ml of sodium carbonate solution (7.5% solution in water) was added to destroy the residual reagent. The mixture was shaken for 15 s and then left to stand at room temperature for 2 h. The absorbances were measured at 760 nm. Results were expressed as mg.g–1 of dry extract. The samples were measured in triplicate.
Cell viability
Mitochondrial reduction of MTT (3-[4,5-dimethyldiazol-2-yl]-2,5-diphenyl-tetrazolium bromide) was determined spectrophotometrically at λmax= 570 nm, following the exposure to H2O2 or to extracts tested in supplemented medium (1% FBS). Trypan blue uptake in situ was determined by exposure of the cells to 0.1% trypan blue in phosphate buffered solution (PBS, pH 7.4, 5 min). The cells were washed with PBS and examined by light microscopy.
Apoptosis/necrosis detection and caspase 3 assay
The apoptotic changes were assessed by ethidium bromide and acridine orange (EB/AO) staining assay (Ribble et al., 2005; Račková et al., 2009a). Briefly, the cells were grown in 3 cm Petri dishes and treated as described above. EB/AO dye mix (100 µg.ml–1 EB and 100 µg.ml–1 AO, 20 µl) was added to each dish at the end of incubation time and the cells were viewed under the fluorescence microscope. Each image (50–200× magnification) was collected with XDS-2 fluorescence microscope running standard software. Early and late apoptotic cells were detected by their bright green and orange nucleus with condensed or fragmented chromatin, respectively, whereas necrotic cells were detected by their intact red nuclei, and live cells had a normal green nucleus. For detection of caspase 3 activation, luminiscence assay using Caspase-Glo 3/7 Assay kit (Promega) was performed according to the manufacturer’s protocol. Fifty thousand cells were used for each measurement.
Intracellular oxidants
Simultaneous oxidation of incorporated 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA) and dihydroethidin (DHE) (probes with differing specificity to oxidizing species) was followed in the β cells exposed to H2O2 stress (Račková et al., 2009a; Halliwell and Whiteman, 2004). In brief, the cells were grown in 96-well plates to a density of 4×104 cells per well and incubated with 15 µmol.l–1 H2DCF-DA in supplemented phenol red and serum free RPMI-1640 for 30 min at 37 °C. After H2DCF-DA loading, the cells were washed and medium was replaced by RPMI-1640 containing 1% FBS and H2O2 with or without the substances tested. DHE (10 µmol) was added to the wells at the incubation time of 1 hour, followed by additional incubation under the same conditions for 20 minutes. Images were collected by fluorescence microscope and analyzed for reaction intensity.
Statistical analysis
Each experiment was performed at least three times. Results are expressed as mean value ± standard deviation (SD). Statistical analysis was performed using unpaired Student's t-test using X-Plot v. 2.81 and statistical significance is expressed as *p<0.05, **p<0.01, ***p<0.001 vs respective control cells.
Results
Determination of total phenolic content
The determined total phenolic content of n-hexane and ethanolic extract amounted to 50.74 and 28.75 mg.g–1 of dry extract, respectively. The results are represented as miligrams of gallic acid equivalent (GAE) per one gram of dry extract.
Radical scavenging activity and composition of extracts isolated from Z. officinale
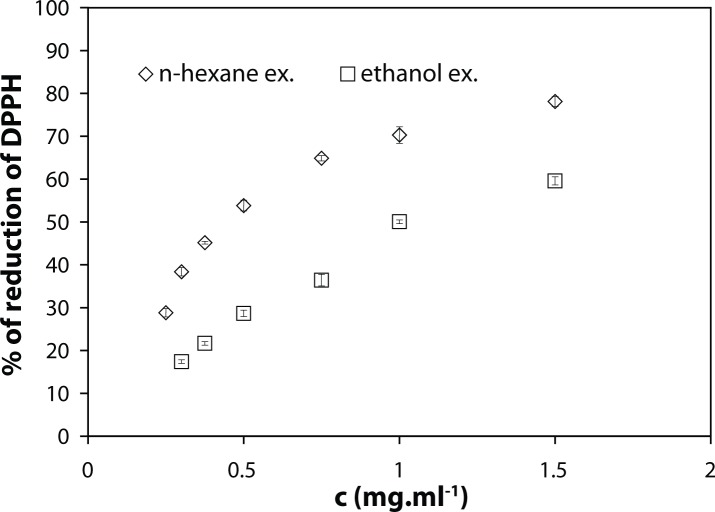
The n-hexane extract showed better reactivity with DPPH ability than ethanolic extract. This was the most evident at the concentration of 750 µg.ml–1 of both n-hexane and ethanolic extract tested, causing reduction of DPPH by 64.9±0.6% and 36.4±1.3%, respectively (Figure 1). This outcome was further supported by corresponding IC50 values: 1.08±0.04 mg.ml–1 and 0.47±0.01 mg.ml–1, respectively.
Figure 1.
DPPH radical reducing efficacies of the n-hexane and ethanolic extract isolated from Zinginber officinale Rosc. Results are expressed as the mean ± S.D., n = 3.
Sample constituents of ethanol extract are given in Table 1: 7-gingerol (16.83%) was identified as the main compound in our analysis, however, 6-gingerol (7.69%) was found as the second major compound. The n-hexane extract profile of ginger (Table 1) shows alpha-curcumene as the main compound (19.09%), other major compounds were alpha-zingiberene (15.25%), beta-sesquiphellandrene (7.163%), tau-muurolol (5.00%) and 4-gingerol (4.64%).
Table 1.
Compounds of Zingiber officinale Rosc.
| n-Hexane extract | Ethanolic extract | ||
|---|---|---|---|
| compound | Peak area [%] | compound | Peak area [%] |
| borneol | 1.25 | tumerone | 0.49 |
| tau-muurolol | 5.00 | folic acid | 0.50 |
| alpha-zingiberene | 15.25 | ar-tumerone | 1.30 |
| alpha-curcumene | 19.09 | phtallic acic, butyloctyl ester | 0.92 |
| beta-sesqui-phellandrene | 7.16 | diepicedrene-1-oxide | 2.08 |
| beta-cedrene-9-alpha-ol | 1.37 | 4-gingerol | 0.8 |
| zingerone | 1.54 | 6-gingerol | 7.69 |
| 4-gingerol | 4.64 | 7-gingerol | 16.83 |
| 6-gingerol | 0.31 | ||
| 7-gingerol | 1.02 | ||
| 8-gingerol | 0.89 | ||
Cytotoxicity of H2O2 and extracts tested
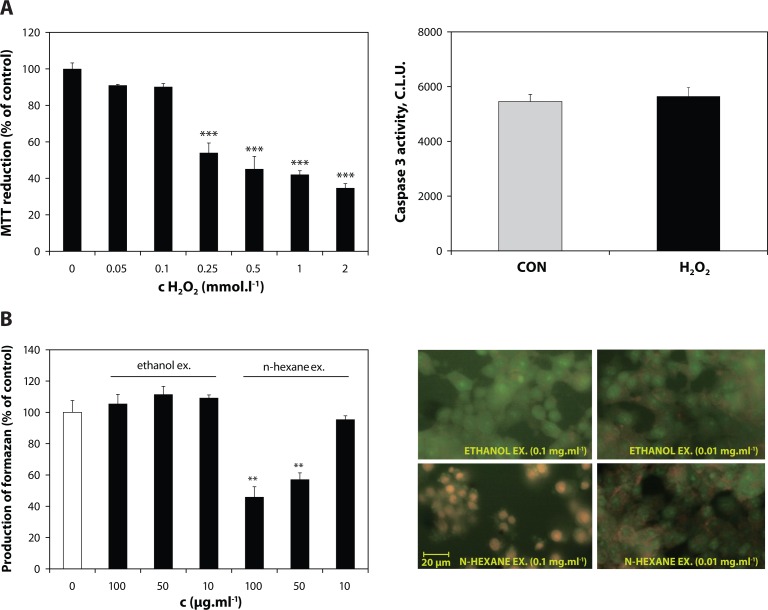
Exposure to H2O2 (0.5 mmol.l–1) during 1 hour caused a concentration-dependent decrease of MTT reduction by INS-1E β cells (Figure 2A). Viability decline was further confirmed by positive staining with Trypan blue as well as increased cell permeability for ethidium bromide dye (in AO/EB staining assay). A significant increase in number of the cells with EB-positive intact nuclei was observed following H2O2 injury. Furthermore, a lack of caspase 3 activation was confirmed within exposure time to H2O2 (Figure 2A), followed by a drop of caspase 3 activity in the next 2 hours (data not shown). In addition, incubation with H2O2 (0.5 and 2 mmol.l–1) promoted intracellular oxidation preferably of DHE (with a minor portion of H2DCF oxidation) (Figure 4).
Figure 2.
Cytotoxicity of H2O2 and the Z. officinale fractions tested in β cells. A: Metabolic activity of INS-1E cells exposed to H2O2 in medium (1% FBS) for 1 hour. Caspase 3 activity in the cells exposed to 0.5 mmol.l–1 H2O2. B: AO/EB uptake and MTT viability of the INS-1E β cells incubated with n-hexane and ethanolic extract for 1 hour. Cytotoxic effect appears as a bright staining of nuclear region of the cells accompanied by membrane blebbing (left bottom image). Results are expressed as the mean ± S.D., n = 3.
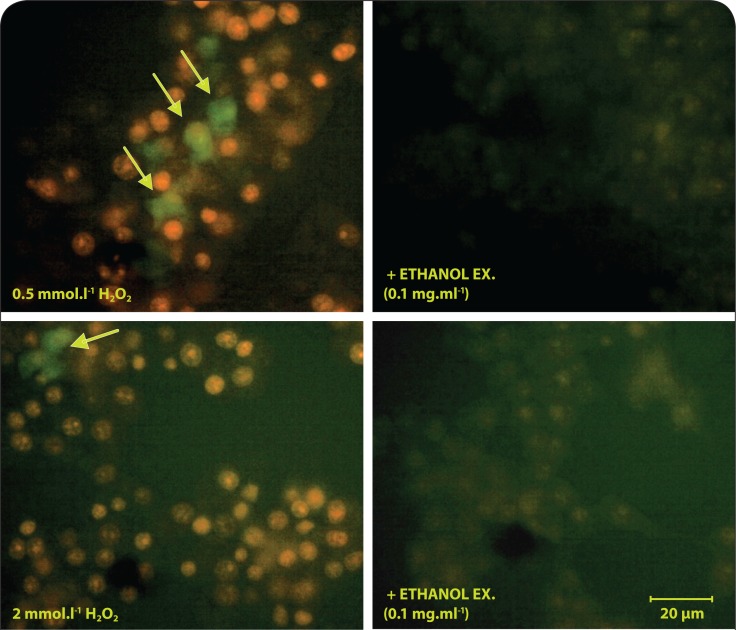
Figure 4.
Antioxidant effect of ginger ethanol extract in INS-1E β cells. Fluorescent images indicate the significant prevalence of the cellular DHE oxidation by superoxide, compared to H2DCF oxidation by RO•, R = alkyl, alkoxyl, H, NO• (green fluorescence indicated in arrows).
Unlike the n-hexane extract, causing at the conc. >50 µg.ml–1 injury of β cells (Figure 2B), ethanolic ginger extract did not show any notable cytotoxicity at the concentration range tested. The reduction of MTT viability by n-hexane extract was accompanied by nuclear shrinkage and membrane blebbing confirmed by AO/EB staining assay (Figure 2B).
Reduction of intracellular oxidants and protection of β cells against injury by H2O2
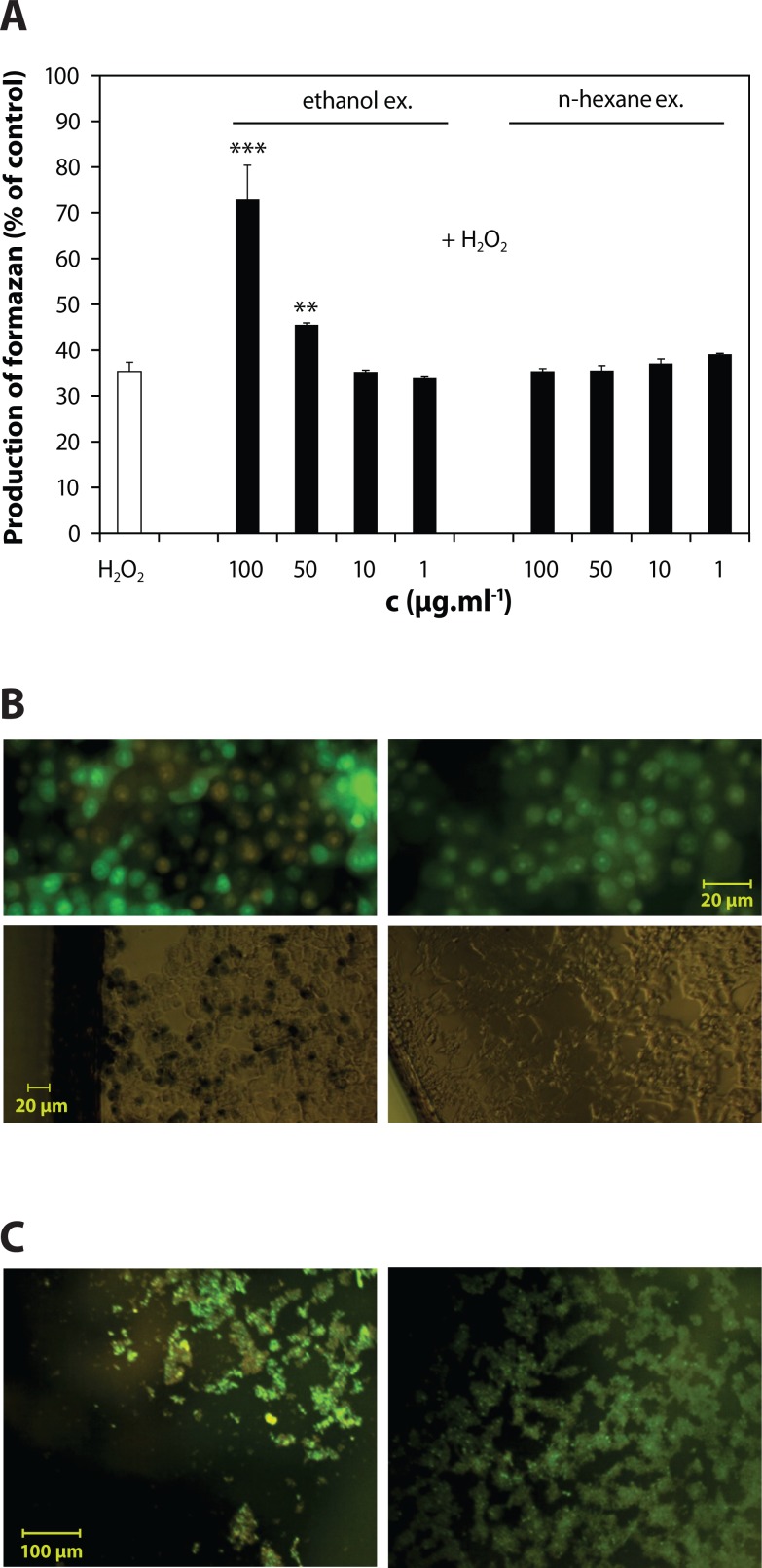
Only ethanolic extract Z. officinale showed a significant dose-dependent protection of metabolic activity of the cells exposed 0.5 mmol.l–1 H2O2 (causing 64.7%-decrease of MTT viability) (Figure 3A). This was accompanied with abolishment of Trypan blue and ethidium bromide uptake by the cells, as shown by microscopic images, suggesting an efficient viability protection (Figure 3B). The loss of cell adherence was also significantly prevented (Figure 3C). The maximum protective effect of ginger ethanolic extract was seen at concentration 100 µg.ml–1 (72.9±7.5% of control).
Figure 3.
Protection of metabolic activity of INS-1E β cells and in situ protective effects of Z. officinale ethanol extract. A: Significant protection of metabolic activity of H2O2-stressed cells by ethanolic extract in comparison to lack of efficacy of n-hexane extract. B: Protection of viability and C: adherence of INS-1E cells by ethanolic extract (0.1 mg.ml–1). Dead cells appear as red-fluorescence positive cells. Results are expressed as the mean ± S.D., n = 3.
The protective effects of ethanolic extract were accompanied by a significant avoidance of H2DCF and DHE oxidation in the stressed cells, appearing as a lack of both ethidium-positive nuclei and dichlorofluorescein fluorescence (Figure 4).
Discussion
n-Hexane extract possessed higher content of polyphenols than ethanolic extract
The determined content of polyphenols in ethanolic extract agrees with previously estimated value 21.24 mg GAE.g–1 of dry weight of ginger ethanolic extract (Liu et al., 2008). Furthermore, El-Ghorab et al. (2010) reported on the value 67.5 mg GAE.g–1 of dry n-hexane ginger extract, which is also in good conformity with our result.
n-Hexane extract showed better radical-reducing capability than ethanolic extract
Phenolic compounds represent a substantial portion of spice antioxidants. Although the oxidative-stress reducing activities of putative antioxidants have been attributed to various mechanisms, DPPH radical-scavenging activity is an appropriate indicator of potential antioxidant efficacy. In analogy with the data reported by El-Ghorab et al. (2010), the results of DPPH assay of both extracts tested corresponded to the differences in their phenolic contents. The n-hexane extract showed clearly more potent hydrogen-donating ability than ethanolic extract. However, both determined IC50 values are considerably higher than our values reported for substances with strong antioxidant activities – curcumin and Curcuma longa extract (3.33 µg.ml–1 and 2.34 µg.ml–1, respectively (Račková et al., 2009b).
The contents of gingerols as the main phenolic constituents were further confirmed by GC-MS analyses in both extracts tested. Unlike the studies reporting on 6-gingerol as a major compound in the varieties of Zingiber (Ojewole, 2006; El-Ghorab et al., 2010; Jolad et al., 2004), 7-gingerol was identified as the main compound in our analysis, however, 6-gingerol was found as the second major compound. In accordance with reports by Natta et al. (2008) and Sacchetti et al. (2005), the constituent's profile of n-hexane extract shows alpha-curcumene as the main compound, other major compounds being alpha-zingiberene, beta-sesquiphellandrene, tau-muurolol and 4-gingerol. The synergism mediated by a greater variety of phenolics (4-,6-,7-,8-gingerol, 6-paradol, Table 1) in n-hexane extract may account for its better efficacy in DPPH assay (El-Ghorab et al., 2010; Liu et al., 2008). In this regard, the antioxidant synergism of various nucleophiles (represented in the n-hexane extract particularly by secondary and tertiary alcohols, Table 1) with 3,4-dihydroxy-polyphenols has been also proposed (Saito & Kawabata, 2004). This can also explain an increased reactivity of n-hexane extract with Folin-Ciocalteu′s reagent which not only measures total phenols but also reflects the total reducing capacity of a sample.
On the other hand, the actual content of 8- and 10-gingerols (thermally labile compounds loosely detectable by GC-MS) in n-hexane extract could be underestimated (Bilehal et al., 2010). In support of this assumption, both zingerone (retention time (Rt) = 58.27 min) and aldehydes (retention time (Rt) (octanal) = 7.18 min; Rt (decanal) = 14.67 min), decomposition products of thermo-labile higher gingerols were detected in chromatogram of n-hexane extract.
Hydrogen peroxide caused necrosis in INS-1E β cells
Hydrogen peroxide is a highly reactive compound, present in the majority of the oxidative processes, responsible for pancreatic β cell demise in diabetes type 1 and 2. During insulitis, activated phagocytes can produce as much as 47 nmol of H2O2 per 106 cells within 30 min corresponding to 1 ml solution of H2O2 with the concentration of 47µmol/l (Anderson, 1992). In type 2 diabetes, excessive glucose metabolism may lead to generation of superoxide anions, which are spontaneously dismutated to H2O2 (Nishikawa & Araki, 2007; Leverve et al., 2003). It has been shown that mitochondria are primary targets for H2O2 damage that may eventually lead to impaired glucose metabolism and decreased insulin secretion (Maechler et al., 1999).
Exposure to H2O2 caused a decrease of MTT reduction, followed by increase of Trypan blue uptake by INS-1E β cells suggesting both viability decline and injury of metabolic function of the cells (Janjic & Wollheim, 1992). Abundance of EB-positive cells with intact nuclei along with a deficiency of caspase 3 activation suggested that H2O2 injury caused mainly necrotic death of INS-1E β cells.
n-Hexane extract exerted increased cytotoxicity in INS-1E β cells
Neither of the ginger substances tested did exert notable cytotoxicities at lower concentrations applied. However, the loss of viability caused by high-dose n-hexane extract was accompanied by presence of apoptotic markers. In paradox, enhanced cytotoxic effects of Z. officinale n-hexane extract may be related to its stronger intrinsic redox activities. The cytotoxic effect of natural phenolic compounds has been documented in pancreatic βTC1, HIT as well as INS-1E cell lines, explained by prooxidant effect of these substances (Bortolotti et al., 2009; Lapidot et al., 2002). Furthermore, increased cytotoxicity of n-hexane extract may be associated with higher lipophilicity of its components (Moridani et al., 2003). Correspondingly, zerumbone, a natural cyclic sesquiterpene isolated from Zingiber zerumbet Smith (Southeast Asian ginger) has been shown to induce apoptosis in pancreatic carcinoma cells (Zhang et al., 2012). This suggests a promising potential of the n-hexane extract constituents in treatment of pancreatic cancer.
Ethanolic extract protected INS-1E β cells against injury by H2O2 along with suppression of intracellular oxidants
Neither H2O2 nor O2 •– can oxidize H2DCF, but peroxyl, alkoxyl, NO2·, carbonate (CO3 •–) and ·OH radicals can, as can peroxynitrite (Halliwell & Whiteman, 2004). Dihydroethidium (dihydroethidine) (DHE) is frequently used as a probe for O2 •–, being oxidized to a fluorescent product, ethidium, which tends to intercalate into nuclear DNA. Apparently, exposure to H2O2 induced oxidation preferably of DHE (with a minor portion of H2DCF oxidation) suggesting that superoxide radicals are the major oxidizing species in the present model. The H2DCF can effectively determine the intracellular oxidants only if they are decomposed to radicals, e.g. by means of transition metals. The presence of extracellular iron was shown as necessary for the generation of a signal from H2DCF upon addition of H2O2 to the cells (Tampo et al., 2003). Furthermore, the cellular peroxidase level and heme protein content are another determinants for the signal generation from this probe (Ohashi et al., 2002).
Exposure to H2O2 was shown to result in the depolarization of mitochondrial membrane potential followed by the inhibition of the hyper-polarization effect of glucose (Maechler et al., 1999). In compliance with our result, mitochondrial depolarization was shown to be responsible for the enhancement of superoxide production in many cellular systems (Budd et al., 1997).
Only ethanolic extract Z. officinale showed a significant protection of the cells exposed to H2O2. Its protective efficacy is comparable to our previously reported MTT viability protection efficacy by 68.9% of control, determined for synthetic pyridoindole (a novel antioxidant standard possessing anti-diabetic potential) at its maximum non-toxic concentration (100 µmol.l–1) in INS-1E cells exposed to H2O2 (Račková et al., 2011; Stefek et al., 2002).
The protective effects of ethanolic extract were accompanied by a significant avoidance of both superoxide-dependent DHE- and superoxide-independent H2DCF-oxidation in the stressed cells. Accordingly, Z. officinale extracts were shown to exert both superoxide and hydrogen peroxide scavenging activity (Khanom et al., 2003; Yang et al., 2009).
In conclusion, the present study suggests that ethanolic extract isolated from Zingiber officinale Rosc. is a promising substance for the in depth study of its protective effects against cytotoxic conditions imposed by diabetes in pancreatic β cells. A higher content of phenolic constituents and increased intrinsic antiradical capability of diverse ginger extract preparations may not be obviously related to their improved biological efficacy under conditions of oxidative stress, but, paradoxically, accounts for an enhanced cytotoxicity. In view of INS-1E cells as an insulinoma cell line, however, the remarkable proapoptotic effect of Z. officinale constituents may indicate their prospective antitumor efficacies.
Acknowledgements
The work was supported by The Agency of the Ministry of Education of the Slovak Republic for the Structural Funds of EU, OP R&D of ERDF as a part of the Project: „Evaluation of natural substances and their selection for prevention and treatment of lifestyle diseases“ (ITMS 26240220040).
REFERENCES
- 1.Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological ant toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol. 2008;46:409–420. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 2.Anderson R. A peroxidase-independent method for the quantitation of extracellular hydrogen peroxide generated by activated phagocytes in vitro . J Immunol Methods. 1992;155:49–55. doi: 10.1016/0022-1759(92)90270-4. [DOI] [PubMed] [Google Scholar]
- 3.Bhandari U, Kanojia R, Pillai KK. Effect of ethanolic extract of Zingiber officinale on dyslipidaemia in diabetic rats. J Ethnopharmacol. 2005;97:227–230. doi: 10.1016/j.jep.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Bilehal DC, Sung DD, Kim YH. Influence of the Solvent, Hydrodistillation–Headspace Solvent Microextraction and Composition of Korean Ginger. Food Anal Methods. 2010 doi: 10.1007/s12161-010-9133-9. [DOI] [Google Scholar]
- 5.Bortolotti C, Kunit T, Moder A, Hufnag C, Schmidt S, Hartl A, Langelueddecke Ch, Fürst J, Geibel JP, Ritter M, Jakab M. The Phytostilbene Resveratrol Induces Apoptosisin INS-1E Rat Insulinoma Cells. Cell Physiol Biochem. 2009;23:245–254. doi: 10.1159/000218171. [DOI] [PubMed] [Google Scholar]
- 6.Budd SL, Castilho RF, Nicholls DG. Mitochondrial membrane potential and hydroethidine-monitored superoxide generation in cultured cerebellar granule cells. FEBS Lett. 1997;415:21–24. doi: 10.1016/s0014-5793(97)01088-0. [DOI] [PubMed] [Google Scholar]
- 7.El-Ghorab AH, Nauman M, Anjum FM, Hussain S, Nadeem M. A comparative study on chemical composition and antioxidant activity of ginger (Zingiber officinale) and cumin (Cuminum cyminum) J Agric Food Chem. 2010;58:8231–8237. doi: 10.1021/jf101202x. [DOI] [PubMed] [Google Scholar]
- 8.Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? British J Pharmacol. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain V, Prasad V, Pal R, Singh S. Standardization and stability studies of neuroprotective lipid soluble fraction obtained from Curcumae longa . J Pharm Biomed Anal. 2007;44:1079–1086. doi: 10.1016/j.jpba.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Janjic D, Wollheim CB. Islet cell metabolism is reflected by the MTT (tetrazolium) colorimetric assay. Diabetologia. 1992;35:482–485. doi: 10.1007/BF02342448. [DOI] [PubMed] [Google Scholar]
- 11.Jolad SD, Lantz RC, Solyom AM, Chen GJ, Bates RB, Timmermann BN. Fresh organically grown ginger (Zingiber officinale): composition and effects on LPS-induced PGE2 production. Phytochemistry. 2004;63:1937–1954. doi: 10.1016/j.phytochem.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Khanom IF, Kayahara H, Hirota M, Tadasa K. Superoxide Scavenging and Tyrosinase Inhibitory Active Compound in Ginger (Zingiber officinale Roscoe) Pakistan J Biol Sci. 2003;6:1996–2000. [Google Scholar]
- 13.Lako J, Trenerry VC, Wahlqvist Wattanapenpaiboo N, Sotheeswaran S, Premier R. Phytochemical flavonols, carotenoids and the antioxidant properties of wide selection of Fijian fruit, vegetables and other readily available foods. Food Chem. 2007;101:1727–1741. [Google Scholar]
- 14.Lantz RC, Chen GJ, Sarihan M, Sólyom AM, Jolad SD. The effect of extract from ginger rhizome on inflammatory mediator production. Phytomedicine. 2007;14:123–128. doi: 10.1016/j.phymed.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Lapidot T, Walker MD, Kanner J. Antioxidant and Prooxidant Effects of Phenolics on Pancreatic β-Cells in vitro . J Agric Food Chem. 2002;50:7220–7225. doi: 10.1021/jf020615a. [DOI] [PubMed] [Google Scholar]
- 16.Leverve XM, Guigas B, Detaille D, Batandier C, Koceir EA, Chauvin C, Fontaine E, Wiernsperger NF. Mitochondrial metabolism and type-2 diabetes: a specific target of metformin. Diabetes Metab. 2003;6:S88–S94. doi: 10.1016/s1262-3636(03)72792-x. [DOI] [PubMed] [Google Scholar]
- 17.Liu H, Qui N, Ding H, Yao R. Polyphenols contents and antioxidant capacity of 68 Chinese herbals suitable for medical or food uses. Food Res Int. 2008;41:363–370. [Google Scholar]
- 18.Maechler P, Jornot L, Wollheim CB. Hydrogen Peroxide Alters Mitochondrial Activation and Insulin Secretion in Pancreatic Beta Cells. J Biol Chem. 1999;274:27905–27913. doi: 10.1074/jbc.274.39.27905. [DOI] [PubMed] [Google Scholar]
- 19.Merglen A, Theander S, Rubi B, Chaffard G, Wollheim CB, Maechler P. Glucose sensitivity and metabolism-secretion coupling studied during two-year continuous culture in INS-1E insulinoma cells. Endocrinology. 2004;145:667–678. doi: 10.1210/en.2003-1099. [DOI] [PubMed] [Google Scholar]
- 20.Moridani MY, Siraki A, O'Brien PJ. Quantitative structure toxicity relationships for phenols in isolated rat hepatocytes. Chem Biol Interactions. 2003;145:213–223. doi: 10.1016/s0009-2797(02)00258-2. [DOI] [PubMed] [Google Scholar]
- 21.Natta L, Orapin K, Krittika N, Pantip B. Essential oil from five Zingiberaceae for anti food-borne bacteria. Int Food Res J. 2008;15:337–346. [Google Scholar]
- 22.Nishikawa T, Araki E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid Redox Signal. 2007;9:343–353. doi: 10.1089/ars.2006.1458. [DOI] [PubMed] [Google Scholar]
- 23.Ohashi T, Mizutani A, Murakami A, Kojo S, Ishii T, Taketani S. Rapid oxidation of dichlorodihydrofluorescin with heme and hemoproteins: formation of the fluorescein is independent of the generation of reactive oxygen species. FEBS Lett. 2002;511:21–27. doi: 10.1016/s0014-5793(01)03262-8. [DOI] [PubMed] [Google Scholar]
- 24.Ojewole JAO. Analgesic, antiinflammatory and hypoglycaemic effects of ethanol extract of Zingiber officinale (Roscoe) rhizomes (Zingiberaceae) in mice and rats. Phytotherapy Res. 2006;20:764–772. doi: 10.1002/ptr.1952. [DOI] [PubMed] [Google Scholar]
- 25.Račková L, Cumaoğlu A, Bağrıacık EU, Štefek M, Maechler P, Karasu Ç. Novel hexahydropyridoindole derivative as prospective agent against oxidative damage in pancreatic β cells. Med Chem. 2011;7:711–717. doi: 10.2174/157340611797928370. [DOI] [PubMed] [Google Scholar]
- 26.Račková L, Šnirc V, Jung T, Štefek M, Karasu Ç, Grune T. Metabolism induced oxidative stress is a mediator of glucose toxicity in HT22 neuronal cells. Free Radic Res. 2009a;24:1–11. doi: 10.1080/10715760903104374. [DOI] [PubMed] [Google Scholar]
- 27.Račková L, Košt'álová D, Bezáková L, Fialová S, Bauerová K, Tóth J, Štefek M, Vanko M, Holková I, Obložinský M. Comparative study of two natural antioxidants, curcumin and Curcuma longa extract. J Food Nutr Res. 2009b;48:148–152. [Google Scholar]
- 28.Ribble D, Goldstein NB, Norris DA, Shellman YG. A simple technique for quantifying apoptosis in 96-well plates. BMC Biotechnol. 2005;5:12–19. doi: 10.1186/1472-6750-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sacchetti G, Maietti S, Muzzoli M, Scaglianti M, Manfredini S, Radice M, Bruni R. Comparative avaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005;91:621–632. [Google Scholar]
- 30.Saito S, Kawabata J. Synergistic effects of thiols and amines on antiradical efficiency of protocatechuic acid. J Agric Food Chem. 2004;52:8163–8168. doi: 10.1021/jf048970j. [DOI] [PubMed] [Google Scholar]
- 31.Singh G, Kapoor IPS, Singh P, de Heluani CS, de Lampasona MP, Catala CAN. Chemistry, antioxidant and antimicrobial investigations on essential oil and oleoresins of Zingiber officinale . Food Chem Toxicol. 2008;46:3295–3302. doi: 10.1016/j.fct.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 32.Stefek M, Gajdosik A, Tribulova N, Navarova J, Volkovova K, Weismann P, Gajdosikova A, Drimal J, Mihalova D. The pyridoindole antioxidant stobadine attenuates albuminuria, enzymuria, kidney lipid peroxidation and matrix collagen cross-linking in streptozotocin-induced diabetic rats. Meth Find Exp Clin Pharm. 2002;24:565–571. [PubMed] [Google Scholar]
- 33.Tampo Y, Kotamraju S, Chitambar RC, Kalivendi SV, Keszler A, Joesph J, Kalyanaraman B. Oxidative stress-induced iron signaling is responsible for peroxidedependent oxidation of dichlorodihydrofluorescein in endotheliel cells. Role of transferrin receptor-dependent iron uptake in apoptois. Circ Res. 2003;92:56–63. doi: 10.1161/01.res.0000048195.15637.ac. [DOI] [PubMed] [Google Scholar]
- 34.Wong SP, Leong LP, Koh JHW. Antioxidant activities of aqueous extracts of selected plants. Food Chem. 2006;99:775–783. [Google Scholar]
- 35.Zhang S, Liu Q, Liu Y, Qiao H, Liu Y. Zerumbone, a Southeast Asian Ginger Sesquiterpene, Induced Apoptosis of Pancreatic Carcinoma Cells through p53 Signaling Pathway. Evid Based Complement Alternat Med[online] 2012 Jan 29;2012(2012) doi: 10.1155/2012/936030. [cit. 3 February 2013]. < http://www.hindawi.com/journals/ecam/2012/936030/>. ISSN: 1741–4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang L, Zhou C, Huang K, Song L, Zheng Q, Yu R, Zhang R, Wu Y, Zeng S, Cheng CH, Zhao Y, Li X, Qu J. Antioxidative and cytotoxic properties of diarylheptanoids isolated from Zingiber officinale . Zhongguo Zhong Yao Za Zhi. 2009;34:319–23. [PubMed] [Google Scholar]