Abstract
A methyl group on a sugar residue is a rarely reported event. Until now this kind of modification has been found in the kingdom of animals only in worms and molluscs, whereas it is more frequently present in some species of bacteria, fungi, algae and plants, but not in mammals. The monosaccharides involved as well as the positions of the methyl groups on the sugar vary with the species. Methylation seems to play a role in some recognition events but details are still unknown. This review summarises the current knowledge on methylation of sugars in all kinds of organism.
Keywords: glycan, methylation, sugar
Introduction
Methylation is a modification which has been often found on proteins (linked to amino-, carboxyl- or sulphydryl-groups), DNA and RNA. It is highly important for modulation and regulation of processes, developmental changes, cell signalling and aging. Changes in the methylation pattern (hypermethylation as well as undermethylation) are related to several diseases (Schulz 1998).
On proteins the methylation is known as a regulator in various cellular processes influencing protein-protein interactions, stability, localisation and enzyme activity. Much is known about methylamines, mainly methylated arginine or lysine, especially of histones but also of some other proteins (Peters and Schübeler, 2005). Also carboxy groups of amino acids may form methyl esters. Those methylations play significant roles in cell recognition processes. On structural proteins methyl thioesters with unknown function have been identified (Branscombe Miranda et al., 2004; Zhang et al., 2012). Epigenetic mechanisms are regulated by DNA methylation causing a closed chromatin state with repressed, inactive genes. This modification plays an important role in imprinting, X-inactivation, oncogenesis, inflammatory and immunological processes (Salozhin et al., 2005; Barnes, 2011; Garaud et al., 2011; Poetsch et al., 2011).
Methylation of the “third estate” of building macromolecules, the carbohydrates, occurs in some bacteria, fungi, plants, worms and molluscs. It has not been found in mammals. It is an additional option to modulate the structure of glycan molecules, however, a clear determination of the function is still missing. As summarised in this review, the study of methylation of sugars is primarily restricted to structural phenomenology.
Bacteria
In bacteria methylated structures have been identified for the first time. Up to now various sugars containing differently linked, sometimes multiple, methyl groups have been identified which are important constituents of bacterial glycans, mainly lipopolysaccharides. In the early studies 2-O-Me-L-Rha has been found as constituent of scopamycin A, a metabolite of a strain of Streptomyces aureofaciens (McAlpine et al., 1971) and as part of the antibiotic aranciamycin (Keller-Schierlein et al.,1970). Surprisingly also its mirror image, 2-O-Me-DRha, is synthesized by Mycobacterium tuberculosis (Demareau-Ginsburg and Lederer, 1963). Due to the medical relevance of this organism, the methylation abilities of mycobacteria have been intensively studied. They display frequently 3-O-Me-Rha, but also Rha with di- or trimethylation or methylated talose (reviewed by Schorey and Sweet, 2008). Besides 3-O-Me-Rha, 3,4-di-O-Me-Rha, 3-O-Me- and 2,3-di-O-deoxy talose furthermore 4-O-Me-GlcA, 2,4-di or 3,4-di-Me-GlcA are present in Mycobacterium habana strain TMC5135 (Khoo et al., 1996). Recently also 4-O-Me-Rha has been found in serotype 13 glycopeptidolipid from Mycobacterium intracellulare (Naka et al., 2011). The biological significance of these methylated sugars in Mycobacteria is still not clear. There are some hints pointing to a role in the regulation of the fatty acid metabolism but final proof is still missing (Jackson and Brennan, 2009).
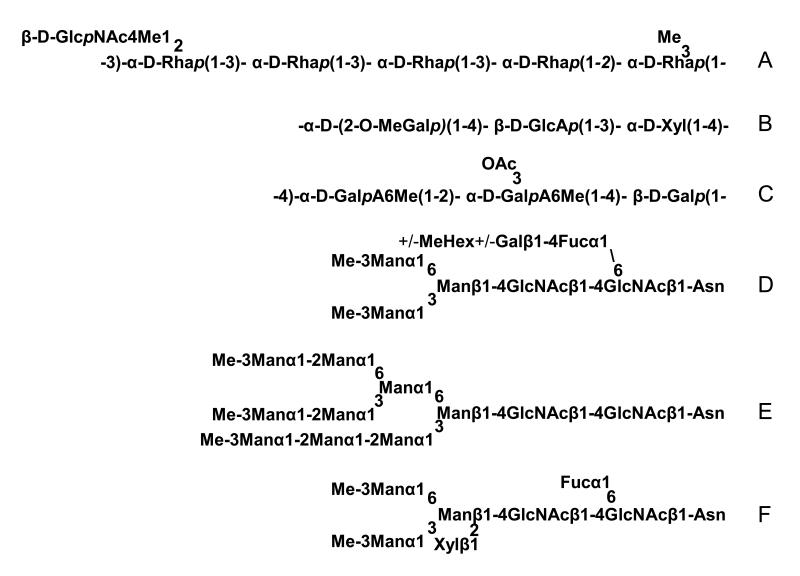
The second group of bacteria which is well investigated for their methylation potential is Rhizobia. They are gram negative symbiotic soil bacteria which are able to fix nitrogen in interaction with their plant host. Rhizobium etli CE3, a symbiont of legumes, displays 2-O-Me-Fuc, 3-O-Me-6-deoxy-L-talose and polymethylated fucose (2,3,4-tri-O-Me-L-Fuc) on the lipopolysaccharide which may facilitate symbiotic interactions in terms of nodules development and nitrogenase activity (Forsberg et al., 2000; Noel et al., 2004). Also in Bradyrhizobium Sp. strain ORS285 2-O-Me-Fuc may play a role in the molecular dialogue between the photosynthetic bacterial symbiont and its tropical aquatic legume host (Aeschynomene spp.) (Renier et al., 2011). In Bradyrhizobium japonicum strain USDA110 2-O-Me-Fuc has been found in the lipooligosaccharide nodulation signal (Sanjuan et al., 1992). In another strain, JS314, a disaccharide containing 4-O-Me-Man has been identified (Carlson and Krishnaiah, 1992). Mesorhizobia, often symbionts of wild herbs and tree legumes, display 3-O-Me-Rha and the very rare 4-O-Me-GlcNAc residue (Fig. 1 A) (Zdorovenko et al., 2009). Recently 3-O-Me-Fuc has been found in Mesorhizobium huakuii strain S-52 capping as a terminal sugar an O-chain (Turska-Szewczuk and Russa, 2011). As part of the capsular polysaccharide from Sinorhizobium fredii HWG35 a dimethylated Gal residue (2,4-di-O-Me-Gal) has been identified (Rodriguez-Carvajal et al., 2005). While the sugar composition of the capsular polysaccharide (repeating units of one neutral and one acidic hexose) seems to influence the ability of this microorganism to form nitrogen-fixing nodules with Asiatic and/or American soybean cultivars, the methylation in particular does not seem to be of functional relevance for this process.
Fig 1. Typical methylated glycan structure.
(A) O-polysaccharide repeating unit of Mesorhizobium loti (Zdorovenko et al., 2009); (B) Polysaccharide repeating unit from the red seaweed Apophloea lyallii (Watt et al., 2002); (C) Polysaccharide repeating unit isolated from fruiting bodies of Lagernaria siceraria (Ghosh et al., 2009); (D) N-glycan from Dugesia japonica (Paschinger et al., 2011); (E) and (F) N-glycans from Arion lusitanicus (Gutternigg et al., 2004).
Another soil inhabitant, the recently newly described gram positive actinomycete Cryptosporangium mongoliense, shows also traces of 3-O-Me-Rha, but no other methylated sugars (Ara et al., 2011).
The plant pathogen Pseudomonas syringae pv. phaseolicola, a phytopathogen, contains 3-OMe-Rha within the O-polysaccharide of the lipopolysaccharide (Zdorovenko et al., 2001), whereas Erwinia carotovora ssp. carotovora GSPB 436 , another phytopathogenic bacterium, terminates the polymer main chain of the O-polysaccharide with 4-O-Me-Man (Senchenkova et al., 2003).
The presence of a methyl phosphate group at O-3 of α-mannopyranose, serves also as the signal for termination of the chain elongation in Klebsiella pneumoniae O3, Hafnia alvei PCM 1223 and Escherichia coli O9/O9a LPS (Kubler-Kielb et al., 2011).
The human pathogen Bacillus anthracis and related organisms are not easy to detect. Their methylated sugars (3-O-Me Rha, 2-O-Me-Rha, 2-O-Me-Fuc) on the surface of the spores help as an identification signal, which distinguishes them clearly from mammalian sugars. Here the methylated sugars are important targets for biodetection technology and enable a rapid detection (Fox et al., 2003).
Novel glycolipids were found as an old heritage, presumable from cyantobacteria, in the sediments of an Antarctic lake (Ace Lake). Docosanyl 3-O-Me-Rha and 3-O-Me-Xyl were identified as the methylated sugars (Sinninghe Damsté et al., 2001).
Fungi
The first description of methylation in fungi was in the early eighties, when the N-glycosylation pathway of the yeast- and mycelial-form cells of the dimorphic fungus Mucor rouxii was investigated. It could be shown that the high mannosidic structures contained 3-O-Me-Man residues, especially in the mycelia cells (Lederkremer and Parodi,1984). Those structures are resistant to α-mannosidase digest, but do not interfere in the cleavage by endoglycosidase H.
In extracellular polysaccharides from mould species (Mucor, Rhizopus, Rhizomucor, Absidia, Syncephalastrum, Thamnidium) 2-O-Me-Man residues were found to be involved in forming an immunodominant carbohydrate (De Ruiter et al., 1994).
A water-soluble extracellular polysaccharide from the edible basidomycete fungus Pleurotus ostreatoroseus Sing, which is known in the Orient for its medicinal properties, was identified to be a partially 3-O-methylated 1,4-linked α-D-galactopyranan (Rosado et al., 2002). While the fruiting bodies of Hericium erinaceus, a traditional Chinese medicinal fungus, contain a fucogalactan with 3-O-Me-Rha residues terminating the polymer main chain (Zhang et al., 2006), the fruiting bodies of Phellinus igniarius, another fungus used in Chinese medicine, contain a neutral polysaccharide with 3-O-Me-Gal as a constituent (Yang et al., 2007). So far, no hints are given on the medical properties or the immunological behaviour of the methylated glycans of these fungi.
Amoebae
Lysosomal enzymes isolated from the slime mould Dictyostelium discoideum display one or two methyl phosphate groups on high mannosidic N-glycans which contain 6 or 7 mannose residues (Gabel et al., 1984). It is speculated that the corresponding pathway for the biosynthesis of phosphorylated high mannosidic N-glycans must therefore be different to the one in higher organisms.
Algae and Plants
Methylation of sugars in plants is a rather frequent modification. Several sugars have been found to be involved, with Gal in algae and GalA in higher plants as the most common ones. Also the linkage of the methyl group is variable. Due to the availability of reasonable amounts of material, most of the studies could be performed using NMR-techniques.
Extracellular proteoglycan produced by Rhodella grisea (unicellular red alga) contains a high amount of 3-O-Me-Xyl (26 %) and traces of 4-O-Me-Xyl and 2,3-di-O-Me-Rha or Fuc (Capek et al., 2008). Recently, 6-O-methylation on non-terminal Man residues was determined on N-glycans of the red microalga Porphyridium sp. (Levy-Ontman et al., 2011). Especially red seaweed is quite well investigated. Galactans from Cryptonemia species contain 2-O-, 4-O- and 6-O-Me-Gal residues in their galactans besides other unusual forms of Gal (3,6-anhydro-D- and L-Gal and 3,6-anhydro-2-O-Me-L-Gal as a minor constituent). Furthermore the galactans are to some extend sulfated (Zibetti et al., 2009). Sulfated polysaccharides containing similar methylated Gal residues (2-O-Me-L-Gal and 6-O-Me-DGal) were also isolated from Georgiella confluens and Bostrychia montagnei (Kolender and Matulewicz, 2002; Duarte et al., 2002). The main acidic polysaccharides from Jania rubens carry besides the rare occurring sulfated or methoxylated 3,6-anhydro-L-Gal, also some minor fractions with 3-O-Me-D-Gal, 3-O-Me-L-Gal and 2,3-di-O-Me-D-Gal (Navarro and Stortz, 2008). The neutral galacto-glucurono-xylo-glyccan of Apophloea lyallii is built up by repeating units of [-α-D-2-O-Me-Gal-β1,4-D-GlcA-α1,3-D-Xyl-1,4-] (Fig. 1 B). In a minor fraction 3-O-Me-Gal was found (Watt et al., 2002).
Regarding mosses and ferns there is not much information. Just Selaginella apoda L., a lycophyte known as meadow spike moss, has been found to contain in 3-O-Me-Gal in its primary cell walls (Popper et al., 2001).
Although occurring in algae quite frequently, methylated Gal is a rare compound in higher plants. It has been found just as 3-O-Me-Gal in polysaccharides of Acanthus ebracteatus, a mangrove, and Salvia officinalis L., an herb. Both are used as medical plants in traditional medicine (Capek and Hříbalová, 2004; Hokputsa et al., 2004; Capek 2008).
Most other studies revealed methylated uronic acids in the polysaccharides of higher plants. 4-O-Me-GlcA is a constituent of xylans which have been found in the aqueous suspension of sugar beet pulp (Beta vulgaris), alkaline extracts of the pericarp of pricky pear seeds of Opuntia ficus-indica and Tamarix austromongolica, a fast growing tree used for prevention of wind erosion and control of desertification (Dinand and Vignon, 2001; Habibi et al., 2002; Sun et al., 2011).
In most of the investigated higher plants 6-O-Me-GlcA is the only or at least main methylated sugar present. It has been detected in the hot aqueous extract of the dietary fiber of green winter melon Benincasa hispida (Das et al., 2009a); the immunoenhancing heteropolysaccharide isolated from unripe green fruits of the aubergine (egg plant) Solenum melongena (Ojha et al., 2009); the polysaccharide of unripe green tomato (Lycopersicon esculentum) (Chandra et al., 2009); pectic polysaccharide from pods of green bean (Phaseolus vulgaris L.) (Patra et al., 2012); the corm of Amorphophallus campanulatus, where the GalA is in addition acylated on C4 (Das et al., 2009b) and the polysaccharide from the fruits of Lagenaria siceraria (Fig. 1 C), the calabash, a melon used for food but also as a container for liquids in earlier times. It is also known for its healthy effects, where the GalA is in addition O-acetylated on C3 (Ghosh et al., 2009). 6-O-Me-GalA was found together with 2-O-Me-Xyl in a xylan isolated from the stem of the same plant (Ghosh et al., 2008) and in a hot water extract of stems of Amaranthus gangeticus L., which is used mainly as an ornamental and vegetable plant but also in medical treatment (Sarkar et al., 2009). In a heteropolysaccharide extracted from the leaves of Catharanthus rosea, another plant with medical potential, originated from Madagaskar, 6-O-Me-GalA was found together with 6-OMe-Glc (Patra et al., 2010).
Another kind of sugar composition was determined for the pectic polysaccharide rhamnogalacturonan II from red wine. It is a nonasaccharide containing a backbone from made from Rha, Ara, Gal and apiose with branching of Ara, Rha and 2-O-Me-Fuc which is O-acetylated in some cases. A similar structure, a heptasaccharide also with 2-O-Me-Fuc was presented for Arabidopsis thaliana (Glushka et al., 2003).
Worms
Caenorhabditis elegans, the model worm, a non-parasitic soil nematode, is well investigated for its glycosylation pattern and the related modifications. It displays a rather complex glycan pattern including modifications with phosphorylcholine and a small amount of terminal linked 2-O-Me-Fuc in N- as well as in O-glycans (Guérardel et al., 2001; Haslam et al., 2002; Paschinger et al., 2008). To date, other sugars have not been found to be methylated in this species.
Another nematode, Toxocara canis, a parasite of dogs and other canid animals all over the world, contains two O-linked trisaccharide structures on the excretory-secretory antigens composed of terminal 2-O-Me-Fuc, a Gal residue, which is 4-O-methylated in some cases and a GalNAc linked to the protein. In Toxocara cati, a close relative of T. canis, the same trisaccharide is present in a dimethylated version (Khoo et al., 1991). Both structures are highly antigenic.
The annelid Alvinella pompejana, found strictly around deep-sea hydrothermal vents, lives in directly secreted tubes build from organo-mineral material with a carbohydrate part containing 2-O-Me-L-Fuc, 3-O-Me-L-Fuc and 2,4-di-O-Me-L-Fuc (Talmont and Fournet,1991). No information is given on the position of these sugars within the glycan chain.
The platyhelminth Dugesia japonica displays 3-O-Me-Man in terminal position on N-glycan cores or terminating Man5GlcNAc2-structures. A fucose residue, extended by a Gal residue and in some cases with a further methyl hexose, linked to the innermost GlcNAc is present in some of the glycans and seems not to influence the methylation status (Fig. 1 D) (Paschinger et al., 2011; Natsuka et al., 2011).
More studies are necessary on nematodes and other worms to make a statement whether different kinds of methylation patterns (methylated Fuc and/or methylated hexoses) are characteristic features for different phyla of worms.
Molluscs
Methylation in snails is a frequent modification in N- as well as in O-glycans in most of the investigated species. So far the methyl groups have been found mainly linked to hexoses: Man and Gal, but also reports on methylated GlcNAc and GlcA are available. For the first time methylation in gastropods (3-O-Me-Man, 3-O-Me-Gal) was reported in 1977 in the hemocyanin, the oxygen-carrier of arthoropods and molluscs, of Helix pomatia (Hall et al., 1977). Later analysis of the hemocyanin of Lymnea stagnalis revealed as the lowest molecular mass N-glycan a core made from two GlcNAc and three Man residues containing one Xyl linked β1,2 to the β-Man and 3-O-bound methyl groups linked to the two terminal Man residues (van Kuik et al., 1986). Terminal 3-O-Me-Gal residues on larger structures were confirmed for Lymnea stagnalis and found on a diphosphonopentaosylceramide of the sea hare Aplysia kurodai (van Kuik et al., 1987, Araki et al., 1986). The hemocyanins of Rapana thomasiana and of the functional unit RvH1 of Rapana venosa also were found to contain 3-O-Me-Gal, the latter together with some terminal 3-O-Me-GlcNAc residues (Stoeva et al., 1995; Dolashka-Angelova et al., 2003).
Analysis of other molluscs revealed more variations: Tridacnin, a lectin isolated from the marine clam Hippopus hippopus, contains terminal 6-O-Me-Man and terminal dimethylated Man residues (Puanglarp et al., 1995). A novel glycosphingolipid from spermatozoa of the fresh water bivalve Hyriopsis schlegelii was identified carrying 4-O-Me-GlcA on its glycan (Hori et al., 1983).
At that time all methylated sugars described in molluscs were determined in terminal position of the glycans. In the mid-nineties, due to the increasing technical opportunities, it became possible to identify also minor compounds of the glycan spectrum which are present in very low amounts. Again, the hemocyanin of Helix pomatia was under investigation using 500/600-MHz 1H-NMR spectroscopy. With the advanced technology 21 more N-glycan structures could be determined in detail. Those showed a high degree of methylated Gal residues, mainly 3-O-Gal, and also methylation of Gal on the C4 was shown. In addition for the first time in molluscs, some of the methylated Gal residues were found not to be in terminal position of the glycan, but were elongated by methylated Gal residues (Lommerse et al.,1997).
Further studies on gastropods revealed mostly similar patterns. The hemocyanin of Haliotis tuberculata was found to contain 3-O-Me-Man and 3-O-Me-Gal and the major Biomphalaria glabrata shell matrix protein was shown to display a core structure terminated by two 3-O-Me-Man residues (Marxen et al., 2003; Idakieva et al., 2004). In a later study the N-glycan spectrum of hemolymph glycoproteins of the latter organism was analysed showing a broad pattern of different structures containing 3-O-Me-Man and 3-O-Me-Gal as methylated constituents. While 3-O-Me-Man was determined as terminal sugar of some antennae a methylated hexose elongated by an amino sugar and one hexose is not further determined (Lehr et al., 2007). In agreement with the data of Lommerse et al., 1997 it can be speculated that the 3-O-Me-Man is a terminating signal for further elongation whereas the 3-O-Me-Gal may be further elongated. An elongation of methylated sugars seems to be a very rare occasion in gastropods, as these two reports are so far the only published data. However, we also had some hints on internal methylated sugars in large glycans of Arion lusitanicus but the amounts were too low to determine the structures in detail.
In the course of our own studies we analysed the N-glycosylation patterns of whole tissue extracts derived from Arion lusitanicus, Limax maximus, Cepaea hortensis, Planorbarius corneus, Arianta arbustorum and Achatina fulica and found very frequently terminal located 3-O-Me-Man residues on high mannosidic and paucimannosidic structures and to a lesser extend methylated Gal on larger glycans (Fig. 1 E and F) (Gutternigg et al., 2004; Gutternigg et al., 2007). The O-glycosylation patterns of the same species and additionally of Biomphalaria glabrata, Clea helena and Helix pomatia revealed a high amount of methylated Gal and some methylated Man (Stepan et al., unpublished results). The methylated Man was identified as exclusively 3-O-methylated whereas the galactose occurs 3-O- as well as 4-O-methylated (Stepan et al., 2010). The distribution of the two types of methylated Gal residues varies within the different species. While 3-O-Me-Man occurs in every investigated snail. 3-O-Me-Gal is absent in Achatina fulica and Arion lusitanicus; 4-O-Me-Gal is missing in Arianta arbustorum and Biomphalaria glabrata. It is not possible to correlate the kind and amount of methylation with the occurrence of the snail (water or land living species) or with appearance (shell carrying or slug).
The N-glycan structure of the egg extracellular coat of the mollusc bivalve Unio elongatulus was determined as Glc1Man9GlcNAc2 , containing no methyl group (Di Patrizi et al., 2001) and the major egg glycolipoproteins from the perivitellin fluid of the apple snail Pomacea canaliculata also do not display methyl groups (Dreon et al., 2004). This correlates with our own observations where we did not find any methylation in eggs of Arion lusitanicus (Gutternigg et al., 2004). This may be due to different expression levels of methyltransferase(s) in different developmental stages. More studies are needed to confirm this theory.
Methyltransferases
Knowing so many glycan structures modified by methyl groups it is rather surprising that most of the corresponding methyltransferases are still unknown. There are only few data on methyltransferases acting on sugar residues. All these data have been generated from bacterial sources, mainly mycobacterial strains. For Mycobacterium smegmatis a methyltransferase (mtf1) was identified which methylates Rha at the C3 position. This methylation is a requirement for any further methylation of the glycopeptidolipid (Weisman and Ballou,1984; Patterson et al., 2000). A second putative gene (mtf2) located in the same gene cluster was identified to methylate the fatty acid of the glycopeptidolipid rather than the sugars (Jeevarajah et al., 2002). Furthermore also the two genes responsible for the methylation of Rha in C4 and C2 position, rmt4 and rmt2 respectively, have been identified and their sequentially action – first 4-O-methylation, then 2-O-methylation, both after the initial 3-O-methylation – was shown (Jeevarajah et al., 2004). One more bacterial methyltransferase transferring a methyl group to the C2 position of a galacturonosyl residue of a teichuronic type polyssaccharide was identified in Rhizobium meliloti (Ruiz and Ugalde,1998).
It is not completely clear if the methylation is always performed as the final modification step after the glycan has been biosynthesized (directly from S-adenosylmethionine as donor), or if there is also the option that a glycosyltransferase (in the case of mycobacteria the rhanmosyltransferase) is able to transfer the already methylated sugar residue (from a nucleotide methyl sugar) onto the growing oligosaccharide chain (Miyamoto et al., 2006; Schorey and Sweet, 2008).
Function
Structural analysis of glycans and their modifications is carried out by a broad range of analytical methods, including gas chromatography with different derivatisation protocols, liquid-chromatography with or without prior labelling often followed by mass spectrometry and several NMR techniques. (For more information on methodical details see Geyer and Geyer 1994; Gerwig and Vliegenthart 2000; Mechref et al. 2009, North et al. 2009; Stepan et al. 2011). These methods are often challenging when the material is limited and the glycans are only semi-purified, but the analysis of the function of a small modification of the glycan is even more difficult.
In some of the above mentioned studies of bacteria, the glycosylation abilities are correlated with cell signaling, recognition or adhesion function. The mycobacterial glycopeptidolipids are involved in pathogenicity, the immunological response to infection and colony morphology. The immune response of a host via Toll-like receptor 2 is influenced by the glycopeptidolipid profile of Mycobacterium intracellulare and M. avium, for sure by a modification by acetylation, but there are also some hints that methylation on Rha may play a role for the effect (Sweet at al., 2008; Naka et al., 2011).
It is obvious that in plants Gal (for algae) and GalA (for higher plants) are the primary sugars decorated with methyl groups. Even when many of the investigated plants are known for some medical effect no correlation with this modification of the glycan has been documented so far. Perhaps methylation may occur also in many other plants but they are not investigated in detail as they do not show medical relevance.
In fungi (diverse mould species) the 2-O-Me-Man residues decorated glycan reacts with rabbit IgG antibodies. After removal of the terminal 2-O-Me-Man residues by an exo-α-D-mannosidase prepared from Trichoderma harzianum, the antigenicity is abolished (De Ruiter et al., 1994). Other investigated fungi are known for their medical usability, but no correlation of this attribute to methylation of glycan chains is given.
Glycosylation plays an important role in the communication between helminth parasites such as Schistosomes and their snail host. For example imitating the immunogenic surface coat of the parasite by synthetic sugar-albumin conjugates a down-regulation of extracellular-signal regulated kinase activities, protein kinase C activities and phagocytosis can be detected (Plows et al., 2005). Methylated sugars have not been used for such studies so far. Comparison of the N-glycosylation patterns of glycoproteins derived from the hemolymph of Biomphalaria glabrata strains which are different susceptible to Schistosoma mansoni infection, showed that Xyl and terminal Fuc residues are responsible for the cross reactivity with schistosomal glycconjugates. The methyl hexoses, present on snail glycans, seem not to be important for the antigenicity of the structures (Lehr et al., 2010).
Glycan chains of the functional unit RtH2-e from Rapana thomasiana hemocyanin, some of them contain terminal 3-O-Me-Gal, are involved in the antigenicity of this protein (Siddiqui et al., 2007). These experiments were carried out with a mixture of glycans of Rapana, so the influence of methylation cannot be determined. But immunological cross reaction between α-macroglobulin and hemocyanin, both occurring in the hemolymph of Helix pomatia, seems to be related to the carbohydrate content of these two glycoproteins, especially to Xyl and 3-OMe-Gal (Siddiqui et al., 2009). This is the only demonstration that a methylated structure is involved in an antigenic recognition event.
Conclusion
Methylation of sugar residues is a rare modification. As far as we know, mammals do not carry this modification on their glycans, but many other organisms display methylated structures which are heterogenous in terms of involved sugars as well as in terms of the linkage of the methyl group. Methylation on hexose residues is rather frequent but the decorated sugar may also be a Fuc, Rha, Xyl, amino sugar or uronic acid.
The type of methylation pattern correlates to some extend with the species. For typical examples see Fig. 1. Whereas in bacteria and plants the methylated sugars occur often within the oligosaccharide chain, in all other organisms the methylated sugars are mainly in terminal position. Especially 3-O-Me-Man is terminally located and seems to be a stop signal for a further elongation of the glycan chain. This can be seen in plants as well as in gastropods. Some methylated monosaccharides are very frequent in some species, others are rare in all phyla. In molluscs, Man and Gal are the typically methylated residues. In plants Gal, together with uronic acids (GalA and GlcA), are very frequent; however also Xyl and Fuc are present. Bacteria show the whole spectrum of methylated sugar residues, even when methylated hexoses (Man, Gal and Glc) are only minor compounds (Tab.1). For all other organisms the data are too limited to make a statement on frequency.
Table 1. Methylated sugars analysed in detail so far.
No methylation has been found so far in arthropods. However, it has to be kept in mind that only a few species are well investigated: those insects which are used as expression systems, mainly lepidoptera, some hymenoptera species (wasp, bee, hornet) which are of medical relevance due to their venom and Drosophila melanogaster, the model insect. All other insects and other arthropods still wait for the elucidation of their biochemical and molecular biological properties. Let us see which surprises are hidden there!
Also the determination and characterisation of methyltransferases as well as detailed investigation of the function of the methylation on sugars is still an open field for further investigation. Even when the current data are rather limited and do not allow valid predictions, a function of such a modification, which alters the chemical behaviour of a sugar rendering it more hydrophobic, is very likely. The frequently occurring “methyl sugar”, fucose (a deoxyhexose) is indeed an important structural feature in many recognition events.
Acknowledgements
Our studies on snails were supported by the Austrian Science Fund (FWF) [P20393-B11 and P22118-B20]. I want to thank Prof. Iain B.H. Wilson for reading the manuscript and Dr. Herwig Stepan for fruitful discussions.
Abbreviations
- Ara
arabinose
- Fuc
fucose
- Gal
galactose
- GalA
galacturonic acid
- Glc
glucose
- GlcA
glucuroonic acid
- GlcNAc
N-acetylglucosamine
- Hex
hexose
- Man
mannose
- Me
methyl
- Rha
rhamnose
- Tal
talose
- Xyl
xylose
Footnotes
Dedicated to Prof. Dr. Rudolf Geyer on the occasion of his 65th birthday
References
- Ara I, Tsetseg B, Daram D, Suto M, Ando K. Cryptosporangium mongoliense sp. nov. isolated from soil. Int. J. Syst. Evol. Microbiol. 2011 doi: 10.1099/ijs.0.038307-0. IJSEM Papers in Press. Published December 2, 2011 as doi:10.1099/ijs.0.038307-0. [DOI] [PubMed] [Google Scholar]
- Araki S, Satake M, Ando S, Hayashi A, Fujii N. Characterization of a diphosphopentaosylceramide containing 3-O-methylgalactose from the skin of Aplysia kurodai (sea hare) J. Biol. Chem. 1986;261:5138–5144. [PubMed] [Google Scholar]
- Barnes P. Pathophysiology of allergic inflammation. Immunol. Rev. 2011;242:31–50. doi: 10.1111/j.1600-065X.2011.01020.x. [DOI] [PubMed] [Google Scholar]
- Garaud S, Youinou P, Renaudineau Y. DNA methylation and B-cell autoreactivity. Adv. Exp. Med. Biol. 2011;711:50–60. doi: 10.1007/978-1-4419-8216-2_5. [DOI] [PubMed] [Google Scholar]
- Branscombe Miranda T, Lowenson JD, Clarke S. A new type of protein methylation activated by tyrphostin A25 and vanadate. FEBS Lett. 2004;577:181–186. doi: 10.1016/j.febslet.2004.09.080. [DOI] [PubMed] [Google Scholar]
- Capek P, Hříbalová V. Water-soluble polysaccharides from Salvia officinalis L. possessing immunomodulatory activity. Phytochem. 2004;65:1983–1992. doi: 10.1016/j.phytochem.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Capek P. An arabinogalactan containing 3-O-methyl-D-galactose residues isolated from the aerial parts of Salvia officinalis L. Carbohydr. Res. 2008;343:1390–1393. doi: 10.1016/j.carres.2008.03.026. [DOI] [PubMed] [Google Scholar]
- Capek P, Matulová M, Combourieu B. The extracellular proteoglycan produced by Rhodella grisea. Int. J. Biol. Macromol. 2008;43:390–393. doi: 10.1016/j.ijbiomac.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Carlson RW, Krishnaiah BS. Structures of the oligosaccharides obtained from the core regions of the lipopolysaccharides of Bradyrhizobium japonicum 61A101c and its symbiotically defective lipopolysaccharide mutant, JS314. Carbohydr. Res. 1992;231:205–219. doi: 10.1016/0008-6215(92)84020-s. [DOI] [PubMed] [Google Scholar]
- Chandra K, Ghosh K, Ojha AK, Islam SS. Chemical analysis of a polysaccharide of unripe (green) tomato (Lycopersicon esculentum) Carbohydr. Res. 2009;344:2188–2194. doi: 10.1016/j.carres.2009.08.029. [DOI] [PubMed] [Google Scholar]
- Das D, Mondal S, Roy SK, Islam SS. Structural characterization of dietary fiber of green chalcumra (Benincasa hispida) fruit by NMR spectroscopic analysis. Nat. Prod. Commun. 2009a;4:547–552. [PubMed] [Google Scholar]
- Das D, Mondal S, Roy SK, Maiti D, Bhunia B, Maiti TK, Islam SS. Isolation and characterization of a heteropolysaccharide from the corm of Amorphophallus campanulatus. Carbohydr. Res. 2009b;344:2581–2585. doi: 10.1016/j.carres.2009.09.025. [DOI] [PubMed] [Google Scholar]
- Demarteau-Ginsburg H, Lederer E. On the chemical structure of mycoside B. Biochem. Biophys. Acta. 1963;70:442–451. doi: 10.1016/0006-3002(63)90774-1. [DOI] [PubMed] [Google Scholar]
- De Ruiter GA, Van Bruggen-Van der Lugt AW, Mischnick P, Smid P, Van Boom JH, Notermans SHW, Rombouts FM. 2-O-Methyl-D-mannose residues are immunodominat in extracellular polysaccharides of Mucor racemosus and related molds. J. Biol. Chem. 1994;269:4299–4306. [PubMed] [Google Scholar]
- Dinand E, Vignon MR. Isolation and NMR characterization of a (4-O-methyl-D-glucurono)-D-xylan from sugar beet pulp. Carbohydr. Res. 2001;330:285–288. doi: 10.1016/s0008-6215(00)00273-1. [DOI] [PubMed] [Google Scholar]
- Di Patrizi L, Capone A, Focarelli R, Rosati F, Gutiérrez Gallego R, Gerwig GJ, Vliegenthart JFG. Structural characterization of the N-glycans of gp273, the ligand for sperm-egg interaction in the mollusc bivalve Unio elongatulus. Glycoconj. J. 2001;18:511–518. doi: 10.1023/a:1019617728660. [DOI] [PubMed] [Google Scholar]
- Dolashka-Angelova P, Beck A, Dolashki A, Beltramini M, Stevanovic S, Salvato B, Voelter W. Characterization of the carbohydrate moieties of the functional unit RvH1-a of Rapana venosa haemocyanin using HPLC/electrospray ionization MS and glycosidase digestion. Biochem. J. 2003;374:185–192. doi: 10.1042/BJ20030291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreon MS, Heras H, Pollero RJ. Characterization of the major egg glycolipoproteins from the perivitellin fluid of the apple snail Pomacea canaliculata. Mol. Reprod. Dev. 2004;68:359–364. doi: 10.1002/mrd.20078. [DOI] [PubMed] [Google Scholar]
- Duarte MER, Noseda MD, Cardoso MA, Tulio S, Cerezo AS. The structure of a galactan sulphate from the red seaweed Bostrychia montagnei. Carbohydr. Res. 2002;337:1137–1144. doi: 10.1016/s0008-6215(02)00095-2. [DOI] [PubMed] [Google Scholar]
- Forsberg LS, Bhat UR, Carlson RW. Structural characterization oft he O-antigenic polysaccharide of the lipopolysaccharide from Rhizobium etli strain CE3. A unique O-acetylated glycan of discrete size, containing 3-O-methyl-6-deoxy-L-talose and 2,3,4-tri-O-methyl-L-fucose. J. Biol. Chem. 2000;275:18851–18863. doi: 10.1074/jbc.M001090200. [DOI] [PubMed] [Google Scholar]
- Fox A, Stewart GC, Waller LN, Fox KF, Harley WM, Price RL. Carbohydrates and glycoproteins of Bacillus anthracis and related bacilli: targets for biodetection. J. Microbiol. Meth. 2003;54:143–152. doi: 10.1016/s0167-7012(03)00095-2. [DOI] [PubMed] [Google Scholar]
- Gabel CA, Costello CE, Reinhold VN, Kurz L, Kornfeld S. Identification of methylphosphomannosyl residues as components of the high mannose oligosaccharides of Dictyostelium discoideum glycoproteins. J. Biol. Chem. 1984;259:13762–13769. [PubMed] [Google Scholar]
- Garaud S, Youinou P, Renaudineau Y. DNA methylation and B-cell autoreactivity. Adv. Exp. Med. Biol. 2011;711:50–60. doi: 10.1007/978-1-4419-8216-2_5. [DOI] [PubMed] [Google Scholar]
- Gerwig GJ, Vliegenthart JF. Analysis of glycoprotein-derived glycopeptides. EXS. 2000;88:159–186. doi: 10.1007/978-3-0348-8458-7_11. [DOI] [PubMed] [Google Scholar]
- Geyer H, Geyer R. Saccharide linkage analysis using methylation and other techniques. Methods Enzymol. 1994;230:86–108. doi: 10.1016/0076-6879(94)30009-7. [DOI] [PubMed] [Google Scholar]
- Ghosh K, Chandra K, Roy SK, Mondal S, Maiti D, Das D, Ojha AK, Islam SS. Structural studies of a methyl galacturonosyl-methoxyxylan isolated from the stem of Lagenaria siceraria (Lau) Carbohydr. Res. 2008;343:341–349. doi: 10.1016/j.carres.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Ghosh K, Chandra K, Ojha AK, Sarkar S, Islam SS. Structural identification and cytotoxic activity of a polysaccharide from the fruits of Lagenaria siceraria (Lau) Carbohydr. Res. 2009;344:693–698. doi: 10.1016/j.carres.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Glushka JN, Terrell M, York WS, O’Neill MA, Gucwa A, Darvill AG, Albersheim P, Prestegard JH. Primary structure of the 2-O-methyl-α-L-fucose-containing side chain of the pectic polysaccharide, rhamnogalacturonan II. Carbohydr. Res. 2003;338:341–352. doi: 10.1016/s0008-6215(02)00461-5. [DOI] [PubMed] [Google Scholar]
- Guérardel Y, Balanzino L, Maes E, Leroy Y, Coddeville B, Oriol R, Strecker G. The nematode Caenorhabditis elegans synthesizes unusual O-linked glycans: identification of glucose substituted mucin-type O-glycans and short chondroitin-like oligosaccharides. Biochem. J. 2001;357:167–182. doi: 10.1042/0264-6021:3570167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutternigg M, Ahrer K, Grabher-Meier H, Bürgmayr S, Staudacher E. Neutral N-glycans of the gastropod Arion lusitanicus. Eur. J. Biochem. 2004;271:1348–1356. doi: 10.1111/j.1432-1033.2004.04045.x. [DOI] [PubMed] [Google Scholar]
- Gutternigg M, Bürgmayr S, Pöltl G, Rudolf J, Staudacher E. Neutral N-glycan patterns of the gastropods Limax maximus, Cepaea hortensis, Planorbarius corneus, Arianta arbustorum and Achatina fulica. Glycoconj. J. 2007;24:475–489. doi: 10.1007/s10719-007-9040-5. [DOI] [PubMed] [Google Scholar]
- Habibi Y, Mahrouz M, Vignon MR. Isolation and structure of D-xylans from pericarp seeds of Opuntia ficus-indica prickly pear fruits. Carbohydr. Res. 2002;337:1593–1598. doi: 10.1016/s0008-6215(02)00186-6. [DOI] [PubMed] [Google Scholar]
- Hall RL, Wood EJ, Kamberling JP, Gerwig GJ, Vliegenthart JFG. 3-OMethyl sugars as constituents of glycoproteins. Biochem. J. 1977;165:173–176. doi: 10.1042/bj1650173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam S, Gems D, Morris HR, Dell A. The glycomes of Caenorhabditis elegans and other model organisms. Biochem. Soc. Symp. 2002;69:117–134. [PubMed] [Google Scholar]
- Hokputsa S, Harding SE, Inngjerdingen K, Jumel K, Michaealsen TE, Heinze T, Koschella A, Paulsen BS. Bioactive polysaccharides from the stems of the Thai medicinal plant Acanthus ebracteatus: their chemical and physical features. Carbohydr. Res. 2004;339:753–762. doi: 10.1016/j.carres.2003.11.022. [DOI] [PubMed] [Google Scholar]
- Hori T, Sugita M, Ando S, Tsukada K, Shiota K, Tsuzuki M, Itasaka O. Isolation and characterization of a 4-O-methylglucuronic acid-containing glycosphinogolipid from spermatozoa of a fresh water bivalve, Hyriopsis schlegelii. J. Biol. Chem. 1983;258:2239–2245. [PubMed] [Google Scholar]
- Idakieva K, Stoeva S, Voelter W, Gielens C. Glycosylation of Rapana thomasiana hemocyanin. Comparison with other prosobranch (gastropod) Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004;138:221–228. doi: 10.1016/j.cbpc.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Jackson M, Brennan PJ. Polymethylated polysaccharides from Mycobacterium species revisted. J. Biol. Chem. 2009;284:1949–1953. doi: 10.1074/jbc.R800047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeevarajah D, Patterson JH, McConville MJ, Billman-Jacobe H. Modification of glycopeptidolipids by an O-methyltransferase of Mycobacterium smegmatis. Microbiology. 2002;148:3079–3087. doi: 10.1099/00221287-148-10-3079. [DOI] [PubMed] [Google Scholar]
- Jeevarajah D, Patterson JH, Taig E, Sargeant T, McConville MJ, Billman-Jacobe H. Methylation of GPLs in Mycobacterium smegmatis and Mycobacterium avium. J. Bacteriol. 2004;186:6792–6799. doi: 10.1128/JB.186.20.6792-6799.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller-Schierlein W, Sauerbier J, Vogler U, Zähner H. Metabolits of microorganisms. Aranciamycin. Helv. Chim. Acta. 1970;53:779–789. doi: 10.1002/hlca.19700530413. [DOI] [PubMed] [Google Scholar]
- Khoo KH, Maizels RM, Page AP, Taylor GW, Rendell NB, Dell A. Characterization of nematode glycoproteins: the major O-glycans of Toxocara excretory-secretory antigens are O-methylated trisaccharides. Glycobiology. 1991;1:163–171. doi: 10.1093/glycob/1.2.163. [DOI] [PubMed] [Google Scholar]
- Khoo K-H, Chatterjee D, Dell A, Morris HR, Brennan PJ, Draper P. Novel O-methylated terminal glucuronic acid characterizes the polar glycopeptidolipids of Mycobacterium habana strain TMC 5135. J. Biol. Chem. 1996;271:12333–12342. doi: 10.1074/jbc.271.21.12333. [DOI] [PubMed] [Google Scholar]
- Kolender AA, Matulewicz MC. Sulfated polysacccharides from the red seaweed Georgiella confluens. Carbohydr. Res. 2002;337:57–68. doi: 10.1016/s0008-6215(01)00283-x. [DOI] [PubMed] [Google Scholar]
- Kubler-Kielb J, Whitfield C, Katzenellenbogen E, Vinogradov E. Identification of the methyl phosphate substituent at the non-reducing terminal mannose residue of the O-specific polysaccharides of Klebsiella pneumonia O3, Hafnia alvei PCM 1223 and Escherichia coli O9/O9a LPS. Carbohydr. Res. 2011;347:186–188. doi: 10.1016/j.carres.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederkremer GZ, Parodi AJ. 3-O-Methylation of mannose residues. A novel reaction in the processing of N-linked oligosaccharides occurring in Mucor rouxii. J Biol Chem. 1984;259:12514–12518. [PubMed] [Google Scholar]
- Lehr T, Geyer H, Maaß K, Doenhoff MJ, Geyer R. Structural characterization of N-glycans from the freshwater snail Biomphalaria glabrata cross-reacting with Schistosoma mansoni glycoconjugates. Glycobiology. 2007;17:82–103. doi: 10.1093/glycob/cwl048. [DOI] [PubMed] [Google Scholar]
- Lehr T, Frank S, Natsuka S, Geyer H, Beuerlein K, Doenhoff MJ, Hase S, Geyer R. N-Glycosylation patterns of hemolymph glycoproteins from Biomphalaria glabrata strains expressing different susceptibility to Schistosoma mansoni infection. Exp. Parasitol. 2010;126:592–602. doi: 10.1016/j.exppara.2010.06.031. [DOI] [PubMed] [Google Scholar]
- Levy-Ontman O, Arad S, Harvey DJ, Parosns TB, Fairbanks A, Tekoah Y. Unique N-glycan moieties of the 66-kDa cell wall glycoprotein from the red microalga Porphyridium sp. J. Biol. Chem. 2011;286:21340–21352. doi: 10.1074/jbc.M110.175042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommerse JPM, Thomas-Oates JE, Gielens C, Préaux G, Kamerling JP, Vliegenthart JFG. Primary structure of 21 novel monoantennary and diantennary N-linked carbohydrate chains from αD-hemocanain of Helix pomatia. Eur. J. Biochem. 1997;249:195–222. doi: 10.1111/j.1432-1033.1997.00195.x. [DOI] [PubMed] [Google Scholar]
- Marxen JC, Nimtz M, Becker W, Mann K. The major soluble 19.6 kDa protein of the organic shell matrix of the freshwater snail Biomphalaria glabrata is an N-glycosylated dermatopontin. Biochim. Biophys. Acta. 2003;1650:92–98. doi: 10.1016/s1570-9639(03)00203-6. [DOI] [PubMed] [Google Scholar]
- McAlpine JB, Corcoran JW, Egan RS. Scopamycin. II. Identification of the sugar moiety of scopamycin A as 2-O-methyl-L-rhamnose. J. Antibiotics. 1971;XXIV:51–56. [PubMed] [Google Scholar]
- Mechref Y, Kang P, Novotny MV. Solid-phase permethylation for glycomic analysis. Methods Mol. Biol. 2009;534:53–64. doi: 10.1007/978-1-59745-022-5_4. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Mukai T, Nakata N, Maeda Y, Kai M, Naka T, Yano I, Makino M. Identification and characterization of the genes involved in glycosylation pathways of mycobacterial glycopeptidolipid biosynthesis. J. Bacteriol. 2006;188:86–95. doi: 10.1128/JB.188.1.86-95.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka T, Nakata N, Maeda S, Yamamoto R, Doe M, Mizuno S, Niki M, Kobayashi K, Ogura H, Makino M, Fujiwara N. Structure and host recognition of serotype 13 glycopeptidolipid from Mycobactrium intracellulare. J. Bacteriol. 2011;193:5766–5774. doi: 10.1128/JB.05412-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsuka S, Hirohata Y, Nakakita S, Sumiyoshi W, Hase S. Structural analysis of N-glycans of the planarian Dugesia japonica. FEBS J. 2011;278:452–460. doi: 10.1111/j.1742-4658.2010.07966.x. [DOI] [PubMed] [Google Scholar]
- Navarro DA, Stortz CA. The system of xylogalactans from the red seaweed Jania rubens (Corallinales, Rhodophyta) Carbohydr. Res. 2008;343:2613–2622. doi: 10.1016/j.carres.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Noel KD, Box JM, Bonne VJ. 2-O-Methylation of fucosyl residues of a rhizobial lipopolysaccharide is increased in response to host exudate and is eliminated in a symbiotically defective mutant. Appl. Environ. Microbiol. 2004;70:1537–1544. doi: 10.1128/AEM.70.3.1537-1544.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North SJ, Hitchen PG, Haslam SM, Dell A. Mass spectrometry in the analysis of N-and O-linked glycans. Curr. Opin. Struct. Biol. 2009;19:498–506. doi: 10.1016/j.sbi.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojha AK, Chandra K, Ghosh K, Bhunia B, Maiti TK, Islam SS. Structural analysis of an immunoenhancing heteropolysaccharide isolated from the green (unripe) fruits of Solenum melongena (Brinjal) Carbohydr. Res. 2009;344:2357–2363. doi: 10.1016/j.carres.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Paschinger K, Gutternigg M, Rendić D, Wilson IBH. The N-glycosylation pattern of Caenorhabditis elegans. Carbohydr. Res. 2008;343:2041–2049. doi: 10.1016/j.carres.2007.12.018. [DOI] [PubMed] [Google Scholar]
- Paschinger K, Razzazi-Fazeli E, Furukawa K, Wilson IBH. Presence of galactosylated core fucose on N-glycans in the planaria Dugesia japonica. J. Mass Spectrom. 2011;46:561–567. doi: 10.1002/jms.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra S, Maity KK, Bhunia SK, Dey B, Das D, Mondal S, Bhunia B, Maiti TK, Islam SS. Structural characterization of an immunoenhancing heteropolysaccharide isolatd from hot water extract of the fresh leaves of Catharanthus rosea. Carbohydr. Polymers. 2010;81:584–591. [Google Scholar]
- Patra P, Das D, Behera B, Maiti TK, Islam SS. Structure elucidation of an immunoenhancing pectic polysaccharide isolated from aqueous extract of pods of green bean (Phaseolus vulgaris L.) Carbohydr. Polymers. 2012;87:2169–2175. [Google Scholar]
- Patterson JH, McConville MJ, Haites RE, Coppel RL, Billman-Jacobe H. Identification of a methyltransferase from Mycobacterium smegmatis involved in glycopeptidolipid synthesis. J. Biol. Chem. 2000;275:24900–24906. doi: 10.1074/jbc.M000147200. [DOI] [PubMed] [Google Scholar]
- Peters AHFM, Schübeler D. Methylation of histones: playing memory with DNA. Curr. Opin. Cell Biol. 2005;17:230–238. doi: 10.1016/j.ceb.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Plows LD, Cook RT, Davies AJ, Walker AJ. Carbohydrates that mimic schistosome surface coat components affect ERK and PKC signaling in Lymnea stagnalis haemocytes. Int. J. Parasitol. 2005;35:293–302. doi: 10.1016/j.ijpara.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Poetsch AR, Clarke C. Transcriptional regulation by DNA methylation. Cancer Treatment Rev. 2011;37:S8–S12. doi: 10.1016/j.ctrv.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Popper ZA, Sadler IH, Fry SC. 3-O-Methyl-D-galactose residues in lycophyte primary cell walls. Phytochem. 2001;57:711–719. doi: 10.1016/s0031-9422(01)00140-6. [DOI] [PubMed] [Google Scholar]
- Puanglarp N, Oxley D, Currie GJ, Bacic A, Craik DJ, Yellowlees D. Structure of the N-linked oligosaccharides from tridacnin, a lectin found in the haemolymph of the giant clam Hippopus hippopus. Eur. J. Biochem. 1995;232:873–880. [PubMed] [Google Scholar]
- Renier A, Maillet F, Fardoux J, Poinsot VR, Giraud E, Nouwen N. Photosynthetic Bradyrhizobium Sp. Strain ORS285 synthesizes 2-O-methylfucosylated lipochitooligosaccharides for nod gene? Dependent interaction with Aeschynomene plants. Mol. Plant Microbe Interact. 2011;24:1440–1447. doi: 10.1094/MPMI-05-11-0104. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Carvajal MA, Rodrigues JA, Soria-Diaz ME, Tejero-Mateo P, Buendia-Claveria A, Gutiérrez R, Ruiz-Sainz JE, Thomas-Oates J, Gil-Serrano AM. Structural analysis of the capsular polysaccharide from Sinorhizobium fredii HWG35. Biomacromol. 2005;6:1148–1456. doi: 10.1021/bm049264u. [DOI] [PubMed] [Google Scholar]
- Rosado RR, Carbonero ER, Kemmelmeier C, Tischer CA, Gorin PAJ, Iacomini M. A partially 3-O-methylated (1-4)-linked α-D-galactan and α-D-mannan from Pleurotus ostreatoroseus Sing. FEMS Microbiol Lett. 2002;212(2):261–265. doi: 10.1111/j.1574-6968.2002.tb11276.x. [DOI] [PubMed] [Google Scholar]
- Ruiz OA, Ugalde RA. Partial characterization and photolabeling of a Rhizobium meliloti polysaccharide methyltransferase with S-adenosylmethionine. Int. Microbiol. 1998;1:225–230. [PubMed] [Google Scholar]
- Salozhin SV, Prokhorchuk EB, Georgiev GP. Methylation of DNA – one of the major epigenetic markers. Biochemistry (Moscow) 2005;70:525–532. doi: 10.1007/s10541-005-0146-8. [DOI] [PubMed] [Google Scholar]
- Sanjuan J, Carlson RW, Spaink HP, Bhat UR, Barbour WM, Glushka J, Stacey G. A 2-O-methylfucose moiety is present in the lipo-oligosaccharide nodulation signal of Bradyrhizobium japonicum. Proc. Natl. Acad. Sci. USA. 1992;89:8789–8793. doi: 10.1073/pnas.89.18.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar R, Nandan CK, Mandal S, Patra P, Das D, Islam SS. Structural characterization of a heteropolysaccharide isolated from hot water extract of the stems of Amaranthus tricolor Linn. (Amaranthus gangeticus L.) Carbohydr. Res. 2009;344:2412–2416. doi: 10.1016/j.carres.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Schorey JS, Sweet L. The mycobacterial glycopeptidolipids: structure, function, and their role in pathogenesis. Glycobiology. 2008;18:832–841. doi: 10.1093/glycob/cwn076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz WA. DNA methylation in urological malignancies (review) Int. J. Oncol. 1998;13:151–167. [PubMed] [Google Scholar]
- Senchenkova SN, Knirel YA, Shashkov AS, Ahmed M, Mavridis A, Rudolph K. Structure of the O-polysaccharide of Erwinia carotovora ssp. carotovora GSPB 436. Carbohydr. Res. 2003;338:2025–2027. doi: 10.1016/s0008-6215(03)00326-4. [DOI] [PubMed] [Google Scholar]
- Siddiqui NI, Idakieva K, Demarsin B, Doumanova L, Compernolle F, Gielens C. Involvement of glycan chains in the antigenicity of Rapana thomasiana hemocyanin. Biochem. Biophys. Res. Commun. 2007;361:705–711. doi: 10.1016/j.bbrc.2007.07.098. [DOI] [PubMed] [Google Scholar]
- Siddiqui NI, Yigzaw Y, Préaux G, Gielens C. Involvement of glycans in the immunological cross-reaction between α-macroglobulin and hemocyanin of the gastropod Helix pomatia. Biochimie. 2009;91:508–516. doi: 10.1016/j.biochi.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Sinninghe Damsté JS, van Dongen BE, Rijpstra WIC, Schouten S, Volkman JK, Geenevasen JAJ. Novel intact glycolipids in sediments from an Antarctic lake (Ace Lake) Org. Geochem. 2001;32:321–332. [Google Scholar]
- Stepan H, Bleckmann C, Geyer H, Geyer R, Staudacher E. Determination of 3-O- and 4-O-methylated monosaccharide constituents in snail glycans. Carbohydr. Res. 2010;345:1504–1507. doi: 10.1016/j.carres.2010.03.027. [DOI] [PubMed] [Google Scholar]
- Stepan H, Staudacher E. Optimization of monosaccharide determination using anthranilic acid and 1-phenyl-3-methyl-5-pyrazolone for gastropod analysis. Anal. Biochem. 2011;418:24–29. doi: 10.1016/j.ab.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeva S, Rachev R, Severov S, Voelter W, Genov N. Carbohydrate content and monosaccharide composition of Rapana thomasiana grosse (Gastropoda) hemocyanin and its structural subunits. Comparison with gastropodan hemocyanins. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1995;110:761–765. doi: 10.1016/0305-0491(94)00201-5. [DOI] [PubMed] [Google Scholar]
- Sun Y-C, Wen J-L, Xu F, Sun R-C. Structural and thermal characterization of hemicelluloses isolated by organic solvents and alkaline solutions from Tamarix austromongolica. Biores. Technol. 2011;102:5947–5951. doi: 10.1016/j.biortech.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Sweet L, Zhang W, Torres-Fewell H, Serianni A, Boggess W, Schorey J. Mycobacterium avium glycopeptidolipids require specific acetylation and methylation patterns for signaling through Toll-like receptor 2. J. Biol. Chem. 2008;283:33221–33231. doi: 10.1074/jbc.M805539200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmont F, Fournet B. Isolation and characterization of methylated sugars from the tube of the hydrothermal vent tubiculous annelid worm Alvinella pompejana. FEBS Lett. 1991;281:55–58. doi: 10.1016/0014-5793(91)80357-9. [DOI] [PubMed] [Google Scholar]
- Turska-Szewczuk A, Russa R. Structural studies of the O-specific polysaccharide from the lipopolysaccharide of Mesorhizobium huakuii strain S-52, the symbiotic partner or Astragalus sinicus. Carbohydr. Res. 2011;346:1065–1069. doi: 10.1016/j.carres.2011.03.017. [DOI] [PubMed] [Google Scholar]
- v.Kuik JA, Sijbesma RP, Kamerling JP, Vliegenthart JFG, Wood EJ. Primary structure of a low-molecular-mass N-linked oligosaccharide from hemocyanin of Lymnea stagnalis. 3-O-methyl-D-mannose as a constituent of the xylose-containing core structure in an animal glycoprotein. Eur. J. Biochem. 1986;160:621–625. doi: 10.1111/j.1432-1033.1986.tb10083.x. [DOI] [PubMed] [Google Scholar]
- v.Kuik JA, Sijbesma RP, Kamerling JP, Vliegenthart JFG, Wood EJ. Primary structure determination of seven novel N-linked carbohydrate chains derived from hemocyanin of Lymnea stagnalis. 3-O-methyl-galactose and N-acetyl-D-galactosamine as constituents of xylose-containing N-linked oligosaccharides in an animal glycoprotein. Eur. J. Biochem. 1987;169:399–411. doi: 10.1111/j.1432-1033.1987.tb13626.x. [DOI] [PubMed] [Google Scholar]
- Watt DK, O’Neill SA, Percy AE, Brasch DJ. Isolation and characterisation of a partially methylated galacto-glucurono-xylo-glycan, a unique polysaccharide from the red seaweed Apophloea lyallii. Carbohydr. Polymers. 2002;50:283–294. [Google Scholar]
- Weismann LS, Ballou CE. Biosynthesis of the mycobacterial methylmannose polysaccharide. Identification of a 3-O-methyltransferase. J. Biol. Chem. 1984;259:3464–3469. [PubMed] [Google Scholar]
- Yang Y, Zhang J, Liu Y, Tang Q, Zhao Z, Xia W. Structural elucidation of a 3-O-methyl-D-galactose-containing neutral polysaccharide from the fruiting bodies of Phellinus igniarius. Carbohydr. Res. 2007;342:1063–1070. doi: 10.1016/j.carres.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Zdorovenko EL, Ovod VV, Zatonsky GV, Shashkov AS, Kocharova NA, Knirel YA. Location of the O-methyl groups in the O-polysaccharide of Pseudomonas syringae pv. phaseolicola. Carbohydr. Res. 2001;330:505–510. doi: 10.1016/s0008-6215(01)00005-2. [DOI] [PubMed] [Google Scholar]
- Zdorovenko EL, Valueva OA, Kachala VV, Shashkov AS, Kocharova NA, Knirel YA, Kutkowska J, Turska-Szweczuk A, Urbanik-Sypniewska T, Choma A, Russa R. Structure of the O-polysaccharides of the lipopolysaccharides of Mesorhizobium loti HAMBI 1148 and Mesorhizobium amorphae ATCC 19655 containing two O-methylated monosaccharides. Carbohydr. Res. 2009;344:2519–2527. doi: 10.1016/j.carres.2009.08.040. [DOI] [PubMed] [Google Scholar]
- Zhang A, Zhang J, Tang Q, Jia W, Yang Y, Liu Y, Fan J, Pan Y. Structural elucidation of a novel fucogalactan that contains 3-O-methyl rhamnose isolated from the fruiting bodies of the fungus, Hericium erinaceus. Carbohydr. Res. 2006;341:645–649. doi: 10.1016/j.carres.2005.11.038. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wen H, Shi X. Lysine methylation: beyond histones. Acta Biochim. Biophys. Sin. 2012;44:14–27. doi: 10.1093/abbs/gmr100. [DOI] [PubMed] [Google Scholar]
- Zibetti RGM, Duarte MER, Noseda MD, Colodi FG, Ducatti DRB, Ferreira LG, Cardoso MA, Cerezo AS. Galactans from Cryptonemia species. Part II: Studies on the system of galactans of Cryptonemia seminervis (Halymeniales) and on the structure of major fractions. Carbohydr. Res. 2009;344:2364–2374. doi: 10.1016/j.carres.2009.09.003. [DOI] [PubMed] [Google Scholar]