Summary
The second messenger cyclic AMP (cAMP) operates in discrete subcellular regions within which proteins that synthesize, break down or respond to the second messenger are precisely organized. A burgeoning knowledge of compartmentalized cAMP signaling is revealing how the local control of signaling enzyme activity impacts upon disease. The aim of this Cell Science at a Glance article and the accompanying poster is to highlight how misregulation of local cyclic AMP signaling can have pathophysiological consequences. We first introduce the core molecular machinery for cAMP signaling, which includes the cAMP-dependent protein kinase (PKA), and then consider the role of A-kinase anchoring proteins (AKAPs) in coordinating different cAMP-responsive proteins. The latter sections illustrate the emerging role of local cAMP signaling in four disease areas: cataracts, cancer, diabetes and cardiovascular diseases.
Introduction
Cyclic AMP (cAMP) is a chemical second messenger that couples extracellular signals to intracellular responses in all cell types (Sutherland, 1971). cAMP signaling proteins are constrained within well-organized subcellular environments (Zaccolo and Pozzan, 2002). This sophisticated mode of molecular organization permits cells to differentially use this ubiquitous second messenger, while simultaneously modulating a plethora of individual cellular events (Buxton and Brunton, 1983; Keely, 1977).
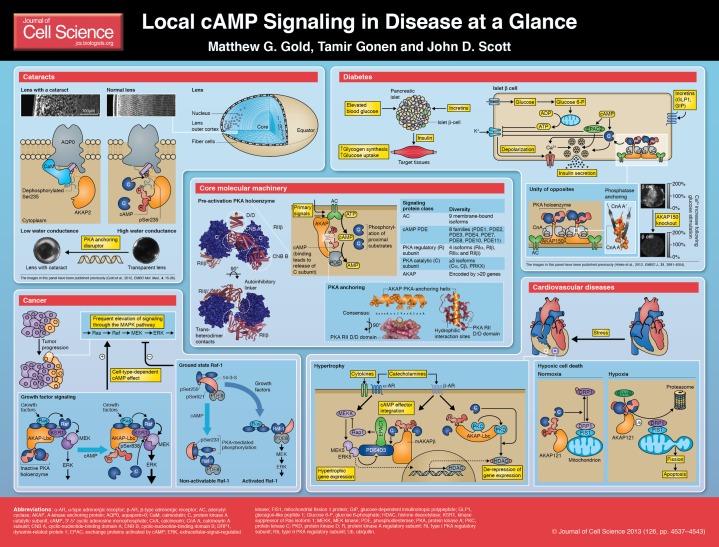
The core molecular machinery involved in cAMP signaling is illustrated in the central panel of the poster. Adenylyl cyclases (ACs) synthesize cAMP in response to upstream primary signals. Primary signaling molecules, such as hormones, prostaglandins and neurotransmitters, initiate the cAMP response, in many cases, by activating members of the large G-protein-coupled receptor (GPCR) family (Roth and Marshall, 2012). All nine membrane-bound AC isoforms have been implicated in localized cAMP signaling (Dessauer, 2009). Termination of cAMP action is achieved by various families of phosphodiesterase (PDE) enzymes that catalyze the hydrolysis of cAMP to 5′AMP (Francis et al., 2011). Collectively, the opposing effects of AC and PDE activity create a dynamic environment in which pools of cAMP accumulate and dissipate at discrete locations in the cell. The major intracellular receptors for cAMP, the protein kinase A (PKA) family, exchange proteins activated by cAMP (EPACs) and cyclic-nucleotide-gated channels, are discussed in Box 1. The subcellular coordination of cAMP signaling proteins is achieved through protein–protein and protein–lipid interactions. An important component of this organization is the assembly of macromolecular complexes by A-kinase anchoring proteins (AKAPs) (Wong and Scott, 2004) (Box 2).
Box 1. Intracellular receptors for cAMP.
There are three classes of intracellular receptors for cAMP: members of the cAMP-dependent protein kinase A (PKA) family, exchange proteins activated by cAMP (EPACs) (Bos, 2006) and cyclic-nucleotide-gated channels (Matulef and Zagotta, 2003). PKA holoenzymes consist of two regulatory (R) and two catalytic (C) subunits. There is diversity with regard to both regulatory (Taylor et al., 2012) and catalytic (Søberg et al., 2013) subunits, and the holoenzymes are classified according to whether they assemble from regulatory subunits of type I (RI) or type II (RII). Their distinguishing characteristics include the activation of type I holoenzymes at lower cAMP concentrations and a more organized subcellular distribution for type II holoenzymes (Taylor et al., 2012). Cooperative binding of cAMP to each of the dimerized regulatory subunits leads to the release of the catalytic subunit and ensuing phosphorylation of substrates in the vicinity. Recent high-resolution structures have established the molecular basis of cooperative cAMP activation (Kim et al., 2007; Wu et al., 2007; Zhang et al., 2012). In the case of type RIIβ PKA holoenzyme (illustrated on the poster), cooperativity arises from allosteric changes that are propagated both in cis between the two cyclic-nucleotide-binding (CNB) domains of each regulatory subunit, and in trans through trans-heterodimer contacts (Zhang et al., 2012).
Box 2. Coordination of cAMP signaling proteins by AKAPs.
There are approaching 50 human AKAPs (Welch et al., 2010), taking into account multiple splice variants that possess different subcellular targeting properties (Josefsen et al., 2010). All AKAPs anchor PKA through an amphipathic helix that mediates a tight protein–protein interaction with the dimerization and docking domain (D/D) of the PKA regulatory subunit dimer. AKAP–PKA complexes are directed to discrete subcellular locations by further protein–protein or protein–lipid interactions with the anchoring proteins (Wong and Scott, 2004). The majority of AKAPs selectively bind the RII subunits of PKAs, with a few exceptions such as the RI-selective AKAP sphingosine kinase interacting protein (SKIP) (Means et al., 2011). Accordingly, type I PKA holoenzymes exhibit a more homogeneous and cytoplasmic distribution than the type II PKA holoenzymes that remain tethered via AKAPs to a variety of cell membranes and intracellular organelles (Wong and Scott, 2004). Several structural studies have established that anchoring helices of AKAPs dock into a shallow hydrophobic groove that is formed by the dimerization of the D/D domains of RI (Sarma et al., 2010) or RII (Gold et al., 2006; Kinderman et al., 2006). In addition, high-throughput sequence–function approaches (Fowler et al., 2010) have confirmed a role for hydrophilic interaction sites on the D/D surface (Gold et al., 2013). In the past decade, it has emerged that other components of the cAMP signaling cascade, such as GPCRs (Fraser et al., 2000), ACs (Bauman et al., 2006; Kapiloff et al., 2009; Li et al., 2012b; Piggott et al., 2008) and PDEs (Dodge et al., 2001; Dodge-Kafka et al., 2005; Terrenoire et al., 2009; Willoughby et al., 2006) also interact with AKAPs.
As the clustering of several enzymes in a signaling pathway has a profound influence on the scope, duration and flow of information within cells, it is no surprise that alterations in AKAP-mediated signaling can contribute to the etiology of several common diseases (Marx et al., 2002; Tröger et al., 2012). The intent of this article and the accompanying poster is to highlight some recently described misregulations in local cyclic AMP signaling that have been linked to pathophysiological consequences in four areas: cataracts, diabetes, cancer and cardiovascular diseases.
Cataracts
A clouding of the ocular lens, known as a cataract, is the leading global cause of blindness (Brian and Taylor, 2001). The lens relies upon the passage of water and nutrients through channel and gap-junction proteins to ensure the delivery of essential nutrients and chemical signals while minimizing light scattering (Mathias et al., 2007). The water channel aquaporin-0 (AQP0) constitutes more than 60% of the total membrane protein in lens fiber cells (Bloemendal et al., 1972). This six-transmembrane helical protein multimerizes to assemble pores for water conduction across biological membranes (Gonen and Walz, 2006). The C-terminal cytoplasmic tail of AQP0 tail is a locus for regulating water conductance through the channel; binding of Ca2+/calmodulin (CaM) to a helical segment between its residues 225 and 243 decreases water permeability (Reichow and Gonen, 2008; Varadaraj et al., 2005), whereas phosphorylation at Ser235 by PKA or CaM kinase II opposes CaM binding to sustain water conductance (Ball et al., 2004; Gold et al., 2012; Reichow and Gonen, 2008; Lindsey Rose et al., 2008).
Recently, it has become clear that AKAPs that are associated with AQP0 provide an efficient means to ensure appropriate PKA-mediated phosphorylation of the water pore (Gold et al., 2012). AKAP2 is the predominant AKAP in the lens, where it is tethered to the inner face of membranes in fiber cells that reside in the equatorial cortex. AKAP2 directly interacts with AQP0, including at a site spanning residues 238–246 in the C-terminal tail of the water channel (Gold et al., 2012). This interaction enables AKAP2 to target PKA for phosphorylation of the consensus phosphorylation site Ser235 (Gold et al., 2012). Ser235 phosphorylation abrogates binding of the AQP0 tail to a negatively charged binding cleft in CaM, thus favoring the active open conformation of the water channel (see Cataracts panel of the poster). Application of a PKA-anchoring helix peptide, derived from an AKAP with a high-affinity for PKA, releases PKA from its native anchoring sites, and led to the development of cataracts in the outer cortex of murine lenses (Gold et al., 2012). Thus, phosphorylation of AQP0 by anchored PKA provides a homeostatic mechanism to sustain open water pores and fluid flow in the lens (Gold et al., 2012). This model is consistent with previous studies, which showed that pharmacological inhibition of the catalytic (C) subunit of PKA leads to cortical cataracts (Calvin et al., 2003). Moreover, this AQP0–AKAP2–PKA signaling axis is potentially involved in the pathology of the autosomal dominant cataractogenic AQP0 mutation Arg233Lys (Lin et al., 2007). This mutation corresponds to part of the substrate recognition sequence of PKA for residue Ser235 (Zetterqvist and Ragnarsson, 1982). Accordingly, PKA-mediated phosphorylation of an AQP0 peptide (residues 223–242) that harbours the Arg233Lys mutation is reduced by ∼80% in comparison with the wild-type sequence (Gold et al., 2012). Future studies could examine whether further lens proteins, including gap junction proteins (Liu et, al., 2011), are subject to regulation by the AKAP2–PKA complex and whether AKAP2 nucleates additional cAMP signaling components. Possible therapeutic approaches might include enhancing the phosphorylation of Ser235 on AQP0 by the topical delivery of a phosphatase inhibitor into the lens.
Diabetes
Type 2 diabetes mellitus (T2DM) is a global pandemic with over 300 million people living with the disease worldwide (Ashcroft and Rorsman, 2012). Reduced insulin release from β-cells in pancreatic islets is the key feature of T2DM (Ashcroft and Rorsman, 2012). Signaling processes involving second messengers are fundamental to the normal function of β-cells. Raised circulating blood glucose leads to higher ATP concentration in β-cells, which inhibits ATP-sensitive K+ (KATP) channels. The resulting membrane depolarization activates voltage-gated Ca2+ channels and the subsequent influx of this second messenger leads to fusion of insulin vesicles with the cell membrane and insulin secretion (Ashcroft and Rorsman, 2012). The consequent elevation of the blood insulin concentration triggers glycogen synthesis and glucose uptake in tissues, including skeletal muscle, liver and adipose tissue (summarized in the Diabetes panel of the poster). cAMP signals potentiate multiple aspects of insulin release by β-cells. cAMP accumulates in β-cells following both glucose stimulation and activation of receptors by the incretins glucagon-like peptide-1 (GLP1, also known as ZGLP1) and glucose-dependent insulinotropic polypeptide (GIP). KATP channel currents and vesicle fusion coupled to Ca2+ entry are important control points in the β-cell. Both PKA and EPAC2 (also known as RAPGEF4) (Box 1) are thought to contribute to the regulation of these processes downstream of cAMP accumulation. GLP1 promotes the closure of KATP channels through both PKA- (Gromada et al., 2004) and Epac2-dependent mechanisms (Leech et al., 2011). It is well established that PKA phosphorylation amplifies cellular influx of Ca2+ through voltage-gated Ca2+ channels (Gao et al., 1997; Sculptoreanu et al., 1993). In addition, PKA upregulates the release of Ca2+ from intracellular stores that is triggered by the initial influx of extracellular Ca2+, and increases the size of a pool of secretory granules that are highly sensitive to Ca2+ (Leech et al., 2011). EPAC2 is also thought to stimulate the exocytotic insulin release process (Shibasaki et al., 2007). The Ca2+/calmodulin (CaM)-activated cyclase AC8 (also known as ADCY8) contributes to β-cell cAMP synthesis and is under the control of the endoplasmic reticulum stress-related protein Wolfram syndrome 1 (Fonseca et al., 2012). Research is now directed to understanding how these different cAMP signaling proteins are coordinated in β-cells.
PKA anchoring is necessary for incretin-stimulated insulin release (Lester et al., 1997). Recently, the contribution of AKAP150 (encoded by Akap5; the human ortholog is also known as AKAP79) to the modulation of glucose homeostasis in mice has been reported (Hinke et al., 2012). This dimeric (Gao et al., 2011; Gold et al., 2011) protein co-anchors PKA with multiple signaling components, including AC (Bauman et al., 2006) and the Ca2+/CaM-activated protein phosphatase calcineurin (Coghlan et al., 1995) (see Diabetes panel of the poster). AKAP150 binds to calcineurin through a constitutive interaction motif (PIAIIITD) in its C-terminus and a Ca2+/CaM-dependent site in its N-terminal region. Results from mass spectrometry of intact protein complexes (Gold et al., 2011) and crystal structures of calcineurin-anchoring peptide complexes (Li et al., 2012a; Li et al., 2007) are consistent with the binding of two copies of this phosphatase per AKAP150 polypeptide, although it remains to be established whether this is always the physiological stoichiometry. AKAP150-null mice exhibit impaired insulin secretion with decreases in both glucose-stimulated Ca2+ entry (illustrated in the Diabetes panel) and cAMP production (Hinke et al., 2012). These data are consistent with a central role for AKAP150 in coordinating cAMP signaling in pancreatic β-cells. Follow-up rescue experiments with AKAP150 knock-in mice that are unable to bind to either PKA or calcineurin indicate that phosphatase anchoring is more important to the function of AKAP150 than the anchoring of PKA (Hinke et al., 2012). Coordination of cAMP signaling is also important in insulin target tissues, as AKAP150-null mice possess elevated insulin sensitivity in skeletal muscle (Hinke et al., 2012). Current anti-diabetic drugs are thought to operate in part by modulating cAMP signaling (Miller et al., 2013; Rehmann, 2012; Zhang et al., 2009). Emerging discoveries concerning the coordination of cAMP signaling proteins might offer new avenues for therapeutic intervention.
Cancer
A fundamental trait of cancer cells is sustained chronic proliferation (Hanahan and Weinberg, 2011), and the upregulation of signaling through the proliferative Ras–Raf–MEK–ERK pathway is a frequent adaptation of cancerous cells. For example, somatic B-Raf missense mutations are harbored by 66% of malignant melanomas (Davies et al., 2002). Twenty years ago, it was discovered that cAMP inhibits signaling through the mitogen-activated protein kinase (MAPK) cascade in Rat-1 and NIH3T3 fibroblasts (Burgering et al., 1993; Cook and McCormick, 1993; Wu et al., 1993). It has since been established that cAMP can both stimulate and inhibit MAPK signaling depending on the cell type. For example, cAMP potentiates MAPK signals in rat pheochromocytoma PC12 cells (Dumaz and Marais, 2005). Subsequent investigations have addressed the underlying mechanism of cAMP-mediated regulation of the MAPK cascade with a growing awareness of the importance that the spatiotemporal synchronization cAMP signaling affords.
An example concerning an activator role for cAMP (see Cancer panel of the poster) involves kinase suppressor of Ras (KSR) proteins that augment signal transmission through the MAPK cascade by interacting with both MEK and with Raf to stimulate signal relay (Brennan et al., 2011). The PKA-anchoring protein AKAP-Lbc (also known as AKAP13) interacts with KSR and with Raf isoforms, thereby providing a means to target PKA for the phosphoregulation of MAPK signaling (Smith et al., 2010). Accordingly, biochemical experiments and live-cell imaging of kinase activity revealed that AKAP-Lbc is able to augment the activation of MEK and ERK by directing PKA to phosphorylate residue Ser838 of KSR1. This residue is positioned adjacent to the MEK-binding site of KSR1, although the mechanism of MEK activation by Ser838 phosphorylation has not been determined (Smith et al., 2010). The necessity of AKAP-Lbc for phosphorylation at Ser838 of KSR1 highlights the importance of the intracellular architecture in governing which proteins are phosphorylated by PKA, and this activation mechanism might be a feature of cells whose proliferation is potentiated by cAMP.
A model that explains the inhibitory effect of cAMP on MAPK signaling (see Cancer panel) concerns the Raf isoform Raf-1 (Dumaz and Marais, 2005). Raf-1 is phosphorylated by PKA at three sites (Ser43, Ser233 and Ser259) in its N-terminal domain (Burgering et al., 1993) and all three of these sites can block its activation by Ras. Phosphorylation of Raf-1 Ser43 directly interferes with Ras binding (Wu et al., 1993), whereas phosphorylation of Ser233 and Ser259 results in the recruitment of dimeric 14-3-3 proteins that prevent Ras binding (Dumaz and Marais, 2003; Light et al., 2002). In the absence of Raf-1 Ser233 phosphorylation, 14-3-3 proteins are thought to span the phosphorylated Raf-1 residues Ser259 and Ser621 in a conformation that is permissive to Ras binding. By constitutively interacting with Raf-1, the phosphodiesterase PDE8A counteracts the phosphorylation of Raf-1 by PKA (Maurice, 2013), and this signaling module is pertinent to melanomas that harbor oncogenic Ras mutations that operate through Raf-1 (Marquette et al., 2011). Such melanoma cells have elevated cAMP PDE activity owing to overexpression of PDE4 enzymes, which prevent PKA inhibition and thus permit the reactivation of Raf-1 (Marquette et al., 2011). Accordingly, the PDE4-selective inhibitor rolipram suppresses the activity of Raf-1, thereby inhibiting proliferation and inducing apoptosis in melanoma cells (Marquette et al., 2011). Further elucidation of these signaling processes will help to illuminate other avenues for the disruption of cancer cell proliferation.
Cardiovascular diseases
cAMP signaling also governs many aspects of cardiac function (Diviani et al., 2013; Perera and Nikolaev, 2013). Significantly, cAMP is the predominant second messenger downstream of sympathetic heart stimulation by catecholamines. The importance of coordinating cardiac cAMP signaling is exemplified by mutations in Yotiao (also known as AKAP9) that cause long-QT syndrome by rendering this AKAP incapable of targeting PKA to the slowly activating cardiac K+ channel (Chen et al., 2007; Marx et al., 2002). There has been considerable interest in determining how cAMP regulates adaptive cardiac changes to stress, including activation of hypertrophic gene expression and responses to low oxygen concentration (hypoxia). Pathological cardiac hypertrophy (Hill and Olson, 2008), which is an increase in cardiac mass that results from stress-induced cardiac myocyte growth, is a major factor underlying heart failure. Two signaling complexes have been identified that mediate the effects of catecholamines on hypertrophic gene expression in tandem with other extracellular signals (see Cardiovascular disease panel of the poster). Muscle-specific AKAP β (mAKAPβ, also known as AKAP6) coordinates the interaction of two cAMP receptors (EPAC1 and PKA) with PDE4D3. Components of the MAPK signaling cascade are also brought into the complex through their association with PDE4D3. As PKA is more sensitive to cAMP than EPAC1, and PKA activates PDE4D3 to reduce the cAMP concentration in the vicinity of the mAKAP complex, highly elevated levels of cAMP are required to activate mAKAP-associated EPAC1. Upon its activation, EPAC1 attenuates hypertrophic signals that lead to ERK5 activation by inhibiting MAPK signaling through Rap1 (Dodge-Kafka et al., 2005). AKAP-Lbc also modulates gene expression by governing the nuclear export of the general transcription repressor histone deacetylase 5 (HDAC5) (Carnegie et al., 2008) through phosphorylation. AKAP-Lbc harbors multiple protein interaction sites, and enables release of active protein kinase D (PKD) by co-anchoring it with PKA and protein kinase C (PKC) (Carnegie et al., 2004). Following its activation, PKD translocates to the nucleus where it phosphorylates HDAC5, resulting in its nuclear export (Carnegie et al., 2008).
Oxygen supply is a major factor in heart disease. Sir James Black developed the first blockbuster drug, propranolol, guided by the notion that reducing the myocardial need for oxygen could protect against injurious myocardial infarction (Stapleton, 1997). Current research efforts aim to understand the adaptive responses to cardiac hypoxia that include the induction of apoptosis. Components of the cAMP signaling cascade are also coordinated to regulate this process (see Cardiovascular disease panel). AKAP121 (also known as AKAP1) enables PKA-mediated phosphorylation of dynamin-related protein 1 (DRP1), which prevents it from associating with the fission factor FIS1 on the mitochondrial membrane (Kim et al., 2011). Hypoxia results in the increased expression and activity of the ubiquitin ligase SIAH2, which then ubiquitylates AKAP121, leading to its breakdown and consequent dephosphorylation of DRP1. Upon its dephosphorylation, DRP1 is able to interact with FIS1 to trigger mitochondrial fission and apoptosis (Kim et al., 2011). Intriguingly, another AKAP, mAKAP, binds to the transcription factor hypoxia-inducible factor 1α (Wong et al., 2008). It will be interesting to explore whether PKA has similar protective properties against ischemia within the mAKAP complex.
Conclusions and perspectives
A common feature of the four diseases outlined in this article, in addition to their augmentation by defects in local cAMP signaling, is their prevalence in older people. The incidence and economic burden of these disorders is set to increase as average global lifespans rise. Further diseases that impinge on the area of perturbed local cAMP signaling include obesity (Cummings et al., 1996; Czyzyk et al., 2008), asthma (Gerthoffer et al., 2013), and neurodegenerative and behavioral disorders (Bernstein et al., 2013; Millar et al., 2005; Reissner, 2013; Renthal et al., 2009; Richter et al., 2011). There is a growing awareness among structural biologists and pharmacologists that the higher-order topology of signaling protein complexes should be considered when contemplating therapeutic intervention (Blundell et al., 2011). In cases where local cAMP signaling is an important element of the pathophysiology of a condition, targeting AKAP–enzyme interactions might offer a means to therapeutically intervene and manage some of these pathological events. Selectively disrupting PKA by targeting the helices that mediate AKAP anchoring (Christian et al., 2011; Gold et al., 2013; Wells and McClendon, 2007) is one potential approach.
However, there are several challenges and misconceptions that must be overcome before we can confidently proceed in this direction. One unforeseen outcome of the drive to develop drugs that inhibit kinases (Davis et al., 2011) is the realization that protein–protein interactions can profoundly influence drug action (Prince and Ahn, 2010). For example, the association of a kinase with endogenous binding partners can confer resistance to ATP-analog inhibitors, which provides an explanation for why kinases, such as Akt (PKB) and B-Raf, can become refractory to certain anticancer drugs (Brennan et al., 2011; Knight et al., 2010; Okuzumi et al., 2010; Okuzumi et al., 2009; Poulikakos et al., 2010). The same might be true for the PKC inhibitor ruboxistaurin, which is being developed to manage diabetic microcirculatory complications and neuropathies (Short and Tuttle, 2005; Tuttle et al., 2005), as structural analogs of this compound have been found to be ineffective against PKC that is associated with AKAP150 (Hoshi et al., 2010; Prince and Ahn, 2010). Importantly, despite their abundance and therapeutic relevance, targeting protein–protein interactions with drugs has long been avoided (Mullard, 2012). However, recent advances in drug discovery argue that targeting protein-protein interfaces could yield compounds with greater selectivity and fewer side effects (Filippakopoulos et al., 2010; Higueruelo et al., 2013; Sperandio et al., 2010; Yu et al., 2007). For example, small molecules have been developed that bind competitively to histone binding bromodomains (Filippakopoulos et al., 2010). Therefore, it may be necessary to screen for new pharmacological compounds that act on their target enzymes in the context of their association with endogenous binding partners.
Supplementary Material
Footnotes
Funding
Research in the laboratories of T.G. and J.D.G. is funded by the Howard Hughes Medical Institute; and the National Institutes of Health [grant number GM48321 to J.D.S.]. M.G.G. is a University College London Independent Research Fellow. Deposited in PMC for release after 12 months.
A high-resolution version of the poster is available for downloading in the online version of this article at jcs.biologists.org. Individual poster panels are available as JPEG files at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.133751/-/DC1.
References
- Ashcroft F. M., Rorsman P. (2012). Diabetes mellitus and the β cell: the last ten years. Cell 148, 1160–1171 10.1016/j.cell.2012.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball L. E., Garland D. L., Crouch R. K., Schey K. L. (2004). Post-translational modifications of aquaporin 0 (AQP0) in the normal human lens: spatial and temporal occurrence. Biochemistry 43, 9856–9865 10.1021/bi0496034 [DOI] [PubMed] [Google Scholar]
- Bauman A. L., Soughayer J., Nguyen B. T., Willoughby D., Carnegie G. K., Wong W., Hoshi N., Langeberg L. K., Cooper D. M., Dessauer C. W. et al. (2006). Dynamic regulation of cAMP synthesis through anchored PKA-adenylyl cyclase V/VI complexes. Mol. Cell 23, 925–931 10.1016/j.molcel.2006.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein H. G., Dobrowolny H., Schott B. H., Gorny X., Becker V., Steiner J., Seidenbecher C. I., Bogerts B. (2013). Increased density of AKAP5-expressing neurons in the anterior cingulate cortex of subjects with bipolar disorder. J. Psychiatr. Res. 47, 699–705 10.1016/j.jpsychires.2012.12.020 [DOI] [PubMed] [Google Scholar]
- Bloemendal H., Zweers A., Vermorken F., Dunia I., Benedetti E. L. (1972). The plasma membranes of eye lens fibres. Biochemical and structural characterization. Cell Differ. 1, 91–106 10.1016/0045-6039(72)90032-2 [DOI] [PubMed] [Google Scholar]
- Blundell T. L., Harrison S. C., Stroud R. M., Yokoyama S., Kay L. E., Rossman M. G., Berman H. M., Wlodawer A., Conti E., Kobilka B. et al. (2011). Celebrating structural biology. Nat. Struct. Mol. Biol. 18, 1304–1316 10.1038/nsmb1211-1304 [DOI] [PubMed] [Google Scholar]
- Bos J. L. (2006). Epac proteins: multi-purpose cAMP targets. Trends Biochem. Sci. 31, 680–686 10.1016/j.tibs.2006.10.002 [DOI] [PubMed] [Google Scholar]
- Brennan D. F., Dar A. C., Hertz N. T., Chao W. C., Burlingame A. L., Shokat K. M., Barford D. (2011). A Raf-induced allosteric transition of KSR stimulates phosphorylation of MEK. Nature 472, 366–369 10.1038/nature09860 [DOI] [PubMed] [Google Scholar]
- Brian G., Taylor H. (2001). Cataract blindness—challenges for the 21st century. Bull. World Health Organ. 79, 249–256 [PMC free article] [PubMed] [Google Scholar]
- Burgering B. M., Pronk G. J., van Weeren P. C., Chardin P., Bos J. L. (1993). cAMP antagonizes p21ras-directed activation of extracellular signal-regulated kinase 2 and phosphorylation of mSos nucleotide exchange factor. EMBO J. 12, 4211–4220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton I. L., Brunton L. L. (1983). Compartments of cyclic AMP and protein kinase in mammalian cardiomyocytes. J. Biol. Chem. 258, 10233–10239 [PubMed] [Google Scholar]
- Calvin H. I., Wu K., Li W., Guo L., Banerjee U., Fu S. C. (2003). Induction of cortical cataracts in cultured mouse lenses with H-89, an inhibitor of protein kinase A. Curr. Eye Res. 27, 269–278 10.1076/ceyr.27.5.269.17224 [DOI] [PubMed] [Google Scholar]
- Carnegie G. K., Smith F. D., McConnachie G., Langeberg L. K., Scott J. D. (2004). AKAP-Lbc nucleates a protein kinase D activation scaffold. Mol. Cell 15, 889–899 10.1016/j.molcel.2004.09.015 [DOI] [PubMed] [Google Scholar]
- Carnegie G. K., Soughayer J., Smith F. D., Pedroja B. S., Zhang F., Diviani D., Bristow M. R., Kunkel M. T., Newton A. C., Langeberg L. K. et al. (2008). AKAP-Lbc mobilizes a cardiac hypertrophy signaling pathway. Mol. Cell 32, 169–179 10.1016/j.molcel.2008.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Marquardt M. L., Tester D. J., Sampson K. J., Ackerman M. J., Kass R. S. (2007). Mutation of an A-kinase-anchoring protein causes long-QT syndrome. Proc. Natl. Acad. Sci. USA 104, 20990–20995 10.1073/pnas.0710527105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian F., Szaszák M., Friedl S., Drewianka S., Lorenz D., Goncalves A., Furkert J., Vargas C., Schmieder P., Götz F. et al. (2011). Small molecule AKAP-protein kinase A (PKA) interaction disruptors that activate PKA interfere with compartmentalized cAMP signaling in cardiac myocytes. J. Biol. Chem. 286, 9079–9096 10.1074/jbc.M110.160614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan V. M., Perrino B. A., Howard M., Langeberg L. K., Hicks J. B., Gallatin W. M., Scott J. D. (1995). Association of protein kinase A and protein phosphatase 2B with a common anchoring protein. Science 267, 108–111 10.1126/science.7528941 [DOI] [PubMed] [Google Scholar]
- Cook S. J., McCormick F. (1993). Inhibition by cAMP of Ras-dependent activation of Raf. Science 262, 1069–1072 10.1126/science.7694367 [DOI] [PubMed] [Google Scholar]
- Cummings D. E., Brandon E. P., Planas J. V., Motamed K., Idzerda R. L., McKnight G. S. (1996). Genetically lean mice result from targeted disruption of the RII beta subunit of protein kinase A. Nature 382, 622–626 10.1038/382622a0 [DOI] [PubMed] [Google Scholar]
- Czyzyk T. A., Sikorski M. A., Yang L., McKnight G. S. (2008). Disruption of the RIIbeta subunit of PKA reverses the obesity syndrome of Agouti lethal yellow mice. Proc. Natl. Acad. Sci. USA 105, 276–281 10.1073/pnas.0710607105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H., Bignell G. R., Cox C., Stephens P., Edkins S., Clegg S., Teague J., Woffendin H., Garnett M. J., Bottomley W. et al. (2002). Mutations of the BRAF gene in human cancer. Nature 417, 949–954 10.1038/nature00766 [DOI] [PubMed] [Google Scholar]
- Davis M. I., Hunt J. P., Herrgard S., Ciceri P., Wodicka L. M., Pallares G., Hocker M., Treiber D. K., Zarrinkar P. P. (2011). Comprehensive analysis of kinase inhibitor selectivity. Nat. Biotechnol. 29, 1046–1051 10.1038/nbt.1990 [DOI] [PubMed] [Google Scholar]
- Dessauer C. W. (2009). Adenylyl cyclase—A-kinase anchoring protein complexes: the next dimension in cAMP signaling. Mol. Pharmacol. 76, 935–941 10.1124/mol.109.059345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diviani D., Maric D., Pérez López I., Cavin S., Del Vescovo C. D. (2013). A-kinase anchoring proteins: molecular regulators of the cardiac stress response. Biochim. Biophys. Acta 1833, 901–908 10.1016/j.bbamcr.2012.07.014 [DOI] [PubMed] [Google Scholar]
- Dodge K. L., Khouangsathiene S., Kapiloff M. S., Mouton R., Hill E. V., Houslay M. D., Langeberg L. K., Scott J. D. (2001). mAKAP assembles a protein kinase A/PDE4 phosphodiesterase cAMP signaling module. EMBO J. 20, 1921–1930 10.1093/emboj/20.8.1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge-Kafka K. L., Soughayer J., Pare G. C., Carlisle Michel J. J., Langeberg L. K., Kapiloff M. S., Scott J. D. (2005). The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature 437, 574–578 10.1038/nature03966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumaz N., Marais R. (2003). Protein kinase A blocks Raf-1 activity by stimulating 14-3-3 binding and blocking Raf-1 interaction with Ras. J. Biol. Chem. 278, 29819–29823 10.1074/jbc.C300182200 [DOI] [PubMed] [Google Scholar]
- Dumaz N., Marais R. (2005). Integrating signals between cAMP and the RAS/RAF/MEK/ERK signalling pathways. Based on the anniversary prize of the Gesellschaft für Biochemie und Molekularbiologie Lecture delivered on 5 July 2003 at the Special FEBS Meeting in Brussels. FEBS J. 272, 3491–3504 10.1111/j.1742-4658.2005.04763.x [DOI] [PubMed] [Google Scholar]
- Filippakopoulos P., Qi J., Picaud S., Shen Y., Smith W. B., Fedorov O., Morse E. M., Keates T., Hickman T. T., Felletar I. et al. (2010). Selective inhibition of BET bromodomains. Nature 468, 1067–1073 10.1038/nature09504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca S. G., Urano F., Weir G. C., Gromada J., Burcin M. (2012). Wolfram syndrome 1 and adenylyl cyclase 8 interact at the plasma membrane to regulate insulin production and secretion. Nat. Cell Biol. 14, 1105–1112 10.1038/ncb2578 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Fowler D. M., Araya C. L., Fleishman S. J., Kellogg E. H., Stephany J. J., Baker D., Fields S. (2010). High-resolution mapping of protein sequence-function relationships. Nat. Methods 7, 741–746 10.1038/nmeth.1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis S. H., Blount M. A., Corbin J. D. (2011). Mammalian cyclic nucleotide phosphodiesterases: molecular mechanisms and physiological functions. Physiol. Rev. 91, 651–690 10.1152/physrev.00030.2010 [DOI] [PubMed] [Google Scholar]
- Fraser I. D., Cong M., Kim J., Rollins E. N., Daaka Y., Lefkowitz R. J., Scott J. D. (2000). Assembly of an A kinase-anchoring protein-beta(2)-adrenergic receptor complex facilitates receptor phosphorylation and signaling. Curr. Biol. 10, 409–412 10.1016/S0960-9822(00)00419-X [DOI] [PubMed] [Google Scholar]
- Gao T., Yatani A., Dell'Acqua M. L., Sako H., Green S. A., Dascal N., Scott J. D., Hosey M. M. (1997). cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron 19, 185–196 10.1016/S0896-6273(00)80358-X [DOI] [PubMed] [Google Scholar]
- Gao S., Wang H. Y., Malbon C. C. (2011). AKAP5 and AKAP12 Form Homo-oligomers. J. Mol. Signal. 6, 3 10.1186/1750-2187-6-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerthoffer W. T., Solway J., Camoretti-Mercado B. (2013). Emerging targets for novel therapy of asthma. Curr. Opin. Pharmacol. 13, 324–330 10.1016/j.coph.2013.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M. G., Lygren B., Dokurno P., Hoshi N., McConnachie G., Taskén K., Carlson C. R., Scott J. D., Barford D. (2006). Molecular basis of AKAP specificity for PKA regulatory subunits. Mol. Cell 24, 383–395 10.1016/j.molcel.2006.09.006 [DOI] [PubMed] [Google Scholar]
- Gold M. G., Stengel F., Nygren P. J., Weisbrod C. R., Bruce J. E., Robinson C. V., Barford D., Scott J. D. (2011). Architecture and dynamics of an A-kinase anchoring protein 79 (AKAP79) signaling complex. Proc. Natl. Acad. Sci. USA 108, 6426–6431 10.1073/pnas.1014400108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M. G., Reichow S. L., O'Neill S. E., Weisbrod C. R., Langeberg L. K., Bruce J. E., Gonen T., Scott J. D. (2012). AKAP2 anchors PKA with aquaporin-0 to support ocular lens transparency. EMBO Mol. Med. 4, 15–26 10.1002/emmm.201100184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M. G., Fowler D. M., Means C. K., Pawson C. T., Stephany J. J., Langeberg L. K., Fields S., Scott J. D. (2013). Engineering AKAP-selective regulatory subunits of PKA through structure-based phage selection. J. Biol. Chem. 288, 17111–17121 10.1074/jbc.M112.447326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonen T., Walz T. (2006). The structure of aquaporins. Q. Rev. Biophys. 39, 361–396 10.1017/S0033583506004458 [DOI] [PubMed] [Google Scholar]
- Gromada J., Brock B., Schmitz O., Rorsman P. (2004). Glucagon-like peptide-1: regulation of insulin secretion and therapeutic potential. Basic Clin. Pharmacol. Toxicol. 95, 252–262 10.1111/j.1742-7843.2004.t01-1-pto950502.x [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R. A. (2011). Hallmarks of cancer: the next generation. Cell 144, 646–674 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- Higueruelo A. P., Jubb H., Blundell T. L. (2013). Protein-protein interactions as druggable targets: recent technological advances. Curr. Opin. Pharmacol 10.1016/j.coph.2013.05.009 [DOI] [PubMed] [Google Scholar]
- Hill J. A., Olson E. N. (2008). Cardiac plasticity. N. Engl. J. Med. 358, 1370–1380 10.1056/NEJMra072139 [DOI] [PubMed] [Google Scholar]
- Hinke S. A., Navedo M. F., Ulman A., Whiting J. L., Nygren P. J., Tian G., Jimenez-Caliani A. J., Langeberg L. K., Cirulli V., Tengholm A. et al. (2012). Anchored phosphatases modulate glucose homeostasis. EMBO J. 31, 3991–4004 10.1038/emboj.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi N., Langeberg L. K., Gould C. M., Newton A. C., Scott J. D. (2010). Interaction with AKAP79 modifies the cellular pharmacology of PKC. Mol. Cell 37, 541–550 10.1016/j.molcel.2010.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsen K., Lee Y. C., Thams P., Efendic S., Nielsen J. H. (2010). AKAP 18 alpha and gamma have opposing effects on insulin release in INS-1E cells. FEBS Lett. 584, 81–85 10.1016/j.febslet.2009.10.086 [DOI] [PubMed] [Google Scholar]
- Kapiloff M. S., Piggott L. A., Sadana R., Li J., Heredia L. A., Henson E., Efendiev R., Dessauer C. W. (2009). An adenylyl cyclase-mAKAPbeta signaling complex regulates cAMP levels in cardiac myocytes. J. Biol. Chem. 284, 23540–23546 10.1074/jbc.M109.030072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keely S. L. (1977). Activation of cAMP-dependent protein kinase without a corresponding increase in phosphorylase activity. Res. Commun. Chem. Pathol. Pharmacol. 18, 283–290 [PubMed] [Google Scholar]
- Kim C., Cheng C. Y., Saldanha S. A., Taylor S. S. (2007). PKA-I holoenzyme structure reveals a mechanism for cAMP-dependent activation. Cell 130, 1032–1043 10.1016/j.cell.2007.07.018 [DOI] [PubMed] [Google Scholar]
- Kim H., Scimia M. C., Wilkinson D., Trelles R. D., Wood M. R., Bowtell D., Dillin A., Mercola M., Ronai Z. A. (2011). Fine-tuning of Drp1/Fis1 availability by AKAP121/Siah2 regulates mitochondrial adaptation to hypoxia. Mol. Cell 44, 532–544 10.1016/j.molcel.2011.08.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinderman F. S., Kim C., von Daake S., Ma Y., Pham B. Q., Spraggon G., Xuong N. H., Jennings P. A., Taylor S. S. (2006). A dynamic mechanism for AKAP binding to RII isoforms of cAMP-dependent protein kinase. Mol. Cell 24, 397–408 10.1016/j.molcel.2006.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight Z. A., Lin H., Shokat K. M. (2010). Targeting the cancer kinome through polypharmacology. Nat. Rev. Cancer 10, 130–137 10.1038/nrc2787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech C. A., Dzhura I., Chepurny O. G., Kang G., Schwede F., Genieser H. G., Holz G. G. (2011). Molecular physiology of glucagon-like peptide-1 insulin secretagogue action in pancreatic β cells. Prog. Biophys. Mol. Biol. 107, 236–247 10.1016/j.pbiomolbio.2011.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester L. B., Langeberg L. K., Scott J. D. (1997). Anchoring of protein kinase A facilitates hormone-mediated insulin secretion. Proc. Natl. Acad. Sci. USA 94, 14942–14947 10.1073/pnas.94.26.14942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Zhang L., Rao A., Harrison S. C., Hogan P. G. (2007). Structure of calcineurin in complex with PVIVIT peptide: portrait of a low-affinity signalling interaction. J. Mol. Biol. 369, 1296–1306 10.1016/j.jmb.2007.04.032 [DOI] [PubMed] [Google Scholar]
- Li H., Pink M. D., Murphy J. G., Stein A., Dell'Acqua M. L., Hogan P. G. (2012a). Balanced interactions of calcineurin with AKAP79 regulate Ca2+-calcineurin-NFAT signaling. Nat. Struct. Mol. Biol. 19, 337–345 10.1038/nsmb.2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Chen L., Kass R. S., Dessauer C. W. (2012b). The A-kinase anchoring protein Yotiao facilitates complex formation between adenylyl cyclase type 9 and the IKs potassium channel in heart. J. Biol. Chem. 287, 29815–29824 10.1074/jbc.M112.380568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light Y., Paterson H., Marais R. (2002). 14-3-3 antagonizes Ras-mediated Raf-1 recruitment to the plasma membrane to maintain signaling fidelity. Mol. Cell. Biol. 22, 4984–4996 10.1128/MCB.22.14.4984-4996.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey Rose K. M., Wang Z., Magrath G. N., Hazard E. S., Hildebrandt J. D., Schey K. L. (2008). Aquaporin 0-calmodulin interaction and the effect of aquaporin 0 phosphorylation. Biochemistry 47, 339–347 10.1021/bi701980t [DOI] [PubMed] [Google Scholar]
- Lin H., Hejtmancik J. F., Qi Y. (2007). A substitution of arginine to lysine at the COOH-terminus of MIP caused a different binocular phenotype in a congenital cataract family. Mol. Vis. 13, 1822–1827 [PubMed] [Google Scholar]
- Liu J., Ek Vitorin J. F., Weintraub S. T., Gu S., Shi Q., Burt J. M., Jiang J. X. (2011). Phosphorylation of connexin 50 by protein kinase A enhances gap junction and hemichannel function. J. Biol. Chem. 286, 16914–16928 10.1074/jbc.M111.218735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquette A., André J., Bagot M., Bensussan A., Dumaz N. (2011). ERK and PDE4 cooperate to induce RAF isoform switching in melanoma. Nat. Struct. Mol. Biol. 18, 584–591 10.1038/nsmb.2022 [DOI] [PubMed] [Google Scholar]
- Marx S. O., Kurokawa J., Reiken S., Motoike H., D'Armiento J., Marks A. R., Kass R. S. (2002). Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science 295, 496–499 10.1126/science.1066843 [DOI] [PubMed] [Google Scholar]
- Mathias R. T., Kistler J., Donaldson P. (2007). The lens circulation. J. Membr. Biol. 216, 1–16 10.1007/s00232-007-9019-y [DOI] [PubMed] [Google Scholar]
- Matulef K., Zagotta W. N. (2003). Cyclic nucleotide-gated ion channels. Annu. Rev. Cell Dev. Biol. 19, 23–44 10.1146/annurev.cellbio.19.110701.154854 [DOI] [PubMed] [Google Scholar]
- Maurice D. H. (2013). PDE8A runs interference to limit PKA inhibition of Raf-1. Proc. Natl. Acad. Sci. USA 110, 6248–6249 10.1073/pnas.1303920110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means C. K., Lygren B., Langeberg L. K., Jain A., Dixon R. E., Vega A. L., Gold M. G., Petrosyan S., Taylor S. S., Murphy A. N. et al. (2011). An entirely specific type I A-kinase anchoring protein that can sequester two molecules of protein kinase A at mitochondria. Proc. Natl. Acad. Sci. USA 108, E1227–E1235 10.1073/pnas.1107182108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar J. K., Pickard B. S., Mackie S., James R., Christie S., Buchanan S. R., Malloy M. P., Chubb J. E., Huston E., Baillie G. S. et al. (2005). DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science 310, 1187–1191 10.1126/science.1112915 [DOI] [PubMed] [Google Scholar]
- Miller R. A., Chu Q., Xie J., Foretz M., Viollet B., Birnbaum M. J. (2013). Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature 494, 256–260 10.1038/nature11808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullard A. (2012). Protein-protein interaction inhibitors get into the groove. Nat. Rev. Drug Discov. 11, 173–175 10.1038/nrd3680 [DOI] [PubMed] [Google Scholar]
- Okuzumi T., Fiedler D., Zhang C., Gray D. C., Aizenstein B., Hoffman R., Shokat K. M. (2009). Inhibitor hijacking of Akt activation. Nat. Chem. Biol. 5, 484–493 10.1038/nchembio.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuzumi T., Ducker G. S., Zhang C., Aizenstein B., Hoffman R., Shokat K. M. (2010). Synthesis and evaluation of indazole based analog sensitive Akt inhibitors. Mol. Biosyst. 6, 1389–1402 10.1039/c003917a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera R. K., Nikolaev V. O. (2013). Compartmentation of cAMP signalling in cardiomyocytes in health and disease. Acta Physiol. (Oxf.) 207, 650–662 10.1111/apha.12077 [DOI] [PubMed] [Google Scholar]
- Piggott L. A., Bauman A. L., Scott J. D., Dessauer C. W. (2008). The A-kinase anchoring protein Yotiao binds and regulates adenylyl cyclase in brain. Proc. Natl. Acad. Sci. USA 105, 13835–13840 10.1073/pnas.0712100105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulikakos P. I., Zhang C., Bollag G., Shokat K. M., Rosen N. (2010). RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 464, 427–430 10.1038/nature08902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince J. T., Ahn N. G. (2010). The case of the disappearing drug target. Mol. Cell 37, 455–456 10.1016/j.molcel.2010.02.010 [DOI] [PubMed] [Google Scholar]
- Rehmann H. (2012). Epac2: a sulfonylurea receptor? Biochem. Soc. Trans. 40, 6–10 10.1042/BST20110640 [DOI] [PubMed] [Google Scholar]
- Reichow S. L., Gonen T. (2008). Noncanonical binding of calmodulin to aquaporin-0: implications for channel regulation. Structure 16, 1389–1398 10.1016/j.str.2008.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner K. J. (2013). Proteomic analyses of PKA and AKAP signaling in cocaine addiction. Neuropsychopharmacology 38, 251–252 10.1038/npp.2012.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W., Kumar A., Xiao G., Wilkinson M., Covington H. E., 3rd, Maze I., Sikder D., Robison A. J., LaPlant Q., Dietz D. M. et al. (2009). Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins. Neuron 62, 335–348 10.1016/j.neuron.2009.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter S., Gorny X., Marco-Pallares J., Krämer U. M., Machts J., Barman A., Bernstein H. G., Schüle R., Schöls L., Rodriguez-Fornells A. et al. (2011). A Potential Role for a Genetic Variation of AKAP5 in Human Aggression and Anger Control. Front Hum. Neurosci 5, 175 10.3389/fnhum.2011.00175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth B. L., Marshall F. H. (2012). NOBEL 2012 Chemistry: Studies of a ubiquitous receptor family. Nature 492, 57 10.1038/492057a [DOI] [PubMed] [Google Scholar]
- Sarma G. N., Kinderman F. S., Kim C., von Daake S., Chen L., Wang B. C., Taylor S. S. (2010). Structure of D-AKAP2:PKA RI complex: insights into AKAP specificity and selectivity. Structure 18, 155–166 10.1016/j.str.2009.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sculptoreanu A., Scheuer T., Catterall W. A. (1993). Voltage-dependent potentiation of L-type Ca2+ channels due to phosphorylation by cAMP-dependent protein kinase. Nature 364, 240–243 10.1038/364240a0 [DOI] [PubMed] [Google Scholar]
- Shibasaki T., Takahashi H., Miki T., Sunaga Y., Matsumura K., Yamanaka M., Zhang C., Tamamoto A., Satoh T., Miyazaki J. et al. (2007). Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc. Natl. Acad. Sci. USA 104, 19333–19338 10.1073/pnas.0707054104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short R. A., Tuttle K. R. (2005). Clinical evidence for the influence of uric acid on hypertension, cardiovascular disease, and kidney disease: a statistical modeling perspective. Semin. Nephrol. 25, 25–31 10.1016/j.semnephrol.2004.09.005 [DOI] [PubMed] [Google Scholar]
- Smith F. D., Langeberg L. K., Cellurale C., Pawson T., Morrison D. K., Davis R. J., Scott J. D. (2010). AKAP-Lbc enhances cyclic AMP control of the ERK1/2 cascade. Nat. Cell Biol. 12, 1242–1249 10.1038/ncb2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søberg K., Jahnsen T., Rognes T., Skålhegg B. S., Laerdahl J. K. (2013). Evolutionary paths of the cAMP-dependent protein kinase (PKA) catalytic subunits. PLoS ONE 8, e60935 10.1371/journal.pone.0060935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperandio O., Reynès C. H., Camproux A. C., Villoutreix B. O. (2010). Rationalizing the chemical space of protein-protein interaction inhibitors. Drug Discov. Today 15, 220–229 10.1016/j.drudis.2009.11.007 [DOI] [PubMed] [Google Scholar]
- Stapleton M. P. (1997). Sir James Black and propranolol. The role of the basic sciences in the history of cardiovascular pharmacology. Tex. Heart Inst. J. 24, 336–342 [PMC free article] [PubMed] [Google Scholar]
- Sutherland E. W. (1971). [Nobel prize in physiology or medicine 1971: the action of hormones outlined]. Lakartidningen 68, 4991–4995 [PubMed] [Google Scholar]
- Taylor S. S., Ilouz R., Zhang P., Kornev A. P. (2012). Assembly of allosteric macromolecular switches: lessons from PKA. Nat. Rev. Mol. Cell Biol. 13, 646–658 10.1038/nrm3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrenoire C., Houslay M. D., Baillie G. S., Kass R. S. (2009). The cardiac IKs potassium channel macromolecular complex includes the phosphodiesterase PDE4D3. J. Biol. Chem. 284, 9140–9146 10.1074/jbc.M805366200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tröger J., Moutty M. C., Skroblin P., Klussmann E. (2012). A-kinase anchoring proteins as potential drug targets. Br. J. Pharmacol. 166, 420–433 10.1111/j.1476-5381.2011.01796.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle K. R., Bakris G. L., Toto R. D., McGill J. B., Hu K., Anderson P. W. (2005). The effect of ruboxistaurin on nephropathy in type 2 diabetes. Diabetes Care 28, 2686–2690 10.2337/diacare.28.11.2686 [DOI] [PubMed] [Google Scholar]
- Varadaraj K., Kumari S., Shiels A., Mathias R. T. (2005). Regulation of aquaporin water permeability in the lens. Invest. Ophthalmol. Vis. Sci. 46, 1393–1402 10.1167/iovs.04-1217 [DOI] [PubMed] [Google Scholar]
- Welch E. J., Jones B. W., Scott J. D. (2010). Networking with AKAPs: context-dependent regulation of anchored enzymes. Mol. Interv. 10, 86–97 10.1124/mi.10.2.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J. A., McClendon C. L. (2007). Reaching for high-hanging fruit in drug discovery at protein-protein interfaces. Nature 450, 1001–1009 10.1038/nature06526 [DOI] [PubMed] [Google Scholar]
- Willoughby D., Wong W., Schaack J., Scott J. D., Cooper D. M. (2006). An anchored PKA and PDE4 complex regulates subplasmalemmal cAMP dynamics. EMBO J. 25, 2051–2061 10.1038/sj.emboj.7601113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W., Scott J. D. (2004). AKAP signalling complexes: focal points in space and time. Nat. Rev. Mol. Cell Biol. 5, 959–970 10.1038/nrm1527 [DOI] [PubMed] [Google Scholar]
- Wong W., Goehring A. S., Kapiloff M. S., Langeberg L. K., Scott J. D. (2008). mAKAP compartmentalizes oxygen-dependent control of HIF-1alpha. Sci. Signal. 1, ra18 10.1126/scisignal.2000026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Dent P., Jelinek T., Wolfman A., Weber M. J., Sturgill T. W. (1993). Inhibition of the EGF-activated MAP kinase signaling pathway by adenosine 3′,5′-monophosphate. Science 262, 1065–1069 10.1126/science.7694366 [DOI] [PubMed] [Google Scholar]
- Wu J., Brown S. H., von Daake S., Taylor S. S. (2007). PKA type IIalpha holoenzyme reveals a combinatorial strategy for isoform diversity. Science 318, 274–279 10.1126/science.1146447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., van Berkel T. J., Biessen E. A. (2007). Therapeutic potential of VIVIT, a selective peptide inhibitor of nuclear factor of activated T cells, in cardiovascular disorders. Cardiovasc. Drug Rev. 25, 175–187 10.1111/j.1527-3466.2007.00011.x [DOI] [PubMed] [Google Scholar]
- Zaccolo M., Pozzan T. (2002). Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science 295, 1711–1715 10.1126/science.1069982 [DOI] [PubMed] [Google Scholar]
- Zetterqvist O., Ragnarsson U. (1982). The structural requirements of substrates of cyclic AMP-dependent protein kinase. FEBS Lett. 139, 287–290 10.1016/0014-5793(82)80872-7 [DOI] [PubMed] [Google Scholar]
- Zhang C. L., Katoh M., Shibasaki T., Minami K., Sunaga Y., Takahashi H., Yokoi N., Iwasaki M., Miki T., Seino S. (2009). The cAMP sensor Epac2 is a direct target of antidiabetic sulfonylurea drugs. Science 325, 607–610 10.1126/science.1172256 [DOI] [PubMed] [Google Scholar]
- Zhang P., Smith-Nguyen E. V., Keshwani M. M., Deal M. S., Kornev A. P., Taylor S. S. (2012). Structure and allostery of the PKA RIIβ tetrameric holoenzyme. Science 335, 712–716 10.1126/science.1213979 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.