Abstract
Hydroxamate-based histone deacetylase inhibitors (Hb-HDACIs), such as vorinostat, belinostat and panobinostat, have been previously shown to have a wide range of activity in hematologic malignancies such as cutaneous T-cell lymphoma and multiple myeloma. Recent data show that they synergize with a variety of cytotoxic and molecular targeted agents in many different solid tumors, including breast, prostate, pancreatic, lung and ovarian cancer. Hb-HDACIs have a quite good toxicity profile and are now being tested in phase I and II clinical trials in solid tumors with promising results in selected neoplasms, such as hepatocarcinoma. This review will focus on their clinical activity and safety in patients with advanced solid neoplasms.
Keywords: hydroxamate-based histone deacetylase inhibitors, histone deacetylase inhibitors, vorinostat, belinostat, panobinostat, pracinostat, abexinostat, resminostat
1. Introduction
Over the past decade, considerable research interest has been focused on developing innovative drugs able to target specific mechanisms strictly related to cancer initiation and progression. The major aim of this novel approach has been to produce anticancer agents with specific selectivity for cancer cells in order to obtain more effective treatments with less toxicities than classical approaches, such as chemotherapy and radiotherapy. This has been made possible by the extraordinary progress in the understanding of molecular pathways involved in cancer development, including those regulating the cellular epigenome in hematological and solid tumor malignancies [1]. Epigenetics refers to the heritable changes in gene expression caused by complex mechanisms regulating histone modification and chromatin remodeling, DNA methylation, loss of imprinting, and microRNAs interference, without changes in the DNA nucleotide sequence [2,3]. Dysregulation of these pathways has been related to the development of many different pathologies, including cellular aging and cancer [2,4]. A great deal of attention has been devoted in the last years to the study of histone acetylation, one of the best described epigenetic process, which represents a key regulatory mechanism regulating gene expression. Essentially, increased acetylation of histones is associated with an increased transcriptional activity, whereas deacetylation is associated with silencing of gene expression [5]. Histone deacetylases (HDACs) are a class of enzymes that influence gene expression by altering the acetylation status of nucleosomal histones as well as of a number of non-histone proteins the regulation of multiple cell pathways [6]. The HDAC protein family consists of 18 members and is divided into four classes based on size, cellular localization, number of catalytic active sites, homology to yeast HDAC proteins and susceptibility to different inhibitors [7,8]. Classes I, II, and IV are zinc (Zn2+)-dependent enzymes [9], while class III HDACs, or sirtuins, do not contain zinc and require nicotinamide adenine dinucleotide (NAD+) for their catalytic activity [10]. The Zn2+-dependent HDACs have a tube-like catalytic pocket with a Zn2+ at its end [11].
HDACs are over-expressed in several solid tumors, making them an attractive target of anti-cancer drugs [12]. Altered HDAC activity has been associated with a variety of cancers, including ovarian, gastric, lung, breast, pancreatic, colorectal and prostate cancer [13,14,15,16,17,18,19]. New drug discovery programs using a wide range of procedures, including structure-based drug design and high-throughput screening, has allowed to identify multiple categories of HDAC inhibitors (HDACIs).
1.1. Rational for Combining HDACIs with Other Anticancer Agents
Compared to normal cells, transformed cells are particularly sensitive to HDAC inhibition, indicating a pivotal role for HDACs on maintenance of the neoplastic phenotype [20,21,22]. These enzymes seem to confer a survival advantage to cancer cells by regulating the expression of genes involved in growth, differentiation and apoptosis [23]. For this reason, agents able to interfere with cell growth and survival are potential enhancer of the HDACIs anticancer activity.
According to this general assumption, most of the chemotherapeutic and targeted agents used for cancer treatment might synergize with HDACIs. Actually, experimental evidences support the ability of HDACIs to increase the anticancer activities of a plethora of agents, both in vitro and in vivo [24]. However, the mechanisms underlying the additive or synergistic effect of the combinations remain in many cases unknown. In most clinical trials the rationale for the combination is based on the preclinical anticancer activity of the compounds, but the specific molecular mechanism is usually not described [25].
In recent years, the greater understanding of the molecular alterations involved in cell transformation and the knowledge of the mechanisms of action of many anticancer agents, including HDACIs, have allowed a better characterization of the pathways involved in the synergism observed when some HDACIs are combined with other agents. For example, the recognized ability of HDACIs to affect the expression of genes involved in DNA-damage response is probably the cause of their synergism with cytotoxic agents that specifically determine DNA-damage, such as antimetabolites, platinum derivatives, alchilant agents and topoisomerase II inhibitors [26,27]. The synergism of HDACIs with taxanes might be explained by an increased microtubules stabilization due to the inhibition of HDAC-6, enzyme responsible for α-tubulin deacetylation [28]. Other compounds that synergize with HDACIs are the proteosome inhibitors such as bortezomib, marizomib, and carfilzomib [29]. The proteosome system is widely utilized by cancer cells to degrade the excess of misfolded proteins accumulated inside the cells during their rapid turnover, a process indispensable for cancer cell survival. In case of hampered proteosome activity, such as in presence of bortezomib, cells activate the formation of aggresome, an escape pathway that allows the degradation of misfolded proteins and that depends on the HDAC6-mediated deacetylation of α-tubulin [30]. The synergism between bortezomib and HDACIs is, therefore, due to the simultaneous inhibition of proteosome and aggresome, a dual block that determines intracellular accumulation of misfolded proteins and, as a consequence, cell apoptosis [31].
Another well described anticancer effect of HDACIs is the inhibition of angiogenesis, resulting from the downregulation of genes involved in angiogenesis, such as the vascular endothelial growth factor (VEGF) and the endothelial nitric oxide synthase (eNOS) [32,33,34]. This effect is the rationale for the association of HDACIs with antiangiogenetic agents, such as bevacizumab [35].
1.2. Structural Requirements of Hb-HDACIs
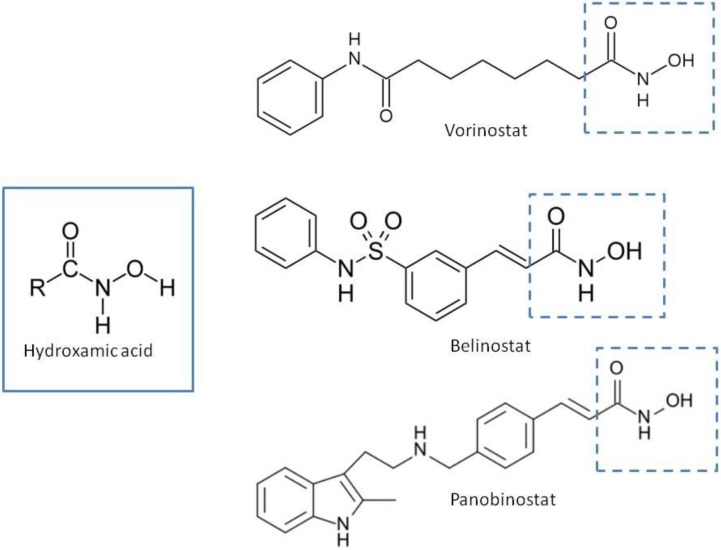
Natural and synthetic HDACIs can be structurally divided into four major structural classes: hydroxamic acids (Hb-HDACIs), small molecular weight carboxylates, benzamides and cyclic peptides [36]. Hb-HDACIs typically contain a metal-binding moiety represented by the hydroxamic acid group (Figure 1).
Figure 1.
Chemical structures of Hb-HDACIs. Three different Hb-HDACIs, vorinostat, belinostat and panobinostat, are depicted in their planar formulae. The hydroxamic acid group, which characterizes this class of HDACIs, is represented in the square. It is responsible for the binding of these compounds to the zinc ion located into the catalytic domain of the enzyme.
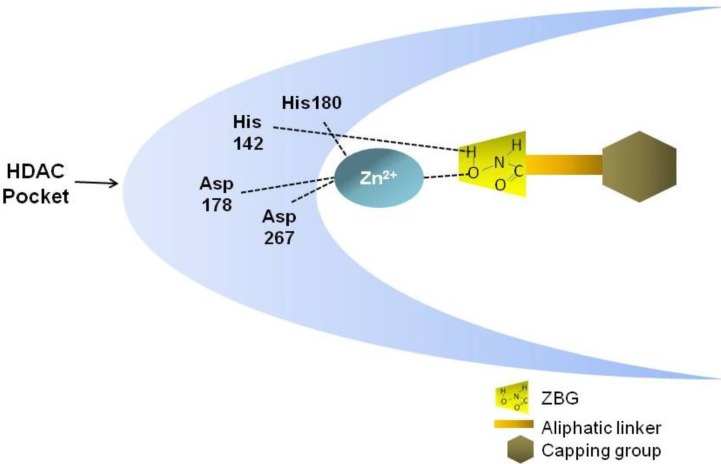
The general structure of these compounds consists of three structural motifs that differently interact with the catalytic pocket of the HDAC enzyme: the hydroxamic acid group able to chelate the Zn2+ metal ions, an aliphatic linker, and a capping group that interacts with the residues at the entrance of the active site (Figure 2) [37]. By inserting in the HDAC catalytic pocket, Hb-HDACIs block the substrate access to the enzyme, inhibiting its activity with the consequence of an accumulation of acetylated histone and nonhistone proteins [38].
Figure 2.
General structure of Hb-HDACI and its interaction with the HDAC enzyme. Hb-HDACIs contain three distinct structural motifs: a zinc binding group represented by the hydroxamic acid, an aliphatic linker that occupies the cavity of the HDAC enzyme, and a surface recognition cap group. The hydroxamic acid interacts with the zinc ion and the histidine 142 in the HDAC pocket inhibiting the enzyme activity.
The Hb-HDACIs such as abexinostat (PCI-24781/CRA-024781), belinostat (PXD101), CHR-2845, CHR-3996, dacinostat (NVPLAQ824), givinostat (ITF2357), panobinostat (LBH-589), pracinostat (SB939), resminostat (4SC AG), trichostatin A and vorinostat (SAHA) can directly inhibit the HDAC enzyme by docking in its catalytic pocket, while others HDACIs, such as the cyclic tetrapeptide romidepsin (a non-Hb-HDACI), are prodrugs that require to be converted in a reduced intermediate to be active [39]. The compounds belonging to the class Hb-HDACIs and investigated in clinical trials are listed in Table 1. These agents show different toxicity profiles in solid tumors and have been administered by different routes (Table 2).
Table 1.
Hb-HDACIs in clinical trials for cancer treatment. The hydroxamic acid group, which characterizes this class of HDACIs, is represented in the red dotted square.
| Hb-HDACIs | Structure | Chemical name | Formula | Molecular mass |
|---|---|---|---|---|
| Vorinostat | 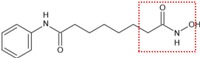 |
N-hydroxy-N'-phenyl-octanediamide | C14H20N2O3 | 264.32 g/mol |
| Belinostat (PXD101) | 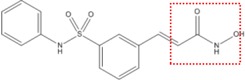 |
(2E)-N-Hydroxy-3-[3-(phenylsulfamoyl) phenyl]prop-2-enamide | C15H14N2O4S | 318.35 g/mol |
| Panobinostat (LBH589) | 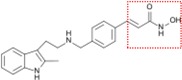 |
(2E)-N-hydroxy-3-[4-({[2-(2-methyl-1H-indol-3l)ethyl]amino}methyl) phenyl]acrylamide | C21H23N3O2 | 349.42 g/mol |
| Pracinostat (SB939) | 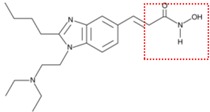 |
(E)-3-(2-butyl-1-(2-(diethylamino)ethyl)-1H-benzo[d]imidazol-5-yl)-N-hydroxyacrylamide | C20H30N4O2 | 358.48 g/mol |
| Abexinostat (PCI-24781 /CRA-024781) |
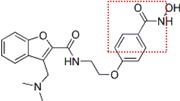 |
3-[(Dimethylamino) methyl]-N-{2-[4-(hydroxycarbamoyl) phenoxy]ethyl}-1-benzofuran-2-carboxamide | C21H23N3O5 | 397.42 g/mol |
| JNJ-26481585 | 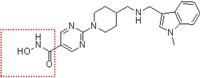 |
N-hydroxy-2-(4-(((1-methyl-1H-indol-3-yl)methylamino) methyl)piperidin-1-yl) pyrimidine-5-carboxamide |
C21H26N6O2 | 394.47 g/mol |
| Dacinostat (LAQ824) | 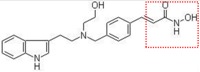 |
(E)-3-(4-(((2-(1H-indol-3-yl) ethyl)(2-hydroxyethyl)amino) methyl)phenyl)-N-hydroxyacrylamide |
C22H25N3O3 | 379.45 g/mol |
| Resminostat (RAS2410/4SC-201) | 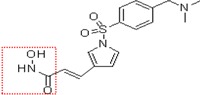 |
(E)-3-(1-((4-((dimethyl amino) methyl)phenyl)sulfonyl)-1H-pyrrol-3-yl)-N-hydroxyacrylamide |
C16H19N3O4S | 349.10 g/mol |
| CHR-3996 | 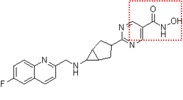 |
2-(6-(((6-fluoroquinolin-2-yl) methyl)amino) bicycle [3,1,0]hexan-3-yl)-N-hydroxypy rimidine-5-carboxamide |
C21H20FN5O2 | 393.16 g/mol |
Table 2.
Hb-HDACIs in the clinic.
| HDACIs | Routes of administration | Side effects | FDA approval |
|---|---|---|---|
| Vorinostat | Oral | Anorexia, fatigue, dehydration, diarrhea, and myelosuppression | Cutaneous T-cell lymphoma |
| Belinostat (PXD101) | Oral, i.v. | Lethargy/fatigue, nausea and vomiting | Granted orphan drug and fast track designations for relapsed or refractory peripheral T-cell lymphoma |
| Panobinostat (LBH589) | Oral, i.v | Fatigue, nausea, diarrhea and myelosuppression | Not approved |
| Pracinostat (SB939) | Oral | Fatigue, nausea, vomiting, anorexia and diarrhoea | Not approved |
| Abexinostat (PCI-24781/ CRA-024781) | Oral, i.v. | Under evaluation in clinical trials | Not approved |
| JNJ-26481585 | Oral | Under evaluation in clinical trials | Not approved |
| Dacinostat (LAQ824) | i.v. | Nausea, vomiting and fatigue | Not approved |
| Resminostat (RAS2410/4SC-201) | Oral | Under evaluation in clinical trials | Granted orphan drug designation in relapsed/refractory Hodgkin’s lymphoma and hepatocellular carcinoma |
| CHR-3996 | Oral | Thrombocytopenia, fatigue, increase of plasma creatinine and atrial fibrillation | Not approved |
Abbreviations: FDA, United States Food and Drug Administration; i.v., intravenous.
Several clinical trials have been completed and many others are ongoing using Hb-HDACIs in patients with different haematological malignancies, leading to approval of vorinostat on October 6, 2006 by the U.S. Food and Drug Administration (FDA) for the treatment of advanced forms of cutaneous T-cell lymphoma (CTCL) that have failed multiple other systemic treatment options [40,41,42,43]. Among Hb-HDACIs reported in literature [44,45], here we will focus only on those which are currently under investigation in phase I and phase II clinical trials for solid tumors, such as vorinostat, belinostat, panobinostat and other novel “second-generation” molecules, such as pracinostat, abexinostat, JNJ-26481585, dacinostat, and resminostat, which show promising clinical activity in this field.
2. Vorinostat in Solid Tumors
The discovery of hexamethylene bisacetamide (HMBA), a polar compound able to induce differentiation of transformed cells [39,46], and the attempt to overcome its severe side effects [46,47], especially thrombocytopenia, have brought to the development of vorinostat (SAHA), a second-generation compound endowed with greater potency (about 2,000-fold) and less toxicity than HMBA. This new Hb-HDACI has been tested in phase I/II clinical trials either as single agent or in combination therapy.
2.1. Vorinostat as a Single Agent
Oral vorinostat administered as 200 mg twice daily or 400 mg daily was a well-tolerated agent in phase I trials [48,49]. Pharmacokinetic analysis shows that the bioavailability of vorinostat ranges from 34.9% to 52.3% (only slightly improved with food), with a half-life of 91–127 minutes and an inhibition time of HDAC ≥ 10 hours [48]. Predominant toxicities of vorinostat include anorexia, fatigue, dehydration, diarrhea, and myelosuppression. It has also been suggested that the approved dose of vorinostat (single 400 mg p.o. daily dose) should be reduced in patients with hepatic dysfunction [49].
In the last few years, the clinical activity of Hb-HDACIs has been mainly tested in hematological malignancies with few studies testing their activity in solid tumors. In phase II trials (Table 3), no responses to oral vorinostat have been observed in patients with relapsed or refractory non small cell lung (NSCLC), colorectal, prostate, head and neck cancer [8,50,51,52,53,54]. A modest clinical activity of vorinostat has been reported in ovarian cancer, primary peritoneal carcinoma and recurrent glioblastoma multiforme [8,55,56]. In these phase II trials, vorinostat was well tolerated: anorexia, fatigue, nausea and vomiting, diarrhea, anemia and thrombocytopenia were frequently observed, but none of these drug-related toxicities was severe.
Table 3.
Vorinostat in published phase II clinical trials in solid tumors.
| Disease | Regimen | No. pts. | PFS | Efficacy | Ref. |
|---|---|---|---|---|---|
| Metastatic head and neck cancer | Vorinostat: 400 mg orally daily | 13 | SD 2 pts. OR 0% | [51] | |
| Metastatic breast cancer | Vorinostat: 400 mg orally daily | 14 | SD 4 pts | [52] | |
| Relapsed NSCLC | Vorinostat: 400 mg or 300 mg once daily on days 1–14 of the 21 day cycle | 16 | Median: 2.3 m. | SD 57% | [53] |
| Metastatic prostate cancer | Vorinostat: 400 mg orally daily | 27 | Median: 2.8 m. | SD 2 pts. | [54] |
| Ovarian or primary peritoneal carcinoma | Vorinostat: 400 mg orally daily | 27 | PR 1 pt. | [55] | |
| Recurrent GBM | Vorinostat: 200 mg p.o.twice a day for 14 days, followed by a 7-day rest period | 66 | 6-m.-PFS: 9 of the first 52 patients | OR 2 pts. | [56] |
| Metastatic colorectal cancer |
Vorinostat: 800 or 1,400 mg/day once a day × 3 days, every 2 weeks +5-FU: preceded by leucovorin, at 400 mg/m2 followed by a 46 h infusion at 2.400 mg/m2 on days 2–3 |
58 | PFS rate did not reach the threshold of 27 out of 43 patients | PR 1 pt. | [57] |
| Untreated stage IIIB or IV NSCLC |
Vorinostat: 400 mg or Placebo on days 1 through 14 of each 3-week cycle +Carboplatin: AUC 6 + Paclitaxel: 200 mg/m2, both on day 3 of each 21-day cycle |
94 | Median: Vorinostat: 6.0 m. Placebo: 4.1 m. P = ns |
RR Vorinostat: 34% Placebo: 12.5% P = 02 |
[58] |
| Advanced thyroid cancer | Vorinostat: 400 mg orally daily | 19 | SD 56% OR 0 | [59] | |
| Recurrent GBM | Vorinostat: 400 mg daily for 14 days of a 21-day cycle + Bortezomib: 1.3 mg/m2 intravenously on days 1,4, 8, and 11 of a 21-day cycle | 37 | Median: 1.5 m. | PR 1 pt. | [60] |
Abbreviations: 5-FU, 5-fluorouracil; 6-m.-PFS, progression free survival at 6 months; AUC, area under the curve; CB, clinical benefit; GBM, glioblastoma multiforme; LV, leucovorin; m., months; MBC, metastatic breast cancer; No., number; NSCLC, non small cell lung cancer; OR, objective response; PFS, progression free survival; PR, partial response; pts., patients; Ref. , references; RR, objective response rate.
2.2. Vorinostat in Combination Therapy
Even if vorinostat demonstrated a low clinical activity in solid tumors, in consideration of its acceptable safety profile and the oral route of administration, further studies were carried out to investigate this drug in association with other antineoplastic agents in patients affected by different types of advanced solid tumors. Vorinostat has been tested in combination with 5-FU, leucovorin (LV) with or without oxaliplatin in patients with metastatic colorectal cancer [61,62,63]. The rational of these association is based on the reported anticancer synergisms between vorinostat and both 5-FU and oxaliplatin in vitro and in vivo colorectal cancer models [63,64]. However, the combination of these drugs did not allow to obtain significant improvements in response rate or time to disease progression, the best results being represented by disease stabilization in some patients [61,62,63].
The combination of vorinostat with docetaxel, in patients with advanced or recurrent cancer, resulted in a very high toxicity without any clinical activity, leading to an early closure of the trial [28]. Similarly, vorinostat, in combination with weekly doxorubicin in solid tumors showed a minimal activity at the expenses of a high incidence of hematological toxicity and thromboembolic events [65]. On the other hand, oral vorinostat at 200 mg for 7 days in combination with weekly vinorelbine at 25 mg/m2 was a well tolerated regimen, even if two cases of grade 3 hyperglycemia were observed at the first dose level [66]. More promising results have been obtained in patients with metastatic NSCLC and breast cancer [67,68]. In preclinical models, vorinostat, when co-administered with platinum agents, has been shown to increase platinum adduct formation by relaxing the chromatin structure, thus allowing a more efficient anticancer activity of the alkylating agents [69]. Vorinostat has also been reported to synergize with taxanes through the inhibition of HDAC-6, responsible of α-tubulin de-acetylation [70]. These mechanisms could lead to more stable microtubules, resulting in the arrest of cell division [28]. In NSCLC, vorinostat in combination with carboplatin and paclitaxel allowed to obtain a response rate of 34% as compared to 12.5% of placebo, with the vorinostat arm showing a trend toward a longer progression-free survival (6.0 months vs. 4.1 months) and overall survival (13.0 months vs. 9.7 months) [58]. However, a higher incidence of toxicity, including nausea/vomiting, fatigue, hyponatremia, and thrombocytopenia in the vorinostat arm, prompted 26% of patients to discontinue therapy [58].
Oral vorinostat at 400 mg daily for 3 out of 4 weeks and oral tamoxifen at 20 mg daily, in patients with endocrine-resistant advanced breast cancer, obtained an objective response rate of 19%, a clinical benefit rate of 40%, and a median time to disease progression of 10.3 months [68]. The favorable toxicity profile of this combination allowed up to 2 years treatment for responding patients [58].
Good results are coming from trials testing vorinostat in combination with chemotherapy and targeted therapy such as bevacizumab, the anti-vascular endothelia growth factor monoclonal antibody [71]. In patients with metastatic breast cancer, vorinostat (200 or 300 mg p.o. BID) on days 1–3, 8–10, and 15–17, plus paclitaxel (90 mg/m2) on days 2, 9, 16, and bevacizumab (10 mg/kg) on days 2 and 16 every 28 days, allowed to obtain a response rate of 55%, with only an increased incidence of diarrhea with respect to the expected toxicity profile of paclitaxel and bevacizumab [72].
Vorinostat has also been tested in combination with bevacizumab and CPT-11 in patient with recurrent glioblastoma [73]. Dose-limiting toxicities were represented by mucositis, fatigue and diarrhea, with a trend for better progression free survival and overall survival for patients treated with the higher doses of vorinostat [73]. However, vorinostat in combination with the proteasome inhibitor bortezomib [74] had only a minimal clinical activity in the same category of patients [60]. Vorinostat was also combined with flavopiridol, an inhibitor of cyclin-dependent kinases [75]. In a phase I study, eight out of 34 patients with solid tumors refractory to standard therapy had stable disease for an average 5.5 months, with no objective responses being observed [76]. In another phase I study vorinostat was associated with the novel proteasome inhibitor marizomib in 22 patients with melanoma, pancreatic carcinoma or NSCLC. The combination was well tolerated with a stable disease observed in 61% of patients, including 39% with a minimal tumor size reduction [77].
3. Belinostat in Solid Tumors
Belinostat (PXD101) is a novel and potent class I and II Hb-HDACI, active at nanomolar concentrations [78,79]. Belinostat demonstrated a high antitumor activity both in vitro and in vivo, either alone or in combination with antineoplastic agents such as paclitaxel, docetaxel and carboplatin [79,80]. Belinostat, tested in phase II clinical trials for solid tumors, has shown an encouraging clinical activity (Table 4). The response rate and the safety profile of belinostat administered as a second line therapy in patient with recurrent or refractory malignant pleural mesothelioma was evaluated in a phase II study [81]. In this trial, among 13 patients evaluable for response assessment, only two patients had stabilization of disease, with no partial responses.
Table 4.
Belinostat in published phase II clinical trials in solid tumors.
| Disease | Regimen | No. pts. | PFS | Efficacy | Ref. |
|---|---|---|---|---|---|
| Recurrent or refractory malignant pleural mesothelioma | Belinostat: 1 g/m2 IV on days 1 to 5 of a 21-day cycle | 13 | Median: 1 m. | SD 2 pts. | [81] |
| Recurrent or refractory advanced thymic epithelial tumors | Belinostat: 1 g/m2 IV on days 1 to 5 of a 21-day cycle | 41 | 6-m.- PFS: 46% | RR 8% CB 68% |
[82] |
| Unresectable hepatocellular carcinoma | Belinostat: 1,400 mg/m2 per day, on days 1–5 every 3 weeks | 54 | Median: 2.64 m. | PR 2.4% SD 45.2% |
[83] |
| Platinum resistant EOC and LMP ovarian tumors | Belinostat: 1 g/m2 IV on days 1 to 5 of a 21-day cycle | 32 |
EOC: Median: 2.3 m. LMP: Median: 13.4 m. |
EOC: SD 9/15 pts LMP: PR 2/12 pts | [84] |
| Previously treated ovarian, fallopian tube, or primary peritoneal carcinoma |
Belinostat: 1g/m² IV daily for 5 days of a 21-day cycle +Carboplatin: AUC 5 on day three of 21-day cycles |
29 | Median: 3.3 m. | RR 7.4% CR 3.7% PR 3.7% SD 44.4% | [85] |
| Previously treated ovarian cancer |
Belinostat: 1g/m² IV daily for 5 days of a 21-day cycle +Carboplatin: AUC 5 +Paclitaxel: 175 mg/m² both on day 3 of each 21-day cycle |
35 | 6-m. PFS: 48% | RR 43% | [86] |
Abbreviations: 6-m.-PFS, progression free survival at 6 months; AUC, area under the curve; CB, clinical benefit; CR, complete response; EOC, epithelial ovarian cancer; LMP, micropapillary; m., months; No., number; PFS, progression free survival; pts, patients; Ref., references; RR, objective response rate.
3.1. Belinostat as a Single Agent
In a phase I study, belinostat, administered i.v. on days 1 to 5 every 21 days and tested on 48 patients with advanced malignancies, showed a good tolerability profile with the maximum tolerated dose set at 1,000 mg/m2/d i.v. [87]. At this dose level, the most common adverse events were represented by lethargy/fatigue, nausea and vomiting. Interestingly, 39% of patients treated with belinostat achieved a stable disease and showed an increment of a caspase-dependent cleavage of cytokeratin 18 (a serum marker of apoptosis in epithelial tumors) during treatment [87,88]. Belinostat in monotherapy has also been tested in 41 patients with advanced thymic epithelial malignancies after a first line platinum chemotherapy regimen [82]. Interestingly, two patients affected by thymoma showed a partial response, 25 patients achieved a stable disease, while patients affected by thymic carcinoma did not respond to treatment. A phase I–II study in patients with unresectable hepatocellular carcinoma, belinostat at 1,400 mg/m2 per day, on days 1–5 every 3 weeks, allowed to obtain stable disease 45.2% of patients, with a median progression free survival of 2.64 months (95% CI, 1.55–3.17 months) [83]. Belinostat, at the standard dose of 1,000 mg/m2/d on days 1–5 of a 21 day cycle, was tested in patients with platinum resistant metastatic or recurrent epithelial ovarian cancer (EOC) and micropapillary/LMP ovarian tumors [84]. Generally, belinostat was very well tolerated with fatigue and nausea representing the most frequent side effects. Nine out of 15 patients with platinum resistant EOC had stable disease and among 12 patients with micropapillary/LMP tumors, two partial responses have been observed. Similar promising results have been obtained by the combination of belinostat and carboplatin in patients with platinum-resistant ovarian, fallopian tube, or primary peritoneal carcinoma: the overall response rate was 7.4%, with 44.4% of patients reaching stable disease [85].
3.2. Belinostat in Combination Therapy
Belinostat at the maximum dose of 1,000 mg/m2 in association with carboplatin and/or paclitaxel was a well tolerated regimen in patients affected by solid tumors, the most common toxicities being represented by neutropenia, thrombocytopenia and anemia, peripheral sensory neuropathy, fatigue, vomiting and myalgia [89]. In a phase II study carried out on 35 women with recurrent ovarian cancer, belinostat in combination with carboplatin and paclitaxel showed an overall response rate of 43% and a well tolerated profile [86].
4. Panobinostat in Solid Tumors
Panobinostat (LBH589) is a novel cinnamic hydroxamic acid class of HDACIs, formulated as oral capsule or i.v. solution [90]. Panobinostat improves its anticancer activity through the hyper-acetylation of histone and non-histone proteins thus modifying chromatin structure, gene expression, transcriptional activity and apoptosis [91,92].
Efficacy of Panobinostat in Solid Tumor
Panobinostat is mainly metabolized by the human cytocrome P450 CYP3A4 and strong CYP3A4 inhibitors, such as ketoconazole, are able to increase panobinostat AUC by ~2-fold with a subsequent higher incidence of gastrointestinal adverse events [93].
In a phase I trial in patients affected by solid tumors or cutaneous T-cell lymphoma, oral panobinostat at 20 mg/m2, once daily on three alternating days of each week on a 28-day cycle, was considered as the maximum tolerated dose to be tested in phase II trials [94]. Dose limiting toxicities were represented by thrombocytopenia, anemia and, to a lesser extent, by diarrhea and nausea [94].
Panobinostat has also been tested in other phase I trials in combination with antineoplastic drugs, such as gemcitabine, docetaxel, paclitaxel, carboplatin, everolimus and bevacizumab [95,96,97,98]. In these trials oral panobinostat was used at different schedules: 30 mg three times per week every other week [35], 20 mg on three alternating days of weeks 1–2 every 21 days [95], 10 mg on three alternating days of weeks 1–2 every 21 days [98], or weekly [96,97]. Limited clinical activity has been shown by the association of panobinostat with paclitaxel and carboplatin [96], with gemcitabine [98] or with everolimus and bevacizumab [97]. In a phase I trial accruing patients with castration-resistant prostate cancer, panobinostat was administered as monotherapy (20 mg orally on three alternating days of weeks 1–2, every 4 weeks) or in combination with docetaxel 75 mg/m2 every 3 weeks plus prednisone 5 mg daily [95]. All 16 patients who received panobinostat monotherapy and two out of eight patients treated with panobinostat in combination with docetaxel had progressive disease [95]. The best promising clinical activity has been shown by the combination of panobinostat with bevacizumab in patients with recurrent high-grade glioma: among 12 enrolled patients, three patients had partial response and seven patients reached stable disease [35].
In phase II trials (Table 5), panobinostat did not show any significant clinical activity, either in monotherapy in patients with advanced refractory renal carcinoma [99], or in combination with bortezomib in patients with advanced refractory pancreatic cancer [100].
Table 5.
Panobinostat in published phase II clinical trials in solid tumors.
| Disease | Regimen | No. pts. | PFS | Efficacy | Ref. |
|---|---|---|---|---|---|
| Refractory metastatic renal cell carcinoma. | Panobinostat: 45 mg orally twice a week | 20 | OR 0 | [99] | |
| Previously treated advanced pancreatic cancer |
Panobinostat: 20 mg orally three times weekly +Bortezomib: 1.3 mg/m2 IV twice weekly, both during the first two weeks, followed by a 9-day rest period. |
Median: 2.1 m. | OR 0 | [100] |
Abbreviations: m., months; MBC, metastatic breast cancer; No., number; OR, objective response; PFS, progression free survival; pts, patients; Ref., references.
5. Other Hb-HDACIs in Solid Tumors
5.1. Pracinostat
Pracinostat (SB939) is a potent Hb-HDACI, able to inhibit HDACs class I, II, and IV, developed with the aim to increase the pharmacokinetic properties and anti-tumor activities of this class of drugs [101]. In two different phase I clinical trials performed in patients with advanced solid tumors, the recommended dose of pracinostat was fixed at 60 mg orally given either for five consecutive days every 2 weeks or 3 times a week for 3 weeks in a 4-week cycle [102,103]. Pracinostat showed a promising clinical acivity [102,103], and was well tolerated, with the most common side effects being represented by fatigue, nausea, vomiting, anorexia and diarrhoea.
5.2. Abexinostat
Abexinostat (PCI-24781/CRA-024781) is a Hb-HDACI with a high antitumor activity both in vitro and in vivo in human solid tumor xenografts [104]. Great interest has been devoted to the potential activity of abexinostat either alone or in combination with other anti-cancer agents in experimental models of soft tissue sarcomas [105,106,107], malignant peripheral nerve sheath tumors [108], glioblastoma [109] and gallbladder carcinoma [110]. The drug is currently tested in patients with refractory advanced solid tumor patient with promising clinical activity, but full data have not yet been published.
5.3. JNJ-26481585
JNJ-26481585 is an oral powerful pan-HDACI endowed with in vitro antitumor activity against lung, breast, colon, prostate, brain, and ovarian cancer cell lines and in vivo as a single agent in xenografts tumor models [111]. Interestingly, JNJ-26481585 was more active in inhibiting cell proliferation of Ras mutant than Ras wild-type colorectal cancer cells [111].
5.4. Dacinostat
Dacinostat (LAQ824) is a water soluble synthetic cinnamyl Hb-HDACI, able to inhibit HDAC at less than 0.15 μM [112]. It has been shown to be active in vitro and in vivo against multiple human solid tumors [21,113,114,115,116,117,118,119,120]. In a phase I study in patients with advanced solid tumors, dacinostat was administered i.v. as a 3-h infusion on days 1, 2, and 3 every 21 days and the recommended dose was set from 24 to 72 mg/m2 [121]. The most common toxicities of dacinostat were similar to those of other HDACIs, and represented by nausea, vomiting and fatigue. The clinical activity of dacinostat was limited, with the best response represented by disease stabilization in three out of 39 patients enrolled in the study [121].
5.5. Resminostat
The oral pan-Hb-HDACI resminostat (RAS2410/4SC-201) [122] has been used in a phase I trial in patients with advanced refractory solid tumors [123]. Nausea, vomiting and fatigue represented the most common drug-related toxicities. The recommended dose for phase II evaluation was 600 mg once daily, on day 1–5 in a 14-day cycle [123]. Resminostat is currently being tested in phase I/II clinical trials in hepatocellular carcinoma in monotherapy or in combination with sorafenib (SHELTER trial) [124] and as a second-line treatment option in patients with KRAS tumor mutations in combination with the FOLFIRI regimen colorectal cancer, but data have not yet been published.
5.6. CHR-3996
CHR-3996 is a new potent compound in development for clinical trials in solid tumors [125]. In a phase I trial in patients with refractory solid tumors, the recommended phase II dose was set at 40 mg/d orally once daily [126]. The drug was generally well tolerated, with dose limiting toxicities being represented by thrombocytopenia, fatigue, increase of plasma creatinine and atrial fibrillation [126].
6. Conclusions
Since the first HDACI was approved for clinical use in 2006, several novel Hb-HDCAIs have been tested in clinical trials, either as single agents or in combination therapies. Although many studies are still ongoing (Table 6), the available results disappointed the high expectancies based on the principle that increased function of HDAC is required for cancer cell survival and proliferation.
Table 6.
Ongoing phase II clinical trials of Hb-HDACIs in solid tumors [127].
| Disease | Compound | Regimen | No. Pts. | End-Point | ClinicalTrials. gov Identifier |
|---|---|---|---|---|---|
| Relapsed/refractorysarcomas-age 4–21 years | Vorinostat |
Vorinostat: orally on a daily × 4 schedule +Etoposide: at a fixed dose i.v. daily × 3 days |
50 | DLT MTD RR |
NCT01294670 |
| HER2-positive locally recurrent or metastatic breast cancer |
Vorinostat |
Vorinostat: 300 mg × 4 days on, then 3 days off +Lapatinib: 1,250 mg daily |
47 | CB | NCT01118975 |
| Metastatic RCC | Vorinostat | Vorinostat: escalating doses PO BID on days 1–14 +Bevacizumab: IV on day 1 of a 21-day cycle | 42 | MTD PFS |
NCT00324870 |
| Advanced soft tissue sarcomas | Vorinostat | Vorinostat: orally once daily for 14 days + Bortezomib: IV on days 1, 4, 8, 11 of a 21-day cycle | 45 | RR | NCT00937495 |
| Metastatic NSCLC | Belinostat |
Belinostat: dose escalation with starting dose 1 g/m2 i.v. on days 1-5 of a 21-day cycle +Carboplatin AUC 6 +Paclitaxel 200 mg/m2 |
35 | MTD | NCT01310244 |
| Recurrent GBM | Panobinostat |
Panobinostat: orally three times per week every other week +Bevacizumab: i.v. on days 1 and 15 of a 28-day cycle |
67 | MTD PFS |
NCT00859222 |
| Advanced sarcomas | Abexinostat | Abexinostat: orally on days 1–5 +Doxorubicin on day 4 of a 21-day cycle | 47 | MTD RR |
NCT01027910 |
| K-Ras mutated advanced CRC | Resminostat |
Resminostat: orally +OLFIRI i.v. |
80 | MTD PFS |
NCT01277406 |
| HCC pretreated with sorafenib | Resminostat | orally | 60 | PFS | NCT00943449 |
Abbreviations: CB, clinical benefit rate; CRC, colorectal carcinoma; DLT, dose limiting toxicity; GBM, glioblastoma multiforme; HCC, hepatocellular carcinoma; MBC, metastatic breast cancer; MDT, maximum tolerated dose; NSCLC, non small cell lung cancer; PFS, progression free survival; RCC, renal cell carcinoma; RR, objective response rate.
Generally, HDACIs seem to be more active in hematological malignancies than in solid tumors. The reasons for this discrepancy are unknown. A possible explanation might be related to the pharmacokinetic profile of HDACIs. In fact, these compounds have a relatively short half-life, ranging from 91.6 to 127 minutes when given orally and from 34.7 to 42.4 minutes when administered intravenously [128]. This short half-life might be responsible for higher drug concentrations in plasma than in tumor microenvironment, resulting in a more pronounced HDAC inhibition in blood rather than in tissue cancer cells. Moreover, solid tumors usually show persistent activation of the signal transducer and activator of transcription (STAT) signaling [129], a pathway that has been reported to be associated with resistance to HDCAIs [130].
Altogether, clinical trials evidence indicates that single agent Hb-HDCAIs show a very limited therapeutic activity. However, when combined with other agents, Hb-HDCAIs are able to increase their anti-tumor activity. We have to wait for the conclusion of current studies to estimate the real clinical efficacy of Hb-HDCAIs.
Conflict of Interest
The authors declare no conflict of interest
References
- 1.Bird A. Perceptions of epigenetics. Nature. 2007;447:396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- 2.Berger S.L., Kouzarides T., Shiekhattar R., Shilatifard A. An operational definition of epigenetics. Genes Dev. 2009;23:781–783. doi: 10.1101/gad.1787609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Engeland M., Derks S., Smits K.M., Meijer G.A., Herman J.G. Colorectal cancer epigenetics: Complex simplicity. J. Clin. Oncol. 2011;29:1382–1391. doi: 10.1200/JCO.2010.28.2319. [DOI] [PubMed] [Google Scholar]
- 4.Jones P.A., Baylin S.B. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wade P.A. Transcriptional control at regulatory checkpoints by histone deacetylases: Molecular connections between cancer and chromatin. Hum. Mol. Genet. 2001;10:693–698. doi: 10.1093/hmg/10.7.693. [DOI] [PubMed] [Google Scholar]
- 6.Marks P.A., Xu W.S. Histone deacetylase inhibitors: Potential in cancer therapy. J. Cell. Biochem. 2009;107:600–608. doi: 10.1002/jcb.22185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregoretti I.V., Lee Y.M., Goodson H.V. Molecular evolution of the histone deacetylase family: Functional implications of phylogenetic analysis. J. Mol. Biol. 2004;338:17–31. doi: 10.1016/j.jmb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Lane A.A., Chabner B.A. Histone deacetylase inhibitors in cancer therapy. J. Clin. Oncol. 2009;27:5459–5468. doi: 10.1200/JCO.2009.22.1291. [DOI] [PubMed] [Google Scholar]
- 9.De Ruijter A.J., van Gennip A.H., Caron H.N., Kemp S., van Kuilenburg A.B. Histone deacetylases (hdacs): Characterization of the classical hdac family. Biochem. J. 2003;370:737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blander G., Guarente L. The sir2 family of protein deacetylases. Annu. Rev. Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- 11.Bertrand P. Inside hdac with hdac inhibitors. Eur. J. Med. Chem. 2010;45:2095–2116. doi: 10.1016/j.ejmech.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 12.Yoo C.B., Jones P.A. Epigenetic therapy of cancer: Past, present and future. Nat. Rev. Drug Discov. 2006;5:37–50. doi: 10.1038/nrd1930. [DOI] [PubMed] [Google Scholar]
- 13.Khabele D., Son D.S., Parl A.K., Goldberg G.L., Augenlicht L.H., Mariadason J.M., Rice V.M. Drug-induced inactivation or gene silencing of class I histone deacetylases suppresses ovarian cancer cell growth: Implications for therapy. Cancer Biol. Ther. 2007;6:795–801. doi: 10.4161/cbt.6.5.4007. [DOI] [PubMed] [Google Scholar]
- 14.Song J., Noh J.H., Lee J.H., Eun J.W., Ahn Y.M., Kim S.Y., Lee S.H., Park W.S., Yoo N.J., Lee J.Y., et al. Increased expression of histone deacetylase 2 is found in human gastric cancer. APMIS. 2005;113:264–268. doi: 10.1111/j.1600-0463.2005.apm_04.x. [DOI] [PubMed] [Google Scholar]
- 15.Bartling B., Hofmann H.S., Boettger T., Hansen G., Burdach S., Silber R.E., Simm A. Comparative application of antibody and gene array for expression profiling in human squamous cell lung carcinoma. Lung Cancer. 2005;49:145–154. doi: 10.1016/j.lungcan.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Saji S., Kawakami M., Hayashi S., Yoshida N., Hirose M., Horiguchi S., Itoh A., Funata N., Schreiber S.L., Yoshida M., et al. Significance of hdac6 regulation via estrogen signaling for cell motility and prognosis in estrogen receptor-positive breast cancer. Oncogene. 2005;24:4531–4539. doi: 10.1038/sj.onc.1208646. [DOI] [PubMed] [Google Scholar]
- 17.Tinari N., de Tursi M., Grassadonia A., Zilli M., Stuppia L., Iacobelli S., Natoli C. An epigenetic approach to pancreatic cancer treatment: The prospective role of histone deacetylase inhibitors. Curr. Cancer Drug Targets. 2012;12:439–452. doi: 10.2174/156800912800190884. [DOI] [PubMed] [Google Scholar]
- 18.Mariadason J.M. Hdacs and hdac inhibitors in colon cancer. Epigenetics. 2008;3:28–37. doi: 10.4161/epi.3.1.5736. [DOI] [PubMed] [Google Scholar]
- 19.Abbas A., Gupta S. The role of histone deacetylases in prostate cancer. Epigenetics. 2008;3:300–309. doi: 10.4161/epi.3.6.7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nebbioso A., Clarke N., Voltz E., Germain E., Ambrosino C., Bontempo P., Alvarez R., Schiavone E.M., Ferrara F., Bresciani F., et al. Tumor-selective action of hdac inhibitors involves trail induction in acute myeloid leukemia cells. Nat. Med. 2005;11:77–84. doi: 10.1038/nm1161. [DOI] [PubMed] [Google Scholar]
- 21.Atadja P., Gao L., Kwon P., Trogani N., Walker H., Hsu M., Yeleswarapu L., Chandramouli N., Perez L., Versace R., et al. Selective growth inhibition of tumor cells by a novel histone deacetylase inhibitor, nvp-laq824. Cancer Res. 2004;64:689–695. doi: 10.1158/0008-5472.CAN-03-2043. [DOI] [PubMed] [Google Scholar]
- 22.Ungerstedt J.S., Sowa Y., Xu W.S., Shao Y., Dokmanovic M., Perez G., Ngo L., Holmgren A., Jiang X., Marks P.A. Role of thioredoxin in the response of normal and transformed cells to histone deacetylase inhibitors. Proc. Natl. Acad. Sci. USA. 2005;102:673–678. doi: 10.1073/pnas.0408732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnstone R.W. Histone-deacetylase inhibitors: Novel drugs for the treatment of cancer. Nat. Rev. Drug Discov. 2002;1:287–299. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- 24.Miller C.P., Singh M.M., Rivera-Del Valle N., Manton C.A., Chandra J. Therapeutic strategies to enhance the anticancer efficacy of histone deacetylase inhibitors. J. Biomed. Biotechnol. 2011;2011:514261. doi: 10.1155/2011/514261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bots M., Johnstone R.W. Rational combinations using hdac inhibitors. Clin. Cancer Res. 2009;15:3970–3977. doi: 10.1158/1078-0432.CCR-08-2786. [DOI] [PubMed] [Google Scholar]
- 26.Stiborova M., Eckschlager T., Poljakova J., Hrabeta J., Adam V., Kizek R., Frei E. The synergistic effects of DNA-targeted chemotherapeutics and histone deacetylase inhibitors as therapeutic strategies for cancer treatment. Curr. Med. Chem. 2012;19:4218–4238. doi: 10.2174/092986712802884286. [DOI] [PubMed] [Google Scholar]
- 27.Venkitaraman A.R. Modifying chromatin architecture during the response to DNA breakage. Crit. Rev. Biochem. Mol. Biol. 2010;45:2–13. doi: 10.3109/10409230903325446. [DOI] [PubMed] [Google Scholar]
- 28.Schneider B.J., Kalemkerian G.P., Bradley D., Smith D.C., Egorin M.J., Daignault S., Dunn R., Hussain M. Phase I study of vorinostat (suberoylanilide hydroxamic acid, nsc 701852) in combination with docetaxel in patients with advanced and relapsed solid malignancies. Invest. New Drugs. 2012;30:249–257. doi: 10.1007/s10637-010-9503-6. [DOI] [PubMed] [Google Scholar]
- 29.Spratlin J.L., Pitts T.M., Kulikowski G.N., Morelli M.P., Tentler J.J., Serkova N.J., Eckhardt S.G. Synergistic activity of histone deacetylase and proteasome inhibition against pancreatic and hepatocellular cancer cell lines. Anticancer Res. 2011;31:1093–1103. [PMC free article] [PubMed] [Google Scholar]
- 30.Kawaguchi Y., Kovacs J.J., McLaurin A., Vance J.M., Ito A., Yao T.P. The deacetylase hdac6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell. 2003;115:727–738. doi: 10.1016/S0092-8674(03)00939-5. [DOI] [PubMed] [Google Scholar]
- 31.Nawrocki S.T., Carew J.S., Pino M.S., Highshaw R.A., Andtbacka R.H., Dunner K., Jr., Pal A., Bornmann W.G., Chiao P.J., Huang P., et al. Aggresome disruption: A novel strategy to enhance bortezomib-induced apoptosis in pancreatic cancer cells. Cancer Res. 2006;66:3773–3781. doi: 10.1158/0008-5472.CAN-05-2961. [DOI] [PubMed] [Google Scholar]
- 32.Michaelis M., Michaelis U.R., Fleming I., Suhan T., Cinatl J., Blaheta R.A., Hoffmann K., Kotchetkov R., Busse R., Nau H., et al. Valproic acid inhibits angiogenesis in vitro and in vivo. Mol. Pharmacol. 2004;65:520–527. doi: 10.1124/mol.65.3.520. [DOI] [PubMed] [Google Scholar]
- 33.Deroanne C.F., Bonjean K., Servotte S., Devy L., Colige A., Clausse N., Blacher S., Verdin E., Foidart J.M., Nusgens B.V., et al. Histone deacetylases inhibitors as anti-angiogenic agents altering vascular endothelial growth factor signaling. Oncogene. 2002;21:427–436. doi: 10.1038/sj.onc.1205108. [DOI] [PubMed] [Google Scholar]
- 34.Qian D.Z., Kato Y., Shabbeer S., Wei Y., Verheul H.M., Salumbides B., Sanni T., Atadja P., Pili R. Targeting tumor angiogenesis with histone deacetylase inhibitors: The hydroxamic acid derivative lbh589. Clin. Cancer Res. 2006;12:634–642. doi: 10.1158/1078-0432.CCR-05-1132. [DOI] [PubMed] [Google Scholar]
- 35.Drappatz J., Lee E.Q., Hammond S., Grimm S.A., Norden A.D., Beroukhim R., Gerard M., Schiff D., Chi A.S., Batchelor T.T., et al. Phase I study of panobinostat in combination with bevacizumab for recurrent high-grade glioma. J. Neurooncol. 2012;107:133–138. doi: 10.1007/s11060-011-0717-z. [DOI] [PubMed] [Google Scholar]
- 36.Drummond D.C., Noble C.O., Kirpotin D.B., Guo Z., Scott G.K., Benz C.C. Clinical development of histone deacetylase inhibitors as anticancer agents. Annu. Rev. Pharmacol. Toxicol. 2005;45:495–528. doi: 10.1146/annurev.pharmtox.45.120403.095825. [DOI] [PubMed] [Google Scholar]
- 37.Chen P.C., Patil V., Guerrant W., Green P., Oyelere A.K. Synthesis and structure–activity relationship of histone deacetylase (hdac) inhibitors with triazole-linked cap group. Bioorg. Med. Chem. 2008;16:4839–4853. doi: 10.1016/j.bmc.2008.03.050. [DOI] [PubMed] [Google Scholar]
- 38.Witter D.J., Belvedere S., Chen L., Secrist J.P., Mosley R.T., Miller T.A. Benzo[b]thiophene-based histone deacetylase inhibitors. Bioorg. Med. Chem. Lett. 2007;17:4562–4567. doi: 10.1016/j.bmcl.2007.05.091. [DOI] [PubMed] [Google Scholar]
- 39.Marks P.A., Breslow R. Dimethyl sulfoxide to vorinostat: Development of this histone deacetylase inhibitor as an anticancer drug. Nat. Biotechnol. 2007;25:84–90. doi: 10.1038/nbt1272. [DOI] [PubMed] [Google Scholar]
- 40.Howman R.A., Prince H.M. New drug therapies in peripheral T-cell lymphoma. Expert Rev. Anticancer Ther. 2011;11:457–472. doi: 10.1586/era.11.4. [DOI] [PubMed] [Google Scholar]
- 41.Lemoine M., Younes A. Histone deacetylase inhibitors in the treatment of lymphoma. Discov. Med. 2010;10:462–470. [PubMed] [Google Scholar]
- 42.Prince H.M., Bishton M.J., Harrison S.J. Clinical studies of histone deacetylase inhibitors. Clin. Cancer Res. 2009;15:3958–3969. doi: 10.1158/1078-0432.CCR-08-2785. [DOI] [PubMed] [Google Scholar]
- 43.Grant S., Easley C., Kirkpatrick P. Vorinostat. Nat. Rev. Drug Discov. 2007;6:21–22. doi: 10.1038/nrd2227. [DOI] [PubMed] [Google Scholar]
- 44.Federico M., Bagella L. Histone deacetylase inhibitors in the treatment of hematological malignancies and solid tumors. J. Biomed. Biotechnol. 2011;2011:475641. doi: 10.1155/2011/475641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ververis K., Hiong A., Karagiannis T.C., Licciardi P.V. Histone deacetylase inhibitors (hdacis): Multitargeted anticancer agents. Biologics. 2013;7:47–60. doi: 10.2147/BTT.S29965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andreeff M., Stone R., Michaeli J., Young C.W., Tong W.P., Sogoloff H., Ervin T., Kufe D., Rifkind R.A., Marks P.A. Hexamethylene bisacetamide in myelodysplastic syndrome and acute myelogenous leukemia: A phase II clinical trial with a differentiation-inducing agent. Blood. 1992;80:2604–2609. [PubMed] [Google Scholar]
- 47.Richon V.M., Webb Y., Merger R., Sheppard T., Jursic B., Ngo L., Civoli F., Breslow R., Rifkind R.A., Marks P.A. Second generation hybrid polar compounds are potent inducers of transformed cell differentiation. Proc. Natl. Acad. Sci. USA. 1996;93:5705–5708. doi: 10.1073/pnas.93.12.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kelly W.K., Richon V.M., O’Connor O., Curley T., MacGregor-Curtelli B., Tong W., Klang M., Schwartz L., Richardson S., Rosa E., et al. Phase I clinical trial of histone deacetylase inhibitor: Suberoylanilide hydroxamic acid administered intravenously. Clin. Cancer Res. 2003;9:3578–3588. [PubMed] [Google Scholar]
- 49.Ramalingam S.S., Kummar S., Sarantopoulos J., Shibata S., LoRusso P., Yerk M., Holleran J., Lin Y., Beumer J.H., Harvey R.D., et al. Phase i study of vorinostat in patients with advanced solid tumors and hepatic dysfunction: A national cancer institute organ dysfunction working group study. J. Clin. Oncol. 2010;28:4507–4512. doi: 10.1200/JCO.2010.30.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vansteenkiste J., van Cutsem E., Dumez H., Chen C., Ricker J.L., Randolph S.S., Schoffski P. Early phase II trial of oral vorinostat in relapsed or refractory breast, colorectal, or non-small cell lung cancer. Invest. New Drugs. 2008;26:483–488. doi: 10.1007/s10637-008-9131-6. [DOI] [PubMed] [Google Scholar]
- 51.Blumenschein G.R., Jr., Kies M.S., Papadimitrakopoulou V.A., Lu C., Kumar A.J., Ricker J.L., Chiao J.H., Chen C., Frankel S.R. Phase ii trial of the histone deacetylase inhibitor vorinostat (zolinza, suberoylanilide hydroxamic acid, saha) in patients with recurrent and/or metastatic head and neck cancer. Invest. New Drugs. 2008;26:81–87. doi: 10.1007/s10637-007-9075-2. [DOI] [PubMed] [Google Scholar]
- 52.Luu T.H., Morgan R.J., Leong L., Lim D., McNamara M., Portnow J., Frankel P., Smith D.D., Doroshow J.H., Wong C., et al. A phase II trial of vorinostat (suberoylanilide hydroxamic acid) in metastatic breast cancer: A california cancer consortium study. Clin. Cancer Res. 2008;14:7138–7142. doi: 10.1158/1078-0432.CCR-08-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Traynor A.M., Dubey S., Eickhoff J.C., Kolesar J.M., Schell K., Huie M.S., Groteluschen D.L., Marcotte S.M., Hallahan C.M., Weeks H.R., et al. Vorinostat (nsc# 701852) in patients with relapsed non-small cell lung cancer: A wisconsin oncology network phase II study. J. Thorac. Oncol. 2009;4:522–526. doi: 10.1097/JTO.0b013e3181952478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bradley D., Rathkopf D., Dunn R., Stadler W.M., Liu G., Smith D.C., Pili R., Zwiebel J., Scher H., Hussain M. Vorinostat in advanced prostate cancer patients progressing on prior chemotherapy (national cancer institute trial 6862): Trial results and interleukin-6 analysis: A study by the department of defense prostate cancer clinical trial consortium and university of chicago phase 2 consortium. Cancer. 2009;115:5541–5549. doi: 10.1002/cncr.24597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Modesitt S.C., Sill M., Hoffman J.S., Bender D.P. A phase II study of vorinostat in the treatment of persistent or recurrent epithelial ovarian or primary peritoneal carcinoma: A gynecologic oncology group study. Gynecol. Oncol. 2008;109:182–186. doi: 10.1016/j.ygyno.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 56.Galanis E., Jaeckle K.A., Maurer M.J., Reid J.M., Ames M.M., Hardwick J.S., Reilly J.F., Loboda A., Nebozhyn M., Fantin V.R., et al. Phase II trial of vorinostat in recurrent glioblastoma multiforme: A north central cancer treatment group study. J. Clin. Oncol. 2009;27:2052–2058. doi: 10.1200/JCO.2008.19.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fakih M.G., Groman A., McMahon J., Wilding G., Muindi J.R. A randomized phase II study of two doses of vorinostat in combination with 5-fu/lv in patients with refractory colorectal cancer. Cancer Chemother. Pharmacol. 2012;69:743–751. doi: 10.1007/s00280-011-1762-1. [DOI] [PubMed] [Google Scholar]
- 58.Ramalingam S.S., Maitland M.L., Frankel P., Argiris A.E., Koczywas M., Gitlitz B., Thomas S., Espinoza-Delgado I., Vokes E.E., Gandara D.R., et al. Carboplatin and paclitaxel in combination with either vorinostat or placebo for first-line therapy of advanced non-small-cell lung cancer. J. Clin. Oncol. 2010;28:56–62. doi: 10.1200/JCO.2009.24.9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woyach J.A., Kloos R.T., Ringel M.D., Arbogast D., Collamore M., Zwiebel J.A., Grever M., Villalona-Calero M., Shah M.H. Lack of therapeutic effect of the histone deacetylase inhibitor vorinostat in patients with metastatic radioiodine-refractory thyroid carcinoma. J. Clin. Endocrinol. Metab. 2009;94:164–170. doi: 10.1210/jc.2008-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Friday B.B., Anderson S.K., Buckner J., Yu C., Giannini C., Geoffroy F., Schwerkoske J., Mazurczak M., Gross H., Pajon E., et al. Phase II trial of vorinostat in combination with bortezomib in recurrent glioblastoma: A north central cancer treatment group study. Neuro Oncol. 2012;14:215–221. doi: 10.1093/neuonc/nor198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilson P.M., El-Khoueiry A., Iqbal S., Fazzone W., LaBonte M.J., Groshen S., Yang D., Danenberg K.D., Cole S., Kornacki M., et al. A phase I/II trial of vorinostat in combination with 5-fluorouracil in patients with metastatic colorectal cancer who previously failed 5-fu-based chemotherapy. Cancer Chemother. Pharmacol. 2010;65:979–988. doi: 10.1007/s00280-009-1236-x. [DOI] [PubMed] [Google Scholar]
- 62.Fakih M.G., Fetterly G., Egorin M.J., Muindi J.R., Espinoza-Delgado I., Zwiebel J.A., Litwin A., Holleran J.L., Wang K., Diasio R.B. A phase I, pharmacokinetic, and pharmacodynamic study of two schedules of vorinostat in combination with 5-fluorouracil and leucovorin in patients with refractory solid tumors. Clin. Cancer Res. 2010;16:3786–3794. doi: 10.1158/1078-0432.CCR-10-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fakih M.G., Pendyala L., Fetterly G., Toth K., Zwiebel J.A., Espinoza-Delgado I., Litwin A., Rustum Y.M., Ross M.E., Holleran J.L., et al. A phase I, pharmacokinetic and pharmacodynamic study on vorinostat in combination with 5-fluorouracil, leucovorin, and oxaliplatin in patients with refractory colorectal cancer. Clin. Cancer Res. 2009;15:3189–3195. doi: 10.1158/1078-0432.CCR-08-2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fazzone W., Wilson P.M., Labonte M.J., Lenz H.J., Ladner R.D. Histone deacetylase inhibitors suppress thymidylate synthase gene expression and synergize with the fluoropyrimidines in colon cancer cells. Int. J. Cancer. 2009;125:463–473. doi: 10.1002/ijc.24403. [DOI] [PubMed] [Google Scholar]
- 65.Munster P.N., Marchion D., Thomas S., Egorin M., Minton S., Springett G., Lee J.H., Simon G., Chiappori A., Sullivan D., et al. Phase I trial of vorinostat and doxorubicin in solid tumours: Histone deacetylase 2 expression as a predictive marker. Br. J. Cancer. 2009;101:1044–1050. doi: 10.1038/sj.bjc.6605293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gandia P., Arellano C., Chalret du Rieu Q., Lochon I., Campone M., Pierga J.Y., Poublanc M., Hennebelle I., Filleron T., Chatelut E., et al. Unexpected high levels of vorinostat when combined with vinorelbine in patients with advanced cancer. Curr. Clin. Pharmacol. 2011;6:274–279. doi: 10.2174/157488411798375921. [DOI] [PubMed] [Google Scholar]
- 67.Ramalingam S.S., Parise R.A., Ramanathan R.K., Lagattuta T.F., Musguire L.A., Stoller R.G., Potter D.M., Argiris A.E., Zwiebel J.A., Egorin M.J., et al. Phase I and pharmacokinetic study of vorinostat, a histone deacetylase inhibitor, in combination with carboplatin and paclitaxel for advanced solid malignancies. Clin. Cancer Res. 2007;13:3605–3610. doi: 10.1158/1078-0432.CCR-07-0162. [DOI] [PubMed] [Google Scholar]
- 68.Munster P.N., Thurn K.T., Thomas S., Raha P., Lacevic M., Miller A., Melisko M., Ismail-Khan R., Rugo H., Moasser M., et al. A phase II study of the histone deacetylase inhibitor vorinostat combined with tamoxifen for the treatment of patients with hormone therapy-resistant breast cancer. Br. J. Cancer. 2011;104:1828–1835. doi: 10.1038/bjc.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim M.S., Blake M., Baek J.H., Kohlhagen G., Pommier Y., Carrier F. Inhibition of histone deacetylase increases cytotoxicity to anticancer drugs targeting DNA. Cancer Res. 2003;63:7291–7300. [PubMed] [Google Scholar]
- 70.Hubbert C., Guardiola A., Shao R., Kawaguchi Y., Ito A., Nixon A., Yoshida M., Wang X.F., Yao T.P. Hdac6 is a microtubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 71.Amit L., Ben-Aharon I., Vidal L., Leibovici L., Stemmer S. The impact of bevacizumab (avastin) on survival in metastatic solid tumors- a meta-analysis and systematic review. PLoS One. 2013;8:e51780. doi: 10.1371/journal.pone.0051780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramaswamy B., Fiskus W., Cohen B., Pellegrino C., Hershman D.L., Chuang E., Luu T., Somlo G., Goetz M., Swaby R., et al. Phase I-II study of vorinostat plus paclitaxel and bevacizumab in metastatic breast cancer: Evidence for vorinostat-induced tubulin acetylation and hsp90 inhibition in vivo. Breast Cancer Res. Treat. 2012;132:1063–1072. doi: 10.1007/s10549-011-1928-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chinnaiyan P., Chowdhary S., Potthast L., Prabhu A., Tsai Y.Y., Sarcar B., Kahali S., Brem S., Yu H.M., Rojiani A., et al. Phase I trial of vorinostat combined with bevacizumab and cpt-11 in recurrent glioblastoma. Neuro Oncol. 2012;14:93–100. doi: 10.1093/neuonc/nor187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Du X.L., Chen Q. Recent advancements of bortezomib in acute lymphocytic leukemia treatment. Acta Haematol. 2013;129:207–214. doi: 10.1159/000345260. [DOI] [PubMed] [Google Scholar]
- 75.Kaur G., Stetler-Stevenson M., Sebers S., Worland P., Sedlacek H., Myers C., Czech J., Naik R., Sausville E. Growth inhibition with reversible cell cycle arrest of carcinoma cells by flavone l86–8275. J. Natl. Cancer Inst. 1992;84:1736–1740. doi: 10.1093/jnci/84.22.1736. [DOI] [PubMed] [Google Scholar]
- 76.Dickson M.A., Rathkopf D.E., Carvajal R.D., Grant S., Roberts J.D., Reid J.M., Ames M.M., McGovern R.M., Lefkowitz R.A., Gonen M., et al. A phase I pharmacokinetic study of pulse-dose vorinostat with flavopiridol in solid tumors. Invest. New Drugs. 2011;29:1004–1012. doi: 10.1007/s10637-010-9447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Millward M., Price T., Townsend A., Sweeney C., Spencer A., Sukumaran S., Longenecker A., Lee L., Lay A., Sharma G., et al. Phase I clinical trial of the novel proteasome inhibitor marizomib with the histone deacetylase inhibitor vorinostat in patients with melanoma, pancreatic and lung cancer based on in vitro assessments of the combination. Invest. New Drugs. 2012;30:2303–2317. doi: 10.1007/s10637-011-9766-6. [DOI] [PubMed] [Google Scholar]
- 78.Khan N., Jeffers M., Kumar S., Hackett C., Boldog F., Khramtsov N., Qian X., Mills E., Berghs S.C., Carey N., et al. Determination of the class and isoform selectivity of small-molecule histone deacetylase inhibitors. Biochem. J. 2008;409:581–589. doi: 10.1042/BJ20070779. [DOI] [PubMed] [Google Scholar]
- 79.Plumb J.A., Finn P.W., Williams R.J., Bandara M.J., Romero M.R., Watkins C.J., la Thangue N.B., Brown R. Pharmacodynamic response and inhibition of growth of human tumor xenografts by the novel histone deacetylase inhibitor pxd101. Mol. Cancer Ther. 2003;2:721–728. [PubMed] [Google Scholar]
- 80.Qian X., LaRochelle W.J., Ara G., Wu F., Petersen K.D., Thougaard A., Sehested M., Lichenstein H.S., Jeffers M. Activity of pxd101, a histone deacetylase inhibitor, in preclinical ovarian cancer studies. Mol. Cancer Ther. 2006;5:2086–2095. doi: 10.1158/1535-7163.MCT-06-0111. [DOI] [PubMed] [Google Scholar]
- 81.Ramalingam S.S., Belani C.P., Ruel C., Frankel P., Gitlitz B., Koczywas M., Espinoza-Delgado I., Gandara D. Phase II study of belinostat (pxd101), a histone deacetylase inhibitor, for second line therapy of advanced malignant pleural mesothelioma. J. Thorac. Oncol. 2009;4:97–101. doi: 10.1097/JTO.0b013e318191520c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Giaccone G., Rajan A., Berman A., Kelly R.J., Szabo E., Lopez-Chavez A., Trepel J., Lee M.J., Cao L., Espinoza-Delgado I., et al. Phase II study of belinostat in patients with recurrent or refractory advanced thymic epithelial tumors. J. Clin. Oncol. 2011;29:2052–2059. doi: 10.1200/JCO.2010.32.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yeo W., Chung H.C., Chan S.L., Wang L.Z., Lim R., Picus J., Boyer M., Mo F.K., Koh J., Rha S.Y., et al. Epigenetic therapy using belinostat for patients with unresectable hepatocellular carcinoma: A multicenter phase I/II study with biomarker and pharmacokinetic analysis of tumors from patients in the mayo phase II consortium and the cancer therapeutics research group. J. Clin. Oncol. 2012;30:3361–3367. doi: 10.1200/JCO.2011.41.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mackay H.J., Hirte H., Colgan T., Covens A., MacAlpine K., Grenci P., Wang L., Mason J., Pham P.A., Tsao M.S., et al. Phase II trial of the histone deacetylase inhibitor belinostat in women with platinum resistant epithelial ovarian cancer and micropapillary (lmp) ovarian tumours. Eur. J. Cancer. 2010;46:1573–1579. doi: 10.1016/j.ejca.2010.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dizon D.S., Blessing J.A., Penson R.T., Drake R.D., Walker J.L., Johnston C.M., Disilvestro P.A., Fader A.N. A phase ii evaluation of belinostat and carboplatin in the treatment of recurrent or persistent platinum-resistant ovarian, fallopian tube, or primary peritoneal carcinoma: A gynecologic oncology group study. Gynecol. Oncol. 2012;125:367–371. doi: 10.1016/j.ygyno.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dizon D.S., Damstrup L., Finkler N.J., Lassen U., Celano P., Glasspool R., Crowley E., Lichenstein H.S., Knoblach P., Penson R.T. Phase II activity of belinostat (pxd-101), carboplatin, and paclitaxel in women with previously treated ovarian cancer. Int. J. Gynecol. Cancer. 2012;22:979–986. doi: 10.1097/IGC.0b013e31825736fd. [DOI] [PubMed] [Google Scholar]
- 87.Steele N.L., Plumb J.A., Vidal L., Tjornelund J., Knoblauch P., Rasmussen A., Ooi C.E., Buhl-Jensen P., Brown R., Evans T.R., et al. A phase 1 pharmacokinetic and harmacodynamic study of the histone deacetylase inhibitor belinostat in patients with advanced solid tumors. Clin. Cancer Res. 2008;14:804–810. doi: 10.1158/1078-0432.CCR-07-1786. [DOI] [PubMed] [Google Scholar]
- 88.Takada M., Kataoka A., Toi M., Bando H., Toyama K., Horiguchi S., Ueno T., Linder S., Saji S., Hayashi Y., et al. A close association between alteration in growth kinetics by neoadjuvant chemotherapy and survival outcome in primary breast cancer. Int. J. Oncol. 2004;25:397–405. [PubMed] [Google Scholar]
- 89.Lassen U., Molife L.R., Sorensen M., Engelholm S.A., Vidal L., Sinha R., Penson R.T., Buhl-Jensen P., Crowley E., Tjornelund J., et al. A phase I study of the safety and pharmacokinetics of the histone deacetylase inhibitor belinostat administered in combination with carboplatin and/or paclitaxel in patients with solid tumours. Br. J. Cancer. 2010;103:12–17. doi: 10.1038/sj.bjc.6605726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.George P., Bali P., Annavarapu S., Scuto A., Fiskus W., Guo F., Sigua C., Sondarva G., Moscinski L., Atadja P., et al. Combination of the histone deacetylase inhibitor lbh589 and the hsp90 inhibitor 17-aag is highly active against human cml-bc cells and aml cells with activating mutation of flt-3. Blood. 2005;105:1768–1776. doi: 10.1182/blood-2004-09-3413. [DOI] [PubMed] [Google Scholar]
- 91.Marks P.A., Richon V.M., Breslow R., Rifkind R.A. Histone deacetylase inhibitors as new cancer drugs. Curr. Opin. Oncol. 2001;13:477–483. doi: 10.1097/00001622-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 92.Bolden J.E., Peart M.J., Johnstone R.W. Anticancer activities of histone deacetylase inhibitors. Nat. Rev. Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 93.Hamberg P., Woo M.M., Chen L.C., Verweij J., Porro M.G., Zhao L., Li W., van der Biessen D., Sharma S., Hengelage T., et al. Effect of ketoconazole-mediated cyp3a4 inhibition on clinical pharmacokinetics of panobinostat (lbh589), an orally active histone deacetylase inhibitor. Cancer Chemother. Pharmacol. 2011;68:805–813. doi: 10.1007/s00280-011-1693-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morita S., Oizumi S., Minami H., Kitagawa K., Komatsu Y., Fujiwara Y., Inada M., Yuki S., Kiyota N., Mitsuma A., et al. Phase I dose-escalating study of panobinostat (lbh589) administered intravenously to japanese patients with advanced solid tumors. Invest. New Drugs. 2012;30:1950–1957. doi: 10.1007/s10637-011-9751-0. [DOI] [PubMed] [Google Scholar]
- 95.Rathkopf D., Wong B.Y., Ross R.W., Anand A., Tanaka E., Woo M.M., Hu J., Dzik-Jurasz A., Yang W., Scher H.I. A phase I study of oral panobinostat alone and in combination with docetaxel in patients with castration-resistant prostate cancer. Cancer. Chemother. Pharmacol. 2010;66:181–189. doi: 10.1007/s00280-010-1289-x. [DOI] [PubMed] [Google Scholar]
- 96.Jones S.F., Infante J.R., Thompson D.S., Mohyuddin A., Bendell J.C., Yardley D.A., Burris H.A., 3rd. A phase I trial of oral administration of panobinostat in combination with paclitaxel and carboplatin in patients with solid tumors. Cancer Chemother. Pharmacol. 2012;70:471–475. doi: 10.1007/s00280-012-1931-x. [DOI] [PubMed] [Google Scholar]
- 97.Strickler J.H., Starodub A.N., Jia J., Meadows K.L., Nixon A.B., Dellinger A., Morse M.A., Uronis H.E., Marcom P.K., Zafar S.Y., et al. Phase I study of bevacizumab, everolimus, and panobinostat (lbh-589) in advanced solid tumors. Cancer Chemother. Pharmacol. 2012;70:251–258. doi: 10.1007/s00280-012-1911-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jones S.F., Bendell J.C., Infante J.R., Spigel D.R., Thompson D.S., Yardley D.A., Greco F.A., Murphy P.B., Burris H.A., 3rd. A phase I study of panobinostat in combination with gemcitabine in the treatment of solid tumors. Clin. Adv. Hematol. Oncol. 2011;9:225–230. [PubMed] [Google Scholar]
- 99.Hainsworth J.D., Infante J.R., Spigel D.R., Arrowsmith E.R., Boccia R.V., Burris H.A. A phase II trial of panobinostat, a histone deacetylase inhibitor, in the treatment of patients with refractory metastatic renal cell carcinoma. Cancer Invest. 2011;29:451–455. doi: 10.3109/07357907.2011.590568. [DOI] [PubMed] [Google Scholar]
- 100.Wang H., Cao Q., Dudek A.Z. Phase II study of panobinostat and bortezomib in patients with pancreatic cancer progressing on gemcitabine-based therapy. Anticancer Res. 2012;32:1027–1031. [PubMed] [Google Scholar]
- 101.Novotny-Diermayr V., Sangthongpitag K., Hu C.Y., Wu X., Sausgruber N., Yeo P., Greicius G., Pettersson S., Liang A.L., Loh Y.K., et al. Sb939, a novel potent and orally active histone deacetylase inhibitor with high tumor exposure and efficacy in mouse models of colorectal cancer. Mol. Cancer Ther. 2010;9:642–652. doi: 10.1158/1535-7163.MCT-09-0689. [DOI] [PubMed] [Google Scholar]
- 102.Yong W.P., Goh B.C., Soo R.A., Toh H.C., Ethirajulu K., Wood J., Novotny-Diermayr V., Lee S.C., Yeo W.L., Chan D., et al. Phase I and pharmacodynamic study of an orally administered novel inhibitor of histone deacetylases, sb939, in patients with refractory solid malignancies. Ann. Oncol. 2011;22:2516–2522. doi: 10.1093/annonc/mdq784. [DOI] [PubMed] [Google Scholar]
- 103.Razak A.R., Hotte S.J., Siu L.L., Chen E.X., Hirte H.W., Powers J., Walsh W., Stayner L.A., Laughlin A., Novotny-Diermayr V., et al. Phase I clinical, pharmacokinetic and pharmacodynamicstudy of sb939, an oral histone deacetylase (hdac) inhibitor, in patients with advanced solid tumours. Br. J. Cancer. 2011;104:756–762. doi: 10.1038/bjc.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Buggy J.J., Cao Z.A., Bass K.E., Verner E., Balasubramanian S., Liu L., Schultz B.E., Young P.R., Dalrymple S.A. Cra-024781: A novel synthetic inhibitor of histone deacetylase enzymes with antitumor activity in vitro and in vivo. Mol. Cancer Ther. 2006;5:1309–1317. doi: 10.1158/1535-7163.MCT-05-0442. [DOI] [PubMed] [Google Scholar]
- 105.Lopez G., Liu J., Ren W., Wei W., Wang S., Lahat G., Zhu Q.S., Bornmann W.G., McConkey D.J., Pollock R.E., et al. Combining pci-24781, a novel histone deacetylase inhibitor, with chemotherapy for the treatment of soft tissue sarcoma. Clin. Cancer Res. 2009;15:3472–3483. doi: 10.1158/1078-0432.CCR-08-2714. [DOI] [PubMed] [Google Scholar]
- 106.Yang C., Choy E., Hornicek F.J., Wood K.B., Schwab J.H., Liu X., Mankin H., Duan Z. Histone deacetylase inhibitor (hdaci) pci-24781 potentiates cytotoxic effects of doxorubicin in bone sarcoma cells. Cancer Chemother. Pharmacol. 2011;67:439–446. doi: 10.1007/s00280-010-1344-7. [DOI] [PubMed] [Google Scholar]
- 107.Yang C., Choy E., Hornicek F.J., Wood K.B., Schwab J.H., Liu X., Mankin H., Duan Z. Histone deacetylase inhibitor pci-24781 enhances chemotherapy-induced apoptosis in multidrug-resistant sarcoma cell lines. Anticancer Res. 2011;31:1115–1123. [PMC free article] [PubMed] [Google Scholar]
- 108.Lopez G., Torres K., Liu J., Hernandez B., Young E., Belousov R., Bolshakov S., Lazar A.J., Slopis J.M., McCutcheon I.E., et al. Autophagic survival in resistance to histone deacetylase inhibitors: Novel strategies to treat malignant peripheral nerve sheath tumors. Cancer Res. 2011;71:185–196. doi: 10.1158/0008-5472.CAN-10-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Singh M.M., Manton C.A., Bhat K.P., Tsai W.W., Aldape K., Barton M.C., Chandra J. Inhibition of lsd1 sensitizes glioblastoma cells to histone deacetylase inhibitors. Neuro Oncol. 2011;13:894–903. doi: 10.1093/neuonc/nor049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kitamura T., Connolly K., Ruffino L., Ajiki T., Lueckgen A., DiGiovanni J., Kiguchi K. The therapeutic effect of histone deacetylase inhibitor pci-24781 on gallbladder carcinoma in bk5. Erbb2 mice. J. Hepatol. 2012;57:84–91. doi: 10.1016/j.jhep.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Arts J., King P., Marien A., Floren W., Belien A., Janssen L., Pilatte I., Roux B., Decrane L., Gilissen R., et al. Jnj-26481585, a novel “second-generation” oral histone deacetylase inhibitor, shows broad-spectrum preclinical antitumoral activity. Clin. Cancer Res. 2009;15:6841–6851. doi: 10.1158/1078-0432.CCR-09-0547. [DOI] [PubMed] [Google Scholar]
- 112.Catley L., Weisberg E., Tai Y.T., Atadja P., Remiszewski S., Hideshima T., Mitsiades N., Shringarpure R., LeBlanc R., Chauhan D., et al. Nvp-laq824 is a potent novel histone deacetylase inhibitor with significant activity against multiple myeloma. Blood. 2003;102:2615–2622. doi: 10.1182/blood-2003-01-0233. [DOI] [PubMed] [Google Scholar]
- 113.Fuino L., Bali P., Wittmann S., Donapaty S., Guo F., Yamaguchi H., Wang H.G., Atadja P., Bhalla K. Histone deacetylase inhibitor laq824 down-regulates her-2 and sensitizes human breast cancer cells to trastuzumab, taxotere, gemcitabine, and epothilone b. Mol. Cancer Ther. 2003;2:971–984. [PubMed] [Google Scholar]
- 114.Remiszewski S.W., Sambucetti L.C., Bair K.W., Bontempo J., Cesarz D., Chandramouli N., Chen R., Cheung M., Cornell-Kennon S., Dean K., et al. N-hydroxy-3-phenyl-2-propenamides as novel inhibitors of human histone deacetylase with in vivo antitumor activity: Discovery of (2e)-n-hydroxy-3-[4-[[(2-hydroxyethyl)[2-(1h-indol-3-yl)ethyl]amino]methyl]phenyl]-2-propenamide (nvp-laq824) J. Med. Chem. 2003;46:4609–4624. doi: 10.1021/jm030235w. [DOI] [PubMed] [Google Scholar]
- 115.Chen L., Meng S., Wang H., Bali P., Bai W., Li B., Atadja P., Bhalla K.N., Wu J. Chemical ablation of androgen receptor in prostate cancer cells by the histone deacetylase inhibitor laq824. Mol. Cancer Ther. 2005;4:1311–1319. doi: 10.1158/1535-7163.MCT-04-0287. [DOI] [PubMed] [Google Scholar]
- 116.Bluethner T., Niederhagen M., Caca K., Serr F., Witzigmann H., Moebius C., Mossner J., Wiedmann M. Inhibition of histone deacetylase for the treatment of biliary tract cancer: A new effective pharmacological approach. World J. Gastroenterol. 2007;13:4761–4770. doi: 10.3748/wjg.v13.i35.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Egler V., Korur S., Failly M., Boulay J.L., Imber R., Lino M.M., Merlo A. Histone deacetylase inhibition and blockade of the glycolytic pathway synergistically induce glioblastoma cell death. Clin. Cancer Res. 2008;14:3132–3140. doi: 10.1158/1078-0432.CCR-07-4182. [DOI] [PubMed] [Google Scholar]
- 118.Haefner M., Bluethner T., Niederhagen M., Moebius C., Wittekind C., Mossner J., Caca K., Wiedmann M. Experimental treatment of pancreatic cancer with two novel histone deacetylase inhibitors. World J. Gastroenterol. 2008;14:3681–3692. doi: 10.3748/wjg.14.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hurtubise A., Bernstein M.L., Momparler R.L. Preclinical evaluation of the antineoplastic action of 5-aza-2'-deoxycytidine and different histone deacetylase inhibitors on human ewing’s sarcoma cells. Cancer Cell Int. 2008;8:16. doi: 10.1186/1475-2867-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kato Y., Salumbides B.C., Wang X.F., Qian D.Z., Williams S., Wei Y., Sanni T.B., Atadja P., Pili R. Antitumor effect of the histone deacetylase inhibitor laq824 in combination with 13-cis-retinoic acid in human malignant melanoma. Mol. Cancer Ther. 2007;6:70–81. doi: 10.1158/1535-7163.MCT-06-0125. [DOI] [PubMed] [Google Scholar]
- 121.De Bono J.S., Kristeleit R., Tolcher A., Fong P., Pacey S., Karavasilis V., Mita M., Shaw H., Workman P., Kaye S., et al. Phase I pharmacokinetic and pharmacodynamic study of laq824, a hydroxamate histone deacetylase inhibitor with a heat shock protein-90 inhibitory profile, in patients with advanced solid tumors. Clin. Cancer Res. 2008;14:6663–6673. doi: 10.1158/1078-0432.CCR-08-0376. [DOI] [PubMed] [Google Scholar]
- 122.Mandl-Weber S., Meinel F.G., Jankowsky R., Oduncu F., Schmidmaier R., Baumann P. The novel inhibitor of histone deacetylase resminostat (ras2410) inhibits proliferation and induces apoptosis in multiple myeloma (mm) cells. Br. J. Haematol. 2010;149:518–528. doi: 10.1111/j.1365-2141.2010.08124.x. [DOI] [PubMed] [Google Scholar]
- 123.Brunetto A.T., Ang J.E., Lal R., Olmos D., Frentzas S., Mais A., Hauns B., Mollenhauer M., Lahu G., de Bono J.S. A first-in-human phase I study of 4sc-201, an oral histone deacetylase (hdac) inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 2009;27:3530. [Google Scholar]
- 124.Bitzer M., Horger M., Ganten T.M., Lauer U.M., Woerns M.A., Siveke J.T., Dollinger M.M., Gerken G., Wege H., Giannini E.G., et al. Efficacy, safety, tolerability, and pk of the hdac inhibitor resminostat in sorafenib-refractory hepatocellular carcinoma (hcc): Phase II shelter study. J. Clin. Oncol. . 2012;30:Abstract 4115. [Google Scholar]
- 125.Moffat D., Patel S., Day F., Belfield A., Donald A., Rowlands M., Wibawa J., Brotherton D., Stimson L., Clark V., et al. Discovery of 2-(6-{[(6-fluoroquinolin-2-yl)methyl]amino}bicyclo [3.1.0]hex-3-yl)-n-hydroxypyrim idine-5-carboxamide (chr-3996), a class I selective orally active histone deacetylase inhibitor. J. Med. Chem. 2010;53:8663–8678. doi: 10.1021/jm101177s. [DOI] [PubMed] [Google Scholar]
- 126.Banerji U., van Doorn L., Papadatos-Pastos D., Kristeleit R., Debnam P., Tall M., Stewart A., Raynaud F., Garrett M.D., Toal M., et al. A phase I pharmacokinetic and pharmacodynamic study of chr-3996, an oral class I selective histone deacetylase inhibitor in refractory solid tumors. Clin. Cancer Res. 2012;18:2687–2694. doi: 10.1158/1078-0432.CCR-11-3165. [DOI] [PubMed] [Google Scholar]
- 127.Clinicaltrials.Gov, U.S. National institutes of health. [(accessed on 15 April 2013)]. Available online: http://www.clinicaltrial.gov/
- 128.Kelly W.K., O’Connor O.A., Krug L.M., Chiao J.H., Heaney M., Curley T., MacGregore-Cortelli B., Tong W., Secrist J.P., Schwartz L., et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J. Clin. Oncol. 2005;23:3923–3931. doi: 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bromberg J. Stat proteins and oncogenesis. J. Clin. Invest. 2002;109:1139–1142. doi: 10.1172/JCI15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fantin V.R., Loboda A., Paweletz C.P., Hendrickson R.C., Pierce J.W., Roth J.A., Li L., Gooden F., Korenchuk S., Hou X.S., et al. Constitutive activation of signal transducers and activators of transcription predicts vorinostat resistance in cutaneous t-cell lymphoma. Cancer Res. 2008;68:3785–3794. doi: 10.1158/0008-5472.CAN-07-6091. [DOI] [PubMed] [Google Scholar]