Abstract
In this review, we will examine various molecular biomarkers for their potential to serve as independent prognostic factors for predicting survival outcome in postoperative patients with progressive intrahepatic cholangiocarcinoma. Specific rodent models of intrahepatic cholangiocarcinoma that mimic relevant cellular, molecular, and clinical features of the human disease are also described, not only in terms of their usefulness in identifying molecular pathways and mechanisms linked to cholangiocarcinoma development and progression, but also for their potential value as preclinical platforms for suggesting and testing novel molecular strategies for cholangiocarcinoma therapy. Last, recent studies aimed at addressing the role of desmoplastic stroma in promoting intrahepatic cholangiocarcinoma progression are highlighted in an effort to underline the potential value of targeting tumor stromal components together with that of cholangiocarcinoma cells as a novel therapeutic option for this devastating cancer.
Intrahepatic cholangiocarcinoma is a primary epithelial cancer of the hepatobiliary tract that exhibits characteristics of cholangiocyte differentiation. This highly malignant and progressive hepatobiliary cancer accounts for approximately 10%–15% of all primary liver malignancies, with more than 90% of intrahepatic cholangiocarcinomas being classified as well-differentiated to moderately differentiated adenocarcinomas. During the past several years, intrahepatic cholangiocarcinoma has become a malignancy of increasing importance1 and one that continues to present significant biologic and therapeutic challenges. Globally, the incidence and mortality rates for intrahepatic cholangiocarcinoma have been steadily increasing during the past 2–3 decades, with notable increases having been reported to have occurred in the United States, the United Kingdom, and Australia.2,3 The cause for this increase remains unclear, and the vast majority of patients diagnosed with intrahepatic cholangiocarcinoma present with advanced disease most often developed without an identifiable etiology.3 Curative surgical resection offers the only hope for long-term survival, but recurrence rates remain high, and only a relatively few patients are suitable candidates for curative surgical therapies. Patients with unresectable intrahepatic cholangiocarcinoma will typically die within less than 12–24 months of diagnosis. Moreover, advanced intrahepatic cholangiocarcinoma is for the most part unresponsive to systemic chemotherapy and radiotherapy regimens, with existing preoperative (radiologic, pathologic, and laparoscopic) staging strategies having been reported not to allow an accurate determination for predicting long-term prognosis in operable patients.4 Only by increasing our understanding of the molecular mechanisms underlying the progression of intrahepatic cholangiocarcinoma and by identifying more effective prognostic biomarkers that might also serve as therapeutic targets, can we hope to devise and test novel strategies aimed at improving the survival and quality of life of patients with this devastating cancer.
This review will highlight recent findings suggesting the prognostic value of select molecular biomarkers as predictors of progression and poor outcome in human intrahepatic cholangiocarcinoma. In addition, specific rodent models of intrahepatic cholangiocarcinogenesis and tumor progression will be briefly described to illustrate their preclinical relevance to the human disease. Last, this review will address what is currently known about the role of tumor microenvironment and stromal fibroblastic cells in promoting intrahepatic cholangiocarcinoma progression, with suggested strategies for combined therapeutic targeting of tumor stroma cells and malignant cholangiocytes as a potentially effective and testable strategy for intrahepatic cholangiocarcinoma therapy.
Biomarkers Correlated With Poor Outcome and Progression in Human Intrahepatic Cholangiocarcinoma
Macroscopically, intrahepatic cholangiocarcinoma, also known as peripheral cholangiocarcinoma, has been subclassified into mass-forming, periductular-infiltrating, mass-forming plus periductular-infiltrating, and intraductal papillary types.5–7 Of these, mass-forming and mass-forming plus periductular infiltrating intrahepatic cholangiocarcinomas are the most common types, with the mass-forming plus periductular infiltrating type showing the poorest survival outcome.5,7 In comparison, patients with the intraductal papillary type were shown to have a significantly better survival rate than those with non-intraductal papillary tumors, with aggressive curative resection being associated with a longer survival.6
Other clinicopathologic features reported to be significant prognostic factors for poor survival outcome in intrahepatic cholangiocarcinoma after surgical resection include large tumor size, multifocal tumors, positive resection margin, vascular invasion, perineural invasion, intrahepatic metastasis, and perihepatic lymph node metastasis.1,3,7 Clinically, high levels of carbohydrate antigen19-9 (CA19-9) also appear to be a poor prognostic indicator for intrahepatic cholangiocarcinoma.4 However, although these various clinicopathologic features relate to poor survival in intrahepatic cholangiocarcinoma patients after surgical resection, they do not provide any insight as to mechanisms of tumor progression or recurrence, and they do not suggest possibilities for the development of novel therapeutic strategies aimed at improving survival rates. In this regard, identifying molecular biomarkers that can act not only as independent prognostic factors for predicting survival outcomes in intrahepatic cholangiocarcinoma, but that also might be linked mechanistically to the pathogenesis of the progressive malignant disease and that might further serve as potential targets for cholangiocarcinoma therapy can have important implications for advancing our understanding and treatment of this highly lethal primary liver cancer.
Table 1 lists a number of molecular biomarkers that have been reported to significantly correlate with overall poor survival rates for intrahepatic cholangiocarcinoma after surgical resection. It is beyond the scope of this review to provide a comprehensive and detailed analysis for each of these molecular factors as they might relate to cholangiocarcinogenesis and tumor progression. Moreover, it is still not yet clear as to the extent to which the various molecular biomarkers depicted in Table 1 are causally related to cholangiocarcinoma progression versus those that merely represent a surrogate marker of prognosis.13 The reproducibility and accuracy of these various molecular biomarkers as significant independent predictors of survival outcome in intrahepatic cholangiocarcinoma patients still need to be extended and further validated in carefully controlled multi-institutional trials before they can be adopted into clinical practice. Nevertheless, specific classes of molecular biomarkers (mucins [MUCs], matrix metalloproteinases [MMPs], and CCN proteins) represented in Table 1 will be expanded on below for their potential prognostic significance and relevance to intrahepatic cholangiocarcinoma progression. The role of specific tumor stromal proteins as prognostic factors and mediators of intrahepatic cholangiocarcinoma progression will be discussed in another section of this review.
Table 1. Select Molecular Biomarkers of Poor Prognosis in Intrahepatic Cholangiocarcinoma After Surgical Resection.
| Biomarkera | Method | Expression level | Tumor tissue location | Prognostic value | Reference |
|---|---|---|---|---|---|
| MUC1 | IHC, real-time RT-PCR | Overexpressed | Carcinoma cell membrane and cytoplasm | Independent prognostic factor for poor survival by MVA | 8,9 |
| MUC4 | IHC | Overexpressed | Carcinoma cell membrane and cytoplasm | Independent prognostic factor for poor survival by MVA | 10,11 |
| MMP-7 | IHC | Overexpressed | Carcinoma cell cytoplasm | Prognostic factor for poor survival by UVA | 12 |
| Fascin | IHC, RT-PCR | Overexpressed | Carcinoma cell membrane and cytoplasm | Independent prognostic factor for poor survival by MVA | 13 |
| Survivin | IHC | Overexpressed | Carcinoma cell nucleus | Independent prognostic factor for poor survival by MVA | 14 |
| Cyclin D1 | IHC | Overexpressed | Carcinoma cell nucleus | Prognostic factor for poor survival by UVA, but not by MVA | 15 |
| VEGF-C | IHC | Overexpressed | Carcinoma cell cytoplasm | Independent prognostic factor for poor survival by MVA | 16 |
| Tenascin | IHC | Overexpressed | Carcinoma cells and stroma at invasive front | Prognostic factor for poor survival by UVA, but not by MVA | 17 |
| Laminin gamma 2 chain | IHC | Overexpressed | Stroma around carcinoma cells at invasive front | Prognostic factor for poor survival by UVA, but not by MVA | 18 |
| aPKC-i | IHC | Overexpressed | Carcinoma cell cytoplasm and weak membranous staining at luminal surface | Independent prognostic factor for poor survival by MVA | 19 |
| Aquaporin-1 | IHC | Low or negative | Carcinoma cells | Independent prognostic factor for poor survival by MVA | 20 |
| CTGF | IHC | Low or negative | Carcinoma cells | Prognostic factor for poor survival by UVA | 21 |
| Syndecan-1 | IHC | Low or negative | Carcinoma cells | Independent prognostic factor for poor survival by MVA | 22 |
aPKC-i, atypical protein kinase C-iota; IHC, immunohistochemistry; MVA, statistically significant by multivariate analysis; UVA, statistically significant by univariate analysis.
Molecular biomarkers correlated with poor overall survival based on Kaplan–Meier method.
Mucins
MUCs represent a heterogeneous family of heavily O-glycosylated high molecular weight glycoproteins whose aberrant apoprotein expression levels and abnormal glycosylation states have been correlated with epithelial cancer progression and prognosis. MUC1 oncoprotein is a transmembrane mucin thought to play an important role in intrahepatic cholangiocarcinoma progression4,23,24 and has significant potential as a molecular target for cholangiocarcinoma therapy.24 MUC1 has been demonstrated to be a statistically significant risk factor for predicting poor survival outcome after surgery in mass-forming intrahepatic cholangiocarcinoma.8,9 Cytoplasmic and cell membrane MUC1 has further been shown to be more frequently detected in invasive tubular cholangiocarcinoma (Figure 1C, D, and F) than in the less aggressive intraductal papillary type.8,25 In contrast, mucinous intraductal papillary-type intrahepatic cholangiocarcinomas can exhibit immunophenotypic features of intestinal differentiation, including goblet cell metaplasia, MUC2 immunostaining (Figure 1A, B, and E),25,26 and ectopic expression of intestinal cytokeratin 20.25 MUC2, which is primarily expressed in goblet cells of the normal small intestine and colon, has further been shown in intrahepatic papillary ductal neoplasms to be closely related to aberrant expression of the caudal-related homeodomain intestine-specific transcription factor CDX226 and to correlate with a more favorable prognosis.25,27 Notably, MUC2 expression is either not detected or only rarely detected in invasive mass-forming intrahepatic cholangiocarcinomas (Figure 1E).25,28 Moreover, our results shown in Figure 1G demonstrate a strong positive correlation between an increasing MUC1 immunoreactivity and Ki-67 nuclear labeling indices in tubular versus intestinal-type papillary intrahepatic cholangiocarcinomas compared with large and small bile duct hyperplasias in primary sclerosing cholangitis livers without cholangiocarcinoma, and with intrahepatic bile ducts of normal adult human liver. Interestingly, multivariate analysis has also demonstrated the Ki-67 index, a marker of cell proliferative activity, to be a significant independent risk factor for poor prognosis in intrahepatic cholangiocarcinoma.15
Figure 1.
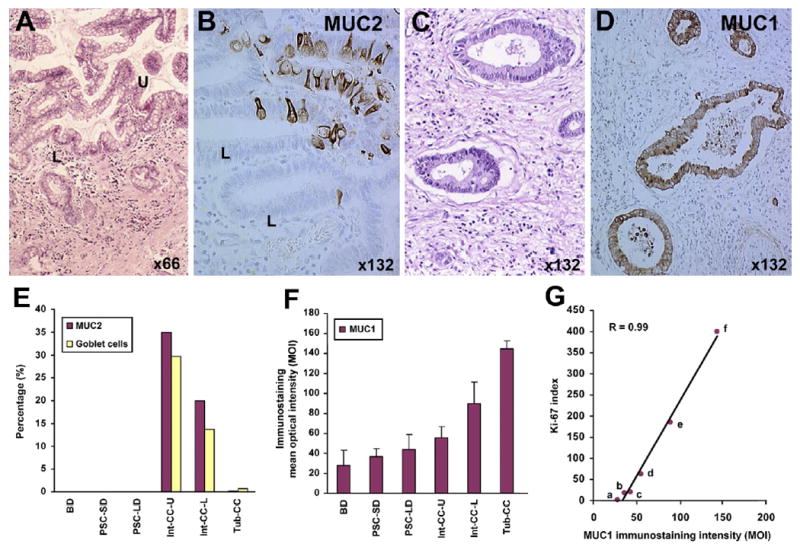
Differential expression of MUC1 and MUC2 in human intestinal-type (Int-CC) versus tubular-type (Tub-CC) human intrahepatic cholangiocarcinoma. (A) Low-grade papillary intestinal-type cholangiocarcinoma exhibiting an extensive goblet cell metaplasia. U, upper region of neoplastic papillae; L, lower or “cryptic” region of neoplastic papillae. (B) MUC2-positive neoplastic epithelial cells within the neoplastic papillae of a low-grade Int-CC. (C) Well-differentiated Tub-CC showing a prominent desmoplastic stroma. (D) Neoplastic glands of a Tub-CC exhibiting uniformly strong positive cell membrane and cytoplasmic immunoreactivity for MUC1. (E) Distribution of “intestinal” differentiation biomarkers (goblet cells and MUC2-positive cells) in Int-CC versus Tub-CC, compared with intrahepatic bile ducts of normal adult liver (BD), as well as with small (SD, ≤500 μm in diameter) and large (LD, ≥1000 μm in diameter) hyperplastic intrahepatic bile ducts in primary sclerosing cholangitis (PSC) liver. Note distribution of MUC2-positive cells closely parallels that of metaplastic goblet cells. (F) Comparison of levels of MUC1 immunostaining, reflected by mean optical density intensity (MOI) values, exhibited by malignant neoplastic epithelial cells of Tub-CC versus Int-CC (U and L) and relative to MOI values determined for non-neoplastic BD, PSC-SD, and PSC-LD, respectively. (G) Linear regression curve for MUC1 immunostaining intensity (MOI) values versus nuclear Ki-67 labeling indices in Tub-CC (f), Int-CC-L (e), and-U (d), and PSC-LD (c) PSC-SD (b), and BD (a). R, correlation coefficient.
Like MUC1, MUC4 is another transmembrane mucin that has been recently found to have prognostic value for predicting overall survival rate for mass-forming intrahepatic cholangiocarcinoma after surgical resection10,11 and also has potential as a therapeutic target.29 MUC4 functions as a novel intramembrane ligand and modulator for the ErbB2 receptor tyrosine kinase pathway that has been shown to potentiate growth factor signaling by ErbB2, to suppress tumor cell apoptosis, and to promote tumor progression.23,30 Shibahara et al10 reported that patients with MUC4 and ErbB2 double-positive intrahepatic cholangiocarcinomas had a significantly worse outcome after surgical resection than those with MUC4 and ErbB2 double-negative tumors. Moreover, patients with MUC4 and MUC1 positive expression showed a significantly worse outcome when compared with those with MUC1-positive/MUC4-negative tumors, whereas MUC4-negative/MUC1-negative expression yielded the best outcome in this series.
High expression of the gel-forming secreted mucin MUC5AC has also been linked to shorter survival in intrahepatic cholangiocarcinoma patients,20,31 although MUC5AC expression has been reported by Boonla et al9 not to be an independent prognostic factor for survival in liver fluke-associated intrahepatic cholangiocarcinoma. On the other hand, median survival has been reported to be worse in cholangiocarcinoma patients with high serum MUC5AC than in those having low-level serum MUC5AC.31 Biliary MUC4 and serum MUC5AC also seem to have significant potential as specific tumor-associated MUCs in biliary tract cancer, although as pointed out by Alvaro,31 their sensitivity as diagnostic and prognostic indicators is yet to be realized. It is also noteworthy that down-regulation of aquaporin-1 has been shown to be inversely correlated with MUC5AC expression in intrahepatic cholangiocarcinoma, as well as to be an independent prognostic factor for poor survival in such patients undergoing surgical resection for this cancer.20 In comparison, low rather than elevated expression of MUC6 in intrahepatic cholangiocarcinoma also independently correlates with poor prognosis after surgical resection.32
Matrix Metalloproteinases
Increased expression and activity of various matrix metalloproteinases (MMPs), most notably MMP-2, -7, and -9, are associated with tumor invasion and metastasis in malignant neoplasms, including intrahepatic cholangiocarcinoma.12,28,33,34 Relative to other MMPs, MMP-7, in particular, appears to have significant potential as a specific prognostic factor for poor survival in cholangiocarcinoma patients after surgery.12,34 Unlike MMP-2 and -9, which are expressed in both the carcinoma and tumor stromal cells of intrahepatic cholangiocarcinoma, MMP-7 is mainly expressed in malignant cholangiocytes,34 suggesting its intrinsic value as a hepatobiliary tumor cell marker. MMP-7 was also seen to be more frequently expressed in invasive non-papillary cholangiocarcinomas than in the papillary type showing a lesser depth of tumor invasion and infrequent metastasis.34 Among serum levels of carcinoembryonic antigen, CA19-9, MMP-7, and MMP-9, only serum MMP-7 was determined to be significantly higher in cholangiocarcinoma patients compared with patients diagnosed with benign biliary tract diseases.35 However, it should also be noted that in at least one reported study, increased MMP-9 immunoreactivity significantly correlated with poor survival and lymph node metastasis in surgically resected cases of intrahepatic cholangiocarcinoma, with lymph node recurrence being more common in patients with MMP-9 positive tumors than in those with MMP-9 negative tumors.33
CCN Proteins
CCN proteins comprise a family of gene products encoded by the connective tissue growth factor (CTGF), cysteine-rich 61, nephroblastoma overexpressed (Nov) gene or CCN gene. CTGF is a highly profibrogenic and mitogenic factor that is transcriptionally regulated by transforming growth factor–β (TGF-β) and other fibrogenic growth factors.21 In fibrotic liver, CTGF is expressed in fibroblasts, myofibroblasts, hepatic stellate cells, and cholangiocytes and is believed to play an important role in hepatic stellate cell activation and progression of fibrosis. Sedlaczek et al36 have shown proliferating cholangiocytes are a major source of CTGF in rat biliary fibrosis. In the presumably only reported study to date concerning CTGF in intrahepatic cholangiocarcinoma, Gardini et al21 demonstrated that patients with intrahepatic cholangiocarcinoma expressing high levels of CTGF had a better prognosis with less chance of tumor recurrence than low or negative expressers. CTGF was determined to be a significant independent prognostic indicator of both tumor recurrence and overall survival for the intrahepatic cholangiocarcinoma patients analyzed in this series, irrespective of vascular or perineural invasion. Presumably, cholangiocarcinomas producing high levels of CTGF might be expected to exhibit a pronounced desmoplastic response, which, in turn, might circumvent the ability of malignant cholangiocytes to invade and metastasize. However, specific mechanisms or pathways related to CTGF as a potential prognostic biomarker for intrahepatic cholangiocarcinoma have not yet been identified.
WISP1v, a splice variant of Wnt-inducible secreted protein 1 and another member of the CCN protein family, was shown by Tanaka et al37 to be overexpressed in 49% of analyzed cases of human intrahepatic cholangiocarcinoma compared with adjacent uninvolved liver tissue and to significantly associate with lymphatic and perineural invasion as well as with poor clinical prognosis and reduced survival. In situ hybridization and laser capture microdissection combined with reverse transcription-polymerase chain reaction (RT-PCR) localized WISP1v mRNA to the fibroblast-enriched tumor stroma rather than to the malignant cholangiocytes of the tumor. In addition, WISP1v mRNA was detected in 4 of 5 analyzed cases of intraductal papillary cholangiocarcinomas with duct wall invasion, but not in 11 analyzed cases of intraductal papillary tumors without duct wall invasion. In vitro analysis further revealed the ability of transfected WISP1v to stimulate human HuCCT1 cholangiocarcinoma cell migration, which was dependent on activation of the p38 mitogen-activated protein kinase (MAPK) pathway. These findings suggest that WISP1v-mediated signaling plays a role in promoting the invasive phenotype in cholangiocarcinoma cells, leading to progression to a more aggressive malignancy.
Preclinical Animal Models of Intrahepatic Cholangiocarcinogenesis and Tumor Progression Recapitulating Key Features of the Human Disease
Animal models that recapitulate key cellular, molecular, and clinical features of human intrahepatic cholangiocarcinoma progression are highly desirable, because such models would not only facilitate studies aimed at elucidating mechanisms of cholangiocarcinoma cell growth, invasion, and metastasis, but also because they could serve as valuable preclinical platforms for testing new molecular strategies for cholangiocarcinoma therapy. Table 2 lists established rodent models of intrahepatic cholangiocarcinoma development and progression that have been demonstrated to exhibit phenotypic features and molecular alterations also expressed in human cholangiocarcinoma subtypes. Each of these model systems has value for investigating mechanisms regulating cholangiocarcinoma tumor growth and progression, as well as for use in testing chemoprevention and/or target-based strategies for cholangiocarcinoma therapy.
Table 2. Preclinical Rodent Models of Intrahepatic Cholangiocarcinoma (ICC) Mimicking Phenotypic Features and Molecular Alterations of the Human Disease.
| Model | Species | Tumor | Phenotypic features | Molecular alterations | Reference | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Development (weeks) | Incidence (%) | Classification | |||||
| Furan | Rat | >52 | 70–100 | Int-type tubular adenocarcinoma | MF, WD, Des, CK19+, mucin+, weakly metastatic | phos-ErbB2/Neu ↑, phos-Met ↑, COX-2 ↑, TFG-β1 ↑ | 38,39 |
| Furan-derived transplantable C611B cholangiocarcinoma cell model | Rat | 4–6 | 100 | Tubular adenocarcinoma | MF, WD, Des, CK19+, mucin+ | phos-ErbB2/Neu ↑, phos-Met ↑, COX-2 ↑, cyclin D1 ↑, telomerase ↑, phos-Akt ↑, phos-p42/44 MAPK ↑ | 23, 40–42 |
| Thioacetamide | Rat | 16–24 | 80–100 | Int-type tubular adenocarcinoma | MF, WD, Des, CK19+, CK7+, mucin+, non-metastatic | ErbB2/Neu ↑, EGFR ↑, Met ↑, MUC1 ↑, MUC4 ↑, MMP-2 ↑, MMP-9 ↑, SCF ↑, c-Kit ↑ | 28, 43, 44 |
| 3′ Me-DAB | Rat | 16–22 | NR | Tubular adenocarcinoma | Focal and MF, Des, mucin+ | TFG-β1 ↑, telomerase ↑ | 45 |
| Orthotopic BDEneu cell transplantation model of ICC progression | Rat | 1–4 | 100 | Ductal carcinoma | MF, WD-to-MD, Des, CK19+, highly metastatic | Mutationally-activated ErbB2/Neu ↑, COX-2 ↑, MUC1 ↑, telomerase ↑, cyclin D1 ↑, phos-Akt ↑, phos-p42/44 MAPK ↑, amphiregulin ↑, caveolin ↑, tenascin ↑ | 42, 46, 47 |
| Ov+DMN | Hamster | 26–35 | 100 | Tubular adenocarcinoma and cystadenocarcinoma | Microscopic and macroscopic foci, Des | Tenascin ↑ | 48 |
| p53 −/− plus chronic CCl4 | Mouse | ≥16 | 40 | Ductal carcinoma | Des, CK19+, weakly metastatic | phos-Met ↑, ErbB2/Neu ↑, E-Cadherin ↓ | 49 |
| Smad4Co/CoPtenCo/CoAlb-Cre | Mouse | 16–40 | 100 | Ductal carcinoma | Des, CK19+, mucin+ | PTEN ↓, SMAD ↓, MUC5 AC ↑, cyclin D1 ↑, phos-Akt ↑, phos-mTOR ↑, phos-p42/44 MAPK ↑, phos-FOXO1 ↑ | 50 |
| BK5.ErbB2a | Transgenic mouse | 12–32 | 25–30 | Focal papillary carcinoma | — | ErbB2/Neu ↑ | 51 |
3′ Me-DAB, 3′-methyl 4-dimethylazobenzene; Ov+DMN, Opisthorchis viverrini + dimethylnitrosamine; CCL4, carbon tetrachloride; NR, not reported; Int-type, intestinal-type; MF, mass-forming; WD, well-differentiated; MD, moderately differentiated; Des, desmoplastic; CK, cytokeratin; phos, phosphorylated; EGFR, epidermal growth factor receptor; SCF, stem cell factor; Akt, serine/threonine Akt/PKB; PTEN, phosphatase and tensin homolog deleted on chromosome 10; m-TOR, mammalian target of rapamycin; FOXO, forkhead box O; ↑, increased; ↓, decreased.
Preferentially develops gallbladder adenocarcinoma at 100% incidence exhibiting phos-ErbB2/Neu ↑, phos-EGFR ↑, COX-2 ↑, and phos-MAPK ↑.
In this context, we recently described a unique “patient-like” rat model of intrahepatic cholangiocarcinoma that closely mimics the disease.42,46 In this model, oncogenic neu-transformed rat cholangiocytes (BDEneu cells) are orthotopically transplanted via bile duct inoculation into the livers of young adult syngeneic Fischer 344 male rats, resulting during a 25-day period in a rapid exponential growth of invasive cholangiocarcinoma, which, as also observed in humans with advanced intrahepatic cholangiocarcinoma, is paralleled by progressive increases in tumor-induced bile duct obstruction with elevated serum bilirubin levels, and in the development of gross peritoneal metastases. Notably, with this model, we demonstrated bile duct obstruction to be a potent stimulus for intrahepatic cholangiocarcinoma tumor growth and progression.46 We further correlated high MUC1 expression in BDEneu cells with their significantly enhanced tumorigenic potential when compared with either non-tumorigenic or low tumorigenic cell lines derived from the same parent rat cholangiocyte cell line that was used to generate the BDEneu cell line.42,46 Data from Affymetrix (Santa Clara, CA) microarray analysis (Table 3), validated by laser capture microdissection combined with real-time RT-PCR, quantitative immunohistochemistry, and/or Western blotting (Figure 2), has further demonstrated up-regulation of amphiregulin, a potent ErbB growth factor ligand, and of caveolin-1, the major structural protein in caveolae implicated in malignant cell metastasis, to each significantly correlate with tumor progression in the BDEneu rat orthotopic tumor model.47
Table 3. Amphiregulin (Areg) and Caveolin-1 (Cav) Gene Expression Correlated with Rat BDEneu Cholangiocarcinoma Progressiona.
| Gene symbol | Average expression (Log2) BDEneu liver tumor at 10 days | Average expression (Log2) BDEneu liver tumor at 15 days | Average expression (Log2) BDEneu liver tumor at 25 days | Average expression (Log2) BDEneu Met at 25 days | Jonckheere-Terpstra trend P valueb | Spearman rank correlation |
|---|---|---|---|---|---|---|
| Areg | 5.9 | 7.2 (2.4) | 8.1 (4.4)c | 8.0 (4.2) | 1.06E-02 | 0.843 |
| Cav | 8.9 | 9.6 (1.6) | 9.9 (1.9) | 10.7 (3.3) | 1.96E-02 | 0.791 |
Gene expression determined by microarray analysis of total RNA extracted from whole tumor tissue samples (n = 3).
Trend analysis performed on tumor samples only, excluding peritoneal metastases (Met).
Number in parentheses indicates geometric fold increases in gene expression levels compared with those of liver tumors at 10 days.
Figure 2.
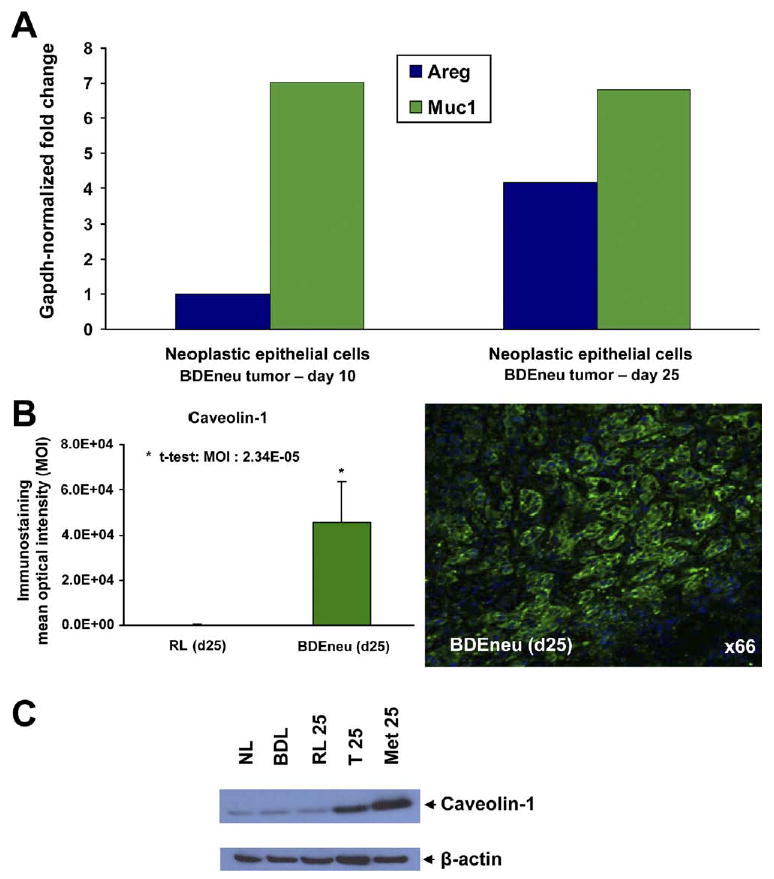
(A) Real-time RT-PCR gene expression measurements of amphiregulin (Areg) and mucin1 (Muc1) mRNA expressed in neoplastic biliary ducts obtained by laser capture microdissection from rat BDEneu intrahepatic cholangiocarcinomas at 10 and 25 days after inoculation. Glyceraldehyde 3-phosphate dehydrogenase–normalized fold changes are plotted. Note that amphiregulin mRNA is up-regulated in the malignant cholangiocytes in day 25 liver tumor compared with day 10 tumor, whereas MUC1 is equally expressed in both. (B) Representative photomicrograph depicting strongly positive immunofluorescence staining for caveolin-1 overexpressed in neoplastic cholangiocytes of a rat BDEneu intrahepatic cholangiocarcinoma, together with corresponding mean MOI values ± standard deviation for caveolin-1 immunostaining in tissue sections from day 25 BDEneu liver tumors (n = 3) compared with pair-matched cancer-free right liver lobe tissue samples obtained from the same animals as the tumor. Caveolin-1 immunoreactivity was not detected in either normal (day 10 liver) or hyperplastic bile ducts (day 25 liver) observed in the corresponding pair-matched rat liver lobe tissue samples. However, a strong positive immunoreactivity was observed in the portal vein and artery branches of the rat liver lobe tissue samples, as well as in the BDEneu tumor vasculature (data not shown). (C) Representative Western blot demonstrating prominently overexpressed caveolin-1 protein in whole tumor lysates prepared from day 25 rat BDEneu liver tumor (T 25) and associated peritoneal metastasis (Met 25) relative to caveolin-1 protein levels expressed in normal adult rat liver (NL), cholestatic liver at 21 days after bile duct ligation (BDL), and right liver lobe without cancer (RL) from the same rat as the BDEneu tumor.
Both amphiregulin mRNA (Table 3, Figure 2A) and protein (data not shown) and caveolin-1 mRNA (Table 3) and protein (Figure 2B, C) were each determined to be significantly increased in hepatic tumors and associated peritoneal metastases formed at 25 days after initial bile duct inoculation of BDEneu cells into liver when compared with day 10 liver tumors without evidence of gross peritoneal tumors (see time course data46). The human relevance and functional significance of these latter findings still need to be determined. However, amphiregulin has been shown to be differentially overexpressed in human biliary cancers compared with normal epithelium,52 as well as to be significantly expressed in cultured human cholangiocarcinoma cells,53 hepatocellular carcinoma cells,53,54 pancreatic cancer cells,53 and colon cancer cells.53,55 Amphiregulin was further demonstrated to behave as a mitogenic and anti-apoptotic growth factor and to contribute to the transformed phenotype of human hepatocellular carcinoma cells.54 Furthermore, amphiregulin was reported to be an independent prognostic marker for liver metastasis when detected in primary lesions of human colorectal cancer55 and represents a promising target for epithelial cancer therapy.53
Stage-specific caveolin-1 overexpression has been reported to correlate with cancer progression, metastasis, and poor clinical prognosis in human hepatocarcinoma,56 but to our knowledge, it has not been previously investigated in either human or experimental models of intrahepatic cholangiocarcinoma. Clearly, the role of caveolin-1 as a possible promoter of intrahepatic cholangiocarcinoma progression and metastasis still needs to be assessed. However, targeting caveolin-1 expression has been suggested as a novel means of preventing metastasis.57 In this regard, the BDEneu model appears to be ideally suited for testing this proposed strategy.
Role of Tumor Stroma in Intrahepatic Cholangiocarcinoma Progression
It is well-recognized that unlike hepatocellular carcinoma, intrahepatic cholangiocarcinoma typically exhibits an excessive desmoplastic reaction characterized by abundant extracellular matrix (ECM) proteins and cancer- associated fibroblasts (CAFs) predominately expressing a myofibroblast-like phenotype.58,59 As exemplified in Figure 3A and B, intrahepatic cholangiocarcinoma is most often characterized by nests of cytokeratin 19–positive malignant ductal carcinoma cells typically surrounded by an abundance of myofibroblastic-like cells strongly immunoreactive for α–smooth muscle actin (α-SMA). The origin of the α-SMA–positive CAFs in stroma of intrahepatic cholangiocarcinoma remains unknown, but they are likely arising from different sources, including portal or periductal fibroblasts and hepatic stellate cells,59 also potentially from bone marrow–derived progenitor cells,60 and by epithelial-tomesenchymal transition.61,62 Regardless of origin, α-SMA–positive CAFs produce ECM proteins and growth factors known to affect tumorigenic growth, invasion, metastasis, and tumor microvascular environment. However, to date, studies aimed at specifically addressing the role played by tumor stromal components in promoting intracellular cholangiocarcinoma progression have been limited and often circumstantial or descriptive. Nevertheless, the available data from these limited studies support the importance of tumor stroma in intrahepatic cholangiocarcinoma progression and in patient survival.
Figure 3.
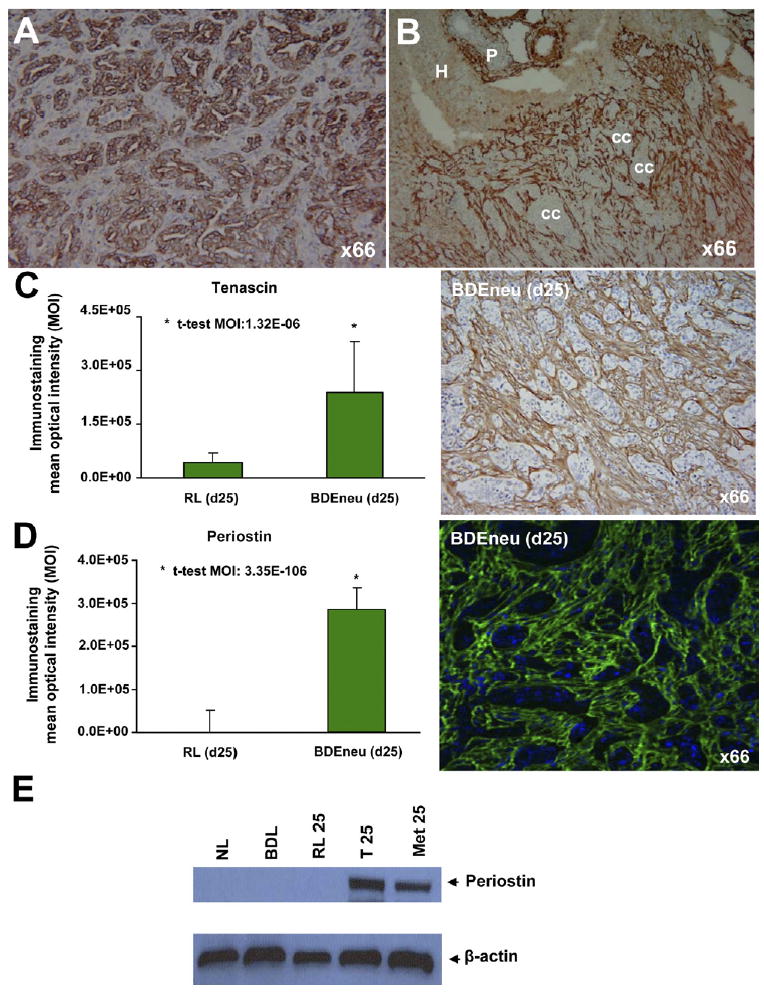
(A) Representative photomicrograph of rat BDEneu liver tumor tissue section exemplifying positive cytoplasmic immunoreactivity for “biliary” cytokeratin 19 as a characteristic phenotypic feature of neoplastic cholangiocytes in intrahepatic cholangiocarcinoma. (B) Photomicrograph depicting abundant α-SMA–positive intratumoral stromal cells surrounding cholangiocarcinoma cell nests (cc) in an invasive rat BDEneu liver tumor. P, portal area; H, hepatocytes. Representative photomicrographs depicting strong positive immunostaining reactions, together with corresponding MOI values (mean ± standard deviation), for the stromal proteins tenascin (C) and periostin (D) in tissue sections from day 25 BDEneu liver tumors compared with respective cancer-free pair-matched right liver lobe tissue samples. Tenascin and periostin immunostaining was either not detected or only marginally detected in the analyzed non-cancerous liver tissue sections with or without bile duct hyperplasia. (E) Representative Western blot demonstrating profound differential overexpression of periostin in day 25 rat BDEneu intrahepatic cholangiocarcinoma (T 25) and associated peritoneal metastatic tumor (Met 25) compared with normal adult rat liver (NL), 21 day bile duct–ligated liver (BDL), and pair-matched right liver lobe without cancer (RL 25).
Intrahepatic cholangiocarcinoma patients with surgically resected tumors having a high expression of α-SMA exhibited poorer survival times than those with low α-SMA expression tumors.58,59 Multivariate analysis further revealed high α-SMA to be an independent prognostic factor for intrahepatic cholangiocarcinoma.59 In this same study, it was further found that co-culturing of hepatic stellate cells with 2 different cholangiocarcinoma cell lines stimulated a significant increase in in vitro cholangiocarcinoma cell growth and invasion, suggesting activated hepatic stellate cells to be involved in the progression of intrahepatic cholangiocarcinoma.
Fibrogenic growth factors associated with activation of CAFs, including TGF-β, fibroblast growth factor (FGF), and platelet-derived growth factor (PDGF), have been detected in increased amounts in bile from cholangiocarcinoma patients.63 Moreover, conditioned medium from α-SMA expressing fibroblast cultures derived from human cholangiocarcinoma tissue was further shown to promote proliferation of cultured non-tumorigenic H69 biliary epithelial cells and of various human cholangiocarcinoma cell lines via both secreted substances and cell-to-cell contact.58 In addition, stromal-derived growth factor-1 (SDF-1), secreted by the embryonic lung fibroblast cell line WI-38 interacting with its receptor CXCR4 expressed in 2 cultured human intrahepatic cholangiocarcinoma cell lines (HuCCT1 and CCKS-1), was found to stimulate cholangiocarcinoma cell migration in vitro, suggesting that SDF-1 expressed by stromal fibroblasts might be involved in intrahepatic cholangiocarcinoma cell invasion.64 Furthermore, tumor necrosis factor–α acted synergistically to increase SDF-1–stimulated HuCCT1 and CCKS-1 cell migration. Of further note, hepatocyte growth factor (HGF) produced by fibroblasts in co-culture with GB-d1 human gallbladder carcinoma cells was also shown to enhance cancer cell invasion in vitro.65
As noted, tenascin, a high molecular weight glycoprotein of the ECM, expressed by activated CAFs and induced by TGF-β1,66 is abundantly elaborated into the desmoplastic stroma of intrahepatic cholangiocarcinomas17 (Figure 3C) and potentially associated with their invasiveness.17 In terms of a possible mechanism, it has been demonstrated that tenascin produced by cultured human colon cancer myofibroblasts might act through its epidermal growth factor (EGF)–like repeats to confer a permissive and priming signal for the proinvasive activity of HGF on human colon cancer cells in vitro via activation of Rac signaling.66
Periostin represents another TGF-β1 inducible secretory protein recently demonstrated by us to be dramatically overexpressed in the desmoplastic stroma of rat BDEneu intrahepatic cholangiocarcinomas and associated peritoneal metastases46 (Figure 3D, E). Our global microarray analysis further demonstrated periostin to be the most highly overexpressed gene in the BDEneu liver tumors (data not shown). To our knowledge, there are to date no published accounts concerning the role played by periostin in human cholangiocarcinogenesis. However, periostin has been reported to be involved in the development and progression of various other human cancers, such as breast, lung, colon, pancreatic, and ovarian malignancies.67 With respect to other gastrointestinal cancers, periostin, which is secreted from tumor stromal cells,68,69 has been shown to have growth and survival promoting effects on colorectal cancer cells.70 In the case of pancreatic cancer, periostin was reported to create a tumor-supportive microenvironment for cancer cell growth, invasiveness, and resistance to hypoxia-induced cell death68 and correlated with epithelial-to-mesenchymal transition.69
Galectin-1, an endogenous β-galactoside-binding lectin, was shown to be intensely expressed in the desmoplastic stroma of human intrahepatic cholangiocarcinomas.71 Up-regulation of galectin-1 in tumor stroma correlated with histologic dedifferentiation of intrahepatic cholangiocarcinoma and significantly correlates with perineural and vascular invasion. More recently, galectin-1 was identified as a new functional receptor for tissue plasminogen activator (tPA), with galectin-1 activation of tPA catalytic activity having been demonstrated to mediate proliferation and invasion of cultured pancreatic cancer cells and of tumor-derived fibroblasts.72 These data also support targeting galectin-1 as a potential therapeutic strategy for desmoplastic cancers such as intrahepatic cholangiocarcinoma and pancreatic ductal adenocarcinoma.
Desmoplastic stroma might also contribute to the progression of cholangiocarcinoma by impeding angiogenesis. Although cholangiocarcinoma cells produce angiogenic factors such as vascular endothelial growth factor (VEGF),73 these tumors are often relatively hypovascularized. It has been recently proposed that excessive amounts of ECM proteins together with CAFs and inflammatory cells might limit tumor neovascularization, leading to poor therapeutic drug delivery.74 Diminished vascularity of intrahepatic cholangiocarcinomas has also been shown to be related in part to an overexpression of thrombospondin-1 (TSP-1), a multifunctional ECM protein that functions as an antiangiogenic factor, together with a decrease in VEGF.75 Survival analysis showed that intrahepatic cholangiocarcinoma patients with positive TSP-1 expression had a tendency for shorter survival than those negative for TSP-1; TSP-1 expression correlated with the desmoplastic response in the tumor.75,76 These results also suggest that neoangiogenesis is not directly related to intrahepatic cholangiocarcinoma progression, but that enhanced expression of TSP-1 might be contributing to enhanced tumor aggressiveness by possibly contributing to a hypoxic microenvironment.76 Activated CAFs might also be contributing to the fibrotic/hypoxia milieu by amplifying the production of endostatin by carcinoma cells.77
Interestingly, lymphangiogenesis was also reported not to be playing a direct role in lymphatic metastasis linked to VEGF-C overexpression in human intrahepatic cholangiocarcinomas, although lymphatic invasion via preexisting lymphatic vessels significantly correlated with VEGF-C expression.16 Furthermore, postoperative survival rates of patients with VEGF-C positive intrahepatic cholangiocarcinoma were significantly worse than those negative for VEGF-C expression.16
Concluding Remarks
Significant progress has been made during the past several years in defining cellular interactions and molecular pathways associated with the pathogenesis of intrahepatic cholangiocarcinoma and, as highlighted in this review, leading to the identification of various select molecular markers having potential as either prognostic indicators and/or therapeutic targets for this lethal cancer. However, translation of these findings into effective clinical strategies for the treatment of advanced intrahepatic cholangiocarcinoma is at present far from being realized. The limitations that continue to hinder the development and testing of new target-based strategies for intrahepatic cholangiocarcinoma therapy include the fact that despite its rising incidence, this cancer is relatively uncommon in most regions of the world, thus making clinical trials a challenge. Moreover, although a number of experimental animal models are now available for use as preclinical platforms for testing novel molecular therapeutic strategies against intrahepatic cholangiocarcinoma, there remains a real need to assess and validate their ability to accurately predict effective therapeutic activity against the human disease. Furthermore, it is becoming increasingly evident that the tumor stroma, and most notably CAFs, are likely playing a key role in promoting intrahepatic cholangiocarcinoma progression, although specific mechanisms whereby stromal/cancer cell crosstalk and select ECM proteins are acting to mediate malignant tumor aggressiveness, invasion, and metastasis in intrahepatic cholangiocarcinoma still need to be clarified. Nevertheless, it also seems apparent that target-based strategies that combine targeting of both malignant cholangiocytes and stromal CAFs are most likely to yield a positive therapeutic response for desmoplastic, hypovascularized intrahepatic cholangiocarcinoma.
In this regard, the recently reported findings of Olive et al74 are instructive. These authors showed that administration of IPI-926, a drug that depletes tumor-associated stromal tissue by inhibition of the Hedgehog cellular signaling pathway, improved the vascular delivery and increased efficacy of the anticancer drug gemcitabine when co-administered in a de novo mouse model of pancreatic cancer. This finding suggests novel testable strategies in which targeting of CAFs with agents such as those that inhibit TGF-β, PDGF, or Hedgehog signaling pathways or that block the production of ECM proteins like periostin or tenascin are administered in combination with agents designed to selectively interact with molecular targets such as MUC1, MUC4, or possibly amphiregulin and caveolin-1, which are significantly overexpressed in malignant cholangiocytes and have been shown to be associated with intrahepatic cholangiocarcinoma progression.
Acknowledgments
Funding: Supported by National Institutes of Health Grants R01 CA 83650 and R01 CA 39225 (A.E.S).
Abbreviations used in this paper
- CAFs
cancer-associated fibroblasts
- CCN
connective tissue growth factor, cysteine-rich 61, nephroblastoma
- CTGF
connective tissue growth factor
- ECM
extracellular matrix
- EGF
epidermal growth factor
- FGF
fibroblast growth factor
- HGF
hepatocyte growth factor
- MAPK
mitogen-activated protein kinase
- MMP
matrix metalloproteinase
- MOI
mean optical intensity
- MUCs
mucins
- PDGF
platelet-derived growth factor
- RT-PCR
reverse transcription-polymerase chain reaction
- α-SMA
α–smooth muscle actin
- SDF-1
stromal-derived growth factor-1
- TGF-β
transforming growth factor–β
- tPA
tissue plasminogen activator
- TSP-1
thrombospondin-1
- VEGF
vascular endothelial growth factor
Footnotes
Conflicts of interest: The authors disclose no conflicts.
References
- 1.Hammill CW, Wong LL. Intrahepatic cholangiocarcinoma: a malignancy of increasing importance. J Am Coll Surg. 2008;207:594–603. doi: 10.1016/j.jamcollsurg.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 2.Khan SA, Toledano MB, Taylor-Robinson SD. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB. 2008;10:77–82. doi: 10.1080/13651820801992641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blechacz BRA, Gores GJ. Cholangiocarcinoma. Clin Liver Dis. 2008;12:131–150. doi: 10.1016/j.cld.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Briggs CD, Neal CP, Mann CD, et al. Prognostic molecular markers in cholangiocarcinoma: a systematic review. Eur J Cancer. 2009;45:33–47. doi: 10.1016/j.ejca.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 5.Hirohashi K, Uenishi T, Kubo S, et al. Macroscopic types of intrahepatic cholangiocarcinoma: clinicopathologic features and surgical outcomes. Hepatogastroenterology. 2002;49:326–329. [PubMed] [Google Scholar]
- 6.Yeh CN, Jan YY, Yeh TS, et al. Hepatic resection of the intraductal papillary type of peripheral cholangiocarcinoma. Ann Surg Oncol. 2004;11:606–611. doi: 10.1245/ASO.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 7.Guglielmi A, Ruzzenente A, Campagnaro T, et al. Intrahepatic cholangiocarcinoma: prognostic factors after surgical resection. World J Surg. 2009;33:1247–1254. doi: 10.1007/s00268-009-9970-0. [DOI] [PubMed] [Google Scholar]
- 8.Matsumura N, Yamamoto M, Aruga A, et al. Correlation between expression of MUC1 core protein and outcome after surgery in mass-forming intrahepatic cholangiocarcinoma. Cancer. 2002;94:1770–1776. doi: 10.1002/cncr.10398. [DOI] [PubMed] [Google Scholar]
- 9.Boonla C, Sripa B, Thuwajit P, et al. MUC1 and MUC5AC mucin expression in liver fluke-associated intrahepatic cholangiocarcinoma. World J Gastroenterol. 2005;11:4939–4946. doi: 10.3748/wjg.v11.i32.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shibahara H, Tamada S, Higashi M, et al. MUC4 is a novel prognostic factor of intrahepatic cholangiocarcinoma-mass forming type. Hepatology. 2004;39:220–229. doi: 10.1002/hep.20031. [DOI] [PubMed] [Google Scholar]
- 11.Yeh CN, Pang ST, Wu RC, et al. Prognostic value of MUC4 for mass-forming intrahepatic cholangiocarcinoma after hepatectomy. Oncology Reports. 2009;21:49–56. [PubMed] [Google Scholar]
- 12.Miwa S, Miyagawa S, Soeda J, et al. Matrix metalloproteinase-7 expression and biologic aggressiveness of cholangiocellular carcinoma. Cancer. 2002;94:428–434. doi: 10.1002/cncr.10235. [DOI] [PubMed] [Google Scholar]
- 13.Iguchi T, Aishima S, Taketomi A, et al. Fascin overexpression is involved in carcinogenesis and prognosis of human intrahepatic cholangiocarcinoma: immunohistochemical and molecular analysis. Hum Pathol. 2009;40:174–180. doi: 10.1016/j.humpath.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 14.Javle MM, Tan D, Yu J, et al. Nuclear survivin expression predicts poor outcome in cholangiocarcinoma. Hepatogastroenterology. 2004;51:1653–1657. [PubMed] [Google Scholar]
- 15.Sugimachi K, Aishima S, Taguchi K, et al. The role of overexpression and gene amplification of cyclin D1 in intrahepatic cholangiocarcinoma. J Hepatol. 2001;35:74–79. doi: 10.1016/s0168-8278(01)00079-4. [DOI] [PubMed] [Google Scholar]
- 16.Aishima S, Nishihara Y, Iguchi T, et al. Lymphatic spread is related to VEGF-C expression and D2-40-positive myofibroblasts in intrahepatic cholangiocarcinoma. Mod Pathol. 2008;21:256–264. doi: 10.1038/modpathol.3800985. [DOI] [PubMed] [Google Scholar]
- 17.Aishima S, Taguchi K, Terashi T, et al. Tenascin expression at the invasive front is associated with poor prognosis in intrahepatic cholangiocarcinoma. Mod Pathol. 2003;16:1019–1027. doi: 10.1097/01.MP.0000086860.65672.73. [DOI] [PubMed] [Google Scholar]
- 18.Aishima S, Matsuura S, Terashi T, et al. Aberrant expression of laminin gamma 2 chain and its prognostic significance in intrahepatic cholangiocarcinoma according to growth morphology. Mod Pathol. 2004;17:938–945. doi: 10.1038/modpathol.3800143. [DOI] [PubMed] [Google Scholar]
- 19.Li Q, Wang JM, Liu C, et al. Correlation of aPKC-ι and E-cadherin expression with invasion and prognosis of cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2008;7:70–75. [PubMed] [Google Scholar]
- 20.Aishima S, Kuroda Y, Nishihara Y, et al. Down-regulation of aquaporin-1 in intrahepatic cholangiocarcinoma is related to tumor progression and mucin expression. Hum Pathol. 2007;38:1819–1825. doi: 10.1016/j.humpath.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 21.Gardini A, Corti B, Fiorentino M, et al. Expression of connective tissue growth factor is a prognostic marker for patients with intrahepatic cholangiocarcinoma. Dig Liver Dis. 2005;37:269–274. doi: 10.1016/j.dld.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Harada K, Masuda S, Hirano M, et al. Reduced expression of syndecan-1 correlates with histologic dedifferentiation, lymph node metastasis, and poor prognosis in intrahepatic cholangiocarcinoma. Hum Pathol. 2003;34:857–863. doi: 10.1016/s0046-8177(03)00336-8. [DOI] [PubMed] [Google Scholar]
- 23.Sirica AE. Role of ErbB family receptor tyrosine kinases in intrahepatic cholangiocarcinoma. World J Gastroenterol. 2008;14:7033–7058. doi: 10.3748/wjg.14.7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology. 2005;41:5–15. doi: 10.1002/hep.20537. [DOI] [PubMed] [Google Scholar]
- 25.Zen Y, Fujii T, Itatsu K, et al. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology. 2006;44:1333–1343. doi: 10.1002/hep.21387. [DOI] [PubMed] [Google Scholar]
- 26.Ishikawa A, Sasaki M, Ohira S, et al. Aberrant expression of CDX2 is closely related to the intestinal metaplasia and MUC2 expression in intraductal papillary neoplasm of the liver in hepatolithiasis. Lab Invest. 2004;84:629–638. doi: 10.1038/labinvest.3700087. [DOI] [PubMed] [Google Scholar]
- 27.Higashi M, Yonezawa S, Ho JJ, et al. Expression of MUC1 and MUC2 mucin antigens in intrahepatic bile duct tumors: its relationship with a new morphological classification of cholangiocarcinoma. Hepatology. 1999;30:1347–1355. doi: 10.1002/hep.510300609. [DOI] [PubMed] [Google Scholar]
- 28.Jan YY, Yeh TS, Yeh JN, et al. Expression of epidermal growth factor receptor, apomucins, matrix metalloproteinases, and p53 in rat and human cholangiocarcinoma: appraisal of an animal model of cholangiocarcinoma. Ann Surg. 2004;240:89–94. doi: 10.1097/01.sla.0000129492.95311.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh AP, Chaturvedi P, Batra SK. Emerging roles of MUC4 in cancer: a novel target for diagnosis and therapy. Cancer Res. 2007;67:433–436. doi: 10.1158/0008-5472.CAN-06-3114. [DOI] [PubMed] [Google Scholar]
- 30.Workman HC, Sweeney C, Carraway KL., III The membrane mucin Muc4 inhibits apoptosis induced by multiple insults via ErbB2-dependent and ErbB2-independent mechanisms. Cancer Res. 2009;69:2845–2852. doi: 10.1158/0008-5472.CAN-08-2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alvaro D. Serum and bile biomarkers for cholangiocarcinoma. Curr Opin Gastroenterol. 2009;25:279–284. doi: 10.1097/mog.0b013e328325a894. [DOI] [PubMed] [Google Scholar]
- 32.Thuwajit P, Chawengrattanachot W, Thuwajit C, et al. Enhanced expression of mucin 6 glycoprotein in cholangiocarcinoma tissue from patients in Thailand as a prognostic marker for survival. J Gastroenterol Hepatol. 2008;23:771–778. doi: 10.1111/j.1440-1746.2008.05332.x. [DOI] [PubMed] [Google Scholar]
- 33.Shirabe K, Shimada M, Kajiyama K, et al. Expression of matrix metalloproteinase-9 in surgically resected intrahepatic cholangiocarcinoma. Surgery. 1999;126:842–846. [PubMed] [Google Scholar]
- 34.Itatsu K, Zen Y, Yamaguchi J, et al. Expression of matrix metalloproteinase 7 is an unfavorable postoperative prognostic factor in cholangiocarcinoma of the perihilar, hilar, and extrahepatic bile ducts. Hum Pathol. 2008;39:710–719. doi: 10.1016/j.humpath.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 35.Leelawat K, Sakchinabut S, Narong S, et al. Detection of serum MMP-7 and MMP-9 in cholangiocarcinoma patients: evaluation of diagnostic accuracy. BMC Gastroenterol. 2009;9:30. doi: 10.1186/1471-230X-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sedlaczek N, Jia JD, Bauer M, et al. Proliferating bile duct epithelial cells are a major source of connective tissue growth factor in rat biliary fibrosis. Am J Pathol. 2001;158:1239–1244. doi: 10.1016/S0002-9440(10)64074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka S, Sugimachi K, Kameyama T, et al. Human WISP1v, a member of the CCN family, is associated with invasive cholangiocarcinoma. Hepatology. 2003;37:1122–1129. doi: 10.1053/jhep.2003.50187. [DOI] [PubMed] [Google Scholar]
- 38.Radaeva S, Ferreira-Gonzalez A, Sirica AE. Overexpression of C-NEU and C-MET during rat liver cholangiocarcinogenesis: a link between biliary intestinal metaplasia and mucin-producing cholangiocarcinoma. Hepatology. 1999;29:1453–1462. doi: 10.1002/hep.510290524. [DOI] [PubMed] [Google Scholar]
- 39.Sirica AE, Lai GH, Zhang Z. Biliary cancer growth factor pathways, cyclo-oxygenase-2 and potential therapeutic strategies. J Gastroenterol Hepatol. 2001;16:363–372. doi: 10.1046/j.1440-1746.2001.02438.x. [DOI] [PubMed] [Google Scholar]
- 40.Lai GH, Sirica AE. Establishment of a novel rat cholangiocarcinoma cell culture model. Carcinogenesis. 1999;20:2335–2340. doi: 10.1093/carcin/20.12.2335. [DOI] [PubMed] [Google Scholar]
- 41.Sirica AE, Lai GH, Endo K, et al. Cyclooxygenase-2 and ERBB-2 in cholangiocarcinoma: potential therapeutic targets. Semin Liver Dis. 2002;22:303–313. doi: 10.1055/s-2002-34507. [DOI] [PubMed] [Google Scholar]
- 42.Lai GH, Zhang Z, Shen XN, et al. erbB-2/neu transformed rat cholangiocytes recapitulate key cellular and molecular features of human bile duct cancer. Gastroenterology. 2005;129:2047–2057. doi: 10.1053/j.gastro.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 43.Yeh CN, Maitra A, Lee KF, et al. Thioacetamide-induced intestinal-type cholangiocarcinoma in rat: an animal model recapitulating the multi-stage progression of human cholangiocarcinoma. Carcinogenesis. 2004;25:631–636. doi: 10.1093/carcin/bgh037. [DOI] [PubMed] [Google Scholar]
- 44.Mansuroglu T, Ramadori P, Dudás J, et al. Expression of stem cell factor and its receptor c-Kit during the development of intrahepatic cholangiocarcinoma. Lab Invest. 2009;89:562–574. doi: 10.1038/labinvest.2009.15. [DOI] [PubMed] [Google Scholar]
- 45.Lu JP, Mao JQ, Li MS, et al. In situ detection of TGF betas, TGF beta receptor II mRNA and telomerase activity in rat cholangiocarcinogenesis. World J Gastroenterol. 2003;9:590–594. doi: 10.3748/wjg.v9.i3.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sirica AE, Zhang Z, Lai GH, et al. A novel “patient-like” model of cholangiocarcinoma progression based on bile duct inoculation of tumorigenic rat cholangiocyte cell lines. Hepatology. 2008;47:1178–1190. doi: 10.1002/hep.22088. [DOI] [PubMed] [Google Scholar]
- 47.Dumur CI, Almenara JA, Campbell DJ, et al. Genome-wide gene expression profiling in a novel “patient-like” rat model of intrahepatic cholangiocarcinoma progression closely mimicking the human disease. Proceedings of the 100th Annual Meeting of the American Association for Cancer Research; April 18–22, 2009; Denver, CO. Philadelphia. AACR; 2009. abstract nr 1432. [Google Scholar]
- 48.Sithithaworn P, Ando K, Limviroj W, et al. Expression of tenascin in bile duct cancer of hamster liver by combined treatment of dimethylnitrosamine with Opisthorchis viverrini infections. J Helminthol. 2002;76:261–268. doi: 10.1079/JOH2002129. [DOI] [PubMed] [Google Scholar]
- 49.Farazi PA, Zeisberg M, Glickman J, et al. Chronic bile duct injury associated with fibrotic matrix microenvironment provokes cholangiocarcinoma in p53-deficient mice. Cancer Res. 2006;66:6622–6627. doi: 10.1158/0008-5472.CAN-05-4609. [DOI] [PubMed] [Google Scholar]
- 50.Xu X, Kobayashi S, Qiao W, et al. Induction of intrahepatic cholangiocellular carcinoma by liver-specific disruption of Smad4 and Pten in mice. J Clin Invest. 2006;116:1843–1852. doi: 10.1172/JCI27282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kiguchi K, Carbajal S, Chan K, et al. Constitutive expression of ErbB-2 in gallbladder epithilium results in development of adenocarcinoma. Cancer Res. 2001;61:6971–6976. [PubMed] [Google Scholar]
- 52.Hansel DE, Rahman A, Hidaigo M, et al. Identification of novel cellular targets in biliary tract cancers using global gene expression technology. Am J Pathol. 2003;163:217–229. doi: 10.1016/S0002-9440(10)63645-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yotsumoto F, Yagi H, Suzuki SO, et al. Validation of HB-EGF and amphiregulin as targets for human cancer therapy. Biochem Biophys Res Commun. 2008;365:555–561. doi: 10.1016/j.bbrc.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 54.Castillo J, Erroba E, Perugorría MJ, et al. Amphiregulin contributes to the transformed phenotype of human hepatocellular carcinoma cells. Cancer Res. 2006;66:6129–6138. doi: 10.1158/0008-5472.CAN-06-0404. [DOI] [PubMed] [Google Scholar]
- 55.Yamada M, Ichikawa Y, Yamagishi S, et al. Amphiregulin is a promising prognostic marker for liver metastases of colorectal cancer. Clin Cancer Res. 2008;14:2351–2356. doi: 10.1158/1078-0432.CCR-07-4499. [DOI] [PubMed] [Google Scholar]
- 56.Zhang ZB, Cai L, Zheng SG, et al. Overexpression of caveolin-1 in hepatocellular carcinoma with metastasis and worse prognosis: correlation with vascular endothelial growth factor, microvessel density and unpaired artery. Pathol Oncol Res. 2009 doi: 10.1007/s12253-008-9144-7. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 57.van Golen KL. Is caveolin-1 a viable therapeutic target to reduce cancer metastasis? Expert Opin Ther Targets. 2006;10:709–721. doi: 10.1517/14728222.10.5.709. [DOI] [PubMed] [Google Scholar]
- 58.Chuaysri C, Thuwajit P, Paupairoj A, et al. Alpha-smooth muscle actin-positive fibroblasts promote biliary cell proliferation and correlate with poor survival in cholangiocarcinoma. Oncol Rep. 2009;21:957–969. doi: 10.3892/or_00000309. [DOI] [PubMed] [Google Scholar]
- 59.Okabe H, Beppu T, Hayashi H, et al. Hepatic stellate cells may relate to progression of intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2009;16:2555–2564. doi: 10.1245/s10434-009-0568-4. [DOI] [PubMed] [Google Scholar]
- 60.Worthley DL, Ruszkiewicz A, Davies R, et al. Human gastrointestinal neoplasia-associated myofibroblasts can develop from bone marrow-derived cells following allogeneic stem cell transplantation. Stem Cells. 2009;27:1463–1468. doi: 10.1002/stem.63. [DOI] [PubMed] [Google Scholar]
- 61.Rygiel KA, Robertson H, Marshall HL, et al. Epithelial-mesenchymal transition contributes to portal tract fibrogenesis during human chronic liver disease. Lab Invest. 2008;88:112–123. doi: 10.1038/labinvest.3700704. [DOI] [PubMed] [Google Scholar]
- 62.Lee MJ, Yu GR, Yoo HJ, et al. ANXA8 down-regulation by EGF-FOXO4 signaling is involved in cell scattering and tumor metastasis of cholangiocarcinoma. Gastroenterology. 2009;137:1138–1150. doi: 10.1053/j.gastro.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 63.Su WC, Shiesh SC, Liu HS, et al. Expression of oncogene products HER2/Neu and Ras and fibrosis-related growth factors bFGF, TGF-beta, and PDGF in bile from biliary malignancies and inflammatory disorders. Dig Dis Sci. 2001;46:1387–1392. doi: 10.1023/a:1010619316436. [DOI] [PubMed] [Google Scholar]
- 64.Ohira S, Sasaki M, Harada K, et al. Possible regulation of migration of intrahepatic cholangiocarcinoma cells by interaction of CXCR4 expressed in carcinoma cells with tumor necrosis factor-α and stromal-derived factor-1 released in stroma. Am J Pathol. 2006;168:1155–1168. doi: 10.2353/ajpath.2006.050204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matsumoto K, Nakamura T. Hepatocyte growth factor and the Met system as a mediator of tumor-stromal interactions. Int J Cancer. 2006;119:477–483. doi: 10.1002/ijc.21808. [DOI] [PubMed] [Google Scholar]
- 66.De Wever O, Nguyen QD, Van Hoorde L, et al. Tenascin-C and SF/HGF produced by myofibroblasts in vitro provide convergent pro-invasive signals to human colon cancer cells through RhoA and Rac. FASEB J. 2004;18:1016–1018. doi: 10.1096/fj.03-1110fje. [DOI] [PubMed] [Google Scholar]
- 67.Ruan K, Bao S, Ouyang G. The multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci. 2009;66:2219–2230. doi: 10.1007/s00018-009-0013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Erkan M, Kleeff J, Gorbachevski A, et al. Periostin creates a tumor-supportive microenvironment in the pancreas by sustaining fibrogenic stellate cell activity. Gastroenterology. 2007;132:1447–1464. doi: 10.1053/j.gastro.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 69.Kanno A, Satoh K, Masamune A, et al. Periostin, secreted from stromal cells, has biphasic effect on cell migration and correlates with the epithelial to mesenchymal transition of human pancreatic cancer cells. Int J Cancer. 2008;122:2707–2718. doi: 10.1002/ijc.23332. [DOI] [PubMed] [Google Scholar]
- 70.Bao S, Ouyang G, Bai X, et al. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell. 2004;5:329–339. doi: 10.1016/s1535-6108(04)00081-9. [DOI] [PubMed] [Google Scholar]
- 71.Shimonishi T, Miyazaki K, Kono N, et al. Expression of endogenous galectin-1 and galectin-3 in intrahepatic cholangiocarcinoma. Hum Pathol. 2001;32:302–310. doi: 10.1053/hupa.2001.22767. [DOI] [PubMed] [Google Scholar]
- 72.Roda O, Ortiz-Zapater E, Martínez-Bosch N, et al. Galectin-1 is a novel functional receptor for tissue plasminogen activator in pancreatic cancer. Gastroenterology. 2009;136:1379–1390. doi: 10.1053/j.gastro.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 73.Ogasawara S, Yano H, Higaki K, et al. Expression of angiogenic factors, basic fibroblast growth factor and vascular endothelial growth factor, in human biliary tract carcinoma cell lines. Hepatol Res. 2001;20:97–113. doi: 10.1016/s1386-6346(00)00117-0. [DOI] [PubMed] [Google Scholar]
- 74.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kawahara N, Ono M, Taguchi KI, et al. Enhanced expression of thrombospondin-1 and hypovascularity in human cholangiocarcinoma. Hepatology. 1998;28:1512–1517. doi: 10.1002/hep.510280610. [DOI] [PubMed] [Google Scholar]
- 76.Aishima S, Taguchi K, Sugimachi K, et al. The role of thymidine phosphorylase and thrombospondin-1 in angiogenesis and progression of intrahepatic cholangiocarcinoma. Int J Surg Pathol. 2002;10:47–56. doi: 10.1177/106689690201000108. [DOI] [PubMed] [Google Scholar]
- 77.Erkan M, Reiser-Erkan C, Michalski CW, et al. Cancer-stellate cell interactions perpetuate the hypoxia-fibrosis cycle in pancreatic ductal adenocarcinoma. Neoplasia. 2009;11:497–508. doi: 10.1593/neo.81618. [DOI] [PMC free article] [PubMed] [Google Scholar]


