Abstract
Purpose
The celiac lymph node axis acts as a gateway for metastatic systemic spread. The need for prophylactic celiac nodal coverage in chemoradiation therapy for esophageal cancer is controversial. Given the improved ability to evaluate lymph node status before treatment via positron emission tomography (PET) and endoscopic ultrasound, we hypothesized that prophylactic celiac node irradiation may not be needed for patients with localized esophageal carcinoma.
Methods and Materials
We reviewed the radiation treatment volumes for 131 patients who underwent definitive chemoradiation for esophageal cancer. Patients with celiac lymph node involvement at baseline were excluded. Median radiation dose was 50.4 Gy. The location of all celiac node failures was compared with the radiation treatment plan to determine whether the failures occurred within or outside the radiation treatment field.
Results
At a median follow-up time of 52.6 months (95% CI 46.1–56.7 months), 6 of 60 patients (10%) without celiac node coverage had celiac nodal failure; in 5 of these patients, these failures represented the first site of recurrence. Of the 71 patients who had celiac coverage, only 5 patients (7%) had celiac region relapse. In multivariate analyses, having a pretreatment-to-posttreatment change in standardized uptake value on PET >52% (odds ratio [OR] 0.198, p=0.0327) and having failure in the clinical target volume (OR 10.72, p=0.001) were associated with risk of celiac region relapse. Of those without celiac coverage, the 6 patients that later developed celiac failure had a worse median overall survival time compared to the other 54 patients who did not fail (median OS time: 16.5 months vs. 31.5 months, p=0.041). Acute and late toxicities were similar in both groups.
Conclusions
While celiac lymph node failures occur in approximately 1 of 10 patients, the lack of effective salvage treatments and subsequent low morbidity may justify prophylactic treatment in distal esophageal cancer patients.
Keywords: Esophageal cancer, celiac lymph node, failure patterns
INTRODUCTION
Esophageal cancer is the eighth most common cause of cancer; with 482,000 new cases and 407,000 deaths estimated worldwide in 2008, it is the sixth most common cause of death from cancer (1). Localized esophageal carcinomas are highly aggressive and difficult to eradicate with definitive chemoradiation at the doses currently used (2). Although surgery continues to be the standard approach for early-stage esophageal cancers, 3- to 5-year survival rates are poor, ranging from 6% to 35% (3, 4). A trimodality approach comprising chemotherapy, radiation therapy (RT), and surgery has produced improved survival rates (5, 6).
Because the prevalence of esophageal cancer, specifically adenocarcinoma, in North America continues to increase in parallel with the increasing prevalence of obesity, gastroesophageal reflux disease, and Barrett′s metaplasia, understanding the pattern by which the disease spreads is imperative (7). A historical review of adenocarcinoma of the stomach revealed high rates of local abdominal lymph node failure after surgery (8). That study prompted clinicians to extend the radiation field to cover abdominal lymph nodes, including the celiac nodes, for patients with gastric and distal esophageal carcinomas. Other recent studies of failure patterns in patients with such tumors included one by Nakamura et al. (9), who found that 42.1% of all patients with T1–2N0M0 disease had evidence of lymph node involvement at surgery; moreover, 46% of those with lower esophageal tumors and 70% of those with abdominal esophageal tumors had lymph node involvement. The celiac nodes in particular were involved in more than 12% of patients with lower thoracic esophageal tumors. These investigators recommended that the celiac nodes be covered within the radiation field because of the high risk of metastasis there. They also concluded that the involvement of distal regional nodes was unpredictable regardless of tumor location.
Although disease in the celiac nodes was previously classified as distant metastasis (M1), they are currently considered regional rather than distant (10). Given the proximity of the esophagus and celiac nodes to several critical organs in the abdomen, and the poor prognosis associated with involved celiac nodes, evaluating these nodes for disease, and treating them appropriately, is critical to controlling disease spread and overall survival.
Improvements in imaging, including the use of positron emission tomography (PET) and endoscopic ultrasound (EUS), have helped to eliminate some of the uncertainty in the clinical assessment of celiac node status. The use of EUS with fine needle aspiration (FNA) has drastically improved the detection rate of celiac node involvement in patients with esophageal carcinomas, with sensitivity and specificity as high as 83% and 98% (11, 12). Other studies have also supported the use of EUS to detect celiac node involvement (13, 14). With better disease staging techniques, the need to include the celiac nodes in the radiation field as prophylaxis for all patients with distal esophageal carcinoma comes into question. To address this, we reviewed cases of esophageal cancer treated at a single institution, documented the occurrence of celiac node failure, and compared the rates and locations of failure between those who had had prophylactic celiac coverage and those who had not. We hypothesized that prophylactic celiac coverage could be eliminated for at least some patients in the era of PET and EUS for staging. We further sought to identify factors that might predict celiac node failure.
METHODS AND MATERIALS
We retrospectively identified 131 patients with unresectable esophageal cancer treated at The University of Texas MD Anderson Cancer Center from January 2002 through January 2009. All patients had received definitive chemoradiation therapy. Patients who had been treated with surgery were excluded. Those who had celiac node involvement before treatment were also excluded (n=35). All patients underwent thorough nodal evaluation for staging including both a PET scan and EBUS with nodal biopsies. Staging was based on the American Joint Committee Classification (AJCC) 6th edition criteria. Failures within the celiac nodes were documented from post-treatment PET, computed tomography (CT), or PET/CT scans and the locations compared with the original CT-based radiation treatment plans. The institutional review board of MD Anderson Cancer Center approved this post hoc analysis.
Radiation Planning and Treatment
All patients had undergone 4D CT–based treatment simulation and planning to account for respiratory motion as follows. CT images were acquired first while the patient was free-breathing, and 4D images were acquired immediately thereafter. During the 4D CT image acquisition, patient respiration was monitored with an external Real-Time Position Management Respiratory Gating System (Varian Medical Systems, Palo Alto, CA). Each 4D CT image set consisted of 10 CT data sets representing 10 equally divided breathing phases in a complete respiratory cycle. The 4D CT images provided quantitative, time-dependent information about internal organ motion in 3 dimensions, allowing quantitative description of internal organ motion for both treatment targets and normal organs.
The gross tumor volume (GTV) was contoured on the planning CT scans by the attending radiation oncologist using all available resources, including data from PET/CT fusion scans, EUS images, and diagnostic CT images. The GTV was expanded to the clinical target volume (CTV) by extending the radiation coverage 3 cm superiorly, 3 cm inferiorly, 1 cm laterally, and 3 cm into the gastric mucosa. The planning target volume (PTV) was then generated by using a uniform 0.5-cm expansion beyond the borders of the CTV. Those patients whose celiac nodes were within the PTV were categorized as having had celiac coverage; those patients whose celiac nodes were marginal or outside the radiation treatment field were designated as not having had celiac coverage. Celiac coverage was physician dependent. Those with larger, more distal esophageal tumors with nodal involvement (other than the celiac axis) tended to receive prophylactic nodal coverage. Organs at risk (e.g., heart, lung, and liver) were outlined. All patients were to be treated to a total dose of 50.4 Gy, given in 28 fractions, with concurrent fluorouracil with a taxane or platinum-based chemotherapy. All radiation was to be delivered as intensity-modulated radiation therapy; treatment plans were generated with a Pinnacle planning system (Phillips Medical Systems, Andover, MA).
Documentation of Nodal Failures
Celiac failures were assessed based on serial post-treatment images that included PET scans, CT scans, and EUS images. Failure locations were documented by fusing post-treatment PET scans with the treatment-planning CT scans. Local and distant failures were considered separately; local failure was defined as disease recurrence within the radiation treatment field (GTV, CTV, PTV); distant failures were those occurring outside the field. Celiac failures were categorized separately and were not considered in these local or distant categories, as we wanted to observe if local and distant failure patterns were related to celiac node involvement. All patients in the study, regardless of whether the celiac nodes were covered or not, had no evidence of celiac nodal disease (confirmed by imaging or pathologic evaluation) before treatment.
Toxicity
Gastrointestinal toxicities were scored with the Common Terminology Criteria for Adverse Events v4.03. Data were collected from electronic notes in which symptoms and toxicities had been documented by physicians. Fifty patients were randomly selected from both groups and evaluated for nausea/vomiting and frequency of percutaneous endoscopic gastrostomy (PEG) tube placement during and after treatment.
Statistical Analysis
Continuous variables were summarized by descriptive statistics such as means, standard deviations, medians, and ranges. Categorical variables were tabulated by frequency and percentage. Fisher′s exact test or chi-square test and Wilcoxon′s rank-sum test were used to compare patient characteristics between patients with and without failure. Logistic regression models were fit for multivariate analyses to evaluate associations of celiac failure with clinical factors, initially including clinical factors having a P value <0.15 in univariate analysis. The backward selection procedure was used for model selection. (Only univariate analysis was used for celiac node failures in patients without radiation coverage owing to small numbers of patients.) The Kaplan-Meier product-limit method and log-rank test were applied to estimate survival probabilities and to compare survival, respectively. SAS 9.2 (SAS Institute Inc., Cary, NC 27513) and S-Plus 8.0 (TIBCO software Inc., Palo Alto, CA 94304) were used for statistical analyses.
RESULTS
Patient Characteristics
Patient characteristics are listed in Table 1. The median age of the 131 patients was 67 years (range, 30–83 years); most (113, or 86%) were men. Baseline body mass index (BMI) was 27.1 (range, 15.5–36.9), that majority of the patients (79%) had T3 tumors; 11.5% had T2, 7% had T4, and 2% had T1 tumors. Similarly, most patients (65%) had N1 disease, and 88% had M0 disease. Tumors were located mostly in the lower thoracic esophagus (76%) and most (77%) were adenocarcinomas. Slightly more than half of all tumors (53%) were poorly differentiated, and the median tumor length was 5 cm (range, 1–11 cm). Nearly all patients (99%) had received concurrent chemotherapy, and 41% had received induction chemotherapy as well. The median radiation dose was 50.4 Gy (range, 39.0–66.0 Gy), with 83.2% receiving 50.4 Gy. The planned radiation fields covered the celiac nodes in 71 patients (54%) and did not in 60 patients (46%). Patients with celiac coverage tended to have greater BMI. They also had higher staged tumors more commonly located in the lower thoracic esophagus, with mostly poorly differentiated, adenocarcinoma type tumors (Table 1).
Table 1.
Patient Characteristics
| Characteristic | Celiac Coverage | No Celiac Coverage | All Patients (%) |
|---|---|---|---|
| Sex | |||
| Female | 7 (39%) | 11 (61%) | 18 (14%) |
| Male | 64 (57%) | 49 (43%) | 113 (86%) |
| Age, years median (range) | 67 (30, 81) | 68 (44, 83) | 67 (30, 83) |
| Baseline Body Mass Index median (range) | 27.1 (16.2, 63.9) | 24.9 (15.5, 36.4) | 27.1 (15.5, 36.9) |
| Tumor Status | |||
| T1 | 3 (100%) | 0 (0%) | 3 (2%) |
| T2 | 8 (53%) | 7 (47%) | 15 (12%) |
| T3 | 56 (54%) | 48 (46%) | 104 (79%) |
| T4 | 4 (44%) | 5 (56%) | 9 (7%) |
| Lymph Node Status | |||
| N0 | 24 (53%) | 21 (47%) | 45 (34%) |
| N1 | 46 (54%) | 39 (46%) | 85 (65%) |
| NX | 1 (100%) | 0 (0%) | 1 (1%) |
| Metastatic Status | |||
| M0 | 64 (56%) | 51 (44%) | 115 (8 8%) |
| M1a | 2 (33%) | 4 (67%) | 6 (5%) |
| M1b | 5 (50%) | 5 (50%) | 10 (8%) |
| Primary Tumor Site | |||
| Cervical | 0 (0%) | 2 (100%) | 2 (1.5%) |
| Upper Thoracic | 0 (0%) | 8 (100%) | 8 (6%) |
| Mid-Thoracic | 5 (23%) | 17 (77%) | 22 (17%) |
| Lower Thoracic | 66 (67%) | 33 (33%) | 99 (76%) |
| Tumor Histology | |||
| Adenocarcinoma | 67 (66%) | 34 (34%) | 101 (7 7%) |
| Squamous Cell | 4 (14%) | 25 (86%) | 29(22%) |
| Neuroendocrine | 0 (0%) | 1 (100%) | 1 (1%) |
| Tumor Grade | |||
| G1 Well Diff. | 1 (50%) | 1 (50%) | 2 (2%) |
| G2 Moderate Diff. | 28 (48%) | 30 (52%) | 58 (44%) |
| G3 Poorly Diff. | 42 (60%) | 28 (40%) | 70 (53%) |
| GX Undetermined | 0 (0%) | 1 (100%) | 1 (1%) |
| Tumor Length, cm median (range) | 5 (1, 10) | 6 (1, 11) | 5 (1, 11) |
| Induction Chemotherapy | |||
| No | 42 (54.5%) | 35 (45.5%) | 77 (59%) |
| Yes | 29 (54%) | 25 (46%) | 54 (41%) |
| Concurrent Chemotherapy | |||
| No | 0 (0%) | 1 (100%) | 1 (1%) |
| Yes | 60 (46%) | 70 (54%) | 130 (99%) |
| Radiation Dose, Gy median (range) | 50.4 (45.0, 59.4) | 50.4 (39.0, 66.0) | 50.4 (39.0, 66.0) |
| Pretreatment SUV median (range) | 10.8 (0, 34) | 12.4 (0, 51) | 11.7 (0, 51) |
| Posttreatment SUV median (range) | 4.3 (0, 19.9) | 4.8 (0, 13.0) | 4.4 (0, 19.9) |
| Celiac Nodes within RT Field | |||
| No | 0 (0%) | 60 (10 0%) | 60 (46%) |
| Yes | 71 (100%) | 0 (0%) | 71 (54%) |
| Celiac Lymph Node Metastasis | |||
| No | 54 (45%) | 66 (55%) | 120 (9 2%) |
| Yes | 6 (54.5%) | 5 (45.5%) | 11 (8%) |
Diff., differentiated; SUV, standardized uptake value; RT, radiation therapy
Predictors of Celiac Failure for All Patients
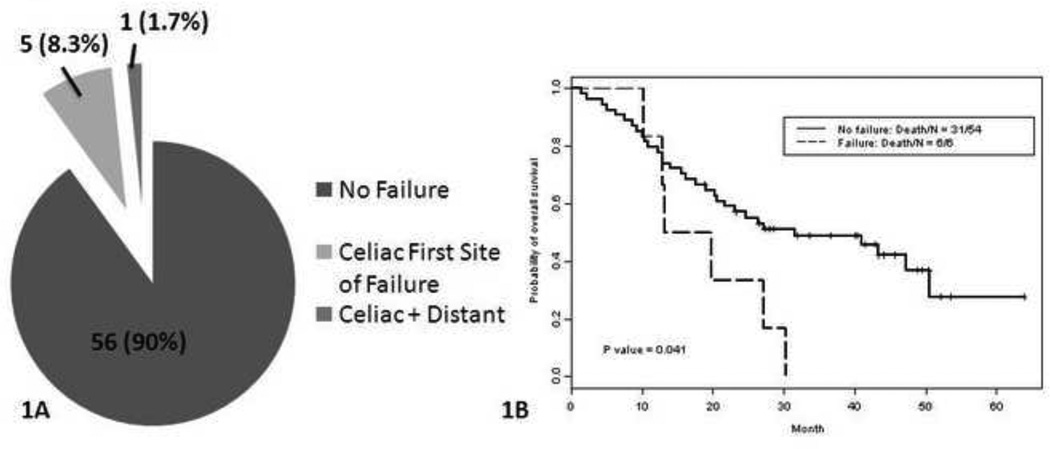
Overall, 11 patients (8%) experienced celiac node metastasis after treatment: 6 of the 60 patients without celiac coverage (10%) and 5 of the 71 patients with prophylactic celiac node coverage (7%, p=0.75) (Fig. 1A). Five of the 11 patients presented initially with suggestive isolated celiac failure.
Figure 1.
A, Pie chart illustrates rates of celiac failures and non-failures according to coverage of the celiac nodes in the radiation treatment fields. B, Kaplan-Meier OS curves for patients who did not have celiac nodes covered in the treatment field according to whether or not celiac failure occurred.
When looking at pre- and post-treatment predictors to identify those that may benefit from elective nodal irradiation, under univariate analysis of the entire group (i.e., those with or without prophylactic celiac coverage), the following factors were associated with failure in the celiac nodes: a baseline BMI ≥30, post-treatment standardized uptake value on PET (SUV) >8.7 and a pretreatment-to-posttreatment change in SUV for the primary tumor ≤52% (p=0.0160); and having failure within the GTV, CTV, PTV, or distantly. Having N1 disease did not predict celiac failure (Table 2). In multivariate analysis, the only factors that remained significant with regard to risk of celiac failure were an SUV change (for the primary tumor) >52% (OR 0.198, 95% CI 0.045–0.875, p=0.0327) and failure within the CTV (OR 10.72, 95% CI 2.599–44.25, p=0.001) (Table 2). Celiac nodal radiation was not associated with celiac nodal failure (p=0.75).
Table 2.
Univariate and multivariate analysis of factors associated with celiac node failures in all 131 patients
| Celiac Node Failure |
P Value (univariate) |
P Value (multivariate) |
Odds Ratio (95% CI) |
||
|---|---|---|---|---|---|
| No | Yes | ||||
| Celiac Node within RT Field | |||||
| No | 54 (90.0%) | 6 (10.0%) | 0.75 | — | — |
| Yes | 66 (93.0%) | 5 (7.0%) | |||
| Sex | |||||
| Female | 18 (100%) | 0 (0%) | 0.3598 | — | — |
| Male | 102 (90.3%) | 11 (9.7%) | |||
| Baseline BMI | |||||
| ≥30 | 25 (83.3%) | 5 (16.7%) | 0.0350 | — | — |
| <30 | 95 (94.1%) | 6 (5.9%) | |||
| Tumor Status | |||||
| T1/T2 | 17 (94.4%) | 1 (5.6%) | 1.000 | — | — |
| T3/T4 | 103 (91.2%) | 10 (8.8%) | |||
| Lymph Node Status | |||||
| N0 | 43 (95.6%) | 2 (4.4%) | 0.3845 | — | — |
| N1 | 76 (89.4%) | 9 (10.6%) | |||
| NX | 1 (100%) | 0 (0%) | |||
| Metastatic Status | |||||
| M0 | 104 (90.4%) | 11 (9.6%) | 0.3581 | — | — |
| M1 | 16 (100%) | 0 (0%) | |||
| Primary Tumor Site | |||||
| Cervical | 2 (100%) | 0 (0%) | 0.874 | — | — |
| Upper Thoracic | 8 (100%) | 0 (0%) | |||
| Mid-Thoracic | 21 (95.5%) | 1 (4.5%) | |||
| Lower Thoracic | 89 (89.9%) | 10 (10.1%) | |||
| Tumor Histology | |||||
| Adenocarcinoma | 92 (91.1%) | 9 (8.9%) | 1.000 | — | — |
| Squamous & Other | 28 (93.3%) | 2 (6.7%) | |||
| Tumor Grade | |||||
| G1/G2 | 54 (90.0%) | 6 (10.0%) | 0.7535 | — | — |
| G3/GX | 66 (93.0%) | 5 (7.0%) | |||
| Tumor Length | |||||
| >5 cm | 63 (92.6%) | 5 (7.4%) | 0.7552 | — | — |
| ≤5 cm | 55 (90.2%) | 6 (9.8%) | |||
| Induction Chemotherapy | |||||
| No | 72 (93.5%) | 5 (6.5%) | 0.3594 | — | — |
| Yes | 48 (88.9%) | 6 (11.1%) | |||
| Pretreatment SUV | |||||
| ≤6 (median) | 22 (88.0%) | 3 (12.0%) | 0.6937 | — | — |
| >6 (median) | 90 (91.8%) | 8 (8.2%) | |||
| Posttreatment SUV | |||||
| >8.7 (median) | 10 (66.7%) | 5 (33.3%) | 0.0038 | — | — |
| ≤8.7 (median) | 104 (94.5%) | 6 (5.5%) | |||
| SUV Change | |||||
| >52% | 69 (95.8%) | 3 (4.2%) | 0.0160 | 0.0327 | 0.198 |
| ≤52% | 32 (80%) | 8 (20%) | (0.045–0.875) | ||
| GTV Failure | |||||
| No | 73 (96.1%) | 3 (3.9%) | 0.0518 | — | — |
| Yes | 47 (85.5%) | 8 (14.5%) | |||
| CTV Failure | |||||
| No | 106 (96.4%) | 4 (3.6%) | 0.0002 | 0.001 | 10.72 |
| Yes | 14 (66.7%) | 7 (33.3%) | (2.599–44.25) | ||
| PTV Failure | |||||
| No | 112 (93.3%) | 8 (6.7%) | 0.0504 | — | — |
| Yes | 8 (72.7%) | 3 (27.3%) | |||
| Distant Failure | |||||
| No | 61 (98.4%) | 1 (1.6%) | 0.0097 | — | — |
| Yes | 59 (85.5%) | 10 (14.5%) | |||
Abbreviations: RT, radiation therapy; BMI, body mass index; iSUV, initial standardized uptake value; GTV, gross tumor volume; CTV, clinical target volume; PTV: planning target volume
Predictors of Celiac Failure for Patients Without Nodal Coverage
For the 6 patients who experienced celiac failure who had not had this area covered in the radiation field, univariate analysis of potential risk factors for failure were a posttreatment SUV >8.7 (p=0.0126) and an SUV change ≤52% (p=0.0089) (Table 3). Five of these 6 individuals experienced first failure at the celiac nodes before experiencing further abdominal metastasis, most often in the liver (Table 4). The only patient who experienced a first failure at a non-celiac node survived longer than the others (34.1 months vs. median 16.4 months) (Table 4).
Table 3.
Univariate analysis of factors associated with celiac node failures in 60 patients without celiac node coverage
| Celiac Node Failure |
P Value (univariate) |
||
|---|---|---|---|
| No | Yes | ||
| Baseline BMI | |||
| ≥30 | 7 (77.8%) | 2 (22.2%) | 0.218 |
| <30 | 47 (92.2%) | 4 (7.8%) | |
| Pre-treatment iSUV | |||
| ≤6 (median) | 6 (87.5%) | 1 (14.3%) | 0.5621 |
| >6 (median) | 45 (90.0%) | 5 (10.0%) | |
| Post-treatment iSUV | |||
| ≤8.7 (median) | 47 (94.0%) | 3 (6.0%) | 0.0126 |
| >8.7 (median) | 3 (50.0%) | 3 (50.0%) | |
| (continuous, cm) | 4.5 (0,10.4) | 8.8 (2.5–13) | 0.0222 |
| iSUV Change | |||
| ≤52% | 11 (68.8%) | 5 (31.3%) | 0.0089 |
| >52% | 34 (97.1%) | 1 (2.9%) | |
| (continuous, %) | 73.1 (−39, 100) | 13.1 (−31.3, 75.2) | 0.0112 |
| GTV Failure | |||
| No | 34 (97.1%) | 1 (2.9%) | 0.0731 |
| Yes | 20 (80%) | 5 (20%) | |
| CTV Failure | |||
| No | 47 (92.2%) | 4 (7.8%) | 0.218 |
| Yes | 7 (77.8%) | 2 (22.2%) | |
| PTV Failure | |||
| No | 51 (92.7%) | 4 (7.3%) | 0.0735 |
| Yes | 3 (60%) | 2 (40%) | |
| Distant Failure | |||
| No | 31 (96.9%) | 1 (3.1%) | 0.0884 |
| Yes | 23 (82.1%) | 5( 17.9%) | |
Abbreviations: BMI, body mass index; iSUV, initial standardized uptake value; GTV, gross tumor volume; CTV, clinical target volume; PTV, planned tumor volume
Table 4.
Patterns of disease progression
| Patient No. |
First Site of Disease Recurrence |
Next Site of Disease Metastasis |
Overall Survival Time, months |
|---|---|---|---|
| 1 | Celiac node | Abdominal nodes | 22.7 |
| 2 | Celiac node & esophagus | No follow-up | 14.6 |
| 3 | Celiac node & lung | Liver | 29.1 |
| 4 | Celiac node & esophagus | Liver and peritoneum | 16.4 |
| 5 | Celiac node & esophagus | Liver, peritoneum, pancreas | 15.3 |
| 6 | Retroperitoneal nodes | Celiac nodes, then liver | 34.1 |
Overall Survival
At a median follow-up time of 52.6 months (95% CI 46.1–56.7 months), the median OS time for the entire cohort was 23.1 months (95% CI 20.2–37.5 months). For all patients, the median OS time was significantly shorter for those who experienced celiac node failure (13.3 months, 95% CI 12.83–not reached) than for those who did not experience celiac node failure (24.6 months, 95% CI 20.6–42.6 months, p=0.022). Two-year OS survival rates were also worse for those who had celiac failure (27.3%, 95% CI 10.4%– 71.6%) than for those who did not (51.6%, 95% CI 43.3%–61.6%, p=0.022). No differences in median or 2-year OS were seen for those with or without celiac coverage (p=0.385).
For the 6 patients without celiac coverage and later had failure there, the median OS time and 2-year OS rate were also worse (16.5 months, 95% CI 12.83 months–not reached; 33.4%, 95% CI 10.8%–100%) than for those who were not covered and did not experience celiac failure (31.5 months, 95% CI 20.6 months–not reached; 57.1%, 95% CI 45.3%–72.1%. p=0.041 for 2-year OS) (Fig. 1B).
Toxicity among Patients with or without Celiac Node Coverage
No difference was found between patients who had or had not had prophylactic celiac coverage in terms of rates of acute or late nausea/vomiting, dysphagia, odynophagia, esophagitis, abdominal pain, constipation, diarrhea, and need for PEG tube placement (Table 5).
Table 5.
Toxicity Profile in Patients Covered vs. Not Covered
| Grade, n (%) | Fisher's Exact p-value | ||||
|---|---|---|---|---|---|
| Toxicity | Any | 1–2 | 3 | None | |
| Nausea/Vomiting | |||||
| With Coverage | 26 (52) | 24 (48) | 2 (4) | 24 (48) | 0.625 |
| Without Coverage | 22 (44) | 19 (38) | 3 (6) | 28 (56) | |
| Dysphagia | |||||
| With Coverage | 15 (30) | 14 (28) | 1 (2) | 35 (70) | 1.000 |
| Without Coverage | 15 (30) | 13 (26) | 2 (4) | 35 (70) | |
| Odynophagia | |||||
| With Coverage | 5 (10) | 5 (10) | 0 (0) | 45 (90) | 0.079 |
| Without Coverage | 13 (26) | 11 (22) | 2 (4) | 37 (74) | |
| Esophagitis | |||||
| With Coverage | 19 (38) | 17 (34) | 2 (4) | 31 (62) | 0.444 |
| Without Coverage | 20 (40) | 20 (40) | 0 (0) | 30 (60) | |
| Abdominal Pain | |||||
| With Coverage | 2 (4) | 2 (4) | 0 (0) | 48 (96) | 1.000 |
| Without Coverage | 2 (4) | 2 (4) | 0 (0) | 48 (96) | |
| Constipation | |||||
| With Coverage | 3 (6) | 3 (6) | 0 (0) | 47 (94) | 0.121 |
| Without Coverage | 9 (18) | 9 (18) | 0 (0) | 41 (82) | |
| Diarrhea | |||||
| With Coverage | 5 (10) | 5 (10) | 0 (0) | 45 (90) | 0.436 |
| Without Coverage | 3 (6) | 2 (4) | 1 (2) | 47 (94) | |
| PEG Tube Placement | |||||
| With Coverage | 5 (10) | - | - | 45 (90) | - |
| Without Coverage | 9 (18) | - | - | 41 (82) | |
Abbreviation: PEG, percutaneous endoscopic gastrostomy.
DISCUSSION
To our knowledge, this is the first report of rates of failure in the celiac nodes after chemoradiation therapy for esophageal cancer according to whether those nodes had been covered or not in the original radiation treatment plan, in combination with assessment of potential predictive factors for such failure. Our finding that 8.4% of the study cohort (11/131) experienced celiac node failure (7% of whom had had prophylactic coverage and 10% of whom had not) is similar to other studies indicating celiac failure rates of about 10% after surgery (15). Although the celiac failure rates were no different for those who had had coverage of that region and those who had not, this does not suggest that celiac nodes should not be covered within the radiation field. Rather, those patients who received prophylactic celiac nodal coverage appear to have had more advanced disease, which may have prompted the treating physician to cover areas they suspected of harboring occult disease.
In terms of predictors of celiac failure, we found that regardless of celiac node coverage, patients with a posttreatment SUV >8.7 and a pretreatment-to-posttreatment change in SUV for the primary tumor of ≤52% were far more likely to develop celiac failure. These findings support the idea that disease that persists after definitive chemoradiation may be more likely to metastasize. We did not find that pretreatment T or N status predicted celiac failure, although others have (16). We also found tumor location (proximal vs. distal esophagus) to not influence celiac failure, although others have found celiac failure rates as high as 17.4% among patients with lower esophageal tumors (15). For patients without celiac coverage, predictors of celiac failure included pretreatment-to-posttreatment change in SUV, again suggesting that more biologically aggressive disease may be more likely to reappear as distant failure.
Among the 6 patients without celiac coverage and later experienced celiac failure, the celiac nodes were the first site of any metastasis in 5 (83%). Disease quickly spread to other abdominal structures, most often the liver, in these patients, suggesting that the celiac axis acts as a “gateway” to the abdomen, possibly leading to poorer OS among those with celiac node failure. Another possibility could be that celiac nodal involvement is a “marker” for widespread microscopic disease that may later become evident. These findings support those of others that celiac node involvement predicts worse outcomes and poorer OS (17, 18). These findings also support coverage of celiac nodes within the radiation fields for esophageal cancer, especially among patients at high risk of distant metastasis. The relatively mild gastrointestinal toxicity in this study, and the lack of difference between the covered and not-covered groups, suggests that covering the celiac nodes would be prudent (4, 5). In this case, slightly worse dose-volume histograms for organs such as the stomach in those with celiac coverage did not translate to worse clinical outcomes.
Because we evaluated patients who underwent definitive chemoradiation without surgery, the frequency of failure at the primary tumor site was fairly high. Three of the 6 patients with celiac node failure who had not had coverage of this site also had simultaneous residual disease at the primary site. If we were to expand our analysis to patients treated with surgery, we would expect the rate of primary disease recurrence to be much lower and thus distant metastasis—including metastasis to the celiac nodes—would be the more common pattern of failure. Thus controlling celiac node disease would remain an important aspect of improving outcomes.
Due to improvements in imaging we have greatly improved the accuracy of disease staging and thus often allowing for smaller/better tailored treatment fields. For esophageal tumors, the use of EUS-FNA in addition to PET has greatly improved the ability to identify nodes suspected of harboring disease, including those in the celiac axis (19), with accuracy rates as high as 98% (95% CI 90%–100%) (18). With these improvements in imaging, radiation coverage of celiac nodes can potentially be reserved for those patients with suspected or proven involvement of lymph nodes, for those with more distal esophageal tumors, and perhaps for those with N1 or greater initial staging, as such patients may be more likely to experience distant failure.
Among the limitations of this study were its retrospective nature, with the associated biases, and the fact that techniques for disease staging improved considerably over the 7-year time span of the study. Another potential weakness is the heterogeneity of the patient population in terms of age (range, 30–83 years) and the presence of comorbid conditions, which precluded at least some of the patients in this study from undergoing surgery. Moreover, the small number of patients, particularly those who experienced celiac node relapse without having had radiation field coverage, precluded multivariate analyses of this subgroup. Some of the strengths of our study include the relative consistency of the treatment dose and technique used over the period of study and the relatively long follow-up time. We were able to report rates and outcomes of celiac failure among those with or without celiac coverage, but the single-institution nature of this study may limit the generalizability of our findings to patients treated at other centers.
In summary, approximately 1 in 10 patients without prophylactic celiac nodal coverage will fail in this area. Predictors of celiac failure included having a small change in tumor SUV after treatment relative to before. OS was poorer in those with celiac failure than those without it. These findings, in combination with the low morbidity associated with celiac coverage and current lack of salvage treatments, indicate that prophylactic celiac node coverage may continue to be used, particularly for the treatment of distal esophageal tumors, assuming all critical dose volume constraints can be achieved.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA: Cancer Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Minsky BD, Pajak TF, Ginsberg RJ. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–1174. doi: 10.1200/JCO.2002.20.5.1167. [DOI] [PubMed] [Google Scholar]
- 3.Kelsen DP, Winter KA, Gunderson LL, et al. Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol. 2007;25(24):3719–3725. doi: 10.1200/JCO.2006.10.4760. [DOI] [PubMed] [Google Scholar]
- 4.Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335(7):462–467. doi: 10.1056/NEJM199608153350702. [DOI] [PubMed] [Google Scholar]
- 5.Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26(7):1086–1092. doi: 10.1200/JCO.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaast A, van Hagen P, Hulshof M, et al. Effect of preoperative concurrent chemoradiotherapy on survival of patients with resectable esophageal or esophagogastric junction cancer: Results from a multicenter randomized phase III study. J Clin Oncol. 2010;28(15s) [abstract] [Google Scholar]
- 7.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. Journal of the National Cancer Institute. 2008;100(16):1184–1187. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunderson LL, Sosin H. Adenocarcinoma of the stomach: areas of failure in a re-operation series (second or symptomatic look) clinicopathologic correlation and implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 1982;8(1):1–11. doi: 10.1016/0360-3016(82)90377-7. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura T, Hatooka S, Kodaira T, et al. Determination of the irradiation field for clinical T1-T3N0M0 thoracic/abdominal esophageal cancer based on the postoperative pathological results. Jpn J Clin Oncol. 2009;39(2):86–91. doi: 10.1093/jjco/hyn131. [DOI] [PubMed] [Google Scholar]
- 10.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 11.Catalano MF, Alcocer E, Chak A, et al. Evaluation of metastatic celiac axis lymph nodes in patients with esophageal carcinoma: accuracy of EUS. Gastrointest Endosc. 1999;50(3):352–356. doi: 10.1053/ge.1999.v50.98154. [DOI] [PubMed] [Google Scholar]
- 12.Parmar KS, Zwischenberger JB, Reeves AL, et al. Clinical impact of endoscopic ultrasound-guided fine needle aspiration of celiac axis lymph nodes (M1a disease) in esophageal cancer. Ann Thorac Surg. 2002;73(3):916–920. doi: 10.1016/s0003-4975(01)03560-3. discussion 20–1. [DOI] [PubMed] [Google Scholar]
- 13.Giovannini M, Monges G, Seitz JF, et al. Distant lymph node metastases in esophageal cancer: impact of endoscopic ultrasound-guided biopsy. Endoscopy. 1999;31(7):536–540. doi: 10.1055/s-1999-60. [DOI] [PubMed] [Google Scholar]
- 14.Reed CE, Mishra G, Sahai AV, et al. Esophageal cancer staging: improved accuracy by endoscopic ultrasound of celiac lymph nodes. Ann Thorac Surg. 1999;67(2):319–321. doi: 10.1016/s0003-4975(99)00031-4. discussion 22. [DOI] [PubMed] [Google Scholar]
- 15.Seto Y, Fukuda T, Yamada K, et al. Celiac lymph nodes: distant or regional for thoracic esophageal carcinoma? Dis Esophagus. 2008;21(8):704–707. doi: 10.1111/j.1442-2050.2008.00842.x. [DOI] [PubMed] [Google Scholar]
- 16.Eloubeidi MA, Wallace MB, Reed CE, et al. The utility of EUS and EUS-guided fine needle aspiration in detecting celiac lymph node metastasis in patients with esophageal cancer: a single-center experience. Gastrointest Endosc. 2001;54(6):714–719. doi: 10.1067/mge.2001.119873. [DOI] [PubMed] [Google Scholar]
- 17.Hiele M, De Leyn P, Schurmans P, et al. Relation between endoscopic ultrasound findings and outcome of patients with tumors of the esophagus or esophagogastric junction. Gastrointest Endosc. 1997;45(5):381–386. doi: 10.1016/s0016-5107(97)70148-2. [DOI] [PubMed] [Google Scholar]
- 18.Eloubeidi MA, Wallace MB, Hoffman BJ, et al. Predictors of survival for esophageal cancer patients with and without celiac axis lymphadenopathy: impact of staging endosonography. Ann Thorac Surg. 2001;72(1):212–219. doi: 10.1016/s0003-4975(01)02616-9. discussion 9–20. [DOI] [PubMed] [Google Scholar]
- 19.Heidemann J, Schilling MK, Schmassmann A, et al. Accuracy of endoscopic ultrasonography in preoperative staging of esophageal carcinoma. Dig Surg. 2000;17(3):219–224. doi: 10.1159/000018838. [DOI] [PubMed] [Google Scholar]