Abstract
Background
Mastocytosis is a clonal disorder associated with an increased mast cell burden. We have recently demonstrated the ability of human mast cells to express and be activated through multiple serotonin receptors; to synthesize and release serotonin; and that mastocytosis patients may have abnormal serotonin levels. As serotonin has been implicated in the genesis of clinical symptoms found in association with some chronic diseases, we have now determined the whole blood serotonin levels in 29 patients diagnosed with mastocytosis, and correlated these levels with multiple clinical and laboratory parameters.
Materials and methods
Patients with mastocytosis were categorized according to disease variant. Blood serotonin values were determined and correlated with values reported for normal subjects; and clinical and laboratory features of the disease.
Results
Total blood serotonin levels followed a bimodal distribution in line with our earlier report, unlike the normal distribution reported for normal individuals. Serotonin levels did not correlate with platelet numbers, liver function tests or serum tryptase levels. Patients with lower serotonin values had greater rates of fatigue (P = 0·0001), migraine headaches (P = 0·0028), psychiatric symptoms (P = 0·0001), diarrhoea (P = 0·0407), flushing (0·0085), and abdominal and bone pain (P = 0·0001).
Conclusions
Our study suggests that low blood serotonin levels help define a sub-group of patients with mastocytosis that are more likely to present with neurological and gastrointestinal complaints, and suggests that the use of pharmacologic agents that alter blood serotonin levels could be explored in selected patients.
Keywords: 5HT, mast cells, mastocytosis, platelets, serotonin, tryptase
Introduction
Mastocytosis is a clonal systemic disease associated with a pathological increase in tissue mast cells and symptoms typical of mediator release [1,2]. In 2007, we communicated that human mast cells synthesize and release serotonin [3], a bioamine that functions as a neurotransmitter, tissue mediator and vasoactive agent which can either apparently up regulate or down regulate a given biological response [4,5]. In the 2007 letter, we also noted that blood serotonin levels in patients with mastocytosis had an abnormal distribution [3]. As blood serotonin levels have been used to help diagnose and monitor the treatment of carcinoid disease [6,7], evaluate agents used to treat psychiatric disorders [8,9] and Raynaud's phenomenon [10], and follow provocation of irritable bowel syndrome [11], we elected to determine if the abnormal blood serotonin levels in patients with mastocytosis correlated with the clinical and laboratory parameters of the disease and might have some diagnostic and therapeutic value.
As we will show, we first verified blood serotonin levels in patients with mastocytosis [3] are distributed in a bimodal manner. We then found that patients with a low blood serotonin level were more likely to suffer from flushing, headaches, fatigue, pain, psychiatric symptoms, and diarrhoea. These complaints did not parallel tryptase levels, which are thought to reflect mast cell burden.
Materials and methods
Patients
Thirty-eight patients with mastocytosis were evaluated at the National Institute of Allergy and Infectious Diseases (NIAID) after informed consent was obtained, as part of a study of the natural history of mastocytosis approved by the NIAID Institutional Review Board. Seven of these patients who were on cytoreductive treatment (cladribine, imatinib) were excluded from the analysis, as were two patients who were on serotonin modifying agents. Initial evaluation included a history and medical records review, a physical examination and standard laboratory tests. Patients were then classified into mastocytosis variants according to the World Health Organization (WHO) consensus classification [2,12]. In the group enrolled, 24 had indolent systemic mastocytosis (ISM), three fell within the smouldering indolent systemic mastocytosis variant (SISM), and two had aggressive systemic mastocytosis (ASM).
Determination of blood serotonin and serum tryptase levels
Blood samples were collected prior to food and medication intake. Total blood serotonin levels (ng mL−1) were determined by liquid chromatography with tandem mass spectrometry at Mayo Medical Laboratories (Rochester, MN, USA). The threshold for serotonin detection was 50 ng mL−1. Levels reported as below the level of detection (BLD) were assigned the intermediate value of 25 ng mL−1 when required by analysis. A normal distribution of serotonin blood levels was obtained from a published report of 470 normal volunteers [13]. Serum tryptase levels (ng dL−1) were determined with a commercial Fluoroenzyme Immunoassay (Pharmacia Immuno CAP) (Mayo Medical Laboratories, Rochester, MN, USA).
Symptoms
Symptoms were recorded in the chart as part of the initial screening visit by independent staff physicians and reviewed retrospectively. Pain was defined as persistent complaint of pain longer than six months duration without an identifiable cause; and included complaints of musculoskeletal and/or abdominal pain. Psychiatric symptoms included depression, anxiety, emotional instability, and memory loss. Migraine headache was diagnosed by physicians and/or self-reported by the patients as headache with migraine characteristics (aura, photophobia, positive response to migraine-specific medications). Diarrhoea was self-reported as loose stools more than three per day, intermittent or persistent, with or without weight loss not associated with food allergy or anaphylaxis, and without identifiable infectious cause. Osteoporosis/osteopenia was documented by dual energy X-ray absorptiometry (DEXA) scan scores and/or a history of bone fractures. Fatigue was self-reported as present and lasting longer than six months. Pruritus and flushing were reported by the patient and were not associated with an allergic reaction. Organomegaly was confirmed by computer tomography as a part of the initial visit. Skin involvement with mastocytosis was documented by physical examination and biopsy. Anaphylaxis was reported by the referring physician and was defined as respiratory/cardiovascular collapse that required epinephrine administration and/or resuscitation.
Statistical analysis
For the purpose of graphical presentation, the patients were ranked by their blood serotonin values and divided into even terciles. The first tercile was designated as Group 1, (nine patients, serotonin values < 50 ng mL−1); those in the second tercile were designated as within Group 2 (10 patients, serotonin values 68–200 ng mL−1); and those in the third tercile were designated as within Group 3 (10 patients, serotonin values 210–500 ng mL−1). If blood serotonin levels follow a normal distribution with a mean 130·2 and standard deviation of 42·3 [13], tercile one corresponds to the lower 3% of normals, tercile two has normal values, and tercile three corresponds to the upper 3% of normals, indicating the mastocytosis sample had a large fraction of both abnormally low and high values.
A non-parametric approach was used to avoid the statistical problems inherent with values below the limit of detection, and because the serotonin values in this study were not consistent with a normal distribution Spearman rank correlations were computed to assess the association between blood serotonin levels and the presence of each symptom. Spearman correlation and linear regression were used to assess the correlation of serotonin levels to both platelet numbers and tryptase determinations. P-values less than 0·05 were considered statistically significant.
Results
Patients' characteristics
Twenty-nine patients were entered into the analysis (Table 1). Of these individuals, 14 (48%) were women, (mean age 47), and 15 (52%) were men (mean age 49). All patients were positive for the KIT D816V mutation, which was detected in bone marrow. Twenty-four (83%) patients were diagnosed with ISM, three patients (10%) had SISM and two patients (7%) had ASM.
Table 1.
Patients' characteristics
| Characteristics | N (%) | Age, year (range) |
|---|---|---|
| Total | 29 (100%) | 48 (23–77) |
| Female | 14 (48%) | 47 (32–58) |
| Male | 15 (52%) | 49 (23–77) |
| Mutation in KIT | 29 (100%) | |
| Mastocytosis classification: | ||
| Indolent Systemic Mastocytosis (ISM) | 24 (83%) | |
| Indolent Systemic Mastocytosis – Smouldering ISM (SISM) | 3 (10%) | |
| Aggressive Systemic (ASM) | 2 (7%) |
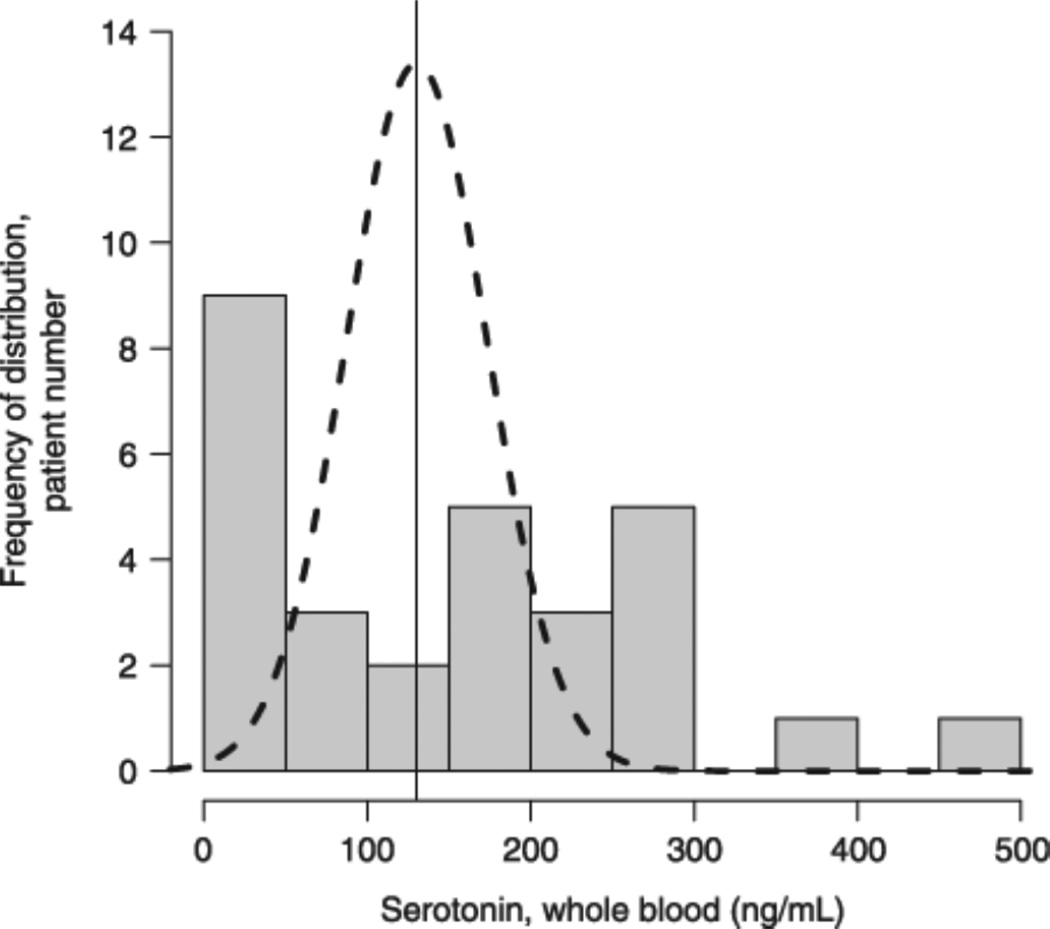
Serotonin blood level in patients with mastocytosis deviates from a normal distribution
We have corresponded that blood serotonin levels obtained from a group of patients with mastocytosis did not follow the normal distribution as reported in normal individuals [13]. In the current study we revisited this question and determined blood serotonin levels from the patients entered into this study. We again observed a bimodal distribution (Fig. 1). To assure that the blood serotonin measurements are reasonably constant over time, we repeated the serotonin determinations in six patients with an interval between determinations of one day to one year. For each patient the first serotonin value represents day 0, with the second value obtained after the time interval stated. Blood serotonin values obtained in each patient remained similar in that in no instance would the repeat value have placed a specific patient in a different tercile (Table 2).
Figure 1.
Serotonin blood level distribution. Total blood serotonin levels were determined in a cohort of patients with mastocytosis and compared with published values obtained from normal volunteers which followed Gaussian distribution: theoretical curve based on mean ± SD (130·2 ± 42·3) [13]; bars represent number of mastocytosis patients with serotonin blood levels per each value bracket shown on the X axis.
Table 2.
Repeated measurements of serotonin blood levels demonstrate reproducibility of the test
| Patient | Serotonin (ng mL−1) | Measurement interval |
|---|---|---|
| 1 | < 50, < 50 | 6 months |
| 2 | 139, 152 | 1 day |
| 2 | 152, 180 | 1 year |
| 3 | 195, 172 | 6 months |
| 4 | < 50, < 50 | 1 year |
| 5 | 269, 231 | 1 year |
| 6 | 312, 275 | 1 year |
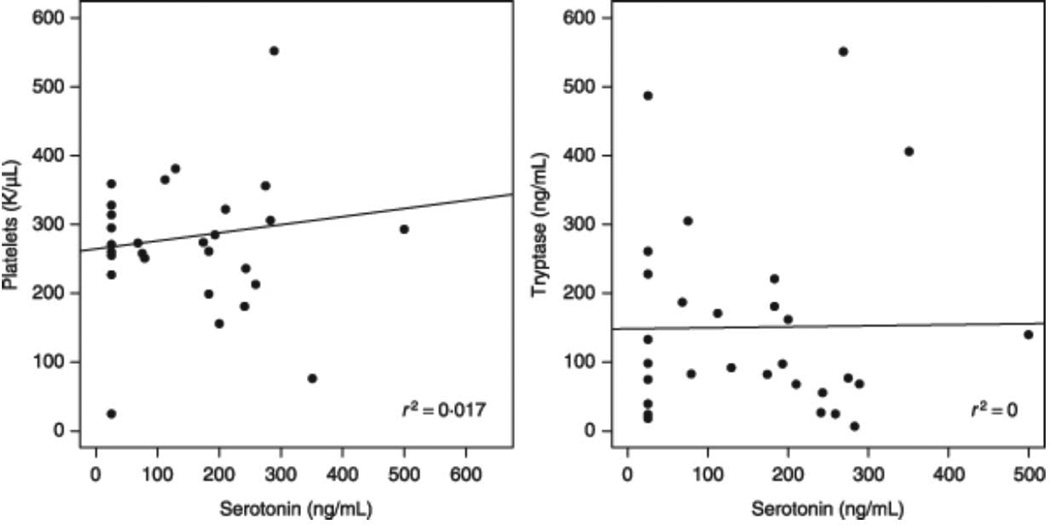
Blood serotonin levels did not correlate with platelet number, liver function tests or serum tryptase levels
We again found that platelet numbers did not correlate with blood serotonin levels (Fig. 2). Because the production and metabolism of serotonin can reportedly be affected by liver function, we examined measures of liver function including albumin, total bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase and creatinine. None of these measures correlated with blood serotonin levels. Tryptase is considered a surrogate marker of the mast cell burden in patients with mastocytosis. Thus, we next analysed the relationship between serum tryptase and blood serotonin levels, and found no statistical correlation (Fig. 2). The analysis of all laboratory parameters was performed on a continuous sample and within each group with similar results. There were insufficient patients within the variants of mastocytosis to include severity of the disease as a variable.
Figure 2.
Correlation of serotonin to tryptase and platelets. Serotonin values were plotted against platelets and tryptase, the line represents the linear regression. Correlations are not statistically significant.
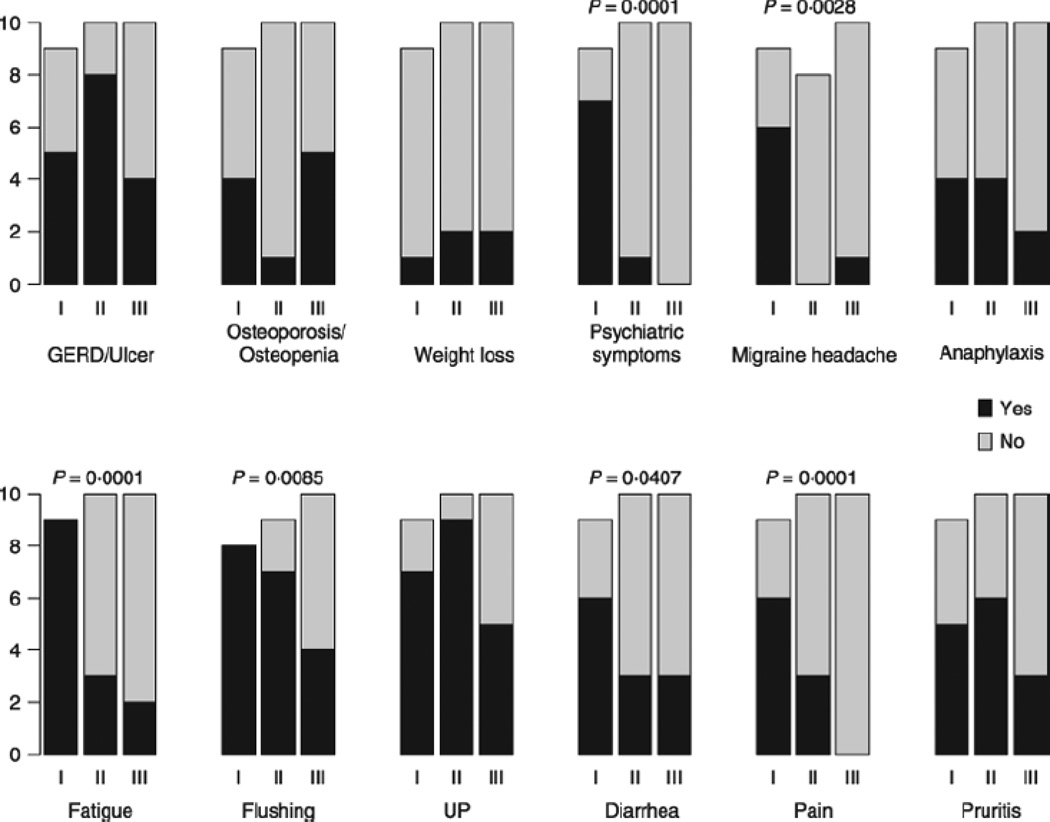
Association of lower serotonin values with clinical symptoms
Associations between serotonin level and clinical symptoms are represented graphically in Fig. 3 by serotonin tercile; continuous serotonin values were analysed by Spearman rank correlation and were statistically significant for the following clinical categories: psychiatric symptoms (P = 0·0001), migraine headache (P = 0·0028), pain (P = 0·0001), fatigue (P = 0·0001), flushing (P = 0·0085), and diarrhoea (P = 0·0407); all significant correlations were negative, indicating that lower values of serotonin were associated with higher rates of symptoms. Patients with pain complained of abdominal and/or musculoskeletal discomfort. Gastroesophageal reflux disease (GERD), weight loss, osteoporosis, anaphylaxis, pruritus, and urticaria pigmentosa (UP) were not associated with serotonin blood levels. We found 100% (9/9) of the Group I patients had a combination of at least six of these 12 symptoms, compared to only 20% (2/10) of Group II and 10% (1/10) of Group III.
Figure 3.
Distribution and analysis of symptoms within groups. Groups with low (I), normal (II) and high (III) levels of serotonin were compared based on their clinical symptoms. Bars represent the number of patients where the grey portion is assigned to patients without symptoms, and the black portion is assigned to patients with symptoms. The association between continuous serotonin values and presence of symptoms is evaluated with Spearman rank correlation, P-values of statistically significant associations (P < 0·05) are shown above bars.
Discussion
Serotonin has been studied extensively as a neuromediator involved in the pathogenesis of depression, anxiety, migraine, chronic pain and irritable bowel syndrome [8–19]. Blood serotonin levels have been used to diagnose and monitor treatment of carcinoid disease and response in psychiatric disorders to specific pharmacologic agents [6–8]. Reduced levels of serotonin reported in patients with migraine attacks have been attributed to a dysfunction in the enzymes involved in serotonin biosynthesis, a dysfunction in serotonin release, or abnormal uptake by platelets and lymphocytes [20].
In this study of patients with mastocytosis, we found that distribution of the serotonin levels was abnormal. Most patients had either low or high blood serotonin levels. Moreover, analysis of these subjects demonstrated that symptoms tended to occur in patients whose serotonin blood level was low. However, not all findings correlated with low blood serotonin levels. UP, GERD, pruritus, osteoporosis, and anaphylaxis did not correlate with blood serotonin levels. It is also unlikely, given the clear abnormal distribution of serotonin levels in this study of patients with mastocytosis, that this would normalize if all mastocytosis patients in a given population were analysed. However, if a population of patients with mastocytosis and relatively few symptoms were analysed, then it might be expected that most serotonin levels would fall within the mid and upper terciles (Fig. 3) and the abnormal distribution as evidenced in this study (Fig. 1) would not necessarily be observed.
We found no explanation for the abnormal blood serotonin levels in patients with mastocytosis. Blood serotonin measurements did not correlate with measures of liver function. Thus, it is unlikely that abnormal serotonin levels were due to mastocytosis–associated liver damage. Patients with low serotonin levels had a higher incidence of diarrhoea, but had normal protein levels and no other signs of malabsorption. This suggests that diarrhoea is more likely to be secondary to any serotonin abnormality, in agreement with studies examining mast cells and serotonin in anaphylaxis-induced diarrhoea [21]. There is evidence of some role for serotonin in the pathogenesis of diarrhoea-predominant irritable bowel syndrome (IBS) as it is relieved with serotonergic medications [22]. This observation may be relevant to mastocytosis. There was also a lack of correlation of platelet number to blood levels of serotonin and to mast cell tryptase levels [23,24,] in spite of our report that human mast cells are capable of serotonin synthesis and release [3].
Recently we demonstrated that mast cells are capable of a functional response to serotonin through multiple serotonin receptors [25]. Others have reported the high expression in mast cells of mastocytosis patients of a monoamine transporter that has high affinity for serotonin (VMAT) [26]. As mast cells in mastocytosis often carry an activating mutation in KIT that can affect activation and releasability [27,28], it is possible that patients with mastocytosis could have abnormalities in mast cells relating to serotonin storage and release, or degradation of serotonin by mast cell products and, depending on these circumstances, have abnormal blood levels of serotonin which might in turn contribute to clinical symptoms such as migraine, chronic fatigue and diarrhoea. It could also be that low serotonin production predisposes for a more severe form of mastocytosis. An interesting control group would be patients with symptomatic allergies. If serotonin blood levels were also low in a subset of allergic patients, this would point to a link between mast cell activation and decrease in serotonin levels. It would also be of interest to determine if mast cell ablative therapy would lead to a rise in serotonin blood levels.
Here for the first time we present the evidence that low serotonin levels are associated with a specific symptom complex. These observations suggest further work is needed, including validation with a prospective study enrolling more patients, to understand the basis of these abnormal levels, with the hope that these observations may lead the way to specific therapy directed to alleviating some of the symptoms and consequences of mastocytosis.
Conclusion
Our study suggests that low blood serotonin levels help define a sub-group of patients with mastocytosis that are more likely to present with neurological and gastrointestinal complaints; and suggests that the use of pharmacologic agents that alter blood serotonin levels could be explored in selected patients.
Acknowledgements
This study was funded by the Division of Intramural Research and the Division of Clinical Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
References
- 1.Akin C, Metcalfe DD. Systemic mastocytosis. Annu Rev Med. 2004;55:419–432. doi: 10.1146/annurev.med.55.091902.103822. Links. [DOI] [PubMed] [Google Scholar]
- 2.Valent P, Akin C, Escribano L, Födinger M, Hartmann K, Brockow K, et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest. 2007;37:435–453. doi: 10.1111/j.1365-2362.2007.01807.x. [DOI] [PubMed] [Google Scholar]
- 3.Kushnir-Sukhov NM, Brown JM, Wu Y, Kirshenbaum A, Metcalfe DD. Human mast cells are capable of serotonin synthesis and release. J Allergy Clin Immunol. 2007;119:498–499. doi: 10.1016/j.jaci.2006.09.003. (Letter) [DOI] [PubMed] [Google Scholar]
- 4.Barnes PJ. Histamine and serotonin. Pulm Pharmacol Ther. 2001;14:329–339. doi: 10.1006/pupt.2000.0318. [DOI] [PubMed] [Google Scholar]
- 5.Menard G, Turmel V, Bissonnette EY. Serotonin modulates the cytokine network in the lung: involvement of prostaglandin E2. Clin Exp Immunol. 2007;150:340–348. doi: 10.1111/j.1365-2249.2007.03492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlin RS, Degg TJ, Allen KR, Bax ND, Barth JH. Evaluation of whole blood serotonin and plasma and urine 5-hydroxyindole acetic acid in diagnosis of carcinoid disease. Ann Clin Biochem. 2002;39:577–582. doi: 10.1177/000456320203900605. [DOI] [PubMed] [Google Scholar]
- 7.Allen KR, Degg TJ, Anthoney DA, Fitzroy-Smith D. Monitoring the treatment of carcinoid disease using blood serotonin and plasma 5-hydroxyindoleacetic acid: three case examples. Ann Clin Biochem. 2007;44:300–307. doi: 10.1258/000456307780480936. [DOI] [PubMed] [Google Scholar]
- 8.Chalon SA, Granier LA, Vandenhende FR, Bieck PR, Bymaster FP, Joliat MJ, et al. Duloxetine increases serotonin and norepinephrine availability in healthy subjects: a double-blind, controlled study. Neuropsychopharmacology. 2003;28:1685–1693. doi: 10.1038/sj.npp.1300209. [DOI] [PubMed] [Google Scholar]
- 9.Humble M, Bejerot S, Bergqvist PB, Bengtsson F. Reactivity of serotonin in whole blood: relationship with drug response in obsessive-compulsive disorder. Biol Psychiatry. 2001;49:360–368. doi: 10.1016/s0006-3223(00)00956-2. [DOI] [PubMed] [Google Scholar]
- 10.Arnekio-Nobin B, Elmer O, Akesson A. Effect of long-term ketanserin treatment on 5-HT levels, platelet aggregation and peripheral circulation in patients with Raynaud's phenomenon. A double-blind, placebo-controlled cross-over study. Int Angiol. 1988;7:19–25. [PubMed] [Google Scholar]
- 11.Zuo XL, Li YQ, Yang XZ, Guo M, Guo YT, Lu XF, et al. Plasma and gastric mucosal 5-hydroxytryptamine concentrations following cold water intake in patients with diarrhoea-predominant irritable bowel syndrome. J Gastroenterol Hepatol. 2006;22:2330–2337. doi: 10.1111/j.1440-1746.2006.04772.x. [DOI] [PubMed] [Google Scholar]
- 12.Valent P, Horny H-P, Li CY, Longley JB, Metcalfe DD, Parwaresch RM, et al. Mastocytosis. World Health Organization (WHO) classification of tumours. In: Jaffe ES, Harris NL, Stein H, Varderman JW, editors. Pathology and Genetics. Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001. pp. 291–302. [Google Scholar]
- 13.Jernej B, Banovic M, Cicin-Sain L, Hranilovic D, Balija M, Oreskovic D, et al. Physiological characteristics of platelet/circulatory serotonin: study on a large human population. Psychiatry Res. 2000;94:153–162. doi: 10.1016/s0165-1781(00)00129-3. [DOI] [PubMed] [Google Scholar]
- 14.Barbara G, De Giorgio R, Stanghellini V, Cremon C, Salvioli B, Corinaldesi R. New pathophysiological mechanisms in irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20(Suppl. 2):1–9. doi: 10.1111/j.1365-2036.2004.02036.x. [DOI] [PubMed] [Google Scholar]
- 15.Lance JW. Current concepts of migraine pathogenesis. Neurology. 1993;43:S11–S15. [PubMed] [Google Scholar]
- 16.Humphrey PP, Feniuk W, Perren MJ, Beresford IJ, Skingle M, Whalley ET. Serotonin and migraine. Ann N Y Acad Sci. 1990;600:587–598. doi: 10.1111/j.1749-6632.1990.tb16912.x. [DOI] [PubMed] [Google Scholar]
- 17.Smith NL. Serotonin mechanisms in pain and functional syndromes. management implications in co morbid fibromyalgia, headache, and irritable bowel syndrome – case study and discussion. J Pain Palliat Care Pharmacother. 2004;18:31–45. doi: 10.1300/j354v18n04_04. [DOI] [PubMed] [Google Scholar]
- 18.Zemlan FP, Behbehani MM, Murphy RM. Serotonin receptor subtypes and the modulation of pain transmission. Prog Brain Res. 1988;77:349–355. doi: 10.1016/s0079-6123(08)62801-0. Links. [DOI] [PubMed] [Google Scholar]
- 19.Cady RK. Pathophysiology of migraine: implications to patient care. Tex Dent J. 2006;123:142–151. [PubMed] [Google Scholar]
- 20.Nagata E, Shibata M, Hamada J, Shimizu T, Katoh Y, Gotoh K, et al. Plasma 5-hydroxytryptamine (5-HT) in migraine during an attack-free period. Headache. 2006;46:592–596. doi: 10.1111/j.1526-4610.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- 21.Finkelman FD. Anaphylaxis: lessons from mouse models. J Allergy Clin Immunol. 2007;120:506–515. doi: 10.1016/j.jaci.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 22.Wood JD. Enteric nervous system, serotonin, and the irritable bowel syndrome. Curr Opin Gastroenterol. 2001;17:91–97. doi: 10.1097/00001574-200101000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Akin C, Metcalfe DD. Surrogate markers of disease in mastocytosis. Int Arch Allergy Immunol. 2002;127:133–136. doi: 10.1159/000048184. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz LB, Min HK, Ren S, Xia HZ, Hu J, Zhao W, et al. Tryptase precursors are preferentially and spontaneously released, whereas mature tryptase is retained by HMC-1 cells, Mono-Mac-6 cells, and human skin-derived mast cells. J Immunol. 2003;170:5667–5673. doi: 10.4049/jimmunol.170.11.5667. [DOI] [PubMed] [Google Scholar]
- 25.Kushnir-Sukhov NM, Gilfillan AM, Coleman JW, Brown JM, Bruening S, Toth M, et al. 5-hydroxytryptamine induces mast cell adhesion and migration. J Immunol. 2006;177:6422–6432. doi: 10.4049/jimmunol.177.9.6422. [DOI] [PubMed] [Google Scholar]
- 26.Anlauf M, Schafer MK, Schwark T, von Wurmb-Schwark N, Brand V, Sipos B, et al. Vesicular monoamine transporter 2 (VMAT2) expression in hematopoietic cells and in patients with systemic mastocytosis. J Histochem Cytochem. 2006;54:201–213. doi: 10.1369/jhc.5A6739.2005. [DOI] [PubMed] [Google Scholar]
- 27.Metcalfe DD, Akin C. Mastocytosis: molecular mechanisms and clinical disease heterogeneity. Leuk Res. 2001;25:577–582. doi: 10.1016/s0145-2126(01)00046-7. [DOI] [PubMed] [Google Scholar]
- 28.Metcalfe DD. Regulation of normal and neoplastic human mast cell development in mastocytosis. Trans Am Clin Climatol Assoc. 2005;116:185–203. [PMC free article] [PubMed] [Google Scholar]