Abstract
Background:
Preterm birth is a major cause of neurodevelopmental disorders. Allopregnanolone, a key metabolite of progesterone, has neuroprotective and developmental effects in the brain. The objectives of this study were to measure the neuroactive steroid concentrations following preterm delivery in a neonatal guinea pig model and assess the potential for postnatal progesterone replacement therapy to affect neuroactive steroid brain and plasma concentrations in preterm neonates.
Methods:
Preterm (62-63 days) and term (69 days) guinea pig pups were delivered by cesarean section and tissue was collected at 24 hours. Plasma progesterone, cortisol, allopregnanolone, and brain allopregnanolone concentrations were measured by immunoassay. Brain 5α-reductase (5αR) expression was determined by Western blot. Neurodevelopmental maturity of preterm neonates was assessed by immunohistochemistry staining for myelination, glial cells, and neurons.
Results:
Brain allopregnanolone concentrations were significantly reduced after birth in both preterm and term neonates. Postnatal progesterone treatment in preterm neonates increased brain and plasma allopregnanolone concentrations. Preterm neonates had reduced myelination, low birth weight, and high mortality compared to term neonates. Brain 5αR expression was also significantly reduced in neonates compared to fetal expression.
Conclusions:
Delivery results in a loss of neuroactive steroid concentrations resulting in a premature reduction in brain allopregnanolone in preterm neonates. Postnatal progesterone therapy reestablished neuroactive steroid levels in preterm brains, a finding that has implications for postnatal growth following preterm birth that occurs at a time of neurodevelopmental immaturity.
Keywords: allopregnanolone, preterm birth, neonate, fetus, progesterone
Introduction
Preterm birth (birth at <37 weeks gestation) is a major contributor to the burden of disease and mortality in newborn infants. Due to the vulnerability of immature organ systems to injury and the disruption of normal developmental processes, preterm infants are at a greater risk of poor short- and long-term health outcomes.1 A physiological consequence of preterm birth is the premature removal of the fetus from the influence of placentally derived hormones, including progesterone. Progesterone is essential for the establishment and maintenance of pregnancy, with high concentrations of progesterone and its metabolites present during gestation, which fall rapidly following removal of the placenta at birth.2,3 The neuroactive steroid allopregnanolone (3α,5α-tetrahydroxyprogesterone) is synthesized from progesterone by the enzymes 5α-reductase types 1 and 2 (5αR1 and 5αR2) and 3α-hydroxysteroid dehydrogenase, which are present in the placenta and within the brain as well as other endocrine organs.4 Previous studies in sheep have shown that neuroactive steroid concentrations decline rapidly in line with reductions in progesterone following term birth despite continued expression of 5αRs in the brain. Although there have been no studies on neuroactive steroids with preterm birth, the loss of the placenta may be expected to lead to a similar drop in allopregnanolone concentrations. The expression of allopregnanolone synthetic enzymes is developmentally regulated and altered by stressors during gestation and the postnatal period. Expression of 5αR2 and concentrations of allopregnanolone increase in the fetal brain in response to hypoxic/ischemic events in utero5 and in the neonate.6 Changes in neuroactive steroid synthetic enzyme expression following preterm birth have not been previously studied.
Neuroactive progesterone metabolites have important actions within the developing brain that may be affected by preterm birth. Allopregnanolone binds to the γ-aminobutyric acid type A receptor, reducing neural excitability and exerting neuroprotective and antiseizure effects within the brain.7 Neuroactive steroids also influence neural development, axonal and dendritic outgrowth, myelin synthesis, and inhibit the excessive gliosis that impairs recovery following injury.8 Important neuroprotective actions of progesterone are mediated by its metabolites. Inhibition of allopregnanolone synthesis in the late-gestation guinea pig reduces myelination and enhances the expression of glial fibrillary acidic protein (GFAP)-positive astrocytes, a marker of brain injury.9 Low allopregnanolone concentrations increase apoptosis in the fetal sheep brain, an increase that is ameliorated by the administration of a synthetic allopregnanolone analog.10 Additionally, stress during gestation in rats reduces brain allopregnanolone turnover from progesterone and results in offspring with poor cognitive functioning.11 Allopregnanolone administration also improves morphological and functional recovery in adult animal models of hypoxic/ischemic and traumatic brain injury12 as well as enhancing myelination and myelinating oligodendrocytes in brain slice culture systems.13
We hypothesize that the loss of placental progesterone at the time of premature birth reduces neuroactive steroid concentrations and contributes to poor neurodevelopmental outcomes after preterm birth. Despite the potentially important protective and neurodevelopmental actions of neuroactive steroids in the immature brain, the changes in neuroactive steroid concentrations following preterm birth have not been previously studied. The establishment of a small animal model of premature delivery in the guinea pig enables the study of the neuroactive steroid system in an animal that maintains relatively high concentrations of placentally derived progesterone until term.14 The initial aim of this study was therefore to develop a preterm neonatal model in the guinea pig and to describe the effects of preterm delivery on brain development at this age by measuring markers of myelination, astrocyte maturity and activation, and neuronal development. Further objectives of this study were to examine the effect of preterm birth on neuroactive steroid levels and key synthetic enzymes in the brains of neonatal guinea pigs. The specific aims were to identify the gestational age- and sex-specific changes in brain allopregnanolone concentrations in immature preterm guinea pig neonates, describe the brain development of neonatal guinea pigs delivered prematurely at this age, and determine the effect of postnatal progesterone therapy on allopregnanolone concentrations in the preterm neonatal brain.
Materials and Methods
Animals
Animal procedures were conducted with approval from the University of Newcastle Animal Care and Ethics Committee, in accordance with Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. The University of Newcastle Research Support Unit provided time-mated outbred tricolor guinea pigs that were housed indoors under controlled 12-hour light/dark cycles. Pregnant animals were provided with ascorbic acid-fortified water and commercial guinea pig pellets ad libitum.
Preterm and Term Cesarean-Section Delivery
Pregnant guinea pigs (term, n = 11; preterm, n = 23) were randomly allocated to preterm and term groups for delivery at gestational day 62 to 63 (preterm) and gestational day 69 or after 2 days of the pubic symphysis being greater than 1.5 cm open (67-69 days). The mean gestational age of the term group was 68.2 days. Spontaneous term delivery occurs at 71 ± 0.5 days in this colony.15 Single male and single female neonates in each treatment group were used from each litter. To aid neonatal respiratory function, all pregnant guinea pigs received β-methasone sodium phosphate/β-methasone acetate subcutaneously (sc, 1mg/kg; Celestone chronodose; Schering-Plough, North Ryde, New South Wales, Australia) at 24 and 12 hours prior to cesarean-section delivery. Pregnant guinea pigs were anesthetized under 1% to 3% isoflurane in oxygen, the uterus was exposed via midline abdominal incision, fetuses were removed, and umbilical cord was tied off and cut. The maternal guinea pig was then killed by intracardiac sodium pentobarbital (200 mg/kg). Neonates were dried and suction was applied to remove excess fluid from the upper respiratory tract. Surfactant (50 μL, Curosurf, 80 mg/mL poractant alfa; Douglas Pharmaceuticals, Baulkham Hills, New South Wales, Australia) was administered into the oropharynx and continuous positive airway pressure was applied using a modified mask and Neopuff Infant T-Piece Resuscitator (Fisher & Paykel Healthcare Australia, Melbourne, Australia), with a positive-end expiratory pressure of 7 cm H2O, peak inspiratory pressure of 20 cm H2O, and inspired oxygen concentration of 50%. With the establishment of stable respiration, neonates were placed in a humidified incubator (Thermocare, Nevada) maintained at ∼34°C. Neonatal respiration, posture/muscle tone, and activity were monitored and recorded. Neonates were fed at 2-hour intervals using commercial milk formula (Wombaroo Food Products, Adelaide, South Australia, Australia).
Progesterone Treatment
Preterm neonates were randomly assigned to receive vehicle (22.5%, w/v (2-hydroxypropyl)-β-cyclodextrin) or progesterone (16 mg/1.6 mL/kg in (2-hydroxypropyl)-β-cyclodextrin) injections at 1 hour (intraperitoneally) and then 6 hours postdelivery (sc). Progesterone dose used in this study was based on that used by Goss et al.16
Tissue and Plasma Collection
At 24 hours after delivery, neonates were euthanized by carbon dioxide inhalation. After euthanasia, plasma was collected via cardiac puncture and stored at −80°C for steroid analysis. Brains were removed from the skull within 2 to 3 minutes of euthanasia, weighed, hemisected, and divided coronally into 3 regions (rostral, middle, and caudal). The right hemisphere was snap frozen and stored at −80°C, and left hemisphere immersion fixed in paraformaldehyde (PFA; 4%, w/v PFA in 0.1 mol/L phosphate buffer) before paraffin embedding. The middle block of the right hemisphere, including tissue from the cerebral cortex, corpus callosum, thalamus, and hippocampus, was crushed and homogenized and used in this study for brain radioimmunoassay of allopregnanolone and 5αR Western blot analyses. Neonatal sex, body, and organ weights were recorded at the time of tissue collection.
Samples for fetal steroid concentrations and brain immunoblotting were obtained from a study of gestational neuroactive steroids9 and are presented for comparison between fetal and neonatal values. Fetal animals used for these studies did not receive β-methasone treatment, with tissue collected, as above, from animals killed in utero at 65 to 68 days of gestation. Fetal samples were run concurrently with those from the present study.
Steroid Radioimmunoassay and Enzyme Immunoassay
Allopregnanolone concentrations in plasma and brain (middle tissue block from the right hemisphere) were determined via radioimmunoassay.17 Brain and plasma samples were treated with acidified 50% methanol and brain samples homogenized. Brain and plasma samples were then added to Sep-Pak C18 cartridges (Waters, Milford, Massachusetts) for steroid extraction using graded methanol washes. Allopregnanolone antibody cross-reactivity to progesterone was minimized by oxidation of nonsaturated steroids using 5% potassium permanganate.18 Steroids were then reextracted using diethyl-ether/n-hexane (50%, v/v). Allopregnanolone concentration in each sample was quantified by radioimmunoassay using an antibody supplied by Dr R.H. Purdy (Department of Psychiatry, Veterans Administration Hospital, San Diego, California) and tritium-labeled allopregnanolone (5α-[9, 11, 12,3H(N)]; PerkinElmer Life and Analytical Sciences, Boston, Massachusetts). Extraction efficiency was determined by the addition of tritiated allopregnanolone prior to extraction and used to correct for extraction loss. The limit of detection for allopregnanolone was 35.0 ± 2.5 pg/tube. The average recovery was 57.7 ± 1.1% for plasma samples and 57.8 ± 2.6% for brain samples. The intra-assay coefficient for plasma was 10.6% and 10.9% in brain assays.
Progesterone and cortisol were measured in guinea pig plasma by immunoassay carried out by Hunter Area Pathology Service using the Beckman Coulter UniCel Dx1800 Access Immunoassay System. The intra-assay coefficients of variance were 4.3% and 7.9% for cortisol and progesterone assays, respectively.
Western Blot Analysis
The expression of 5αR, types 1 and 2, was determined in the brain using Western blot.9 Brain protein was extracted by homogenization in protein extraction buffer (50 mmol/L Tris-HCl, 150 mmol/L NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate with protease and phosphatase inhibitors). Sodium dodecyl sulfate polyacrylamide gel electrophoresis was performed using NuPAGE Novex 12% Bis/Tris gels (Invitrogen, Mt Waverley, Australia) before transfer to polyvinylidene fluoride membranes, which were then blocked with bovine serum albumin (BSA)/skim milk, incubated overnight in primary antibodies (5αR1, NB100-1491, Novus Biologicals, Littleton, Colorado; 5αR2, Ab27469, AbCam, Cambridge, UK), followed by secondary antibody incubation (P0449; DakoCytomation, Glostrup, Denmark) and chemiluminescence detection. Protein expression of 5αR1 (∼26 kDa) and 5αR2 (∼29 kDa) was quantified using Multigauge v2.4 software (Fuji Photo Film, Tokyo, Japan), normalized to β-actin and internal controls.
Immunohistochemistry
Immunohistochemistry was performed on 8-μm sections from paraffin-embedded brains that were dewaxed, incubated in Reveal It Solution (ImmunoSolution Pty Ltd, Everton Park, Queensland, Australia), blocked (0.1 mol/L phosphate-buffered saline pH 7.2 with 0.5%, w/v BSA, 0.05%, w/v saponin, and 0.05%, v/v sodium azide), and incubated in primary antibody (GFAP, G3893; myelin basic protein [MBP], M9434; microtubule-associated protein 2 [MAP-2], M9942, Sigma-Aldrich). Incubations in secondary antibodies (E0354, DakoCytomation; B7139, Sigma-Aldrich) and streptavidin–biotin–horseradish peroxidase complex (RPN1051V; GE Healthcare Life Sciences, Rydalmere, NSW, Australia) with 3,3′-diaminobenzidine tetrahydrochloride chromagen were then performed. The GFAP and MBP immunostaining was examined in the hippocampus and subcortical white matter regions. The MAP-2 was measured in the CA1 region of the hippocampus, subgranular zone of the dentate gyrus, and cortex, adjacent to the pial surface. Staining density was analyzed using ImageJ 1.40 (National Institutes of Health, Bethesda, Maryland) on binary 8-bit images captured at 40× magnification, with the percentage of area coverage recorded for 4 fields of view per region on 2 consecutive sections per animal.
Statistical Analyses
Statistical analyses were performed using Prism v4.0b for MacOSX (GraphPad Software Inc, La Jolla, California). Data are presented as group mean ± standard error of the mean. Data were analyzed by 2-way analysis of variance (ANOVA) for neonatal sex and gestational age at delivery, with Bonferroni post hoc comparison between groups. Where no sex differences were identified, data were analyzed by 1-way ANOVA with Bonferroni post hoc analysis. Data that exhibited unequal variance (Bartlett test for equal variance P < .05) were log or square root transformed. Plasma cortisol data were not normally distributed and were analyzed by Kruskal-Wallis with post hoc Dunn multiple comparison. Survival data were analyzed by Fisher exact test. P < .05 was considered statistically significant and indicated on graphs by letters and symbols (*P < .05, **P < .01, and †P < .001).
Results
Neonatal Animals
Regular observations of preterm neonates showed periods of apnea, forelimb spasticity, and irregular respiration, not present in term neonates. In addition, relative activity and motility were markedly lower in preterm animals. The gestational age at delivery, mean body, and organ weights of preterm and term neonates that survived the initial 24-hour period are shown in Table 1. There were no significant differences in gestational age between male and female or preterm vehicle and progesterone-treated neonates. Preterm animals had a significantly greater mortality rate of 60% at 24 hours following delivery, compared to a 3% mortality rate in term neonates (P < .001). Male neonates made up a higher proportion of those animals that did not reach 24 hours (67%). When examined by neonatal sex and drug treatment, male neonates had survival rates of 40% with vehicle and 62% with progesterone treatment. Preterm female neonatal survival at 24 hours was 50% with vehicle and 38% with progesterone administration. However, these values for male and female preterm survival with progesterone treatment were not significantly different between groups. Preterm neonates had significantly lower body weights than term animals at birth and 1-day postnatal age (P < .05). There were no differences in preterm body weights with progesterone treatment. Brain weights were significantly lower in preterm and preterm progesterone-treated groups when compared to term (P < .001). No differences in brain-to-liver weight ratio (BLR) were identified with gestational age or progesterone treatment. Male preterm progesterone-treated animals had significantly lower liver weights than those in term males. No significant differences in organ or body weights were present between male and female neonates of the same gestational ages.
Table 1.
Animal Characteristics and Organ Weights of Preterm and Term Neonatal Guinea Pigs.a
| n | Litters | GA Delivery | Birth Weight | PM Weight | Brain Weight | Liver Weight | BLR | |
|---|---|---|---|---|---|---|---|---|
| Term | 22 | 11 | 68.2 ± 0.16b | 87.3 ± 2.50b | 83.3 ± 2.58b | 2.3 ± 0.03 | 3.6 ± 0.15 | 0.66 ± 0.02 |
| Female | 10 | 8 | 68.3 ± 0.21b | 86.3 ± 3.32b | 82.9 ± 3.37b | 2.3 ± 0.03 | 3.5 ± 0.16 | 0.67 ± 0.03 |
| Male | 12 | 9 | 68.2 ± 0.24b | 88.2 ± 3.76b | 83.7 ± 3.95b | 2.3 ± 0.04 | 3.7 ± 0.24a | 0.65 ± 0.04 |
| Preterm | 20 | 19 | 62.5 ± 0.15 | 69.0 ± 1.92 | 64.1 ± 1.70 | 2.1 ± 0.03 | 3.0 ± 0.11 | 0.72 ± 0.02 |
| Female | 10 | 9 | 62.4 ± 0.22 | 67.3 ± 2.88 | 62.3 ± 2.52 | 2.1 ± 0.04 | 2.9 ± 0.13 | 0.72 ± 0.03 |
| Male | 10 | 10 | 62.6 ± 0.22 | 70.7 ± 2.58 | 66.0 ± 2.26 | 2.2 ± 0.03 | 3.1 ± 0.03ab | 0.73 ± 0.04 |
| Preterm Prog | 16 | 15 | 62.6 ± 0.16 | 67.1 ± 2.06 | 63.2 ± 1.97 | 2.1 ± 0.04 | 2.9 ± 0.14 | 0.73 ± 0.03 |
| Female | 8 | 8 | 62.7 ± 0.29 | 69.9 ± 3.59 | 66.2 ± 3.54 | 2.1 ± 0.05 | 3.1 ± 0.26 | 0.71 ± 0.04 |
| Male | 8 | 7 | 62.5 ± 0.20 | 64.3 ± 1.73 | 60.3 ± 1.24 | 2.1 ± 0.06 | 2.8 ± 0.12b | 0.76 ± 0.04 |
Abbreviations: GA delivery, gestational age at delivery; PM, postmortem; BLR, brain-to-liver weight ratio; Prog, progesterone replacement; SEM, standard error of the mean.
aValues expressed as mean ± SEM; weight (g). Different lower case letters indicate significant difference in male liver weights. Liver weights and BLR exhibited unequal variance by Bartlett test and were transformed by square root for analysis.
b P < .05 term is significantly different from preterm groups.
Plasma Steroid Concentrations
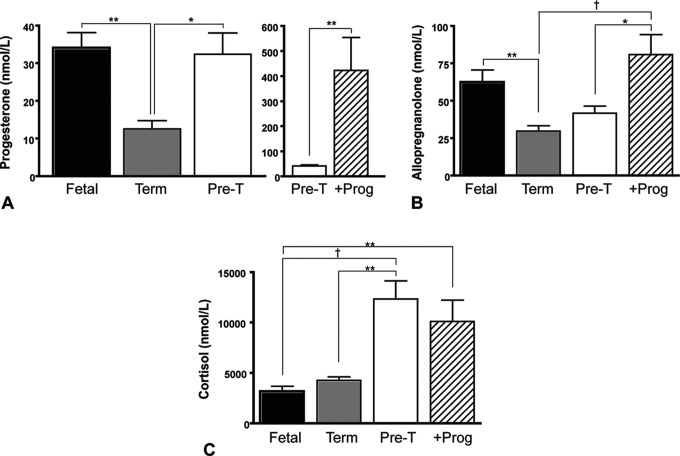
Figure 1 shows plasma progesterone, allopregnanolone, and cortisol concentrations of fetal, term neonates, and preterm (pre-T) neonates. Additionally, plasma steroid levels in preterm animals that received postnatal progesterone replacement (+Prog) are also shown. Analysis of these data showed no sex difference (2-way ANOVA); and therefore, the data are presented with combined neonatal sex. Plasma progesterone concentrations in term neonates at 24 hours after birth were significantly lower than those measured in late gestation fetal plasma (P < .01; Figure 1A). Preterm neonates had similar progesterone concentrations to fetal guinea pigs. Postnatal progesterone treatment significantly increased plasma progesterone concentrations compared to vehicle-treated preterm animals (P < .01; Figure 1A, right panel).
Figure 1.
Plasma steroid concentrations in fetal (n = 9), term (n = 12), preterm (pre-T; n = 9), and preterm progesterone-treated (+Prog; n = 9) neonates. A, Plasma progesterone in fetal guinea pigs, term, and preterm neonates (left-hand panel) and preterm compared to preterm progesterone-treated neonates (right-hand panel; note difference in scaling in this panel). B, Plasma allopregnanolone. C, Plasma cortisol. Each bar represents mean ± SEM. Significant differences between groups *P < .05, **P < .01, and † P < .001. SEM indicates standard error of the mean.
Plasma allopregnanolone concentrations in term neonates were significantly lower than late-gestation fetal concentrations (P < .01; Figure 1B). Plasma allopregnanolone concentrations in preterm neonates at 24 hours were not different from fetal levels. Following postnatal progesterone treatment in preterm neonates, plasma concentrations of allopregnanolone rose to levels above those seen in term (P < .001) and preterm (P < .05) levels.
Cortisol concentrations in term neonates were unchanged from fetal levels (Figure 1C). Cortisol concentrations were markedly higher in preterm neonates compared to fetal (P < .001) and term (P < .01) animals. Preterm neonates that received progesterone treatment also had significantly higher plasma cortisol than fetal animals (P < .01).
Allopregnanolone Concentrations in the Brain
Concentrations of allopregnanolone in the brains of term and preterm guinea pig neonates were significantly reduced compared to fetal brain concentrations (P < .001; Figure 2, left-hand panel). There were no differences in allopregnanolone concentrations between term and preterm neonatal brains or between male and female neonates in these groups. Postnatal progesterone administration in preterm neonates resulted in markedly increased brain allopregnanolone concentrations compared to vehicle-treated preterm neonates (P < .001; Figure 2, right-hand panel).
Figure 2.
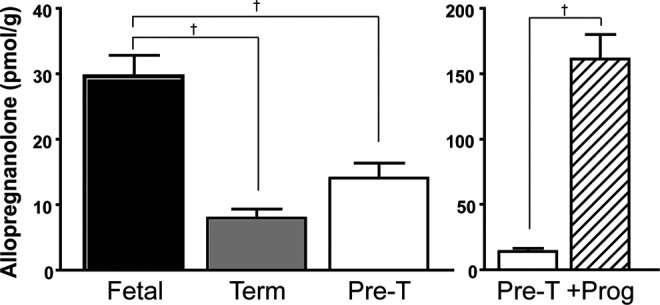
Brain allopregnanolone concentrations in fetal (n = 12), term (n = 9), preterm (pre-T; n = 11, left-hand panel), and preterm compared to progesterone-treated neonates (+Prog; n = 10, right-hand panel), with increased brain allopregnanolone concentrations following progesterone treatment (note difference in scaling in the right-hand panel). Each bar represents mean ± SEM. †, significant differences between groups (P < .001). SEM indicates standard error of the mean.
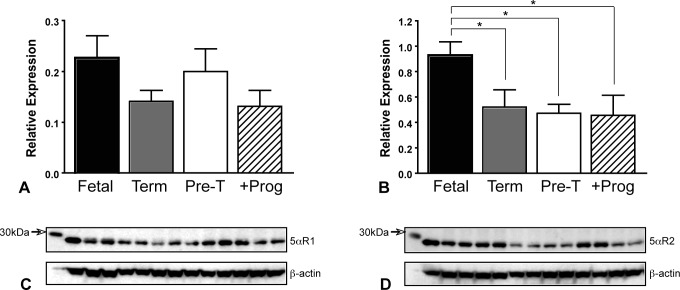
Brain 5αR Expression
The expression of 5αR2 was significantly reduced in term, preterm, and preterm progesterone-treated animals when compared to expression in fetal brains (P < .05; Figure 3B). No differences in the expression of the 5αR1 (Figure 3A) were observed between fetal, term, and preterm groups. No sex differences were present for either isoform.
Figure 3.
Expression of 5α-reductase enzymes in fetal (n = 16), term (n = 11), preterm (n = 12; pre-T), and preterm progesterone-treated neonatal guinea pig (n = 8; +Prog) brains. A, Relative expression and (C) representative Western blots (with β-actin loading control) of 5α-reductase type 1. B, Relative expression and (D) representative Western blots (with β-actin loading control) of 5α-reductase type 2. Bars represent mean ± SEM. *, significant differences between groups (P < .05). SEM indicates standard error of the mean.
Myelin Basic Protein Expression
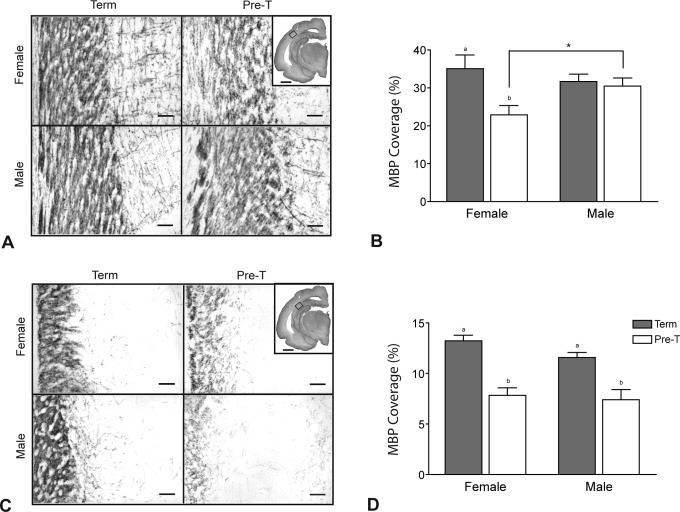
Representative micrographs of MBP immunostaining in the subcortical white matter tracts and the CA1 region of the hippocampus of neonates delivered at term and preterm are shown in Figure 4A and C, respectively. Densitometric analysis of MBP immunostaining shows that, in the subcortical white matter region (Figure 4B) staining was significantly reduced in female preterm neonates when compared to female term neonates (P < .05). This difference between term and preterm animals was not observed in male neonates. Furthermore, female preterm neonates had significantly lower MBP coverage compared to male preterm neonates (P < .05) in this region. The density of MBP immunostaining in the CA1 region of the hippocampus (Figure 4D) was reduced in male and female preterm neonates compared to term animals of the same sex. No differences with neonatal sex were identified in the hippocampus.
Figure 4.
The MBP immunostaining in male and female neonates delivered at term or preterm (pre-T) showing immaturity in preterm myelination. A, Representative photomicrographs and (B) density of MBP coverage in the subcortical white matter region. C, Representative photomicrographs and (D) density of MBP coverage in the CA1 region of the hippocampus. Regions analyzed are indicated on inset micrographs in (A) and (C; scale bars indicate 2.0 mm). Main scale bars represent 0.1 mm. Density of staining is expressed as mean percentage of area coverage ± SEM (n = 6; term, gray bars; preterm, open bars). Different lower case letters indicate significant differences between preterm and term groups (P < .05). *, significant difference between male and female neonates at the same age (P < .05). MBP indicates myelin basic protein; SEM, standard error of the mean.
Glial Fibrillary Acidic Protein Expression
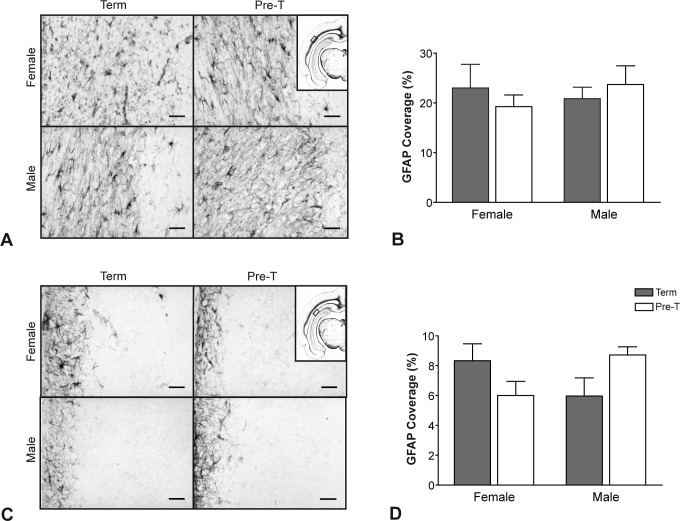
Astrocyte activation in preterm delivery was assessed with GFAP immunostaining (Figure 5). Comparison of representative images in the subcortical white matter (Figure 5A) shows no differences in GFAP staining between term and preterm neonates nor were there major differences in hippocampus (Figure 5C) between the groups of neonates. When quantified, GFAP expression did not differ between preterm and term neonates or by neonatal sex in the subcortical white matter region (Figure 5B). There was, however, a significant interaction effect between neonatal sex and age of gestation in the hippocampal CA1 region (P = .0185; Figure 4D).
Figure 5.
The GFAP immunostaining in male and female neonates delivered at term or preterm (pre-T). A, Representative photomicrographs and (B) density of GFAP coverage in the subcortical white matter region. C, Representative photomicrographs and (D) density of GFAP coverage in the CA1 region of the hippocampus. Regions analyzed are indicated on inset micrographs in (A) and (C). Main scale bars represent 0.1 mm. Density of staining is expressed as mean percentage of area coverage ± SEM (n = 6; term, gray bars; preterm, open bars). Two-way ANOVA identified significant interaction between age at delivery and neonatal sex in GFAP staining in the CA1 region of the hippocampus (P = .0185). ANOVA indicates analysis of variance; GFAP, glial fibrillary acidic protein; SEM, standard error of the mean.
Microtubule-Associated Protein 2 Expression
Representative micrographs showing MAP-2 immunostaining, examined in proliferating neurons in the subgranular zone of the dentate gyrus, are shown in Figure 6A. The density of MAP-2 staining in the subgranular zone of the dentate gyrus (Figure 6B) shows that gestational age at delivery had no effect on MAP-2 expression in this region with no differences identified between preterm and term neonates. Neonatal sex had no effect on the density of MAP-2 immunostaining.
Figure 6.
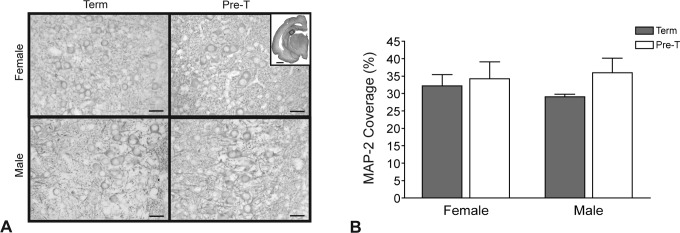
The MAP-2 immunostaining in male and female neonates delivered at term or preterm. A, Representative photomicrographs and (B) density of MAP-2 coverage in the subgranular zone of the dentate region. Scale bars represent 0.1 mm. Density of staining is expressed as mean percentage of area coverage ± SEM (n = 6; term, gray bars; preterm, open bars). MAP-2 indicates microtubule-associated protein 2; SEM, standard error of the mean.
Discussion
This study describes the characteristics of prematurely delivered guinea pig neonates and the changes in neuroactive steroid concentrations that result from preterm delivery. The key finding of this study was a confirmation that the reduction in brain allopregnanolone concentrations that occurs following birth at term gestation, also occurs following preterm birth, prematurely depriving these neonates of normal allopregnanolone levels. In these preterm neonates, we have also demonstrated, for the first time, the efficacy of postnatal progesterone treatment in reestablishing brain and plasma allopregnanolone concentrations. Furthermore, these changes were shown in a clinically relevant model of premature birth, with demonstrated neurodevelopmental immaturity and reduced myelination at this preterm gestational age. These findings may have clinical implications on neurodevelopment and the potential for neuroactive steroid therapy following premature birth.
The transition from fetal to neonatal life in this novel guinea pig model is associated with a loss of progesterone, and a reduction in allopregnanolone concentrations are consistent with previous findings in other species at term, with allopregnanolone concentrations known to fall markedly with term delivery in neonatal sheep.19 This fall in neuroactive steroid supply has not been previously shown in conjunction with preterm birth. Surprisingly, in the current study, plasma progesterone concentrations declined rapidly after birth in term animals, but a similar same reduction was not present in preterm neonates. The mechanisms involved in maintaining these concentrations at 24 hours after birth, despite the removal of the placenta as a source of progesterone, remain unclear. Variations in the concentrations of progesterone and its precursor pregnenolone mediated by gestational age, in utero concentrations, or contribution of adrenal steroidogenesis may be responsible for the differences in neuroactive steroid concentrations at term and preterm gestations. Pregnenolone sulfate concentrations are also relatively high in the fetal circulation and may function as a reserve of pregnenolone in preterm neonates.20 The trend toward lower allopregnanolone concentrations in preterm plasma also suggests alterations in progesterone metabolism in the preterm guinea pig despite maintenance of plasma progesterone concentrations. Without the continued supply of placentally derived steroids, these concentrations of progesterone are likely to decline on the neonatal period, thus reducing the synthesis of neuroactive metabolites and resulting in a prolonged deficit of neuroactive steroid concentrations. The effect of this reduction in long-term neurodevelopmental processes in the preterm neonate is still to be determined but may contribute to the high risk of adverse outcomes following preterm birth.
Importantly, higher plasma concentrations of progesterone at 24 hours in preterm neonates did not maintain brain allopregnanolone concentrations at fetal concentrations. The finding that 5αR2 expression was reduced within the brain suggests that both the endogenous supply of progesterone and the capacity of the brain to synthesize neuroactive progesterone metabolites are compromised in the preterm neonates. This may impact neurodevelopmental processes in the preterm neonate as trophic proliferative actions of allopregnanolone in the immature brain are potentially reduced.13 Previous studies of allopregnanolone production in fetal sheep have identified 5αR2 as the more important isoform in regulating neuroactive steroid levels within the fetal brain and in controlling responses to stress.5 The reduced expression of this enzyme in preterm neonates may potentially blunt this endogenous stress response. Additionally, exogenous glucocorticoids such as β-methasone may also influence 5αR expression with repeated β-methasone exposure reducing 5αR2 messenger RNA in the brains of male fetal guinea pigs.21 This potential neuroendocrine interaction is an important consideration due to the administration of β-methasone to pregnant animals in the current study to aid fetal respiratory development before delivery. The interaction of endogenous and exogenous glucocorticoids with the neuroactive steroid synthetic pathway may have influenced neuroactive steroid levels in the preterm brain. However, both term and preterm animals received β-methasone treatment in order to reduce these potential effects as a source of variation.
Observations of the condition and general activity of preterm neonates indicate considerable immaturity of animals delivered at this age compared to term. Furthermore, the reduced survival suggests greater stress and the presence of other adverse factors, such as hypoxia, in the preterm group that may mediate vulnerability to injury in the preterm brain. An increased stress response is supported by the finding of elevated plasma cortisol concentrations in preterm neonates, potentially due to poorer health in these animals.22 Although difficult to avoid, the stress observed in preterm infants in this model may reasonably mimic aspects of preterm neonatal outcomes observed clinically. The trend toward an increase in male mortality may also be relevant to the clinical disadvantage23 often faced by male preterm neonates. The potential interaction between glucocorticoids, increased stress in these preterm neonates, and neuroactive progesterone metabolites should be a consideration in the future studies of functional and neuropathological findings after preterm birth in this model.
Given the changes in progesterone availability and reduced expression of the neuroactive steroid synthetic enzymes, 5αR, following delivery, a major objective of this study was to determine the efficacy of progesterone replacement therapy in increasing preterm brain allopregnanolone concentrations that are reduced after birth. We have shown that this reduction occurs prematurely in preterm neonates, relative to the brain development of animals delivered at this age. The present findings demonstrate that administration of progesterone to preterm neonates increases plasma progesterone concentrations and the concentration of its neuroactive metabolite, allopregnanolone. Despite the reduced expression of 5αR2, the exogenous administration of progesterone, at this dose, was also sufficient to raise the brain allopregnanolone concentrations to levels above those of fetal concentrations. This finding provides support for the potential augmentation of allopregnanolone via endogenous synthetic mechanisms, including the metabolism of progesterone by 5αR, following indirect administration of precursor steroids in preterm infants. Progesterone therapy during the preterm postnatal period may therefore be useful in replacing neuroactive steroids that have the potential to influence brain maturation in preterm neonates during vulnerable periods of neurodevelopment. This finding also indicates that care should be exercised in the administration of progesterone and steroids in general in preterm infants, due to the potential negative effects of excessive production of neuroactive metabolites, including high concentrations of allopregnanolone in the preterm brain. Therefore, further studies to determine appropriate therapeutic doses for progesterone therapy are necessary. Progesterone and synthetic progestins have been investigated for use as preventative treatments for preterm birth.24 However, there is currently little information on the effect of such treatments on fetal and neonatal allopregnanolone and other neuroactive steroid concentrations. The finding that neuroactive steroid levels in the preterm neonate are at least partially dependent on progesterone concentrations suggests that the actions of these treatments in the fetal and neonatal brain should also be considered. Pilot data from clinical trials in extremely preterm neonates, examining the effects of postnatal replacement of placental hormones on bone mineralization25 and antenatal progesterone therapy on postnatal lung function,26 support the investigation of these steroid hormones for use in preterm neonates, with potential positive effects on maturation of vulnerable organ systems after preterm birth. Larger studies are required to assess neurodevelopmental effects of these therapies in vulnerable preterm populations,27 as the status of neuroactive steroid metabolites and their potential actions were not assessed in these trials. The further study on the neuroactive effects of pre- and postnatal steroid treatments on vulnerable infants is at least warranted in the clinical setting.
An important finding of this study was the reduction in MBP expression in preterm neonates compared to those delivered at term. Neurodevelopmental markers were examined to establish the immaturity of the guinea pig brain using this new model of prematurity. Previous studies have shown reduced myelination as a result of intrauterine growth restriction in fetal guinea pigs28; however, this is the first study looking at myelination with preterm delivery in this species. Reduced myelination at the time of delivery may lead to delay or disrupted myelination due to the altered conditions in the ex utero environment. The irreversible loss of myelinating oligodendrocytes can result in permanent perturbation of myelin development, severe white matter damage, and lasting neurological effects.29 Evidence from imaging studies in human preterm infants has shown that white matter injury and myelination disorders in the early preterm postnatal period are likely to persist to term equivalent age and are not rectified by catch-up growth.30 Additionally, myelin formation and maturation are influenced by both progesterone and its neuroactive steroid metabolites such as allopregnanolone.10,31,32 The reduced concentrations of allopregnanolone following preterm birth may therefore disrupt the long-term progression of myelination and catch-up growth required in these developmentally immature brains. The finding of short-term efficacy of progesterone replacement therapy in augmenting preterm brain allopregnanolone concentrations supports the further examination of this therapy for effects on myelination.
Neurodevelopmental processes associated with delivery at early gestational ages were further examined by assessing GFAP and MAP-2 expression. Expression of GFAP, a marker of reactive gliosis, is altered in the late gestation fetal guinea pig brain exposed to reduced concentrations of allopregnanolone in utero.9 The observation in this study that GFAP expression was affected by neonatal sex and gestational age, with an increase in GFAP in male preterm brains, may suggest increased vulnerability to injury in this group. Indeed, endogenous protective processes have been reported in cultured astrocytes from female neonatal mice that are more resistant to injury and cell death than male astrocytes.33 The finding that MAP-2 expression in the subgranular zone was not affected by gestational age suggests that neuronal proliferation is advanced at the age of preterm delivery used in this study. The possibility that there are long-term neurodevelopmental effects of a deficit in progesterone or allopregnanolone exposure in preterm neonates requires further investigation. Evidence from studies in immature rat cerebellar cells has shown that progesterone can enhance neuron and dendrite growth,34 processes that are likely to be important to postnatal growth. Examination of this postnatal development, the effects of preterm deficit in progesterone and allopregnanolone concentrations, and replacement of these neuroactive steroids for an extended postnatal period are therefore important future studies.
This study establishes a model of preterm delivery in the guinea pig, which demonstrates a premature reduction in brain concentrations of the key neuroactive steroid, allopregnanolone. The current findings demonstrate that this reduction can be reversed by postnatal treatment with progesterone. The development of this model, with immature neurodevelopment at delivery, will enable future investigations into progesterone administration during the immediate preterm postnatal period. Progesterone and its neuroactive steroid metabolites may have important actions on postnatal processes of myelination and perturbation of other key neurodevelopmental pathways associated with preterm birth.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Health and Medical Research Council of Australia (project grant ID#455527).
References
- 1. Behrman R, Butler A. Preterm Birth: Causes, Consequences. Washington, DC: National Academies Press; 2007. [PubMed] [Google Scholar]
- 2. Gilbert Evans SE, Ross LE, Sellers EM, Purdy RH, Romach MK. 3alpha-reduced neuroactive steroids and their precursors during pregnancy and the postpartum period. Gynecol Endocrinol. 2005;21(5):268–279. [DOI] [PubMed] [Google Scholar]
- 3. Dolling M, Seamark R. Progestagen metabolites in fetal sheep plasma: the effect of fetal nephrectomy. J Dev Physiol. 1979;1(5):399–413. [PubMed] [Google Scholar]
- 4. Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21(1):1–56. [DOI] [PubMed] [Google Scholar]
- 5. Nguyen PN, Billiards SS, Walker DW, Hirst JJ. Changes in 5alpha-pregnane steroids and neurosteroidogenic enzyme expression in fetal sheep with umbilicoplacental embolization. Pediatr Res. 2003;54(6):840–847. [DOI] [PubMed] [Google Scholar]
- 6. Billiards SS, Walker DW, Canny BJ, Hirst JJ. Endotoxin increases sleep and brain allopregnanolone concentrations in newborn lambs. Pediatr Res. 2002;52(6):892–899. [DOI] [PubMed] [Google Scholar]
- 7. Mellon SH. Neurosteroids: biochemistry, modes of action, and clinical relevance. J Clin Endocrinol Metab. 1994;78(5):1003–1008. [DOI] [PubMed] [Google Scholar]
- 8. Mellon SH. Neurosteroid regulation of central nervous system development. Pharmacol Ther. 2007;116(1):107–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kelleher M, Palliser H, Walker D, Hirst J. Sex-dependent effect of a low neurosteroid environment and intrauterine growth restriction on foetal guinea pig brain development. J Endocrinol. 2011;208(3):301–309. [DOI] [PubMed] [Google Scholar]
- 10. Yawno T, Hirst JJ, Castillo-Melendez M, Walker DW. Role of neurosteroids in regulating cell death and proliferation in the late gestation fetal brain. Neuroscience. 2009;163(3):838–847. [DOI] [PubMed] [Google Scholar]
- 11. Paris J, Frye C. Juvenile offspring of rats exposed to restraint stress in late gestation have impaired cognitive performance and dysregulated progestogen formation. Stress. 2011;14(1):23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. He J, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, enhances behavioral recovery and decreases neuronal loss after traumatic brain injury. Restor Neurol Neurosci. 2004;22(1):19–31. [PubMed] [Google Scholar]
- 13. Ghoumari A, Baulieu E, Schumacher M. Progesterone increases oligodendroglial cell proliferation in rat cerebellar slice cultures. Neuroscience. 2005;135(1):47–58. [DOI] [PubMed] [Google Scholar]
- 14. Heap R, Deanesly R. Progesterone in systemic blood and placentae of intact and ovarectomized pregnant guinea-pigs. J Endocrinol. 1966;34(4):417–423. [DOI] [PubMed] [Google Scholar]
- 15. Palliser H, Zakar T, Symonds I, Hirst J. Progesterone receptor isoform expression in the guinea pig myometrium from normal and growth restricted pregnancies. Repro Sci. 2010;17(8):776–782. [DOI] [PubMed] [Google Scholar]
- 16. Goss CW, Hoffman SW, Stein DG. Behavioral effects and anatomic correlates after brain injury: a progesterone dose-response study. Pharmacol Biochem Behav. 2003;76(2):231–242. [DOI] [PubMed] [Google Scholar]
- 17. Barbaccia M, Roscetti G, Trabucchi M, Ambrosio C, Massotti M. Cyclic AMP-dependent increase of steroidogenesis in brain cortical minces. Eur J Pharmacol. 1992;219(3):485–486. [DOI] [PubMed] [Google Scholar]
- 18. Bicikova M, Lapcik O, Hampl R, et al. A novel radioimmunoassay of allopregnanolone. Steroids. 1995;60(2):210–213. [DOI] [PubMed] [Google Scholar]
- 19. Nguyen PN, Billiards SS, Walker DW, Hirst JJ. Changes in 5alpha-pregnane steroids and neurosteroidogenic enzyme expression in the perinatal sheep. Pediatr Res. 2003;53(6):956–964. [DOI] [PubMed] [Google Scholar]
- 20. Klak J, Hill M, Parizek A, et al. Pregnanolone isomers, pregnenolone and their polar conjugates around parturition. Physiol Res. 2003;52(2):211–221. [PubMed] [Google Scholar]
- 21. McKendry A, Palliser H, Yates D, Walker D, Hirst J. The effect of betamethasone treatment on neuroactive steroid synthesis in a foetal guinea pig model of growth restriction. J Neuroendocrinol. 2010;22(3):166–174. [DOI] [PubMed] [Google Scholar]
- 22. Hughes D, Murphy J, Dyas J, Robinson J, Riad-Fahmy D, Hughes I. Blood spot glucocorticoid concentrations in ill preterm infants. Arch Dis Child. 1987;62(10):1014–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vatten L, Skjaerven R. Offspring sex and pregnancy outcome by length of gestation. Early Hum Dev. 2004;76(1):47–54. [DOI] [PubMed] [Google Scholar]
- 24. Mackenzie R, Walker M, Armson A, Hannah M. Progesterone for the prevention of preterm birth among women at increased risk: a systematic review and meta-analysis of randomized controlled trials. Am J Obstet Gynecol. 2006;194(5):1234–1242. [DOI] [PubMed] [Google Scholar]
- 25. Trotter A, Maier L, Grill HJ, Kohn T, Heckmann M, Pohlandt F. Effects of postnatal estradiol and progesterone replacement in extremely preterm infants. J Clin Endocrinol Metab. 1999;84(12):4531–4535. [DOI] [PubMed] [Google Scholar]
- 26. Dodd J, Crowther C, McPhee A, Flenady V, Robinson J. Progesterone after previous preterm birth for prevention of neonatal respiratory distress syndrome (PROGRESS): a randomised controlled trial. BMC Pregnancy Childbirth. 2009;9:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trotter A, Steinmacher J, Kron M, Pohlandt F. Neurodevelopmental follow-up at five years corrected age of extremely low birth weight infants after postnatal replacement of 17β-estradiol and progesterone. J Clin Endocrinol Metab. 2012;97(3):1041–1047. [DOI] [PubMed] [Google Scholar]
- 28. Tolcos M, Bateman E, O'Dowd R, et al. Intrauterine growth restriction affects the maturation of myelin. Exp Neurol. 2011;232(1):53–65. [DOI] [PubMed] [Google Scholar]
- 29. Kinney HC. Human myelination and perinatal white matter disorders. J Neurol Sci. 2005;228(2):190–192. [DOI] [PubMed] [Google Scholar]
- 30. Inder TE, Warfield SK, Wang H, Huppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115(2):286–294. [DOI] [PubMed] [Google Scholar]
- 31. Ghoumari A, Ibanez C, El-Etr M, et al. Progesterone and its metabolites increase myelin basic protein expression in organotypic slice cultures of rat cerebellum. J Neurochem. 2003;86(4):848–859. [DOI] [PubMed] [Google Scholar]
- 32. Baulieu EE, Schumacher M. Progesterone as a neuroactive neurosteroid, with special reference to the effect of progesterone on myelination. Hum Reprod. 2000;(15 suppl 1):1–13. [DOI] [PubMed] [Google Scholar]
- 33. Liu M, Hurn P, Reoselli C, Alkayed N. Role of P450 aromatase in sex-specific astrocytic cell death. J Cereb Blood Flow Metab. 2007;27(1):135–141. [DOI] [PubMed] [Google Scholar]
- 34. Tsutsui K. Progesterone biosynthesis and action in the developing neuron. Endocrinology. 2008;149(6):2757–2761. [DOI] [PubMed] [Google Scholar]