Abstract
Objective
To examine change in health-related quality of life (HRQoL) in association with clinical outcomes of neuropsychiatric (NP) events in SLE.
Methods
An international study evaluated newly diagnosed SLE patients for NP events attributed to SLE and non-SLE causes. Outcome of events was determined by physician-completed 7-point scale and compared to patient-completed SF-36 questionnaires. Statistical analysis used linear mixed-effects regression models with patient specific random effects.
Results
274 patients (92% female; 68% Caucasian), from a cohort of 1400, had ≥ 1 NP event where the interval between assessments was 12.3 ± 2 months. The overall difference in change between visits in mental component summary (MCS) scores of the SF-36 was significant (p<0.0001) following adjustments for gender, ethnicity, center and previous score. A consistent improvement in NP status (N=295) was associated with an increase in the mean(SD) adjusted MCS score of 3.66(0.89) in SF-36 scores. Between paired visits where NP status consistently deteriorated (N=30), the adjusted MCS score decreased by 4.00(1.96). For the physical component summary (PCS) scores the corresponding changes were +1.73(0.71) and −0.62(1.58) (p<0.05) respectively. Changes in SF-36 subscales were in the same direction (p<0.05; with the exception of role physical). Sensitivity analyses confirmed these findings. Adjustment for age, education, medications, SLE disease activity, organ damage, disease duration, attribution and characteristics of NP events did not substantially alter the results.
Conclusion
Changes in SF-36 summary and subscale scores, in particular those related to mental health, are strongly associated with the clinical outcome of NP events in SLE patients.
Keywords: Systemic lupus erythematosus, Neuropsychiatric, Inception cohort, Health related quality of life, SF-36
The prevalence of neuropsychiatric (NP) disease in patients with systemic lupus erythematosus (SLE) varies from 21% to 95% and recent studies indicate a frequency at the lower end of this range (1–5). There is a spectrum of potential NP events as reflected by the American College of Rheumatology (ACR) case definitions for 19 NP syndromes (6). Only 13% to 38% of all NP events are attributable to SLE depending upon the stringency of the decision rules for determining attribution (7–11). Regardless of attribution, both cross-sectional (3, 8) and longitudinal (7, 11) studies have demonstrated that NP events are independently associated with lower self-report health related quality of life (HRQoL).
Clinical trials are necessary to address the optimal management of NP events in SLE patients. Challenges to planning and executing such studies include the lack of validated outcome measures for the diverse NP events. Specific outcomes for individual NP events in addition to generic outcomes to allow a comparison with change across all NP events and other manifestations of SLE are required. The objective of the present study was to determine if the SF-36 is an appropriate outcome measure for the study of NP events in SLE. Specifically, we examined the association between changes in patient derived SF-36 summary and subscale scores and physician determined outcomes of NP events in an international, longitudinal cohort study of NP events in SLE patients.
Patients and Methods
Research network
This prospective study was performed by the Systemic Lupus International Collaborating Clinics (SLICC) (12) between October 1999 and May 2009. The study was approved by the Capital Health Research Ethics Board, Halifax, Nova Scotia, Canada and by the institutional research ethics boards of participating centers in accordance with the Declaration of Helsinki’s guidelines for research in humans.
Patients
Patients fulfilled the American College of Rheumatology (ACR) classification criteria for SLE (13) and provided written informed consent. The date of diagnosis was the time when four or more of the ACR criteria were first recognized and patients were enrolled up to 15 months thereafter. Data collection included age, gender, ethnicity, education, medication use, SLE Disease Activity Index (SLEDAI) (14) and SLICC/ACR damage index (SDI) (15). Health related quality of life (HRQOL) was measured by the SF-36 (16). Laboratory data included a complete blood count, serum creatinine, urinalysis, anti-dsDNA, C3 and C4.
Neuropsychiatric (NP) events
An enrollment window within which all NP events were captured extended from 6 months prior to the diagnosis of SLE up to the enrollment date. NP events were characterized using the ACR nomenclature and case definitions for 19 NP syndromes (6). Screening for NP syndromes was done by clinical evaluation and investigations were performed if clinically warranted.
Patients were reviewed annually following enrollment with a 6-month window around the anticipated assessment date. New NP events since the previous study visit and their attribution were determined. For the purpose of this study, patients were required to have an NP event(s) at one assessment, a physician defined outcome of the event at a subsequent assessment and a completed SF-36 questionnaire at both assessments. Those patients who developed a new or recurring NP event(s) between these assessments were excluded as such events would not have been reflected in the previous SF-36 scores.
Supplementary information was recorded as per the ACR glossary for NP syndromes (6) to identify other potential causes (“exclusions”) or contributing factors (“associations”) for each of the NP events (6). These “non-SLE factors” were used in part to determine the attribution of NP events. Patients could have more than one type of NP event and repeated episodes of the same event within the enrollment window or at follow-up annual assessments were recorded once. The date of onset of the NP event was the time of the first episode within the assessment period.
Attribution of NP events
All NP events were recorded and attribution to SLE was determined by decision rules of different stringency (models A and B) as described in detail elsewhere (8, 10). NP events which fulfilled the criteria for model A (the most stringent) or for model B (the least stringent) were attributed to SLE. By definition, all NP events attributed to SLE using model A were included in the group of NP events attributed to SLE using model B. Those events which did not fulfill these criteria were attributed to non-SLE causes.
Outcome of NP events
A physician generated 7-point Likert scale for NP events comparing the change in NP status between the onset of the event and time of study assessment was available for each NP event (1=patient demise, 2=much worse, 3=worse, 4=no change, 5=improved, 6=much improved, 7=resolved). Due to the occurrence of multiple concurrent NP events in the same patient, there were 5 possible patient level outcomes for each pair of assessments: 0 – no difference (single event) or no consistent change (multiple events) in NP status; 1 – all events improve; 2 - all events worsen; 3 – some but not all events improve and none worsen; 4 – some but not all events worsen and none improve. For the purpose of the primary analyses the patients’s paired visits with outcomes 3 and 4, of which there were only 71, were combined with those patients paired visits with NP events without a consistent change, outcome 0.
A second outcome measure was based on a patient generated SF-36 questionnaire which provided mental (MCS) and physical (PCS) component summary scores, subscale scores (16) and SF-6D scores (17). The difference between the scores at the paired visits was used as the response variable. The physician determined outcome of NP status defined explanatory variables for the regression analyses, which were also adjusted for other relevant variables. The SF-36 scores were not available to the physicians at the time of their evaluation of NP status.
Statistical analyses
Linear mixed-effects models with random intercepts were used so that patient/visits involving the same patient are not assumed to be independent. Specifically, conditional on this random intercept, we assume the variance of the change in health scores is constant and that the patients’ scores are uncorrelated.
Results
Patients
A total of 274 patients (20% of all patients) who had ≥ 1 NP event with a documented clinical outcome and 2 completed SF-36 questionnaires at the appropriate assessments were identified from a cohort of 1400 newly diagnosed SLE patients. Patients were predominantly women (92.3%) with a mean ± SD age of 37.1 ± 13.2 years and a wide ethnic distribution although predominantly Caucasian (Table 1).
Table 1.
Demographic and clinical manifestations of 274 SLE patients
| Number of Patients | 274 | |
| Gender (%) | Female Male |
253 (92.3) 21 (7.7) |
| Age (years) (mean ± SD) at enrollment | 37.1 ± 13.1 | |
| Ethnicity (%) | Caucasian | 68 |
| Hispanic | 5 | |
| Asian | 8 | |
| Black | 16 | |
| Other | 3 | |
| Region (%) | Canada | 34 |
| US | 26 | |
| Mexico | 3 | |
| Europe | 32 | |
| Asia | 5 | |
| Single/Married/Other (%) | 39/43/18 | |
| Post secondary education (%) | 67 | |
| Disease duration (months) (mean ± SD) at enrollment | 5.9 ± 4.0 | |
| Number of ACR criteria (mean ± SD) | 4.8 ±1.0 | |
| Cumulative ACR manifestations (%) | ||
| Malar rash | 37 | |
| Discoid rash | 9 | |
| Photosensitivity | 43 | |
| Oral/nasopharyngeal ulcers | 42 | |
| Serositis | 26 | |
| Arthritis | 71 | |
| Renal disorder | 24 | |
| Neurological disorder | 9 | |
| Hematologic disorder | 53 | |
| Immunologic disorder | 70 | |
| Antinuclear antibody | 95 | |
| SLEDAI score (mean ± SD) at enrollment | 5.1 ± 5.4 | |
| SLICC/ACR score (mean ± SD) at enrollment | 0.31 ± 0.70 | |
| Medications (%) | ||
| Antimalarials | 69 | |
| Immunosuppressive drugs | 34 | |
| Oral corticosteroids | 62 | |
| Pulse IV corticosteroids | 4 | |
| Anticoagulants including warfarin, ASA | 25 | |
| Antidepressants | 19 | |
| Antiseizure drugs | 9 | |
| Other psychoactive drugs | 7 | |
At enrollment the mean disease duration was 5.9 ± 4.0 months. The prevalence of individual ACR classification criteria at baseline reflected an unselected patient population and “neurologic disorder”, which includes seizures and psychosis only, was present in 25 (9%) of the 274 patients. The mean SLEDAI and SDI scores at enrollment were 5.1 ± 5.4 and 0.31 ± 0.70 respectively indicating moderate global disease activity and minimal cumulative organ damage respectively. Therapy at enrollment reflected the typical range of lupus medications. The mean interval between assessments was 12.3 ± 1.94 months.
The characteristics of the patients that did not contribute to the analysis were generally similar to those that did. For example, 88% were female and their age at enrollment was 33.6 ± 13.3 years. Their mean SLEDAI and SDI scores at enrollment were 5.5 ± 5.5 and 0.29 ± 0.75. However some regions, such as Canada for example, were under-represented in the sample that did not contribute to the analysis (Canada 19%; US 30%; Mexico 16%; Europe 23%; Asian 13%) and this has some implications for their ethnicity (Caucasian 42%; Hispanic 20%; Asian 19%; Black 16%; other 4%). All analyses adjust for region and ethnicity effects and hence the main impact of any difference would be to limit the power for testing interaction effects.
Frequency, attribution of NP events
In 274 patients there were 587 pairs of patient visits that met the criteria in order to contribute to the analysis. One hundred and twenty four of these patients contributed a single pair of visits and 150 contributed two or more pairs. Multiple events at the previous study visit were common and, in total, 912 events were included in the analysis, which encompassed 17 of the 19 ACR case definitions (Table 2); Guillain-Barré syndrome and aseptic meningitis did not occur in any patient.
Table 2.
The number of NP events by attribution over the period of study (N=912)
| SLE NP events (model A; more stringent) |
SLE NP events (model B; less stringent ) |
Non-SLE NP events |
Total NP events |
|||||
|---|---|---|---|---|---|---|---|---|
| Event types | # | % | # | % | # | % | # | % |
| Headache | 0 | 0 | 0 | 0 | 437 | 66 | 437 | 48 |
| Mood Disorders | 34 | 24 | 77 | 30 | 105 | 16 | 182 | 20 |
| Seizure Disorders | 12 | 9 | 22 | 9 | 4 | 1 | 26 | 3 |
| Cognitive Dysfunction | 14 | 10 | 37 | 15 | 26 | 4 | 63 | 7 |
| Anxiety Disorder | 0 | 0 | 0 | 0 | 58 | 9 | 58 | 6 |
| Cerebrovascular Disease | 20 | 14 | 31 | 12 | 0 | 0 | 31 | 3 |
| Acute Confusional State | 1 | 1 | 2 | 1 | 4 | 1 | 6 | 1 |
| Polyneuropathy | 9 | 6 | 15 | 6 | 16 | 2 | 31 | 3 |
| Mononeuropathy | 21 | 15 | 31 | 12 | 0 | 0 | 31 | 3 |
| Neuropathy, Cranial | 12 | 9 | 12 | 5 | 4 | 1 | 16 | 2 |
| Psychosis | 1 | 1 | 2 | 1 | 0 | 0 | 2 | <0.5 |
| Myelopathy | 8 | 6 | 14 | 6 | 0 | 0 | 14 | 2 |
| Movement Disorder | 1 | 1 | 2 | 1 | 2 | <0.5 | 4 | <0.5 |
| Aseptic Meningitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Demyelinating Syndrome | 3 | 2 | 5 | 2 | 0 | 0 | 5 | 1 |
| Guillain-Barré Syndrome | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Autonomic Disorder | 2 | 1 | 2 | 1 | 0 | 0 | 2 | <0.5 |
| Plexopathy | 1 | 1 | 1 | 0 | 2 | 0 | 3 | <0.5 |
| Myasthenia Gravis | 0 | 0 | 1 | <0.5 | 0 | 0 | 1 | <0.5 |
| Total (%) NP Events | 139 | 15% | 254 | 28% | 658 | 72% | 912 | 100% |
Attribution Model A: NP events which had their onset within the enrollment window and had no “exclusions” or “associations” and were not one of the NP events identified by Ainiala (18) were attributed to SLE.
Attribution Model B: NP events which had their onset within 10 years of the diagnosis of SLE and were still present within the enrollment window and had no “exclusions” and were not one of the NP events identified by Ainiala (18) were attributed to SLE.
The most frequent events were headache (437 (48%) of 912 events), mood disorders (182 (20%)), cognitive dysfunction (63 (6.9%)), anxiety disorder (58 (6.4%)), cerebrovascular disease (31 (3.4%)), polyneuropathy (31 (3.4%)), mononeuropathy (31 (3.4%) and seizures (26 (2.9%)). The remaining 11 NP syndromes had a prevalence of less than 6% of all NP events.
NP events attributed to SLE using alternate attribution models varied from 15% (model A) to 28% (model B) of the 912 NP events (Table 2). Of these, 91% affected the central nervous system and 9% involved the peripheral nervous system; 83% were diffuse and 17% were focal events. Using attribution models A and B respectively, the most frequent NP events attributed to SLE were mood disorders (24–30%), mononeuropathy (15–12%), cerebrovascular disease (14–12%) and cognitive dysfunction (10–15%).
Physician determined outcome scores for NP events
A summary of NP outcomes at individual patient visits is provided in Table 3.
Table 3.
Outcome of NP events in SLE patients at individual physician generated assessments
| Outcome of NP events$ | ||||||
|---|---|---|---|---|---|---|
| No. of NP Events: |
0 | 1 | 2 | 3 | 4 | Total pairs of patient visits |
| 1 | 130 | 204 | 27 | 0 | 0 | 361 |
| 2 | 35 | 71 | 3 | 38 | 7 | 154 |
| 3 | 19 | 17 | 0 | 15 | 2 | 53 |
| 4 | 4 | 2 | 0 | 5 | 1 | 12 |
| ≥ 5 | 3 | 1 | 0 | 2 | 1 | 7 |
| Total | 191 | 295 | 30 | 60 | 11 | 587 |
Outcomes determined by physician evaluation for each patient between assessments: 0 – no difference (single event) or no consistent change (multiple events) in NP status; 1 – all events improve; 2 - all events worsen; 3 – some but not all events improve and none worsen; 4 – some but not all events worsen and none improve.
Change in SF-36 summary and subscale scores and outcome of NP events
The overall difference in the change in MCS scores in 3 patient groups (all NP events improved, all NP events deteriorated and NP events without a consistent change) reached statistical significance (p<0.0001) following adjustments for gender, ethnicity, research center and previous SF-36 MCS score (Table 4).
Table 4.
The mean (±SEM) difference* in change in SF-36 mental component summary (MCS), physical component summary (PCS) and SF-6D scores between patients visits whose NP events all improve (1) or all worsen (2) compared to patients lacking a consistent change in NP events (0, 3, 4).
| MCS | PCS | SF-6D | |
|---|---|---|---|
| All NP events improve (N=295) (1) | 3.66 ± 0.89 | 1.73 ± 0.71 | 0.035 ±0.009 |
| NP events without consistent change (N=262) (0, 3, 4) | 0 | 0 | 0 |
| All NP events worsen (N=30) (2) | −4.00 ± 1.97 | −0.62 ± 1.58 | −0.029 ± 0.019 |
| Global significance | <0.0001 | 0.035 | <0.0001 |
adjusted for previous SF-36 score and other demographic variables.
Patients whose NP status consistently improved (N=295) had their estimated adjusted mean (SD) MCS score increase by 3.66 (0.89) more than in patients without a consistent change (N=262) over the same interval. In contrast, for patients whose NP status consistently deteriorated (N=30), the MCS score decreased by an estimated adjusted mean of 4.00 (1.96). For the SF-36 physical component summary (PCS) scores the corresponding changes associated with improvement and deterioration in NP status were +1.73 (0.71) and −0.62 (1.58) (p<0.05) respectively. The results are also shown for SF-6D (29) in Table 4, and qualitatively similar results are obtained using this measure as the SF-36 MCS. Sensitivity analyses were conducted by modifying the definition of clinical change in NP status. Expanding the definition of improvement in NP status, by using all 5 categories of change as in table 3, and refitting the models, did not substantially change the estimated effects associated with consistent improvement or worsening (Online supplementary file Table SI).
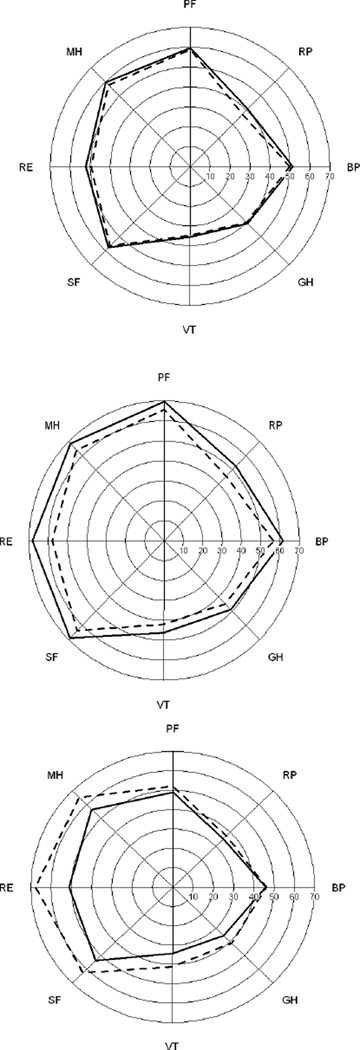
Changes in the eight SF-36 subscales were in the same direction as the summary scores (p<0.05; with the exception of role physical)) (Table 5). Unadjusted mean SF-36 subscale scores for the patient/visits for each of the three groups in Table 4 are shown in Figure 1 using a ‘spydergram’ (19).
Table 5.
The mean (±SEM) difference* in change in SF-36 subscale scores between patient visits whose NP events all improve (1) or all worsen (2) compared to patients lacking a consistent change in NP events (0, 3, 4).
| Role Emotion |
Social Function |
Vitality | Mental Health | |
|---|---|---|---|---|
| (1) | 10.58 ± 3.46 | 7.50 ± 1.92 | 5.77 ± 1.56 | 6.52 ± 1.45 |
| (0, 3, 4) | 0 | 0 | 0 | 0 |
| (2) | −11.06 ± 7.67 | −5.67 ± 4.24 | −4.53 ± 3.43 | −6.68 ± 3.19 |
| Global | ||||
| Significance | 0.0010 | 0.0001 | 0.0001 | <0.0001 |
|
Bodily Pain |
General Health |
Physical Function |
Role Physical |
|
| (1) | 6.17 ± 1.75 | 4.88 ± 1.29 | 5.34 ± 1.54 | 6.77 ± 3.28 |
| (0, 3, 4) | 0 | 0 | 0 | 0 |
| (2) | −3.10 ± 3.90 | −6.18 ± 2.90 | −6.71 ± 3.46 | −3.69 ± 7.28 |
| Global | ||||
| Significance | 0.0006 | <0.0001 | 0.0001 | 0.075 |
adjusted for previous SF-36 score and other demographic variables.
Figure 1.
Unadjusted mean SF-36 subscale scores in patient/visits in three groups: patients lacking a consistent change in NP events (top panel; Outcomes 0, 3 and 4; N=262); patients whose NP events all improve (middle panel; Outcome 1; N=295); patient whose NP events all worsen (bottom panel; Outcome 2; N=30). Dashed lines denote subscale scores from the first visit of the pair and solid lines denote the second set of scores. The SF-36 subscales are BP = Bodily pain, RP = Role physical, PF = Physical function, MH = Mental health, RE = Role emotion, SF = Social function, VT = Vitality, GH= General health.
Additional variables at the first of the two visits were considered for adjustment: age, education, medications, global SLE disease activity, cumulative organ damage, attribution of NP events, diffuse/focal classification of NP events and disease duration. Since headache was such a common event, whether or not it was present was also investigated as a potential variable for adjustment. In each instance the additional variables were entered into the statistical model both with and without an interaction with change in NP status. Again, no substantial changes were observed.
For the MCS, the only notable predictor was SLEDAI (non-NP score estimated without the NP variables) where there was evidence of an interaction with change in NP status (p<0.01, 2df test). The dominant interaction with the change in SF-36 score occurred in patients whose NP status deteriorated compared to patients without a consistent change. The mean change in SF-36 MCS was estimated to decrease by approximately 2 points for each 1 unit increase in SLEDAI. Since the standard deviation of SLEDAI (non-NP) across the entire sample of patient visits is 4.4, this could translate into an important quantitative effect. However, there were only 30 patients who had a consistent deterioration in NP status between their two assessments (table 2) and hence this estimate is uncertain, with an approximate 95% confidence interval of (0.7, 3.7) and should be interpreted with some caution. The comparable (non-significant) estimated effect of SLEDAI (non-NP) on the mean difference in SF-36 MCS score between patients with improved NP status and those with no consistent change was estimated to be a decrease of 0.3 per unit change in SLEDAI (non-NP) with a confidence interval of (–0.1,0.7).
For PCS, the only strong additional potential predictor was the patients’ age (p<0.0001). A strong and negative association between age and change in PCS score was observed as one might expect but this did not affect estimates linked to the change in NP status.
Discussion
Different outcome measures have been used in the assessment of patients treated for NPSLE. These include clinical assessment (20, 21), neuropsychological evaluation of cognition (22, 23) and neuroimaging (21, 24). Although NP events in SLE patients are known to impact negatively on HRQoL (3, 7, 8, 11), the use of standardized measures of HRQoL have not been validated in the assessment of change in NP status in SLE. We have examined the change in SF-36 summary and subscale scores in SLE patients who have had a clinically significant change in NP status over 1 year. In essence we examined the face validity of SF-36 scores for measuring the clinical outcome of NP events. The results indicate that SF-36 scores, in particular those which reflect self-report mental health, change in the appropriate direction in association with both clinical improvement and deterioration in a variety of NP events.
The study was conducted within a large, international, inception cohort of SLE patients. The characteristics of the entire cohort have been published elsewhere (7, 8) and are similar to the subset of patients who were eligible for the current analysis. In contrast to many previous studies of NPSLE, ours was prospective with the specific objective of identifying the characteristics, attribution and outcome of NP events using a predefined annual data collection protocol. The multi-center, international study design provides a basis for assuming that our findings are applicable to the broader community of SLE patients.
Predefined outcomes in studies of therapeutic interventions for NPSLE have been variable and largely determined by the specific NP event under study. Thus, in the evaluation of interventions to improve cognitive performance (22, 23) sequential neuropsychological assessment was the primary outcome of brain health. The efficacy of treating neurological manifestations of NPSLE have been determined by sequential neuroimaging alone (24, 25) or used in combination with clinical variables to provide a composite outcome (26, 27). To our knowledge none of the previous approaches have been validated. Given the diversity of NP events in SLE and the lack of clinical trial evidence to support specific interventions, there is a need to determine optimal outcome measures.
Rather than developing a measure for change in a specific NP manifestation, we wished to validate a change in patient self-report HRQoL as a generic measure for improvement and deterioration in NP status over time. This was a logical step following upon the previous work by ourselves and others showing that NP events have been associated with lower SF-36 scores in both cross-sectional and longitudinal studies of SLE patients (3, 7, 8, 11, 28). Physician determined change in NP status was significantly correlated with change in patient self-report SF-36 scores. Not surprisingly the magnitude of change in SF-36 scores was greater for the mental component summary score than the physical score. Although the SF-36 may be affected by other variables such as global disease activity, cumulative organ damage and concurrent medications, adjustment for these and other variables in the analysis did not negate the association with NP events with the possible exception of SLE disease activity outside of the nervous system in patients who had deterioration in NP status. It was also apparent that attribution of NP events to SLE and non-SLE causes did not change the results, indicating that this is a valid outcome for all NP events in SLE patients, even though the majority of events are not attributed to SLE.
Previous studies have indicated that in SLE patients the minimum clinically important difference (MCID) for SF-36 MCS and PCS summary scores is 2.5 – 5.0 and for subscale scores is 5.0–10.0 (29, 30). The adjusted differences in SF-36 scores in our analysis, which would be expected to be less than unadjusted changes, fall within the MCID for the MCS score and most of the subscale scores of the SF-36. Furthermore the estimated standard error associated with a single observation of a change in SF-36 summary scores between visits, with adjustment for gender, ethnicity, institution and previous score, is approximately 10. Therefore if a clinical trial was designed to detect a difference in mean change of 3.66 (the mean difference in MCS scores between patients whose NP events all improve compared to patients without a consistent change in NP events) between two equal sized treatment groups, the sample size to achieve 80% power when testing at the 5% level would be approximately 120 patients per group. If no adjustment for previous score was planned, then approximately 155 patients per group would be required.
There are limitations to the current analysis. First, some NP events were either absent or underrepresented which emphasizes the relatively low frequency of some NP manifestations. In previous studies (2–5, 18) approximately half of the 19 NP syndromes in the ACR case definitions occurred in only 1–2% of SLE patients. Second, patients with severe or very active NP disease may have been unable to complete the SF-36 at the time when NP disease was at it worst. In such cases, the magnitude of change in scores would likely have been even higher that what was found. Third, some psychosocial variables such as fibromyalgia, helplessness and low socioeconomic status were not available in our dataset. Fourth, the physician assessment of some NP events (e.g. headache and mood disorders) is dependent in part on patients’ subjective complaints which are potential confounders in the interpretation of parallel changes in SF-36 scores. However, for those NP events (e.g. cerebovascular disease, myelopathy and neuropathies) with more objective outcomes, there were comparable changes in SF-36 scores. Finally, the small number of patients (30) with outcome 2 (all worsen) for NP events, which may have been due to the lengthy interval between assessments that provided ample time for treatment, limits the gereralizability of the findings for this group.
Despite these limitations the results of our study indicate that changes in SF-36 summary and subscale scores, in particular those related to mental health, are strongly associated with the clinical outcome of NP events in SLE patients. Thus, changes in SF-36 should be an outcome measure in any clinical trial or study which examines the efficacy of therapeutic interventions for NP disease in SLE patients.
Supplementary Material
Acknowledgements
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non-exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in Annals of the Rheumatic Diseases and any other BMJPGL products to exploit all subsidiary rights, as set out in our licence (http://group.bmj.com/products/journals/instructions-for-authors/licence-forms).
Financial support:
J.G. Hanly (Canadian Institutes of Health Research grant MOP-57752, Capital Health Research Fund), M.B. Urowitz (Canadian Institutes of Health Research grant MOP-49529, Lupus Foundation of Ontario, Ontario Lupus Association, Lupus UK, Lupus Foundation of America, Lupus Alliance Western New York, Conn Smythe Foundation, Tolfo Family (Toronto), D. Jackson (MRC(UK) grant U.1052.00.006.00001.01), S.C. Bae (Korea Healthcare technology R & D project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A080588), C. Gordon (Lupus UK, arthritis research campaign, Wellcome Trust Clinical Research Facility in Birmingham, UK), A. Clarke (Fonds de la recherche en sante de Quebec National Scholar, Singer Family Fund for Lupus Research), S. Bernatsky (Canadian Institutes of Health Research Junior Investigator Award; Fonds de la recherche en santé du Québéc Jeune Chercheure; Canadian Arthritis Network Scholar Award; McGill University Health Centre Research Institute), G.S Alarcón (University of Alabama at Birmingham, grant P60AR48095), D.D.Gladman (Canadian Institutes of Health Research), P.R. Fortin (Distinguished Senior Research Investigator of the Arthritis Society and Arthritis Centre of Excellence), IN Bruce (supported by the Manchester Academic Health Sciences Centre and the Manchester NIHR Biomedical Research Centre), M. Petri (Hopkins Lupus Cohort grant AR 43727, Johns Hopkins University General Clinical Research Center grant MO1 RR00052), S Manzi (National Institutes of Health research grants R01 AR46588, K24 AR002213 and M01 RR000056, O. Nived (Swedish Medical Research council grant 13489), G. Sturfelt (Swedish Medical Research council grant 13489), R. Ramsey-Goldman (National Institutes of Health research grants UL1RR025741; K24 AR02318; P60 AR 48098), V. Farewell (MRC(UK) grant U.1052.00.009.00001.01).
References
- 1.Ainiala H, Loukkola J, Peltola J, et al. The prevalence of neuropsychiatric syndromes in systemic lupus erythematosus. Neurology. 2001;57(3):496–500. doi: 10.1212/wnl.57.3.496. [DOI] [PubMed] [Google Scholar]
- 2.Brey RL, Holliday SL, Saklad AR, et al. Neuropsychiatric syndromes in lupus: prevalence using standardized definitions. Neurology. 2002;58(8):1214–1220. doi: 10.1212/wnl.58.8.1214. [DOI] [PubMed] [Google Scholar]
- 3.Hanly JG, McCurdy G, Fougere L, et al. Neuropsychiatric events in systemic lupus erythematosus: attribution and clinical significance. J Rheumatol. 2004;31(11):2156–2162. [PubMed] [Google Scholar]
- 4.Sanna G, Bertolaccini ML, Cuadrado MJ, et al. Neuropsychiatric manifestations in systemic lupus erythematosus: prevalence and association with antiphospholipid antibodies. J Rheumatol. 2003;30(5):985–992. [PubMed] [Google Scholar]
- 5.Sibbitt WL, Jr, Brandt JR, Johnson CR, et al. The incidence and prevalence of neuropsychiatric syndromes in pediatric onset systemic lupus erythematosus. J Rheumatol. 2002;29(7):1536–1542. [PubMed] [Google Scholar]
- 6.The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42(4):599–608. doi: 10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 7.Hanly JG, Su L, Farewell V, et al. Prospective study of neuropsychiatric events in systemic lupus erythematosus. J Rheumatol. 2009;36(7):1449–1459. doi: 10.3899/jrheum.081133. [DOI] [PubMed] [Google Scholar]
- 8.Hanly JG, Urowitz MB, Sanchez-Guerrero J, et al. Neuropsychiatric events at the time of diagnosis of systemic lupus erythematosus: an international inception cohort study. Arthritis Rheum. 2007;56(1):265–273. doi: 10.1002/art.22305. [DOI] [PubMed] [Google Scholar]
- 9.Hanly JG, Urowitz MB, Siannis F, et al. Autoantibodies and neuropsychiatric events at the time of systemic lupus erythematosus diagnosis: results from an international inception cohort study. Arthritis Rheum. 2008;58(3):843–853. doi: 10.1002/art.23218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanly JG, Urowitz MB, Su L, et al. Short-term outcome of neuropsychiatric events in systemic lupus erythematosus upon enrollment into an international inception cohort study. Arthritis Rheum. 2008;59(5):721–729. doi: 10.1002/art.23566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanly JG, Urowitz MB, Su L, et al. Prospective analysis of neuropsychiatric events in an international disease inception cohort of SLE patients. Ann Rheum Dis. 2009 doi: 10.1136/ard.2008.106351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isenberg D, Ramsey-Goldman R. Systemic Lupus International Collaborating Group--onwards and upwards? Lupus. 2006;15(9):606–607. doi: 10.1177/0961203306071868. [DOI] [PubMed] [Google Scholar]
- 13.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 14.Bombardier C, Gladman DD, Urowitz MB, et al. Derivation of the SLEDAI. A disease activity index for lupus patients The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35(6):630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 15.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39(3):363–369. doi: 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- 16.Thumboo J, Fong KY, Ng TP, et al. Validation of the MOS SF-36 for quality of life assessment of patients with systemic lupus erythematosus in Singapore. J Rheumatol. 1999;26(1):97–102. [PubMed] [Google Scholar]
- 17.Brazier J, Usherwood T, Harper R, et al. Deriving a preference-based single index from the UK SF-36 Health Survey. J Clin Epidemiol. 1998;51(11):1115–1128. doi: 10.1016/s0895-4356(98)00103-6. [DOI] [PubMed] [Google Scholar]
- 18.Ainiala H, Hietaharju A, Loukkola J, et al. Validity of the new American College of Rheumatology criteria for neuropsychiatric lupus syndromes: a population-based evaluation. Arthritis Rheum. 2001;45(5):419–423. doi: 10.1002/1529-0131(200110)45:5<419::aid-art360>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 19.Strand V, Crawford B, Singh J, et al. Use of "spydergrams" to present and interpret SF-36 health-related quality of life data across rheumatic diseases. Ann Rheum Dis. 2009;68(12):1800–1804. doi: 10.1136/ard.2009.115550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mok CC, Lau CS, Wong RW. Treatment of lupus psychosis with oral cyclophosphamide followed by azathioprine maintenance: an open-label study. Am J Med. 2003;115(1):59–62. doi: 10.1016/s0002-9343(03)00135-9. [DOI] [PubMed] [Google Scholar]
- 21.Tokunaga M, Saito K, Kawabata D, et al. Efficacy of rituximab (anti-CD20) for refractory systemic lupus erythematosus involving the central nervous system. Ann Rheum Dis. 2007;66(4):470–475. doi: 10.1136/ard.2006.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denburg SD, Carbotte RM, Denburg JA. Corticosteroids and neuropsychological functioning in patients with systemic lupus erythematosus. Arthritis Rheum. 1994;37(9):1311–1320. doi: 10.1002/art.1780370907. [DOI] [PubMed] [Google Scholar]
- 23.Harrison MJ, Morris KA, Horton R, et al. Results of intervention for lupus patients with self-perceived cognitive difficulties. Neurology. 2005;65(8):1325–1327. doi: 10.1212/01.wnl.0000180938.69146.5e. [DOI] [PubMed] [Google Scholar]
- 24.Steens SC, Bosma GP, ten Cate R, et al. A neuroimaging follow up study of a patient with juvenile central nervous system systemic lupus erythematosus. Ann Rheum Dis. 2003;62(6):583–586. doi: 10.1136/ard.62.6.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leung FK, Fortin PR. Intravenous cyclophosphamide and high dose corticosteroids improve MRI lesions in demyelinating syndrome in systemic lupus erythematosus. J Rheumatol. 2003;30(8):1871–1873. [PubMed] [Google Scholar]
- 26.Barile-Fabris L, Ariza-Andraca R, Olguin-Ortega L, et al. Controlled clinical trial of IV cyclophosphamide versus IV methylprednisolone in severe neurological manifestations in systemic lupus erythematosus. Ann Rheum Dis. 2005;64(4):620–625. doi: 10.1136/ard.2004.025528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neuwelt CM, Lacks S, Kaye BR, et al. Role of intravenous cyclophosphamide in the treatment of severe neuropsychiatric systemic lupus erythematosus. Am J Med. 1995;98(1):32–41. doi: 10.1016/S0002-9343(99)80078-3. [DOI] [PubMed] [Google Scholar]
- 28.Tam LS, Wong A, Mok VC, et al. The relationship between neuropsychiatric, clinical, and laboratory variables and quality of life of Chinese patients with systemic lupus erythematosus. J Rheumatol. 2008;35(6):1038–1045. [PubMed] [Google Scholar]
- 29.Thumboo J, Fong KY, Chan SP, et al. A prospective study of factors affecting quality of life in systemic lupus erythematosus. J Rheumatol. 2000;27(6):1414–1420. [PubMed] [Google Scholar]
- 30.Strand V, Crawford B. Improvement in health-related quality of life in patients with SLE following sustained reductions in anti-dsDNA antibodies. Expert Rev Pharmacoecon Outcomes Res. 2005;5(3):317–326. doi: 10.1586/14737167.5.3.317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.