Abstract
Skin, the largest, most exposed organ of the body, provides a protective interface between humans and the environment. One of its primary roles is protection against exposure to sunlight, a major source of skin damage where the UV radiation (UVR) component functions as a complete carcinogen. Melanin pigmentation and the evolution of dark skin is an adaptive protective mechanism against high levels of UVR exposure. Recently, the hypothesis that skin pigmentation balances folate preservation and Vitamin D production has emerged. Both micronutrients are essential for reproductive success. Photodegradation of bioactive folates suggests a mechanism for the increased tendency of populations of low melanin pigmentation residing in areas of high UV exposure to develop skin cancers. Folate is proposed as a cancer prevention target for its role in providing precursors for DNA repair and replication, as well as its ability to promote genomic integrity through the generation of methyl groups needed for control of gene expression. The cancer prevention potential of folate has been demonstrated by large-scale epidemiological and nutritional studies indicating that decreased folate status increases the risk of developing certain cancers. While folate deficiency has been extensively documented by analysis of human plasma, folate status within skin has not been widely investigated. Nevertheless, inefficient delivery of micronutrients to skin and photolysis of folate argue that documented folate deficiencies will be present if not exacerbated in skin. Our studies indicate a critical role for folate in skin and the potential to protect sun exposed skin by effective topical delivery as a strategy for cancer prevention.
Keywords: Cancer prevention, DNA repair, Folate, Folic acid, Skin, Topical delivery strategy, UV light
10.1 Human Skin and UV Radiation
The skin provides a direct interface for the human body with the environment and plays many roles in protection against physical, chemical, and microbial insults. The average human skin area exceeds m2 and is generally less than 2 mm thick (Goldsmith 1991). Skin protects against most solar UV radiation (UVR), regulates body temperature through surface blood flow control and sweating, allows detection of the ambient and physical environments through feel and touch, and participates in social communication through the display of information such as age, health, and ancestry (Jablonski 2004). Human skin is a complex laminar structure comprised of many different cell types organized into the outer epidermal layer and an inner dermal layer. The epidermis provides the barrier properties of the skin and is composed of keratinocytes (structure), melanocytes (pigmentation), Langerhans cells (immune system), and Merkel cells (sense of touch). Keratinocytes are the primary cell type in the epidermis and originate in the stratum basale layer from the continual division of stem cells. They migrate outward through the epidermis while undergoing differentiation until they reach the stratum corneum where they form a layer of dead, flattened, highly keratinized cells called squamous cells. The epidermis is avascular and contributes to the elasticity and toughness of the skin. At the stratum corneum, keratinocytes are continuously shed and replaced (Goldsmith 1991) generating significant metabolic demands for energy and nutrient consumption. The dermis is a dense fibroelastic connective tissue composed of collagen fibers, elastic fibers, and an interfibrillar gel which comprises most of the skin thickness. Fibroblasts are the primary cell type in the dermis and are collagen-rich. Collagen is a major skin component and accounts for the tensile strength of the skin. Interwoven with the collagen is a network of elastic fibers. The dermis appears to be of equal thickness in people with dark or light pigmentation (Taylor 2002).
10.2 UVR, DNA Damage, and Skin Cancer
Skin damage, particularly that derived from sunlight, constitutes a major public health problem. Non-melanoma skin cancers (NMSC) are the most frequent malignancies in the United States with more than 1,000,000 cases diagnosed annually (Karagas et al. 1999). Melanoma skin cancer is the most rapidly increasing type of cancer. Additionally, actinic keratosis (AK), skin lesions that can progress to NMSC are far more prevalent than skin cancers. DNA damage and cellular responses to DNA damage play a central role in skin damage (Ames 2001). Sunlight is the major source of skin damage as it leads to DNA damage directly via formation of pyrimidine dimers and other photoproducts (Ullrich 2002) and indirectly via generation of reactive oxygen species (ROS) and reactive carbonyl species (RCS) by photooxidation and photosensitization reactions (Wondrak et al. 2002a, b) (Wondrak et al. 2003). UVR is a complete carcinogen. UVB (280–320 nm) which penetrates into the epidermis, is responsible for most of the direct DNA damage and is the most 10 Folate in Skin Cancer Prevention 183 effective at initiation of squamous cell carcinoma (SCC). UVA (320–400 nm) which penetrates into the dermis, induces ROS and causes SCC (Pentland et al. 1999; Agar et al. 2004).
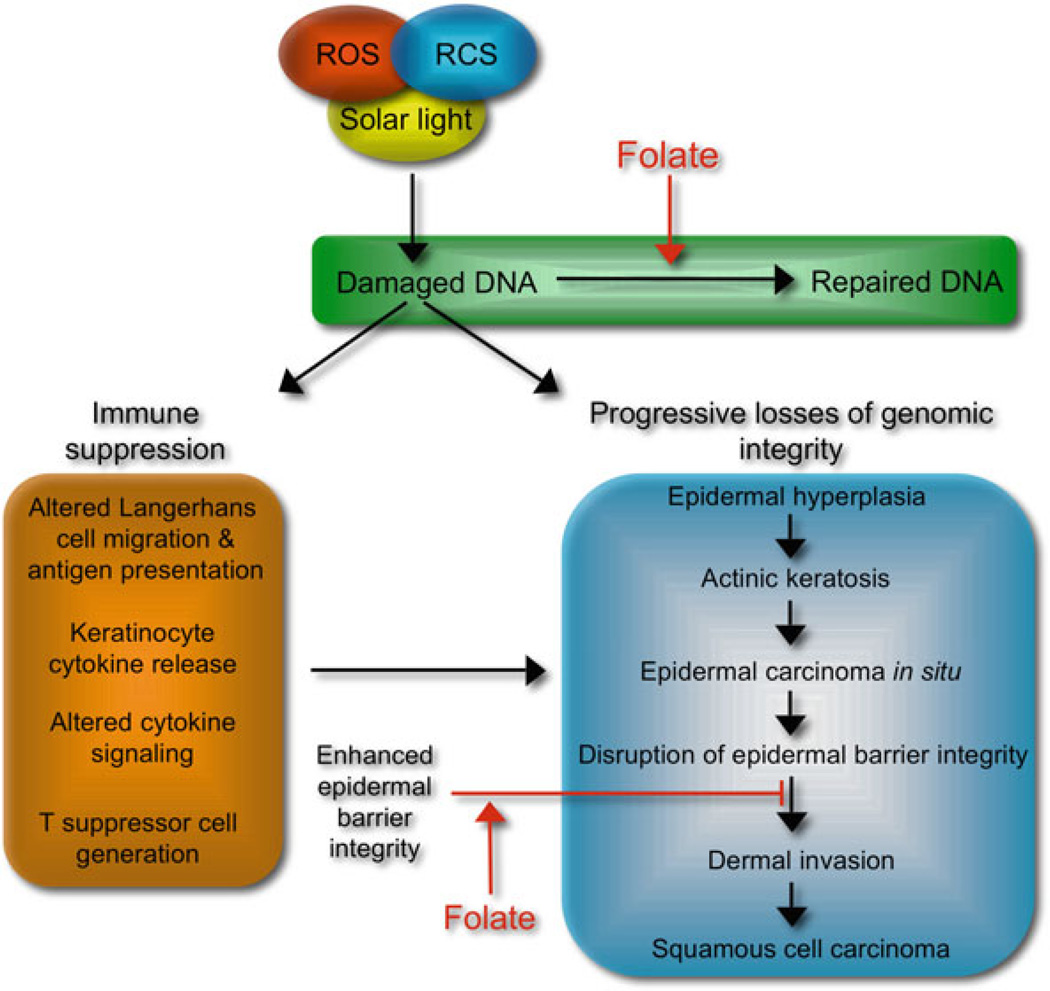
Chronic DNA damage results in progressive losses of genomic integrity and end stage skin damage in the form of skin cancer involving altered growth properties of keratinocytes such as unresponsiveness to terminal differentiation signals leading to epidermal hyperplasia and progressively to actinic keratosis (Jeffes and Tang 2000; Lober et al. 2000). Cell populations present in actinic keratosis lesions progress to transformed cell populations that represent epidermal carcinoma in situ (Guenthner et al., 1999; Hurwitz and Monger 1995; Kobayashi et al., 2000). Subsequent cellular changes occur including induction of matrix proteases that facilitate disruption of the integrity of the epidermal barrier leading to invasion of the dermis, the point at which the damage process is diagnosed as SCC. A second major consequence of DNA damage in skin is the suppression of immune responses that would normally detect and remove damaged cells. The consequences of sunlight-induced DNA damage are depicted in Fig. 10.1. While mechanisms of immune suppression extend beyond DNA damage, DNA damage is a major factor. The consequences of genotoxic stress include altered migration, antigen presentation by Langerhans cells, and stimulation of cytokine release by keratinocytes that likely alters cytokine signaling required for normal immune surveillance including generation of T suppressor cells. Given the complexity of damage pathways and the down stream effects, opportunities to modulate the consequences of genotoxic stress include preventing DNA damage, enhancing DNA repair, and strengthening the integrity of the epidermal barrier to prevent migration of transformed cells from the epidermis (depicted in Fig. 10.1). A compelling body of evidence now indicates that several key micronutrients are candidates for skin damage prevention. This chapter will focus on the role of folate in skin health.
Fig. 10.1.
Sunlight-induced DNA damage pathways and opportunities for micronutrient modulation
10.3 Folate Metabolism
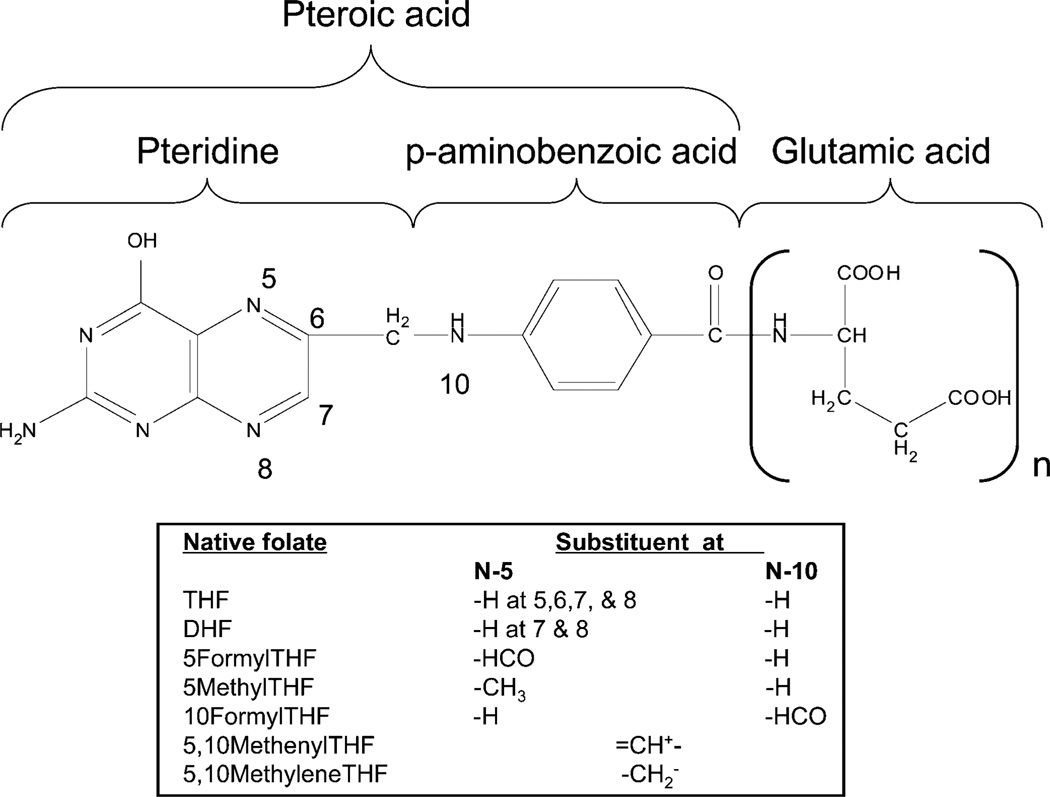
In 1931, it was demonstrated by Lucy Wills that yeast extract was effective in treating tropical macrocytic anemia observed during late pregnancy (Wills 1978). This observation led to the isolation and structural determination of the B vitamin folate, named after the Latin word – folium (leaf). The terms folate and B9 vitamins refer to a large family of chemically similar compounds, the most widely known of which is folic acid. Folic acid or pteroylmonoglutamate (PteGlu), is a synthetic folate analog utilized preferentially due to its enhanced chemical stability and rapid conversion to the bioactive forms upon uptake. Natural folates vary in the one-carbon substituent at the N10 and N5 positions as well as in the number of glutamic acid residues conjugated via gamma glutamyl bonds to form the polyglutamate tail. Natural folates also vary in the oxidation state of the pteridine ring, with the reduced, unsubstituted dihydrofolate (DHF, H2PteGlun) and tetrahydrofolate (THF, H4PteGlun) forms being particularly prone to biological inactivation due to cleavage of the C-9 and N-10 bond (Blakley 1969). The structure of folic acid and its reduced, native forms are illustrated in Fig. 10.2.
Fig. 10.2.
The chemical structure of folic acid and its various derivatives
Humans do not synthesize folate, and thus are dependant upon a variety of dietary sources. The overall folate nutritional status of the population is believed to have been positively influenced since the implementation of the United States federal government’s requirement to fortify cereal-grain products in 1998 (Dietrich et al. 2005); however, this effect may be waning due to current low carbohydrate diet fads. Natural folates are a mixture of reduced folate polyglutamates which vary in the number of glutamic acid residues. The main folate found in foods is 5methyltetrahydrofolate (5-methyl-H4PteGlun, 5MTHF) and the monoglutamyl form is the primary form of folate which enters the circulating plasma (Thien et al. 1977; Herbert et al. 1962).
The transport and metabolism of the synthetic folic acid and it’s active folate metabolites have been extensively characterized. Figure 10.3 depicts an overview of folate metabolism which along with the role of folate in disease processes is expertly reviewed in (Lucock 2000).
Fig. 10.3.
Overview of folate metabolism
In the cell, folates serve to carry and transfer one-carbon units of various oxidation levels during biosynthetic reactions that occur within the cell. Of particular importance is the role that folates play in purine and pyrimidine nucleotide biosynthesis to supply precursors for DNA repair and synthesis. Also of importance is the role that 5MTHF plays in the homocysteine (Hcy) remethylation cycle and de novo methionine biosynthesis. De novo synthesized methionine can then be activated by ATP and the enzyme methionine adenosyltransferase to yield the methyl donor Sadenosylmethionine (SAM). SAM serves as the methyl donor for the majority of methyltransferases that modify DNA, RNA, histones and other proteins, contributing to replicational, transcriptional and translational fidelity, mismatch repair, chromatin remodeling, epigenetic modifications and imprinting, which are all topics of great interest and importance in cancer research and aging.
10.4 UVR at the Earth’s Surface and Photodegradation of Folates
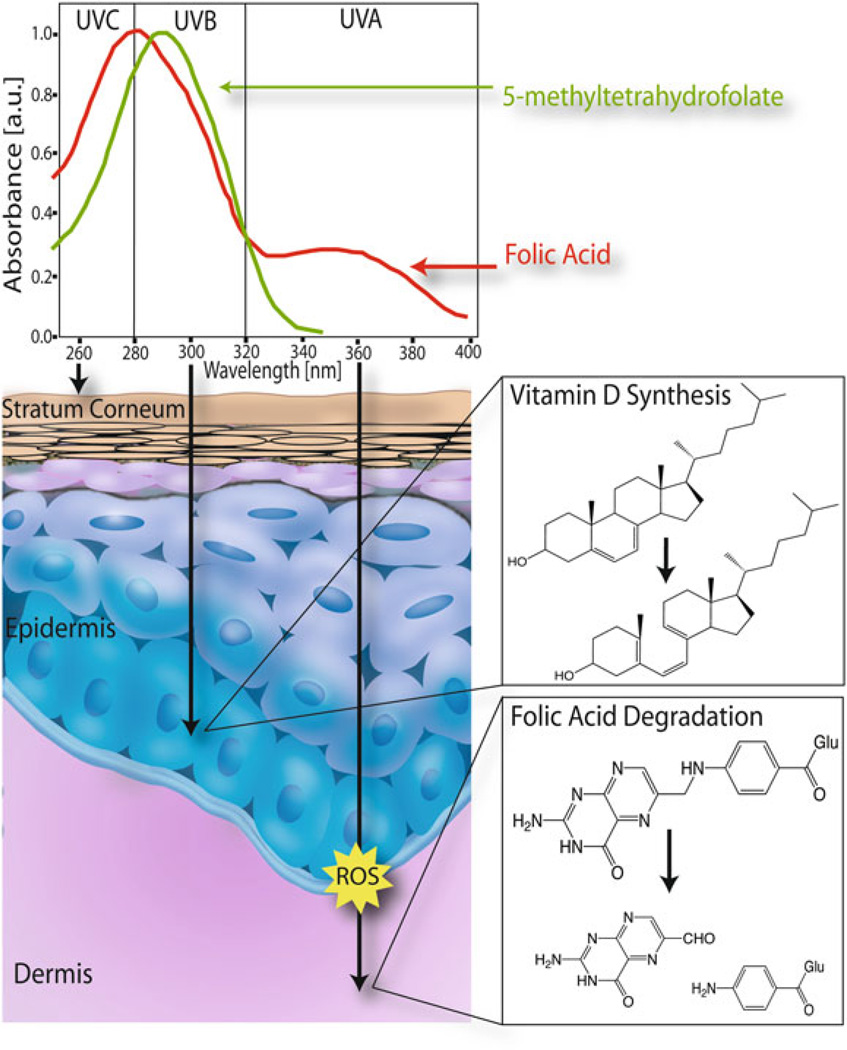
UVR levels reaching the Earth’s surface are affected by numerous factors such as latitude, altitude, season, moisture content, cloud cover, and the depth of the ozone column (Madronich et al. 1998). The shortest UV wavelengths (UV-C, 100–280 nm) are essentially completely blocked or absorbed by atmospheric oxygen (O2) and ozone (O3). Wavelengths in the UV-B range (280–320 nm) are partially absorbed by O3 and thus reach the Earth’s surface while UV-A wavelengths (320–400 nm) are only weakly absorbed by O3 and are most easily transmitted through the atmosphere. At latitudes further away from the Equator, the angle of solar elevation decreases and the thickness of the atmosphere and ozone layer through which sunlight must pass before reaching the Earth’s surface increases which results in attenuation of UVR levels. It is now well established that folic acid is photosensitive. Folic acid has absorption peaks at 280 and 350 nm with shoulders at 300 and 370 nm. The absorption peak at 350 nm indicates that folic acid absorbs UVA radiation which is very prevalent at the Earth’s surface. Folic acid solutions exposed to biologically relevant levels of UVA radiation demonstrated that UVR exposure results in the cleavage of folic acid to form p-aminiobenzoyl-L-glutamic acid and 6-forml pterin (Off et al. 2005). Bioactive folate derivatives are also UV labile. The most common bioactive folate, 5MTHF, has an absorption maximum at 290 nm. Exposure of 5MTHF to UVB at 312 nm results in oxidation to 5-methyldihydrofolate (5MDHF), which upon continued irradiation is further broken down to a pterin residue and paminobenzoylglutamate (Lucock et al. 2003). While the breakdown of 5MTHF results in irreversible loss of vitamin activity by cleavage of the C9-N10 bond, even oxidation to 5MDHF may decrease vitamin activity. UVB does not penetrate deeply into the skin as depicted in Fig. 10.4, and therefore, does not reach the blood stream. Thus, direct photodegradation of folates may not be the mechanism of biological significance. However, the oxidation of 5MTHF to 5MDHF is stimulated by exposure to light in the presence of photosensitizers (Steindal et al. 2006). This implicates ROS in folate degradation. ROS are produced in human tissues by UVA radiation (Fig. 10.4) and possibly radiation of even longer wavelengths which would penetrate the skin and blood vessels to a much greater extent than UVB. ROS production by longer wavelength radiation is enhanced in the presence of photosensitizers, and numerous endogenous photosensitizers are present in both the skin and blood.
Fig. 10.4.
Effects of UV radiation on lightly pigmented skin
10.5 Variations in Human Skin Pigmentation
It has been long recognized that skin colors among indigenous populations show a direct relationship to the intensity of the local UV spectrum. Recently, Jablonski and Chaplin have dramatically advanced the study of the proposed relationships by correlation and regression analyses of quantitative skin color measurements obtained by skin reflectance spectrophotometry with newly available remotely sensed data on levels of UVR, total solar radiation, temperature, humidity, precipitation, and other environmental variables at the Earth’s surface (Jablonski 2004). The strongest association to skin color was found to be latitude, which corresponds to an effect of UVR intensity. A strong correlation was found between latitude and annual average minimal erythemal dose of UVR (UVMED), and thus between annual average UVMED and skin reflectance using UVMED data from the Earth’s surface (Jablonski and Chaplin 2000). A subsequent study of the influence of minimum, maximum, and seasonal levels of UVR showed that skin reflectance was correlated with autumn levels of UVMED, and that skin reflectance could be almost fully modeled as a linear effect of this variable alone (Jablonski 2004). Dark pigmentation was found to be primarily a function of UVMED (Jablonski and Chaplin 2000), with regression analysis demonstrating that autumn UVMED levels have the strongest effect. Interestingly, their data indicated that skin color is more strongly correlated with UVA, which is consistently higher throughout the year at all latitudes, than with UVB (Jablonski 2004).
10.6 The Evolution of Skin Pigmentation and the Micronutrient Hypothesis
The evolution of human skin color has invited many possible explanations. Enhanced resistance to sunburn is clearly a major hypothesis. Devastating consequences for albinos in topical regions of intense UV light exposure including early fatalities from both SCC and acute injury from solar exposure leading to denudation and resultant infections directly support this reasoning (Cohn 1998). The benefits of lightly pigmented skin have been related to the synthesis of vitamin D. It is estimated that most humans utilize sunlight exposure to obtain nearly 100% of their vitamin D which is optimally stimulated by exposure to UVB photons with wavelengths of 295,300 nm (Holick 2003; Holick et al. 1981). The current hypothesis states that light skin pigmentation is necessary in regions of low UVR in order to permit vitamin D biosynthesis (Loomis 1967) and is an adaptation to resist cold injury (Post et al. 1975). Clearly, melanin pigmentation is an adaptation to some attribute of the physical environment. The genetic characteristic of skin pigmentation is believed to be governed by at least three to five loci which would require continued positive selection for its maintenance. This points to a sustained evolutionary pressure which acts to favor retention of pigmentation characteristics (Cohn 1998). Adaptive explanations for a given phenotypic trait require demonstration that the trait increases the real or probable reproductive success of the organism. Adaptive explanations such as resistance to skin cancer or protection from cold injuries attributed to different levels of melanin pigmentation in human skin have suffered from an inability to demonstrate probable or real differences in survivorship and reproduction of different skin color phenotypes (Jablonski 2004). The original hypothesis that dark skin pigmentation arose to protect against skin cancer induced by UV exposure does not account for selective reproductive pressure as fatal repercussions of skin cancers tend to develop after reproductive age. In this reasoning, the adaptive response of skin pigmentation toward the adequate protection or production of micronutrients which play key roles in the success of the reproductive process has gained much credibility. The evolution of dark skin to protect against folate photo-degradation serves a direct reproductive influence as folate is now known to be essential for fetal development and fertility. The attenuation of skin pigment levels to allow for adequate vitamin D production also serves a direct reproductive influence in fetal bone development and maternal bone health. This model in which skin pigmentation balances availability of essential micronutrients is of much interest as shifting world populations result in many people residing in areas of UV exposure that are significantly different from those to which their skin tone has been adapted.
10.7 Melanin and UV Penetrance
Melanocytes in the epidermis produce the skin’s primary pigment, melanin. In mammals there are two types of melanin pigments, eumelanin which is black-brown and pheomelanin which is yellow-reddish (Thody et al. 1991). Melanins are produced in highly organized elliptic membrane bound cytoplasmic organelles of melanocytes called melanosomes. Melanocytes project their dendrites into the neighboring keratinocytes where they then transfer mature melanosomes. Once transferred to the keratinocytes, melanosomes aggregate and are surrounded by a membrane in a melanosome complex (Szabo et al. 1969). The variation in skin color among various races is determined mainly by the number, melanin content, and distribution of melanosomes within the keratinocytes (Jimbow et al. 1976). Natural selection has produced a gradient of skin pigmentations in response to two opposing factors. The first factor results in a cline of photoprotection that grades from darkly pigmented skin at the Equator to lightly pigmented skin near the Poles. The second factor results in a cline of vitamin D photosynthesis that grades from lightly pigmented near the Poles to darkly pigmented at the Equator. In the middle of the two clines we find peoples with enhanced abilities to develop facultative pigmentation according to seasonal UVR levels (Jablonski 2004). The concentration, depth, and distribution of skin melanin is strongly affected by UVR exposure. The tanning response observed in peoples able to develop facultative pigmentation results from distribution of melanosomes throughout the epidermis from the basal layer.Melanin has a broad absorption spectrum stretching from the UV to the near infrared regions. Recently it has been noted that not only the quantity of melanin present but also the depth and distribution of the melanosomes impact the amount of UVR penetrating to the living cells of the epidermis (Nielsen et al. 2006). Within keratinocytes, melanin granules accumulate above the nuclei and act as a sunscreen absorbing harmful UVR before it can reach the nucleus and damage the DNA.
Melanin serves to protect at least in part from mutations that might be caused by UVR exposure (Ohnishi and Mori 1998). In addition to DNA protection, it has also been shown that darkly pigmented skin generates a larger reflectance of wavelengths below 300 nm than lightly pigmented skin due to scattering of light by the spheroid shaped melanosomes (Nielsen et al. 2004). Scattering and absorption of light in this range shows that melanin and melanosomes play an important role as natural sunscreens in moderating UVR related health effects.
10.8 Folate Deficiency
Naturally occurring folates are water soluble, labile compounds that rapidly lose activity in foods over periods of days or weeks, consequently it is estimated that half or even three-quarters of initial folate activity may be lost between harvest and consumption (John Scott 2000). In view of the dietary behavior of many individuals, inadequate folate intake is likely and supplementation of folates is strongly recommended. This usually involves consumption of synthetic folic acid, which is more chemically stable and bioavailable than the natural folates.
10.9 Folate Deficiencies in Skin
Folate deficiency has been extensively documented in human plasma. However, folate status within skin has not been widely investigated. Delivery to the skin via the blood circulation of nutrients taken orally is inherently inefficient since this delivery is distal to other organs, particularly the liver, which removes many agents by first pass metabolism. In addition, the epidermal layer of the skin is non-vascular. The inefficiency of delivery of nutrients to skin argues that documented folate deficiencies will extend to skin. Folate is sensitive to photolysis by exposure to UVR. Exposure to sunlight and UVR in particular is expected to lower folate levels in at least the superficial layers of the skin. Photolysis of folate stores in the skin may be extensive enough to result in systemic depletion of folate. Indeed it has been reported that fair skinned patients undergoing photochemotherapy for dermatological conditions have low serum folate concentrations, suggesting that folate depletion may occur in vivo (Branda and Eaton 1978). However, in another experiment where volunteers were exposed to UVA radiation in a solarium, no connection between UVA exposure and plasma folate status was found (Gambichler et al. 2001). This study has since been criticized for use of non-specific bioassays for folate quantification, which serves to exemplify the point that much research is left to be done (Lucock et al. 2003).
10.10 Folate Deficiencies and Human Disease
Folate deficiencies and genetic folate metabolism alterations have been linked to a wide range of conditions including megaloblastic anemia (Lucock 2000), mood alterations (Godfrey et al. 1990), Alzheimer’s disease (Clarke et al. 1998), and numerous athero/thrombogenic phenomena (Brattstrom and Wilcken 2000). Folate deficiencies have also been shown to play a role in Downs syndrome (Hobbs et al. 2000), neural tube defects (MRC vitamin study research group 1991), pregnancy complications (Rajkovic et al. 1997), and male infertility (Wong et al. 2002), which positions folate as a limiting factor in the evolution of the human species. Finally folate deficiencies have been linked to an array of cancers including those of the colon (Slattery et al. 1999), breast (Zhang et al. 1999), pancreas (Stolzenberg- Solomon et al. 2001), stomach (Fang et al. 1997), cervix (Butterworth 1993), bronchus (Kamei et al. 1993), and blood (Skibola et al. 1999).
10.11 Cancer Prevention by Enhancing Genomic Integrity and Repair
The major putative relationship between cancer and folate status relates to the role of folate in providing precursors for DNA repair and synthesis. Folate may also promote genomic integrity through its role in the generation of methyl groups needed for regulation of gene expression via CpG methylation patterns. The cancer protection potential of folates has been demonstrated by large-scale epidemiological and nutritional studies indicating that decreased folate status increases the risk of developing certain cancers. Consistent with a role in DNA repair, chromosome breaks and centrosome abnormalities have been observed in patients deficient in folate (Heath 1966; Chen et al. 1989). In vitro, DNA strand breakage and uracil misincorporation increased in a time and concentration dependent manner after human lymphocytes were cultured with decreasing amounts of folate (Duthie and Hawdon 1998). Moreover, folate deficiency impaired DNA excision repair in specific tissues excised from animal models (Choi et al. 1998). These data indicate that folic acid deficiency affects the stability of cellular DNA at the chromosomal and molecular levels (Choi and Mason 2000). Folate supplementation, particularity localized to areas of elevated demand, is thus proposed as a strategy to enhance genomic integrity and prevent the development of cancer.
10.12 Cancer Prevention by Enhancing the Epidermal Barrier
A terminally differentiated epidermal barrier with high integrity is crucial to cancer prevention. Several points need to be considered with regard to micronutrients in epidermal barrier development. First, there is a growing body of evidence indicating that a significant percentage of the American population is deficient in a number of micronutrients and the constant turnover of the epidermis makes this tissue particularly vulnerable to micronutrient depletion. Micronutrient deficiencies observed in plasma are observed also in skin (Peng et al. 1993). Second, the constant renewal of the epidermal compartment places an important nutrient requirement on the organism. Thus, the nutritional status of micronutrients such as folic acid whose bioactive forms play important roles in the generation of components necessary for optimal cell growth and protein expression regulation are important to the integrity of the epidermal barrier. Third, the non-vascular nature of epidermal compartment makes micronutrient delivery to this compartment inherently inefficient. The above considerations lead to the hypothesis that optimal micronutrient status will strengthen integrity of the epidermal barrier which in turn can lead to a decrease in skin cancer. Studies have shown that cell populations with altered growth properties within actinic keratosis lesions can be recognized by immune surveillance and removed. Alternatively, cell populations within such lesions can progress to cell populations (carcinoma in situ) that secrete proteases and other factors that allow escape from the epidermis. Thus, the degree of integrity of the epidermal barrier can be a deciding factor between the ultimate fates of removal or escape of abnormal cell populations from the epidermal compartment.
10.13 Generation of Folate-Restricted Skin Cell Culture Model
HaCaT keratinocytes have been used to create a cell culture model of folate deficiency. The HaCaT cell line derives from normal human abdominal skin and exhibits a differentiation profile comparable with normal human keratinocytes despite an altered and unlimited growth potential. HaCaT cells exhibit UV-B type-specific mutations on the p53 tumor suppressor gene (Stampfer et al. 1993), representative of an early stage precancerous skin cell. To analyze the role of folate in skin cells during exposure to UV photodamage or photooxidative stress, we have developed a cell culture method in which folate concentrations in the culture medium are controlled by restricting folate and dialyzing the fetal bovine serum. This significantly reduces growth rates of HaCaT keratinocytes, as shown in Fig. 10.5. Because these cells cannot synthesize folates de novo, folate levels are rapidly depleted during cell division. We have found that through folate restriction the population doubling times of cells in culture are increased in a direct relationship to folate depletion over a critical range of concentrations (Fig. 10.5). Normal growth rates were reestablished upon folate supplementation. Interestingly, under conditions of complete folate deprivation, HaCaT cells survive without dividing for an extended period of time stalled in the S phase of the cell cycle.
Fig. 10.5.
HaCaT keratinocytes were depleted of folate for 14 days. Cell growth was measured after folate repletion at varying concentrations.
10.14 Topical Micronutrient Delivery for Skin Damage Prevention
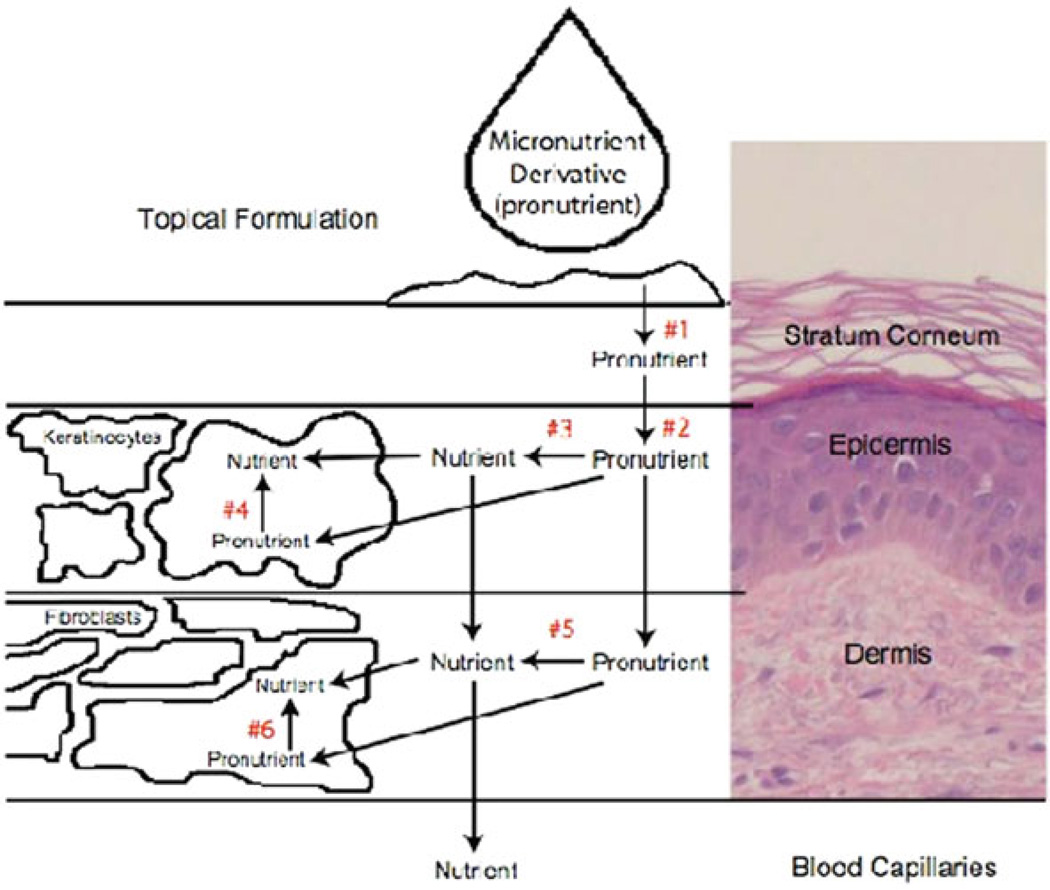
Described here is evidence that folate is a candidate for skin damage prevention involving several different mechanisms. However, a major challenge for the development of micronutrient prevention strategies for skin damage is delivery of micronutrients to skin. Folic acid is a good candidate for topical micronutrient delivery based on the roles for the bioactive forms of this nutrient in maintenance of genomic integrity via enhancement of DNA synthesis, DNA repair, and maintenance of epigenetic regulation. Our studies have demonstrated that a topical micronutrient delivery strategy to limit skin damage is feasible. Since folate is hydrophilic, developing a lipophilic form would enhance delivery through the stratum corneum, which is very lipophilic. Pro-folate compounds designed for targeted delivery were synthesized. Once delivered to the epidermis, the abundant esterases present there rapidly cleave the pro-folates to the parent compound. We designed the delivery properties to provide a slow, continuous supply of folate to skin cells to allow increased uptake by the cells. This strategy takes into consideration two distinct barriers that influence the delivery of a micronutrient to skin, lipophilicity of the stratum corneum and skin metabolic activity. The formulation strategy controls the rate of partitioning of the pro-folate into the stratum corneum by optimizing lipophilicity. Figure 10.6 shows a multiple compartment model that served as the framework for the development of this delivery strategy.
Fig. 10.6.
Tropical micronutriment delivery: a multiple compartment model
The pro-folate must effectively partition from the topical formulation into the stratum corneum. The highly lipophilic nature of the stratum corneum dictates that the profolate be sufficiently lipophilic to effectively partition into the stratum corneum from the delivery vehicle, e.g. a skin cream of lotion (arrow #1 in Fig. 10.6). The pro-folatet must partition from the stratum corneum into the epidermis at an optimal rate to achieve effective delivery to the cellular components of skin. (#2 in Fig. 10.6). Studies of drug structure-penetration relationships have provided useful information concerning this criterion (Tsai et al. 1992; Weber et al. 1994). A correlation between skin permeability and the physicochemical properties of the drug, such as octanol/water partition coefficient (Poct/w) have proven to be of great value in predicting drug transport across skin. A linear correlation between skin permeability of many compounds and their log (Poct/w) has been established (Anderson et al. 1998; Roberts et al. 1978). These findings allow a correlation between prodrug lipophilicity and drug delivery.
10.15 Summary
The discussion presented above suggests that a great deal is yet to be learned regarding folate metabolism in skin. Using the in vitro model described along with experiments in human reconstructed skin models and animal models, addressing this dearth of information should be possible. Furthermore, the strategy to optimize folate status by directed pro-folate topical delivery mechanisms in sun exposed skin may provide an opportunity to prevent skin cancer development and progression.
Acknowledgments
The research described herein was supported in part by the National Institutes of Health and Niadyne, Inc. ELJ and MKJ are principles in Niadyne, Inc. and conflict of interest management is conducted by the University of Arizona Board of Regents
References
- Agar NS, Halliday GM, Barnetson RS, Ananthaswamy HN, Wheeler M, Jones AM. The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: A role for UVA in human skin carcinogenesis. Proc Natl Acad Sci USA. 2004;101:4954–4959. doi: 10.1073/pnas.0401141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames BN. DNA damage from micronutrient deficiencies is likely to be a major cause of cancer. Mutat Res. 2001;475:7–20. doi: 10.1016/s0027-5107(01)00070-7. [DOI] [PubMed] [Google Scholar]
- Anderson BD, Higuchi WI, Raykar PV. Heterogeneity effects on permeability–partition coefficient relationships in human stratum corneum. Pharm Res. 1998;5:566–573. doi: 10.1023/a:1015989929342. [DOI] [PubMed] [Google Scholar]
- Blakley RL. The biochemistry of folic acid and related pteridines. In: Tatum EL, editor. Frontiers of biology. vol 13. New York: North Holland Publishing Company; 1969. [Google Scholar]
- Branda RF, Eaton JW. Skin color and nutrient photolysis: An evolutionary hypothesis. Science. 1978;201:625–626. doi: 10.1126/science.675247. [DOI] [PubMed] [Google Scholar]
- Brattstrom L, Wilcken DE. Homocysteine and cardiovascular disease: Cause or effect? Am J Clin Nutr. 2000;72:315–323. doi: 10.1093/ajcn/72.2.315. [DOI] [PubMed] [Google Scholar]
- Butterworth CE., Jr Folate status, women’s health, pregnancy outcome, and cancer. J Am Coll Nutr. 1993;12:438–441. doi: 10.1080/07315724.1993.10718334. [DOI] [PubMed] [Google Scholar]
- Chen AT, Reidy JA, Annest JL, Welty TK, Zhou HG. Increased chromosome fragility as a consequence of blood folate levels, smoking status, and coffee consumption. Environ Mol Mutagen. 1989;13:319–324. doi: 10.1002/em.2850130407. [DOI] [PubMed] [Google Scholar]
- Choi SW, Mason JB. Folate and carcinogenesis: An integrated scheme. J Nutr. 2000;130:129–132. doi: 10.1093/jn/130.2.129. [DOI] [PubMed] [Google Scholar]
- Choi SW, Kim YI, Weitzel JN, Mason JB. Folate depletion impairs DNA excision repair in the colon of the rat. Gut. 1998;43:93–99. doi: 10.1136/gut.43.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer Disease. Arch Neurol. 1998;55:1449–1455. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- Cohn BA. The vital role of the skin in human natural history. Int J Dermatol. 1998;37:821–824. doi: 10.1046/j.1365-4362.1998.00575.x. [DOI] [PubMed] [Google Scholar]
- Dietrich M, Brown CJ, Block G. The effect of folate fortification of cereal-grain products on blood folate status, dietary folate intake, and dietary folate sources among adult non-supplement users in the united states. J Am Coll Nutr. 2005;24:266–274. doi: 10.1080/07315724.2005.10719474. [DOI] [PubMed] [Google Scholar]
- Duthie SJ, Hawdon A. DNA instability (strand breakage, uracil misincorporation, and defective repair) is increased by folic acid depletion in human lymphocytes in vitro. FASEB J. 1998;12:1491–1497. [PubMed] [Google Scholar]
- Fang JY, Xiao SD, Zhu SS, Yuan JM, Qiu DK, Jiang SJ. Relationship of plasma folic acid and status of DNA methylation in human gastric cancer. J Gastroenterol. 1997;32:171–175. doi: 10.1007/BF02936363. [DOI] [PubMed] [Google Scholar]
- Gambichler T, Bader A, Sauermann K, Altmeyer P, Hoffmann K. Serum folate levels after UVA exposure: a two-group parallel randomised controlled trial. BMC Dermatol. 2001;1:8. doi: 10.1186/1471-5945-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey PS, Toone BK, Carney MW, Flynn TG, Bottiglieri T, Laundy M, Chanarin I, Reynolds EH. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336:392–395. doi: 10.1016/0140-6736(90)91942-4. [DOI] [PubMed] [Google Scholar]
- Goldsmith LA. Physiology, biochemistry, and molecular biology of the skin. 2nd edn. 1 vols. vol. 1. New York: Oxford University Press; 1991. [Google Scholar]
- Guenthner ST, Hurwitz RM, Buckel LJ, Gray HR. Cutaneous squamous cell carcinomas consistently show histologic evidence of in situ changes: a clinicopathologic correlation. J Am Acad Dermatol. 1999;41:443–448. doi: 10.1016/s0190-9622(99)70119-2. [DOI] [PubMed] [Google Scholar]
- Heath CW., Jr Cytogenetic observations in vitamin B12 and folate deficiency. Blood. 1966;27:800–815. [PubMed] [Google Scholar]
- Herbert V, Larrabee AR, Buchanan JM. Studies on the identification of a folate compound of human serum. J Clin Invest. 1962;41:1134–1138. doi: 10.1172/JCI104565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs CA, Sherman SL, Yi P, Hopkins SE, Torfs CP, Hine RJ, Pogribna M, Rozen R, James SJ. Polymorphisms in genes involved in folate metabolism as maternal risk factors for down syndrome. Am J Hum Genet. 2000;67:623–630. doi: 10.1086/303055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF. Vitamin D: a millenium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- Holick MF, MacLaughlin JA, Doppelt SH. Regulation of cutaneous previtamin D3 photosynthesis in man: skin pigment is not an essential regulator. Science. 1981;211:590–593. doi: 10.1126/science.6256855. [DOI] [PubMed] [Google Scholar]
- Hurwitz RM, Monger LE. Solar keratosis: an evolving squamous cell carcinoma. Benign or malignant? Dermatol Surg. 1995;21:184. doi: 10.1111/j.1524-4725.1995.tb00141.x. [DOI] [PubMed] [Google Scholar]
- Jablonski NG. The evolution of human skin and skin color. Annu Rev Anthropol. 2004;33:585-C-581. [Google Scholar]
- Jablonski NG, Chaplin G. The evolution of human skin coloration. J Hum Evol. 2000;39:57–106. doi: 10.1006/jhev.2000.0403. [DOI] [PubMed] [Google Scholar]
- Jeffes EW, 3rd, Tang EH. Actinic keratosis. Current treatment options. Am J Clin Dermatol. 2000;1:167–179. doi: 10.2165/00128071-200001030-00004. [DOI] [PubMed] [Google Scholar]
- Jimbow K, Quevedo WC, Jr, Fitzpatrick TB, Szabo G. Some aspects of melanin biology: 1950–1975. J Invest Dermatol. 1976;67:72–89. doi: 10.1111/1523-1747.ep12512500. [DOI] [PubMed] [Google Scholar]
- John Scott FRJF. Folic acid and folates: the feasibility for nutritional enhancement in plant foods. J Sci Food Agric. 2000;80:795–824. [Google Scholar]
- Kamei T, Kohno T, Ohwada H, Takeuchi Y, Hayashi Y, Fukuma S. Experimental study of the therapeutic effects of folate, vitamin A, and vitamin B12 on squamous metaplasia of the bronchial epithelium. Cancer. 1993;71:2477–2483. doi: 10.1002/1097-0142(19930415)71:8<2477::aid-cncr2820710809>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Karagas MR, Greenberg ER, Spencer SK, Stukel TA, Mott LA. Increase in incidence rates of basal cell and squamous cell skin cancer in New Hampshire, USA. New Hampshire skin cancer study group. Int J Cancer. 1999;81:555–559. doi: 10.1002/(sici)1097-0215(19990517)81:4<555::aid-ijc9>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Nakagawa A, Muramatsu T, Yamashina Y, Shirai T, Hashimoto MW, Ishigaki Y, Lehman TA, Modali R, Boukamp P, Stanek J, Bennett WP, Welsh JA, Metcalf RA, Stampfer MJ, Lober BA, Lober CW, Accola J. Actinic keratosis is squamous cell carcinoma. J Am Acad Dermatol. 2000;43:881–882. doi: 10.1067/mjd.2000.108373. [DOI] [PubMed] [Google Scholar]
- Loomis WF. Skin-pigment regulation of vitamin-D biosynthesis in man. Science. 1967;157:501–506. doi: 10.1126/science.157.3788.501. [DOI] [PubMed] [Google Scholar]
- Lucock M. Folic acid: nutritional biochemistry, molecular biology, and role in disease processes. Mol Genet Metab. 2000;71:121–138. doi: 10.1006/mgme.2000.3027. [DOI] [PubMed] [Google Scholar]
- Lucock M, Yates Z, Glanville T, Leeming R, Simpson N, Daskalakis I. A critical role for B-vitamin nutrition in human developmental and evolutionary biology. Nutr Res. 2003;23:1463–1475. [Google Scholar]
- Madronich S, McKenzie RL, Bjorn LO, Caldwell MM. Changes in biologically active ultraviolet radiation reaching the Earth’s surface. J Photochem Photobiol B. 1998;46:5–19. doi: 10.1016/s1011-1344(98)00182-1. [DOI] [PubMed] [Google Scholar]
- MRC Vitamin Study Research Group. Prevention of neural tube defects: Results of the medical research council vitamin study. Lancet. 1991;338:131–137. [PubMed] [Google Scholar]
- Nielsen KP, Lu Z, Juzenas P, Stamnes JJ, Stamnes K, Moan J. Reflectance spectra of pigmented and nonpigmented skin in the UV spectral region. Photochem Photobiol. 2004;80:450–455. doi: 10.1562/0031-8655(2004)080<0450:RSOPAN>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Nielsen KP, Zhao L, Stamnes JJ, Stamnes K, Moan J. The importance of the depth distribution of melanin in skin for DNA protection and other photobiological processes. J Photochem Photobiol B. 2006;82:194–198. doi: 10.1016/j.jphotobiol.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Off MK, Steindal AE, Porojnicu AC, Juzeniene A, Vorobey A, Johnsson A, Moan J. Ultraviolet photodegradation of folic acid. J Photochem Photobiol B. 2005;80:47–55. doi: 10.1016/j.jphotobiol.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Mori T. Supranuclear melanin caps reduce ultraviolet induced DNA photoproducts in human epidermis. J Invest Dermatol. 1998;110:806–810. doi: 10.1046/j.1523-1747.1998.00178.x. [DOI] [PubMed] [Google Scholar]
- Peng YM, Peng YS, Lin Y, Moon T, Baier M. Micronutrient concentrations in paired skin and plasma of patients with actinic keratoses: effect of prolonged retinol supplementation. Cancer Epidemiol Biomarkers Prev. 1993;2:145–150. [PubMed] [Google Scholar]
- Pentland AP, Schoggins JW, Scott GA, Khan KN, Han R. Reduction of UV-induced skin tumors in hairless mice by selective cox-2 inhibition. Carcinogenesis. 1999;20:1939–1944. doi: 10.1093/carcin/20.10.1939. [DOI] [PubMed] [Google Scholar]
- Post PW, Daniels F, Jr, Binford RT., Jr Cold injury and the evolution of “White” skin. Hum Biol. 1975;47:65–80. [PubMed] [Google Scholar]
- Rajkovic A, Catalano PM, Malinow MR. Elevated homocyst(e)ine levels with preeclampsia. Obstet Gynecol. 1997;90:168–171. doi: 10.1016/S0029-7844(97)00223-8. [DOI] [PubMed] [Google Scholar]
- Roberts MS, Anderson RA, Swarbrick J, Moore DE. The percutaneous absorption of phenolic compounds: the mechanism of diffusion across the stratum corneum. J Pharm Pharmacol. 1978;30:486–490. doi: 10.1111/j.2042-7158.1978.tb13299.x. [DOI] [PubMed] [Google Scholar]
- Skibola CF, Smith MT, Kane E, Roman E, Rollinson S, Cartwright RA, Morgan G. Polymorphisms in the methylenetetrahydrofolate reductase gene are associated with susceptibility to acute leukemia in adults. Proc Natl Acad Sci USA. 1999;96:12810–12815. doi: 10.1073/pnas.96.22.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery ML, Potter JD, Samowitz W, Schaffer D, Leppert M. Methylenetetrahydrofolate reductase, diet, and risk of colon cancer. Cancer Epidemiol Biomarkers Prev. 1999;8:513–518. [PubMed] [Google Scholar]
- Stampfer MR, Fusenig N, Rogan EM, et al. P53 mutations in human immortalized epithelial cell lines. Carcinogenesis. 1993;14:833–839. doi: 10.1093/carcin/14.5.833. [DOI] [PubMed] [Google Scholar]
- Steindal AH, Porojnicu AC, Moan J. Is the seasonal variation in cancer prognosis caused by sun-induced folate degradation? Med Hypotheses. 2006 Dec 29; doi: 10.1016/j.mehy.2006.07.063. EPub ahead of print. [DOI] [PubMed] [Google Scholar]
- Stolzenberg-Solomon RZ, Pietinen P, Barrett MJ, Taylor PR, Virtamo J, Albanes D. Dietary and other methyl-group availability factors and pancreatic cancer risk in a cohort of male smokers. Am J Epidemiol. 2001;153:680–687. doi: 10.1093/aje/153.7.680. [DOI] [PubMed] [Google Scholar]
- Szabo G, Gerald AB, Pathak MA, Fitzpatrick TB. Racial differences in the fate of melanosomes in human epidermis. Nature. 1969;222:1081–1082. doi: 10.1038/2221081a0. [DOI] [PubMed] [Google Scholar]
- Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46:S41–S62. doi: 10.1067/mjd.2002.120790. [DOI] [PubMed] [Google Scholar]
- Thien KR, Blair JA, Leeming RJ, Cooke WT, Melikian V. Serum folates in man. J Clin Pathol. 1977;30:438–448. doi: 10.1136/jcp.30.5.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thody AJ, Higgins EM, Wakamatsu K, Ito S, Burchill SA, Marks JM. Pheomelanin as well as eumelanin is present in human epidermis. J Invest Dermatol. 1991;97:340–344. doi: 10.1111/1523-1747.ep12480680. [DOI] [PubMed] [Google Scholar]
- Tsai R-S, El Tayar N, Carrupt P-A, Testa B. Physicochemical properties and transport behaviour of piribedil: Considerations on its membrane-crossing potential. Int J Pharm. 1992;80:39–49. [Google Scholar]
- Ullrich SE. Photoimmune suppression and photocarcinogenesis. Front Biosci. 2002;7:684–703. doi: 10.2741/A804. [DOI] [PubMed] [Google Scholar]
- Weber H, Meyer-Trumpener K, Lippold BC. Esters of naproxen as potential prodrugs for skin penetration. 2. Penetration behavior in excised mice skin. Arch Pharm. 1994;327:681–686. doi: 10.1002/ardp.19943271102. [DOI] [PubMed] [Google Scholar]
- Wills L. Nutrition classics. British Medical Journal 1:1059–64, 1931. Treatment of “Pernicious anaemia of pregnancy” and “Tropical anaemia” With special reference to yeast extract as a curative agent. By lucy wills. Nutr Rev. 1978;36:149–151. doi: 10.1111/j.1753-4887.1978.tb03735.x. [DOI] [PubMed] [Google Scholar]
- Wondrak GT, Cervantes-Laurean D, Roberts MJ, Qasem JG, Kim M, Jacobson EL, Jacobson MK. Identification of alpha-dicarbonyl scavengers for cellular protection against carbonyl stress. Biochem Pharmacol. 2002a;63:361–373. doi: 10.1016/s0006-2952(01)00915-7. [DOI] [PubMed] [Google Scholar]
- Wondrak GT, Roberts MJ, Jacobson MK, Jacobson EL. Photosensitized growth inhibition of cultured human skin cells: Mechanism and suppression of oxidative stress from solar irradiation of glycated proteins. J Invest Dermatol. 2002b;119:489–498. doi: 10.1046/j.1523-1747.2002.01788.x. [DOI] [PubMed] [Google Scholar]
- Wondrak GT, Roberts MJ, Cervantes-Laurean D, Jacobson MK, Jacobson EL. Proteins of the extracellular matrix are sensitizers of photo-oxidative stress in human skin cells. J Invest Dermatol. 2003;121:578–586. doi: 10.1046/j.1523-1747.2003.12414.x. [DOI] [PubMed] [Google Scholar]
- Wong WY, Merkus HM, Thomas CM, Menkveld R, Zielhuis GA, Steegers-Theunissen RP. Effects of folic acid and zinc sulfate on male factor subfertility: a double-blind, randomized, placebo-controlled trial. Fertil Steril. 2002;77:491–498. doi: 10.1016/s0015-0282(01)03229-0. [DOI] [PubMed] [Google Scholar]
- Zhang S, Hunter DJ, Hankinson SE, Giovannucci EL, Rosner BA, Colditz GA, Speizer FE, Willett WC. A prospective study of folate intake and the risk of breast cancer. JAMA. 1999;281:1632–1637. doi: 10.1001/jama.281.17.1632. [DOI] [PubMed] [Google Scholar]