Abstract
There is currently no cure for muscular dystrophies, although several promising strategies are in basic and clinical research. One such strategy is cell transplantation with satellite cells (or their myoblast progeny) to repair damaged muscle and provide dystrophin protein with the aim of preventing subsequent myofibre degeneration and repopulating the stem cell niche for future use. The present review aims to cover recent advances in satellite cell/myoblast therapy and to discuss the challenges that remain for it to become a realistic therapy.
Keywords: cell therapy, Duchenne muscular dystrophy, mdx mouse, muscular dystrophy, myoblasts, satellite cells, skeletal muscle regeneration
Introduction
Muscular dystrophies comprise a large group of heterogeneous genetic disorders characterized by progressive muscle weakness and degeneration, which vary with respect to severity, the muscle groups affected and the involvement of the heart 1. Duchenne muscular dystrophy (DMD), the most severe form, is caused by mutations in the gene for DMD, leading to a near absence of functional dystrophin protein 2,3. Dystrophin is located beneath the sarcolemma; it functions to assemble the dystroglycan complex at the sarcolemma and to connect the internal cytoplasmic actin filament network and extracellular matrix, thus providing physical strength to the muscle fibre 4. Myofibres lacking dystrophin are easily damaged, leading to satellite cell-mediated repair. However, the repaired/regenerated myofibres in turn degenerate, leading to chronic muscle degeneration and regeneration, as well as exhaustion of the satellite cell pool. This results in the eventual loss of muscle fibres and their replacement by fibrotic and fatty tissue, compromising normal muscle function 5.
Satellite cells are the principal skeletal muscle stem cell. They reside between the sarcolemma and basal lamina of muscle fibres and are mitotically quiescent until required for growth or repair. Upon receiving activation signals, they rapidly proliferate to produce a pool of myoblasts that fuse with each other to form nascent myofibres and/or with damaged fibres to repair them. A small minority do not differentiate but, instead, re-enter quiescence to maintain the stem cell pool 6. Satellite cells are extremely efficient at repairing muscle; several thousand myonuclei can be generated from a small number of transplanted satellite cells contained on a single fibre 7 and even from just a single satellite cell obtained by fluorescence activated cell sorting 8. Transplanted satellite cells can occupy the satellite cell niche and participate in future rounds of regeneration, indicating self-renewal and confirming their stem cell status 7.
There is a body of experimental evidence to support the hypothesis that not all satellite cells are functionally equivalent. Only a minority of satellite cells contributes to muscle regeneration 7,8 and recent data from satellite cell transplantation experiments have suggested that there are two populations of satellite cells. One population is responsible for myonuclei addition during growth and general muscle maintenance throughout life; these satellite cells are present in greater numbers in growing muscle, are diminished with age, and are more numerous in adult males compared to females. The second population is formed by those satellite cells that are activated by severe muscle injury and survive transplantation; they are present in similar numbers from birth to old age and do not differ between male and female mice 9. A subpopulation of satellite cells has been shown to produce distinct daughter cell fates by asymmetrically segregating template and newly-synthesized DNA strands 10; these may correspond to the ‘stem’ satellite cells that are capable of contributing to muscle regeneration and functionally reconstituting the satellite cell compartment 7.
The findings that stem cells other than satellite cells (derived from muscle, bone marrow, the interstitum or the circulation) could also contribute to muscle regeneration led to studies moving away from using satellite cells/myoblasts, towards atypical stem cells 11,12. Of the cells investigated, those with the greatest potential appear to be mesoangioblasts 13,14, pericytes 15,16 and CD133+ cells 17–19, as a result of their ability to migrate through the vasculature (a major limitation of satellite cell/myoblast therapy), to contribute to considerable muscle regeneration and to engraft into the satellite cell niche. Indeed, mesoangioblasts are currently being tested in a clinical trial for DMD, under the direction of Guilo Cossu (Division of Regenerative Medicine, San Raffaele Scientific Institute of Milan, Italy). Other recently described but less well characterized cells, which may also hold some promise, are PW1+ cells and amniotic fluid stem cells. PW1+ muscle resident interstitial cells reportedly have a regenerative capacity similar to satellite cells and can reconstitute the satellite cell niche; however, so far, they have only been isolated from mouse muscles and injected intramuscularly 20. Amniotic fluid stem cells 21 are multipotent cells capable of undergoing myogenesis and proof-of-concept studies have shown that they make some contribution to muscle regeneration in mouse models after local or systemic delivery 22,23. The recent discovery that, in the adult mouse at least, Pax7+ satellite cells are the only cells that can regenerate skeletal muscle (i.e. their conditional genetic ablation completely prevents regeneration 24–27) suggests that the myogenic contribution of other stem cells is either negligible or requires paracrine factors released by satellite cells for them to enter the myogenic programme, or that ablation experiments result in excessive disruption of muscle tissue, in turn perturbing the homeostasis of other stem cells. This may help explain the apparent discordant findings of Dellavalle et al. 16, who elegantly demonstrated the fusion of muscle resident pericytes with developing myofibres, as well as pericytes, entering the satellite cell compartment during postnatal growth. The re-establishment of the satellite cell as the principal endogenous muscle stem cell comes at a time when much effort is focused on cellular therapies. Recent advances in overcoming the limitations of myoblasts, with the aim of improving their regenerative capacity, are the focus of the present review.
Myoblast cell therapy
Failure of early myoblast transplantation clinical trials
Cell therapy (i.e. the delivery of myogenic cells to enact muscle repair) has been considered as a potential therapy for DMD for many years, ever since Partridge et al. 28 demonstrated that donor myoblasts could fuse with host myoblasts, suggesting the possibility of functional restoration in defective fibres. The pivotal discovery that donor heterologous myoblasts could restore dystrophin expression in the dystrophin deficient mdx mouse 29 set the precedent for a number of human clinical trials in DMD patients in the 1990s 30. Disappointingly, little or no dystrophin restoration was observed in the injected muscles and no functional improvements were discerned 31–38. The failure of the trials was subsequently attributed to several factors, including the rapid cell death of the majority of cells within a few hours of transplantation, the limited migratory capacity of transplanted cells and a lack of immune suppression leading to graft rejection 12. It is also now known that myoblasts are not as efficient as their parent satellite cells. Standard culture greatly reduces their regenerative and self-renewal capacity 39.
Overcoming problems
Strategies to overcome some of these problems include improved immunosuppression, the injection of more cells but in smaller volumes to prevent ischaemic necrosis, and high-density injection protocols to aid migration 40. Using these improvements, a recent phase I clinical trial for DMD delivered a large number of allogeneic myoblasts using multiple injections (high-density injection protocol) to the biceps brachii, under continuous immunosuppression by tacrolimus (FK506), to avoid rejection. Long-term expression of donor-derived dystrophin was detected in 27.5% of fibres 1 month after injection and, in 34.5% of fibres, after 18 months 41,42. Although promising, this was only achieved in one patient, repair was localized to the injection sites, long-term immunosuppression is required and the protocol is only applicable to easily accessible small muscle groups 43.
Clinical trials, using autologous myoblasts, for muscular dystrophies that affect only subsets of muscles, namely oculopharnygeal muscular dystrophy (ClinicalTrial.gov identifier: NCT00773227) and facioscapulohumeral muscular dystrophy (under the direction of C. Desnuelle, Centre Hospitalier Universitaire de Nice, France) are currently underway (initiated in 2005). These have the benefit of not requiring immunosuppression. Oculopharnygeal muscular dystrophy is characterized by late onset eyelid drooping (ptosis) and dysphagia (difficulty swallowing) as a result of dystrophy of the pharyngeal muscle. Myoblasts from unaffected limb muscles were grafted into the pharyngeal muscle of patients, following on from promising preclinical trials conducted in the beagle dog 44. The trial is a safety and efficacy trial, with results on any swallowing improvements expected in 2015. A similar trial using autologous myoblasts from non-affected areas is underway for facioscapulohumeral muscular dystrophy, which is characterized by asymmetric muscle weakness, predominantly in the face, scapula and upper arms. The results of this trial are expected soon. However, it would not be possible to treat a muscular dystrophy such as DMD with autologous myoblasts because the regenerated myofibres would still lack dystrophin and therefore be prone to continuing bouts of degeneration and regeneration. Because the use of autologous cells may not require immunosuppression of the patient, efforts have been made to genetically modify autologous myoblasts. Vectors such as retroviruses and lentiviruses have been used to heritably insert marker or therapeutic, genes into myoblasts; however, retroviruses can only infect dividing cells, and so the quiescent, more ‘stem-cell’ myoblasts would not be transduced. Lentiviral vectors efficiently infect quiescent cells, including stem cells 45, and, because they integrate into the host genome, give long-term, heritable, gene expression. The drawbacks of lentiviral vectors include possible gene silencing, or mutagenesis 46, as a result of the site at which the virus inserts into the host genome. Although lentiviruses integrate preferentially into active transcription sites 47, the development of third-generation lentiviruses with an advanced self-inactivating design, to reduce transactivation of neighbouring genes 48, physiological promoters (such as muscle creatine kinase or desmin) 49, cell-specific envelope proteins 50 and enhancer-less regulatory elements (e.g. the ubiquitously acting chromatin opening element) 49,51, should reduce the risk of insertional mutagenesis or gene silencing.
A major disadvantage of lentiviruses is that they can carry only a relatively small DNA insert of up to 10 kb 52. Lentiviral vectors have been used to insert either a mini- or micro-dystrophin gene, or constructs designed to skip mutated dystrophin exons, into myoblasts 45,53,54. These genetically-modified myoblasts contribute to regenerated muscle fibres, containing a shorter dystrophin protein, after their intramuscular transplantation in animal models of DMD. Although these engineered mini-dystrophins appear to retain most of the functional properties of full-length dystrophin, they nevertheless miss important domains, such as the nitric oxide synthase-anchoring domain 55, and so an important goal is to insert as large as possible functional dystrophin construct into a lentiviral vector.
Improving myoblast culture conditions
Why is it that myoblasts do not perform as well as satellite cells? When placed in tissue culture, the majority of satellite cells proliferate rapidly, although a minority divide slowly and it is the latter that contribute more extensively to muscle regeneration in vivo 56,57. Selecting for a subpopulation of quiescent myoblasts may improve their engraftment potential. Small, nongranular mouse satellite cells 39 contribute more effectively to muscle regeneration than larger, granular satellite cells from the same preparations 58, and sorting on the basis of satellite cell size and/or specific cell surface markers 59 may be able to enrich for the ‘stem’ satellite cells with enhanced muscle regenerative capacity.
The ability to guide the behaviour and fate of stem cells in culture is hindered by a limited understanding of the niche composition and the regulation that it imposes on satellite cell fate. The niche comprises both biochemical (e.g. growth factors, cytokines, receptor ligands) and biophysical (matrix stiffness, topography, fluidity, temperature, oxygen, pH) factors that direct stem cell fate 60. Modifying any of these factors can have a pronounced impact on muscle regeneration and satellite cell self-renewal.
It is now well recognized that oxygen tension is an important component of stem cell niches. Most tissue culture is performed using atmospheric levels of oxygen (20%) when, in reality, tissue levels are much lower, usually 2–9% (14.4–64.8 mmHg) depending on the tissue; even within a tissue, there is considerable variability depending on the proximity of cells to blood vessels 61–64. The neural stem cell niche has an oxygen tension in the range < 1–8% oxygen, the hematopoietic stem cell niche in the range 1–6% oxygen and the mesenchymal stem cell niche in the range 2–8% oxygen 64, whereas the thymus, kidney medulla and bone marrow can exist at 1% oxygen 63. Low oxygen levels are often referred to hypoxic when, in reality, they are normoxic for the tissue or cell in question. Culture in 20% oxygen can be toxic to cells causing DNA damage and apoptosis 61, whereas low levels of oxygen have been shown to prevent oxidative stress caused by aerobic metabolism, in turn preventing the generation of reactive oxygen species that may cause DNA damage 61,64. However, for myoblast cultures, oxygen levels are routinely uncontrolled 61,65.
Two recent studies highlight the benefits of using more physiological levels of oxygen in the cultivation of myoblasts. Duguez et al. 65 confirmed that atmospheric oxygen is hyperoxic for myoblasts and represses their proliferation, compared to myoblasts cultured in 5% oxygen, and suggested that the mechanism by which this occurs is via increased mitochondrial activation in hyperoxic conditions. We have also observed a decreased proliferation of satellite cell-derived myoblasts at 20% oxygen compared to 5% oxygen (D. Briggs, L. Boldrin and J.E. Morgan, unpublished results). Another study by Liu et al. 66 demonstrated that reducing the oxygen level further (to 1%) increases myoblast quiescence, reduces differentiation and promotes self-renewal. It was elegantly shown that hypoxia upregulates Pax7 through downregulation of miR-1 and miR206, whose expression, in turn, is controlled by the Notch signalling pathway 66. Furthermore, hypoxia conditioning was shown to enhance the efficiency of myoblast transplantation and self-renewal in vivo in cardiotoxin injured mdx mouse muscles 66.
The importance of physiological tissue rigidity has long been suspected but, as a result of the complexity of reflecting this in vitro, has largely been ignored. Using a bioengineering approach, Gilbert et al. 67 created polyethylene glycol hydrogels, which mimic the elasticity of muscle much more closely than standard, rigid, tissue culture plastic. It was demonstrated that soft substrates enhance satellite cell survival, prevent differentiation and promote stemness (increased self-renewal) in vitro and, more importantly, result in extensive muscle regeneration in vivo compared to traditional culture on plastic 67. This was the first study to show high levels of engraftment in mice from a small number of transplanted cultured cells (100% incidence of donor-derived engraftment was obtained from 1000 cells and 10% from just 10 cells), which represents an efficiency comparable to the injection of 10 freshly-isolated satellite cells 67. The use of this artificial niche will allow the influence that other biochemical niche components have on stem cell fate and behaviour to be examined at a single cell level, on a large scale, using time-lapse microscopy and an algorithm that enables automated analysis, garnering previously unobtainable information 68. Eventually, this should allow the selection and subsequent expansion of the stem cell subpopulation of satellite cells (Fig. 1). Transplantation of satellite stem cells rather than myoblasts would dramatically improve donor-derived muscle regeneration.
Figure 1.
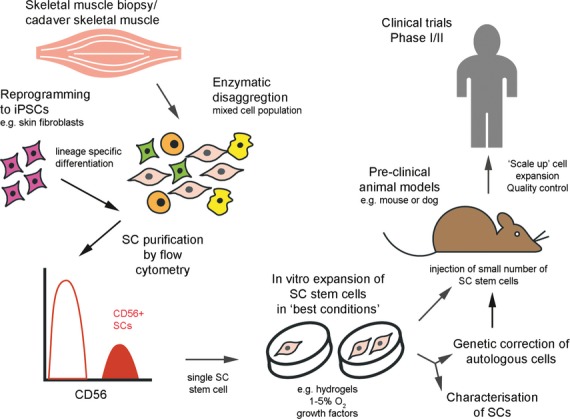
Potential protocol for improving cell therapy for muscular dystrophy. With advancements in the isolation and culture of muscle stem cells, the following may become possible. Skeletal muscle satellite cells (SCs) could be obtained by muscle biopsy or from cadaver muscle and enzymatically disaggregated to a single cell suspension containing an impure population of satellite cells. Satellite stem cells could be purified by flow cytometry. Alternatively, satellite cells could be derived from reprogrammed iPSCs. Culture conditions that allow the expansion of only the stem cell subpopulation of satellite cells would improve transplantation and require only limited cell numbers (e.g. the use of hydrogels and low levels of oxygen). Genetic correction of autologous satellite cells would also be required. Preclinical studies in animal models, such as the dystrophin deficient mdx mouse and golden retriever muscular dystrophy dog, would be performed to confirm safety and efficacy before the therapy enters the clinic. Currently, satellite cells are only deliverable intramuscularly, although further understanding of their biology may allow their modification so that they can be delivered systemically.
Most satellite cell research is carried out using mouse cells because only very low numbers of human satellite cells can be obtained by muscle biopsy, which are then cultured to increase the cell number and thus become myoblasts. Recently, Latil et al. 69 showed that satellite stem cells are enriched in post-mortem tissue, adopting a dormant state and remaining viable for up to 17 days in humans and 14 days in mice. Obtaining satellite cells from post-mortem muscles could provide a large number of human normal and dystrophic satellite cells for research at the single cell level and potentially could provide autologous satellite cells for transplantation.
Modifying the environment
Satellite cells are absolutely necessary for muscle regeneration 24–26,70; however, they do not work alone (Fig. 2). Regeneration is a multistep process requiring resident and infiltrating immune and stromal cells to remove debris, regulate satellite cell proliferation and differentiation, and allow muscle remodelling 71,72. The necessity of the inflammatory response has been demonstrated in many studies, with a reduced entry of monocytes/macrophages into injured muscle causing a delay in regeneration and the persistence of adipocytes 71–74. Moreover, complete depletion strikingly results in no regenerative response, highlighting the importance of inflammation 72–75.
Figure 2.
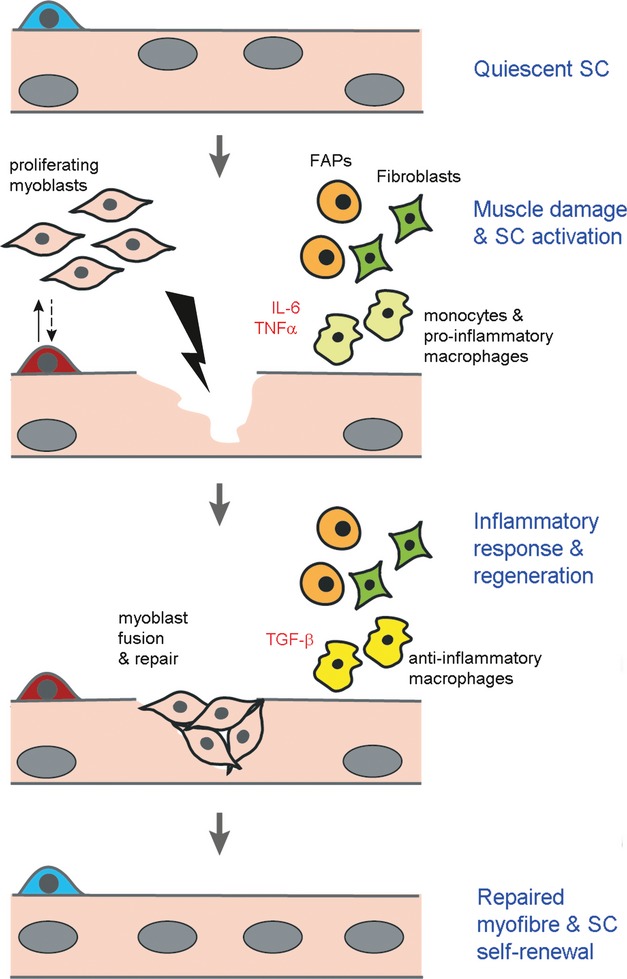
Schematic of satellite cell-mediated muscle regeneration. In response to myofibre damage, satellite cells rapidly activate and proliferate to produce a pool of myoblasts that fuse to repair or replace damaged fibres. Infiltration by immune cells occurs, including neutrophils, monocytes, pro-inflammatory and later anti-inflammatory macrophages, along with stromal cells including fibroblasts and FAPs secrete paracrine and autocrine factors, remove debris and ensure efficient regeneration. The immune and stromal cells do this by controlling the balance between myoblast proliferation and differentiation and ensuring satellite cell self-renewal to replenish the stem cell niche. A hallmark of regenerated fibres in the mouse is the central (i.e. opposed to peripheral) position of nuclei. IL-6, interleukin-6; TNFα, tumour necrosis factor α.
Coinjection of pro-inflammatory (but not anti-inflammatory) macrophages, along with human myoblasts, into regenerating muscle (injured by cryodamage) of Rag2−/−γC−/− immunodeficient mice improves donor-derived muscle regeneration by extending the window of proliferation, increasing migration and delaying differentiation 76. It is suggested that pro-inflammatory macrophages can then switch to an anti-inflammatory phenotype in vivo to stimulate differentiation of the donor myoblasts 76. These results provide the first in vivo evidence for pro-inflammatory macrophages having a supportive role in the regulation of myoblast behaviour after engraftment into pre-injured muscle 76. A similar study, using the coinjection of mouse macrophages and myoblasts, but into the dystrophic environment of mdx mice, also reported improved donor-derived regeneration, which was attributed to improved donor myoblast survival, proliferation and migration 77. The increased survival was considered to be a result of macrophages improving cell adhesion, thereby decreasing ankiosis and having a mitogenic effect by secreting growth factors. This is important in the context of cell therapy because massive early cell death, poor proliferation and migration are some of the main obstacles that need to be overcome for it to become a viable therapy option 77.
Another vital component of the regenerating niche is muscle connective tissue (MCT) cells (stromal cells), including fibroblasts and dual potential fibro/adipoprogenitors (FAPs) 78. Fibroblasts are necessary for extracellular matrix and collagen synthesis and an increase in extracellular matrix is a hallmark of regenerating muscle. The study of MCT fibroblasts had been limited by the lack of specific markers until the recent finding that MCT fibroblasts express the transcription factor Tcf4 79. Using genetic ablation studies, Murphy et al. 25 showed that Tcf4+ fibroblasts are required for efficient regeneration, and that their loss leads to premature satellite cell differentiation, depletion of the myoblast pool and smaller regenerated fibres. Reciprocally, myoblasts promote MCT fibroblast proliferation 25. FAPs have only recently been described but represent a significant fraction of the mononuclear cells present in muscle 80. FAPs are quiescent in healthy muscle but proliferate efficiently in response to damage; their transient expansion during regeneration provides signals that promote the terminal differentiation of proliferating myoblasts 80. A greater understanding of this population of cells may lead to therapeutic strategies for reducing the scarring and fibrosis found in dystrophic muscle, thereby providing an environment amenable to muscle regeneration 80.
The effect of ageing on satellite cell function is a matter of much debate because the loss of skeletal muscle mass and function with increasing age (sarcopenia) is of great importance in ageing western populations. However, despite evidence that the satellite cell niche deteriorates with age 81 and that satellite cells are lost with age 58,82, the regeneration-competent, ‘stem’ satellite cells are retained and those derived from aged donors remain as functional as those from young donors 9,58,83,84. It therefore appears that there are two subpopulations of satellite cell: one that is lost with age and is responsible for maintaining muscle mass, and a second that is retained throughout life 9 and, given the correct environmental cues, can contribute robustly to muscle regeneration.
Improving regeneration
There is a plethora of studies in mice examining ways of augmenting the regenerative potential of myoblasts. Preventing cell death, increasing proliferation and/or migration, and decreasing early differentiation have all been shown to have a positive impact on mouse and human myoblast transplantations in immunodeficient mice. For example, upregulating the heatshock response (Hsp70 protein) improves both mouse and human myoblast survival, leading to increased engraftment 85,86. Reducing hypoxia-related death by overexpressing vascular endothelial growth factor has a similar effect 87. Overexpression of matrix metalloproteinase 9, a proteolytic enzyme that can remodel the extracellular matrix, enhances myoblast migration and engraftment 88. Transforming growth factor-β (TGF-β) is a negative regulator of skeletal muscle development and elevated levels can limit skeletal muscle regeneration 89. Fakhfakh et al. 90 have shown that treatment with oral losartan, a molecule that downregulates TGF-β1 expression, improves the transplantation efficiency of human myoblasts into immunodeficient dystrophic mice, as demostrated by an increase in dystrophin positive fibres 1 month after engraftment compared to nontreated controls 90. Increased myoblast survival was observed 3 days after transplantation (10% versus 6% of radiolabelled cells), which led to increased proliferation and differentiation concomitant with the increased expression of Myf5, MyoD and myogenin 90. Blocking the myostatin signal (another negative regulator of muscle regeneration) with a dominant negative receptor improves the success of human myoblast transplantation by increasing myoblast proliferation and fusion and changing the expression of myogenic regulatory factors 91. However, this approach may not be as straightforward as hoped; a recent clinical trial using ACE-031 (a soluble form of activin receptor type IIB, which binds to myostatin and other members of the TGFβ family) in DMD patients, was terminated early because of safety concerns (http://www.acceleronpharma.com/products/ace-031/; ClinicalTrials.gov Identifier: NCT01099761).
Concerning the limited migratory capacity of human myoblasts in vivo, several studies have linked this with the limited proliferation of the injected myoblasts. When human myoblasts are injected into the cryodamaged muscles of Rag2−/−γC−/− immunodeficient mice, in medium containing serum, which is rich in growth factors, rather than NaCl/Pi, the window of proliferation is extended from 3 to 5 days. This increases migration, leading to enhanced regeneration as a direct result of slower myoblast differentiation 92. Similarly, AG490 (a specific inhibitor of janus tyrosine kinase 2) has been used to block myoblast differentiation, increasing proliferation and cell survival in vivo 93. However, other studies have shown that, although co-injection of insulin-like growth factor 1 and/or basic fibroblast growth factor with human myoblasts improves myoblast migratory capacity and dispersal 94,95, growth factor addition does not improve the transplantation success in undamaged primate muscle 95, in contrast to the enhanced regeneration observed in mice 92,96.
Challenges remaining
How to induce regeneration
A major problem with the intramuscular injection of myoblasts in the human clinical trials was that regeneration (dystrophin positive fibres) appeared to be limited to damaged muscle along the injection trajectory. This was also seen in primate experiments 40,95. In mice, successful engraftments require either pre-treatment of the host muscle with irradiation 97,98, or an injury to be administered to induce or increase muscle damage; with use of the snake venom myotoxins notexin and cardiotoxin 99 or cryodamage 100 being most common. Irradiation limits the host satellite-cell contribution to regeneration and provides an optimal environment for donor mouse cell engraftment 84,98,101. Cryodamage destroys cells near to the injury site but preserves the basal lamina of muscle fibres 102. Following cryodamage, skeletal muscle can regenerate, indicating that at least some satellite cells either survive the injury or migrate into damaged areas 84. Injection of myotoxins destroys muscle fibres but preserves their basal lamina, nerves, blood vessels and satellite cells 84. Neither cryodamage, nor myotoxins are as effective as irradiation for enhancing mouse donor satellite cell-derived muscle regeneration 84. This is not the case for human myoblasts, where cryodamage is at least as effective as irradiation, allowing similar amounts of donor muscle regeneration and engraftment of more total donor cells (including cells outside of muscle fibres) 96,103. The reason for differences between the behaviour of mouse and human myoblasts is not known, suggesting caution with respect to the assumption that what works in mice will also work in humans. For patients in whom it would be unethical to use these pre-treatments, other ways of increasing donor satellite cell or myoblast engraftment might exist. Intense muscle exercise has been shown to greatly improve myofibre regeneration in mdx mice 104. It is possible that exercise (rather than an acute and extensive injury to the host muscle) may be sufficient to promote donor-derived muscle regeneration in patients.
Harnessing the potential of induced pluripotent stem cells (iPSCs)
iPSCs 105 hold great promise for cell therapy; they could potentially yield unlimited numbers of autologous stem/progenitor cells. This is important because myoblasts, particularly dystrophic ones, undergo a limited numbers of doublings before entering senescence and the use of donor heterologous myoblasts requires life-long immunosuppression. A caveat is that patient-derived iPSCs would still need to be genetically corrected before transplantation. The generation and use of human iPSCs does not pose the same ethical dilemma as deriving human embryonic stem cells (ESCs), making them a more attractive candidate 106,107. The technology for both ESCs and iPSCs is limited by the efficiency of cell-lineage-specific differentiation and the efficiency of cell purification to eliminate the risk of teratoma, although many strategies are being devised to overcome these limitations 108. Reprogramming of mouse iPSCs and ESCs into satellite cells/myoblasts has been achieved using various protocols 109–110, although the equivalent reprogramming of human iPSCs and ESCs has lagged behind. Only one reported study, showing reprogramming of human ESCs into myoblasts with limited efficiency 112, was available until Darabi et al. 106, Tedesco et al. 113 and Goudenege et al. 107 published new protocols for deriving myogenic progenitors from iPSCs, based on mesoderm commitment followed by myogenic transcription factor overexpression, within a few months of each other. Darabi et al. 106 and Goudenege et al. 107 demonstrated very efficient reprogramming of both human iPSCs and ESCs using the forced overexpression of different myogenic regulatory factors, specifically MyoD in an adenoviral vector 106 and Pax7 in a lentiviral vector 107. Both methods gave highly efficient myogenic conversion, with cells expressing high levels of the satellite cell marker CD56 and myosin heavy chain upon in vitro differentiation, and notably generating a large number of muscle fibres upon intramuscular transplantation into immunodeficient dystrophic mice 106,107 Darabi et al. 106 also demonstrated a functional improvement in treated muscles, long-term expression of donor-derived dystrophin (11 months) and occupation of the satellite cell niche. Tedesco et al. 113 used a similar strategy but went one step further by deriving mesoangioblast-like cells (no CD56 expression) from human iPSCs generated from limb-girdle muscular dystrophy 2D (sub-type of limb-girdle muscular dystrophy) patient fibroblasts or myoblasts. These cells were then lentivirally transduced with both a therapeutic gene (Sgca, encoding α-sarcoglycan) to correct the genetic defect and with MyoD to induce myogenic differentiation. Importantly, donor cell engraftment into Sgca-null immunodeficent mice, was obtainable using both intramuscular and inter-arterial injections, as indicated by α-sarcoglycan expression 113. However, there are safety concerns with iPSCs, particularly the potential tumourigenicity of cells that are not fully differentiated at the time of transplantation, as well as the genomic integrity of the iPSCs 114.
Concluding remarks
In recent years, there has been both an improved understanding of the biology of satellite cells themselves, together with increasing knowledge on the effect of the host skeletal muscle environment on their function in vivo. In particular, the effect of factors such as microRNAs, growth factors and extracellular matrix components produced by host cells, including myofibres, blood vessel-associated, stromal and inflammatory cells, and the effect of the host satellite cell niche on donor satellite cell engraftment are particularly relevant to improving donor cell engraftment. We envisage that a combination of tissue culture conditions to promote or retain the ‘stem-like’ myoblasts, with modification of the host muscle environment to enhance donor satellite cell migration, proliferation and self-renewal, will be the way forward.
Because satellite cells and their progeny myoblasts 15 do not appear to be systemically deliverable, they could only be used to treat individual muscles, although this might still be of benefit to patients with DMD. If hand or finger muscles could be successfully treated, this could improve the quality of life 115 by allowing the patient, for example, to operate a computer keyboard or touchscreen.
Even in the era of molecular therapies, myoblast or other stem cell therapies are still highly relevant. Although potential treatments for DMD such as exon skipping are promising, exon skipping is neither applicable to all DMD patients, nor will it restore lost muscle fibres. An effective stem cell-based treatment will therefore be a powerful alternative, or adjunct, to other therapies.
Acknowledgments
Jennifer Morgan and Deborah Briggs are supported by a Wellcome Trust University Award, held by Jennifer Morgan. We also gratefully acknowledge support by the Muscular Dystrophy Campaign, Association Francaise contre les myopathies, Duchenne Parent Project (Netherlands), the Medical Research Council and the Biotechnology and Biological Sciences Research Council. The authors declare that there are no conflicts of interest.
Glossary
- DMD
Duchenne muscular dystrophy
- ESC
embryonic stem cell
- FAP
fibro/adipoprogenitor
- iPSC
induced pluripotent stem cell
- MCT
muscle connective tissue
- SC
satellite cell
- TGF-β
transforming growth factor-β
References
- 1.Emery AE. The muscular dystrophies. Lancet. 2002;359:687–695. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 3.Bonilla E, Samitt CE, Miranda AF, Hays AP, Salviati G, DiMauro S, Kunkel LM, Hoffman EP, Rowland LP. Duchenne muscular dystrophy: deficiency of dystrophin at the muscle cell surface. Cell. 1988;54:447–452. doi: 10.1016/0092-8674(88)90065-7. [DOI] [PubMed] [Google Scholar]
- 4.Ervasti JM. Dystrophin, its interactions with other proteins, and implications for muscular dystrophy. Biochim Biophys Acta. 2007;1772:108–117. doi: 10.1016/j.bbadis.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Morgan JE, Zammit PS. Direct effects of the pathogenic mutation on satellite cell function in muscular dystrophy. Exp Cell Res. 2010;316:3100–3108. doi: 10.1016/j.yexcr.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neal A, Boldrin L, Morgan JE. The satellite cell in male and female, developing and adult mouse muscle: distinct stem cells for growth and regeneration. PLoS One. 2012;7:e37950. doi: 10.1371/journal.pone.0037950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rocheteau P, Gayraud-Morel B, Siegl-Cachedenier I, Blasco MA, Tajbakhsh S. A subpopulation of adult skeletal muscle stem cells retains all template DNA strands after cell division. Cell. 2012;148:112–125. doi: 10.1016/j.cell.2011.11.049. [DOI] [PubMed] [Google Scholar]
- 11.Tedesco FS, Dellavalle A, Diaz-Manera J, Messina G, Cossu G. Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J Clin Invest. 2010;120:11–19. doi: 10.1172/JCI40373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Negroni E, Vallese D, Vilquin J-T, Butler-Browne G, Mouly V, Trollet C. Current advances in cell therapy strategies for muscular dystrophies. Expert Opin Biol Ther. 2011;11:157–176. doi: 10.1517/14712598.2011.542748. [DOI] [PubMed] [Google Scholar]
- 13.Sampaolesi M, Torrente Y, Innocenzi A, Tonlorenzi R, D’Antona G, Pellegrino MA, Barresi R, Bresolin N, De Angelis MG, Campbell KP, et al. Cell therapy of alpha-sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts. Science. 2003;301:487–492. doi: 10.1126/science.1082254. [DOI] [PubMed] [Google Scholar]
- 14.Sampaolesi M, Blot S, D’Antona G, Granger N, Tonlorenzi R, Innocenzi A, Mognol P, Thibaud JL, Galvez BG, Barthélémy I, et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444:574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- 15.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 16.Dellavalle A, Maroli G, Covarello D, Azzoni E, Innocenzi A, Perani L, Antonini S, Sambasivan R, Brunelli S, Tajbakhsh S, Cossu G. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun. 2011;2:499. doi: 10.1038/ncomms1508. [DOI] [PubMed] [Google Scholar]
- 17.Torrente Y, Belicchi M, Sampaolesi M, Pisati F, Meregalli M, D’Antona G, Tonlorenzi R, Porretti L, Gavina M, Mamchaoui K, et al. Human circulating AC133(+) stem cells restore dystrophin expression and ameliorate function in dystrophic skeletal muscle. J Clin Invest. 2004;114:182–195. doi: 10.1172/JCI20325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torrente Y, Belicchi M, Marchesi C, Dantona G, Cogiamanian F, Pisati F, Gavina M, Giordano R, Tonlorenzi R, Fagiolari G, et al. Autologous transplantation of muscle-derived CD133+ stem cells in Duchenne muscle patients. Cell Transplant. 2007;16:563–577. doi: 10.3727/000000007783465064. [DOI] [PubMed] [Google Scholar]
- 19.Negroni E, Riederer I, Chaouch S, Belicchi M, Razini P, Di Santo J, Torrente Y, Butler-Browne GS, Mouly V. In vivo myogenic potential of human CD133+ muscle-derived stem cells: a quantitative study. Mol Ther. 2009;17:1771–1778. doi: 10.1038/mt.2009.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell KJ, Pannérec A, Cadot B, Parlakian A, Besson V, Gomes ER, Marazzi G, Sassoon DA. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat Cell Biol. 2010;12:257–266. doi: 10.1038/ncb2025. [DOI] [PubMed] [Google Scholar]
- 21.De Coppi P, Bartsch G, Jr, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 22.Ma X, Zhang S, Zhou J, Chen B, Shang Y, Gao T, Wang X, Xie H, Chen F. Clone-derived human AF-amniotic fluid stem cells are capable of skeletal myogenic differentiation in vitro and in vivo. J Tissue Eng Regen Med. 2012;6:598–613. doi: 10.1002/term.462. [DOI] [PubMed] [Google Scholar]
- 23.Piccoli M, Franzin C, Bertin E, Urbani L, Blaauw B, Repele A, Taschin E, Cenedese A, Zanon GF, André-Schmutz I, et al. Amniotic fluid stem cells restore the muscle cell niche in a HSA-Cre, Smn(F7/F7) mouse model. Stem Cells. 2012;30:1675–1684. doi: 10.1002/stem.1134. [DOI] [PubMed] [Google Scholar]
- 24.Lepper C, Partridge TA, Fan C-M. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138:3639–3646. doi: 10.1242/dev.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, Guenou H, Malissen B, Tajbakhsh S, Galy A. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 2011;138:3647–3656. doi: 10.1242/dev.067587. [DOI] [PubMed] [Google Scholar]
- 27.Relaix F, Zammit PS. Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development. 2012;139:2845–2856. doi: 10.1242/dev.069088. [DOI] [PubMed] [Google Scholar]
- 28.Partridge TA, Grounds M, Sloper JC. Evidence of fusion between host and donor myoblasts in skeletal muscle grafts. Nature. 1978;273:306–308. doi: 10.1038/273306a0. [DOI] [PubMed] [Google Scholar]
- 29.Partridge TA, Morgan JE, Coulton GR, Hoffman EP, Kunkel LM. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989;337:176–179. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- 30.Partridge T. The current status of myoblast transfer. Neurol Sci. 2000;21:S939–S942. doi: 10.1007/s100720070007. [DOI] [PubMed] [Google Scholar]
- 31.Gussoni E, Pavlath GK, Lanctot AM, Sharma KR, Miller RG, Steinman L, Blau HM. Normal dystrophin transcripts detected in Duchenne muscular dystrophy patients after myoblast transplantation. Nature. 1992;356:435–438. doi: 10.1038/356435a0. [DOI] [PubMed] [Google Scholar]
- 32.Huard J, Roy R, Bouchard JP, Malouin F, Richards CL, Tremblay JP. Human myoblast transplantation between immunohistocompatible donors and recipients produces immune reactions. Transplant Proc. 1992;24:3049–3051. [PubMed] [Google Scholar]
- 33.Karpati G, Ajdukovic D, Arnold D, Gledhill RB, Guttmann R, Holland P, Koch PA, Shoubridge E, Spence D, Vanasse M, et al. Myoblast transfer in Duchenne muscular dystrophy. Ann Neurol. 1993;34:8–17. doi: 10.1002/ana.410340105. [DOI] [PubMed] [Google Scholar]
- 34.Tremblay JP, Bouchard JP, Malouin F, Théau D, Cottrell F, Collin H, Rouche A, Gilgenkrantz S, Abbadi N, Tremblay M, et al. Myoblast transplantation between monozygotic twin girl carriers of Duchenne muscular dystrophy. Neuromuscul Disord. 1993;3:583–592. doi: 10.1016/0960-8966(93)90121-y. [DOI] [PubMed] [Google Scholar]
- 35.Tremblay JP, Malouin F, Roy R, Huard J, Bouchard JP, Satoh A, Richards CL. Results of a triple blind clinical study of myoblast transplantations without immunosuppressive treatment in young boys with Duchenne muscular dystrophy. Cell Transplant. 1993;2:99–112. doi: 10.1177/096368979300200203. [DOI] [PubMed] [Google Scholar]
- 36.Mendell JR, Kissel JT, Amato AA, King W, Signore L, Prior TW, Sahenk Z, Benson S, McAndrew PE, Rice R, et al. Myoblast transfer in the treatment of Duchenne’s muscular dystrophy. N Engl J Med. 1995;333:832–838. doi: 10.1056/NEJM199509283331303. [DOI] [PubMed] [Google Scholar]
- 37.Morandi L, Bernasconi P, Gebbia M, Mora M, Crosti F, Mantegazza R, Cornelio F. Lack of mRNA and dystrophin expression in DMD patients three months after myoblast transfer. Neuromuscul Disord. 1995;5:291–295. doi: 10.1016/0960-8966(94)00070-p. [DOI] [PubMed] [Google Scholar]
- 38.Miller RG, Sharma KR, Pavlath GK, Gussoni E, Mynhier M, Lanctot AM, Greco CM, Steinman L, Blau HM. Myoblast implantation in Duchenne muscular dystrophy: the San Francisco study. Muscle Nerve. 1997;20:469–478. doi: 10.1002/(sici)1097-4598(199704)20:4<469::aid-mus10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 39.Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- 40.Skuk D, Tremblay JP. Intramuscular cell transplantation as a potential treatment of myopathies: clinical and preclinical relevant data. Expert Opin Biol Ther. 2011;11:359–374. doi: 10.1517/14712598.2011.548800. [DOI] [PubMed] [Google Scholar]
- 41.Skuk D, Goulet M, Roy B, Chapdelaine P, Bouchard JP, Roy R, Dugré FJ, Sylvain M, Lachance JG, Deschênes L, et al. Dystrophin expression in muscles of duchenne muscular dystrophy patients after high-density injections of normal myogenic cells. J Neuropathol Exp Neurol. 2006;65:371–386. doi: 10.1097/01.jnen.0000218443.45782.81. [DOI] [PubMed] [Google Scholar]
- 42.Skuk D, Goulet M, Roy B, Piette V, Côté CH, Chapdelaine P, Hogrel JY, Paradis M, Bouchard JP, Sylvain M, et al. First test of a ‘high-density injection’ protocol for myogenic cell transplantation throughout large volumes of muscles in a Duchenne muscular dystrophy patient: eighteen months follow-up. Neuromuscul Disord. 2007;17:38–46. doi: 10.1016/j.nmd.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Vilquin J-T, Catelain C, Vauchez K. Cell therapy for muscular dystrophies: advances and challenges. Curr Opin Organ Transplant. 2011;16:640–649. doi: 10.1097/MOT.0b013e32834cfb70. [DOI] [PubMed] [Google Scholar]
- 44.Périé S, Mamchaoui K, Mouly V, Blot S, Bouazza B, Thornell LE, St Guily JL, Butler-Browne G. Premature proliferative arrest of cricopharyngeal myoblasts in oculo-pharyngeal muscular dystrophy: therapeutic perspectives of autologous myoblast transplantation. Neuromuscul Disord. 2006;16:770–781. doi: 10.1016/j.nmd.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 45.Li S, Kimura E, Fall BM, Reyes M, Angello JC, Welikson R, Hauschka SD, Chamberlain JS. Stable transduction of myogenic cells with lentiviral vectors expressing a minidystrophin. Gene Ther. 2005;12:1099–1108. doi: 10.1038/sj.gt.3302505. [DOI] [PubMed] [Google Scholar]
- 46.Wilson CA, Cichutek K. The US and EU regulatory perspectives on the clinical use of hematopoietic stem/progenitor cells genetically modified ex vivo by retroviral vectors. Methods Mol Biol. 2009;506:477–488. doi: 10.1007/978-1-59745-409-4_32. [DOI] [PubMed] [Google Scholar]
- 47.Ciuffi A. Mechanisms governing lentivirus integration site selection. Curr Gene Ther. 2008;8:419–429. doi: 10.2174/156652308786848021. [DOI] [PubMed] [Google Scholar]
- 48.Bokhoven M, Stephen SL, Knight S, Gevers EF, Robinson IC, Takeuchi Y, Collins MK. Insertional gene activation by lentiviral and gammaretroviral vectors. J Virol. 2009;83:283–294. doi: 10.1128/JVI.01865-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang F, Thornhill SI, Howe SJ, Ulaganathan M, Schambach A, Sinclair J, Kinnon C, Gaspar HB, Antoniou M, Thrasher AJ. Lentiviral vectors containing an enhancer-less ubiquitously acting chromatin opening element (UCOE) provide highly reproducible and stable transgene expression in hematopoietic cells. Blood. 2007;110:1448–1457. doi: 10.1182/blood-2006-12-060814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rahim AA, Wong AM, Howe SJ, Buckley SM, Acosta-Saltos AD, Elston KE, Ward NJ, Philpott NJ, Cooper JD, Anderson PN, et al. Efficient gene delivery to the adult and fetal CNS using pseudotyped non-integrating lentiviral vectors. Gene Ther. 2009;16:509–520. doi: 10.1038/gt.2008.186. [DOI] [PubMed] [Google Scholar]
- 51.Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C, SergiSergi L, Benedicenti F, Ambrosi A, Di Serio C, et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- 52.Kumar M, Keller B, Makalou N, Sutton RE. Systematic determination of the packaging limit of lentiviral vectors. Hum Gene Ther. 2001;12:1893–1905. doi: 10.1089/104303401753153947. [DOI] [PubMed] [Google Scholar]
- 53.Kimura E, Li S, Gregorevic P, Fall BM, Chamberlain JS. Dystrophin delivery to muscles of mdx mice using lentiviral vectors leads to myogenic progenitor targeting and stable gene expression. Mol Ther. 2010;18:206–213. doi: 10.1038/mt.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quenneville SP, Chapdelaine P, Skuk D, Paradis M, Goulet M, Rousseau J, Xiao X, Garcia L, Tremblay JP. Autologous transplantation of muscle precursor cells modified with a lentivirus for muscular dystrophy: human cells and primate models. Mol Ther. 2007;15:431–438. doi: 10.1038/sj.mt.6300047. [DOI] [PubMed] [Google Scholar]
- 55.Lai Y, Thomas GD, Yue Y, Yang HT, Li D, Long C, Judge L, Bostick B, Chamberlain JS, Terjung RL, Duan D. Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J Clin Invest. 2009;119:624–635. doi: 10.1172/JCI36612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beauchamp JR, Morgan JE, Pagel CN, Partridge TA. Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J Cell Biol. 1999;144:1113–1122. doi: 10.1083/jcb.144.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ono Y, Masuda S, Nam H, Benezra R, Miyagoe-Suzuki Y, Takeda S. Slow-dividing satellite cells retain long-term self-renewal ability in adult muscle. J Cell Sci. 2012;125:1309–1317. doi: 10.1242/jcs.096198. [DOI] [PubMed] [Google Scholar]
- 58.Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 2007;25:885–894. doi: 10.1634/stemcells.2006-0372. [DOI] [PubMed] [Google Scholar]
- 59.Fukada S, Uezumi A, Ikemoto M, Masuda S, Segawa M, Tanimura N, Yamamoto H, Miyagoe-Suzuki Y, Takeda S. Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem Cells. 2007;25:2448–2459. doi: 10.1634/stemcells.2007-0019. [DOI] [PubMed] [Google Scholar]
- 60.Gilbert PM, Blau HM. Engineering a stem cell house into a home. Stem Cell Res Ther. 2011;2:3. doi: 10.1186/scrt44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Csete M. Oxygen in the cultivation of stem cells. Ann N Y Acad Sci. 2005;1049:1–8. doi: 10.1196/annals.1334.001. [DOI] [PubMed] [Google Scholar]
- 62.Brahimi-Horn MC, Pouysségur J. Oxygen, a source of life and stress. FEBS Lett. 2007;581:3582–3591. doi: 10.1016/j.febslet.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 63.Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol. 2008;9:285–296. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 65.Duguez S, Duddy WJ, Gnocchi V, Bowe J, Dadgar S, Partridge TA. Atmospheric oxygen tension slows myoblast proliferation via mitochondrial activation. PLoS One. 2012;7:e43853. doi: 10.1371/journal.pone.0043853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu W, Wen Y, Bi P, Lai X, Liu XS, Liu X, Kuang S. Hypoxia promotes satellite cell self-renewal and enhances the efficiency of myoblast transplantation. Development. 2012;139:2857–2865. doi: 10.1242/dev.079665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gilbert PM, Corbel S, Doyonnas R, Havenstrite K, Magnusson KEG, Blau HM. A single cell bioengineering approach to elucidate mechanisms of adult stem cell self-renewal. Integr Biol. 2012;4:360–367. doi: 10.1039/c2ib00148a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Latil M, Rocheteau P, Châtre L, Sanulli S, Mémet S, Ricchetti M, Tajbakhsh S, Chrétien F. Skeletal muscle stem cells adopt a dormant cell state post mortem and retain regenerative capacity. Nat Commun. 2012;3:903. doi: 10.1038/ncomms1890. [DOI] [PubMed] [Google Scholar]
- 70.McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011;138:3657–3666. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chazaud B, Brigitte M, Yacoub-Youssef H, Arnold L, Gherardi R, Sonnet C, Lafuste P, Chretien F. Dual and beneficial roles of macrophages during skeletal muscle regeneration. Exerc Sport Sci Rev. 2009;37:18–22. doi: 10.1097/JES.0b013e318190ebdb. [DOI] [PubMed] [Google Scholar]
- 73.Shireman PK, Contreras-Shannon V, Ochoa O, Karia BP, Michalek JE, McManus LM. MCP-1 deficiency causes altered inflammation with impaired skeletal muscle regeneration. J Leukoc Biol. 2007;81:775–785. doi: 10.1189/jlb.0506356. [DOI] [PubMed] [Google Scholar]
- 74.Tidball JG, Wehling-Henricks M. Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J Physiol. 2007;578:327–336. doi: 10.1113/jphysiol.2006.118265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lescaudron L, Peltékian E, Fontaine-Pérus J, Paulin D, Zampieri M, Garcia L, Parrish E. Blood borne macrophages are essential for the triggering of muscle regeneration following muscle transplant. Neuromuscul Disord. 1999;9:72–80. doi: 10.1016/s0960-8966(98)00111-4. [DOI] [PubMed] [Google Scholar]
- 76.Bencze M, Negroni E, Vallese D, Yacoub-Youssef H, Chaouch S, Wolff A, Aamiri A, Di Santo JP, Chazaud B, Butler-Browne G, et al. Proinflammatory macrophages enhance the regenerative capacity of human myoblasts by modifying their kinetics of proliferation and differentiation. Mol Ther. 2012;20:2168–2179. doi: 10.1038/mt.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lesault PF, Theret M, Magnan M, Cuvellier S, Niu Y, Gherardi RK, Tremblay JP, Hittinger L, Chazaud B. Macrophages improve survival, proliferation and migration of engrafted myogenic precursor cells into MDX skeletal muscle. PLoS One. 2012;7:e46698. doi: 10.1371/journal.pone.0046698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moyer AL, Wagner KR. Regeneration versus fibrosis in skeletal muscle. Curr Opin Rheumatol. 2011;23:568–573. doi: 10.1097/BOR.0b013e32834bac92. [DOI] [PubMed] [Google Scholar]
- 79.Mathew SJ, Hansen JM, Merrell AJ, Murphy MM, Lawson JA, Hutcheson DA, Hansen MS, Angus-Hill M, Kardon G. Connective tissue fibroblasts and Tcf4 regulate myogenesis. Development. 2010;138:371–384. doi: 10.1242/dev.057463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature. 2012;490:355–360. doi: 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z. Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol. 2006;294:50–66. doi: 10.1016/j.ydbio.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boldrin L, Zammit PS, Muntoni F, Morgan JE. Mature adult dystrophic mouse muscle environment does not impede efficient engrafted satellite cell regeneration and self-renewal. Stem Cells. 2009;27:2478–2487. doi: 10.1002/stem.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boldrin L, Neal A, Zammit PS, Muntoni F, Morgan JE. Donor satellite cell engraftment is significantly augmented when the host niche is preserved and endogenous satellite cells are incapacitated. Stem Cells. 2012;30:1971–1984. doi: 10.1002/stem.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bouchentouf M, Benabdallah BF, Tremblay JP. Myoblast survival enhancement and transplantation success improvement by heat-shock treatment in mdx mice. Transplantation. 2004;77:1349–1356. doi: 10.1097/01.tp.0000121503.01535.f5. [DOI] [PubMed] [Google Scholar]
- 86.Riederer I, Negroni E, Bigot A, Bencze M, Di Santo J, Aamiri A, Butler-Browne G, Mouly V. Heat shock treatment increases engraftment of transplanted human myoblasts into immunodeficient mice. Transplant Proc. 2008;40:624–630. doi: 10.1016/j.transproceed.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 87.Bouchentouf M, Benabdallah BF, Bigey P, Yau TM, Scherman D, Tremblay JP. Vascular endothelial growth factor reduced hypoxia-induced death of human myoblasts and improved their engraftment in mouse muscles. Gene Ther. 2008;15:404–414. doi: 10.1038/sj.gt.3303059. [DOI] [PubMed] [Google Scholar]
- 88.Morgan J, Rouche A, Bausero P, Houssaïni A, Gross J, Fiszman MY, Alameddine HS. MMP-9 overexpression improves myogenic cell migration and engraftment. Muscle Nerve. 2010;42:584–595. doi: 10.1002/mus.21737. [DOI] [PubMed] [Google Scholar]
- 89.Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, Gamradt M, ap Rhys CM, Holm TM, Loeys BL, Ramirez F, Judge DP, Ward CW, Dietz HC. Angiotensin II type 1 receptor blockade attenuates TGF-β-induced failure of muscle regeneration in multiple myopathic states. Nat Med. 2007;13:204. doi: 10.1038/nm1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fakhfakh R, Lamarre Y, Skuk D, Tremblay JP. Losartan enhances the success of myoblast transplantation. Cell Transplant. 2012;21:139–152. doi: 10.3727/096368911X576045. [DOI] [PubMed] [Google Scholar]
- 91.Fakhfakh R, Michaud A, Tremblay JP. Blocking the myostatin signal with a dominant negative receptor improves the success of human myoblast transplantation in dystrophic mice. Mol Ther. 2011;19:204–210. doi: 10.1038/mt.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Riederer I, Negroni E, Bencze M, Wolff A, Aamiri A, Di Santo JP, Silva-Barbosa SD, Butler-Browne G, Savino W, Mouly V. Slowing down differentiation of engrafted human myoblasts into immunodeficient mice correlates with increased proliferation and migration. Mol Ther. 2012;20:146–154. doi: 10.1038/mt.2011.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gérard C, Dufour C, Goudenege S, Skuk D, Tremblay JP. AG490 improves the survival of human myoblasts in vitro and in vivo. Cell Transplant. 2012;21:2665–2676. doi: 10.3727/096368912X655028. [DOI] [PubMed] [Google Scholar]
- 94.Lafreniere JF, Mills P, Tremblay JP, El Fahime E. Growth factors improve the in vivo migration of human skeletal myoblasts by modulating their endogenous proteolytic activity. Transplantation. 2004;77:1741–1747. doi: 10.1097/01.tp.0000131175.60047.eb. [DOI] [PubMed] [Google Scholar]
- 95.Lafreniere JF, Caron MC, Skuk D, Goulet M, Cheikh AR, Tremblay JP. Growth factor coinjection improves the migration potential of monkey myogenic precursors without affecting cell transplantation success. Cell Transplant. 2009;18:719–730. doi: 10.3727/096368909X470900. [DOI] [PubMed] [Google Scholar]
- 96.Brimah K, Ehrhardt J, Mouly V, Butler-Browne GS, Partridge TA, Morgan JE. Human muscle precursor cell regeneration in the mouse host is enhanced by growth factors. Hum Gene Ther. 2004;15:1109–1124. doi: 10.1089/hum.2004.15.1109. [DOI] [PubMed] [Google Scholar]
- 97.Morgan JE, Hoffman EP, Partridge TA. Normal myogenic cells from newborn mice restore normal histology to degenerating muscles of the mdx mouse. J Cell Biol. 1990;111:2437–2449. doi: 10.1083/jcb.111.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morgan JE, Pagel CN, Sherrratt T, Partridge TA. Long-term persistence and migration of myogenic cells injected into pre-irradiated muscles of mdx mice. J Neurol Sci. 1993;115:191–200. doi: 10.1016/0022-510x(93)90224-m. [DOI] [PubMed] [Google Scholar]
- 99.Harris JB. Myotoxic phospholipases A2 and the regeneration of skeletal muscles. Toxicon. 2003;42:933–945. doi: 10.1016/j.toxicon.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 100.Morgan JE, Coulton GR, Partridge TA. Muscle precursor cells invade and repopulate freeze-killed muscles. J Muscle Res Cell Motil. 1987;8:386–396. doi: 10.1007/BF01578428. [DOI] [PubMed] [Google Scholar]
- 101.Gross JG, Bou-Gharios G, Morgan JE. Potentiation of myoblast transplantation by host muscle irradiation is dependent on the rate of radiation delivery. Cell Tissue Res. 1999;298:371–375. doi: 10.1007/s004419900062. [DOI] [PubMed] [Google Scholar]
- 102.Grounds MD, Yablonka-Reuveni Z. Molecular and cell biology of skeletal muscle regeneration. Mol Cell Biol Hum Dis Ser. 1993;3:210–256. doi: 10.1007/978-94-011-1528-5_9. [DOI] [PubMed] [Google Scholar]
- 103.Ehrhardt J, Brimah K, Adkin C, Partridge T, Morgan J. Human muscle precursor cells give rise to functional satellite cells in vivo. Neuromuscul Disord. 2007;17:631–638. doi: 10.1016/j.nmd.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 104.Bouchentouf M, Benabdallah BF, Mills P, Tremblay JP. Exercise improves the success of myoblast transplantation in mdx mice. Neuromuscul Disord. 2006;16:518–529. doi: 10.1016/j.nmd.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 105.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 106.Darabi R, Arpke RW, Irion S, Dimos JT, Grskovic M, Kyba M, Perlingeiro RC. Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell. 2012;10:610–619. doi: 10.1016/j.stem.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Goudenege S, Lebel C, Huot NB, Dufour C, Fujii I, Gekas J, Rousseau J, Tremblay JP. Myoblasts derived from normal hESCs and dystrophic hiPSCs efficiently fuse with existing muscle fibers following transplantation. Mol Ther. 2012;20:2153–2167. doi: 10.1038/mt.2012.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu SM, Hochedlinger K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol. 2011;13:497–505. doi: 10.1038/ncb0511-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chang H, Yoshimoto M, Umeda K, Iwasa T, Mizuno Y, Fukada S, Yamamoto H, Motohashi N, Miyagoe-Suzuki Y, Takeda S, et al. Generation of transplantable, functional satellite-like cells from mouse embryonic stem cells. FASEB J. 2009;23:1907–1919. doi: 10.1096/fj.08-123661. [DOI] [PubMed] [Google Scholar]
- 110.Mizuno Y, Chang H, Umeda K, Niwa A, Iwasa T, Awaya T, Fukada S, Yamamoto H, Yamanaka S, Nakahata T, Heike T. Generation of skeletal muscle stem/progenitor cells from murine induced pluripotent stem cells. FASEB J. 2010;24:2245–2253. doi: 10.1096/fj.09-137174. [DOI] [PubMed] [Google Scholar]
- 111.Darabi R, Pan W, Bosnakovski D, Baik J, Kyba M, Perlingeiro RCR. Functional myogenic engraftment from mouse iPS cells. Stem Cell Rev. 2011;7:948–957. doi: 10.1007/s12015-011-9258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Barberi T, Bradbury M, Dincer Z, Panagiotakos G, Socci ND, Studer L. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med. 2007;13:642–648. doi: 10.1038/nm1533. [DOI] [PubMed] [Google Scholar]
- 113.Tedesco FS, Gerli MF, Perani L, Benedetti S, Ungaro F, Cassano M, Antonini S, Tagliafico E, Artusi V, Longa E, et al. Transplantation of genetically corrected human iPSC-derived progenitors in mice with limb-girdle muscular dystrophy. Sci Transl Med. 2012;4:140ra89. doi: 10.1126/scitranslmed.3003541. [DOI] [PubMed] [Google Scholar]
- 114.Hussein SMI, Elbaz J, Nagy AA. Genome damage in induced pluripotent stem cells: assessing the mechanisms and their consequences. BioEssays. 2013;35:152–162. doi: 10.1002/bies.201200114. [DOI] [PubMed] [Google Scholar]
- 115.Patel K, Morgan J. 185th ENMC International Workshop: stem/precursor cells as a therapeutic strategy for muscular dystrophies 3–5 June 2011, Naarden, The Netherlands. Neuromuscul Disord. 2012;22:447–452. doi: 10.1016/j.nmd.2011.09.008. [DOI] [PubMed] [Google Scholar]


