Abstract
STUDY QUESTION
Is a vaginal preparation of sildenafil citrate capable of alleviating acute menstrual pain in patients with primary dysmenorrhea (PD)?
SUMMARY ANSWER
A vaginal preparation of sildenafil citrate is capable of alleviating acute menstrual pain in patients with PD with no observed adverse effects.
WHAT IS KNOWN ALREADY
Oral preparations of nitric oxide (NO) donor drugs augment relaxant effects of NO on myometrial cells, reverse the vasoconstriction caused by prostaglandins and successfully alleviate pain, but the incidence of side effects is too high for routine clinical use. Sildenafil citrate inhibits type 5-specific phosphodiesterase (PDE5), thus preventing the degradation of cyclic guanosine monophosphate (cGMP) in the muscle and augmenting the vasodilatory effects of NO. Therefore, by inhibiting PDE5, the tissue remains relaxed and more blood can circulate through. It has been used previously in a vaginal form with no observed side effects, and it enhances endometrial blood flow.
STUDY DESIGN, SIZE, DURATION
A double-blind, randomized, controlled trial comparing vaginal preparation of sildenafil citrate (100 mg single dose) to a placebo in 62 PD patients at the time of painful menstruation was conducted. The primary outcome was total pain relief over 4 consecutive hours (TOPAR4) comparing sildenafil citrate to placebo, where higher TOPAR4 scores represent better pain relief. Secondary outcomes were pain relief as measured by the visual analog scale (VAS) and uterine artery pulsatility index (PI). Subjects were recruited from December 2007 to January 2011. The trial was stopped due to closeout of the funding for the study.
PARTICIPANTS, SETTINGS, METHODS
Participants were women in good health, were aged 18–35 years and suffered from moderate to severe PD. They were randomized to either vaginal placebo or 100 mg vaginal sildenafil citrate in a 1:1 ratio using random permuted blocks having a block size of 4. At baseline and 1, 2, 3, and 4 h post-treatment, patients were asked to provide assessment of their degree of pain using two scales: (i) pain on the 5-level ordinal scale used for TOPAR4 calculation and (ii) pain level on the VAS. The study ended 4 h after treatment initiation.
MAIN RESULTS AND THE ROLE OF CHANCE
Twenty-five subjects completed the study. Using the TOPAR4 score, the sildenafil citrate group had significantly better pain relief compared with the placebo group [mean (SD): 11.9 (3.2) versus 6.4 (2.1), respectively; difference in means = 5.3; 95% CI: (2.9,7.6); P < 0.001)]. On the VAS, sildenafil citrate provided better pain relief than placebo at each time point. At the 2-h time point, the PI was significantly lower in the sildenafil citrate group compared with the placebo group [mean (SD): 1.6 (0.6) versus 2.3 (0.5), respectively; difference in means = −0.7; 95% CI: (−1.2, −0.1); P = 0.01)].
LIMITATIONS, REASONS FOR CAUTION
Since we did not meet our sample size due to the loss of funding and could not confirm our primary hypothesis, larger studies of longer duration, likely multi-center, are needed to confirm the findings from this study.
WIDER IMPLICATIONS OF THE FINDINGS
A number of medications have been investigated to improve the treatment options for PD, but most have proven unsuccessful or to have an unfavorable risk/benefit ratio. Since PD is a condition that most women suffer from and seek treatment for at some point in their lives, our study offers hope that vaginal sildenafil citrate is a safe and effective option for patients who do not desire or are unresponsive to treatments now available on the market.
STUDY FUNDING/COMPETING INTERESTS
Funding for this study was provided by National Institutes of Health (NIH) grants RO3 TW007438 and K24 HD01476. The authors report no relevant conflicts of interest.
TRIAL REGISTRATION NUMBER
NCT00123162 (Clinical trials.gov).
Keywords: primary dysmenorrhea, sildenafil citrate
Introduction
Primary dysmenorrhea (PD) is by far the most common cause of pelvic pain in women. This has both psychological and economic impact, as dysmenorrhea accounts for 600 million lost work hours and $2 billion annually in the USA (Dawood, 1984). It is most commonly attributable to excess endometrial prostaglandin production at the time of menstruation, which leads to abnormal uterine contractions, increased intrauterine pressure, vasoconstriction of small uterine vessels and increased sensitivity of pain receptors, which consequently causes pelvic pain (Pulkkinen, 1983; Hauksson et al., 1988). During uterine contractions, endometrial blood flow decreases suggesting that ischemia due to the hypercontractility exacerbates the pain (Hauksson et al., 1988; Akerlund, 1994).
Nitric oxide (NO) donor drugs augment relaxant effects of NO on myometrial cells, reverse the vasoconstriction caused by prostaglandins and improve uterine blood flow in patients with PD (Moya et al., 2000; Facchinetti et al., 2002). Although these drugs cause vasodilatation and successfully alleviate pain, the incidence of side effects may be too high for routine clinical use with oral route for this indication (Facchinetti et al., 2002). Sildenafil citrate (sildenafil) augments the effect of NO because it inhibits type 5-specific phosphodiesterase (PDE5) and decreases the breakdown of cyclic guanosine monophosphate (cGMP) generated by soluble guanylyl cyclase. It causes relaxation of female rabbit clitoral and vaginal muscle strips, and inhibits mechanical uterine contractions in rat myometrium (Park et al., 1998; Traish et al., 1999; Buhimschi et al., 2004). Enhanced genital blood flow has been demonstrated with sildenafil (Berman et al., 2001). Several authors studied endometrial development following treatment with vaginal sildenafil and concluded that it enhances endometrial blood flow (Sher and Fisch, 2000, 2002; Paulus et al., 2002).
The vagina is an effective route for drug administration intended mainly for local action because delivering medication in close proximity to the target organ decreases the incidence of side effects. The major advantages of vaginal route include accessibility, good blood supply and the ability to bypass first-pass liver metabolism (Back et al., 1987; Chakmakjian and Zachariah, 1987). For these reasons, intravaginal drug delivery is particularly appropriate for drugs associated with women's health issues as it increases safety. The main problem with NO donor drugs is reduced tolerability in the form of a headache, but similar effects with bromocriptine were ameliorated with vaginal administration without loss of treatment efficacy (Vermesh et al., 1988).
To date vaginal sildenafil has not been used in the treatment of PD. We hypothesized that vaginal delivery of sildenafil citrate would induce local vasodilatation of the uterine vessels resulting in amelioration of pain in PD patients while minimizing side effects as the majority of the active substance probably would not reach the systemic circulation. We further hypothesized that the uterine artery pulsatility index (PI) would be decreased. Here we have compared pain relief achieved by sildenafil citrate with a placebo and measured the uterine artery PI by color Doppler ultrasound.
Materials and Methods
Human subjects
The study was approved by the Institutional Review Board at Penn State Hershey Medical Center, USA, and by the Ethics Committee at Nova Gradiska General Hospital in Croatia where the trial was conducted. We obtained an IND from the U.S. Food and Drug Administration (FDA IND # 72 008), and the Croatian Central Ethics Committee granted approval for the use of vaginal preparation of sildenafil citrate. Subjects were recruited from the Ob/Gyn Department at Nova Gradiska General Hospital from December 2007 to January 2011. The trial was stopped due to closure of the funding for the study. Women who participated in the study were either presenting at the clinic for their annual Ob/Gyn examination, complaining of dysmenorrhea, or were referred to the clinic for evaluation of dysmenorrhea. All subjects gave written informed consent.
Inclusion criteria
Women in good health, aged 18–35 years, and who suffered from moderate to severe PD (onset < 3 years after menarche) were enrolled in the study. Subjects had regular (25–31-day) menstrual cycles for the 3-month period preceding enrollment, with symptoms of moderate to severe PD during those cycles. The pain associated with PD was abdominal or pelvic, could radiate to the back and along the thighs and could begin up to 1 day before menses and last for the first 3 days of bleeding. The pain could be accompanied with systemic symptoms including nausea, vomiting, diarrhea, headache, fatigue, nervousness and dizziness.
Exclusion criteria
Exclusion criteria were known or suspected hypersensitivity to trial drug; known or suspected secondary dysmenorrhea (major abdominal or pelvic surgery, endometriosis, pelvic inflammatory disease (PID), ovarian cysts, pathological vaginal secretion, chronic abdominal pain, inflammatory bowel disease, irritable bowel syndrome); resting hypotension (BP < 90/50) or hypertension (BP > 170/110); known or suspected chronic disease, malignancy; serious eye conditions; concomitant long-term treatments; and undiagnosed vaginal bleeding (within the past six cycles). Patients enrolled simultaneously into other investigative studies that required medications or would otherwise prevent compliance with the protocol were excluded.
Design
The study was a double-blind, randomized, controlled trial of a 100 mg single vaginal dose of sildenafil citrate compared with a single vaginal dose of placebo in PD patients. The primary hypothesis was that the 100 mg single dose of sildenafil citrate would have at least a 6.5 unit improvement in a total pain relief over 4 h (TOPAR4) compared with a single dose of placebo in the treatment of moderate-to-severe PD. TOPAR4 (Korn et al., 2004) is a calculated value; it is a weighted sum of pain relief scores (where 0, none; 1, a little; 2, some; 3, a lot and 4, complete) up to Hour 4 calculated by multiplying the pain relief score at each time point by the duration of time since the preceding assessment, and summing these weighed values up to Hour 4. TOPAR has been used as a method to evaluate the response to a drug for acute pain and also the pain related to PD (Mehlisch et al., 1984; Mehlisch, 1998; Malmstrom et al., 2002).
The dosage of 100 mg was extrapolated from the maximum recommended oral daily dose for men. Because we could not know how the drug would act in the vaginal form, and rapid absorption and quick pain relief were of outmost importance, we decided on 100 mg. This dosage of 100 mg also has been used in previous trials with vaginal sildenafil, divided into four daily doses of 25 mg through several days (Paulus et al., 2002). The bioavailability and local action of drugs administered vaginally can be very low, justifying the need for a higher single dose (Chakmakjian and Zachariah, 1987, Chowdary and Rao, 2004).
The visual analog scale (VAS) was a secondary outcome measure of pain relief. The VAS is a validated pain scale (Littman et al., 1985) used widely in clinical trials assessing the intensity of pain, especially in trials with patients suffering from PD (Dmitrovic et al., 2012). The VAS uses an analog linear scale to assess pain intensity. The scale is 100 mm long; the extremes of the scale are to the left ‘no pain’ (i.e. 0 mm) and to the right ‘worst pain I have ever felt’ (i.e. 100 mm). The score itself is determined by measuring the distance from the left side of the scale to the point that the patient marked as their level of pain. Another secondary outcome measure was the uterine artery color Doppler pulsatility index assessed before and after the administration of the randomized drug.
Study visits
Initial screening was done over the phone, when study design and aims were explained to the subjects. Women who satisfied the phone inclusion criteria and were willing to participate had a screening visit. At the screening visit, inclusion and exclusion criteria were determined and informed consent signed. Eligible subjects were then seen at the study visit where randomization was performed and a study drug was administered.
At the screening visit the following tests were done in order to determine if the subjects were eligible for inclusion in the study: a full physical exam including blood pressure assessment and a Pap smear (if no results within a year were available); transvaginal ultrasound to determine uterine size and/or abnormalities, endometrial thickness, and ovarian size and morphology to exclude causes of secondary dysmenorrhea; a phlebotomy for glucose, complete blood count (CBC), alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase, creatinine, total bilirubin, blood urea nitrogen (BUN) and an electrocardiography (EKG). After evaluation of the screening visit results, women who satisfied the study requirements were called and counseled how to schedule a study visit.
The study visit was not scheduled in advance because it is difficult for most women to predict the exact day of their next menstrual bleeding. Within 6 months of the screening visit, patients were instructed to call the coordinator when they got their period and were experiencing pain due to PD. The study coordinator, depending on the availability of the facilities and the study personnel, scheduled subjects for that day or instructed the subjects to take their usual pain medication pill and call during the next day if still in pain, or to call during their next cycle. The subjects were allowed to take their usual pain medication up to 6 h prior to entering the study. If they were in need of another pain medication, i.e. rescue pain medication, during the course of the 4 h study, their participation in the study was terminated at the time of their taking a rescue pain medication.
Upon arrival for the study visit, regardless if it was the first, second or third day of their cycle, subjects were asked to rate their pain using two scales: (i) pain on the 5-level ordinal scale used for TOPAR4 calculation and (ii) pain level on the VAS. They were only allowed to participate in the study if they rated their pain as moderate or severe, and marked the scale on the VAS that corresponded to >35 mm. TOPAR4 and VAS were then presented to subjects at the administration of the treatment, and at each hour following the administration of the treatment up to a maximum of 4 h.
Next, a baseline examination measuring blood pressure was performed along with an EKG and a color Doppler transvaginal ultrasound using an Aloka 2000 color Doppler ultrasound with 7.5 MHz vaginal transducer. The transducer was inserted into the vagina; uterus and ovaries were visualized. The scanning technique was as follows: after excluding uterine and ovarian pathology with the conventional ultrasound, uterine arteries were visualized laterally in the transverse section of the cervicocorporeal junction. Measurements of the PI (PI = (maximal systolic flow − minimal diastolic flow)/mean flow) were made after at least three consecutive blood flow velocity waveforms were analyzed (Scholtes et al., 1989).
Following that, subjects were randomized to one of the two treatment arms and the study medication was administered vaginally by study personnel. At baseline and 1, 2, 3 and 4 h post-treatment, subjects were asked to provide assessment of degree of pain using two scales: (i) pain on the 5-level ordinal scale used for TOPAR4 calculation and (ii) pain level on the VAS. Two hours after the administration of the randomized treatment, color Doppler ultrasound and the measurement of uterine artery PI was repeated. All ultrasound measurements at baseline and after study drug administration were made by the same examiner (R.D.) who was blinded to treatment assignment. The study ended 4 h after treatment initiation, and the rescue pain medications were then allowed, if needed.
Randomization
Subjects were randomized to either vaginal placebo or 100 mg of vaginal sildenafil citrate in a 1:1 ratio using random permuted blocks having a block size of 4. The randomization scheme was generated by our biostatistician at Penn State (A.R.K.) and the randomization list was sent to the Pliva Pharmaceutical Company in Croatia (Pliva d.d., Zagreb), who manufactured the vaginal sildenafil citrate and matching placebo and packed the drugs into individual boxes marked by the randomization number. The drugs were then sent to the research site, where they were used—in consecutive order when a subject was eligible and ready to be randomized. Both participants and care providers in the study were blinded to the treatment, and the randomization list was kept at Penn State and at Pliva Pharmaceutical Company until the end of recruitment.
Sample size and data analysis
Assuming a standard deviation of 7 units for the primary outcome of pain relief assessed using TOPAR4, a sample size of 26 subjects per treatment group provided 90% statistical power to detect a difference of 6.5 units in TOPAR4 between the sildenafil citrate and placebo groups using a two-sided test having a significance level of 0.05. We anticipated subject drop-out as high as 15%; therefore, we had targeted to recruit a total of 31 subjects per treatment group.
A modified intention-to-treat (mITT) approach, defined prior to study initiation, was used for efficacy analyses. All subjects who recorded at least one primary outcome pain score post-dose were included in the mITT. Missing pain relief scores (TOPAR4) and pain severity scores (VAS) were imputed using the last-observation-carried-forward approach. Baseline values were not imputed nor carried forward. All hypotheses tests were two-sided and all analyses were performed using the SAS statistical software, version 9.2 (SAS Institute, Inc., Cary, NC, USA).
For the primary hypothesis of the overall analgesic effect, an analysis of covariance model with factors for treatment group and baseline pain intensity were used to compare the mean TOPAR4 scores for a single dose of 100 mg of sildenafil versus placebo.
Linear mixed-effects models (Laird and Ware, 1982) were fitted to the data to assess between-group (treatment) and within-group (hour) differences for all continuous secondary outcomes. Linear mixed-effects models are an extension of regression that accounts for the within-subject correlation of repeated measurements over time. Residual diagnostics were assessed to ensure that parametric modeling assumptions were met for the analysis of covariance and linear mixed-effects models.
Results
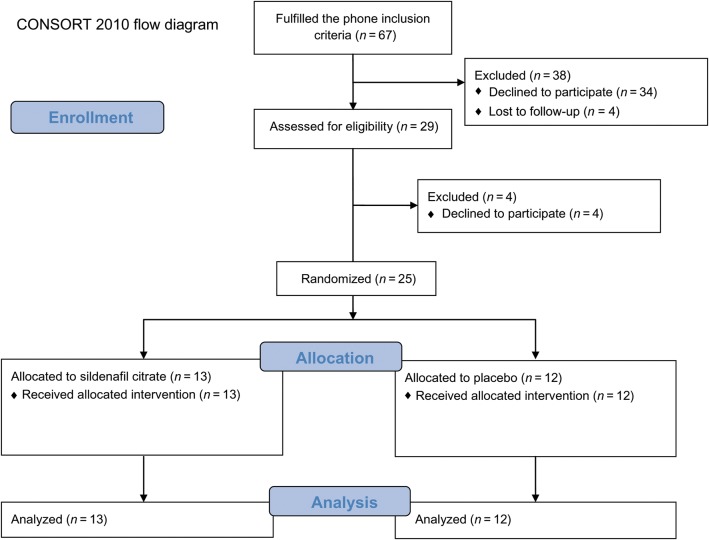
Twenty-nine women were screened; 25 were randomized and completed the study (Fig. 1). All baseline descriptive characteristics were comparable between the groups (Table I). In Table II, mean TOPAR4 score, VAS scores at 0, 1, 2, 3 and 4 h and PI values at 0 and 2 h are shown, as well as the differences between the treatments, which demonstrate that sildenafil provided better pain relief than placebo overall (TOPAR4 results) and at each post-treatment time point (VAS results). Placebo also provided significant pain relief compared with baseline at the 2, 3 and 4-h time points (Fig. 2). We confirmed that the VAS changes from baseline for sildenafil were significantly greater than the changes from baseline for placebo at all 4 time points (data not shown).
Figure 1.
Flow diagram for the study.
Table I.
Baseline characteristics of the study subjects.
| Sildenafil |
Placebo |
P-value* | |||
|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||
| Biometric | |||||
| Age (years) | 13 | 23.7 (5.7) | 12 | 23.3 (4.8) | 0.84 |
| Weight (kg) | 13 | 56.6 (4.7) | 12 | 60.1 (9.4) | 0.25 |
| BMI (kg/m2) | 13 | 20.5 (2.0) | 12 | 21.9 (3.6) | 0.25 |
| Systolic blood pressure (mmHg) | 13 | 110.4 (8.3) | 12 | 108.3 (8.3) | 0.54 |
| Diastolic blood pressure (mmHg) | 13 | 68.5 (10.1) | 12 | 67.5 (7.5) | 0.79 |
| Liver profile | |||||
| Bilirubin (µmol/l) | 13 | 12.4 (5.7) | 12 | 12.2 (3.5) | 0.91 |
| Alkaline phosphatase (J/l) | 12 | 53.4 (11.5) | 12 | 62.7 (20.6) | 0.19 |
| Alaninetransaminase (J/l) | 13 | 14.5 (4.9) | 12 | 20.7 (13.3) | 0.13 |
| Glucose (nmol/l) | 13 | 4.9 (0.7) | 12 | 5.0 (0.3) | 0.48 |
| Renal profile | |||||
| Blood urea nitrogen (µmol/l) | 13 | 239.9 (42.3) | 12 | 224.3 (66.7) | 0.49 |
| Creatinine (µmol/l) | 13 | 79.5 (5.7) | 12 | 78.1 (8.8) | 0.62 |
| Complete blood count | |||||
| White blood cells (×109/l) | 13 | 7.1 (2.4) | 10 | 6.4 (1.6) | 0.41 |
| Red blood cells (×1012/l) | 13 | 4.4 (0.3) | 11 | 4.4 (0.3) | 0.64 |
| Hemoglobin (g/l) | 13 | 132.0 (9.8) | 11 | 132.4 (7.3) | 0.92 |
| Platelet count (×109/l) | 13 | 268.7 (47.9) | 11 | 234.8 (79.0) | 0.21 |
| Conventional ultrasound | |||||
| Endometrial thickness (mm) | 10 | 7.1 (2.7) | 10 | 6.6 (2.3) | 0.65 |
| Total ovarian volume (cm3) | 11 | 11.3 (4.2) | 12 | 12.7 (5.7) | 0.53 |
*P-values are from two-sample t-tests.
Table II.
Comparison of the effects of study treatments on pain relief (TOPAR4), pain assessed on a visual analogue scale (VAS) and uterine artery pulsatility (PI).
| Sildenafil, mean (SD) (n = 13) | Placebo, mean (SD) (n = 12) | Difference in means (95% CI) | P-value* | |
|---|---|---|---|---|
| TOPAR4 | 11.9 (3.2) | 6.4 (2.1) | 5.3 (2.9, 7.6) | <0.001 |
| VAS | ||||
| 0 h | 91.2 (11.2) | 95.6 (5.3) | −4.4 (−20.1, 11.4) | 0.58 |
| 1 h | 65.2 (21.2) | 88.6 (10.9) | −23.4 (−39.2, −7.7) | 0.004 |
| 2 h | 23.4 (25.3) | 72.5 (11.8) | −49.1 (−64.9, −33.3) | <0.001 |
| 3 h | 13.8 (25.3) | 58.5 (19.8) | −44.7 (−60.5, −29.0) | <0.001 |
| 4 h | 8.8 (25.0) | 51.4 (26.6) | −42.6 (−58.3, −26.8) | <0.001 |
| PI | ||||
| 0 h | 2.4 (0.5) | 2.7 (0.9) | −0.3 (−0.8, 0.2) | 0.21 |
| 2 h | 1.6 (0.6) | 2.3 (0.5) | −0.7 (−1.2, −0.1) | 0.01 |
TOPAR4, total pain relief over 4 consecutive hours.
*P-values are from a linear mixed-effects model.
Figure 2.
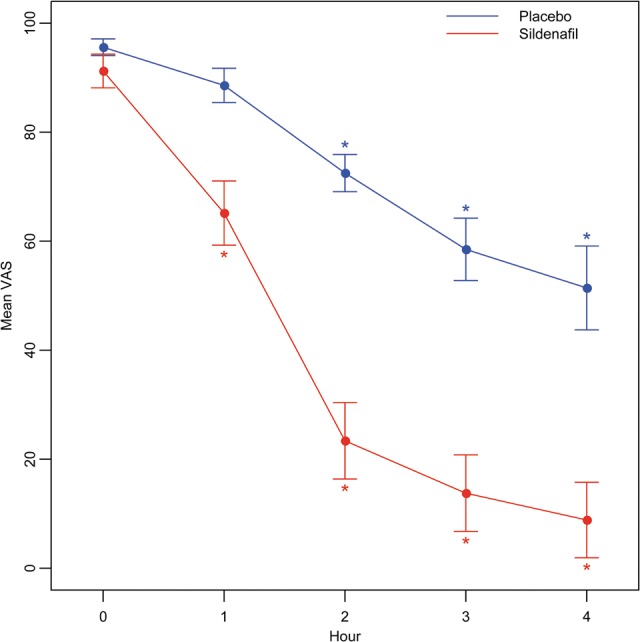
Mean VAS scores for placebo and sildenafil groups, at designated time points in the study. *A significant change from baseline within the respective group (P < 0.05 from linear mixed-effects model).
Although the mean Doppler PI was significantly higher in placebo compared with sildenafil at the 2-h time point (Table II), the changes from baseline at 2 h were not significantly different between the two groups (Table III). No significant association between the change in uterine blood flow and pain relief was found.
Table III.
Changes from baseline in the uterine artery pulsatility index.
| Difference in means (95% CI) | P-value* | |
|---|---|---|
| Sildenafil: 2 h change from baseline | −0.74 (−1.15, −0.32) | 0.001 |
| Placebo: 2 h change from baseline | −0.41 (−0.84, 0.02) | 0.06 |
| Sildenafil versus placebo: 2 h change from baseline | −0.33 (−0.93, 0.26) | 0.26 |
*P-values are from a linear mixed-effects model.
Patients did not report any side effects from any of the treatments.
Discussion
In this study, we have shown that vaginal sildenafil citrate is capable of alleviating acute menstrual pain in patients with moderate to severe PD, although placebo also provided significant pain relief as is common in such studies. Although this was the first study to investigate the effect of sildenafil in the treatment of PD, both findings were expected as both NO donor drugs and placebo have been shown to help patients with dysmenorrhea (Fedele et al., 1989; Moya et al., 2000; Facchinetti et al., 2002). Non-steroidal anti-inflammatory drugs (NSAIDs), which are the treatment of choice in PD, reduce the level of endometrial prostaglandins, but are effective in only 75% of patients. NO donor drugs, on the other hand, have a different mechanism of action, thought to counteract the vasoconstriction caused by prostaglandins, therefore ameliorating the pain in PD. However, we have shown that both sildenafil and placebo improve uterine blood flow as measured by color Doppler ultrasound, and therefore could not confirm this as a probable mechanism on pain relief. If future studies confirm these findings, sildenafil may become another treatment option for patients with PD. Also, although NO donor drugs have been associated with adverse reactions, especially headache, this was the first study to investigate the effect of vaginal drug that acts similarly to NO donor drugs in PD, and no side effects were reported by patients. Therefore, vaginal route may also be a significant delivery route for patients with PD, as it may reduce the severity of side effects.
Unfortunately, we were not able to meet our sample size. There were several barriers to recruitment. When designing this study, we did not want to dispatch study drugs for patients to use in the privacy of their homes because at that time there were no reports of using single 100 mg vaginal sildenafil tablets in any clinical setting, and we wanted to be able to monitor patients for adverse events on site (and these were also requirements of the IND and our IRB approvals). However, this proved to be a major obstacle in the recruitment for the study because patients were unwilling to abstain from self-medication, as well as come to the study site and participate in procedures at the time of their greatest pain. We had 67 initial phone conversations with patients who were interested in participating in the study and who satisfied the phone inclusion criteria, but only 29 came for the screening visit.
Though we did not meet our sample size and were not able to confirm the primary hypothesis that the 100 mg single dose of sildenafil citrate would have at least a 6.5 unit improvement in TOPAR4 over placebo, the difference we found (placebo group 6.4, sildenafil group 11.9, difference = 5.3) is close to that hypothesized. With more data, we believe our hypothesis could be confirmed. Sildenafil appears to be capable of improving uterine blood flow, as did placebo, which may be one of the mechanisms of pain relief, but we did not find a significant association between the change in uterine blood flow and pain relief. A recent study that used sildenafil 100 mg to improve endometrial thickness and lower high uterine artery impedance concludes that sildenafil citrate treatment improves these parameters and may be useful for the patients with a thin endometrium (Takasaki et al., 2010), which is confirmed by our findings.
When designing this trial, and bearing in mind that sildenafil citrate has never been used for this indication, and in this route and dosage, we felt it was safer to use placebo instead of another active drug that has never been used in vaginal form either. In the majority of studies of dysmenorrhea, placebo has been shown to help with pain in a significant number of patients, which was confirmed in our study. As our study progressed, we became aware there were no side effects to either one of the vaginally administered compounds. Now that this study is completed and no side effects were reported, future studies of pain relief in PD patients having a sildenafil arm, a placebo arm and another active drug arm should be considered to assess efficacy and safety of vaginally delivered medications. When treating pain, a placebo effect clearly exists and is mostly associated with psychosocial context surrounding the patients. The effects are non-specific and may come only from the knowledge that a treatment is being given, but usually also include some physical reactions, specifically the activation of endogenous opioids and dopamine (Fedele et al., 1989; Finniss et al., 2010; Hrobjartsson and Gotzsche, 2004). Placebo response in PD as well as in endometriosis is strong (Fedele et al., 1989; Ziaei et al., 2001; Koninckx et al., 2008). In studies of erectile dysfunction in men, the investigators used several methods (TOPAR4, VAS, color Doppler ultrasound) to determine the difference in patient pain relief perception with sildenafil citrate compared with placebo; however, with any of these methods placebo proved to be almost equally effective as the study drug (Safarinejad, 2004; Tignol et al., 2004).
Our period of observation after study drug administration may have been too short to observe later effects. The 4-h period for this study was chosen for two reasons. With sildenafil citrate, maximum median plasma concentrations are reached within 60 min and half-life is ∼4 h (McDonough, 2002). Maximal effect of the drug is expected to be seen in the first 2 h following the administration of sildenafil citrate, and the possible effects of the drug will therefore be seen within the 4-h time frame. The nature of the pain in PD is acute and needs immediate attention. Therefore, rapid absorption and quick pain relief are of outmost importance, and the drug that is used for acute pain relief in PD has to be effective within that time frame. In a study by Hale et al. (2010), it was shown that sildenafil significantly increases uterine volumetric blood flow and vascular impedance in non-pregnant, nulliparous women over the 3-h monitoring period (Hale et al., 2010), which was confirmed in our study. In addition, we were using an unapproved form of the drug for an unapproved indication and did not want to prolong the study beyond the minimum we needed to confirm our hypothesis, if safety issues arise; and also, in case the sildenafil did not help, we did not want our patients to suffer unnecessary pain.
Sildenafil citrate has not been studied in relation to menstrual bleeding. Since, in the above mentioned study (Hale et al., 2010), it has been shown that sildenafil causes vasodilatation and increases uterine blood flow, and, similarly to NO donors, may have platelet inhibitory proprieties, this may lead to heavier menstrual bleeding when administered at time of menstruation. We did not investigate nor follow the possible change in menstrual bleeding pattern in our study because of its short duration, but if studies of larger duration are to follow, this probably should be taken into account.
We did not consider repeated dosages of sildenafil citrate in patients with dysmenorrhea in this study for safety concerns because, unfortunately, there is a paucity of data on repeated dosage and pharmacokinetics of oral 100 mg of sildenafil, and none for the vaginal administration. However, it has been shown that the plasma concentration of sildenafil is dose proportional (Nichols et al., 2002), and therefore, if we would administer another 100 mg dose of sildenafil 4 h after the initial dose, it is reasonable to assume that the mean plasma concentration after several doses would rise above the one found after a single dose, and possibly increase the chance of unwanted side effects. Therefore, at present time and with no data to support otherwise, a 100 mg single vaginal dose of sildenafil should probably be taken no more than twice daily, to avoid a rise in plasma concentrations above the side effects threshold. If pain persists 4 h after the administration of the sildenafil, NSAIDs, which are better studied, should be used.
Following the end of our study, the patients were free to leave the facility and we did not follow them further. In the light of our results, we now recognize that further follow-up would have enhanced the data, but at the time of study design, we did not know if our hypothesis would stand. The care providers and patients were blinded to the allocation treatment, and did not know if the improvement in symptoms came from placebo or sildenafil. Considering our subjects were young girls in pain, who felt uncomfortable in the hospital, and the pharmacokinetics of sildenafil with the half-life of 4 h, we felt that 4 h of follow-up on site was sufficient to answer our questions. The patients who felt no pain when leaving were instructed to take another pain medication if the pain reappears, but we have no follow-up data. We understand now that we probably should have called them in 12 h, with the follow-up questions on pain.
Ultrasound has been the most important diagnostic tool in gynecology in the past decades, but it has limited value in the diagnosis of PD given that many causes of secondary dysmenorrhea evade ultrasound detection (i.e. pelvic adhesions, minimal endometriosis, etc.). Changes in uterine artery indices in patients with PD, however, have been documented previously (Dmitrovic, 2000; Altunyurt et al., 2005). Color Doppler ultrasound comparisons have been used in clinical trials that studied the relaxing effects of NO in PD patients (Paulus et al., 2002). Intra-observer (4.1%) and interobserver (11.8%) variability are lowest with transvaginal approach, suggesting that transvaginal color Doppler ultrasound is reproducible and the results obtained from clinical use are reliable (Steer et al., 1995). In this study we were able, after administration of both our study drug and placebo, to show improvement in uterine blood flow with color Doppler ultrasound suggesting that these changes may be attributed to the pain relief itself.
A number of medications have been investigated daily to improve the treatment options for PD, but most have proven unsuccessful or have an unfavorable risk/benefit ratio (Moya et al., 2000). Since PD is a condition that most women suffer from and seek treatment for at some points in their lives, the quest for new medications is justified. Our small study offers hope that vaginal sildenafil citrate is a safe and effective option for patients who do not desire or are unresponsive to treatments now available on the market. Larger studies of longer duration, likely multi-center, are needed to confirm the findings from this study. Since our study found the medication to be safe for this indication, without adverse effects, recruitment in the future would be enhanced by allowing home administration of the medication.
Authors' roles
R.D. designed and conducted the study, helped with the statistical analysis and wrote the manuscript draft. A.R.K. analyzed the data, wrote statistical section of the manuscript and edited the final version. R.S.L. helped with the design and statistical analysis, monitored the conduction of the study and edited the final version of the manuscript.
Funding
Funding for this study was provided by National Institutes of Health (NIH) (grants RO3 TW007438 and K24 HD01476).
Conflict of interest
None declared.
Acknowledgements
Funding for this study was provided by National Institutes of Health (NIH) grants RO3 TW007438 and K24 HD01476. We acknowledge the excellent supervision of this study by Ivana Segedin from Nova Gradiska General Hospital and the statistical programming support by Christina Stetter from the Department of Public Health Sciences, Penn State College of Medicine.
References
- Akerlund M. Vascularization of human endometrium. Uterine blood flow in healthy condition and in primary dysmenorrhoea. Ann N Y Acad Sci. 1994;734:47–56. doi: 10.1111/j.1749-6632.1994.tb21735.x. [DOI] [PubMed] [Google Scholar]
- Altunyurt S, Gol M, Sezer O, Demir N. Primary dysmenorrhea and uterine blood flow: a color Doppler study. J Reprod Med. 2005;50:251–255. [PubMed] [Google Scholar]
- Back DJ, Grimmer SF, Rogers S, Stevenson PJ, Orme ML. Comparative pharmacokinetics of levonorgestrel and ethinyloestradiol following intravenous, oral and vaginal administration. Contraception. 1987;36:471–479. doi: 10.1016/0010-7824(87)90095-3. [DOI] [PubMed] [Google Scholar]
- Berman JR, Berman LA, Lin H, Flaherty E, Lahey N, Goldstein I, Cantey-Kiser J. Effect of sildenafil on subjective and physiologic parameters of the female sexual response in women with sexual arousal disorder. J Sex Marital Ther. 2001;27:411–420. doi: 10.1080/713846815. [DOI] [PubMed] [Google Scholar]
- Buhimschi CS, Garfield RE, Weiner CP, Buhimschi IA. The presence and function of phosphodiesterase type 5 in the rat myometrium. Am J Obstet Gynecol. 2004;190:268–274. doi: 10.1016/j.ajog.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Chakmakjian ZH, Zachariah NY. Bioavailability of progesterone with different modes of administration. J Reprod Med. 1987;32:443–448. [PubMed] [Google Scholar]
- Chowdary KP, Rao YS. Mucoadhesive microspheres for controlled drug delivery. Biol Pharm Bull. 2004;27:1717–1724. doi: 10.1248/bpb.27.1717. [DOI] [PubMed] [Google Scholar]
- Dawood MY. Ibuprofen and dysmenorrhea. Am J Med. 1984;77:87–94. doi: 10.1016/s0002-9343(84)80025-x. [DOI] [PubMed] [Google Scholar]
- Dmitrovic R. Transvaginal color Doppler study of uterine blood flow in primary dysmenorrhea. Acta Obstet Gynecol Scand. 2000;79:1112–1116. doi: 10.1034/j.1600-0412.2000.0790121112.x. [DOI] [PubMed] [Google Scholar]
- Dmitrovic R, Kunselman AR, Legro RS. Continuous compared with cyclic oral contraceptives for the treatment of primary dysmenorrhea: a randomized controlled trial. Obstet Gynecol. 2012;119:1143–1150. doi: 10.1097/AOG.0b013e318257217a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchinetti F, Sgarbi L, Piccinini F, Volpe A. A comparison of glyceryl trinitrate with diclofenac for the treatment of primary dysmenorrhea: an open, randomized, cross-over trial. Gynecol Endocrinol. 2002;16:39–43. [PubMed] [Google Scholar]
- Fedele L, Marchini M, Acaia B, Garagiola U, Tiengo M. Dynamics and significance of placebo response in primary dysmenorrhea. Pain. 1989;36:43–47. doi: 10.1016/0304-3959(89)90110-3. [DOI] [PubMed] [Google Scholar]
- Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010;375:686–695. doi: 10.1016/S0140-6736(09)61706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale SA, Jones CW, Osol G, Schonberg A, Badger GJ, Bernstein IM. Sildenafil increases uterine blood flow in nonpregnant nulliparous women. Reprod Sci. 2010;17:358–365. doi: 10.1177/1933719109354648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauksson A, Akerlund M, Melin P. Uterine blood flow and myometrial activity at menstruation, and the action of vasopressin and a synthetic antagonist. Br J Obstet Gynaecol. 1988;95:898–904. doi: 10.1111/j.1471-0528.1988.tb06577.x. [DOI] [PubMed] [Google Scholar]
- Hrobjartsson A, Gotzsche PC. Placebo interventions for all clinical conditions. Cochrane Database Syst Rev. 2004:CD003974. doi: 10.1002/14651858.CD003974.pub2. [DOI] [PubMed] [Google Scholar]
- Koninckx PR, Craessaerts M, Timmerman D, Cornillie F, Kennedy S. Anti-TNF-alpha treatment for deep endometriosis-associated pain: a randomized placebo-controlled trial. Hum Reprod. 2008;23:2017–2023. doi: 10.1093/humrep/den177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn S, Vassil TC, Kotey PN, Fricke JR., Jr Comparison of rofecoxib and oxycodone plus acetaminophen in the treatment of acute pain: a randomized, double-blind, placebo-controlled study in patients with moderate to severe postoperative pain in the third molar extraction model. Clin Ther. 2004;26:769–778. doi: 10.1016/s0149-2918(04)90076-8. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Littman GS, Walker BR, Schneider BE. Reassessment of verbal and visual analog ratings in analgesic studies. Clin Pharmacol Ther. 1985;38:16–23. doi: 10.1038/clpt.1985.127. [DOI] [PubMed] [Google Scholar]
- Malmstrom K, Fricke JR, Kotey P, Kress B, Morrison B. A comparison of rofecoxib versus celecoxib in treating pain after dental surgery: a single-center, randomized, double-blind, placebo- and active-comparator-controlled, parallel-group, single-dose study using the dental impaction pain model. Clin Ther. 2002;24:1549–1560. doi: 10.1016/s0149-2918(02)80059-5. [DOI] [PubMed] [Google Scholar]
- McDonough PG. Safety data for sildenafil. Fertil Steril. 2002;78:1353–1354. doi: 10.1016/s0015-0282(02)04358-3. [DOI] [PubMed] [Google Scholar]
- Mehlisch DR. Comparison of bromfenac and naproxen sodium in the management of primary dysmenorrhea. Prim Care Update Ob Gyns. 1998;5:195. doi: 10.1016/s1068-607x(98)00126-7. [DOI] [PubMed] [Google Scholar]
- Mehlisch D, Frakes L, Cavaliere MB, Gelman M. Double-blind parallel comparison of single oral doses of ketoprofen, codeine, and placebo in patients with moderate to severe dental pain. J Clin Pharmacol. 1984;24:486–492. doi: 10.1002/j.1552-4604.1984.tb02757.x. [DOI] [PubMed] [Google Scholar]
- Moya RA, Moisa CF, Morales F, Wynter H, Ali A, Narancio E. Transdermal glyceryl trinitrate in the management of primary dysmenorrhea. Int J Gynaecol Obstet. 2000;69:113–118. doi: 10.1016/s0020-7292(00)00185-5. [DOI] [PubMed] [Google Scholar]
- Nichols DJ, Muirhead GJ, Harness JA. Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: absolute bioavailability, food effects and dose proportionality. Br J Clin Pharmacol. 2002;53(Suppl 1):5S–12S. doi: 10.1046/j.0306-5251.2001.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Moreland RB, Goldstein I, Atala A, Traish A. Sildenafil inhibits phosphodiesterase type 5 in human clitoral corpus cavernosum smooth muscle. Biochem Biophys Res Commun. 1998;249:612–617. doi: 10.1006/bbrc.1998.9206. [DOI] [PubMed] [Google Scholar]
- Paulus WE, Strehler E, Zhang M, Jelinkova L, El-Danasouri I, Sterzik K. Benefit of vaginal sildenafil citrate in assisted reproduction therapy. Fertil Steril. 2002;77:846–847. doi: 10.1016/s0015-0282(01)03272-1. [DOI] [PubMed] [Google Scholar]
- Pulkkinen MO. Prostaglandins and the non-pregnant uterus. The pathophysiology of primary dysmenorrhea. Acta Obstet Gynecol Scand Suppl. 1983;113:63–67. doi: 10.3109/00016348309155200. [DOI] [PubMed] [Google Scholar]
- Safarinejad MR. Oral sildenafil in the treatment of erectile dysfunction in diabetic men: a randomized double-blind and placebo-controlled study. J Diabetes Complications. 2004;18:205–210. doi: 10.1016/S1056-8727(03)00056-4. [DOI] [PubMed] [Google Scholar]
- Scholtes MC, Wladimiroff JW, van Rijen HJ, Hop WC. Uterine and ovarian flow velocity waveforms in the normal menstrual cycle: a transvaginal Doppler study. Fertil Steril. 1989;52:981–985. doi: 10.1016/s0015-0282(16)53162-8. [DOI] [PubMed] [Google Scholar]
- Sher G, Fisch JD. Vaginal sildenafil (Viagra): a preliminary report of a novel method to improve uterine artery blood flow and endometrial development in patients undergoing IVF. Hum Reprod. 2000;15:806–809. doi: 10.1093/humrep/15.4.806. [DOI] [PubMed] [Google Scholar]
- Sher G, Fisch JD. Effect of vaginal sildenafil on the outcome of in vitro fertilization (IVF) after multiple IVF failures attributed to poor endometrial development. Fertil Steril. 2002;78:1073–1076. doi: 10.1016/s0015-0282(02)03375-7. [DOI] [PubMed] [Google Scholar]
- Steer CV, Williams J, Zaidi J, Campbell S, Tan SL. Intra-observer, interobserver, interultrasound transducer and intercycle variation in colour Doppler assessment of uterine artery impedance. Hum Reprod. 1995;10:479–481. doi: 10.1093/oxfordjournals.humrep.a135966. [DOI] [PubMed] [Google Scholar]
- Takasaki A, Tamura H, Miwa I, Taketani T, Shimamura K, Sugino N. Endometrial growth and uterine blood flow: a pilot study for improving endometrial thickness in the patients with a thin endometrium. Fertil Steril. 2010;93:1851–1858. doi: 10.1016/j.fertnstert.2008.12.062. [DOI] [PubMed] [Google Scholar]
- Tignol J, Furlan PM, Gomez-Beneyto M, Opsomer R, Schreiber W, Sweeney M, Wohlhuter C. Efficacy of sildenafil citrate (Viagra) for the treatment of erectile dysfunction in men in remission from depression. Int Clin Psychopharmacol. 2004;19:191–199. doi: 10.1097/01.yic.0000117902.43995.b0. [DOI] [PubMed] [Google Scholar]
- Traish A, Moreland RB, Huang YH, Kim NN, Berman J, Goldstein I. Development of human and rabbit vaginal smooth muscle cell cultures: effects of vasoactive agents on intracellular levels of cyclic nucleotides. Mol Cell Biol Res Commun. 1999;2:131–137. doi: 10.1006/mcbr.1999.0164. [DOI] [PubMed] [Google Scholar]
- Vermesh M, Fossum GT, Kletzky OA. Vaginal bromocriptine: pharmacology and effect on serum prolactin in normal women. Obstet Gynecol. 1988;72:693–698. [PubMed] [Google Scholar]
- Ziaei S, Faghihzadeh S, Sohrabvand F, Lamyian M, Emamgholy T. A randomised placebo-controlled trial to determine the effect of vitamin E in treatment of primary dysmenorrhoea. BJOG. 2001;108:1181–1183. doi: 10.1111/j.1471-0528.2003.00279.x. [DOI] [PubMed] [Google Scholar]