Abstract
The most abundant polyphenol in green tea, epigallocatechin-3-gallate (EGCg), has recently received considerable attention due to the discovery of numerous health-promoting bioactivities. Despite reports of its poor oral bioavailability, EGCg has been included in many dietary supplement formulations. Conventional preformulation methods have been employed to improve the bioavailability of EGCg. However, these methods have limitations that hinder the development of EGCg as an effective therapeutic agent. In this study, we have utilized the basic concepts of crystal engineering and several crystallization techniques to screen for various solid crystalline forms of EGCg and evaluated the efficacy of crystal engineering for modulating the pharmacokinetics of EGCg. We synthesized and characterized seven previously undescribed crystal forms of EGCg including the pure crystal structure of EGCg. The aqueous solubility profiles of four new EGCg cocrystals were determined. These cocrystals were subsequently dosed at 100 mg EGCg per kg body weight in rats, and the plasma levels were monitored over the course of eight hours following the single oral dose. Two of the EGCg cocrystals were found to exhibit modest improvements in relative bioavailability. Further, cocrystallization resulted in marked effects on pharmacokinetic parameters including Cmax, Tmax, area under curve, relative bioavailability, and apparent terminal half-life. Our findings suggest that modulation of the pharmacokinetic profile of EGCg is possible using cocrystallization and that it offers certain opportunities that could be useful during its development as a therapeutic agent.
Keywords: green tea, EGCg, cocrystal, bioavailability, solubility, pharmacokinetics, Alzheimer’s disease, vitamin B3, crystal engineering, dissolution rate
Introduction
Bioflavonoids are a group of polyphenolic compounds that are common throughout the plant kingdom. They are widely studied and have been found to promote healthy living in epidemiologic studies.1,2 Much of the attention that bioflavonoids have attracted is due mainly to the “French paradox”. This is the dietary anomaly in which people in the Mediterranean culture have a higher fat intake but a lower incidence of cardiovascular disease and increased longevity.3 This phenomenon has been largely attributed to increased dietary intake in bioflavonoids.
One of the most widely studied bioflavonoids is, epigallocatechin-3-gallate (EGCg), the most abundant polyphenol in green tea. It has been studied extensively during the past decade for its therapeutic potential in various cancers,4−8 Alzheimer’s disease,9−12 obesity,13,14 and diabetes.15,16 Despite these promising therapeutic uses, the plasma concentrations of EGCg that are required are prohibitively high. Thus, novel methods for improving the oral bioavailability of EGCg are desirable. In a previous study, we demonstrated that proprietary nanolipidic particles could be used to improve the bioactivity of EGCg for reducing amyloid beta production in a cell model of Alzheimer’s disease while also more than doubling the oral bioavailability.17 Although this could potentially overcome the problems thwarting the clinical translation of EGCg, the technology is limited because it requires an alcohol suspension.
Until now there have been no reports of any of the crystalline forms of EGCg in the Cambridge Structural Database (CSD) including the crystal structure of pure EGCg which makes it worth studying from a structural perspective. Therefore, we undertook the synthesis of cocrystals of EGCg and also crystallized its pure form. This was accomplished by taking advantage of crystal engineering concepts and several crystallization techniques. Cocrystallization, crystals formed from two or more components or “cocrystal formers (CCFs)”, has emerged over the past decade as a materials science approach to generate novel solid forms of active pharmaceutical ingredients with improved physicochemical properties.18−29 We recently reported four new cocrystal forms of another flavonoid, quercetin, which improved solubility by up to 14-fold and oral bioavailability by up to 10-fold.(27) Unlike quercetin, EGCg is highly soluble in water.30 Previous pharmacokinetics experiments have shown that EGCg is absorbed rapidly in the gut following oral administration.31,32 However, membrane permeability of EGCg is reported to be low.33,34 Thus, EGCg would likely fall into the Biopharmaceutics Classification System (BCS) 3: high solubility, low permeability. We hypothesized that new cocrystals of EGCg with reduced water solubility might exhibit pharmacokinetic profiles that are more desirable for drug development. Moreover, we hypothesized that reducing the solubility would decrease the rate of dissolution and slow the absorption of EGCg in vivo. We report herein the results of our structural, solubility, and pharmacokinetic studies.
Experimental Section
Reagents and Materials
Green tea-derived EGCg (>95% purity by HPLC) was purchased from http://www.herbs-tech.com. Isonicotinamide (INM, >99% purity), isonicotinic acid (INA, >99% purity), nicotinamide (NIC, >99% purity), and nicotinic acid (NAC, 98% pure) were purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA).
Preparation of Single Crystals
All crystallization and cocrystallization experiments were either conducted at room temperature or at 4 °C. The solvents were distilled prior to their use. Specific experimental details are as follows.
Form II
EGCg (65.0 mg, 0.14 mmol) was dissolved in 1 mL of acetonitrile (99% pure). The resulting solution was layered on 2.5 mL of dichloromethane and was allowed to stand in the refrigerator. Colorless platelet crystals were harvested after three days.
Form III
EGCg (200.0 mg, 0.436 mmol) was dissolved in 2.5 mL of acetonitrile (99% pure). The solution was layered onto 6 mL of dichloromethane and 2 mL of nitrobenzene and then allowed to stand in a refrigerator. Yellow crystalline needles were harvested after 24 h.
Form IV
The pure crystal line form of EGCg, Form IV, was obtained by heating Forms II or III at 120° for 20–25 min. Form IV crystals can also be reproduced by dissolving EGCg (45.0 mg 0.098 mmol) in 1 mL of acetonitrile (99% pure). The solution was layered on 2.5 mL of dichloromethane and seeded with form IV and was allowed to stand in the refrigerator. After one week colorless needle-like crystals were observed.
EGCgINM·5H2O
EGCg (45.80 mg, 0.099 mmol) and iso-nicotinamide (99% pure, used as received, 12.2 mg, 0.0998 mmol) were dissolved in 5 mL of water. Colorless block-like crystals of EGCgINM·5H2O were harvested in less than 5 min. However to further grow better quality crystals, 1:1 water/methanol (v/v %) solvent was used, and the crystals were obtained in one day.
EGCgNIC·9H2O
EGCg (45.80 mg, 0.099 mmol) and nicotinamide (99% pure, used as received, 12.2 mg, 0.0998 mmol) were dissolved in 2 mL of water. A cluster of crystals were obtained after three days. Looking carefully under the microscope a colorless block like single crystal was separated from the cluster and was used for single crystal X-ray diffraction analysis.
EGCgINA·3H2O
EGCg (45.80 mg, 0.099 mmol) and iso-nicotinic acid (99% pure, used as received, 12.3 mg, 0.0998 mmol) were dissolved in 5 mL of water. Colorless needles of EGCgINA·3H2O were harvested in one day.
EGCgINA
The anhydrous form of EGCgINA·3H2O was obtained by dehydrating the cocrystal.
EGCgNAC·xH2O
EGCg (458.0 mg, 0.99 mmol) and nicotinic acid (99% pure, used as received, 123.0 mg, 0.998 mmol) were slurried in 2 mL of water overnight in a vial. The vial was left in the hood undisturbed, and after two days a cluster of block shaped crystals was obtained.
Thermogravimetric Analysis (TGA)
TGA was performed on a Perkin-Elmer STA 6000 simultaneous thermal analyzer. Open alumina crucibles were used for analysis from 30 to 300 °C at 5 °C/min heating rate under nitrogen purge.
Differential Scanning Calorimetry (DSC)
Thermal analysis was carried out employing a TA Instruments DSC 2920 differential scanning calorimeter. Standard aluminum pans were used for the experiment for all the samples. Temperature calibrations were made using indium as a standard. An empty pan, sealed in the same way as the sample, was used as a reference pan. A typical sample (2–8 mg) was heated in a DSC from 30 to 300 °C at a 5 °C/min heating rate. The experimental data were analyzed using commercially available software (TA Universal Analysis 2000; TA Instruments).
Infrared Spectroscopy (FT-IR)
All of the forms of EGCg were characterized by infrared spectroscopy using a Nicolet Avatar 320 FT-IR instrument. The amount of the sample used was around 2–3 mg. The spectra were measured over the range of 4000–400 cm–1. Data were analyzed by using EZ Omnic software.
Powder X-ray Diffraction (PXRD)
The bulk samples were analyzed by powder X-ray diffractometry. The samples were placed on the sample holder with the help of vacuum grease and exposed, at room temperature, to Cu Kα radiation (λ = 1.54056 Å; 40 kV × 30 mA, Bruker AXS D8 advance). For all the samples, the angular range was 3–0° 2θ, with a step size of 0.05° 2θ. Intensity counts were accumulated for 0.5 s at each step. Data analyses were performed using commercially available software (PowderX and Origin).
Single-Crystal X-ray Data Collection and Structure Determinations
Single crystals of Forms II, III, and IV and cocrystals EGCgINM·5H2O, EGCgNIC·9H2O, EGCgINA·3H2O, and EGCgNAC·xH2O were examined under a microscope, and suitable crystals were selected for single crystal X-ray crystallography. Single crystal X-ray diffraction data on Forms III and IV were collected on a Bruker-AXS SMART APEX CCD diffractometer with monochromatized Mo Kα radiation ((λ = 0.71073 Å), whereas for Form II and cocrystals EGCgINM·5H2O, EGCgNIC·9H2O, EGCgINA·3H2O, and EGCgNAC·xH2O, data were collected on a Bruker-AXS SMART APEX 2 CCD diffractometer with monochromatized Cu Kα radiation (λ = 1.54178 Å). Both of the diffractometers are connected to a KRYO-FLEX low temperature device. Indexing was performed using APEX235 (Difference Vectors method). Data integration and reduction were performed using SaintPlus 6.01.36 Absorption correction was performed by a multiscan method implemented in SADABS.37 Space groups were determined using XPREP implemented in APEX2.35 The structure was solved using SHELXS-97 (direct methods) and refined using SHELXL-97 (full-matrix least-squares on F2) contained in APEX235 and WinGX v1.70.0138−40 programs packages.
EGCgNAC·xH2O
All non-hydrogen atoms were refined anisotropically. Hydrogen atoms of −CH and −CH2 groups were placed in geometrically calculated positions and included in the refinement process using a riding model with isotropic thermal parameters: Uiso(H) = 1.2Ueq (−CH2, −CH). Hydrogen atoms of −OH, −NH groups and water molecules have been found from difference Fourier map and refined using DFIX (d(O–H) = 0.84 Å for hydroxyl groups, DFIX −2.2 antibumping restraints for two H···H distances) and SADI restraints for water molecules (O–H and H···H distances) with Uiso(H) = 1.5Ueq(−OH). Some of water molecules were observed to be disordered in the structure. It was not possible to locate hydrogen atoms of those molecules.
EGCgNIC·9H2O
All non-hydrogen atoms were refined anisotropically. Hydrogen atoms of −CH and −CH2 groups were placed in geometrically calculated positions and included in the refinement process using riding model with isotropic thermal parameters: Uiso(H) = 1.2Ueq(−CH2, −CH). Hydrogen atoms of −OH and −NH groups were placed in geometrically calculated position and refined using AFIX 147 and AFIX 93 correspondingly. Positions of hydrogen atoms H4, H15, and H25a were further refined using a distance restraint (H···O{H-bond acceptor}) of 1.865(8) Å as found from a CSD analysis. Some of the water molecules were observed to be disordered, and it was not possible to locate hydrogen atoms for those molecules.
EGCgINA·3H2O
All non-hydrogen atoms were refined anisotropically. Hydrogen atoms of −CH and −CH2 groups were placed in geometrically calculated positions and included in the refinement process using a riding model with isotropic thermal parameters: Uiso(H) = 1.2Ueq(−CH2, −CH). Hydrogen atoms of −OH groups were placed in geometrically calculated position and refined using AFIX 147 or AFIX 83. Water molecules were refined using distance restraints. The observed residual electron density 1.258 el/ Å3 can probably be attributed to the presence of small satellite crystals and/or disordered chains of INA. Crystal data and refinement conditions are shown in Table 1.
Table 1. Crystallographic Data and Structure Refinement Parameters.
| Form II | Form III | Form IV | EGCgINM·5H2O | |
|---|---|---|---|---|
| formula | C22.5H20.5N1.5O12.3Cl0.5 | C28H25NO14 | C22H18O11 | C28H24N2O17 |
| MW | 561.04 | 599.49 | 458.36 | 660.49 |
| crystal system | monoclinic | orthorhombic | monoclinic | monoclinic |
| space group | C2 | P212121 | P21 | C2 |
| a (Å) | 23.072 (5) | 13.161 (3) | 12.959 (12) | 19.603 (8) |
| b (Å) | 14.908 (4) | 13.169 (3) | 5.669 (5) | 14.491 (6) |
| c (Å) | 15.806 (4) | 14.536 (4) | 13.094 (12) | 10.585 (4) |
| α (deg) | 90 | 90 | 90 | 90 |
| β (deg) | 103.44 (2) | 90 | 107.276 (13) | 92.089 (7) |
| γ (deg) | 90 | 90 | 90 | 90 |
| V (Å3) | 5288.1 (2) | 2519.4 (11) | 918.6 | 3005 (2) |
| Dc (mg m–3) | 1.409 | 1.581 | 1.657 | 1.460 |
| Z | 8 | 4 | 2 | 4 |
| 2θ range | 2.87–67.72 | 2.09–25.35 | 1.65–25.35 | 1.75–25.02 |
| Nref/Npara | 6591/830 | 4602/488 | 1854/370 | 4761/429 |
| T (K) | 100 (2) | 100 (2) | 100 (2) | 298 (2) |
| R1 [I > 2σ(I)] | 0.0596 | 0.0356 | 0.0384 | 0.0671 |
| wR2 | 0.1624 | 0.0865 | 0.0872 | 0.1782 |
| GOF | 1.092 | 1.009 | 1.019 | 0.971 |
| abs coef. | 1.407 | 0.129 | 0.135 | 0.124 |
| EGCgNIC·9H2O | EGCgINA·3H2O | EGCgNAC·xH2O | |
|---|---|---|---|
| formula | C28H24N2O16.75 | C28H29NO16 | C28H28NO18 |
| MW | 656.49 | 547.88 | 666.51 |
| crystal system | triclinic | orthorhombic | monoclinic |
| space group | P1 | P212121 | C2 |
| a (Å) | 10.497 (2) | 7.5068 (2) | 19.9700 (4) |
| b (Å) | 11.769 (2) | 14.0136 (3) | 14.1716 (3) |
| c (Å) | 12.203 (2) | 26.1150 (6) | 10.5170 (2) |
| α (deg) | 72.56 (3) | 90 | 90 |
| β (deg) | 85.80 (3) | 90 | 93.4720 (1) |
| γ (deg) | 83.00 (3) | 90 | 90 |
| V (Å3) | 1426.4 (5) | 2747.23 (11) | 2970.9 (1) |
| Dc (g cm–3) | 1.528 | 1.537 | 1.490 |
| Z | 2 | 4 | 4 |
| 2θ range | 4.25–69.33 | 3.38–66.57 | 5.59–67.72 |
| Nref/Npara | 7923/881 | 4747/426 | 4982/502 |
| T (K) | 173 (2) | 100 (2) | 100 (2) |
| R1 [I > 2σ(I)] | 0.0632 | 0.0598 | 0.0467 |
| wR2 | 0.1701 | 0.1542 | 0.1158 |
| GOF | 1.059 | 0.05642 | 1.038 |
| abs coef. | 1.118 | 1.062 | 1.101 |
Synthesis of Cocrystals in Bulk for Dissolution
The cocrystals EGCgINM·5H2O, EGCgNIC·9H2O, EGCgINA·3H2O, and EGCgINA were made in bulk for dissolution studies using the slurry method. EGCgNAC·xH2O was not included in the dissolution study due to difficulties in reproducing it in bulk powder form. Slurry experiments to reproduce EGCgNAC·xH2O resulted in a glue-like material rather than a powder. For the remaining cocrystals, stoichiometric amounts of the starting materials were stirred overnight in 2–3 mL of water with the help of a magnetic stir bar on a stir plate that produced the cocrystals with 100% yield. The purity of the bulk material was tested by powder X-ray diffractometry (PXRD) and differential scanning calorimetry (DSC). A uniform particle size (between 53 and 75 μm) for the bulk powder was obtained for all the cocrystals and EGCg by sieving using standard ASTM sieves.
Cocrystal Solubility Evaluation
Solubility studies were performed on EGCg, EGCgINM·5H2O, EGCgNIC·9H2O, EGCgINA·3H2O, and EGCgINA using UV/vis/NIR spectrophotometry in water at room temperature. The wavelength used for the determination of EGCg was 305 nm since there is no interference with the CCFs at this wavelength. The dissolution studies were conducted by taking approximately 3 g of the cocrystal in 50 mL of water and stirring with a magnetic stir bar at ca. 125 rpm for 4 h. Aliquots were drawn from the slurry at regular time intervals (5, 10, 15, 20, 25, 30, 45, 60, 75, 90, 120, 150, 180, and 240 min) and filtered using a 0.45 μm nylon filter. The filtrates were diluted appropriately and analyzed to measure the concentration of EGCg (at 305 nm) by using a UV/vis/NIR spectrometer. The remaining undissolved solid was analyzed by PXRD and DSC to confirm phase stability. The solubility measurements were done in replicates of three.
Pharmacokinetic Screening of EGCg Formulations in Rats
All animal studies were conducted in accordance with a University of South Florida IACUC-approved protocol. Male Sprague–Dawley rats (n = 3 per group) weighing 200–250 g were purchased from Harlan Laboratories (Indianapolis, IN). The rats were purchased precannulated by Harlan. The rounded tip catheters were surgically implanted into the jugular vein of the rats making multiple, precise blood draws painless to the animal. The rats were food (not water) deprived for 18 h prior to the start of the experiment. Corn oil was selected as the gavage vehicle because all crystal forms were observed to be insoluble in it. All EGCg forms were sieved to attain a particle size between 53 and 75 μm prior to suspending in corn oil at 20 mg of EGCg per mL. The EGCg formulations were delivered via oral gavage at a dosage of 100 mg EGCg per kg body weight. Blood was collected at the following time points: 0, 5, 10, 30, 60, 120, 240, and 480 min. Because heparin was kept in the catheter lines to prevent clotting, a small amount of blood was drawn and discarded before collecting each sample. Approximately 300 μL of blood was collected in EDTA tubes for each time point. The samples were kept on ice to preserve their integrity and then centrifuged at 4000 rpm for 10 min, after which the plasma was transferred to sterile centrifuge tubes. A preservative solution was added to each plasma sample at 10% (v/v) concentration to ensure the integrity of the EGCg during storage.41 This preservative was comprised of 20% ascorbic acid (to prevent oxidation) and 0.1% EDTA (to scavenge any metal contaminants). The samples were stored at −80 °C until they were analyzed for EGCg content.
Quantification of EGCg in Rat Plasma
To accurately quantify the concentration of EGCg in the plasma, a previously described method was employed using liquid chromatography with tandem mass spectrometry.42−44
Stock Preparation
A 2.00 mg/mL stock solution of EGCg in DMSO was prepared. The standard spiking solutions were prepared by diluting the stock solution to 1000 and 100 μg/mL using acetonitrile–water (1:1, v:v). Both solutions were protected from light using amber vials, and all solutions were stored at −20 ◦C.
Standard Curve Preparation
For this analysis two standard curves were prepared: one with a higher (10–0.100 μg/mL) dynamic range, and the other a lower range (1000–10 ng/mL). Both standard curves were prepared using the appropriate blank plasma containing the preservative. The results indicated that the standard curve performance was within acceptable range for bioanalytical method acceptance (R2 > 0.99).
Pharmacokinetic Calculations
Mean plasma EGCg concentrations and the standard error in the mean (SEM) were graphed using GraphPad PRISM software (GraphPad Software, Inc.). Phoenix WinNonlin Version 6.3 (Pharsight Corporation, Mountain View, CA) was used to conduct a noncompartmental analysis of the pharmacokinetic data and generate the pharmacokinetic parameters. The reported pharmacokinetic parameters included Cmax, Tmax, area under curve (AUC), relative bioavailability (Frel), and apparent terminal half-life (HL_Lambda_z). Relative bioavailability was determined by dividing the mean AUC of each EGCg formulation by the control. All pharmacokinetic parameters are reported as mean and standard deviations.
Statistical Analysis
Two-tailed t-tests were used to assess the statistical significance at each time point for the pharmacokinetic curves. Each EGCg cocrystal was compared to the EGCg control at each time point. The criterion for rejection of the null hypothesis was P < 0.05.
Results
Screening of EGCg Cocrystals and Its Pure Form
The CCFs were selected based on the supramolecular synthon approach. According to this approach the CCFs were identified based on the functional groups present on EGCg and analyzing the frequency for the occurrence of supramolecular synthons (homo and hetero) with other functional moieties. This analysis was carried out via the CSD, an archive of over ∼600 000 organic crystal structures. The CSD also offers a software platform that facilitates statistical analysis of packing motifs, thereby providing empirical information on common functional groups and how they associate at the molecular level. Several fragments of EGCg were identified, and a CSD analysis was conducted to determine if these fragments and/or the whole molecule (EGCg) are susceptible to form cocrystals with carboxylic acids, alcohols, or weak bases. Furthermore, the selected CCFs should be suitable for use in drug products (e.g., FDA approved, GRAS or EAFUS listed). Several potential CCFs were selected based on the above-mentioned criteria. However, we were unable to obtain cocrystals with any other CCFs except those presented herein. In addition, efforts to obtain pure crystalline forms of EGCg via different crystallization techniques led to the isolation of two solvates which upon desolvation resulted in a pure crystalline form of EGCg. Figure 1 illustrates the crystalline forms obtained during the screening process.
Figure 1.
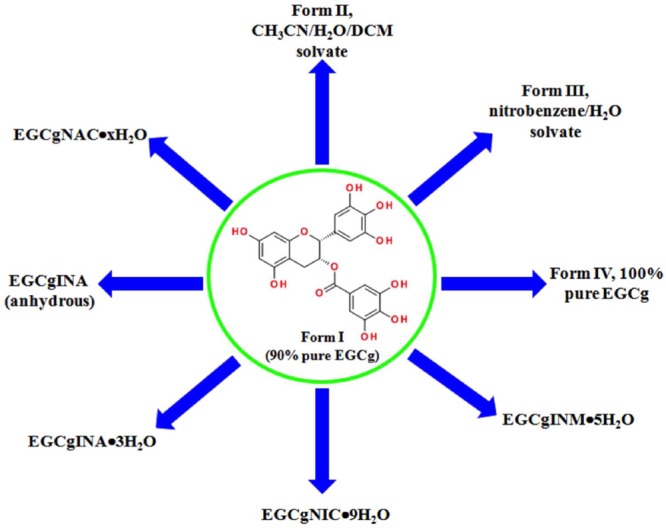
Graphical representation of the new EGCg crystal forms.
Interestingly, all of the isolated cocrystals are in the form of hydrates. EGCg is a large and flexible molecule containing a number of hydrogen bond donors and acceptors. This makes it prone to formation of hydrates. Furthermore, the raw material used is EGCg monohydrate, and dehydration of the starting material is reversible. Except for EGCgINA·3H2O,all cocrystals became amorphous following dehydration. EGCgINA·3H2O upon dehydration yields an anhydrous cocrystal of EGCg and INA. We believe that water plays a pivotal role in crystallizing these cocrystals, but it is difficult to say whether the water molecules are playing a space-filling role in channels or they are an integral part of the crystal lattice.
Crystal Structure Descriptions
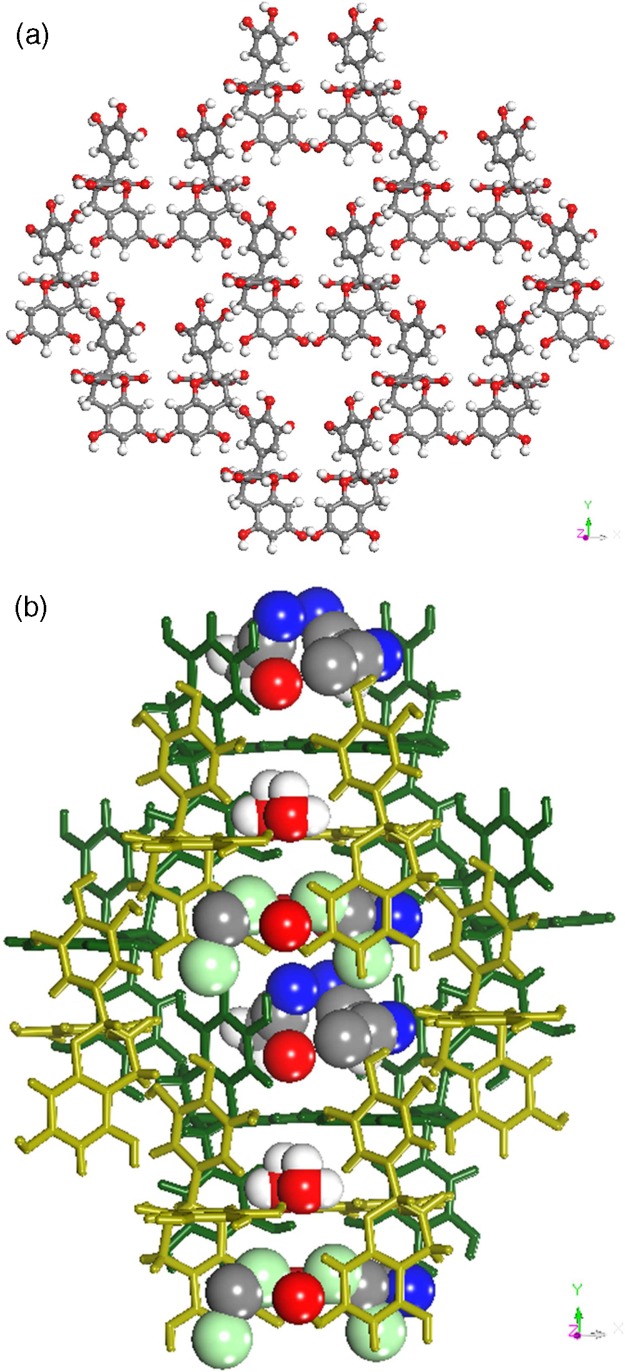
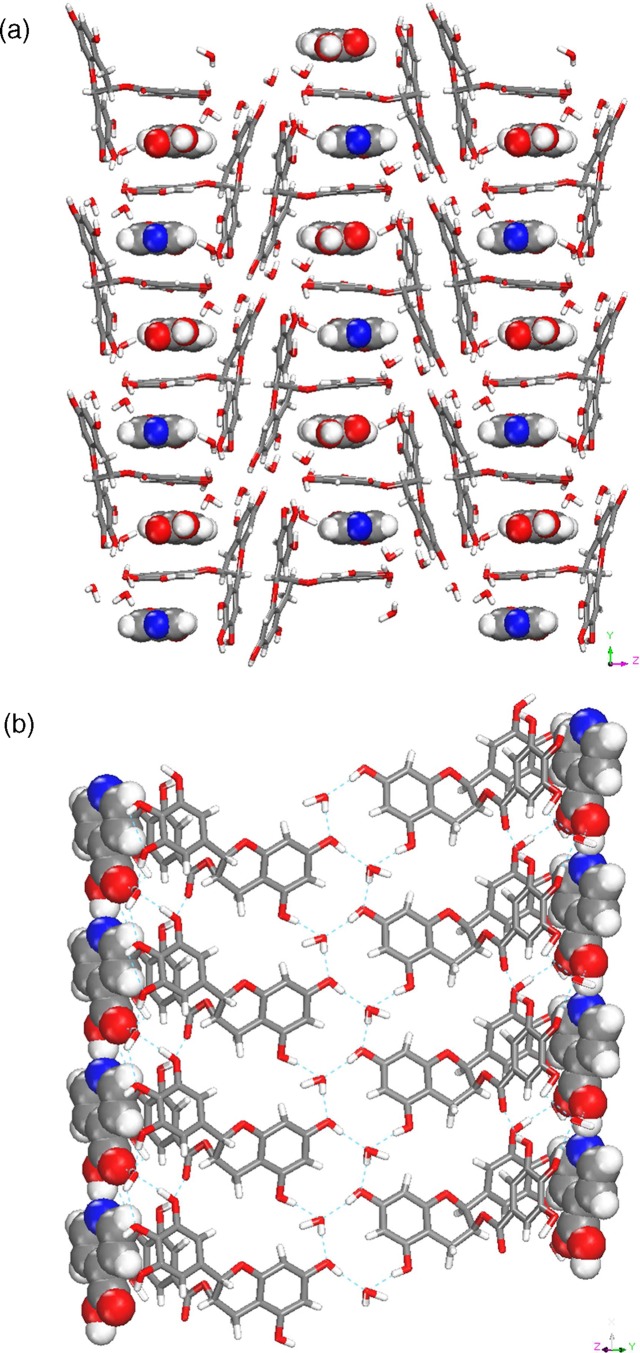
Form II (2C22H18O11·3CH6CN·3H2O·CH2Cl2)
Form II crystallizes in the monoclinic C2 space group and is a high Z’ structure consisting of two EGCg, three acetonitrile, three water, and a disordered dichloromethane molecule in the asymmetric unit. The two symmetry-independent EGCg molecules form sheets with voids that accommodate the solvent molecules, as shown in Figure 2a and b.
Figure 2.
(a) EGCg molecules in Form II forming sheets with channels. (b) A typical channel being occupied by solvent molecules in Form II.
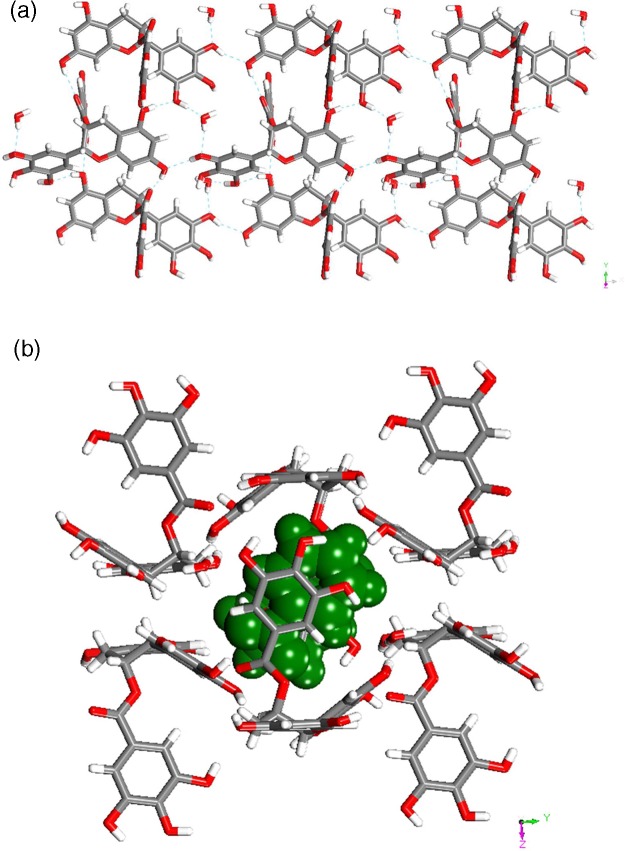
Form III (C22H18O11·C6H5NO2·H2O)
Form III, a solvated form of EGCg with nitrobenzene and water, crystallized in the orthorhombic P21 space group with one molecule of each component in the asymmetric unit. In the crystal structure the EGCg and water molecules form sheets. One of the sheets is shown in Figure 3a.
Figure 3.
(a) Illustration of a representative sheet formed by EGCg and water molecules in Form III. (b) Nitrobenzene molecule sandwiched between sheets of EGCg and water molecules in Form III.
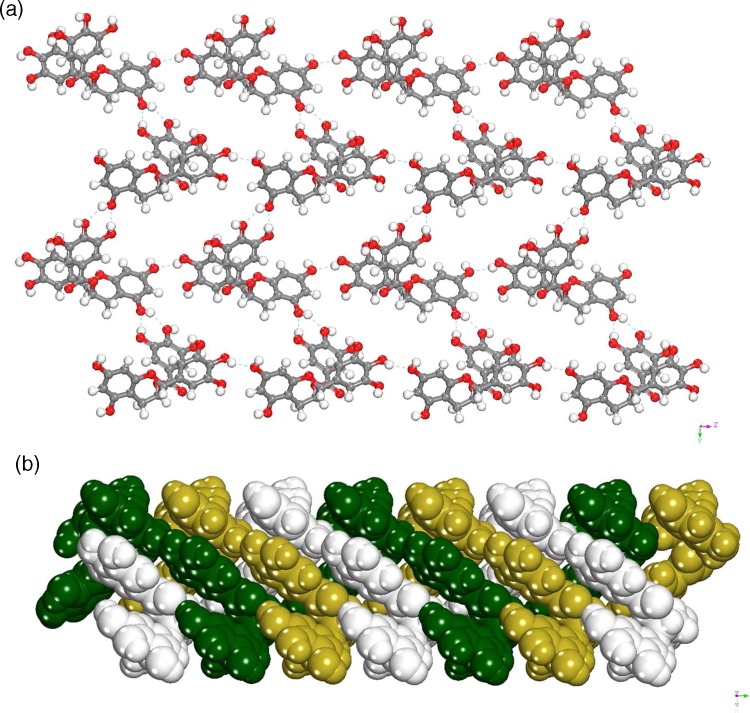
Form IV
Pure EGCg, Form IV, crystallizes in monoclinic P21 space group with one EGCg molecule in asymmetric unit. The EGCg molecules form crinkled sheets with voids. The voids are interpenetrated with other sheets to exhibit 2-fold interpenetration as shown in Figure 4a and b.
Figure 4.
(a) Crystal packing of Form IV; crinkled sheets with cavities were created via several O–H···O H-bonds. (b) Self-interpenetrated 3D structure of Form IV.
EGCgINM·5H2O
The single crystal X-ray structure analysis reveals that EGCgINM·5H2O is a pentahydrate of the 1:1 cocrystal of EGCg and INM. EGCg molecules and INM molecules interact through one point hydrogen bonds (O–H···N, O···N: 2.756 (2) Å) between a hydroxyl group of EGCg molecules and the aromatic nitrogen of INM molecules. These dimeric units are further connected by O–H···O (O···O: 2.662 (4) Å) hydrogen bonds formed between hydroxyl moieties of EGCg and carbonyl moieties of INM molecules and thereby form zigzag chains as illustrated in Figure 5.
Figure 5.
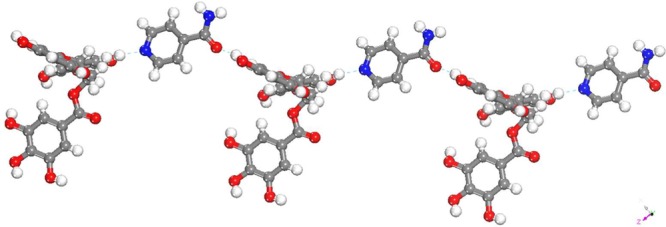
Intermolecular H-bonding between EGCg and INM molecules. Water molecules are removed for clarity.
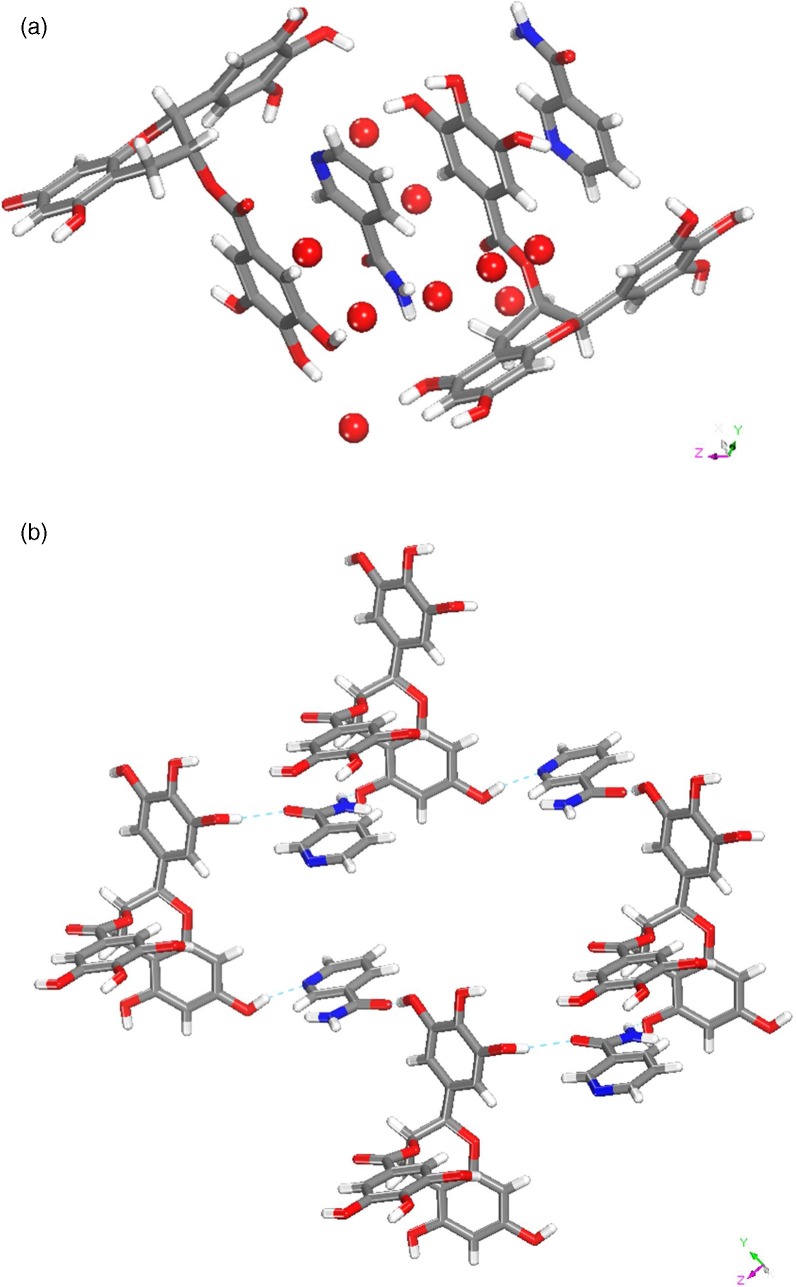
EGCgNIC·9H2O
The crystallization of EGCg and NIC in water results in the formation of a 1:1 cocrystal nonahydrate. It crystallizes in P1 space group with two molecules each of EGCg and NIC molecules and nine water molecules in the asymmetric unit (Figure 6a). The N–H functionality of the NIC molecules interact with one of the O–H moieties present of ring A of EGCg with an N···O bond distance of 2.570 (2) Å (Figure 6b).
Figure 6.
(a) Asymmetric unit of EGCgNIC·9H2O cocrystal. (b) H-bonding between EGCg and NIC molecules.
EGCgINA·3H2O
The cocrystallization of EGCg and INA resulted in the formation of a cocrystal trihydrate which crystallizes in the orthorhombic space group P21P21P21. The INA and EGCg molecules interact with each other through water molecules (Figure 7a). One of the three water molecules interacts with two EGCg and one INA molecule through the following H-bonds: (a) the O–H of the water molecule interacts with the C–O moiety of the INA molecule with a bond distance of 2.739 (3) Å; (b) the O–H of the water molecule interacts with two neighboring EGCg molecules with H-bond distances of 2.704 (5) and 2.752 Å (3) Å. The other water molecule forms a trifurcated bond with three different EGCg molecules as presented in Figure 7b through O–H···O bonds of 2.667 (3), 2690 (2), and 2.712 (2) Å. The INA molecules form head to tail chains through N–H···O bifurcated hydrogen bonds (2.540 (2) and 2.986 (3) Å) (Figure 7a).
Figure 7.
Crystal packing in EGCg·INA·3H2O.
EGCgNAC·xH2O
The 1:1 cocrystal of EGCg and NAC crystallized as a hydrate in monoclinic space group C2. Unlike the other cocrystals the stiochiometry of water in the EGCg-nicotinic acid (NAC) is indefinite. We were unable to determine the number of water molecules in the crystal lattice of EGCg-NAC cocrystals due to poor quality crystals and single crystal data (see Table 1). From the crystal structure it is evident that NAC molecules exist as zwitterions unlike INA in EGCgINA·3H2O. The C–N–C bond angle is 122.76°, and the C–O bond distances are 1.237 and 1.252 Å. One of the water molecules is disordered. Each of the NAC zwitterions interacts two neighboring EGCg molecules via the following H-bonds: (a) an H-bond formed between one of the O–H groups on ring B with one of the carboxylates of the NAC molecules at a distance of 2.588 (3) Å; (b) an H-bond between the N–H of the NAC molecule and one of the O–H functionalities present on ring A of another EGCg molecule (N–H···O: 2.902 (3) Å). The intermolecular H-bonding between EGCg and NAC zwitterions is illustrated in Figure 8.
Figure 8.
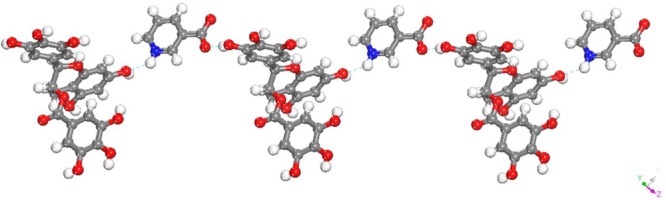
Illustration hydrogen bonding between EGCg and NAC molecules in EGCgNAC·xH2O cocrystal (water molecules are not shown in the figure for clarity).
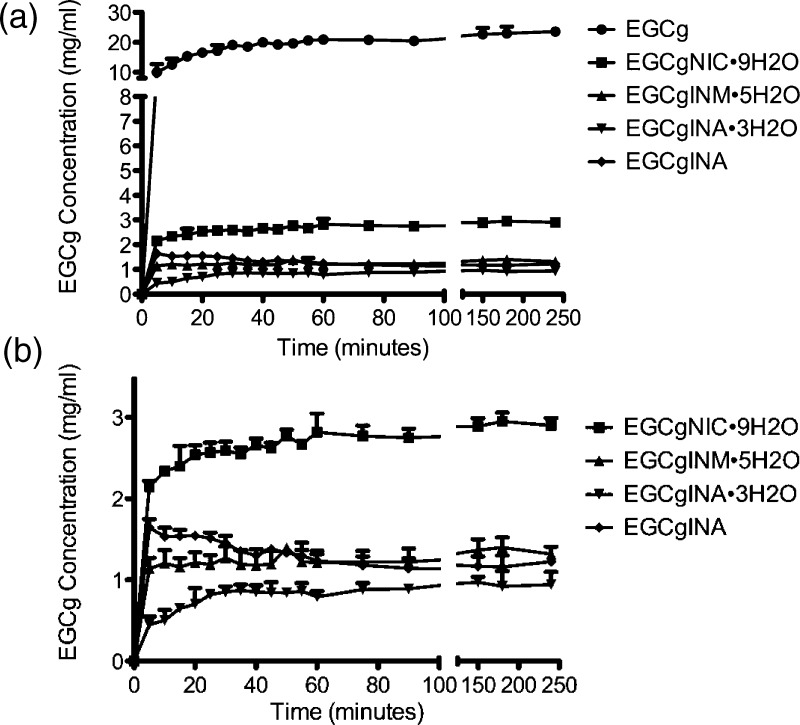
Aqueous Solubility of EGCg Cocrystals
EGCg is relatively soluble in water compared to many other flavonoids such as quercetin. We determined the solubility profiles of the four new EGCg cocrystals reported herein and observed that all of the cocrystals exhibit reduced solubility. The solubility studies were conducted for 4 h because after 1 h it was observed that the solubilities remained almost constant and did not change for the next 3 h. This suggests that the solubilities had reached equilibrium after 1 h. Figure 9 shows the dissolution profiles of EGCg and the EGCg cocrystals. The maximum solubility of EGCg determined experimentally was approximately 23.6 mg/mL during the 4 h dissolution experiment. The EGCg cocrystals were found to exhibit far lower aqueous solubilities when compared to EGCg as illustrated in Figure 9. The cocrystal that exhibited the highest aqueous solubility was EGCgNIC·9H2O (2.95 ± 0.11 mg/mL). EGCgINA (anhydrous), EGCgINM·5H2O, and EGCgINA·3H2O were observed to exhibit solubilities of 1.227 ± 0.036, 1.401 ± 0.122, and 0.97 ± 0.07 mg/mL, respectively. The solubilities of EGCg and its cocrystals are tabulated in Table 2. After the dissolution experiment has concluded (4 h), the identity of the powders was confirmed by PXRD and DSC. EGCgINA anhydrous had converted to the hydrate form, EGCgINA·3H2O, while the remaining cocrystals were found to be stable up to at least 4 h..
Figure 9.
Dissolution profiles of (a) EGCg and its cocrystals and (b) the cocrystals alone in water.
Table 2. Aqueous Solubilities of EGCg and Its Cocrystals.
| compound | aqueous solubility after 4 h (mg/mL) |
|---|---|
| EGCg | 23.60 |
| EGCgINM·5H2O | 1.32 |
| EGCgNIC·9H2O | 2.90 |
| EGCgINA·3H2O | 0.94 |
| EGCgINA | 1.23 |
| EGCgNAC·xH2O | NA |
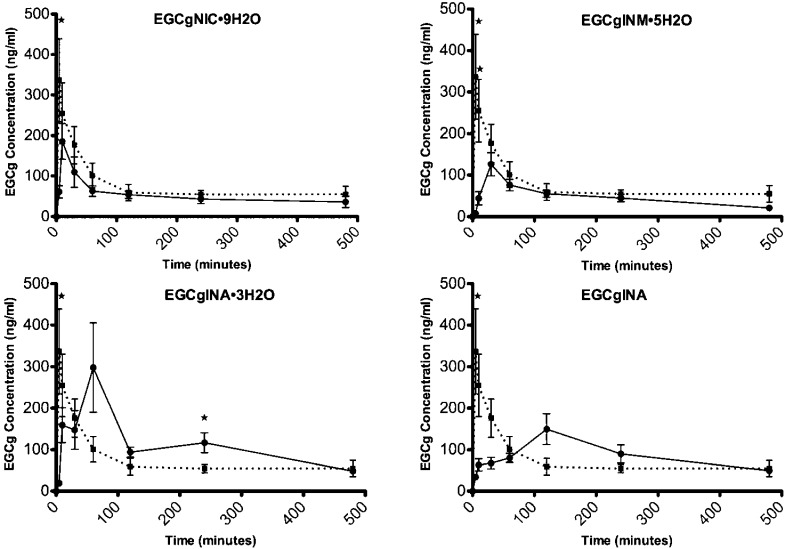
Pharmacokinetics of EGCg Cocrystals
The pharmacokinetic curves for EGCg and the EGCg cocrystals are presented in Figure 10. Each cocrystal is shown separately (solid line) against the EGCg control (dashed line). The EGCg cocrystals exhibited very different pharmacokinetic profiles. The pharmacokinetic parameters are shown in Table 3. EGCg peaked rapidly at 7.50 ± 2.74 min (Tmax) due to its high solubility and consequent high rate of dissolution. The cocrystals peaked in the plasma in the following order: EGCgNIC·9H2O, EGCgINM·5H2O and EGCgINA·3H2O, EGCgINA. Surprisingly, only two of the cocrystal forms resulted in improved bioavailability: EGCgINA·3H2O and EGCgINA. These cocrystals had modest improvements in relative bioavailability at Frel of 1.37 and 1.05, respectively. EGCg peaked more rapidly than all of the cocrystal forms and exhibited the shortest apparent terminal half-life, 249.35 ± 60.73 min. Interestingly, cocrystallization changed the overall shape of the pharmacokinetic curve. This was most apparent with the EGCgINA·3H2O and EGCgINA cocrystals, which exhibited drastic changes in the time to reach maximal plasma concentrations (Tmax) at 30.00 ± 24.49 and 160 ± 61.97 min, respectively. In some cases, these pharmacokinetic profiles might be advantageous to the rapid peak and elimination profile of free EGCg.
Figure 10.
Pharmacokinetic profiles (mean plasma concentration + SEM versus time). The solid line in each panel represents the indicated EGCg cocrystal. The dashed lines are the EGCg control. There were three rats per group (n = 3). ★P < 0.05.
Table 3. Pharmacokinetic Parameters.
| EGCg |
EGCgNIC·9H2O |
EGCgINM·5H2O |
EGCgINA·3H2O |
EGCgINA |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| mean | SD | mean | SD | mean | SD | mean | SD | mean | SD | |
| Tmax (min) | 7.50 | 2.74 | 20.00 | 10.95 | 30.00 | 0.00 | 30.00 | 24.49 | 160.00 | 61.97 |
| Cmax (ng/mL) | 341.50 | 247.10 | 188.33 | 106.26 | 125.95 | 67.91 | 347.17 | 236.92 | 167.40 | 84.66 |
| AUC(0–t) (min·ng/mL) | 39796.33 | 26318.73 | 22064.75 | 3410.81 | 22636.28 | 9177.62 | 54560.42 | 21954.28 | 41937.08 | 12165.97 |
| Frel | 1.00 | NA | 0.55 | NA | 0.57 | NA | 1.37 | NA | 1.05 | NA |
| HL_Lambda_z (min) | 249.35 | 60.73 | 341.71 | 212.97 | 263.74 | 68.31 | 352.61 | 79.85 | 350.83* | 195.13 |
Due to an insufficient number of data points in the elimination phase of the pharmacokinetic profile, Cmax was included in the Lambda_z calculation during the noncompartmental analysis in WinNonlin.
Discussion and Conclusions
EGCg research increased drastically when researchers reported that epidemiological evidence indicated lower rates of certain cancers in populations that consumed the most green tea. Since then, numerous molecular mechanisms for these beneficial properties have been discovered. Not surprisingly, EGCg has become one of the most popular nutraceutical ingredients in the world. However, reports of its poor bioavailability are troublesome to product development.17,45−47 EGCg is highly susceptible to oxidation, first pass metabolism, and rapid efflux.46 Numerous groups have evaluated delivery systems to overcome these limitations with EGCg, but none have tried cocrystallization. Cocrystallization has been used extensively to improve the solubility and/or bioavailability of poorly soluble APIs.22,23,27,48−54 This study is the first report on the bioavailability effects of reducing the solubility of a highly soluble API.
We evaluated the structure, solubility, and pharmacokinetics of four new cocrystals of EGCg, all of which were found to exhibit reduced water solubility. We hypothesized that these changes in solubility would change the pharmacokinetics of EGCg, perhaps improving its bioavailability. When interpreting our data, it is important to note that we used a nonconventional gavage vehicle (corn oil) to prevent presolubilizing of the sieved crystalline forms. Our EGCg control peaked approximately 16.5 min more rapidly than previously published pharmacokinetic data at the same dose in rats using a saline gavage vehicle.55 Furthermore, we previously assessed the pharmacokinetics of an EGCg nanolipidic particle suspension in 10% ethanol.17 For an accurate comparison, we used a 10% ethanol vehicle for the control. This resulted in lower levels of free EGCg measured in the plasma (Cmax = 116.57 ng/mL).17 These variations illustrate the impact that gavage vehicle can have on pharmacokinetics. Nonetheless, the results presented in this paper should be considered a stand-alone comparison of EGCg and EGCg cocrystals in a common vehicle.
At first glance, it seems that cocrystallization might not be the most useful method for improving the bioavailability of EGCg, since only two cocrystals exhibited improved relative bioavailability and the increases were very modest (Table 3). Although we did not monitor the metabolic profile of the circulating EGCg, it is likely that the blunted bioavailability increases were due to the well-known metabolism issues with EGCg,56 which is left unchecked in these cocrystal forms. An additional contributor to the observed relative bioavailability for this experiment is apparent when considering the variability for the EGCg control (Table 3). One of the experimental rats achieved three times higher plasma levels of free EGCg than observed in the other rats. This increased the variability and the Cmax but did not change the Tmax. We decided not to exclude this animal from the analysis. However, if this animal had been excluded as an outlier, the relative bioavailability for the cocrystal forms would have increased. Our pharmacokinetic study design utilized an n = 3. This decision was driven by our desire to minimize the use of animals and based on previous pharmacokinetic studies performed in our lab.17,57 However, the pharmacokinetic study suffered from unexpected levels of variability, which should be considered when drawing conclusions from this data. The pharmacokinetic parameters presented in Table 3 include the means with standard deviations. For the EGCgINA, an asterisk marks the HL_Lambda_z, because there were insufficient data points in the elimination phase such that the Cmax had to be included in the noncompartmental analysis in WinNonlin. This only occurred with the EGCgINA cocrystal because we did not expect such a robust change in the Tmax.
Even though our new forms of EGCg did not lead to large increases in oral bioavailability, this does not mean that cocrystallization cannot benefit the commercialization of EGCg. The ability to have multiple solid forms of EGCg with different physicochemical properties and pharmacokinetic profiles could be very useful. Further examination of Figure 10 indicates that some of the EGCg cocrystals exhibited different pharmacokinetic profiles. The EGCgINA·3H2O and EGCgINA cocrystals not only had the highest relative bioavailabilities of the cocrystals that we evaluated but also resulted in higher concentrations of free EGCg after 60 and 120 min, respectively. This plateau effect could be useful for achieving more sustained levels of EGCg in the blood. Furthermore, because pharmaceutical and nutraceutical products often contain combinations of ingredients with synergistic effects, cocrystallization could be used to select forms with similar pharmacokinetics as the other ingredients. This would allow for optimization of the synergistic activities.
Our results generally support our hypothesis that reducing the solubility reduces the rate of dissolution and delays the time at which maximal plasma levels are reached (Tmax). EGCgNIC·9H2O was found to be the most water-soluble cocrystal form and had the most rapid Tmax after free EGCg. EGCgINM·5H2O and EGCgINA·3H2O also had expected Tmax values based upon the solubulity experiments. However, EGCgINA peaked at 160 min (Table 3) and was found to exhibit similar solubility to that of EGCgINM·5H2O and EGCgINA·3H2O (Figure 9). This phenomenon cannot be explained by our solubility hypothesis. Normally, it might be reasonable to assume that other pharmacokinetic parameters are being changed, like elimination rate or metabolism. This would normally be attributed to some effect of the CCF. However, this cannot be the case for EGCgINA and EGCgINA·3H2O since they have the same CCF. As expected, the hydrate exhibited slightly lower water solubility and peaked later in comparison to the other more soluble EGCg forms. Because anhydrous and hydrous forms were evaluated, we were able to conclude that the presence of INA does not explain the discrepancy observed with the EGCgINA cocrystal. Further studies are required to elucidate the cause of this anomaly. Nonetheless, it is very clear that cocrystallization can be used to generate novel forms of EGCg with modulated dissolution and pharmacokinetic profiles (see also Supporting Information).
In conclusion, the present study demonstrates how crystal engineering concepts can be utilized for isolating new cocrystals of EGCg and that with the aid of several crystallization techniques we were able isolate the first pure (i.e., nonsolvated, nonhydrated) crystal form of EGCg. However, cocrystallization might not be the best option for improving the bioavailability of BCS 3 compounds like EGCg, but it could be very useful when absorption kinetics is critical such as it is in synergistic and/or combination products. For example, selection of a cocrystal form that exhibits a dissolution rate-limited pharmacokinetic profile could achieve sustained plasma levels of free active ingredient similar to other sustained release preformulation techniques like transdermal delivery.58 Sustained plasma levels are preferred so that the therapeutic bioactivity of the active ingredient is maintained for a longer duration. Even though transdermal delivery can also accomplish sustained plasma levels, it has numerous disadvantages to oral formulation such as complicated production, increased cost, and reduced patient compliance due to common side effects like skin irritation at the adhesion site.
This study therefore lends credence to the concept of using cocrystallization to modulate the pharmacokinetics of highly water-soluble compounds. In this study, we evaluated four new solid forms of EGCg with different solubility properties and consequent pharmacokinetic profiles. Although we confirmed that a single cocrystal with reduced solubility relative to a highly soluble pure form is unlikely to produce improved bioavailability, the ability to modulate the pharmacokinetic curve using crystal engineering could be very useful in the clinical translation of active ingredients. In theory, this could result in a superior bioactivity and therapeutic profile. Further studies are required to confirm this potential utility.
Acknowledgments
This work was supported by research grant number ARG2008 from the Johnnie B. Byrd, Sr. Alzheimer’s Center & Research Institute (R.S. and M.Z.).
Glossary
Abbreviations
- EGCg
epigallocatechin 3-gallate
- API
active pharmaceutical ingredient
- INM
isonicotinamide
- INA
isonicotinic acid
- NIC
nicotinamide
- NAC
nicotinic acid
- EGCgNIC·9H2O
EGCg:nicotinamide
- EGCgINM·5H2O
EGCg:isonicotinamide
- EGCgINA·3H2O
EGCg:isonicotinic acid
- EGCgINA
EGCg:isonicotinic acid anhydrous
- EGCgNAC·xH2O
EGCg:nicotinic acid
- PXRD
powder X-ray diffraction
- DSC
differential scanning calorimeter
- FT-IR
Fourier transform infrared spectroscopy
- TGA
thermogravimetric analysis
- AUC
area under curve
Supporting Information Available
IR, DSC, TGA, and PXRD patterns for the reported crystalline forms of EGCg. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Present Address
⊥ Department of Industrial and Physical Pharmacy, College of Pharmacy, Purdue University, West Lafayette, Indiana 47907, United States.
Authors are inventors on USF patent disclosures pertaining to these technologies.
Supplementary Material
References
- Hertog M. G. Epidemiological evidence on potential health properties of flavonoids. Proc. Nutr. Soc. 1996, 551B385–97. [DOI] [PubMed] [Google Scholar]
- Arts I. C. A review of the epidemiological evidence on tea, flavonoids, and lung cancer. J. Nutr. 2008, 13881561S–1566S. [DOI] [PubMed] [Google Scholar]
- Formica J. V.; Regelson W. Review of the biology of Quercetin and related bioflavonoids. Food Chem. Toxicol. 1995, 33121061–80. [DOI] [PubMed] [Google Scholar]
- Hwang J. T.; Ha J.; Park I. J.; Lee S. K.; Baik H. W.; Kim Y. M.; Park O. J. Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett. 2007, 2471115–21. [DOI] [PubMed] [Google Scholar]
- Jung Y. D.; Kim M. S.; Shin B. A.; Chay K. O.; Ahn B. W.; Liu W.; Bucana C. D.; Gallick G. E.; Ellis L. M. EGCG, a major component of green tea, inhibits tumour growth by inhibiting VEGF induction in human colon carcinoma cells. Br. J. Cancer 2001, 846844–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y. C.; Park H. Y.; Hwang H. S.; Kang S. U.; Pyun J. H.; Lee M. H.; Choi E. C.; Kim C. H. (−)-Epigallocatechin-3-gallate (EGCG) inhibits HGF-induced invasion and metastasis in hypopharyngeal carcinoma cells. Cancer Lett. 2008, 2711140–52. [DOI] [PubMed] [Google Scholar]
- Wang X.; Hao M. W.; Dong K.; Lin F.; Ren J. H.; Zhang H. Z. Apoptosis induction effects of EGCG in laryngeal squamous cell carcinoma cells through telomerase repression. Arch. Pharm. Res. 2009, 3291263–9. [DOI] [PubMed] [Google Scholar]
- Wang Y. C.; Bachrach U. The specific anti-cancer activity of green tea (−)-epigallocatechin-3-gallate (EGCG). Amino Acids 2002, 222131–43. [DOI] [PubMed] [Google Scholar]
- Lin C. L.; Chen T. F.; Chiu M. J.; Way T. D.; Lin J. K. Epigallocatechin gallate (EGCG) suppresses beta-amyloid-induced neurotoxicity through inhibiting c-Abl/FE65 nuclear translocation and GSK3 beta activation. Neurobiol. Aging 2009, 30181–92. [DOI] [PubMed] [Google Scholar]
- Obregon D. F.; Rezai-Zadeh K.; Bai Y.; Sun N.; Hou H.; Ehrhart J.; Zeng J.; Mori T.; Arendash G. W.; Shytle D.; Town T.; Tan J. ADAM10 activation is required for green tea (−)-epigallocatechin-3-gallate-induced alpha-secretase cleavage of amyloid precursor protein. J. Biol. Chem. 2006, 2812416419–27. [DOI] [PubMed] [Google Scholar]
- Rezai-Zadeh K.; Arendash G. W.; Hou H.; Fernandez F.; Jensen M.; Runfeldt M.; Shytle R. D.; Tan J. Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008, 1214, 177–87. [DOI] [PubMed] [Google Scholar]
- Rezai-Zadeh K.; Shytle D.; Sun N.; Mori T.; Hou H.; Jeanniton D.; Ehrhart J.; Townsend K.; Zeng J.; Morgan D.; Hardy J.; Town T.; Tan J. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J. Neurosci. 2005, 25388807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J. T.; Park I. J.; Shin J. I.; Lee Y. K.; Lee S. K.; Baik H. W.; Ha J.; Park O. J. Genistein, EGCG, and capsaicin inhibit adipocyte differentiation process via activating AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 2005, 3382694–9. [DOI] [PubMed] [Google Scholar]
- Wolfram S. Effects of green tea and EGCG on cardiovascular and metabolic health. J. Am. Coll. Nutr. 2007, 264373S–388S. [DOI] [PubMed] [Google Scholar]
- Cai E. P.; Lin J. K. Epigallocatechin Gallate (EGCG) and Rutin Suppress the Glucotoxicity through Activating IRS2 and AMPK Signaling in Rat Pancreatic beta Cells. J. Agric. Food Chem. 2009, 57, 9817–27. [DOI] [PubMed] [Google Scholar]
- Lin C. L.; Lin J. K. Epigallocatechin gallate (EGCG) attenuates high glucose-induced insulin signaling blockade in human hepG2 hepatoma cells. Mol. Nutr. Food Res. 2008, 528930–9. [DOI] [PubMed] [Google Scholar]
- Smith A.; Giunta B.; Bickford P. C.; Fountain M.; Tan J.; Shytle R. D. Nanolipidic particles improve the bioavailability and alpha-secretase inducing ability of epigallocatechin-3-gallate (EGCG) for the treatment of Alzheimer’s disease. Int. J. Pharmaceutics 2010, 3891–2207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando S.; Kikuchi J.; Fujimura Y.; Ida Y.; Higashi K.; Moribe K.; Yamamoto K. Physicochemical characterization and structural evaluation of a specific 2:1 cocrystal of naproxen-nicotinamide. J. Pharm. Sci. 2012, 10193214–21. [DOI] [PubMed] [Google Scholar]
- Cherukuvada S.; Babu N. J.; Nangia A. Nitrofurantoin-p-aminobenzoic acid cocrystal: hydration stability and dissolution rate studies. J. Pharm. Sci. 2011, 10083233–44. [DOI] [PubMed] [Google Scholar]
- Gao Y.; Zu H.; Zhang J. Enhanced dissolution and stability of adefovir dipivoxil by cocrystal formation. J. Pharm. Pharmacol. 2011, 634483–90. [DOI] [PubMed] [Google Scholar]
- Goud N. R.; Gangavaram S.; Suresh K.; Pal S.; Manjunatha S. G.; Nambiar S.; Nangia A. Novel furosemide cocrystals and selection of high solubility drug forms. J. Pharm. Sci. 2012, 1012664–80. [DOI] [PubMed] [Google Scholar]
- Jung M. S.; Kim J. S.; Kim M. S.; Alhalaweh A.; Cho W.; Hwang S. J.; Velaga S. P. Bioavailability of indomethacin-saccharin cocrystals. J. Pharm. Pharmacol. 2010, 62111560–8. [DOI] [PubMed] [Google Scholar]
- McNamara D. P.; Childs S. L.; Giordano J.; Iarriccio A.; Cassidy J.; Shet M. S.; Mannion R.; O’Donnell E.; Park A. Use of a glutaric acid cocrystal to improve oral bioavailability of a low solubility API. Pharm. Res. 2006, 2381888–97. [DOI] [PubMed] [Google Scholar]
- Okaniwa M.; Imada T.; Ohashi T.; Miyazaki T.; Arita T.; Yabuki M.; Sumita A.; Tsutsumi S.; Higashikawa K.; Takagi T.; Kawamoto T.; Inui Y.; Yoshida S.; Ishikawa T. Design and synthesis of novel DFG-out RAF/vascular endothelial growth factor receptor 2 (VEGFR2) inhibitors: 2. Synthesis and characterization of a novel imide-type prodrug for improving oral absorption. Bioorg. Med. Chem. 2012, 20154680–92. [DOI] [PubMed] [Google Scholar]
- Rahman Z.; Samy R.; Sayeed V. A.; Khan M. A. Physicochemical and mechanical properties of carbamazepine cocrystals with saccharin. Pharm. Dev. Technol. 2012, 174457–65. [DOI] [PubMed] [Google Scholar]
- Schultheiss N.; Newman A. Pharmaceutical Cocrystals and Their Physicochemical Properties. Cryst. Growth Des. 2009, 962950–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. J.; Kavuru P.; Wojtas L.; Zaworotko M. J.; Shytle R. D. Cocrystals of quercetin with improved solubility and oral bioavailability. Mol. Pharmaceutics 2011, 851867–76. [DOI] [PubMed] [Google Scholar]
- Tsutsumi S.; Iida M.; Tada N.; Kojima T.; Ikeda Y.; Moriwaki T.; Higashi K.; Moribe K.; Yamamoto K. Characterization and evaluation of miconazole salts and cocrystals for improved physicochemical properties. Int. J. Pharmaceutics 2011, 4212230–6. [DOI] [PubMed] [Google Scholar]
- Yadav A. V.; Dabke A. P.; Shete A. S. Crystal engineering to improve physicochemical properties of mefloquine hydrochloride. Drug Dev. Ind. Pharm. 2010, 3691036–45. [DOI] [PubMed] [Google Scholar]
- Shutava T. G.; Balkundi S. S.; Lvov Y. M. (−)-Epigallocatechin gallate/gelatin layer-by-layer assembled films and microcapsules. J. Colloid Interface Sci. 2009, 3302276–83. [DOI] [PubMed] [Google Scholar]
- Ullmann U.; Haller J.; Decourt J. D.; Girault J.; Spitzer V.; Weber P. Plasma-kinetic characteristics of purified and isolated green tea catechin epigallocatechin gallate (EGCG) after 10 days repeated dosing in healthy volunteers. Int. J. Vitam. Nutr. Res. 2004, 744269–78. [DOI] [PubMed] [Google Scholar]
- Swezey R. R.; Aldridge D. E.; LeValley S. E.; Crowell J. A.; Hara Y.; Green C. E. Absorption, tissue distribution and elimination of 4-[(3)h]-epigallocatechin gallate in beagle dogs. Int. J. Toxicol. 2003, 223187–93. [DOI] [PubMed] [Google Scholar]
- Cai Y.; Anavy N. D.; Chow H. H. Contribution of presystemic hepatic extraction to the low oral bioavailability of green tea catechins in rats. Drug Metab. Dispos. 2002, 30111246–9. [DOI] [PubMed] [Google Scholar]
- Hu B.; Ting Y.; Yang X.; Tang W.; Zeng X.; Huang Q. Nanochemoprevention by encapsulation of (−)-epigallocatechin-3-gallate with bioactive peptides/chitosan nanoparticles for enhancement of its bioavailability. Chem. Commun. (Cambridge) 2012, 48182421–3. [DOI] [PubMed] [Google Scholar]
- Bruker APEX2 (Version 2008.1–0); Bruker AXS Inc.: Madison, WI, 2008. [Google Scholar]
- SAINT Data Reduction Software; Bruker AXS Inc.: Madison, WI, 2009. [Google Scholar]
- Sheldrick G. M.SADABS. Program for Empirical Absorption Correction; University of Gottingen: Gottingen, Germany, 2008. [Google Scholar]
- Farrugia L. J. WinGX suite for single crystal small molecule crystallography. J. Appl. Cryst. 1999, 32, 837–838. [Google Scholar]
- Sheldrick G. M.SHELXL-97. Program for the Refinement of Crystal Structures; University of Gottingen: Gottingen, Germany, 1997. [Google Scholar]
- Sheldrick G. M. Phase Annealing in SHELX-90: Direct Methods for Larger Structures. Acta Crystallogr. 1990, A46, 467–473. [Google Scholar]
- Lambert J. D.; Lee M. J.; Diamond L.; Ju J.; Hong J.; Bose M.; Newmark H. L.; Yang C. S. Dose-dependent levels of epigallocatechin-3-gallate in human colon cancer cells and mouse plasma and tissues. Drug Metab. Dispos. 2006, 3418–11. [DOI] [PubMed] [Google Scholar]
- Sparidans R. W.; Lagas J. S.; Schinkel A. H.; Schellens J. H.; Beijnen J. H. Liquid chromatography-tandem mass spectrometric assays for salinomycin in mouse plasma, liver, brain and small intestinal contents and in OptiMEM cell culture medium. J. Chromatogr., B: Analyt. Technol. Biomed. Life Sci. 2007, 8552200–10. [DOI] [PubMed] [Google Scholar]
- Wang M.; Miksa I. R. Multi-component plasma quantitation of anti-hyperglycemic pharmaceutical compounds using liquid chromatography-tandem mass spectrometry. J. Chromatogr., B: Analyt. Technol. Biomed. Life Sci. 2007, 8561–2318–27. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Hop C. E.; Leung K. H.; Pang J. Determination of in vitro permeability of drug candidates through a caco-2 cell monolayer by liquid chromatography/tandem mass spectrometry. J. Mass Spectrom. 2000, 35171–6. [DOI] [PubMed] [Google Scholar]
- Dube A.; Nicolazzo J. A.; Larson I. Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (−)-epigallocatechin gallate. Eur. J. Pharm. Sci. 2010, 412219–25. [DOI] [PubMed] [Google Scholar]
- Kale A.; Gawande S.; Kotwal S.; Netke S.; Roomi W.; Ivanov V.; Niedzwiecki A.; Rath M. Studies on the effects of oral administration of nutrient mixture, quercetin and red onions on the bioavailability of epigallocatechin gallate from green tea extract. Phytother. Res. 2010, 24Suppl 1S48–55. [DOI] [PubMed] [Google Scholar]
- Landis-Piwowar K. R.; Huo C.; Chen D.; Milacic V.; Shi G.; Chan T. H.; Dou Q. P. A novel prodrug of the green tea polyphenol (−)-epigallocatechin-3-gallate as a potential anticancer agent. Cancer Res. 2007, 6794303–10. [DOI] [PubMed] [Google Scholar]
- Sekiguchi K.; Obi N. Studies on Absorption of Eutectic Mixture. I. A Comparison of the Behavior of Eutectic Mixture of Sulfathiazole and that of Ordinary Sulfathiazole in Man. Chem. Pharm. Bull 1961, 9, 866–872. [Google Scholar]
- Hickey M. B.; Peterson M. L.; Scoppettuolo L. A.; Morrisette S. L.; Vetter A.; Guzman H.; Remenar J. F.; Zhang Z.; Tawa M. D.; Haley S.; Zaworotko M. J.; Almarsson O. Performance comparison of a co-crystal of carbamazepine with marketed product. Eur. J. Pharm. Biopharm. 2007, 671112–9. [DOI] [PubMed] [Google Scholar]
- Bak A.; Gore A.; Yanez E.; Stanton M.; Tufekcic S.; Syed R.; Akrami A.; Rose M.; Surapaneni S.; Bostick T.; King A.; Neervannan S.; Ostovic D.; Koparkar A. The co-crystal approach to improve the exposure of a water-insoluble compound: AMG 517 sorbic acid co-crystal characterization and pharmacokinetics. J. Pharm. Sci. 2008, 9793942–56. [DOI] [PubMed] [Google Scholar]
- Cheney M.; Shan N.; Healey E.; Hanna M.; Wojtas L.; Zaworotko M. J.; Sava V.; Song S.; Sanchez-Ramos J. Effects of Crystal Form on Solubility and Pharmacokinetics: A Crystal Engineering Case Study of Lamotrigine. Cryst. Growth Des. 2010, 101394–405. [Google Scholar]
- Stanton M. K.; Kelly R. C.; Colletti A.; Kiang Y. H.; Langley M.; Munson E. J.; Peterson M. L.; Roberts J.; Wells M. Improved pharmacokinetics of AMG 517 through co-crystallization. Part 1: comparison of two acids with corresponding amide co-crystals. J. Pharm. Sci. 2010, 9993769–78. [DOI] [PubMed] [Google Scholar]
- Weyna D. R.; Cheney M. L.; Shan N.; Hanna M.; Zaworotko M. J.; Sava V.; Song S.; Sanchez-Ramos J. R. Improving Solubility and Pharmacokinetics of Meloxicam via Multiple-Component Crystal Formation. Mol. Pharmaceutics 2012, 972094–2102. [DOI] [PubMed] [Google Scholar]
- Cheney M. L.; Weyna D. R.; Shan N.; Hanna M.; Wojtas L.; Zaworotko M. J. Coformer selection in pharmaceutical cocrystal development: a case study of a meloxicam aspirin cocrystal that exhibits enhanced solubility and pharmacokinetics. J. Pharm. Sci. 2011, 10062172–81. [DOI] [PubMed] [Google Scholar]
- Lin L. C.; Wang M. N.; Tseng T. Y.; Sung J. S.; Tsai T. H. Pharmacokinetics of (−)-epigallocatechin-3-gallate in conscious and freely moving rats and its brain regional distribution. J. Agric. Food Chem. 2007, 5541517–24. [DOI] [PubMed] [Google Scholar]
- Warden B. A.; Smith L. S.; Beecher G. R.; Balentine D. A.; Clevidence B. A. Catechins are bioavailable in men and women drinking black tea throughout the day. J. Nutr. 2001, 13161731–7. [DOI] [PubMed] [Google Scholar]
- Smith A. J.; Kavuru P.; Wojtas L.; Zaworotko M. J.; Shytle R. D. Cocrystals of quercetin with improved solubility and oral bioavailability. Mol. Pharmaceutics 2011, 851867–76. [DOI] [PubMed] [Google Scholar]
- Zobrist R. H.; Schmid B.; Feick A.; Quan D.; Sanders S. W. Pharmacokinetics of the R- and S-enantiomers of oxybutynin and N-desethyloxybutynin following oral and transdermal administration of the racemate in healthy volunteers. Pharm. Res. 2001, 1871029–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.