Abstract
Despite the relatively low-grade of most central neurocytomas (CN), evidence suggests an existence of aggressive subset with a propensity for recurrence. Recent studies have found the MIB-1 labeling index to be a prognostic indicator in CN. Here we review our experience with CN to analyze the relationships between extent of resection, adjuvant therapy, tumor histology, and clinical outcomes based on aggressive histology, as defined by MIB-1 labeling. A retrospective review was performed on histologically proven CN surgically resected from 1993 to 2009 at The University of California San Francisco. Recurrence rates were analyzed using Kaplan–Meier with respect to MIB-1 labeling and extent of resection. All MIB-1 labeling indices were analyzed. A total of 18 patients were identified with a mean age of 30 years (range 17–58 years) and median follow-up of 40 months (5–173 months). The treatments were: gross total resection (GTR) alone (17% of patients), subtotal resection (STR) alone (50% of patients), STR plus radiotherapy (XRT: external beam or stereotactic radiosurgery: 28% of patients), or STR plus chemotherapy (5% of patients). The extent of resection and a MIB-1 labeling index > 4% was predictive of recurrence (p<0.01). In the 33% of patients in whom the tumor had recurred, 33% had STR with MIB-1 labeling > 4% with a median time to recurrence of 23.5 months. The 2-year and 4-year recurrence rates in patients with MIB-1 labeling > 4% were 50% and 75% respectively. No patient with a MIB-1 labeling index < 4% who received STR alone had a recurrence. Thus, in patients with CN who were treated with STR, histology demonstrating a MIB-1 labeling index > 4% can be a clinically useful prognostic indicator and can help guide adjuvant treatment.
Keywords: Central neurocytoma, chemotherapy, MIB-1, radiotherapy, recurrence, surgery
1 Introduction
Central neurocytoma (CN) is a rare brain tumor of the ventricular system that most commonly occurs in young adults. Despite the relatively low-grade of most CN1, evidence for a more aggressive subset with a propensity for recurrence and progression is accumulating 2–8. As the mainstay of treatment for CN is surgery, the ability to predict which tumors will be more likely to recur or progress despite initial treatment may help direct adjuvant therapy.
Evidence to help guide appropriate treatment comes largely from case reports, small case series, and a group of analyses performed on one aggregated data set by Rades and colleagues 6,9–14. Without any clinical trials, a variety of treatment approaches have been developed, but there is no authoritative data to guide treatment decisions. The literature appears to support a treatment goal of gross total resection (GTR), with adjuvant radiotherapy (XTR) added for patients who received subtotal resection (STR) to reduce recurrence rates 9,15. Additionally, there is some evidence that MIB-1 labeling indices > 2–3% and histologic atypia indicate a worse prognosis for patients 5,7,13,15,16, and may necessitate a more aggressive primary treatment including XTR or chemotherapy regardless of the extent of resection.
Here we review our institutional experience with CN to analyze the relationships between extent of resection, adjuvant therapy, tumor histology, and clinical outcomes. We show that histology that demonstrates a MIB-1 labeling index >4% in patients with CN who underwent STR can be a clinically useful prognostic indicator.
2 Methods
2.1. Patient population
All patients undergoing neurosurgical intervention at the University of California San Francisco (UCSF) are prospectively enrolled in a database. Using this database, we identified all patients between 1993 and 2009 who underwent evaluation and initial treatment for any neurocytoma at our institution. We then excluded all patients with any other intracranial tumor history or those patients with extraventricular neurocytomas. From this cohort, we evaluated all patients undergoing craniotomy for resection of a histologically proven CN with operative, radiologic, and pathology data, which provided a total of 18 patients. This study was approved by the UCSF Committee on Human Research under the approval CHR# H41955-34759-01.
2.2. Data collection
Clinical information was retrospectively reconstructed using patient medical records, radiologic data and pathologic specimens from both UCSF and outside medical facilities. All clinical assessments were performed by a neurosurgeon. Patient age was defined as the age at the time of surgery. Both the pre-operative, post-contrast T1-weighted MRI and/or the surgeon’s operative note were reviewed to confirm tumor location and site of attachment. Tumors were classified as being intraventricular or extraventricular based on these MRI. The pathologic data was reviewed centrally (by T.T.). Post-operative assessments and imaging were examined for post-operative complications, evidence of recurrence, and treatment for recurrence. No extraventricular or spinal neurocytomas were included in our analysis.
2.3. Statistical analysis
Kaplan–Meier estimates were used to generate survival curves. Differences in time to recurrence or death from disease after initial treatment were analyzed by the log-rank test. Statistical tests were considered significant for p < 0.05. Continuous variables are presented with standard error (SE). All descriptive and statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS version 16.0; Chicago, IL, USA).
3 Results
3.1. Patient demographics and characteristics
A total of 18 patients underwent treatment for CN during the period reviewed. The mean age at time of treatment was 30 years (SE ± 2.9, range 17–58 years) and 56% of patients were male. Extent of resection and adjuvant therapy utilized was: STR (50%), GTR (17%), STR plus XRT (external beam or stereotactic radiosugery) (28%), STR and adjuvant chemotherapy (5%). Median time of follow-up was 40 months (range 5–173 months). The patient demographics are listed in Table 1. Presenting symptoms were primarily representative of increased intracranial pressure (Table 2). Treatments for recurrence included repeated STR followed by temozolomide in one patient, two patients received procarbazine, lomustine (CCNU), and vincristine (PCV) chemotherapy, and one patient received external beam radiation (55 Gy).
Table 1.
Demographics, clinical characteristics and treatment of patients with central neurocytoma
| Characteristic | |
|---|---|
| Age (years) | 30 ±2.9 (range 17–58) |
| Male/female (%) | 56/44 |
| Race (%) | |
| White | 56 |
| Asian | 11 |
| Black | 11 |
| Hispanic | 11 |
| Other | 11 |
| STR (%) | 50 |
| GTR (%) | 17 |
| STR + XRT (%) | 28 |
| STR + chemo (%) | 5 |
| Median follow-up (months) | 40 |
Chemo = chemotherapy, GTR = gross total resection, STR = subtotal resection, XRT = radiotherapy (external beam or stereotactic radiosurgery).
Table 2.
Prevalence of various presenting symptoms in patients with central neurocytomas
| Presenting symptom (n=18) | Prevalence (%) |
|---|---|
| Headache | 60 |
| Vision abnormality | 48 |
| Nausea | 36 |
| Vertigo | 18 |
| Incidental | 12 |
| Balance | 12 |
| Weakness | 12 |
| Syncope | 6 |
| Altered mental status | 6 |
| Dyspraxia | 6 |
| Gait ataxia | 6 |
| Language | 6 |
| Hearing loss | 6 |
3.2. Tumor Characteristics
The mean tumor volume in patients was 83 cm3 (24.5 cm) and the mean largest average diameter was 4.9 cm (0.49 cm). A representative pre-operative MRI of a CN from our series is shown in Figure 1 for a patient who had no recurrence. A pre-operative MRI and a post-operative MRI demonstrating recurrence 4 years later in the same patient in our series is shown in Figure 2. Anatomic localization was 31% in the left lateral ventricle, 31% in the right lateral ventricle, and 38% arose from the midline and extended bilaterally. A total of 54% of the tumors extended beyond the lateral ventricles into the foramen of Monro, 39% into the third ventricle, 15% into the aqueduct of Sylvius, and 8% into the fourth ventricle. On MRI, tumors were generally partially enhancing with 77% of cases demonstrating hydrocephalus and 46% demonstrating shift. Pathology demonstrated positive staining for synaptophysin in 100% of instances, glial fibrillary acidic protein in 64%, and neurofilament in 11%.
Figure 1.
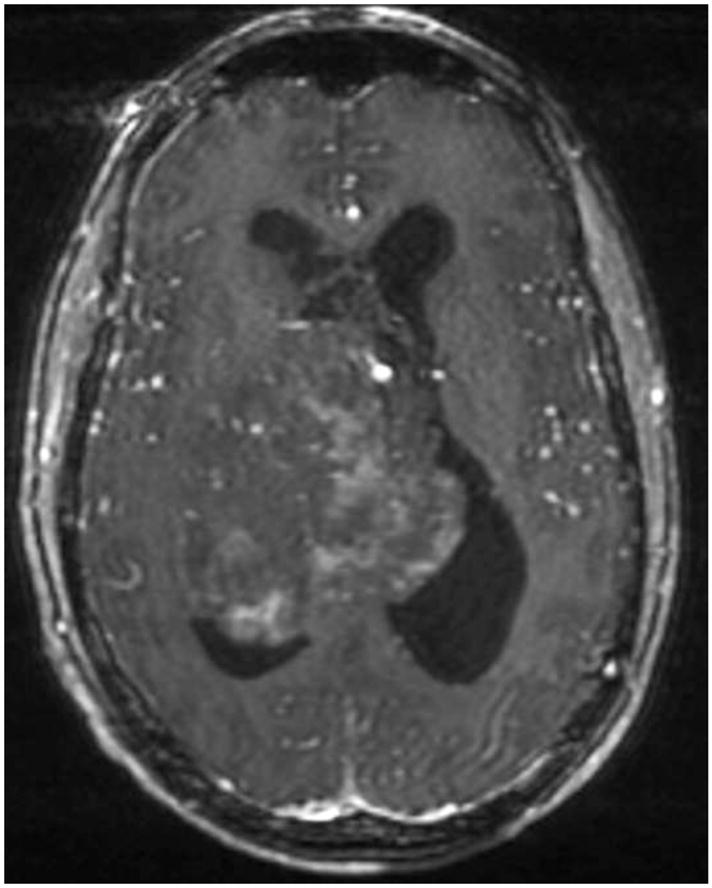
Pre-operative axial T1-weighted post-contrast MRI of a patient in this series who did not have any recurrence after resection.
Figure 2.
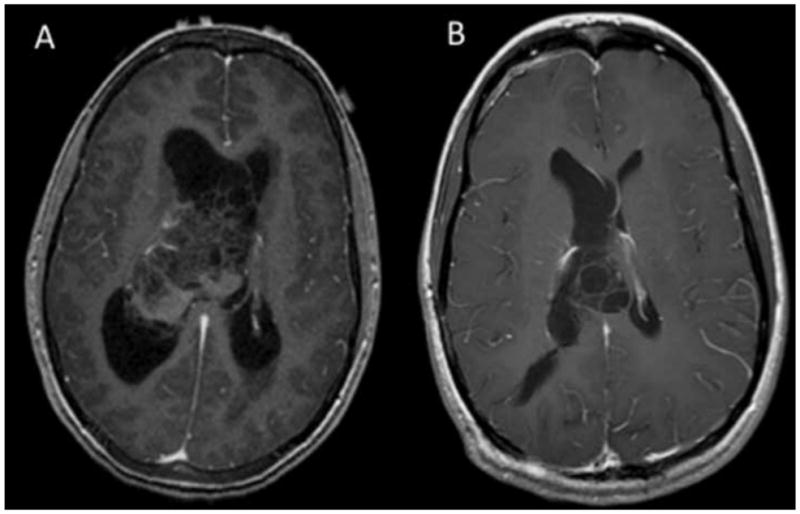
(A) Preoperative axial T1-weighted post-contrast MRI of a patient in this series and (B) post-resection demonstration of recurrence in the same patient 4 years after initial treatment.
3.3. MIB-1 labeling index predicts recurrence in central neurocytoma
The MIB-1 labeling index was available for 10 patients. We found that the average MIB-1 labeling index was 5.4% (0.79%) for recurrent tumors and 1.5% (0.52%) for non-recurrent tumors. The patient population size was not large enough to perform a Cox regression to analyze for interactions between MIB-1 labeling index and extent of resection. A threshold of 4% allowed for a clear prediction of recurrence in our patient group with no recurrences in tumors with a MIB-1 labeling < 4%, and 100% recurrence in tumors with MIB-1 labeling index > 4% (log rank, p < 0.01) (Fig 3). Further, all patients with tumors demonstrating MIB-1 index < 4% who received a STR without adjuvant therapy remained recurrence-free throughout follow-up. Inversely, all patients with STR alone and an MIB-1 index > 4% had a recurrence.
Figure 3.
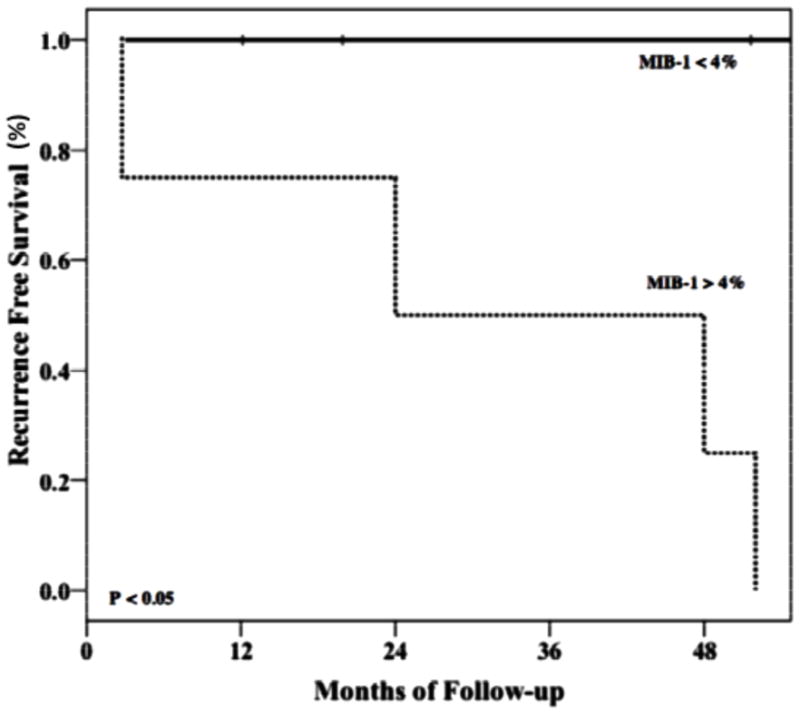
Kaplan–Meier analysis of progression free survival stratified by MIB-1 labeling index > or < 4% demonstrating significant difference in recurrence rates between the two groups (log rank, p < 0.05).
3.4. Recurrence after treatment for central neurocytoma
Recurrence data were available for all patients: 33% of patients had a recurrence of CN after primary treatment, with a median time to recurrence of 23.5 months (mean 26.7 months, 8.1 months). Two patients with recurrence were lost to follow-up after recurrence had been documented at 23 months and 24 months respectively post-primary treatment. No patients had multiple recurrences. One patient with recurrence died of an unknown cause 112 months after initial treatment. Median follow-up time after treatment for recurrence was 28 months (range 12–110 months). Kaplan–Meier analysis demonstrated lower rates of recurrence at 4 years following GTR (0%) and STR with adjuvant XRT (20%) compared to STR alone (48%) (Fig. 4). This difference was not significant, likely due to the low total number of patients in each subgroup.
Figure 4.
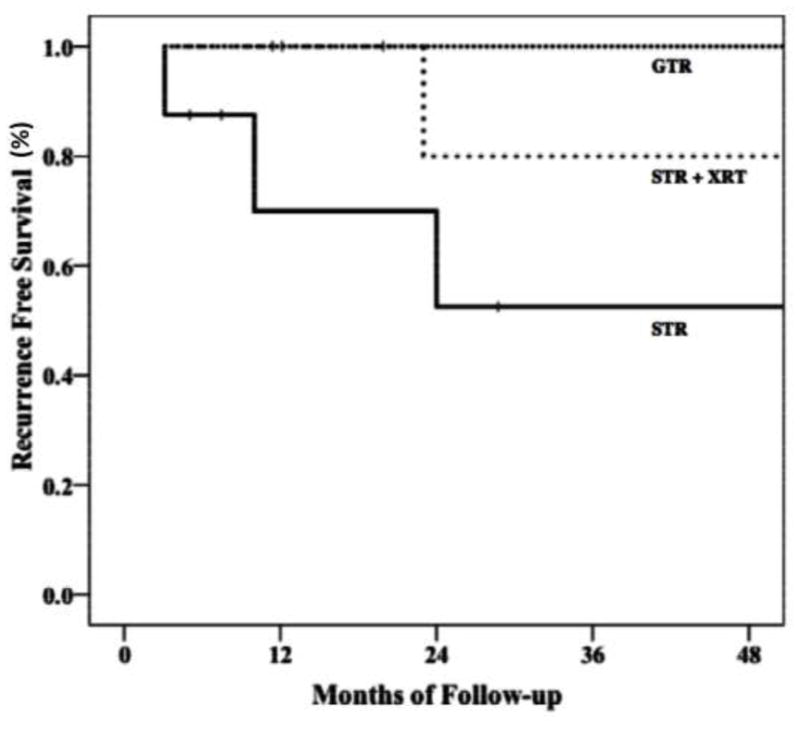
Kaplan–Meier graph of progression free survival by treatment modality including gross-total resection (GTR), subtotal resection (STR), and subtotal resection with adjuvant radiotherapy (STR + XRT) (either external beam or stereotactic radiosurgery) showing a tendency to lower rates of recurrence at 4 years following GTR (0%) and STR with adjuvant XRT (20%) compared to STR alone (48%).
3.5. Morbidity after surgical resection of central neurocytoma
Five patients experienced post-operative neurological or medical complications. One patient who had an extremely large tumor resected, developed pneumonia and pulmonary embolus post-operatively and was treated accordingly. He also developed a permanent hemiparesis. Another patient developed transient language difficulties, hemiparesis, and parasthesias, all of which resolved. A third patient developed transient memory difficulties. A fourth patient had transient memory loss and seizures that resolved and required only temporary medical intervention. The last patient with post-operative complications developed chronic seizures that were treated with levetiracetam. Six patients required ventriculoperitoneal shunting to control their hydrocephalus post-operatively. One of these patients was able to have the shunt removed within 1 year post-treatment. However, three more patients had to have a shunt placed after 1 year or more post-resection. These three patients developed recurrences.
4 Discussion
Despite the classification of CN as a World Health Organization grade II tumor17, there is clearly a tumor subset that has a high recurrence rate. Treatment approaches vary considerably for CN, but generally include surgical resection with adjuvant XRT where where STR only is achieved. In this study we evaluated for the existence of high grade CN.
Our results suggest that a MIB-1 labeling index > 4% is a strong predictor for recurrence after primary treatment. Higher MIB-1 labeling indices are indicative of increased rates of tumor cell proliferation. We found recurrence in all tumors with a MIB-1 labeling index > 4%, whereas tumors with this index < 4% did not recur. Several studies have found a MIB-1 labeling index to be a prognostic indicator with reported cutoffs generally at 2% to 3% 3–5,7,8,13,15,16,18,19; however, some studies have found no such correlation between pathology and disease outcome, indicating that the cutoff of 2% to 3% may be too low19. Soylemezoglu and colleagues 7 first demonstrated a CN histologic subtype with an elevated MIB-1 index as an “atypical central neurocytoma”, portending higher rates of recurrence. It is unclear as to the exact significance of high MIB-1 labeling index in relevance to the underlying tumor biology and phenotype. Increased levels of proliferative markers seen in histologic analysis may be a sign of a more rapidly growing or aggressive tumor, which can be predictive for recurrence particularly following STR. However, the correlation between an elevated MIB-1 index and atypia has yielded conflicting results across different studies 7,18, and may not be indicative of malignant transformation.
The treatment approach for both primary and recurrent CN have varied tremendously across the published literature 6,9,15,20–22. Our institution has favored surgical resection as initial treatment for CN. Similar to the reported literature, we observed that patients receiving GTR had no recurrences although the MIB-1 labeling index was not elevated in any of these patients. Although some literature supports the use of adjuvant radiation therapy for residual tumor post-STR 12,15,20, we pursued adjuvant treatment in 39% of patients post-STR. In 61% of the cases post-STR, patients received no adjuvant therapy, but instead had close clinical and radiologic follow-up, with treatment intervention with recurrence. All recurrence following STR alone occurred in tumors with an MIB-1 labeling index > 4%, whereas no tumor with lower MIB-1 index recurred post-STR. These data suggest that CN with low MIB-1 labeling may receive STR with close follow-up, while those with high MIB-1 indices should receive XRT post-operatively. Our findings differ from those found by a systematic review that reported that adjuvant radiotherapy decreased recurrence rates at early and late time points following STR for CN with MIB-1 indices < 3% 10. The differences seen between our study and the systematic review may reflect the small size and retrospective nature of our series. However, a single institution study has the advantage of applying a consistent methodology in calculation of MIB-1 labeling. Regardless, we observed that recurrences could be controlled by temolozomide or PCV chemotherapy, XRT, or repeat resection with adjuvant XRT.
In conclusion, CN consist of at least two different prognostic groups varying by the degree of proliferative activity as measured by MIB-1 labeling index. Patients should be advised of their differential prognosis depending on tumor histology. GTR should be pursued during initial resection, when possible, to decrease recurrence risk in patients where the tumor is eventually determined to have an elevated MIB-1 labeling index. If STR is performed to preserve function, histology demonstrating a MIB-1 labeling index > 4% should direct patients towards adjuvant therapy. Where the MIB-1 indices < 4% are found following an STR, adjuvant therapy may be pursued but close follow-up may be another reasonable option given the morbidities associated with XRT23–25. In cases of recurrence, chemotherapy, repeat resection, and XRT appear to be viable options alone or in combination, with several regimens in addition to ours already proposed in the literature2,22,26.
Acknowledgments
Gurvinder Kaur and Ari Kane were supported by the Howard Hughes Medical Institute and the Ivy Foundation. Dr. Sughrue and Dr. Oh were supported by a National Research Service Award from the National Institutes of Health and a Neurosurgery Education and Research Foundation grant for the American Association of Neurological Surgeons. Michael Safaee was supported by Doris Duke Foundation and Matthew Sun was supported by UCSF-CTSI Grant Number TL1 RR024129. Dr. Parsa was, in part, supported by the Reza and Georgianna Khatib Endowed Chair in Skull Base Tumor Surgery.
Footnotes
Conflict of Interest/Disclosures
The authors declare that they have no further financial or other conflicts of interest in relation to this research and its publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Choudhari KA, Kaliaperumal C, Jain A, et al. Central neurocytoma: a multi-disciplinary review. Br J Neurosurg. 2009 Dec;23(6):585–595. doi: 10.3109/02688690903254350. [DOI] [PubMed] [Google Scholar]
- 2.Brandes AA, Amista P, Gardiman M, et al. Chemotherapy in patients with recurrent and progressive central neurocytoma. Cancer. 2000 Jan 1;88(1):169–174. doi: 10.1002/(sici)1097-0142(20000101)88:1<169::aid-cncr23>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 3.Favereaux A, Vital A, Loiseau H, Dousset V, Caille J, Petry K. Histopathological variants of central neurocytoma: Report of 10 cases. Ann Pathol. 2000 Dec;20(6):558–563. [PubMed] [Google Scholar]
- 4.Lenzi J, Salvati M, Raco A, Frati A, Piccirilli M, Delfini R. Central neurocytoma: a novel appraisal of a polymorphic pathology. Our experience and a review of the literature. Neurosurg Rev. 2006 Oct;29(4):286–292. doi: 10.1007/s10143-006-0024-x. discussion 292. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa Y, Sugawara T, Seki H, Sakuma T. Central neurocytomas with MIB-1 labeling index over 10% showing rapid tumor growth and dissemination. J Neurooncol. 2006 Sep;79(2):211–216. doi: 10.1007/s11060-006-9129-x. [DOI] [PubMed] [Google Scholar]
- 6.Rades D, Schild SE. Treatment recommendations for the various subgroups of neurocytomas. J Neurooncol. 2006 May;77(3):305–309. doi: 10.1007/s11060-005-9047-3. [DOI] [PubMed] [Google Scholar]
- 7.Soylemezoglu F, Scheithauer BW, Esteve J, Kleihues P. Atypical central neurocytoma. J Neuropathol Exp Neurol. 1997 May;56(5):551–556. doi: 10.1097/00005072-199705000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Kane AJ, Sughrue ME, Rutkowski MJ, et al. Atypia predicting prognosis for intracranial extraventricular neurocytomas. J Neurosurg. 2012 Feb;116(2):349–354. doi: 10.3171/2011.9.JNS10783. [DOI] [PubMed] [Google Scholar]
- 9.Rades D, Fehlauer F. Treatment options for central neurocytoma. Neurology. 2002 Oct 22;59(8):1268–1270. doi: 10.1212/wnl.59.8.1268. [DOI] [PubMed] [Google Scholar]
- 10.Rades D, Fehlauer F, Lamszus K, et al. Well-differentiated neurocytoma: what is the best available treatment? Neuro Oncol. 2005 Jan;7(1):77–83. doi: 10.1215/S1152851704000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rades D, Fehlauer F, Schild S, Lamszus K, Alberti W. Treatment for central neurocytoma: a meta-analysis based on the data of 358 patients. Strahlenther Onkol. 2003 Apr;179(4):213–218. doi: 10.1007/s00066-003-1061-9. [DOI] [PubMed] [Google Scholar]
- 12.Rades D, Schild SE. Value of postoperative stereotactic radiosurgery and conventional radiotherapy for incompletely resected typical neurocytomas. Cancer. 2006 Mar 1;106(5):1140–1143. doi: 10.1002/cncr.21628. [DOI] [PubMed] [Google Scholar]
- 13.Rades D, Schild SE, Fehlauer F. Prognostic value of the MIB-1 labeling index for central neurocytomas. Neurology. 2004 Mar 23;62(6):987–989. doi: 10.1212/01.wnl.0000115392.21898.e3. [DOI] [PubMed] [Google Scholar]
- 14.Rades D, Schild SE, Ikezaki K, Fehlauer F. Defining the optimal dose of radiation after incomplete resection of central neurocytomas. Int J Radiat Oncol Biol Phys. 2003 Feb 1;55(2):373–377. doi: 10.1016/s0360-3016(02)03918-4. [DOI] [PubMed] [Google Scholar]
- 15.Leenstra JL, Rodriguez FJ, Frechette CM, et al. Central neurocytoma: management recommendations based on a 35-year experience. Int J Radiat Oncol Biol Phys. 2007 Mar 15;67(4):1145–1154. doi: 10.1016/j.ijrobp.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 16.Sharma MC, Rathore A, Karak AK, Sarkar C. A study of proliferative markers in central neurocytoma. Pathology. 1998 Nov;30(4):355–359. doi: 10.1080/00313029800169626. [DOI] [PubMed] [Google Scholar]
- 17.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007 Aug;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackenzie IR. Central neurocytoma: histologic atypia, proliferation potential, and clinical outcome. Cancer. 1999 Apr 1;85(7):1606–1610. doi: 10.1002/(sici)1097-0142(19990401)85:7<1606::aid-cncr24>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 19.Bertalanffy A, Roessler K, Koperek O, Gelpi E, Prayer D, Knosp E. Recurrent central neurocytomas. Cancer. 2005 Jul 1;104(1):135–142. doi: 10.1002/cncr.21109. [DOI] [PubMed] [Google Scholar]
- 20.Paek SH, Han JH, Kim JW, et al. Long-term outcome of conventional radiation therapy for central neurocytoma. J Neurooncol. 2008 Oct;90(1):25–30. doi: 10.1007/s11060-008-9622-5. [DOI] [PubMed] [Google Scholar]
- 21.Kulkarni V, Rajshekhar V, Haran RP, Chandi SM. Long-term outcome in patients with central neurocytoma following stereotactic biopsy and radiation therapy. Br J Neurosurg. 2002 Apr;16(2):126–132. doi: 10.1080/02688690220131714. [DOI] [PubMed] [Google Scholar]
- 22.Dodds D, Nonis J, Mehta M, Rampling R. Central neurocytoma: a clinical study of response to chemotherapy. J Neurooncol. 1997 Sep;34(3):279–283. doi: 10.1023/a:1005713909836. [DOI] [PubMed] [Google Scholar]
- 23.al-Mefty O, Kersh JE, Routh A, Smith RR. The long-term side effects of radiation therapy for benign brain tumors in adults. J Neurosurg. 1990 Oct;73(4):502–512. doi: 10.3171/jns.1990.73.4.0502. [DOI] [PubMed] [Google Scholar]
- 24.Niranjan A, Kondziolka D, Lunsford LD. Neoplastic transformation after radiosurgery or radiotherapy: risk and realities. Otolaryngol Clin North Am. 2009 Aug;42(4):717–729. doi: 10.1016/j.otc.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Yousaf I, Byrnes DP, Choudhari KA. Meningiomas induced by high dose cranial irradiation. Br J Neurosurg. 2003 Jun;17(3):219–225. doi: 10.1080/0268869031000153080. [DOI] [PubMed] [Google Scholar]
- 26.Amini E, Roffidal T, Lee A, et al. Central neurocytoma responsive to topotecan, ifosfamide, carboplatin. Pediatr Blood Cancer. 2008 Jul;51(1):137–140. doi: 10.1002/pbc.21551. [DOI] [PubMed] [Google Scholar]


