Abstract
Esthesioneuroblastoma (EN) is a rare sinonasal tumor with varied aggressiveness and potential for intracranial invasion. EN is staged anatomically with radiographic evaluation using the Kadish staging system (stages A, B, and C) and histologically by using Hyam’s criteria (grades 1–4). Here we show that despite radiographic evidence of aggressive features, the prognosis of patients with Kadish stage C EN is best predicted by tumor histology using Hyam’s criteria. We retrospectively analyzed patients with EN with Kadish stage C who were evaluated and treated at our institution between 1995 and 2009. Clinical information was collected using patient medical records, imaging, and review of pathological specimens. Twenty patients with Kadish stage C EN were identified with mean age of 51 years (31–70 years) with a median follow-up of 41.4 months (1.3–175 months). Upon pathological review, 44.4% of patients had low-grade (1/2) and 55.6% had high-grade histology (3/4). About 37.5% of patients with low-grade EN had undergone gross total resection (GTR) and the remaining 62.5% had GTR and adjuvant radiation, whereas 50% of patients with high-grade ER had undergone GTR, 20% had undergone GTR and adjuvant radiation, and 30% had been treated with a subtotal resection (STR) and adjuvant radiation. The 5-year and 10-year survival in patients with low-grade EN was 86% in comparison to 56% and 28% with high-grade EN, respectively. In patients with low-grade EN, the 2-year progression free survival (PFS) was 86% and the 5-year PFS was 65% in comparison to 73% and 49% in patients with high-grade EN, respectively. The patient’s tumor histology (Hyam’s criteria) appeared to be the best way of predicting the prognosis and for selecting patients for adjuvant radiotherapy.
Keywords: Esthesioneuroblastoma, Hyam’s, Kadish, Olfactory neuroblastoma, Prognosis, Recurrence, Survival
1 Introduction
Esthesioneuroblastoma (EN) is a rare sinonasal malignancy with varied aggressiveness, including the possibility for intracranial invasion. EN is typically staged anatomically with radiographic evaluation using the Kadish staging system1, and histologically with Hyam’s criteria2,3. The Kadish staging includes grades A, B, and C representing radiographic findings of tumor in the sinus, paranasal sinuses, and extending beyond the cribriform plate, respectively (Table 1). Hyam’s criteria categorize tumors into four grades, representing a spectrum of relatively benign to malignant histologic features (Table 2). Patients with Kadish stage C typically require neurosurgical involvement.
Table 1.
Kadish staging for esthesioneuroblastoma
| Group A | Tumor is limited to nasal cavity |
| Group B | Tumor is localized to the nasal cavity and paranasal sinuses |
| Group C | Tumor extends beyond the nasal cavity and paranasal sinuses |
Table 2.
Hyam’s grading criteria
| Low-grade | High-grade | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Lobular architecture | + | + | ± | ± |
| Mitotic activity | − | + | ++ | +++ |
| Nuclear pleomorphism | − | ± | + | ++ |
| Rosettes | HW± | HW± | FW± | − |
| Necrosis | − | − | + | ++ |
HW=Homer Wright rosette, FW= Flexner–Wintersteiner rosette. (Reproduced from Morita et al.3 with the kind permission of Wolters Kluwer Health.)
Both Kadish staging and Hyam’s histologic classification have been used to provide a prognosis and to help guide treatment decisions, including the appropriate use of neoadjuvant or adjuvant therapies1–7. We reviewed 956 patients reported in the published literature5. Our analysis demonstrated that histologic tumor grade independently predicted patient survival, and that the biologic behavior of EN could be summarized as representing two patterns: low and high grade. Furthermore, this analysis found that the prognosis was excellent when Kadish stage C tumors were associated with low-grade histology, despite radiographic evidence of aggressive tumor behavior, including intracranial invasion.
Here we validate the findings from our systematic review with a more detailed dataset collected retrospectively from our institutional experience. We show that despite radiographic and anatomic features of aggressive behavior, the prognosis of patients with Kadish stage C tumors is best when based on the biology of the tumor, as reflected by Hyam’s grade. Our findings have implications for post-operative management, particularly regarding the utility of adjuvant therapy after gross total resection (GTR) in patients with Kadish stage C, low-grade tumors.
2 Methods
2.1. Patient Population
We retrospectively identified all patients between 1995 and 2009 who underwent evaluation and initial treatment for EN at our institution, and who had a confirmed pathology with central review. We excluded all patients with any other intracranial tumor history and those patients with Kadish stages A or B. From this cohort of patients with Kadish stage C, we evaluated all patients undergoing primary craniotomy for resection of a histologically proven EN with operative and radiographic data (n = 20 patients). This study was approved by the University of California San Francisco Committee on Human Research under the approval CHR# H41995-34889-01.
2.2. Data Collection
Clinical information was collected retrospectively using patient medical records, radiographic data, and pathologic specimens. All clinical assessments were reviewed by the senior author (A.T.P.). Patient age was defined as age at the time of surgery. Both the pre-operative, post-gadolinium T1-weighted MRI and/or the surgeon’s operative note were reviewed to confirm tumor location and Kadish stage. Tumors were classified using MRI as Kadish stage: A, confined to sinuses; B, extension into the paranasal sinus; or C, extension through the cribriform plate (Fig. 1a). Radiographic patterns of recurrence are demonstrated for a patient with intracranial recurrence (Fig. 2a) and a patient with nodal recurrence (Fig. 2b).
Figure 1.
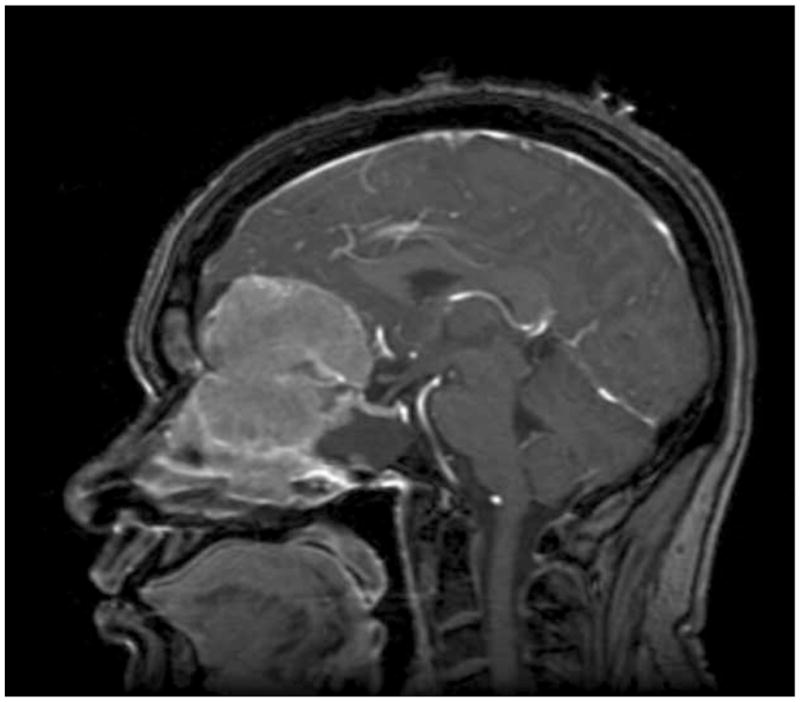
Sagittal, T1-weighted MRI with contrast of Patient 9 demonstrating the typical radiographic appearance of Kadish stage C esthesioneuroblastoma.
Figure 2.
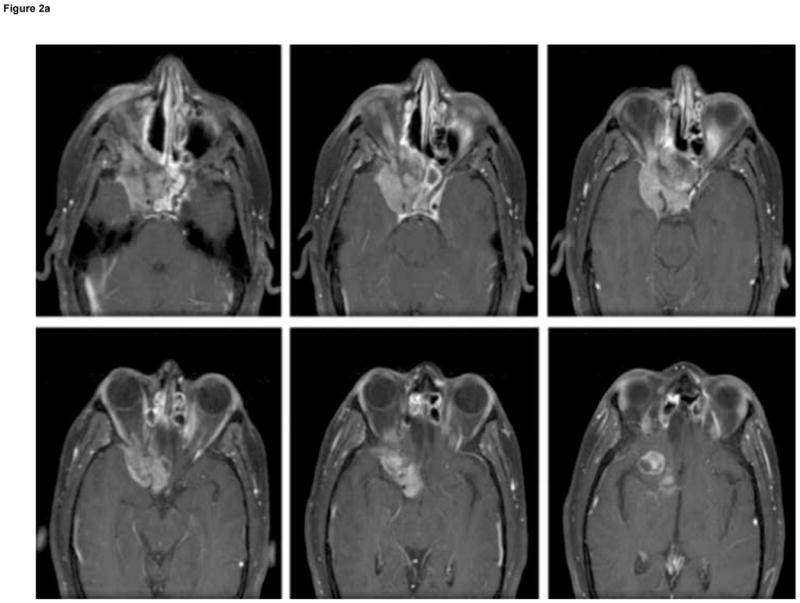
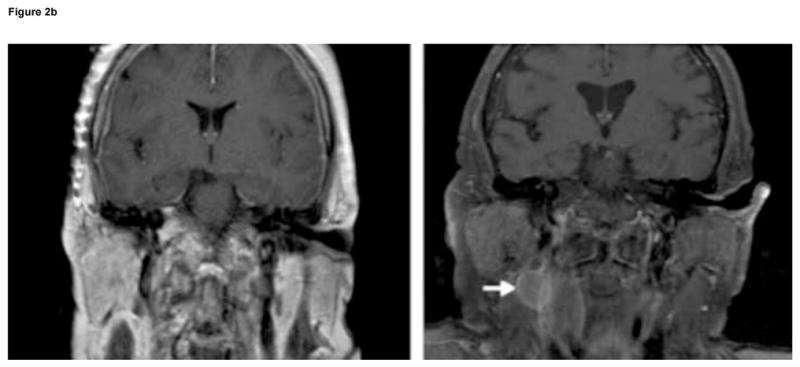
Patterns of recurrence in two patients with high-grade
esthesioneuroblastoma (EN): (A) six sequential axial, T1-weighted, post-gadolinium MRI of Patient 6 with high-grade, aggressive intranasal and intracranial recurrence involving the cavernous sinus and frontal lobe, 41 months after primary resection of a high-grade EN; and (B) coronal, T1-weighted, contrast-enhanced MRI of Patient 14 showing absence of nodal metastasis before initial surgery (left), and evidence of nodal metastasis (white arrow) 48 months after first surgery (right).
Histological features were extracted from pathology reports associated with tumor resection, with confirmation by central review. The tumors were first categorized by Hyam’s classification into grades 1 to 4 and then divided into two groups: low-grade (grades 1 or 2) and high-grade (grades 3 or 4). Extent of resection was assessed based on the surgeon’s operative note in conjunction with the post-operative MRI. Postoperative assessments and imaging were examined for evidence of recurrence, treatment of recurrence, and overall survival (OS).
2.3. Statistical Analysis
Kaplan–Meier estimates were used to generate survival curves. Differences in time to recurrence or death from disease after initial treatment were analyzed by the log-rank test. Statistical tests were considered significant for p < 0.05. Continuous variables are presented as mean ± standard error (SE). All descriptive and statistical analyses were performed using the Statistical Package for the Social Sciences version 16.0 (SPSS, Chicago, IL, USA).
3 Results
3.1. Patient Demographics and Clinical Characteristics
Between 1995 and 2009, 20 patients with Kadish stage C EN were available for review (Table 3a), and their demographic information is summarized in Table 3b. The mean age of these patients was 51±2.5 years, and 86% of the patients were male. A positive smoking history, a known risk factor for EN8, was found in 25% of our patients, and 27% of our patients had a history of occupations that would predispose them to EN such as working in the logging industry8. Orbital involvement was seen in 38% of patients and brain involvement in 43%. Frequently encountered radiologic findings included dural thickening (36%), cystic components (41%), shift/mass effect (29%), and hydrocephalus (12%). The mean tumor volume was 91.9±15.9 mL, and the mean largest diameter was 6.0±0.4 cm. Median post-operative follow-up was 41.4 months and ranged from 1.3 months to 175 months.
Table 3a.
Data for all patients with Kadish stage C esthesioneuroblastoma in this series
| Patient no. | Age/sex | Hyam’s grade | Resection extent | Radiation (cGy) | Chemotherapy | Recurrence (months) | Death (months) | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | 69/M | High | GTR | No | No | No | 22.5 | 22.5 |
| 2 | 70/M | High | GTR | No | No | 13.2 | 27.0 | 27.0 |
| 3 | 46/M | High | GTR | XRT, 6000 | Yes | No | No | 9.8 |
| 4 | 52/M | High | GTR | No | No | No | No | 31.5 |
| 5 | 63/M | High | GTR | IMRT, 6600 | No | No | No | 64.9 |
| 6 | 40/M | High | GTR | No | No | 41.4 | 83.0 | 83.0 |
| 7 | 50M | High | STR | IMRT, 6600 | Yes | No | No | 12.3 |
| 8 | 36/M | High | GTR | No | No | No | No | 27.7 |
| 9 | 42/F | High | STR | XRT | Cisplatin, etoposide | 23.3 | 40.0 | 40.0 |
| 10 | 31/M | High | STR | XRT, 7000 | Cytoxan Vincristine | No | No | 175.4 |
| 11 | 40/M | Low | GTR | IMRT, 6572 | Carboplatin | No | No | 43.7 |
| 12 | 51/F | Low | GTR | XRT | No | No | No | 1.3 |
| 13 | 62/M | Low | GTR | IMRT | No | No | No | 68.2 |
| 14 | 66/F | Low | GTR | No | No | 48.3 | No | 52.5 |
| 15 | 39/M | Low | GTR | No | No | 12.1 | No | 147.3 |
| 16 | 52M | Low | GTR | No | No | No | No | 72.5 |
| 17 | 44/M | Low | GTR | IMRT, 6000 | No | No | No | 63.1 |
| 18 | 63/M | Low | GTR | IMRT, 6600 | Cisplatin | No | 15.9 | 15.9 |
| 19 | 64/M | NA | GTR | No | No | No | No | 1.4 |
| 20 | 49/M | NA | STR | XRT | No | 75.1 | No | 85.1 |
GTR = gross total resection, IMRT = intensity-modulated radiotherapy, STR = subtotal resection, XRT = external beam radiotherapy,.
Table 3b.
Demographic and clinical information for patients with Kadish stage C esthesioneuroblastoma in this series
| Age | 51.4±2.5 (31–70) |
| Gender | 85.7% male |
| Smoking history | 25% |
| Occupational hazard | 27% |
| Mean tumor volume (mL) | 91.9±15.9 |
| Mean diameter (cm) | 6±0.4 |
| Gross total resection | 80% |
| Radiation | 55% |
| Chemotherapy | 30% |
| Median follow-up | 41.4 months |
Upon presentation to our specialty clinics, symptoms were largely referable to the sinonasal tract; presenting symptoms for all patients are described in Table 4. Some of the most common presentations included epistaxis (53%), congestion/nasal obstruction (53%), palpable or disfiguring mass (53%), anosmia (40%), headache (26.7%), and proptosis (20%).
Table 4.
Frequency of presenting symptoms for patients in our series with Kadish stage C esthesioneuroblastoma
| Presenting symptom | Frequency (%) |
|---|---|
| Epistaxis | 53.3 |
| Congestion/nasal obstruction | 53.3 |
| Mass | 53.3 |
| Anosmia | 40.0 |
| Headache | 26.7 |
| Rhinorrea | 20.0 |
| Paresthesias | 20.0 |
| Proptosis | 13.3 |
| Weight loss | 13.3 |
| Cognitive deficit | 6.7 |
| Seizure | 6.7 |
| Papilledema | 6.7 |
| Sinus infection | 6.7 |
| Ageusia | 6.7 |
| Fatigue | 6.7 |
| Vertigo | 6.7 |
3.1. Pathologic features of the tumors
By light microscopy, the tumors demonstrated typical histologic features of EN, including uniform small tumor cells with round nuclear contours and scant cytoplasm, in a prominent neurofibrillary background. Homer Wright rosettes (with central neurofibrillary-like material) were present in some tumors. Mitotic activity varied, as did other cytologic features of malignancy, such as tumor necrosis. Immunohistochemistry demonstrated typical patterns of reactivity for the neuronal markers synaptophysin (100%), neuron specific enolase (91%), chromogranin (72%), and S-100 (72%). EN tested negative for keratin was negative in all instances, which excluded a diagnosis of neuroendocrine carcinoma, sinonasal undifferentiated carcinoma, and pituitary adenoma. Typical histologic features of EN are illustrated in Figure 3.
Figure 3.
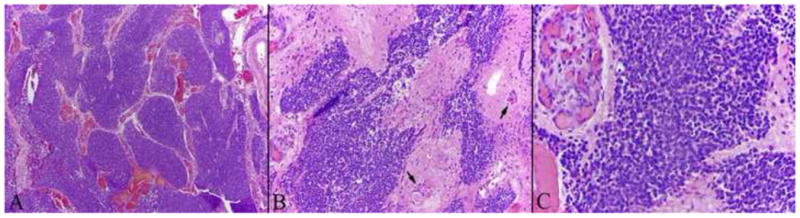
Histopathologic features of esthesioneuroblastoma: (A) solid tumor with prominent lobulated growth pattern with tumor lobules separated by fibrovascular septae (hematoxylin and eosin [H&E], ×100); (B) at higher magnification showing uniform small round blue tumor cells set in a neurofibrillary matrix; the fibrillary areas may be perivascular (arrows) (H&E, ×200); and (C) tumor cells without significant pleomorphism or mitotic activity; large collections of proliferative vessels can be a very prominent feature within the stroma (H&E, ×400).
3.2. The relationship of tumor grade to prognosis
Pathology data and sufficient archived samples available for review to classify tumors by Hyam’s criteria were available for 18 patients with Kadish stage C disease: eight (44.4%) had low-grade and 10 (55.6%) had high-grade histology. The 5-year (86% compared to 56%) and 10-year (86% compared to 28%) OS was higher in patients with low-grade than high-grade tumors, respectively (p=0.24) (Fig. 4). Similarly, the 2-year (86% compared to 73%) and 5-year (65% compared to 49%) progression free survival (PFS) was higher in patients with low-grade than high-grade tumors respectively (p=0.5) (Fig. 5). This analysis was underpowered due to the size of our patient population.
Figure 4.
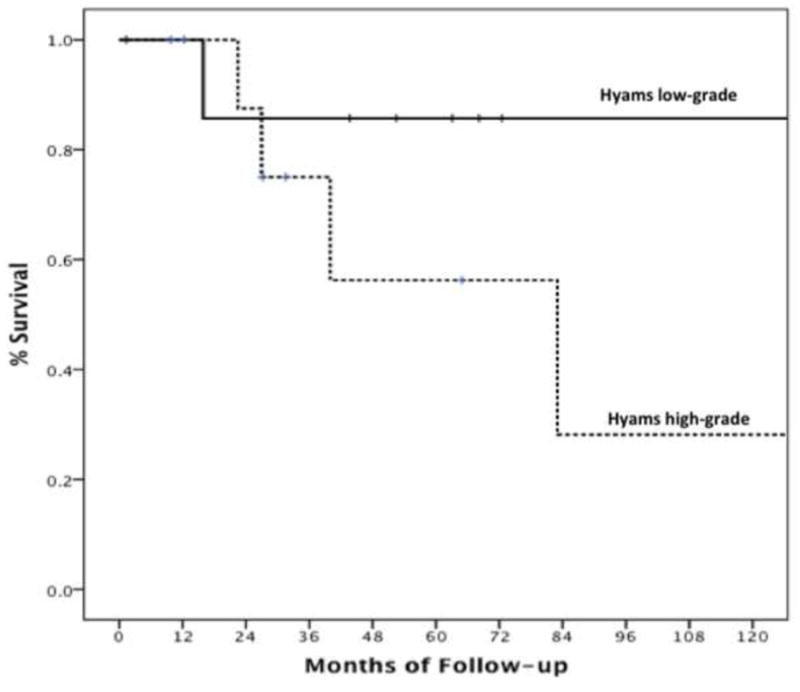
Kaplan–Meier curves demonstrating differences in overall survival between patients with low-grade and high-grade esthesioneuroblastoma (p=0.24).
Figure 5.
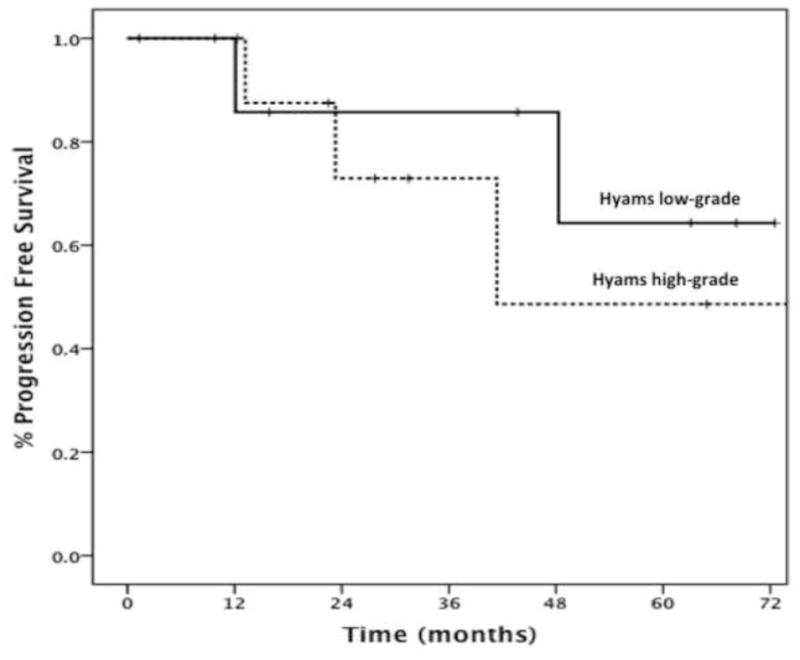
Kaplan–Meier curves demonstrating differences in progression free survival between patients with low-grade and high-grade esthesioneuroblastoma (p=0.5).
3.3. Treatment and outcomes
Nine patients received GTR, four received GTR with adjuvant external beam radiotherapy (XRT), three received GTR with adjuvant XRT and chemotherapy, two received STR with adjuvant XRT, and two received STR with adjuvant XRT and chemotherapy. In the 11 patients who received adjuvant XRT, intensity-modulated radiotherapy (IMRT) was used in six and three-dimensional (3D) conformal radiotherapy (3D-CRT) was used in five patients. The prescription dose range for IMRT was 6,000–6,600 cGy and the dose range for 3D-CRT was 6,000–7,000 cGy. Chemotherapeutic agents varied by patient and included carboplatin (n = 1), cisplatin and etoposide (n = 1), cytoxan and vincristine (n = 1), and cisplatin (n = 1).
Three patients (37.5%) with low-grade EN had GTR alone and the remaining five (62.5%) had GTR and adjuvant treatment (three patients received radiation while two received radiation and chemotherapy). Meanwhile, five patients (50%) with high-grade EN received GTR, two (20%) received GTR and adjuvant treatment (one patient received radiation and second patient received both radiation and chemotherapy), and three (30%) had STR and adjuvant treatment (all received radiation and chemotherapy).
Six patients (30%) had a recurrence of EN during the follow-up period after initial treatment, with a median time to recurrence of 32.4 months. Three of these patients had high-grade tumors with median time to recurrence of 23.3 months in comparison to two additional recurrences in patients with low-grade tumors with an average time to recurrence of 30.4 months. Three recurrences occurred as neck lymph node metastases, either cervical or submandibular (Fig. 2b), and were all treated by radical neck dissection. Two recurrences were intracranial, one arising around the temporal dura and one within the infra-orbital region of the frontal lobe extending into the cavernous sinus (Fig. 2a). Both patients were treated with repeat resection and chemotherapy. Another tumor recurred in the sinus/paranasal sinus/orbital regions and was treated with chemotherapy. Five patients (25%) died during follow-up, with a median time to death of 27 months (range 15.9–83.0 months). Four of five deaths (80%) were in patients with high-grade tumors and one death was in a patient with a low-grade tumor.
4 Discussion
Prior studies have largely utilized the Kadish staging classification to provide a prognosis and to guide treatment decisions. This has resulted in conflicting results between institutional case series leading to different treatment recommendations1,3–7,9–15. In this report, we reviewed our experience with surgical resection, treatment, and post-treatment prognosis of patients with Kadish stage C EN. We found that only a portion of patients with Kadish stage C had high-grade histology, and similar to our previous analysis, tumor grade appears to be a critical prognostic marker.
Although radiographic findings can be determined preoperatively, and help guide patients towards neurosurgical treatment when appropriate, our prior review demonstrated that Kadish grade was not an independent predictor of survival when controlling for histologic grade5. During the 1,045 months of follow-up after initial treatment for our 18 patients, only one patient with a low-grade tumor died. This death occurred in the absence of recurrence and was secondary to radiation treatment effects. In comparison, the four remaining deaths in our series all occurred in patients with high-grade tumors. This difference is reflected in a three-fold increase in 10-year survival for patients with low-grade histology, even in this series of exclusively high stage tumors (Fig. 4). Both our prior review5 and our institutional series found that 45% of patients with Kadish stage C had low-grade histology. These findings indicate that studies utilizing Kadish staging exclusively to divide patients into treatment groups are likely to result in a heterogeneous population, composed of patients with both low-grade and high-grade tumors.
Surgical resection remains the cornerstone of treatment for patients with EN. The utility of adjuvant, or neoadjuvant therapy, is not clearly established due to lack of class I data. While the addition of adjuvant therapies may improve rates of tumor control, they are not without morbidities, as demonstrated in our series by the previously mentioned radiation-associated death in a patient with a low-grade tumor. Both our prior review and this series demonstrate that mortality in EN is largely concentrated in patients with high-grade histology. Patients with low-grade tumors appear to have excellent rates of survival post-treatment with (75%) or without (100%) adjuvant therapy. Although the use of adjuvant therapy does not appear to improve survival for patients with low-grade tumors5, it may reduce recurrences11–13,16–19. In particular, Ward and colleagues in an institutional series recently demonstrated that adjuvant radiation improved disease-free survival at 5 years (83% compared to 27%) and 15 years (83% compared to 0%), but had no effect on OS.19 In an impressive series of 50 patients with mostly stage C EN and a mean of 93 months of follow-up, Loy and colleagues13 demonstrated, across patients with all Kadish stages, a disease free survival of over 80% at 15 years with neoadjuvant chemotherapy and XRT used pre-operatively for stage C patients. In their study, Loy et al. utilized a systematic treatment approach to all Kadish C tumors, thus there were no comparison groups. Although both series are informative, based on our findings, it would be important to perform an analysis based on tumor histology as opposed to exclusively grouping patients based on radiographic findings. Thus, rational modern approaches to the management of patients with stage C and high-grade EN must weigh the potential benefit of improved tumor control against the morbidity associated with adjuvant treatments and the possibility for surgical salvage of recurrent disease. We suggest that the use of adjuvant therapies should be based on tumor histology, not radiographic findings, and that the role of adjuvant therapies in preventing recurrence in low-grade tumors deserves more rigorous analysis.
In conclusion, using a patient’s individual tumor histopathology appears to be the best means of providing a prognosis and selecting patients for more aggressive adjuvant treatments, in those with extensive (Kadish C) disease. Patients with low-grade EN have excellent 10-year survival following initial surgical resection while those with high-grade EN appear to have a very poor prognosis. Prospective trials are necessary to determine appropriate guidelines for the use of adjuvant radiotherapy and chemotherapy, particularly for low-grade tumors, as these therapies are not without morbidity. Accordingly, patients with the clinically more aggressive high-grade tumors would be suitable for future work into the novel molecular therapeutics for this disease.
Acknowledgments
Gurvinder Kaur and Ari Kane were supported by the Howard Hughes Medical Institute and the Ivy Foundation. Dr. Sughrue and Dr. Oh were supported by a National Research Service Award from the National Institutes of Health and a Neurosurgery Education and Research Foundation grant for the American Association of Neurological Surgeons. Michael Sughrue was supported by Doris Duke Foundation and Matthew Sun was supported by UCSF-CTSI Grant Number TL1 RR024129. Dr. Parsa was partially supported by the Reza and Georgianna Khatib Endowed Chair in Skull Base Tumor Surgery.
Footnotes
Conflict of Interest/Disclosures
No portion of this article has been presented or published in any form prior to this submission. The authors declare that they have no financial or other conflicts of interest in relation to this research and its publication
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kadish S, Goodman M, Wang CC. Olfactory neuroblastoma. A clinical analysis of 17 cases. Cancer. 1976 Mar;37(3):1571–1576. doi: 10.1002/1097-0142(197603)37:3<1571::aid-cncr2820370347>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 2.Hyams V. Olfactory neuroblastoma (case 6) In: Batsakis J, Hyams VJ, Morales AR, editors. Special Tumors of the Head and Neck. Chicago: American Society of Clinical Pathologists; 1983. pp. 24–29. [Google Scholar]
- 3.Morita A, Ebersold MJ, Olsen KD, Foote RL, Lewis JE, Quast LM. Esthesioneuroblastoma: prognosis and management. Neurosurgery. 1993 May;32(5):706–714. doi: 10.1227/00006123-199305000-00002. discussion 714–705. [DOI] [PubMed] [Google Scholar]
- 4.Jethanamest D, Morris LG, Sikora AG, Kutler DI. Esthesioneuroblastoma: a population-based analysis of survival and prognostic factors. Arch Otolaryngol Head Neck Surg. 2007 Mar;133(3):276–280. doi: 10.1001/archotol.133.3.276. [DOI] [PubMed] [Google Scholar]
- 5.Kane AJ, Sughrue ME, Rutkowski MJ, et al. Posttreatment prognosis of patients with esthesioneuroblastoma. J Neurosurg. Mar 26; doi: 10.3171/2010.2.JNS091897. [DOI] [PubMed] [Google Scholar]
- 6.Constantinidis J, Steinhart H, Koch M, et al. Olfactory neuroblastoma: the University of Erlangen-Nuremberg experience 1975–2000. Otolaryngol Head Neck Surg. 2004 May;130(5):567–574. doi: 10.1016/j.otohns.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Gruber G, Laedrach K, Baumert B, Caversaccio M, Raveh J, Greiner R. Esthesioneuroblastoma: irradiation alone and surgery alone are not enough. Int J Radiat Oncol Biol Phys. 2002 Oct 1;54(2):486–491. doi: 10.1016/s0360-3016(02)02941-3. [DOI] [PubMed] [Google Scholar]
- 8.DeMonte F, Fourney DR, Garden AS, Diaz EM., Jr . Carcinoma of the Paranasal Sinuses and Olfactory Neuroblastoma. In: Berger MSPM, editor. Textbook of Neuro-Oncology. Philidelphia: Elsevier Saunders; 2004. pp. 371–384. [Google Scholar]
- 9.Zafereo ME, Fakhri S, Prayson R, et al. Esthesioneuroblastoma: 25-year experience at a single institution. Otolaryngol Head Neck Surg. 2008 Apr;138(4):452–458. doi: 10.1016/j.otohns.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 10.Porter AB, Bernold DM, Giannini C, et al. Retrospective review of adjuvant chemotherapy for esthesioneuroblastoma. J Neurooncol. 2008 Nov;90(2):201–204. doi: 10.1007/s11060-008-9645-y. [DOI] [PubMed] [Google Scholar]
- 11.Bachar G, Goldstein DP, Shah M, et al. Esthesioneuroblastoma: The Princess Margaret Hospital experience. Head Neck. 2008 Dec;30(12):1607–1614. doi: 10.1002/hed.20920. [DOI] [PubMed] [Google Scholar]
- 12.Nakao K, Watanabe K, Fujishiro Y, et al. Olfactory neuroblastoma: long-term clinical outcome at a single institute between 1979 and 2003. Acta Otolaryngol Suppl. 2007 Dec;(559):113–117. doi: 10.1080/03655230701599982. [DOI] [PubMed] [Google Scholar]
- 13.Loy AH, Reibel JF, Read PW, et al. Esthesioneuroblastoma: continued follow-up of a single institution’s experience. Arch Otolaryngol Head Neck Surg. 2006 Feb;132(2):134–138. doi: 10.1001/archotol.132.2.134. [DOI] [PubMed] [Google Scholar]
- 14.Resto VA, Eisele DW, Forastiere A, Zahurak M, Lee DJ, Westra WH. Esthesioneuroblastoma: the Johns Hopkins experience. Head Neck. 2000 Sep;22(6):550–558. doi: 10.1002/1097-0347(200009)22:6<550::aid-hed2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 15.McElroy EA, Jr, Buckner JC, Lewis JE. Chemotherapy for advanced esthesioneuroblastoma: the Mayo Clinic experience. Neurosurgery. 1998 May;42(5):1023–1027. doi: 10.1097/00006123-199805000-00040. discussion 1027–1028. [DOI] [PubMed] [Google Scholar]
- 16.Diaz EM, Jr, Johnigan RH, 3rd, Pero C, et al. Olfactory neuroblastoma: the 22-year experience at one comprehensive cancer center. Head Neck. 2005 Feb;27(2):138–149. doi: 10.1002/hed.20127. [DOI] [PubMed] [Google Scholar]
- 17.Kim HJ, Kim CH, Lee BJ, et al. Surgical treatment versus concurrent chemoradiotherapy as an initial treatment modality in advanced olfactory neuroblastoma. Auris Nasus Larynx. 2007 Dec;34(4):493–498. doi: 10.1016/j.anl.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 18.McLean JN, Nunley SR, Klass C, Moore C, Muller S, Johnstone PA. Combined modality therapy of esthesioneuroblastoma. Otolaryngol Head Neck Surg. 2007 Jun;136(6):998–1002. doi: 10.1016/j.otohns.2006.11.051. [DOI] [PubMed] [Google Scholar]
- 19.Ward PD, Heth JA, Thompson BG, Marentette LJ. Esthesioneuroblastoma: Results and Outcomes of a Single Institution’s Experience. Skull Base. 2009 Mar;19(2):133–140. doi: 10.1055/s-0028-1096195. [DOI] [PMC free article] [PubMed] [Google Scholar]


