Abstract
Preeclampsia is associated with increased systemic inflammation and superficial trophoblast invasion, which leads to insufficient utero-placental blood flow. Interleukin (IL)-11 mediates pro- and anti-inflammatory processes and facilitates decidualization. To identify IL-11 expression in vivo at the maternal-placental interface in preeclampsia and control specimens and evaluate regulatory effects of TNF (tumor necrosis factor)-α and IL-1β, cytokines elevated in preeclampsia, on IL-11 levels in first trimester decidual cells in vitro. Placental sections were immunostained for IL-11. Leukocyte-free first trimester decidual cells were incubated with estradiol (E2) ±10−7 mol/L medroxyprogesterone acetate (MPA) ± TNF-α or IL-1β ± inhibitors of the p38MAPK, nuclear factor-kappa B (NFκB), or protein kinase C (PKC) signaling pathways. An ELISA assessed secreted IL-11 levels, and quantitative RT-PCR measured IL-11 mRNA. IL-11 immunoreactivity in placental sections was significantly higher in the cytoplasm of preeclamptic decidual cells versus gestational age-matched controls. Compared to decidual cells, IL-11 immunostaining in neighboring trophoblast is lower, perivascular and not different between control and preeclamptic specimens. TNF-α and IL-1β enhanced levels of IL-11 mRNA and secreted IL-11 in cultured decidual cells. Specific inhibitors of the p38 MAPK and NFκB but not PKC signaling pathways reduced the stimulatory effect of IL-1β. Expression of decidual IL-11 is increased in preeclampsia and suggests a role for IL-11 in the pathogenesis of preeclampsia.
Keywords: Cytokines, Decidua, Endocrinology, Pregnancy
Introduction
The concerted actions of estradiol (E2) and progesterone transform human endometrial stromal cells into decidual cells during the luteal phase of the menstrual cycle and maintain the decidua throughout gestation (Tabanelli et al. 1992). During implantation, blastocyst-derived extravillous trophoblast (EVT) invade the decidua and remodel spiral arteries into low resistance, high capacity vessels that markedly increase uteroplacental blood flow (Pijnenborg et al. 2006). The decidua normally constrains trophoblast invasion which involves sequential attachment to and proteolysis of basement membrane proteins in the peri-decidual extracellular matrix (ECM) (Damsky et al. 1994, Cohen et al. 2006). Shallow EVT invasion leads to incomplete vascular transformation and reduced blood flow to the developing fetal-placental unit (Caniggia et al. 2000, Pijnenborg et al. 2006). Impaired decidual invasion is the primary placental defect of preeclampsia, a leading cause of fetal and maternal morbidity and mortality and a primary contributor to preterm delivery [reviewed in (Sibai et al. 2005)]. Preeclampsia is associated with systemic inflammation (Sibai et al. 2005) and a decidual influx of macrophages (Reister et al. 2001, Abrahams et al. 2004, Lockwood et al. 2006) and dendritic cells (Huang et al. 2008) that promote immune maladaption at the implantation site.
Interleukin-11 (IL-11) belongs to the IL-6 family of cytokines that exert diverse biological effects by binding to surface receptor complexes comprised of a ligand-specific alpha chain with at least one subunit of the gp130 signal transducer (Heinrich et al. 2003). Initially identified as a hematopoiesis-promoting factor capable of enhancing growth of myeloid, erythroid and megakaryocytic progenitor cells, IL-11 was later found to mediate a complex array of pro- and anti-inflammatory effects (Trepicchio & Dorner 1998). In normal mice, uterine IL-11 synthesis peaks during decidualization. Transgenic IL-11 receptor (IL-11Rα) gene knockout mice are infertile because of defective decidualization, which leads to dysregulated trophoblast invasion and proliferation and ends in necrotic loss of the fetus (Robb et al. 1998). Microarray results from control and pseudopregnant IL-11Rα knockout mice suggest that IL-11 regulates changes in the uterine ECM required for decidualization (White et al. 2004). The decidua exhibits the most prominent immunostaining for IL-11 and IL-11Rα at the implantation site of humans and other primates (Dimitriadis et al. 2003). In women, abnormal decidual and villous trophoblast IL-11 expression leads to early pregnancy loss (Chen et al. 2002). Both IL-11 and IL-11Rα mRNA and protein are localized in decidualized stromal cells during the luteal phase of cycling human endometrium (von Rango et al. 2004). In stromal cell monolayers from pre-decidualized human endometrium, IL-11 has been shown to advance progestin-induced morphological and biochemical decidualization markers (Dimitriadis et al. 2002).
Given the complex involvement of IL-11 expression with inflammation, decidualization and trophoblast invasion we posited an association between decidual IL-11 expression and preeclampsia. To test this hypothesis, IL-11 immunohistochemical levels were compared in the decidua of preeclamptic versus gestational age-matched normal placentas. Tumor necrosis factor-alpha (TNF-α) and interleukin-1beta (IL-1β) have been implicated in the early pathogenesis of preeclampsia (Rinehart et al. 1999, Hefler et al. 2001, Bauer et al. 2004, Lockwood et al. 2006) and the previous studies have implicated that the major sources of TNFα and IL-1β are secreted from macrophages in preeclamptic decidua (as paracrine interaction) (Rinehart et al. 1999, Hefler et al. 2001, Reister et al. 2001, Bauer et al. 2004).
Therefore, the effects of these classic pro-inflammatory cytokines were assessed on IL-11 mRNA and protein expression in first trimester decidual cells, the predominant cell type encountered by invading EVT at the implantation site (Dunn et al. 2003). The integral role that progesterone plays in inducing and maintaining decidualization (Tabanelli et al. 1992, Dunn et al. 2003) prompted evaluation of effects of a progestin with and without TNF-α or IL-1β on IL-11 expression in the cultured decidual cells. Incubations were extended to include specific inhibitors of the NF-kappa B (NFκB), p38 MAP kinase (p38 MAPK), or protein kinase C (PKC) pathways since each has been shown to act as intracellular mediators of cytokine effects on IL-11 expression in many cell types (Lacroix et al. 1998, Bamba et al. 2003, Scicchitano et al. 2008).
RESULTS
Immunostaining of IL-11 and IL-11R in decidua of control and preeclamptic specimens
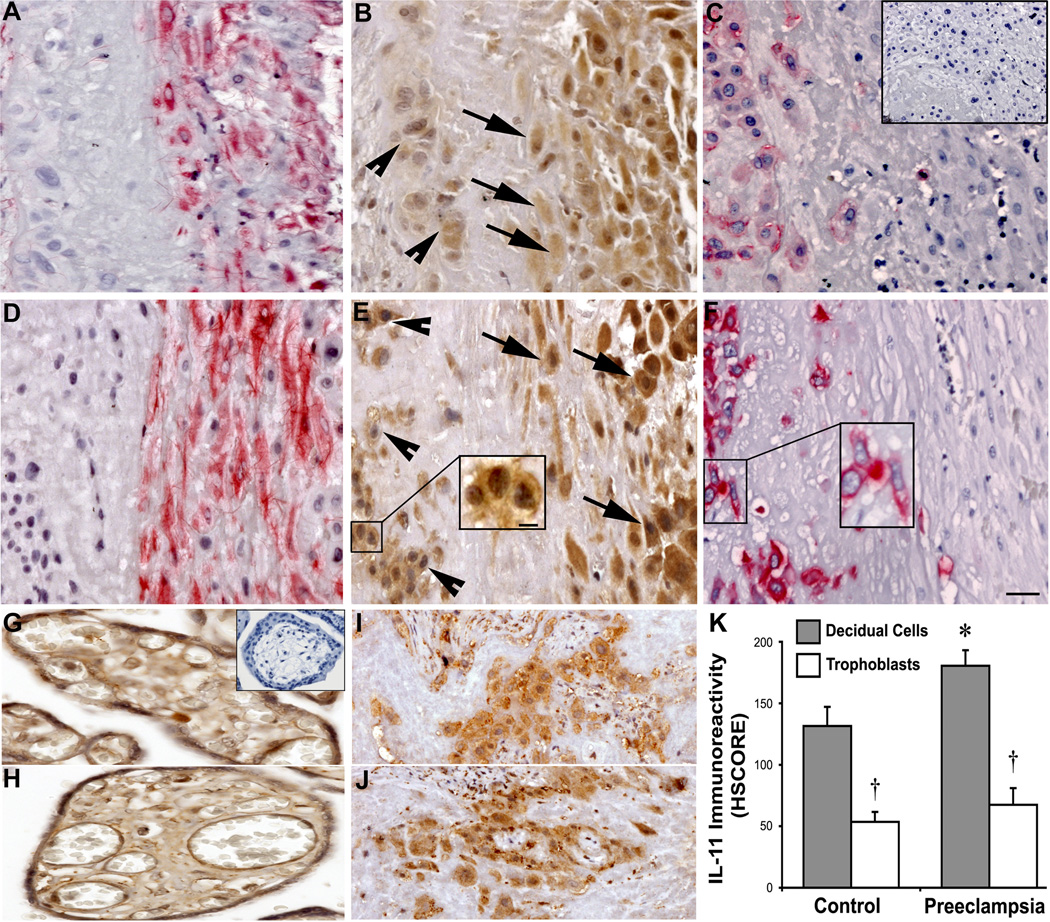
Control and preeclamptic decidua were immunostained for IL-11, vimentin and cytokeratin (Fig. 1). Decidual cells and interstitial cytotrophoblasts were identified by positive (red) vimentin and cytokeratin staining, respectively in control (Fig. 1A, C) and preeclamptic (Fig. 1D, F) specimens. HSCORE analysis revealed that immunoreactivity for IL-11 (brown) was significantly greater in the cytoplasm of decidual cells from preeclamptic tissues (Fig. 1E) compared with control (Fig. 1B) specimens (mean ± SEM HSCORE: 186 ± 13 vs. 121 ± 15, respectively, P < 0.05; Fig 1E). However, IL-11 HSCOREs in the cytoplasm of interstitial trophoblasts were not significantly different between preeclamptic (Fig. 1E) and control (Fig. 1B) tissues (71 ± 15 vs. 52 ± 10, respectively; Fig. 1K). Additionally, no statistical significance for IL-11 HSCORE was observed between chorionic villi of preeclamptic and control tissues (Fig 1 G, H). Within both groups, decidual cells had significantly greater IL-11 HSCOREs than interstitial trophoblasts (186 ± 13 vs. 71 ± 15 in preeclamptic tissues (Fig. 1E), and 121 ± 15 vs. 52 ± 10 in control tissues (Fig. 1B), respectively; p < 0.05). Notably, while IL-11 immunostaining in vimentin-positive decidual cells was homogenous and cytoplasmic, the immunoreactivity in interstitial trophoblasts (Fig. 1E, insert) was mostly peri-membranous.
Figure 1. Immunohistochemical analysis of IL-11 expression in control and preeclamptic decidua.
Representative micrographs of serial sections from control (A, B and C) and preeclamptic (D, E and F) specimens are shown. Vimentin immunostaining (red) identifies decidual cells in control (A) and preeclamptic (D) tissues. Cytokeratin immunostaining (red) identifies trophoblastic cells in control (C) and preeclamptic (F) tissues. IL-11 immunostaining (brown) in decidual cells (arrows) was cytoplasmic and more intense than in interstitial trophoblast (arrowheads), where IL-11 localization was mostly peri-membranous (1E insert). Between groups, IL-11 immunostaining was greater in preeclamptic (E) vs. control (B) decidual cells, while there was no significant difference among interstitial trophoblast. IL-11 intensity HSCOREs in control and preeclamptic specimens (mean ± SEM) are shown (K); *, vs. control decidual cells; †, vs. group-respective decidual cells; p < 0.05. Additionally, no statistical significance for IL-11 HSCORE was observed between chorionic villi of preeclamptic and control tissues (Fig 1 G, H). On the other hand no significant difference was found for the IL-11R expression between preeclamptic (Fig.1J) versus control interstitial cytotrophoblasts (Fig.1I). Parallel staining with a mouse isotype was used as a negative control for IL-11 monoclonal antibody (C and G; inset). Note that IL-11 immunoreactivity in interstitial trophoblast was mostly perimembranous, especially in preeclampsia specimens (E inset). The scale bar in panel F represents 50 µm for all panels, except the panel E insert, where it represents 20 µm.
Normal and preeclamptic decidual tissues were immunostained for. IL-11R expression is predominantly found in the interstitial cytoptrophoblasts while decidual cells have a weak IL11R immunoreactivity. No significant difference was found for the IL-11R expression between preeclamptic (Fig.1J) versus control interstitial cytotrophoblasts (Fig.1I).
IL-11 protein secretion in cultured decidual cells
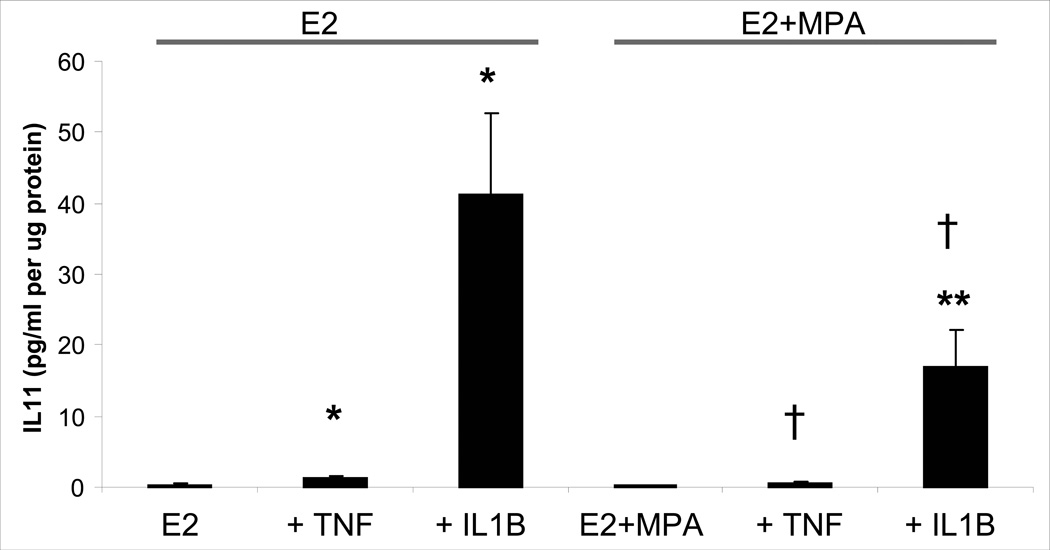
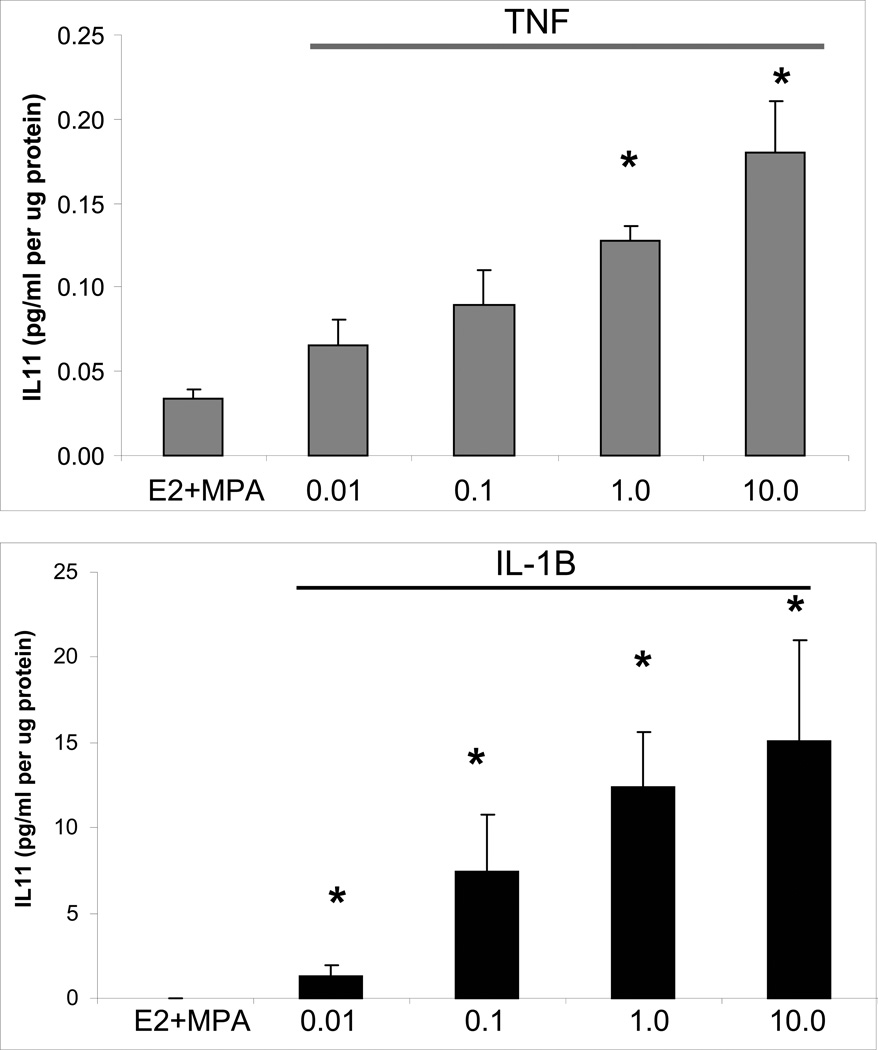
Individual and interactive effects of MPA and cytokines were assessed on levels of IL-11 secreted by leukocyte-free first trimester decidual cells. Figure 2 indicates that secreted basal IL-11 levels were not significantly different between incubations with E2 (0.38 ± 0.20 pg/ml/µg protein) and E2 + MPA (0.17 ± 0.07 pg/ml/µg protein). In the E2-treated cells, TNF-α and IL-1β significantly increased IL-11 output by 5.6 ± 1.6-fold and 385.4 ± 191.6-fold, respectively; (p< 0.05). The addition of MPA reduced the response to each cytokine by half for both TNF-α and IL-1β; p< 0. 05). This inhibition by MPA virtually eliminated the response to TNF-α as secreted IL-11 levels not differ significantly between parallel incubations with E2 + MPA and E2+ MPA + TNF-α. However, the IL-1β mediated increase in IL-11 output in incubations with E2 + MPA was still greater than baseline E2 + MPA (p<0.05) and was reduced compared with E2 + IL-1β. Both IL-1β- and TNF-α-enhanced IL-11 expression was dose dependent over a concentration range of 0.01 to 10.0 ng/ml (Fig. 3).
Figure 2. Effects of E2, MPA, TNF-α and IL-1β on IL-11 output by decidual cell monolayers.
Confluent, leukocyte-free first trimester decidual cells were incubated for 7 days in 10−8 M E2 or E2 + 10−7 M MPA and then switched to defined medium (DM) with corresponding steroid(s) ± 1 ng/ml of TNF-α or IL-1β for 24 hours. IL-11 levels were measured by ELISA in conditioned DM and normalized to total protein levels. Bars represent mean ± SEM of IL-11 levels as pg/ml/µg cell protein (n=14 separate patients’ decidual specimens). * vs. E2, ** vs. E2 + MPA, † vs. corresponding E2 + TNF-α or IL-1β (p<0.05).
Figure 3. Concentration-dependent effects of IL-1β and TNF-α on IL-11 output by decidual cell monolayers maintained in E2 + MPA.
Confluent, leukocyte-free first trimester decidual cells were incubated for 7 days in 10−8 M E2 + 10−7 M MPA, then switched to DM with the steroids ± the indicated amount of IL-1β (0.01 to 10.0 ng/ml) or TNF-α (0.01 to 10.0 ng/ml). IL-11 levels were measured by ELISA in conditioned DM and normalized to cell protein. Bars represent mean ± SEM of IL-11 levels as pg/ml/µg cell protein (n=3 separate patients’ decidual specimens, mean ± SEM). * versus E2 + MPA; p<0.05.
IL-11 mRNA expression in cultured decidual cells
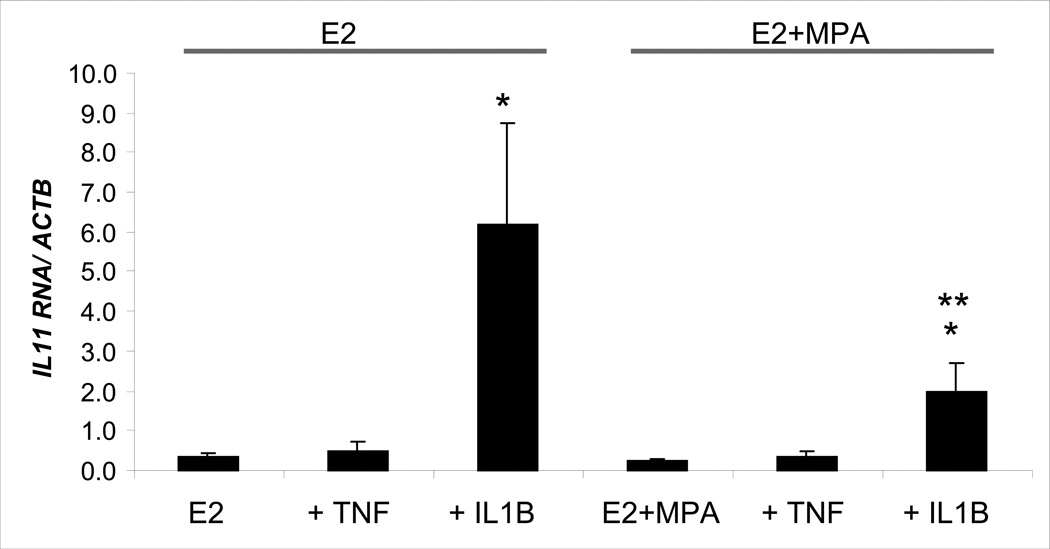
The effects of IL-1β and TNF-α on steady state IL-11 mRNA levels in first trimester decidual cell monolayers were determined by quantitative real-time RT-PCR (Fig. 4). In E2 cultures, TNF-α and IL-1β enhanced IL-11 mRNA levels by 1.55 ± 0.65-fold and 18.8 ± 5.1-fold, respectively, although only the effects of IL-1β were statistically significant. A similar IL-1β-stimulated enhancement of IL-11 mRNA levels was noted in incubations with E2 + MPA, with an increase 7.6 ± 1.3-fold (p<0.05). Consistent with the IL-11 protein results presented above, IL-1β was more effective than TNF-α in up-regulating steady state IL-11 mRNA levels and the cytokine effects were blunted by the addition of MPA.
Figure 4. Effects of E2, MPA, TNF-α and IL-1β on IL-11 mRNA levels in decidual cell monolayers.
Confluent leukocyte-free first trimester decidual cells were incubated for 7 days in 10−8 M E2 or 10−8 M E2 + 10−7 M MPA, then switched to DM with corresponding steroid(s) ± 1 ng/ml of TNF-α or IL-1β for 6 hours. Aliquots of extracted total RNA were used to measure IL-11 mRNA levels by quantitative real-time RT-PCR. Bars represent mean ± SEM of IL-11 mRNA/β-actin mRNA levels (n=5 separate patients’ decidual specimens). * versus E2 or E2 + MPA; ** vs. E2 + IL-1β; p<0.05.
Intracellular mediators of IL-1β-enhanced IL-11 secretion in +cultured decidual cells
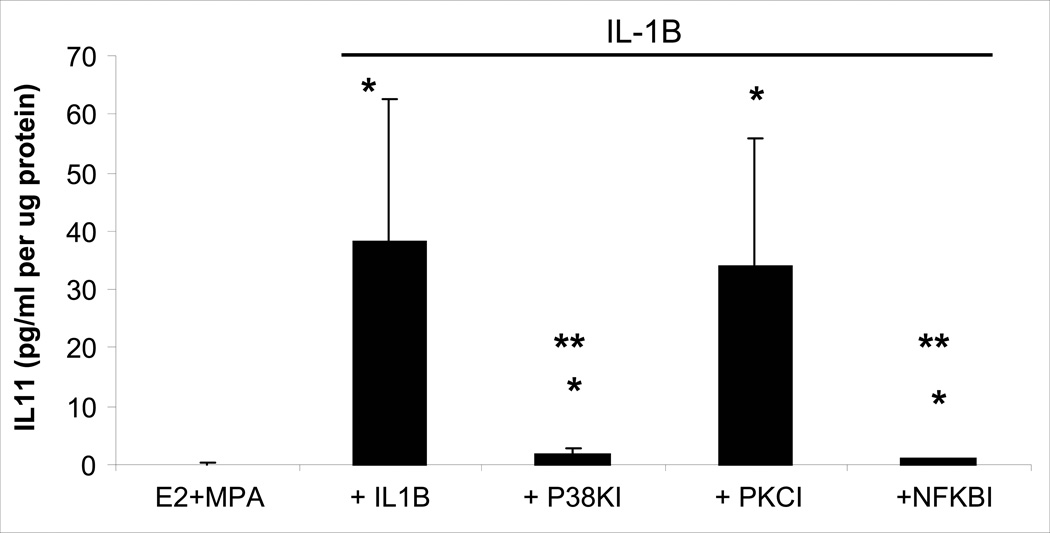
NFκB Activation Inhibitor III at 10−5 M, SB203580 at 10−5 M (p38MAPK inhibitor) and Calphostin C at 10−7 M (PKC inhibitor) were used to infer the intracellular signaling pathways involved in IL-1β-enhanced IL-11 expression. Both the p38 MAPK and NFκB inhibitors virtually eliminated the effects of IL-1β on IL-11 output (Fig. 5). Specifically, the IL-1β-induced increase in IL-11 output was reduced from 60.0 ± 30.9 pg/ml/µg to 2.62 ± 1.27 pg/ml/µg and pg/ml/µg by the p38 MAPK inhibitor and to 1.04 ± 0.29 pg/ml/µg by the NFκB inhibitor (p<0.05; Fig. 5). By contrast, the addition of the PKC inhibitor did not significantly reduce IL-1β-enhanced IL-11 output (Fig 5).
Figure 5. Involvement of NFκB, p38 MAPK and PKC signaling in IL-1β-enhanced IL-11 output by decidual cells maintained in E2 + MPA.
Confluent, leukocyte-free first trimester decidual cells were incubated for 7 days in 10−8 M E2 + 10−7 M MPA, then switched to DM with the steroids alone ± 1ng/ml IL-1β with and without an NFκB inhibitor (Activation Inhibitor III, NFκBI) at 10−5M, or a p38 MAP kinase inhibitor (SB203580; p38KI) at 10−5M, or a PKC inhibitor (Calphostin C, PKCI) at 10−7M. Bars represent mean ± SEM IL-11 levels as pg/ml/µg cell protein as measured by ELISA in conditioned DM and normalized to cell protein. (n=4 separate patients’ decidual specimens). * versus E2 + MPA; p<0.05.
DISCUSSION
Shallow EVT invasion of the basement membrane (BM)-protein-enriched decidualized ECM is the primary placental insult leading to preeclampsia (Sibai et al. 2005). In view of abundant evidence linking local IL-11 expression to decidualization of human endometrium (Dimitriadis et al. 2002, von Rango et al. 2004), IL-11 immunostaining was compared in the decidua of preeclamptic versus gestational-age matched pre-term controls. Immunoreactive IL-11 in decidual cells was primarily localized in the cytoplasm, which suggests in vivo production of IL-11 in decidual cells. Significant increases in intensity and numbers of IL-11 positive decidual cells as indicated by HSCORES in preeclamptic versus control women suggests a role for IL-11 in the inflammatory process of preeclampsia. Although IL-11 immunostaining in adjacent interstitial cytotrophoblast was not significantly greater in the preeclamptic specimens, this staining was much less prominent than that observed in the decidual cells and was primarily perimembranous. This differential localization of IL-11 between the two cell types suggests a potential, previously undisclosed, paracrine interaction in which IL-11 is synthesized and secreted by decidual cells, and then bound to receptors in adjacent interstitial trophoblasts.
By contrast, IL-11 attenuates macrophage-associated inflammation by inhibiting NFκB-mediated effector function, as reflected in reduced expression of IL-12, IL-6, IL-1β, and TNF-α as well as nitric oxide production from activated macrophages (Trepicchio & Dorner 1998). Therefore, unlike the chronic inflammatory action of IL-6, augmented IL-11 expression in decidual cells in response to IL-1β or TNF-α appears to exert an anti-inflammatory effect. Not only is the action of IL-11 inconsistent with macrophage-impaired trophoblast invasion of the decidua, but IL-11 has now been shown to enhance migration of primary EVTs by a mechanism that involves signal transducer and mediator of transcription (STAT) signaling (Paiva et al. 2007). Such increase in EVT migration was observed even at very high IL-11 concentrations (100 ng/ml), arguing strongly against a direct role for IL-11 in impaired EVT invasion that leads to preeclampsia.
In humans and mice, the expression of IL-11 and its receptor IL-11Rα is tightly coupled to the induction and maintenance of decidualization (Robb et al. 1998, Dimitriadis et al. 2002, von Rango et al. 2004, White et al. 2004). The integral role that decidualization plays in restraining the intrinsically invasive EVTs is evident in the uncontrolled invasion with often life-threatening consequences that stems from implantation at sites where the decidua is absent as in ectopic pregnancies including cesarean scar pregnancies or deficient as in placenta accreta (Norwitz 2006, Rosen 2008). Invasion of the decidua involves sequential attachment of EVT-expressed adhesion molecules, particularly integrins, which recognize basement membrane-type proteins in the decidual ECM, followed by their degradation (Damsky et al. 1994, Cohen et al. 2006, Pijnenborg et al. 2006). Several studies have evaluated the role of trophoblast-expressed matrix metalloproteinases (MMPs 2 and 9), which preferentially degrade basement membrane proteins, in promoting EVT invasion of the decidua (Cohen et al. 2006). Recently, we found that IL-1β and TNF-α markedly enhanced MMP-9 expression in leukocyte-free first trimester decidual cell cultures by about 100 and 1,000-fold, respectively, and that decidual cells in placental sections from cases of preeclampsia displayed significantly higher immunohistochemical MMP-9 levels compared with controls (Lockwood et al. 2008a). Preferential degradation of basement membrane proteins in the decidual ECM by this aberrantly large increase in MMP-9 expression in decidual cells is expected to dysregulate the sequential invasion of the decidua by EVTs and interfere with remodeling of the spiral arteries and arterioles required to increase uteroplacental blood flow to the developing fetal-placental unit (Damsky et al. 1994, Pijnenborg et al. 2006).
The absence of a direct inhibitory effect on EVT migration (Paiva et al. 2007), taken together with the crucial role that IL-11 plays in decidualization suggests that excess IL-11 indirectly impedes EVT invasion by augmenting decidual cell expressed basement membrane protein synthesis and/or degradation or by alternative as yet unidentified mechanism(s). One study by Paiva et al. reported that IL-11 inhibits the EVT invasion resulting in shallow placentation (Paiva et al. 2009) which supports our hypothesis that IL-11 may be involved in pathogenesis of preeclampsia by regulating trophoblast invasion. Preeclampsia is associated with placental oxidative stress and activation of NFκB and p38 MAPK pathways in placental villi. The observations in the current study that both the NFκB and p38 MAPK signaling pathways are involved in inflammatory cytokine-enhanced IL-11 expression in first trimester human decidual cells suggests that one or both pathways may be involved in mediating preeclampsia-related pathogenic changes in decidual cells in vivo.
In conclusion, our observations that decidual expression of IL-11 is increased in preeclampsia in vivo and that IL-11 is regulated by inflammatory cytokines IL-1β and TNF-α in cultured decidual cells implicate IL-11 in the pathophysiology of preeclampsia. One possibility is that elevated IL-11 expression may be resultant of the earlier insults leading to preeclampsia and not causative of them. Further studies investigating the mechanistic role of IL-11 in normal and preeclamptic decidual cells are needed to explore these possibilities and to hopefully shed new light on our understanding of the pathogenesis of preeclampsia.
MATERIAL AND METHODS
Patients and tissue
For immunohistochemistry, placental biopsies from the decidual basalis were obtained from preeclamptic (n=9) and gestational age-matched idiopathic preterm labor control (n=8) placentas, under approval of the Yale University School of Medicine Human Investigation Committee (HIC). Table 1 provides relevant clinical details of the patients and controls. Preeclampsia was diagnosed according to standard criteria (2002) as systolic and diastolic blood pressure ≥ 140 and ≥ 90 mmHg, respectively, and proteinuria (≥ 0.3 g protein in a 24-h urine collection), occurring after 20 weeks of gestation in a woman who previously had normal blood pressure. Seven out of the nine preeclampsia patients had severe preeclampsia, defined as systolic blood pressure ≥ 160 mmHg or diastolic blood pressure ≥ 110 mmHg on two occasions at least 6 h apart, with proteinuria > 5 g in a 24-h urine collection. All preeclamptic placental specimens were obtained from cesarean delivery without labor. Control specimens were placentas from idiopathic preterm labor without clinical or histological evidence of chorioamnionitis or chronic villitis, and were obtained after either cesarean or vaginal delivery.
Table 1.
Characteristics of the Women Who Provided Placental Samples.
| Preterm Control (n=8) | Pre-eclampsia (n=9) | P value | |
|---|---|---|---|
| Age, years* | 29.3 ± 3.0 | 29.4 ± 3.8 | NS |
| Nulliparity† | 5 (50) | 5 (50) | NS |
| Gestational age, weeks* | 30.5±1.0 | 29.7±1,2 | NS |
| Systolic blood pressure, mmHg* | 117±6 | 172±7 | <0.001 |
| Diastolic blood pressure, mmHg* | 62±3 | 102±2 | <0.001 |
| Dipstick protein± | 0 (0 to 0) | 4 (2 to 4) | <0.001 |
| 24 hours protein, g | NA | 5 (0.7 to 7.2) | NA |
| IUGR | 0 (0) | 3 (30) | NS |
| HELLP syndrome | 0 (0) | 3 (30) | NS |
| PPROM† | 5 (50) | 0 (0) | <0.05 |
| Birth weight, g* | 1532±202 | 1077±139 | NS |
| Cesarean delivery† | 3 (30) | 8 (80) | NS |
| Histological chorioamnionitis stage II to III† | 0 (0) | 0 (0) | NS |
Data represented as mean±SEM and analyzed by Student’s t-test.
Data represented as n (%) and analyzed by Fischer’s exact test.
Data represented as median and analyzed by Mann-Whitney test.
PPROM, preterm premature rupture of the membranes; IUGR, intrauterine growth restriction; HELLP, syndrome of hemolysis, elevated liver enzymes, and low platelets.
For cell cultures, first-trimester decidual specimens from fourteen uncomplicated, elective terminations between 8 and 12 weeks of gestation were obtained under Institutional Review Board approval at Bellevue Hospital, New York, NY. Decidual tissues were separated from the amnio-chorion, and a small portion of each was formalin-fixed and paraffin-embedded for histological examination for any signs of underlying acute or chronic inflammation. The remainder of each specimen was used for decidual cell isolation and culture.
Immunohistochemistry
IL-11 and IL-11R immunostaining was performed as described previously (Kayisli et al. 2002). Briefly, five-µm formalin-fixed, paraffin-embedded sections were deparaffinize in xylene and rehydrated through a descending ethanol series. Antigen retrieval was then performed by boiling the slides in citrate buffer (10 mM; pH 6.0) for 15 min, followed by endogenous peroxidase quenching with 3% hydrogen peroxide (in 50% methanol/50% distilled water) for 15 min. The slides were then incubated with 5% blocking horse serum (Vector Labs, Burlingame, CA) in Tris-buffered saline (TBS) for 30 min at room temperature in a humidified chamber. Excess serum was then removed, and serial sections were incubated with either mouse monoclonal IL-11 antibody (R&D Systems, Minneapolis, MN) at 10 µg/ml in 1% blocking horse serum in TBS, or monoclonal IL-11R antibody (R&D Systems, Minneapolis, MN) at 10 µg/ml in 1% blocking horse serum in TBS or mouse monoclonal anti-vimentin antibody (DakoCytomation, Carpinteria, CA) at 1:100 dilution in TBS, or anti-cytokeratin antibody (DakoCytomation, Carpinteria, CA) at 1:100 dilution in TBS overnight in a humidified chamber at +4°C. Non-specific mouse IgG isotype antibody was used at the same concentrations as the primary antibodies for negative controls. After washing, the slides were incubated with biotinylated horse anti-mouse secondary antibody (Vector Labs) at 1:400 dilutions for 30 min at room temperature. After washing again in TBS, the antigen-antibody complex was detected with an avidin-biotin peroxidase or alkaline phosphatase kit (Vector Labs). Diaminobenzidine (3, 3-diaminobenzidine tetrahydrochloride dehydrate) and Fast Red (Vector Labs) were used as the chromogens to detect IL-11 and vimentin immunoreactivity, respectively. Slides were then counterstained with hematoxylin and mounted.
The intensity of IL-11 and IL-11R immunostaining was semi-quantitatively evaluated using HSCORE analysis. Immunostaining intensity was categorized into the following scores: 0 (no staining), 1 (weak, but detectable, staining), 2 (moderate staining), and 3+ (intense staining). An HSCORE value was derived for each specimen by calculating the sum of the percentage of cells that stained at each intensity category multiplied by its respective intensity score, using the formula HSCORE = ∑i i*Pi , where i represents the intensity category score, and Pi is the corresponding percentage of cells (Guzeloglu Kayisli et al. 2004). For each slide, five different fields were evaluated microscopically at 200× magnification. HSCORE evaluation was performed independently by two investigators blinded to the source of the samples; the average score of both was then used.
Isolation of decidual cells
Decidual cell isolation was performed as described previously (Lockwood et al. 2008b). Briefly, minced tissues were digested with 0.1% collagenase type IV and 0.01% DNAse in RPMI containing 20ug/ml penicillin/streptomycin and1ul/ml Fungizone (Invitrogen, Carlsbad, CA) in a 37°C shaking water bath for 30 min, and then washed with sterile phosphate buffered saline (PBS) three times and subjected to consecutive filtration through 100µ, 70µ and 40µ Millipore filters. Cells were resuspended in RPMI and then grown to confluence on polystyrene tissue culture dishes. After harvesting with trypsin/EDTA, cells were analyzed by flow cytometric analysis with anti-CD45 and anti-CD14 monoclonal antibodies (BD Pharmingen, San Diego, CA) to determine the presence of leukocytes after each passage. Cultures were found to be leukocyte-free (<1%) after 3–4 passages. Cultured decidual cells were also found to be vimentin-positive and cytokeratin-negative and displayed decidualization-related morphologic and biochemical changes during incubation with a progestin. Decidualization-related biochemical changes were detected as enhanced expression of prolactin and plasminogen activator inhibitor-1 (PAI-1) and inhibited expression of interstitial collagenase and stromelysin-1 as found previously(Lockwood et al. 2008a, Lockwood et al. 2008b, Oner et al. 2008). Cell aliquots were frozen in fetal calf serum/DMSO (9:1) (Sigma-Aldrich, St. Louis, Mo) and kept in liquid nitrogen for future uses.
Experimental incubations
Thawed cells were seeded onto polystyrene culture-treated flasks and incubated in BMS, which consists of basal medium (BM), a phenol red-free 1:1 v:v mix of Dulbecco’s MEM (Invitrogen) and Ham’s F-12 (Flow Labs, Rockville, MD), with 100 U/ml penicillin, 100 ug/ml streptomycin, and 0.25 µg/ml Fungizone supplemented with 10% charcoal-stripped calf serum (S). After two additional passages, confluent cultures were incubated in parallel in BMS containing either 10−8 mol/L estradiol (E2) with or without 10−7mol/L medroxyprogesterone acetate (MPA) (Sigma-Aldrich). For these incubations, MPA substituted for progesterone because of its greater stability in culture (Arici et al. 1999), whereas E2 served as the control incubation for E2 plus MPA. The latter was employed to mimic elevated circulating E2 and progesterone levels during the first trimester of pregnancy. After seven days, the cultures were washed twice with HBSS to remove residual serum elements. The cultures were switched to a defined medium (DM) consisting of BM plus ITS+ (Collaborative Research, Waltham, MA), 5uM FeSO4, 50uM ZnSO4, 1nM CuSO4, 20nM Na2SeO3, trace elements (Invitrogen), 50ug/ml ascorbic acid (Sigma-Aldrich) and 50 ng/ml epidermal growth factor (Becton-Dickinson, Bedford, MA) with either vehicle or steroids or 0.01–10 ng/ml of IL-1β or TNF-α.
In select experiments, after priming with E2 plus MPA in BMS for seven days, the cultures were switched to DM containing the steroids ± 1 ng/ml of IL-1β or TNF-α and ± a signaling pathway inhibitor added at the concentration recommended by the manufacturer. Specifically, these were NFκB inhibitor (Activation Inhibitor III) at 10−5 M, p38 MAPK inhibitor (SB203580) at 10−5 M, and PKC inhibitor (Calphostin C) at 10−7 M (all from EMD Biosciences Inc., Gibbstown, NJ). After the test period, cells were harvested by scraping in ice-cold lysis buffer solution of Tris buffered saline with 1% Triton X-100, 1mM phenylmethanesulfonyl fluoride (Sigma), and Complete protease inhibitor cocktail (Roche, Mannheim, Germany) and then briefly sonicated. Conditioned medium supernatants and cell lysates were stored at .70° C. Total RNA was extracted from five separate, parallel incubations with Trizol Reagent (Sigma-Aldrich).
ELISA
Total cell protein was assessed using a modified Lowry assay (Bio-Rad Laboratories, Inc. Hercules, CA). An ELISA kit (R&D Systems) was used to measure IL-11 levels in the cell-conditioned DM according to the manufacturer’s instructions. The sensitivity of the ELISA is 8 pg/ml, with intra-assay and inter-assay coefficients of variation of 2.4% and 6.9%, respectively. According to the manufacturer there is no significant cross-reactivity or interference by other cytokines in this assay.
Real-time quantitative RT/PCR
To verify that the IL-11 and β-actin probes yielded the correct bands, RNA was extracted from experimental cell incubations and subjected to semi-quantitative RT-PCR using a kit from Invitrogen, performing 35 cycles with the Eppendorf Mastercycler (Eppendorf, Westbury, NY). For quantitative real time RT-PCR, reverse transcription was carried out with AMV reverse transcriptase (Invitrogen). A quantitative standard curve was established (40 pg to 2.5 ng of cDNA) with a Roche Light Cycler (Roche, Indianapolis, IN) by monitoring increasing PCR product fluorescence during amplification. Quantitation of the unknowns was determined with the Roche Light Cycler and normalized to β-actin levels from the corresponding unknowns. Melting curve analysis confirmed the specificity of the amplified products and the absence of primer-dimer formation. All products generated the correct melting temperatures. Products were then run on a 1.2% agarose gel along with a 100 bp DNA ladder and visualized with ethidium bromide. The following primers were synthesized and gel-purified at the Yale DNA Synthesis Laboratory, Critical Technologies.
| Abbrv | Sense (5' to 3') | Antisense (5' to 3') | Size |
| β-actin | CGTACCACTGGCATCGTGAT | GTGTTGGCGTACAGGTCTTTG | 452 bp |
| IL-11 | ACAGTACCCGTATGGG | CCGGTCTCGAACTCTT | 306 bp |
Statistical analysis
Gestational ages of tissue specimens were normally distributed and analyzed by Student’s t test. Immunohistochemistry HSCOREs were also normally distributed (Kolmogorovs-Smirnov test) and analyzed using one-way ANOVA, with post hoc Holm-Sidak testing. Control and treatment groups in culture conditions were compared by the Kruskal-Wallis ANOVA on Ranks test followed by the Student-Newman-Keuls post hoc test. In all comparisons, statistical significance was defined as p < 0.05.
Acknowledgments
This study was kindly supported by a training grant to M Basar and E Kocamaz from The Scientific and Technological Research Council of Turkey (TUBITAK). This study is part of a PhD thesis of Murat Basar.
This work was supported by grants from the National Institutes of Health 2 R01HD33937-05 (to C.J.L.) and 1 R01 HL070004-04 (to C.J.L.)
REFERENCES
- 2002 ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol. 2002 Jan;99(Number 33):159–167. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- Abrahams VM, Kim YM, Straszewski SL, Romero R, Mor G. Macrophages and apoptotic cell clearance during pregnancy. Am J Reprod Immunol. 2004;51:275–282. doi: 10.1111/j.1600-0897.2004.00156.x. [DOI] [PubMed] [Google Scholar]
- Arici A, Marshburn PB, MacDonald PC, Dombrowski RA. Progesterone metabolism in human endometrial stromal and gland cells in culture. Steroids. 1999;64:530–534. doi: 10.1016/s0039-128x(99)00029-x. [DOI] [PubMed] [Google Scholar]
- Bamba S, Andoh A, Yasui H, Makino J, Kim S, Fujiyama Y. Regulation of IL-11 expression in intestinal myofibroblasts: role of c-Jun AP-1- and MAPK-dependent pathways. Am J Physiol Gastrointest Liver Physiol. 2003;285:G529–G538. doi: 10.1152/ajpgi.00050.2003. [DOI] [PubMed] [Google Scholar]
- Bauer S, Pollheimer J, Hartmann J, Husslein P, Aplin JD, Knofler M. Tumor necrosis factor-alpha inhibits trophoblast migration through elevation of plasminogen activator inhibitor-1 in first-trimester villous explant cultures. J Clin Endocrinol Metab. 2004;89:812–822. doi: 10.1210/jc.2003-031351. [DOI] [PubMed] [Google Scholar]
- Caniggia I, Winter J, Lye SJ, Post M. Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta. 2000;21(Suppl A):S25–S30. doi: 10.1053/plac.1999.0522. [DOI] [PubMed] [Google Scholar]
- Chen HF, Lin CY, Chao KH, Wu MY, Yang YS, Ho HN. Defective production of interleukin-11 by decidua and chorionic villi in human anembryonic pregnancy. J Clin Endocrinol Metab. 2002;87:2320–2328. doi: 10.1210/jcem.87.5.8478. [DOI] [PubMed] [Google Scholar]
- Cohen M, Meisser A, Bischof P. Metalloproteinases and human placental invasiveness. Placenta. 2006;27:783–793. doi: 10.1016/j.placenta.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Damsky CH, Librach C, Lim KH, Fitzgerald ML, McMaster MT, Janatpour M, Zhou Y, Logan SK, Fisher SJ. Integrin switching regulates normal trophoblast invasion. Development. 1994;120:3657–3666. doi: 10.1242/dev.120.12.3657. [DOI] [PubMed] [Google Scholar]
- Dimitriadis E, Robb L, Liu YX, Enders AC, Martin H, Stoikos C, Wallace E, Salamonsen LA. IL-11 and IL-11Ralpha immunolocalisation at primate implantation sites supports a role for IL-11 in placentation and fetal development. Reprod Biol Endocrinol. 2003;1:34. doi: 10.1186/1477-7827-1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriadis E, Robb L, Salamonsen LA. Interleukin 11 advances progesterone-induced decidualization of human endometrial stromal cells. Mol Hum Reprod. 2002;8:636–643. doi: 10.1093/molehr/8.7.636. [DOI] [PubMed] [Google Scholar]
- Dunn CL, Kelly RW, Critchley HO. Decidualization of the human endometrial stromal cell: an enigmatic transformation. Reprod Biomed Online. 2003;7:151–161. doi: 10.1016/s1472-6483(10)61745-2. [DOI] [PubMed] [Google Scholar]
- Guzeloglu Kayisli O, Kayisli UA, Luleci G, Arici A. In vivo and in vitro regulation of Akt activation in human endometrial cells is estrogen dependent. Biol Reprod. 2004;71:714–721. doi: 10.1095/biolreprod.104.027235. [DOI] [PubMed] [Google Scholar]
- Hefler LA, Tempfer CB, Gregg AR. Polymorphisms within the interleukin-1 beta gene cluster and preeclampsia. Obstet Gynecol. 2001;97:664–668. doi: 10.1016/s0029-7844(01)01128-0. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SJ, Chen CP, Schatz F, Rahman M, Abrahams VM, Lockwood CJ. Pre-eclampsia is associated with dendritic cell recruitment into the uterine decidua. J Pathol. 2008;214:328–336. doi: 10.1002/path.2257. [DOI] [PubMed] [Google Scholar]
- Kayisli UA, Selam B, Demir R, Arici A. Expression of vasodilator-stimulated phosphoprotein in human placenta: possible implications in trophoblast invasion. Mol Hum Reprod. 2002;8:88–94. doi: 10.1093/molehr/8.1.88. [DOI] [PubMed] [Google Scholar]
- Lacroix M, Siwek B, Marie PJ, Body JJ. Production and regulation of interleukin-11 by breast cancer cells. Cancer Lett. 1998;127:29–35. doi: 10.1016/s0304-3835(97)00542-9. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Matta P, Krikun G, Koopman LA, Masch R, Toti P, Arcuri F, Huang ST, Funai EF, Schatz F. Regulation of monocyte chemoattractant protein-1 expression by tumor necrosis factor-alpha and interleukin-1beta in first trimester human decidual cells: implications for preeclampsia. Am J Pathol. 2006;168:445–452. doi: 10.2353/ajpath.2006.050082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood CJ, Oner C, Uz YH, Kayisli UA, Huang SJ, Buchwalder LF, Murk W, Funai EF, Schatz F. Matrix metalloproteinase 9 (MMP9) expression in preeclamptic decidua and MMP9 induction by tumor necrosis factor alpha and interleukin 1 beta in human first trimester decidual cells. Biol Reprod. 2008a;78:1064–1072. doi: 10.1095/biolreprod.107.063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood CJ, Yen CF, Basar M, Kayisli UA, Martel M, Buhimschi I, Buhimschi C, Huang SJ, Krikun G, Schatz F. Preeclampsia-related inflammatory cytokines regulate interleukin-6 expression in human decidual cells. Am J Pathol. 2008b;172:1571–1579. doi: 10.2353/ajpath.2008.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norwitz ER. Defective implantation and placentation: laying the blueprint for pregnancy complications. Reprod Biomed Online. 2006;13:591–599. doi: 10.1016/s1472-6483(10)60649-9. [DOI] [PubMed] [Google Scholar]
- Oner C, Schatz F, Kizilay G, Murk W, Buchwalder LF, Kayisli UA, Arici A, Lockwood CJ. Progestin-inflammatory cytokine interactions affect matrix metalloproteinase-1 and-3 expression in term decidual cells: implications for treatment of chorioamnionitis470 induced preterm delivery. J Clin Endocrinol Metab. 2008;93:252–259. doi: 10.1210/jc.2007-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva P, Salamonsen LA, Manuelpillai U, Dimitriadis E. Interleukin 11 inhibits human trophoblast invasion indicating a likely role in the decidual restraint of trophoblast invasion during placentation. Biol Reprod. 2009;80:302–310. doi: 10.1095/biolreprod.108.071415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva P, Salamonsen LA, Manuelpillai U, Walker C, Tapia A, Wallace EM, Dimitriadis E. Interleukin-11 promotes migration, but not proliferation, of human trophoblast cells, implying a role in placentation. Endocrinology. 2007;148:5566–5572. doi: 10.1210/en.2007-0517. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–958. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Reister F, Frank HG, Kingdom JC, Heyl W, Kaufmann P, Rath W, Huppertz B. Macrophage-induced apoptosis limits endovascular trophoblast invasion in the uterine wall of preeclamptic women. Lab Invest. 2001;81:1143–1152. doi: 10.1038/labinvest.3780326. [DOI] [PubMed] [Google Scholar]
- Rinehart BK, Terrone DA, Lagoo-Deenadayalan S, Barber WH, Hale EA, Martin JN, Jr, Bennett WA. Expression of the placental cytokines tumor necrosis factor alpha, interleukin 1beta, and interleukin 10 is increased in preeclampsia. Am J Obstet Gynecol. 1999;181:915–920. doi: 10.1016/s0002-9378(99)70325-x. [DOI] [PubMed] [Google Scholar]
- Robb L, Li R, Hartley L, Nandurkar HH, Koentgen F, Begley CG. Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nat Med. 1998;4:303–308. doi: 10.1038/nm0398-303. [DOI] [PubMed] [Google Scholar]
- Rosen T. Placenta accreta and cesarean scar pregnancy: overlooked costs of the rising cesarean section rate. Clin Perinatol. 2008;35:519–529. doi: 10.1016/j.clp.2008.07.003. x. [DOI] [PubMed] [Google Scholar]
- Scicchitano MS, McFarland DC, Tierney LA, Boyce RW, Frazier KS, Schwartz LW, Thomas HC. Role of p38 in regulation of hematopoiesis: effect of p38 inhibition on cytokine production and transcription factor activity in human bone marrow stromal cells. Blood Cells Mol Dis. 2008;40:370–380. doi: 10.1016/j.bcmd.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- Tabanelli S, Tang B, Gurpide E. In vitro decidualization of human endometrial stromal cells. J Steroid Biochem Mol Biol. 1992;42:337–344. doi: 10.1016/0960-0760(92)90137-8. [DOI] [PubMed] [Google Scholar]
- Trepicchio WL, Dorner AJ. The therapeutic utility of Interleukin-11 in the treatment of inflammatory disease. Expert Opin Investig Drugs. 1998;7:1501–1504. doi: 10.1517/13543784.7.9.1501. [DOI] [PubMed] [Google Scholar]
- von Rango U, Alfer J, Kertschanska S, Kemp B, Muller-Newen G, Heinrich PC, Beier HM, Classen-Linke I. Interleukin-11 expression: its significance in eutopic and ectopic human implantation. Mol Hum Reprod. 2004;10:783–792. doi: 10.1093/molehr/gah107. [DOI] [PubMed] [Google Scholar]
- White CA, Robb L, Salamonsen LA. Uterine extracellular matrix components are altered during defective decidualization in interleukin-11 receptor alpha deficient mice. Reprod Biol Endocrinol. 2004;2:76. doi: 10.1186/1477-7827-2-76. [DOI] [PMC free article] [PubMed] [Google Scholar]