Abstract
The complement activation product, C5a, is a key factor for regulation of inflammatory responses. C5a and C5adesArg bind to their receptors, C5aR and C5L2, but the functional roles of C5L2 remain controversial. We screened the patterns of 23 inflammatory mediators in cultures of LPS-activated mouse peritoneal elicited macrophages (PEMs) in the presence or absence of recombinant mouse C5a. Production of most mediators studied was suppressed by C5a, whereas G-CSF production was enhanced. G-CSF gene expression and secretion from PEMs was amplified 2–3-fold by C5a in a dose and time dependent fashion. The degradation product C5adesArg promoted lower levels of G-CSF. The effects of C5a on G-CSF were associated with activation of PI3K/Akt and MEK1/2 signaling pathways. C5a did not enhance G-CSF production in cultures of PEMs from either C5aR-deficient or C5L2-deficient mice, indicating that both C5a receptors are indispensable for mediating the effects of C5a in production of G-CSF. Finally, G-CSF levels in plasma during polymicrobial sepsis after cecal ligation and puncture (CLP) were substantially lower in C5aR-deficient or C5L2-deficient mice as compared to C57BL/6J wild type mice. These findings elucidate the functional characteristics of the C5L2 receptor during the acute inflammatory response.
Keywords: Cecal ligation and puncture, sepsis, macrophages, Akt, MEK1/2
Introduction
Proteolytic cleavage of complement proteins following activation of the classical, alternative, and lectin pathways can generate substantial quantities of the anaphylatoxin, C5a [1]. Rapid inactivation by carboxypeptidase removes the C-terminal arginine, converting C5a to C5adesArg. Both C5a and C5adesArg (with much lower affinity) are ligands for the G-protein coupled C5aR receptor(CD88) [2, 3]. C5aR is abundantly expressed on innate immune cells of the myeloid lineage, lymphocytes and in lower numbers on epithelial and endothelial cells[4–6]. In polymorphonuclear leukocytes (PMNs) and macrophages, ligation of C5a with the C5aR receptor leads to rapid buildup of cytosolic Ca2+, activation of MAPK signaling pathways, chemotaxis, respiratory burst, release of toxic granules and regulation of cytokine expression [2, 3, 7].
A second C5a receptor, C5L2 (GPR77), has been identified [8]. Initially C5a was thought to be a non-signaling “decoy” receptor[8]. Indeed, binding of C5a or C5adesArg to C5L2 does not induce rapid Ca2+ currents[9]. However, accumulating evidence suggests distinct functional roles of C5L2 in disease. For example, both C5aR and C5L2 receptors are critical factors during polymicrobial sepsis after cecal ligation and puncture (CLP) [10]. The expression of C5L2 in PMNs is down-regulated during severe sepsis, which is a marker of poor prognosis [11]. C5L2 determines the outcome of experimental allergic asthma[12]. Polymorphisms of the C5L2 gene may be associated with a higher risk for cardiovascular diseases in some populations [13]. Despite these findings, many aspects of the role of C5L2 in acute and chronic inflammation remain enigmatic.
In this report we describe that C5a promotes the release of G-CSF. These effects required the presence of both C5aR and C5L2 in cultures of macrophages and during polymicrobial sepsis after CLP.
Results and Discussion
Differential regulation of mediator production by C5a
We have recently reported that the production of several cytokines (IL-17, IL-23, IL-27)is suppressed by C5a when present in cultures of LPS-activated peritoneal elicited macrophages (PEMs)[5, 14, 15]. To investigate how broad the spectrum of C5a regulated mediators is, the concentrations of 23 inflammatory mediators were analyzed by a multiplexing bead-based assay (Table 1). Incubation of PEMs from C57BL/6J mice with LPS for 10 h generally increased the release of cytokines and chemokines as compared to untreated control PEMs (Table 1). Simultaneous addition of recombinant mouse C5a (100 nM) to LPS-activated PEMs affected the production of all mediators studied (Table 1). Whereas proinflammatory mediators were suppressed by C5a, the remarkable finding was that only IL-10 and G-CSF were amplified (by 103% and 197%, respectively). No consistent effects on production of the analyzed cytokines was observed, when C5a was used alone in PEMs (data not shown;[5, 15]). It has been reported before that cytokines of the IL-12 family are antagonized by C5a[16, 17]. Furthermore, we have recently described the role of C5a-induced IL-10 in down-modulation of the IL-17A/IL-23 axis [15]. These findings demonstrate that the strong proinflammatory anaphylatoxin C5a in high concentrations can mediate anti-inflammatory effects in primed macrophages, which may be beneficial to prevent excessive inflammation. Reciprocal effects of C5a (as an inducer of proinflammatory cytokines/chemokines) have been noted in other cell types such as alveolar epithelial cells [18], microvascular endothelial cells [19] or blood PMNs [20].
TABLE 1.
Regulation of macrophage-derived mediators by C5a
| Mediator | CTRL (pg/ml) | LPS (pg/ml) | LPS+C5a (%-change) |
|---|---|---|---|
| IL-1α | 36 ± 19 | 388 ± 94 | −30%** |
| IL-1β | 255±166 | 2800±683 | −30%* |
| IL-2 | 44±13 | 83±18 | −23%* |
| IL-3 | 5±2 | 19±5 | −29%* |
| IL-4 | 9±3 | 29±8 | −22%* |
| IL-5 | 6±3 | 15±3 | −27%* |
| IL-6 | 89±46 | 5516±1455 | −64%** |
| IL-9 | 57±17 | 350±74 | −38%** |
| IL-10 | 62±21 | 223±41 | +103%* |
| IL-12(p40) | 157±66 | 12983±4366 | −81%** |
| IL-12(p70) | 73±20 | 1427±344 | −68%* |
| IL-13 | 109±60 | 979±268 | −35%* |
| IL-17 | 43±26 | 1036±375 | −44%* |
| Eotaxin | 461±185 | 2653±613 | −30%*** |
| G-CSF | 38±22 | 375±59 | +197%** |
| GM-CSF | 43±12 | 161±31 | −21%* |
| IFN-γ | 31±9 | 279±39 | −47%* |
| KC | 1148±791 | 26097±2087 | −28%* |
| MCP-1 | 1124±663 | 19722±4038 | −55%** |
| MIP-1α | 457±256 | 21528±6056 | −69%** |
| MIP-1β | 2323±1485 | 130564±37936 | −60%** |
| RANTES | 550±276 | 22212±3888 | −60%** |
| TNF-α | 187±55 | 59126±18211 | −77%** |
Macrophages (PEMs) from C57BL/6J mice were incubated for 10 h with LPS (1 μg/ml) ± rmC5a (100 nM). CTRL denotes supernatants from resting cells. Multiplexing bead-based assay.
Data are from 4 independent experiments. Values represent means ± s.e.m. and values after LPS alone were used as 100% to calculate %-change. Student’s two-tailed t-test, LPS vs. LPS+C5a,
P<0.05,
P<0.01,
P<0.001.
C5a promotes release of G-CSF via Akt and MEK1/2
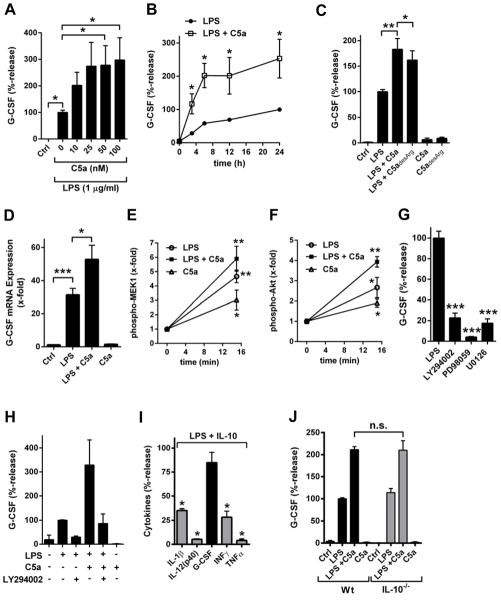
The current studies were focused on G-CSF, which was the mediator most potently enhanced by C5a in PEMs(Table 1). C5a acted dose-dependently to increase G-CSF levels 2–3-fold in cultures of PEMs (Fig. 1A). Higher concentrations of G-CSF were observed at all time points (3–24 h) studied (Fig. 1B). Long-lasting modulation of mediator release by C5a has been also reported for other cytokines [5, 15]. Recombinant C5adesArg in combination with LPS also displayed somewhat diminished ability to amplify G-CSF compared to C5a (Fig. 1C). C5a enhanced G-CSF production on the mRNA level was detected by real time PCR (Fig. 1D). No effects on G-CSF levels were seen when C5a was used alone in the absence of the co-stimulus, LPS (Fig. 1C, 1D).
Figure 1.
C5a-induced amplification of G-CSF production from macrophages. (A) Peritoneal elicited macrophages (PEMs) from C57BL/6J mice were stimulated for 8 h with LPS (1 μg/ml) alone or in combination with different doses of recombinant mouse C5a (10–100 nM) followed by detection of G-CSF by ELISA in supernatant fluids. (B) Time course of G-CSF secretion from PEMs after LPS (1 μg/ml) alone or together with rmC5a (100 nM), ELISA. (C) Comparison of activity of rmC5a (100 nM) and rmC5adesArg (100nM) to enhance G-CSF in supernatants from LPS-activated PEMs, 8h, ELISA. (D) Real time PCR of mRNA for G-CSF from PEMs left as untreated controls (Ctrl), after LPS (1 μg/ml), LPS plus C5a (100 nM) or C5a alone, 6 h.(E) Phospho-MEK1 (serine 217/serine 221)in PEMs after incubation with C5a (100 nM) and LPS (1 μg/ml) alone or in combination. Phosphorylation levels in untreated cells were used as 1-fold, bead-based assay. (F) Phospho-Akt (threonine 308) after stimulation with C5a, LPS or LPS plus C5a, bead-based assay. (G) Relative release of G-CSF from LPS-activated PEMs after blockade of PI3K/Akt with LY294002 (25 μM) or MEK1/2 with PD98059 (50 μM) or U0126 (1 μM), 8 h, ELISA. All experiments were done with cells from C57BL/6J mice. (H) PEMs were treated with combinations of LPS, C5a and LY294002 as indicated followed by quantitation of G-CSF, 8 h, ELISA.(I)PEMs (C57BL/6J) were incubated for 8 h with LPS alone or LPS plus rmIL-10 (10 ng/ml) followed by detection in supernatants of IL-1β, IL-12(p40), G-CSF, INFγ and TNFα. Amounts of cytokines after LPS alone were used as 100% values, bead-based assays.(J)Relative release of G-CSF in PEMs from C57BL/6J mice compared to PEMs from IL-10−/− mice after LPS ± C5a, 8h, ELISA. Data shown in each frame are from three independent experiments, student’s two-tailed t-test;*P<0.05, **P<0.01, ***P<0.001.
C5a is known to activate PI3K/Akt and MEK/ERK kinases in innate immune cells [5, 14, 16, 21–23]. Using bead-based assays with phosphorylation specific antibodies we confirmed that C5a activated MEK1 and Akt (Fig. 1E, 1F). These signaling pathways appeared to be relevant for mediating C5a-enhanced G-CSF production. Blockade of PI3K/Akt with the small molecule inhibitor LY294002, and blockade of MEK1/2 with PD98059 or the highly selective inhibitor, U0126, greatly reduced the release of G-CSF from PEMs activated with LPS or the combination of LPS plus C5a(Fig. 1G, Fig. 1H).
G-CSF production is resistant to the inhibitory effects of C5a-induced IL-10
To further elucidate the mechanism by which C5a differentially suppressed proinflammatory cytokines/chemokines, while up-regulating G-CSF, we studied the role of IL-10. C5a selectively promoted the production of IL-10 from LPS-activated PEMs (Table 1). We have recently reported that C5a-induced IL-10 release is essential for suppression of proinflammatory cytokines such as IL-17A and IL-23 by C5a [15]. Interestingly, the release of G-CSF was resistant to the inhibitory effects of recombinant IL-10, even when used in high concentrations (Fig. 1I). At the same time proinflammatory cytokines (IL-1β, IL-12, IFNγ, TNFα) were greatly reduced by IL-10 (Fig. 1I). When the production of endogenous IL-10 was interrupted using PEMs from IL-10−/− mice, no differences were seen in G-CSF production after LPS alone or in combination with C5a (Fig. 1J). This is in clear contrast to mediators such as IL-12, IL-17A or IL-23 for which the modulatory effects of C5a are mitigated with genetic deficiency or antibody blockade of IL-10 [15, 24, 25].
Dependency of G-CSF release on the presence of C5aR and C5L2
There is an ongoing debate on the precise functional roles of the C5L2 receptor as compared to the C5aR receptor [1]. We used PEMs from C5aR−/− mice and C5L2−/− mice to investigate the roles of both receptors for C5a-mediated amplification of G-CSF. Both C5aR and C5L2 were required for C5a enhancement of G-CSF production in cultures of PEMs from C5aR−/− mice and C5L2−/− mice (Fig. 2A), since the absence of either receptor caused G-CSF levels to fall to baseline levels (with LPS alone).
Figure 2.
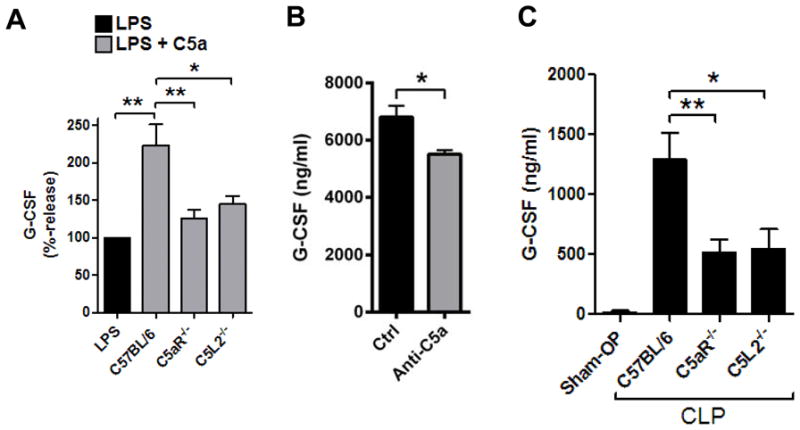
Studies on the production of G-CSF with genetic absence of the C5aR receptor or C5L2 receptor.(A) Relative release of G-CSF from PEMs from C57BL/6J (Wt), C5aR−/− or C5L2−/− mice after LPS (1 μg/ml) alone or together with C5a (100 nM), 8 h, ELISA. Results with LPS alone were used as the 100% value for each mouse strain.(B) Detection of G-CSF in plasma of C57BL/6J mice 12 h after endotoxic shock (LPS 10 mg/kg bodyweight i.p.). Mice were pretreated with neutralizing polyclonal anti-C5a antiserum or control (Ctrl) serum (500 μl i.p., n≥7/group), ELISA.(C) Plasma concentrations of G-CSF in C57BL/6J (Wt) mice after sham-OP (n=7) or cecal ligation and puncture (CLP, n=15) compared to concentrations after CLP in C5aR−/− mice (n=14) or C5L2−/− mice (n=11). Sham-OP was performed only with Wt mice, 24 h, ELISA. Data shown in frame A were from three independent experiments each done in duplicate wells, and data in frame B and C were analyzed using numbers of mice as indicated, student’s two-tailed t-test;*P<0.05, **P<0.01.
To study the effects of C5a on G-CSF production during the acute inflammatory response in vivo, we blocked endogenous C5a during endotoxic shock, which resulted in moderately decreased G-CSF (Fig. 2B). Next, we used the model of polymicrobial sepsis after cecal ligation and puncture (CLP) to study G-CSF production. Sham operated C57BL/6J mice received only anesthesia and laparotomy without manipulation of the cecum. The amounts of G-CSF in plasma of sham-OP mice were low but G-CSF was greatly increased in C57BL/6J mice 24 h after CLP (>1000 ng/ml, Fig. 2C). At the same time, plasma concentrations of G-CSF were substantially lower in C5aR−/− mice and C5L2−/− mice after CLP (Fig. 2C).
Collectively, it appears that neither C5a receptor (C5aR, C5L2) by itself is able to compensate for the genetic absence of the other receptor, respectively. These findings are quite surprising because C5a does not require presence of the C5L2 receptor (as studied in C5L2−/− PEMs)for suppression of other cytokines such as IL-17A, IL-23 or IL-27 [5, 14], suggesting that the effects of C5a on G-CSF production from PEMs are distinct from the regulation of other mediators by C5a (Table 1). Interestingly, a recent study has reported C5aR internalization after ligand binding and co-localization with intracellular C5L2 in human neutrophils, which may suggest a potential cross-talk of the two receptors [26].
Complement activation is an essential arm of innate immune responses, capable of providing immediate clearance of pathogens by direct lysis (membrane attack complex) and facilitating opsonization/phagocytosis of pathogens. C5a directs chemotaxis of PMNs to the site of inflammation and activates such cells. In addition, our data demonstrate that C5a signaling amplifies the production of G-CSF in vitro and in vivo. G-CSF is a critical growth factor for the proliferation of PMNs during hematopoiesis in the bone marrow. Recombinant G-CSF is an FDA-approved pharmaceutical drug for the treatment of chemotherapy related severe neutropenia. In one report C5-deficient mice displayed an impaired mobilization of hematopoietic stem/progenitor cells[27]. C5a promotes the egress of PMNs from the bone marrow into peripheral blood[28]. Our data suggest that C5a via enhanced production of G-CSF may promote the PMN-dependent acute inflammatory response with dependency on both C5aR and C5L2 receptors. Respiratory burst and granule release from PMNs contributes to tissue injury during inflammation (e.g. in the setting of sepsis). In accordance with our findings, the blockade of complement activation using compstatin analogs (which block the C3 convertase) reduced G-CSF levels in human blood during simulated hemodialysis [29]. Moreover, G-CSF appears to upregulate C5aR (CD88) expression on PMNs [28]. In summary, C5a promotes the influx and proliferation of PMNs through multiple mechanisms, whereas different effects are seen with respect to adaptive immunity such as suppression of Th1 and Th17 cytokines.
Concluding remarks
C5a possesses broad-spectrum activities for regulating the release of inflammatory mediators from macrophages. G-CSF is regulated by C5a different than with most other mediators. C5aR and C5L2 are both indispensable for transmission of signaling by C5a in order to amplify G-CSF production in vitro and in vivo.
Materials and methods
Mice
All procedures were in accordance with the U.S. National Institutes of Health guidelines and the University Committee on Use and Care of Animals (UCUCA), University of Michigan. We used male mice of the strains C57BL/6J, IL-10−/− (B6.129P2-Il10tm1Cgn;Jackson Laboratories, Bar Harbor, ME, USA), C5aR−/− and C5L2−/− (both breeding and genotyping were done at the University of Michigan). Mice were housed under specific pathogen free conditions. The generation of C5aR−/− and C5L2−/− mice by targeted gene disruption has been described previously [30, 31]. Animals used were the progeny of at least 10 generations of breeding of the knockout mice on the C57BL/6J background.
Cecal ligation and puncture
The CLP surgery was performed as described elsewhere[32]. Briefly, mice were anaesthetized with ketamine/xylazine i.p. before laparotomy, ligation of 4–5 mm of the distal cecum with puncture (21-G needle) and postoperative administration of 1 ml 0.9% NaCl sub-cutaneously. The severity of the procedure was designed to obtain mortality rates of 20–50% after 7 days. Sham mice received anaesthetics, laparotomy and fluid resuscitation without manipulation of the cecum.
Peritoneal elicited macrophages
Thioglycollate (1.5 ml, 2.4%, i.p.) was injected to mice and macrophages (>80% F4/80+CD11b+) obtained by peritoneal lavage 4 days later[14]. PEMs were cultured at 2×106 cells/ml in RPMI 1640 medium supplemented with 100 U/ml penicillin-streptomycin, 0.1% BSA and 25 mM HEPES at 37°C and 5% CO2. At the end of experiments, non-adherent cells were pelleted by centrifugation (650 g, 5 min, 4°C) and supernatants stored at −80°C until further analysis.
Detection of Proteins
Mouse G-CSF was quantified by ELISA (R&D Systems) according to the instructions of the manufacturer. Bead-based assays were used for detection of phosphorylated MEK1 (serine 217/serine 221) (BioRad) or phosphorylated Akt (threonine 308) (Millipore). Cells were lysed in the presence of protease and phosphatase inhibitors (Bio-Plex Cell Lysis Kit, BioRad) and assays performed according to instructions of the manufacturers. A multiplex bead-based assay (BioPlex Pro, 23-plex group I, BioRad) was used for simultaneous quantification of the following cytokines/chemokines: IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12(p40), IL-12(p70), IL-13, IL-17, eotaxin (CCL11), G-CSF, GM-CSF, IFN-γ, KC (CXCL1), MCP-1 (CCL2), MIP-1α (CCL3), MIP-1β (CCL4), RANTES (CCL5), and TNF-α. All samples from bead-based assays were analyzed on a Luminex-200 instrument as described before [32].
Real time PCR
Total RNA was isolated (RNeasy Mini Kit, Qiagen)and reverse transcribed (TaqMen reagents) followed by real time amplification (SYBR Green Master Mix, Applied Biosystems) on a 7500 Real Time PCR instrument (Applied Biosystems). The 2−ΔΔCT method with normalization to GAPDH and untreated controls was used for calculation of results. Primers for mouse G-CSF (#QT00105140)were purchased from Qiagen. Primer sequences for mouse GAPDH were: (fo) 5′-TACCCCCAATGTGTCCGTCGTG-3′, (re) 5′-CCTTCAGTGGGCCCTCAGATGC-3′.
Reagents
The following reagents were used for the studies: Recombinant mouse C5a (R&D Systems and Hycult); LPS (E. coli, 0111:B4, Sigma-Aldrich); recombinant mouse C5adesArg (Hycult); LY294002 (InvivoGen); PD98059 (InvivoGen); U0126 (InvivoGen). We used a neutralizing anti-C5a antiserum as described before [14].
Statistical Analysis
The GraphPad Prism Version 5.04 software was used for statistical analysis. All values are expressed as mean and error bars represent s.e.m. Data sets were analyzed by two-tailed Student’s t-test across independent experiments. In vitro experiments were performed independently at least 3 times and in vivo experiments were done with numbers of mice as indicated in the figure legends. Differences were considered significant when P<0.05.
Acknowledgments
The authors thank Beverly Schumann, Sue Scott and Robin Kunkel for excellent staff support. This work was supported by grants GM-29507 and GM-61656 (P.A.W.) from the U.S. National Institutes of Health, along with the Deutsche Forschungsgemeinschaft (Project 571701, BO 3482/1-1, M.B.) and the Center of Thrombosis and Hemostasis (CTH) funded by the Federal Ministry of Education and Research of Germany (BMBF, module A/JG to M.B.).
List of abbreviations
- C5a
complement activation fragment C5a
- C5adesArg
C5a lacking the C-terminal arginine
- CLP
cecal ligation and puncture
- PEMs
peritoneal elicited macrophages
- PMNs
polymorphonuclear leukocytes
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Bosmann M, Ward PA. Role of C3, C5 and anaphylatoxin receptors in acute lung injury and in sepsis. Adv Exp Med Biol. 2012;946:147–159. doi: 10.1007/978-1-4614-0106-3_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerard C, Gerard NP. C5A anaphylatoxin and its seven transmembrane-segment receptor. Annual Review of Immunology. 1994;12:775–808. doi: 10.1146/annurev.iy.12.040194.004015. [DOI] [PubMed] [Google Scholar]
- 3.Gerard NP, Gerard C. The chemotactic receptor for human C5a anaphylatoxin. Nature. 1991;349:614–617. doi: 10.1038/349614a0. [DOI] [PubMed] [Google Scholar]
- 4.Wetsel RA. Expression of the complement C5a anaphylatoxin receptor (C5aR) on non-myeloid cells. Immunol Lett. 1995;44:183–187. doi: 10.1016/0165-2478(94)00212-a. [DOI] [PubMed] [Google Scholar]
- 5.Bosmann M, Haggadone MD, Hemmila MR, Zetoune FS, Sarma JV, Ward PA. Complement activation product C5a is a selective suppressor of TLR4-induced, but not TLR3-induced, production of IL-27(p28) from macrophages. J Immunol. 2012;188:5086–5093. doi: 10.4049/jimmunol.1102914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lalli PN, Strainic MG, Yang M, Lin F, Medof ME, Heeger PS. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood. 2008;112:1759–1766. doi: 10.1182/blood-2008-04-151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nature immunology. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okinaga S, Slattery D, Humbles A, Zsengeller Z, Morteau O, Kinrade MB, Brodbeck RM, Krause JE, Choe HR, Gerard NP, Gerard C. C5L2, a nonsignaling C5A binding protein. Biochemistry. 2003;42:9406–9415. doi: 10.1021/bi034489v. [DOI] [PubMed] [Google Scholar]
- 9.Cain SA, Monk PN. The orphan receptor C5L2 has high affinity binding sites for complement fragments C5a and C5a des-Arg(74) Journal of Biological Chemistry. 2002;277:7165–7169. doi: 10.1074/jbc.C100714200. [DOI] [PubMed] [Google Scholar]
- 10.Rittirsch D, Flierl MA, Nadeau BA, Day DE, Huber-Lang M, Mackay CR, Zetoune FS, Gerard NP, Cianflone K, Kohl J, Gerard C, Sarma JV, Ward PA. Functional roles for C5a receptors in sepsis. Nature Medicine. 2008;14:551–557. doi: 10.1038/nm1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huber-Lang M, Sarma JV, Rittirsch D, Schreiber H, Weiss M, Flierl M, Younkin E, Schneider M, Suger-Wiedeck H, Gebhard F, McClintock SD, Neff T, Zetoune F, Bruckner U, Guo RF, Monk PN, Ward PA. Changes in the novel orphan, C5a receptor (C5L2), during experimental sepsis and sepsis in humans. Journal of Immunology. 2005;174:1104–1110. doi: 10.4049/jimmunol.174.2.1104. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Schmudde I, Laumonnier Y, Pandey MK, Clark JR, Konig P, Gerard NP, Gerard C, Wills-Karp M, Kohl J. A Critical Role for C5L2 in the Pathogenesis of Experimental Allergic Asthma. Journal of Immunology. 2010;185:6741–6752. doi: 10.4049/jimmunol.1000892. [DOI] [PubMed] [Google Scholar]
- 13.Zheng YY, Xie X, Ma YT, Yang YN, Fu ZY, Li XM, Ma X, Chen BD, Liu F. Relationship between a novel polymorphism of the C5L2 gene and coronary artery disease. PLoS One. 2011;6:e20984. doi: 10.1371/journal.pone.0020984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosmann M, Patel VR, Russkamp NF, Pache F, Zetoune FS, Sarma JV, Ward PA. MyD88-dependent production of IL-17F is modulated by the anaphylatoxin C5a via the Akt signaling pathway. FASEB J. 2011;25:4222–4232. doi: 10.1096/fj.11-191205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bosmann M, Sarma JV, Atefi G, Zetoune FS, Ward PA. Evidence for anti-inflammatory effects of C5a on the innate IL-17A/IL-23 axis. FASEB J. 2012;26:1640–1651. doi: 10.1096/fj.11-199216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawlisch H, Belkaid Y, Baelder R, Hildeman D, Gerard C, Kohl J. C5a negatively regulates toll-like receptor 4-induced immune responses. Immunity. 2005;22:415–426. doi: 10.1016/j.immuni.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Wittmann M, Zwirner J, Larsson VA, Kirchhoff K, Begemann G, Kapp A, Gotze O, Werfel T. C5a suppresses the production of IL-12 by IFN-gamma-primed and lipopolysaccharide-challenged human monocytes. Journal of immunology (Baltimore, Md: 1950) 1999;162:6763–6769. [PubMed] [Google Scholar]
- 18.Riedemann NC, Guo RF, Sarma VJ, Laudes IJ, Huber-Lang M, Warner RL, Albrecht EA, Speyer CL, Ward PA. Expression and function of the C5a receptor in rat alveolar epithelial cells. J Immunol. 2002;168:1919–1925. doi: 10.4049/jimmunol.168.4.1919. [DOI] [PubMed] [Google Scholar]
- 19.Laudes IJ, Chu JC, Huber-Lang M, Guo RF, Riedemann NC, Sarma JV, Mahdi F, Murphy HS, Speyer C, Lu KT, Lambris JD, Zetoune FS, Ward PA. Expression and function of C5a receptor in mouse microvascular endothelial cells. J Immunol. 2002;169:5962–5970. doi: 10.4049/jimmunol.169.10.5962. [DOI] [PubMed] [Google Scholar]
- 20.Riedemann NC, Guo RF, Hollmann TJ, Gao H, Neff TA, Reuben JS, Speyer CL, Sarma JV, Wetsel RA, Zetoune FS, Ward PA. Regulatory role of C5a in LPS-induced IL-6 production by neutrophils during sepsis. FASEB Journal. 2004;18:370–372. doi: 10.1096/fj.03-0708fje. [DOI] [PubMed] [Google Scholar]
- 21.Bosmann M, Grailer JJ, Zhu K, Matthay MA, Sarma JV, Zetoune FS, Ward PA. Anti-inflammatory effects of beta2 adrenergic receptor agonists in experimental acute lung injury. FASEB J. 2012;26:2137–2144. doi: 10.1096/fj.11-201640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen NJ, Mirtsos C, Suh D, Lu YC, Lin WJ, McKerlie C, Lee T, Baribault H, Tian H, Yeh WC. C5L2 is critical for the biological activities of the anaphylatoxins C5a and C3a. Nature. 2007;446:203–207. doi: 10.1038/nature05559. [DOI] [PubMed] [Google Scholar]
- 23.Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N, Shapiro VS, Dubyak GR, Heeger PS, Medof ME. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28:425–435. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lajoie S, Lewkowich IP, Suzuki Y, Clark JR, Sproles AA, Dienger K, Budelsky AL, Wills-Karp M. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nature immunology. 2010;11:928–935. doi: 10.1038/ni.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Kimura Y, Fang C, Zhou L, Sfyroera G, Lambris JD, Wetsel RA, Miwa T, Song WC. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood. 2007;110:228–236. doi: 10.1182/blood-2006-12-063636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bamberg CE, Mackay CR, Lee H, Zahra D, Jackson J, Lim YS, Whitfeld PL, Craig S, Corsini E, Lu B, Gerard C, Gerard NP. The C5a receptor (C5aR) C5L2 is a modulator of C5aR-mediated signal transduction. The Journal of biological chemistry. 2010;285:7633–7644. doi: 10.1074/jbc.M109.092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee HM, Wu W, Wysoczynski M, Liu R, Zuba-Surma EK, Kucia M, Ratajczak J, Ratajczak MZ. Impaired mobilization of hematopoietic stem/progenitor cells in C5-deficient mice supports the pivotal involvement of innate immunity in this process and reveals novel promobilization effects of granulocytes. Leukemia. 2009;23:2052–2062. doi: 10.1038/leu.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jalili A, Shirvaikar N, Marquez-Curtis L, Qiu Y, Korol C, Lee H, Turner AR, Ratajczak MZ, Janowska-Wieczorek A. Fifth complement cascade protein (C5) cleavage fragments disrupt the SDF-1/CXCR4 axis: further evidence that innate immunity orchestrates the mobilization of hematopoietic stem/progenitor cells. Exp Hematol. 2010;38:321–332. doi: 10.1016/j.exphem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kourtzelis I, Markiewski MM, Doumas M, Rafail S, Kambas K, Mitroulis I, Panagoutsos S, Passadakis P, Vargemezis V, Magotti P, Qu H, Mollnes TE, Ritis K, Lambris JD. Complement anaphylatoxin C5a contributes to hemodialysis-associated thrombosis. Blood. 2010;116:631–639. doi: 10.1182/blood-2010-01-264051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hopken UE, Lu B, Gerard NP, Gerard C. The C5a chemoattractant receptor mediates mucosal defence to infection. Nature. 1996;383:86–89. doi: 10.1038/383086a0. [DOI] [PubMed] [Google Scholar]
- 31.Gerard NP, Lu B, Liu P, Craig S, Fujiwara Y, Okinaga S, Gerard C. An anti-inflammatory function for the complement anaphylatoxin C5a-binding protein, C5L2. Journal of Biological Chemistry. 2005;280:39677–39680. doi: 10.1074/jbc.C500287200. [DOI] [PubMed] [Google Scholar]
- 32.Bosmann M, Russkamp NF, Patel VR, Zetoune FS, Sarma JV, Ward PA. The outcome of polymicrobial sepsis is independent of T and B cells. Shock. 2011;36:396–401. doi: 10.1097/SHK.0b013e3182295f5f. [DOI] [PMC free article] [PubMed] [Google Scholar]