Abstract
Purpose
Although cyclooxygenase (COX)-2 inhibitors could represent the most effective chemopreventive tool against colorectal cancer (CRC), their use in clinical practice is hampered by cardiovascular side effects. Consumption of ω-3-polyunsaturated fatty acids (ω-3-PUFAs) is associated with a reduced risk of CRC. Therefore, in this study, we assessed the efficacy of a novel 99% pure preparation of ω-3-PUFA eicosapentaenoic acid as free fatty acids (EPA-FFA) on polyps in ApcMin/+ mice.
Experimental design
ApcMin/+ and corresponding wild-type mice were fed control diet (Ctrl) or diets containing either EPA-FFA 2.5% or 5%, for 12 weeks while monitoring food intake and body weight.
Results
We found that both EPA-FFA diets protected from the cachexia observed among ApcMin/+ animals fed Ctrl diet (P < 0.0054), without toxic effect, in conjunction with a significant decrease in lipid peroxidation in the treated arms. Moreover, both EPA-FFA diets dramatically suppressed polyp number (by 71.5% and 78.6%, respectively; P < 0.0001) and load (by 82.5% and 93.4%, respectively; P < 0.0001) in both small intestine and colon. In addition, polyps less than 1 mm in size were predominantly found in the EPA-FFA 5% arm whereas those 1 to 3 mm in size were more frequent in the Ctrl arm (P <0.0001). Interestingly, in the EPA-FFA groups, mucosal arachidonic acid was replaced by EPA (P < 0.0001), leading to a significant reduction in COX-2 expression and β-catenin nuclear translocation. Moreover, in the EPA-FFA arms, we found a significant decrease in proliferation throughout the intestine together with an increase in apoptosis.
Conclusions
Our data make 99% pure EPA-FFA an excellent candidate for CRC chemoprevention.
Colorectal cancer (CRC) is the fourth commonest cancer in the United States and Western countries, with a global incidence of 1 million cases every year, and mortality of 500,000 (1). An important strategy for the prevention of CRC could involve pharmacologic interference with the multistep carcinogenesis at an initial stage of tumor development (2). Although cyclooxygenase-2 (COX-2) inhibitors still represent an effective chemopreventive tool against CRC, their use in clinical practice is hampered by cardiovascular and gastrointestinal side effects (3–5).
The role of diet in modulating CRC risk is a well-accepted concept, and natural compounds which have been proven safe over time and easily accessible through the diet represent ideal candidates as chemopreventive agents. The consumption of ω-3-polyunsaturated fatty acids (ω-3-PUFAs) has been associated with a reduced risk of CRC (6). One hypothesis is that their antineo-plastic effects would involve the incorporation of ω-3-PUFAs into cellular membranes replacing the ω-6-PUFA arachidonic acid (AA) with a consequent reduction of pro-inflammatory mediators (7).
In particular, ω-3-PUFAs produce a growth inhibitory effect in colon cancer cells and animal models (8). In clinical studies, dietary supplementation with ω-3-PUFAs significantly reduces crypt proliferation and increases mucosal apoptosis (9). ω-3-PUFAs include the essential fatty acid α-linolenic acid (ALA) and its metabolites eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). There is evidence that these fatty acids have specific effects on disease prevention, but most clinical trials are designed using fish oil-rich meals instead of ω-3-PUFAs supplementation and have failed to differentiate among them. Moreover, differences in dosage, combination, and formulation represent obstacles for reaching a definitive conclusion (10, 11). In addition, commercially available fish oils are supplied as ethyl esters which are up to 5-fold less bioavailable compared to free fatty acids (FFA; refs. 12, 13). Because the FFAs’ formulation does not require hydrolysis by pancreatic lipase, it is more efficiently absorbed, and is subsequently reconstituted into triglycerides in enterocytes. In an attempt to clarify the effectiveness of EPA as a single compound in colon cancer prevention, we evaluated the effects of substituting an innovative formulation of 99% (highly pure) EPA as the free fatty acid (EPA-FFA) for other dietary fats on the development of polyps in the gastrointestinal tract of ApcMin/+ mice, the most widely used animal model to study the chemopreventive potential of dietary nutrients.
Material and Methods
Animal treatments and tissue harvesting
This study was approved by the Institutional Animal Care and Use Committee at the Baylor Research Institute of Dallas. Five-week-old male ApcMin/+ mice on a C57BL/6J background and corresponding wild-type (wt) mice (n = 48) were obtained from The Jackson Laboratory and housed in a temperature and humidity controlled animal facility with a 12-hour light/dark cycle. To avoid gender-related differences during the study protocol, only male animals were used in this study. After 1 week, mice were evenly randomized in 6 groups to be fed the control diet (Ctrl) or diets enriched with EPA-FFA 2.5% or EPA-FFA 5%. The Ctrl diet was based on a modified AIN-93G diet (Research Diets,) where corn oil, which is ω-6-PUFAs predominant, was substituted for soybean oil that contains both ω-3- and ω-6-PUFAs (Supplementary Table 1). EPA-FFA diets were obtained from the Ctrl diet substituting highly purified (99%) EPA-FFA (ALFA, SLA Pharma AG) for corn oil. The 3 diets were isocaloric. To preserve EPA-FFA stability, the food was enriched with antioxidant vitamins, sealed and nitrogen flushed in foil bags, stored at 4°C and used within 3 days after opening. On the basis of animal strain requirements and previous experience, a fixed equal amount of food was provided daily. Mice were allowed to drink tap water ad libitum. Body weight was monitored weekly. After 12 weeks, mice were sacrificed by cervical dislocation under anesthesia with isofluorane (IsoFlo, Burns Vet Supply) and blood was collected by intracardiac puncture and immediately stored at −80°C. The entire gastrointestinal tract was immediately removed, washed with phosphate buffered saline (PBS), and divided into 5 segments [I–IV from proximal small intestine (I) to distal small intestine (IV), and colon]. Each segment was cut longitudinally and rinsed with PBS. Fresh tissue samples were collected and stored at −80°C. Segments were then flattened on filter paper and fixed overnight in 10% buffered formalin. The following day, segments were washed with PBS, and stained with 0.2% methylene blue (Sigma Aldrich) in PBS. The number, location, and size of visible tumors were determined at 10× magnification using an Olympus SZX-ILLB100 microscope by 2 independent and blinded investigators. Polyp size was determined by caliper measurement of the largest (Dim1) and the perpendicular diameter (Dim2). On the basis of Dim1, polyps throughout the intestinal tract were classified into 3 categories (<1 mm, 1–3 mm, >3 mm). Polyp area (A) was calculated according to the equation A = π (Dim1/2) (Dim2/2). Polyp load was expressed as the sum of all the polyp areas.
Histologic and immunohistochemical analysis
Small intestine and colon were Swiss-rolled, formalin-fixed, and paraffin-embedded. One slide for each specimen was stained with hematoxylin and eosin and evaluated independently by 2 blinded pathologists for histologic characterization of the polyps (low grade/high grade dysplasia). For immunohistochemistry (IHC), slides were dewaxed, rehydrated, and subjected to peroxidase inhibition and antigen retrieval with citrate buffer (pH = 6.0) at 98°C for 40 minutes. Before incubation with mouse monoclonal antibodies, samples were layered for 1 hour with goat anti-mouse IgG (Vector Laboratories; dilution 1:50). Slides were incubated with rabbit monoclonal anti-COX-2 (clone SP21, Thermo Scientific; dilution 1:150), mouse monoclonal anti-Ki-67 (clone MM1, Leica Biosystems Newcastle Ltd; dilution 1:1,400) and mouse monoclonal anti-β-catenin (clone 14, BD Transduction; dilution 1:2,500) and processed using the non–biotin-amplified complex (NovoLink Polymer Detection System, Leica Biosystems) according to the manufacturer’s procedure.
COX-2 was quantified according to positive tumor cell percentage and staining intensity (quick score, QS; ref. 14). The Ki-67 proliferation index was expressed as the ratio between positive nuclei and total number of nuclei per crypt analyzed on 8 and 15 full-length, well-orientated, longitudinal crypts from the small intestine and colon, respectively.
Apoptosis analysis
Apoptosis was evaluated with the DeadEnd Fluorimetric TUNEL System (Promega), according to the manufacturer’s recommendations.
Determination of lipid peroxidation
Lipid peroxidation was evaluated on blood samples by malondialdehyde (MDA; ref. 15). The concentrations were expressed as nanomoles of MDA per milliliter of serum.
Mucosal fatty acid analysis
Normal tissue samples were collected from the small intestine (I segment). Each mucosal fatty acid content was determined as described (16). Fatty acid levels are expressed as relative percentages of total fatty acids. Heptadecanoic acid (17:0) was used as internal standard.
Statistical analysis
Sample sizes were based on the average number of polyps and variances in the ApcMin/+ strain. We established that 8 mice in each group would be sufficient to detect a difference in polyp means among the 3 groups with a power of 85% and a minimal reduction of 15% (1-way ANOVA test). Relative weight gain was defined as: (weight at time t – weight at time t0)/weight at time t0.
A generalized linear mixed model was used to evaluate relative weight gain changes over time and among the 3 groups. ANOVA test was used to analyze continuous variables whereas chi-square and Fisher exact tests were applied for categorical variables. Correlation analysis was used to evaluate the relationship between variables. JMP version 8.02 and SAS version 9.2 were used for the statistical analysis. Significance was assigned at P < 0.05.
Results
EPA-FFA diets abrogate the effect of genotype on body weight and protect from lipid peroxidation
The relative weight gain profiles are shown in Supplementary Fig. 1 and Supplementary Table 2. Wild-type and ApcMin/+ mice displayed increasing profiles of relative weight gain when fed either EPA-FFA diets whereas the ApcMin/+ Ctrl arm exhibited a significant fall after 9 weeks (P < 0.0054), corresponding to the period when polyp appearance had significant health impact on the mutant mice. Moreover, we tested whether EPA-FFA supplementation, by altering the pool of total fats, would affect lipid peroxidation. As shown in Figure 1, as expected the highest level of MDA was registered among ApcMin/+ animals fed Ctrl diet. We found that EPA-FFA 5% significantly reduced lipid peroxidation compared to Ctrl diet in both wt and ApcMin/+ mice (P < 0.0001). A reduction was also observed for the EPA-FFA 2.5% arms, although it reached statistical significance only in the ApcMin/+ group (P < 0.0015).
Fig. 1.
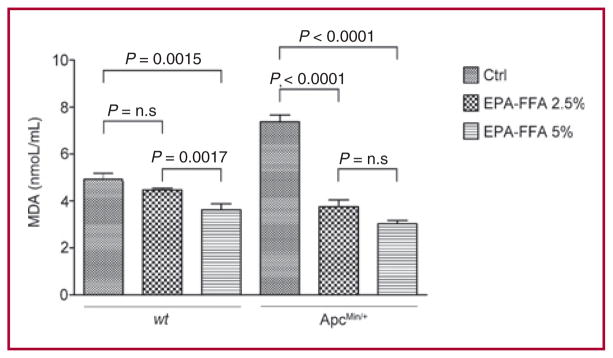
Effect of EPA-FFA dietary supplementation on lipid peroxidation. Significant protection from lipid peroxidation was observed among EPA-FFA 5% treated groups. Data are presented as mean ± SD.
EPA-FFA diets markedly suppress polyp number
We evaluated the effects of EPA-FFA on the development of polyps in the gastrointestinal tract of ApcMin/+ mice, which spontaneously develop multiple intestinal polyps within several weeks of birth (17–19). Our results showed that both EPA-FFA diets markedly suppressed polyp number (Table 1). Similar to previous reports (17, 18, 20), the ApcMin/+ Ctrl group displayed 38.63 ± 7.44 polyps per animal. Both the treated arms showed significantly fewer polyps per animal with a mean of 11.00 ± 2.14 and 8.25 ± 2.55 for the EPA-FFA 2.5% and EPA-FFA 5% respectively, corresponding to reductions of 71.5% (Ctrl vs. EPA-FFA 2.5%; P < 0.0001) and 78.6% (Ctrl vs. EPA-FFA 5%; P < 0.0001). Both EPA-FFA diets were equally effective in reducing the number of polyps (EPA-FFA 2.5% vs. EPA-FFA 5%, P = n.s.). A significant reduction in number of polyps was observed in each segment of the small intestine and colon in the animals fed either EPA-FFA 2.5% or EPA-FFA 5% (P < 0.0009). The overall distribution of polyps throughout the intestinal tract after EPA-FFA administration was proportional to the distribution in the ApcMin/+ mice receiving Ctrl diet (Table 1). All polyps found in the small intestines and colons were characterized by low-grade dysplasia apart from the colons of 2 mice fed the Ctrl diet that developed cancers.
Table 1.
Effect of EPA-FFA dietary supplementation at different concentrations on polyp number in ApcMin/+ mice (mean ± SD)
| Ctrl (n = 8) | EPA-FFA 2.5% (n = 8) | EPA-FFA 5% (n = 8) | |
|---|---|---|---|
| Small intestine | |||
| I Segment | 3.50 ± 0.53 | 1.38 ± 0.74 | 1.75 ± 1.28 |
| II Segment | 5.0 ± 1.2 | 2.13 ± 0.99 | 2.00 ± 0.76 |
| III Segment | 11.88 ± 4.19 | 3.25 ± 1.04 | 1.88 ± 1.13 |
| IV Segment | 17.00 ± 3.25 | 4.00 ± 1.51 | 2.00 ± 0.93 |
| Total small intestine | 37.38 ± 7.07 | 10.75 ± 2.43 | 7.63 ± 2.13 |
| Colon | 1.25 ± 1.49 | 0.25 ± 0.46 | 0.63 ± 0.74 |
| Small intestine + colon | 38.63 ± 7.44 | 11.00 ± 2.14 | 8.25 ± 2.55 |
| % Reduction | 71.5 | 78.6 | |
EPA-FFA significantly affects polyp load and size
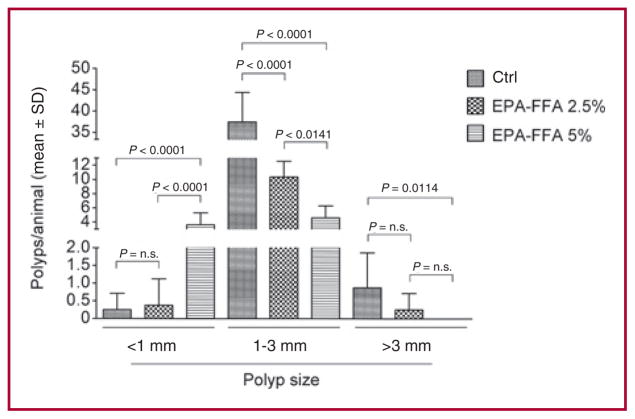
We also examined the effect of EPA-FFA treatment on polyp load and size. Polyp load (mm2) was 74.2 ± 29.3, 13.0 ± 2.7, and 4.9 ± 2.0 in the Ctrl, EPA-FFA 2.5% and EPA-FFA 5% arms, respectively (Ctrl vs. EPA-FFA 2.5%, P < 0.0001; Ctrl vs. EPA-FFA 5%, P < 0.0001; EPA-FFA 2.5% vs. EPA-FFA 5%, P = n.s.) corresponding to a reduction by 82.5% and 93.4% for the EPA-FFA 2.5% and EPA-FFA 5% compared to the Ctrl diet, respectively. As shown in Figure 2, polyps measuring less than 1 mm were significantly more common in the EPA-FFA 5% arm compared to the other groups, whereas polyps 1 to 3 mm in size were predominantly found in the Ctrl group. No polyps greater than 3 mm were found in the EPA-FFA 5% diet group.
Fig. 2.
Effect of EPA-FFA dietary supplementation on polyp size in the intestine of ApcMin/+ mice. The EPA-FFA 5% arm displayed more polyps measuring ≤ 1 mm compared to the other groups. Data are presented as mean ± SD.
Mucosal EPA replaces arachidonic acid after EPA-FFA treatment
The fatty acid composition of nonpolypoid small intestinal tissue was evaluated in ApcMin/+ mice (Table 2). The most representative fatty acids were analyzed, from 16:0 to 22:6 (palmitic acid to DHA). Mucosal EPA content increased significantly in the EPA-FFA 2.5% and EPA-FFA 5% arms (P < 0.0001), in which the amount of EPA was approximately one fourth to one fifth of the total mucosal fatty acid content. Interestingly, the highest EPA incorporation was detected in the EPA-FFA 2.5% group, reaching statistical significance compared to EPA-FFA 5% (P = 0.045). Contrariwise, the percentage of AA present in the cellular membranes was dramatically reduced from 11.7 ± 2.6 in the Ctrl arm to 1.38 ± 0.23 and 1.62 ± 0.24 in the EPA-FFA 2.5% and EPA-FFA 5% groups, respectively (Ctrl vs. EPA-FFA 2.5%, P < 0.0001; Ctrl vs. EPA-FFA 5%, P < 0.0001). A significant negative correlation was found between AA or its precursor (linoleic acid) and EPA or its metabolites (DPA, docosapentaenoic acid, and DHA) suggesting that the pro-inflammatory ω-6-PUFAs were displaced by EPA-FFA (Supplementary Table 3).
Table 2.
Effect of EPA-FFA dietary supplementation at different concentrations on mucosal fatty acid content (mean ± SD)
| Arm | Fatty acid
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| % Palmitic | % Stearic | % Oleic | % Linoleic | % Linolenic | % AA | % EPA | % DPA | % DHA | |
| Ctrl (n = 8) | 21.9 ± 2.6 | 24.1 ± 3.2 | 14.6 ± 4.1 | 24.9 ± 2.1 | 0.14 ± 0.13 | 11.7 ± 2.6 | 0.42 ± 0.32 | 0.11 ± 0.06 | 1.72 ± 0.30 |
| EPA-FFA 2.5% (n = 8) | 19.7 ± 4.4 | 22.39 ± 2.95 | 9.19 ± 5.80 | 17.4 ± 1.7 | 0.16 ± 0.07 | 1.38 ± 0.23 | 24.2 ± 4.9 | 2.40 ± 0.29 | 2.70 ± 0.49 |
| EPA-FFA 5% (n = 8) | 19.2 ± 3.1 | 17.2 ± 2.9 | 12.6 ± 2.8 | 24.4 ± 2.5 | 0.14 ± 0.13 | 1.62 ± 0.24 | 20.5 ± 3.4 | 1.95 ± 0.35 | 2.02 ± 0.25 |
EPA-FFA decreases COX-2 expression and β-catenin nuclear translocation
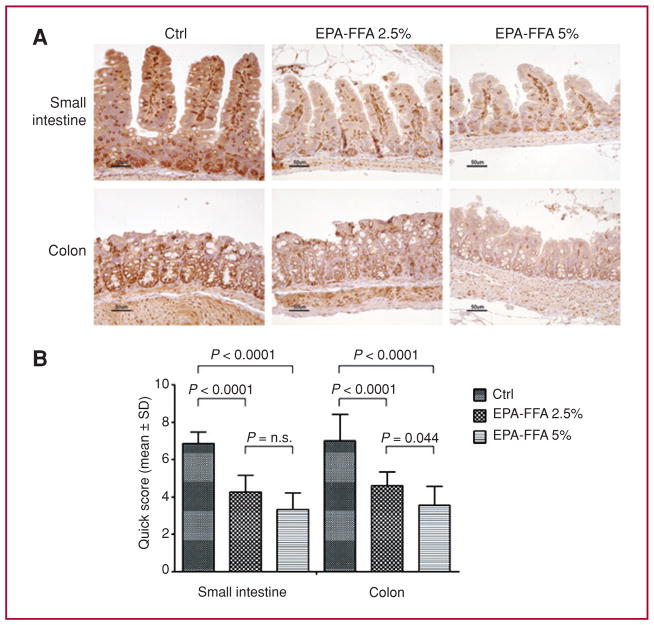
On the basis of the widely accepted concept that ω-3-PUFAs can modulate COX-2 expression, we evaluated the impact of EPA-FFA supplementation on COX-2 and β-catenin expression in the ApcMin/+ mice using IHC (Figs. 3 and Supplementary Fig. 2). Compared to the Ctrl diet, in both the small intestine and colon, the COX-2 QS was markedly reduced by both EPA-FFA 2.5% and EPA-FFA 5%, with no differences between EPA-FFA diets in the small intestine, and a greater effect in the EPA-FFA 5% arm in the colon (EPA-FFA 2.5% vs. EPA-FFA 5%, P = 0.04). Interestingly, in each arm there were no differences in COX-2 QS values between colon and small intestine suggesting that COX-2 inhibition occurs equally in both small and large intestine. Furthermore, nuclear β-catenin translocation was frequently found in the dysplastic areas of the small intestine and colon of the ApcMin/+ animals fed the Ctrl diet, with a reduction of positive nuclei in both EPA-FFA–treated mice (Supplementary Fig. 2). Interestingly, no positive nuclei were found in the colons of any treated animals.
Fig. 3.
EPA-FFA reduces COX-2 expression. A, representative COX-2 IHC in tissues from the small intestine and colon of ApcMin/+ mice. B, quantification of COX-2 staining. A significant reduction in COX-2 expression was observed in the small intestine and colon of EPA-FFA–treated animals. Data are presented as mean ± SD.
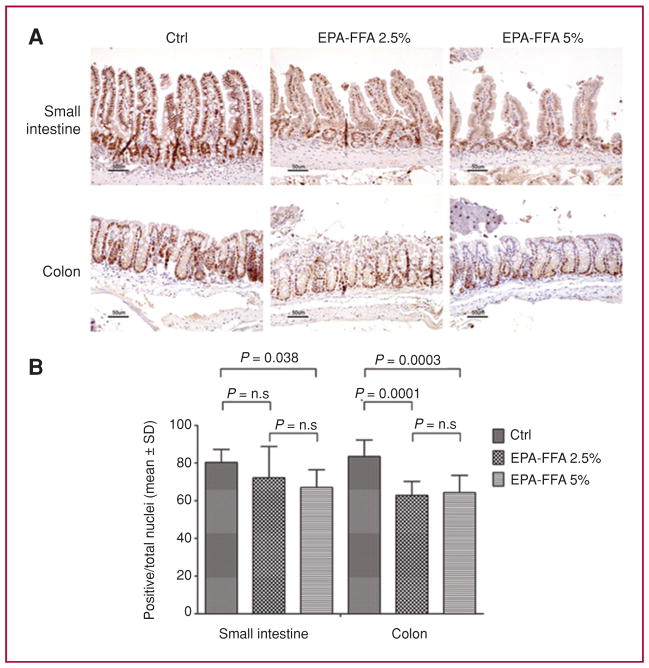
EPA-FFA inhibits cellular proliferation and increases apoptosis
We evaluated cellular proliferation in both the small intestines and colons of ApcMin/+ mice (Fig. 4). In the small intestine, we found a decrease in the Ki-67 proliferation index in both EPA-FFA–treated arms, reaching statistical significance only with EPA-FFA supplementation at the highest concentration (Ctrl vs. EPA-FFA 5%; P = 0.038). In the colon, both EPA-FFA diets significantly inhibited cellular proliferation (Ctrl vs. EPA-FFA 2.5%, P = 0.0001; Ctrl vs. EPA-FFA 5%, P = 0.0003). Finally, an increase in apoptosis was observed among the EPA-FFA–treated animals in both the small intestines and colons of ApcMin/+ mice (Supplementary Fig. 3).
Fig. 4.
EPA-FFA inhibits cellular proliferation. A, representative Ki-67 IHC in tissues from small intestines and colons of ApcMin/+ mice. B, quantification of Ki-67 staining. While a significant reduction of proliferation was observed in the small intestine and colon of animals fed EPA-FFA 5%, the effect of EPA-FFA 2.5% was restricted to the colon. Data are presented as mean ± SD.
Discussion
In this study, we tested the efficacy of an innovative formulation of EPA—99% (highly) purified and supplied as the free fatty acid (EPA-FFA)—and showed that it strongly suppressed polyp number (by 71.5% and 78.6% in EPA-FFA 2.5% and 5% groups compared to the Ctrl) and polyp load (by 82.5% and 93.4% in EPA-FFA 2.5% and 5% groups compared to the Ctrl) in ApcMin/+ mice. In addition, EPA-FFA significantly affected polyp size in a concentration-dependent manner. This was also reflected by protecting against weight loss associated with the mutant genotype. These effects were associated with a suppression of COX-2 expression and reduction of β-catenin nuclear translocation and cellular proliferation, together with an increase in apoptosis. Importantly, the decreased proliferation and enhanced apoptosis found in both small intestines and colons of ApcMin/+ mice fed EPA-FFA 5% could explain why smaller polyps were present in this group. In vitro studies have identified many ‘ideal’ agents for CRC chemo-prevention, which then failed when tested in more complex systems (2, 21, 22). The ApcMin/+ mouse, a model of FAP, is widely used to evaluate the chemopreventive potential of dietary nutrients and chemotherapeutic agents before being tested in clinical settings. Many compounds are effective in reducing polyps in this model (17–19, 23). In particular, nonsteroidal anti-inflammatory drugs (NSAIDs) have significant tumor suppressive activity in animal models, with reductions ranging from 64% to 90% with sulindac and celecoxib (21). In clinical settings, anti-COX-2 drugs, extensively tested in secondary prevention studies, are highly effective; although their long-term administration is associated with a significant increased risk of cardiovascular toxicity (3–5, 24). Many micronutrients such as polyphenols or vitamins, have been identified as possible chemo-preventive agents; although the magnitude of the effects is smaller when compared with synthetic compounds, and the results in the clinical trials have been controversial (21). Importantly, caloric intake, as well as the quality and the relative percentage of macronutrients, are involved in the pathogenesis of CRC. Specifically, the quantity (total fats), quality (animal or vegetable), and type (saturated, monosaturated or polyunsaturated) of fats have been shown to influence polyp formation in ApcMin/+ mice (25).
In the present study, using EPA as the FFA (which is completely absorbable) at the highest purity ever tested, we observed a dramatic suppression of polyp number and polyp burden. Moreover, the EPA-FFA preparation significantly affected polyp size in a concentration-dependent manner. The magnitude of these results suggests that EPA-FFA are as effective as NSAIDs as preventive agents and may be more potent than other nutraceuticals. However on the basis of the design of the study, with the drug administered starting from week 6, we cannot distinguish whether the effects were on suppression of formation or regression of existing polyps.
In the last few decades, there has been an ongoing interest in the role of ω-3-PUFAs in CRC prevention. The antineo-plastic mechanism of ω-3-PUFAs, including EPA, is not fully understood (7). One hypothesis is that EPA incorporates into the cellular membranes competing with the ω-6-PUFA AA as the substrate for COX and lipoxygenases (LO). AA and ω-3-PUFAs are metabolized by COX into prostanoids and by LO to leukotrienes respectively, resulting in opposite effects on tumor growth, apoptosis, angiogenesis, and inflammation (26–28). In addition, ω-3-PUFAs can also be converted into potent anti-inflammatory mediators such as resolvins and protectins (29, 30). It is reasonable to speculate that the dietary balance between ω-3- and ω-6-PUFAs would impact the carcinogenic process.
In the diets of the present study, EPA-FFA (ω-3-PUFA) was substituted for corn oil (ω-6-PUFAs) in the Ctrl diet. Dietary modification in the ω-3-/ω-6-PUFAs ratio was reflected by changes in the phospholipidic composition of the intestinal mucosa. Mucosal AA was replaced by EPA in the ApcMin/+ mice, demonstrating that the EPA-FFA preparation was efficiently incorporated into cellular membranes. To the best of our knowledge, the levels of EPA incorporation measured in the present study are the highest ever reported in animals or humans. Unexpectedly, the EPA-FFA 2.5% group showed a slightly higher EPA incorporation than the EPA-FFA 5% group. As our data suggest that the biological findings are dose dependent, we speculate that the EPA-FFA 2.5% diet may have reached a steady-state level of incorporation in this specific system, but that the 5% diet accumulation might continue in other tissues in which cell turnover is slower (e.g., red blood cells, adipocytes, etc). In CRC, overexpression of COX-2 results in an increased production of PGE2, affecting cell proliferation, apoptosis, migration, and other signaling pathways including NF-kB (31) and Wnt. In particular, Castellone and colleagues identified a pivotal role for crosstalk between the COX-2 and β-catenin pathways (32). In our study, we found AA replacement by EPA in the cellular membranes, and, as a possible consequence, we observed a strong decrease in COX-2 expression accompanied by a decrease in nuclear β-catenin translocation. In addition, a significant inhibition of cellular proliferation and an increase in apoptosis were found. Although this study lacks functional data on COX-2 enzyme activity, our results suggest that the macroscopic effects of EPA-FFA on ApcMin/+ tumorigenesis could be explained by modulation of the 2 major pathways involved in CRC development: COX-2 and β-catenin/Wnt signaling.
In the present study, mutant and wt treated mice displayed comparable weight gain profiles, whereas mice receiving the Ctrl diet showed cachexia or excess in weight gain, respectively. Our data show that EPA-FFA treatment efficiently prevents weight loss in ApcMin/+ mice, abrogating the effect of the genotype. On the contrary, in the wt arms, EPA-FFA treatment contributed to weight gain control. In fact, it has been reported that ω-3-PUFA treatment can modulate adipose tissue function, preventing hyperplasia and hypertrophy, and controlling adipose tissue inflammation (33).
ω-3-PUFAs consumption is safe and well tolerated (34). However, in long-term treatment, some concerns have been raised that higher doses of ω-3-PUFAs would increase lipid peroxidation, which could be controlled by concomitant antioxidant supplementation (7). Importantly, we found significantly decreased lipid peroxidation in treated animals in a dose-dependent manner, which was also found in wt animals. Furthermore, to balance for possible differences in oxidative stress, diets also included an equal amount of vitamin mix (10 gm of mix per kg of diet) containing substantial amounts of antioxidants vitamins, including vitamin E (75 IU).
Previous studies have demonstrated a protective role for ω-3-PUFAs as ethyl esters in ApcMin/+ mice, and reported a 40% to 50% reduction in tumor load (35, 36). A comparable amount of EPA-FFA produces a stronger effect, which makes the highly purified EPA-FFA preparation similar in potency to the most powerful pharmacologic agents. It is likely that the impact of these lipids is influenced by the type (EPA vs. DHA), form (FFA vs. ethyl ester), and purity of the ω-3-PUFAs used.
There is evidence that individual ω-3-PUFAs may have specific and independent effects (10). In ApcMin/+ mice, Petrik et al. reported tumor suppression of 48% in EPA-fed mice, whereas weaker effects were obtained with DHA (36). Moreover, better absorption was reported for EPA over DHA (13). Overall, most clinical trials report a strong and consistent association between EPA intake and reduced risk for colorectal neoplasia, whereas the evidence related to DHA alone has been unconvincing (11).
Commercially available fish oils are mainly supplied as ethyl esters because they are less expensive, more malleable, and conveniently processed for distribution. Conflicting reports have been published on bioavailability of fatty acid ethyl esters. Some reports suggest that EPA supplied as ethyl ester is ineffective compared to free acid or trygliceride (37, 38), whereas others found that ethyl ester and triglyceride are equipotent in humans (13, 39–42) In our study, fish oil supplementation was given as FFAs, which are efficiently absorbed and reconstituted into triglycerides by enterocytes, and do not require hydrolysis by pancreatic lipase. The magnitude of this effect is considerably greater than previous reports using ω-3-PUFA supplementation. We believe that this difference could be explained by the characteristics of the FFA formulation. Although this is the first study using EPA-FFA in animal models, this particular formulation was recently tested in patients who underwent colectomy and ileorectal anastomosis for FAP, and a significant decrease in polyp number and size in the rectum was observed among those who received EPA-FFA for 6 months compared to matched controls (16). Finally, the same formulation has been tested in a clinical trial reporting a greater reduction in cellular proliferation and increase in apoptosis in the normal mucosa of patients with colorectal adenomas than had been seen in an equivalent trial in which ω-3-PUFAs were administered as ethyl esters (9, 43). In conclusion, EPA-FFA is an attractive candidate to reduce cancer risk in an effective, safe, and inexpensive way. More importantly, this intervention has efficacy that resembles what has been obtained with NSAIDs and coxibs, but without the attendant toxicity. The important findings of both the clinical trial and the present study strongly suggest that EPA-FFA could be a successful candidate as a chemopreventive agent in FAP mutation carriers. Also, it is reasonable to speculate that this approach could be considered in relation to sporadic colorectal prevention/recurrence in humans.
Supplementary Material
Translational Relevance.
Today, cyclooxygenase (COX)-2 inhibitors could be the best chemopreventive agents against colorectal cancer, but their cardiovascular side effects outweigh their benefits. A new formulation of highly purified EPA supplied as free fatty acid (EPA-FFA), was administered to ApcMin/+ mice, leading to a dramatic suppression of polyp number and growth. The magnitude of the results is comparable to those obtained with COX-2 inhibitors and better than most nutraceuticals tested in the same animal model. The marked effect of EPA-FFA on polyp development, in the absence of toxicity, makes EPA-FFA an excellent candidate for both CRC chemoprevention and treatment and possibly could be considered for sporadic colorectal prevention/recurrence in humans.
Acknowledgments
We thank Prof. Mark A. Hull for the critical review of the manuscript. The authors had total control over the data and its interpretation, and SLA Pharma AG had no input in the formulation or writing of the manuscript.
Grant Support
SLA Pharma AG.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
Dr. Ricciardiello received a research grant by SLA Pharma AG.
Authors’ Contributions: L. Fini and G. Piazzi—acquisition, analysis, and interpretation of the data, drafting of the manuscript; C. Ceccarelli, G. Graziani, V. Fogliano, and M. Selgrad—analysis and interpretation of the data, and technical support; Y. Daoud—statistical analysis, analysis and interpretation of the data; A. Belluzzi—acquisition of the data, critical revision of the manuscript for important intellectual content; A. Munarini—technical and material support; M. Garcia—technical and material support; A. Gasbarrini and R.M. Genta–analysis and interpretation of the data; C.R. Boland—analysis and interpretation of the data, critical revision of the manuscript for important intellectual content, and obtained funding; and L. Ricciardiello—study concept and design, analysis and interpretation of data, drafting of the manuscript, obtained funding, and study supervision.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Grau MV, Rees JR, Baron JA. Chemoprevention in gastrointestinal cancers: current status. Basic Clin Pharmacol Toxicol. 2006;98:281–7. doi: 10.1111/j.1742-7843.2006.pto_294.x. [DOI] [PubMed] [Google Scholar]
- 3.Baron JA, Sandler RS, Bresalier RS, et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology. 2006;131:1674–82. doi: 10.1053/j.gastro.2006.08.079. [DOI] [PubMed] [Google Scholar]
- 4.Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–84. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 5.Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 6.Tavani A, Pelucchi C, Parpinel M, et al. n-3 polyunsaturated fatty acid intake and cancer risk in Italy and Switzerland. Int J Cancer. 2003;105:113–6. doi: 10.1002/ijc.11018. [DOI] [PubMed] [Google Scholar]
- 7.Dupertuis YM, Meguid MM, Pichard C. Colon cancer therapy: new perspectives of nutritional manipulations using polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care. 2007;10:427–32. doi: 10.1097/MCO.0b013e3281e2c9d4. [DOI] [PubMed] [Google Scholar]
- 8.Chapkin RS, Seo J, McMurray DN, Lupton JR. Mechanisms by which docosahexaenoic acid and related fatty acids reduce colon cancer risk and inflammatory disorders of the intestine. Chem Phys Lipids. 2008;153:14–23. doi: 10.1016/j.chemphyslip.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Courtney ED, Matthews S, Finlayson C, et al. Eicosapentaenoic acid (EPA) reduces crypt cell proliferation and increases apoptosis in normal colonic mucosa in subjects with a history of colorectal adenomas. Int J Colorectal Dis. 2007;22:765–76. doi: 10.1007/s00384-006-0240-4. [DOI] [PubMed] [Google Scholar]
- 10.Anderson BM, Ma DW. Are all n-3 polyunsaturated fatty acids created equal? Lipids Health Dis. 2009;8:33. doi: 10.1186/1476-511X-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nkondjock A, Shatenstein B, Maisonneuve P, Ghadirian P. Specific fatty acids and human colorectal cancer: an overview. Cancer Detect Prev. 2003;27:55–66. doi: 10.1016/s0361-090x(02)00179-4. [DOI] [PubMed] [Google Scholar]
- 12.Gudbrandsen OA, Wergedahl H, Bohov P, Berge RK. The absorption, distribution and biological effects of a modified fatty acid in its free form and as an ethyl ester in rats. Chem Biol Interact. 2009;179:227–32. doi: 10.1016/j.cbi.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Lawson LD, Hughes BG. Human absorption of fish oil fatty acids as triacylglycerols, free acids, or ethyl esters. Biochem Biophys Res Commun. 1988;152:328–35. doi: 10.1016/s0006-291x(88)80718-6. [DOI] [PubMed] [Google Scholar]
- 14.Ceccarelli C, Piazzi G, Paterini P, et al. Concurrent EGFr and Cox-2 expression in colorectal cancer: proliferation impact and tumour spreading. Ann Oncol. 2005;16(Suppl 4):iv74–9. doi: 10.1093/annonc/mdi912. [DOI] [PubMed] [Google Scholar]
- 15.D’Argenio G, Mazzone G, Tuccillo C, et al. Apple polyphenol extracts prevent aspirin-induced damage to the rat gastric mucosa. Br J Nutr. 2008;100:1228–36. doi: 10.1017/S0007114508988747. [DOI] [PubMed] [Google Scholar]
- 16.West NJ, Clark SK, Phillips RK, et al. Eicosapentaenoic acid reduces rectal polyp number and size in familial adenomatous polyposis. Gut. 2010;59:918–25. doi: 10.1136/gut.2009.200642. [DOI] [PubMed] [Google Scholar]
- 17.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–4. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 18.Shoemaker AR, Gould KA, Luongo C, Moser AR, Dove WF. Studies of neoplasia in the Min mouse. Biochim Biophys Acta. 1997;1332:F25–48. doi: 10.1016/s0304-419x(96)00041-8. [DOI] [PubMed] [Google Scholar]
- 19.Yamada Y, Mori H. Multistep carcinogenesis of the colon in Apc(Min/+) mouse. Cancer Sci. 2007;98:6–10. doi: 10.1111/j.1349-7006.2006.00348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCart AE, Vickaryous NK, Silver A. Apc mice: models, modifiers and mutants. Pathol Res Pract. 2008;204:479–90. doi: 10.1016/j.prp.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Half E, Arber N. Colon cancer: preventive agents and the present status of chemoprevention. Expert Opin Pharmacother. 2009;10:211–9. doi: 10.1517/14656560802560153. [DOI] [PubMed] [Google Scholar]
- 22.Marshall JR. Prevention of colorectal cancer: diet, chemoprevention, and lifestyle. Gastroenterol Clin North Am. 2008;37:73–82. vi. doi: 10.1016/j.gtc.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corpet DE, Pierre F. Point: from animal models to prevention of colon cancer. Systematic review of chemoprevention in min mice and choice of the model system. Cancer Epidemiol Biomarkers Prev. 2003;12:391–400. [PMC free article] [PubMed] [Google Scholar]
- 24.Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–52. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 25.Escrich E, Solanas M, Moral R, Costa I, Grau L. Are the olive oil and other dietary lipids related to cancer? Experimental evidence. Clin Transl Oncol. 2006;8:868–83. doi: 10.1007/s12094-006-0150-5. [DOI] [PubMed] [Google Scholar]
- 26.Berquin IM, Edwards IJ, Chen YQ. Multi-targeted therapy of cancer by omega-3 fatty acids. Cancer Lett. 2008;269:363–77. doi: 10.1016/j.canlet.2008.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calviello G, Serini S, Piccioni E. n-3 polyunsaturated fatty acids and the prevention of colorectal cancer: molecular mechanisms involved. Curr Med Chem. 2007;14:3059–69. doi: 10.2174/092986707782793934. [DOI] [PubMed] [Google Scholar]
- 28.Chapkin RS, McMurray DN, Davidson LA, Patil BS, Fan YY, Lupton JR. Bioactive dietary long-chain fatty acids: emerging mechanisms of action. Br J Nutr. 2008;100:1152–7. doi: 10.1017/S0007114508992576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez-Periz A, Horrillo R, Ferre N, et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. 2009;23:1946–57. doi: 10.1096/fj.08-125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohli P, Levy BD. Resolvins and protectins: mediating solutions to inflammation. Br J Pharmacol. 2009;158:960–71. doi: 10.1111/j.1476-5381.2009.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tammali R, Ramana KV, Singhal SS, Awasthi S, Srivastava SK. Aldose reductase regulates growth factor-induced cyclooxygenase-2 expression and prostaglandin E2 production in human colon cancer cells. Cancer Res. 2006;66:9705–13. doi: 10.1158/0008-5472.CAN-06-2105. [DOI] [PubMed] [Google Scholar]
- 32.Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504–10. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 33.Kopecky J, Rossmeisl M, Flachs P, et al. n-3 PUFA: bioavailability and modulation of adipose tissue function. Proc Nutr Soc. 2009:1–9. doi: 10.1017/S0029665109990231. [DOI] [PubMed] [Google Scholar]
- 34.Pellizzon M, Buison A, Ordiz F, Jr, Santa AL, Jen KL. Effects of dietary fatty acids and exercise on body-weight regulation and metabolism in rats. Obes Res. 2002;10:947–55. doi: 10.1038/oby.2002.129. [DOI] [PubMed] [Google Scholar]
- 35.Paulsen JE, Elvsaas IK, Steffensen IL, Alexander J. A fish oil derived concentrate enriched in eicosapentaenoic and docosahexaenoic acid as ethyl ester suppresses the formation and growth of intestinal polyps in the Min mouse. Carcinogenesis. 1997;18:1905–10. doi: 10.1093/carcin/18.10.1905. [DOI] [PubMed] [Google Scholar]
- 36.Petrik MB, McEntee MF, Johnson BT, Obukowicz MG, Whelan J. Highly unsaturated (n-3) fatty acids, but not alpha-linolenic, conjugated linoleic or gamma-linolenic acids, reduce tumorigenesis in Apc (Min/+) mice. J Nutr. 2000;130:2434–43. doi: 10.1093/jn/130.10.2434. [DOI] [PubMed] [Google Scholar]
- 37.Hong DD, Takahashi Y, Kushiro M, Ide T. Divergent effects of eicosapentaenoic and docosahexaenoic acid ethyl esters, and fish oil on hepatic fatty acid oxidation in the rat. Biochim Biophys Acta. 2003;1635:29–36. doi: 10.1016/j.bbalip.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Hudson EA, Tisdale MJ. Comparison of the effectiveness of eicosapentaenoic acid administered as either the free acid or ethyl ester as an anticachectic and antitumour agent. Prostaglandins Leukot Essent Fatty Acids. 1994;51:141–5. doi: 10.1016/0952-3278(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 39.Boustani S, Colette C, Monnier L, Descomps B, Crastes de PA, Mendy F. Enteral absorption in man of eicosapentaenoic acid in different chemical forms. Lipids. 1987;22:711–4. doi: 10.1007/BF02533970. [DOI] [PubMed] [Google Scholar]
- 40.Hamazaki T, Urakaze M, Makuta M, et al. Intake of different eicosapentaenoic acid-containing lipids and fatty acid pattern of plasma lipids in the rats. Lipids. 1987;22:994–8. doi: 10.1007/BF02536438. [DOI] [PubMed] [Google Scholar]
- 41.Krokan HE, Bjerve KS, Mork E. The enteral bioavailability of eicosapentaenoic acid and docosahexaenoic acid is as good from ethyl esters as from glyceryl esters in spite of lower hydrolytic rates by pancreatic lipase in vitro. Biochim Biophys Acta. 1993;1168:59–67. doi: 10.1016/0005-2760(93)90266-c. [DOI] [PubMed] [Google Scholar]
- 42.Lawson LD, Hughes BG. Absorption of eicosapentaenoic acid and docosahexaenoic acid from fish oil triacylglycerols or fish oil ethyl esters co-ingested with a high-fat meal. Biochem Biophys Res Commun. 1988;156:960–3. doi: 10.1016/s0006-291x(88)80937-9. [DOI] [PubMed] [Google Scholar]
- 43.Cheng J, Ogawa K, Kuriki K, et al. Increased intake of n-3 polyunsaturated fatty acids elevates the level of apoptosis in the normal sigmoid colon of patients polypectomized for adenomas/tumors. Cancer Lett. 2003;193:17–24. doi: 10.1016/s0304383502007176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.