Abstract
Human immunodeficiency virus type 1 (HIV-1) entry into host cells is mediated by the trimeric envelope glycoprotein complex (Env). Accordingly, the Env proteins are the targets for neutralizing antibodies (NAbs) and are the focus of vaccines intended to induce NAbs. Because the Env complex is labile, soluble recombinant Env (gp140) trimers require engineering to stabilize them sufficiently for use as immunogens. Trimeric forms of gp140 trimers can be created that are either cleavage-competent or cleavage-defective at the junction between the gp120 and gp41 subunits. As functional trimers are cleaved at this site, the question arises as to whether cleavage affects the antigenic structure of the Env complex in a way that is relevant to vaccine design. Here, we present a comparative analysis of the antigenicity profiles of cleaved and uncleaved gp140 trimers derived from the KNH1144 (subtype A) virus that are otherwise closely sequence-matched. While cleavage did not affect the exposure of NAb epitopes on the gp140 trimers, non-neutralizing antibodies to gp41 epitopes bound much more strongly to uncleaved trimers. Hence cleavage does alter the structure of the HIV-1 Env complex.
Keywords: HIV-1, Env, gp140 trimers, cleavage
INTRODUCTION
Various different forms of human immunodeficiency virus type 1 (HIV-1) Env glycoproteins have been expressed as recombinant proteins for vaccine and/or structural studies over the past 25 years. The Env configurations most commonly studied in recent years have been oligomeric gp140s that contain the gp120 surface glycoprotein and the ectodomain of the gp41 transmembrane glycoprotein; the constructs are truncated immediately before the membrane-spanning domain of gp41, to allow expression of the gp140s as soluble proteins. The secreted gp140s are heterogeneous in that multiple molecular forms can be present, including gp120-gp41 monomers, dimers, trimers, tetramers and higher molecular weight aggregates, the proportions of which vary with the HIV-1 genotype, the nature of the mutations introduced to create the gp140 and probably even the cell type used for protein expression (Binley et al., 2000; Jeffs et al., 2004; Schulke et al., 2002). In general, the goal of expressing a gp140 protein is to make trimers that mimic, as closely as possible, the native configuration of Env as it exists on infectious viruses. Hence a trimer-enriched fraction needs to be purified from the mixture of Env forms that are normally present in gp140 preparations.
The native Env trimer is unstable, because the inter-subunit interactions (gp120-gp41 and gp41-gp41) are non-covalent and weak, a situation that reflects the need for Env to undergo multiple, complex conformational changes during fusion of HIV-1 with its target cells. This instability complicates gp140 expression; the desired trimers tend to dissociate, or in some cases aggregate, rapidly into non-native Env forms (Jeffs et al., 2004). The latter interfere with structural studies and they are unlikely to be immunogens that are capable of inducing broadly neutralizing antibodies at high titers. Hence strategies must be adopted to increase trimer stability. The most common way to do this has been to mutate the cleavage site between gp120 and gp41 to prevent processing by furin-like cellular endoproteases. This device stabilizes the gp120 and gp41 interaction, which remains covalent. The introduction of heterologous trimerization domains at the gp140 C-terminus can help stabilize uncleaved gp140s in a trimeric configuration (Yang et al., 2002; Yang et al., 2000). An alternative way to maintain gp120-gp41 association is the introduction of an intermolecular disulfide bond (SOS) between the two subunits (Binley et al., 2000); together with point substitutions within gp41 (Sanders et al., 2002), this strategy allows the expression and purification of fairly stable, fully cleaved gp140 trimers.
There has been an increasing appreciation that cleaved and uncleaved forms of gp140 trimer are antigenically different. In other words, whether an Env trimer is cleaved or not appears to affect the antibody epitopes it expresses. However, the studies from which these conclusions are drawn are complicated by the presence of non-trimer forms of Env, either on the cell surface or present within unfractionated soluble gp140 preparations. Hence it is not always clear what Env forms are being bound by test MAbs. Moreover, structural studies show that cleavage has only a minimal effect on the subunit topology of other RNA virus fusion glycoproteins (e.g., influenza HA (Stevens et al., 2004), Sindbis virus (Smit et al., 2001)). In the absence of a crystal structure of HIV-1 Env trimers, the precise effects of cleavage on gp120-gp41 structure are unknown. However, to gain more understanding of what cleavage might do, we have now studied epitope exposure on purified, cleaved and uncleaved forms of gp140 from the KNH1144 strain of HIV-1 that differ only at the cleavage site between gp120 and gp41. We conclude that cleavage does indeed affect the topology of gp140 trimers, in that various non-neutralizing MAbs bind to the gp41 moieties of uncleaved but not cleaved trimers. Structural studies may eventually enable these observations to be understood at a molecular level.
RESULTS
Expression and stability of cleaved and uncleaved gp140s from KNH1144
SOSIP gp140 proteins from the KNH1144 subtype A strain lack the consensus epitopes for NAbs 2G12, 2F5 and 4E10. We therefore recreated these epitopes to make cleaved (KNH1144 SOSIP.R6 G-MPER) and uncleaved (KNH1144 SOSIP.IEGR G-MPER) gp140 variants that were otherwise identical in sequence (see Fig. 1A and Methods). MAbs 2G12, 2F5 and 4E10 all bound strongly to the cleaved gp140 (Fig.1B) and also to its uncleaved counterpart (data not shown). These two epitope-modified gp140 proteins were used in all the MAb-binding studies described below.
Fig. 1.
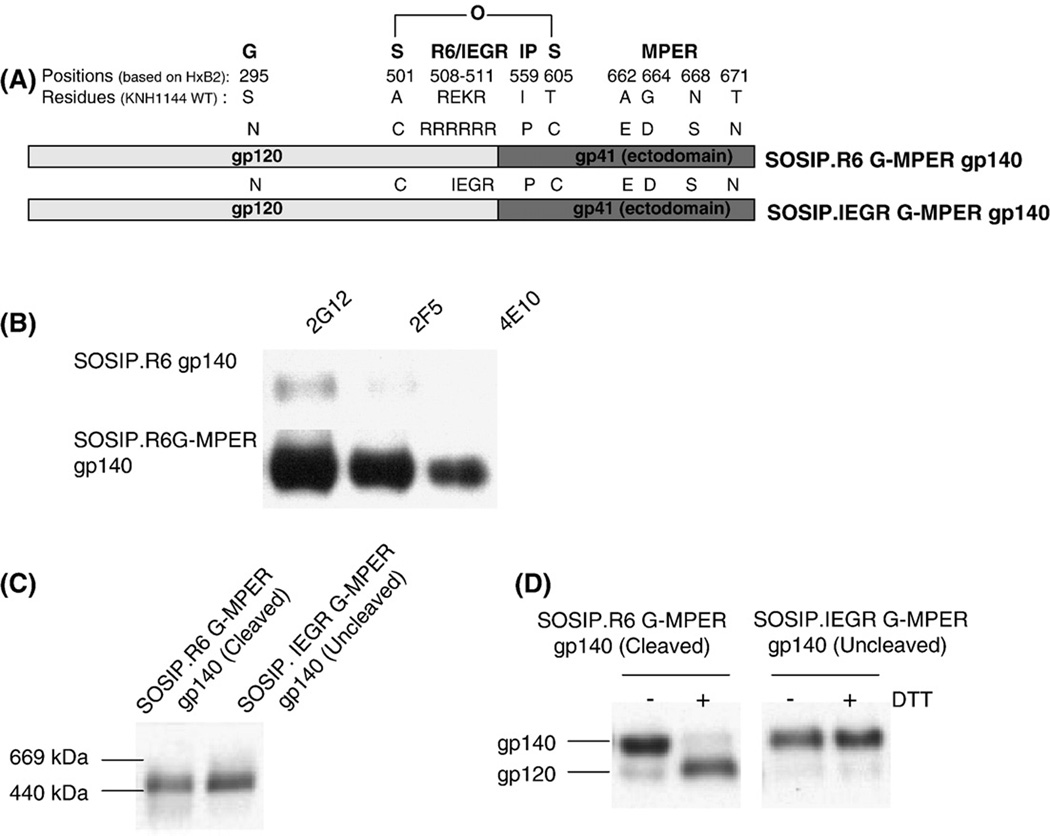
Expression and analysis of soluble, cleaved (SOSIP.R6 G-MPER) and uncleaved (SOSIP.IEGR G-MPER) gp140s from HIV-1 KNH1144. (A) Schematic representation of the various mutations made in the KNH1144 primary sequence to generate a stabilized soluble trimeric gp140 (SOS, IP), to promote (R6) or disrupt (IEGR) cleavage, to introduce the 2G12 epitope (S295N), and to improve 2F5 and 4E10 binding (MPER - A662E, G664D, N668S and T671N). (B) The epitopes for MAbs 2G12, 2F5 and 4E10 were introduced into the KNH1144 SOSIP.R6 G-MPER gp140 sequence by site-directed mutagenesis, to create the SOSIP.R6 G-MPER variant. Binding of these MAbs to the two gp140s was determined by immunoprecipitation. (C) Unpurified cell culture supernatants containing the same cleaved or uncleaved gp140s were analyzed by BN-PAGE and western blotting with MAb CA13 (ARP3119) that binds to the C1 region of gp120. The migration positions of relevant molecular weight standards are marked on the left of the gel. Both the cleaved and uncleaved gp140s migrate as trimers. (D) The same cleaved (lanes 1 and 2) and uncleaved (lanes 3 and 4) gp140s were analyzed by SDS-PAGE in the presence or absence of the reducing agent, DTT, as indicated, followed by western blotting. The SOSIP.R6 G-MPER gp140 is fully cleaved and hence dissociates to gp120 in the presence of DTT (lane 2), whereas the SOSIP.IEGR G-MPER protein is not cleaved (lane 4).
Both the cleaved and uncleaved KNH1144 gp140s were predominantly trimeric after expression in HEK293T cells, as assessed on a non-denaturing, gradient polyacrylamide gel (Fig.1C). Under denaturing conditions, the SOSIP.R6 G-MPER gp140 was >95% cleaved, in that it dissociated to gp120 in the presence of DTT (Fig.1D; the gp41 band is not shown). In contrast, the SOSIP.IEGR G-MPER gp140 was, as intended, completely uncleaved and thereby remained as a gp140 when DTT was present (Fig.1D). Hence the two constructs are suitable templates for further studies of the effect of cleavage on trimer stability and configuration.
Purification of cleaved and uncleaved gp140 trimers
The cleaved and uncleaved gp140s were purified on an oligosaccharide-specific GNA (Galanthus nivalis agglutinin)-lectin affinity column. The eluted proteins were then fractionated, based on their relative hydrodynamic size, using gel filtration chromatography (Fig.2A). The different forms of both cleaved and uncleaved gp140s (monomer, dimer and trimer) were well resolved into distinct peaks on a Superose6 column. The uncleaved trimers migrated very slightly, but consistently, less rapidly than their cleaved counterparts but, overall, the two gp140 preparations had almost identical gel-filtration chromatography profiles.
Fig. 2.
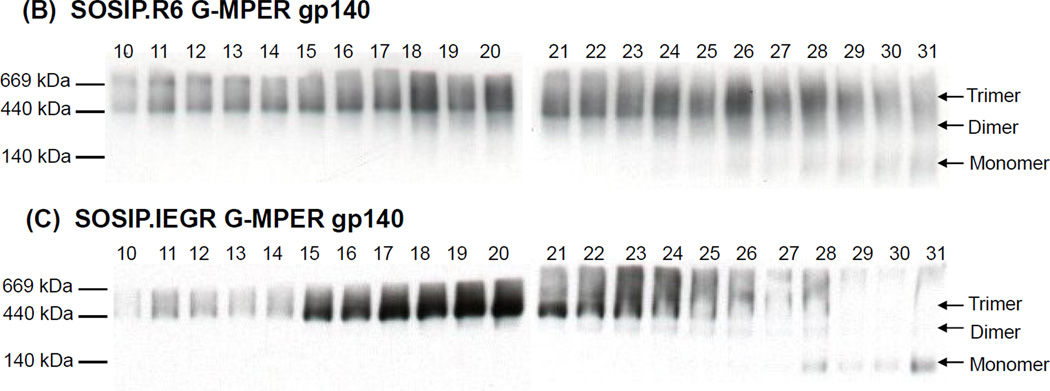
Gel filtration analysis of cleaved and uncleaved gp140s. (A) The SOSIP.R6 G-MPER (cleaved) and SOSIP.IEGR G-MPER (uncleaved) gp140s were purified by lectin-affinity chromatography and then analyzed by size-exclusion chromatography. The separation profile of the two preparations over time (minutes) is shown. The solid arrow indicates the high-molecular aggregate peak, whereas the three dotted arrows show the trimer, dimer and monomer peaks. The precise timings (in minutes) of the three gp140 protomer peaks are listed. (B, C) The gel-filtration chromatography fractions (numbered above the lanes) were analyzed using BN-PAGE and western blotting. The migration positions of relevant molecular weight standards are marked on the left.
A BN-PAGE analysis of fractions eluted from the gel filtration column again showed that the cleaved and uncleaved gp140s were very similar in their migration profiles (Fig.2B,C). However, higher molecular weight, disulphide-linked aggregates were present only in the cleaved gp140 preparation (Fig.2A, lane 1–4). These aggregates form a small shoulder just before the trimer peak (denoted by a red arrow in Fig.2A). Since the purpose of these experiments was to compare cleaved and uncleaved trimers, we did not further analyze the dimeric, monomeric or other forms of gp140 that elute from the gel filtration column in and after fraction 31; we have reported elsewhere on the presence of such Env forms in KNH1144 gp140 preparations (Dey et al., 2007). The peak corresponding to the gp140 trimer fraction was collected from the gel filtration column and concentrated to the desired volume. Subsequent references to ‘purified trimers’ refer to this gp140 fraction, which we analyzed by ultracentrifugation and other procedures as outlined below.
Ultracentrifugation analysis
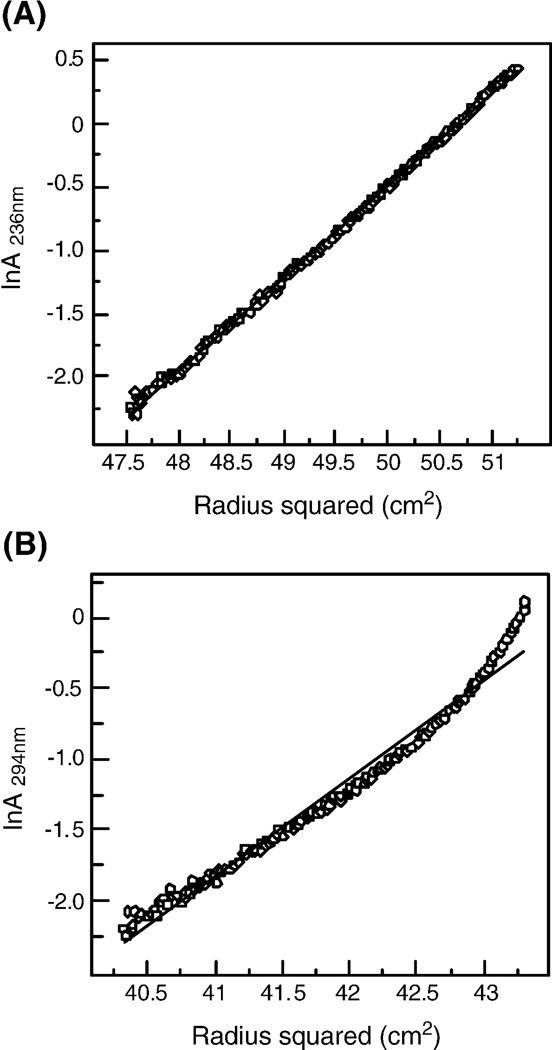
Sedimentation equilibrium measurements were carried out to determine the oligomeric state of the purified, cleaved and uncleaved gp140s. The cleaved gp140 sedimented as a homogeous trimer; its apparent molecular mass is ~460 kDa in the concentration range of 0.1 to 0.4 mg/ml (Fig.3A). The uncleaved gp140 is also trimeric, with an apparent molecular mass of ~435 kDa (Fig.3B). There was no systematic dependence of apparent molecular mass on protein concentration over a 4-fold range of protein concentration studied. Nonetheless, analysis of residual differences from the trimeric model reveals a systematic error, suggesting that the uncleaved gp140 is prone to aggregation.
Fig. 3.
Sedimentation equilibrium analysis of cleaved and uncleaved gp140s. (A) Sedimentation equilibrium data of SOSIP.R6 G-MPER gp140 at 0.2 mg/ml in PBS (pH 7.0). Data are plotted as ln(absorbance) versus the square of the radius from the axis of rotation. The slope of the data is proportional to the molecular mass of the protein oligomer. SOSIP.R6 G-MPER gp140 is a discrete trimer under these conditions. (B) Sedimentation equilibrium data of SOSIP.IEGR G-MPER gp140 at 0.4 mg/ml in PBS (pH 7.0). The slope of the plotted data indicates that the SOSIP.IEGR G-MPER gp140 is a trimeric species.
Stability of cleaved and uncleaved gp140 trimers
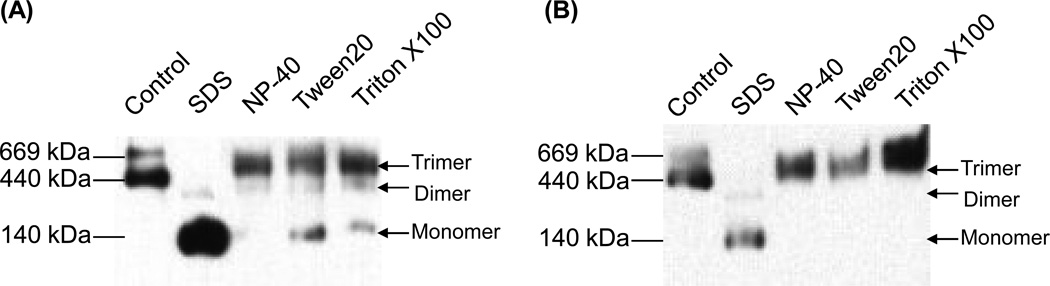
We assessed the stabilities of the purified, cleaved and uncleaved gp140 trimers in the presence of different detergents, to gain an initial understanding of their properties. Both forms of trimer dissociated completely into monomeric gp140 subunits when incubated with the ionic detergent SDS for 1h at room temperature (Fig.4A,B; lane 2). In the presence of non-ionic detergents (NP-40, Tween-20, Triton X-100), the uncleaved gp140 trimers were highly stable (Fig.4B, lanes 3,4,5), whereas the cleaved trimers partially dissociated under the same conditions. Thus, NP-40 treatment of the cleaved trimers caused gp140 dimers to appear (Fig.4A, lane 3), while exposure to Tween-20 and Triton X-100 yielded both gp140 dimers and monomers (Fig.4A, lanes 4,5). Hence cleavage partially destabilizes the gp140 trimer.
Fig. 4.
Detergent stability of cleaved and uncleaved gp140 trimers. Gel filtration-purified (A) KNH1144 cleaved SOSIP.R6 G-MPER and (B) uncleaved SOSIP.IEGR G-MPER gp140 trimers were incubated with 0.1% concentrations of the indicated ionic (SDS) or non-ionic (NP-40, Tween20, Triton X100) detergents for 1h at 25°C, before analysis by BN-PAGE and western blotting.
The gp41 subunits are antigenically different in cleaved and uncleaved gp140s
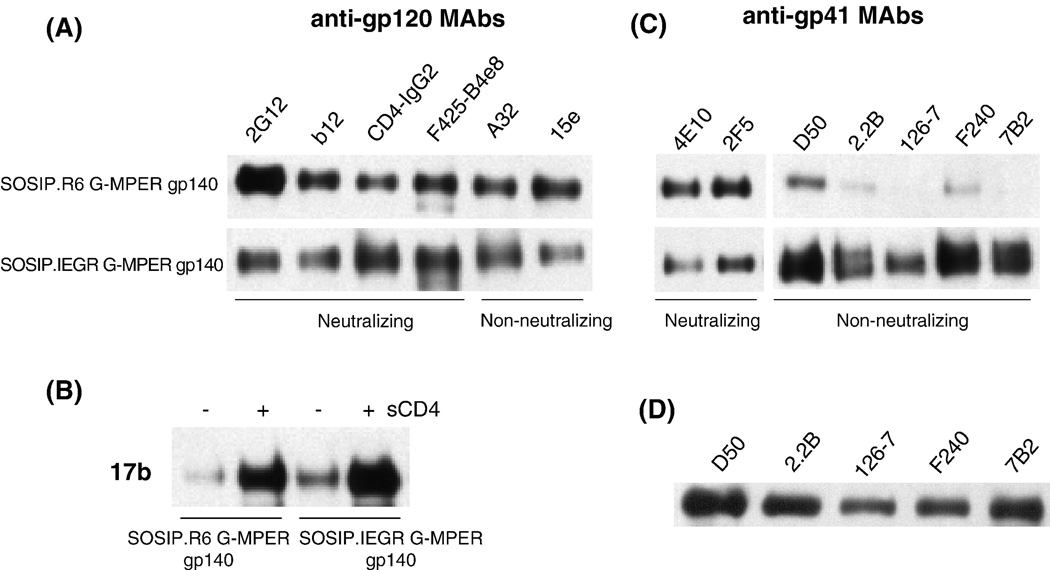
The binding of various neutralizing and non-neutralizing MAbs to the purified, cleaved and uncleaved KNH1144 (G-MPER modified) gp140 trimers was analyzed by immunoprecipitation in the absence of detergent. There was no detectable difference in how CD4-IgG2 or any of the anti-gp120 MAbs (2G12, b12, F425-B4e8, A32, 15e) reacted with the cleaved vs uncleaved trimers (Fig.5A). CD4-induced conformational changes that exposed the 17b epitope also occurred to a similar extent with both forms of trimer (Fig.5B). Hence cleavage does not appear to have a major effect on the topology of the gp120 subunit of trimers. The 2F5 and 4E10 NAbs to the gp41 MPER epitopes also reacted similarly with the cleaved and uncleaved trimers (Fig.5C). However, whereas the non-neutralizing MAbs D50, 2.2B, 126–7 (all to Cluster II epitopes), F240 and 7B2 (both to Cluster I epitopes) all bound strongly to the uncleaved trimers, they did so only weakly or not at all to the cleaved trimers (Fig.5C). To assess whether the limited reactivity of the cleaved gp140s with the non-neutralizing anti-gp41 MAbs was simply due to the absence of the relevant epitopes, we repeated the immunoprecipitation reaction under denaturing conditions; i.e., in the presence of 0.01% SDS. Under these conditions, all the MAbs bound strongly (Fig.5D), confirming that their epitopes are present but are normally occluded within the cleaved gp140 trimer.
Fig. 5.
Reactivity of anti-gp120 and anti-gp41 MAbs with cleaved and uncleaved gp140 trimers. Gel filtration-purified cleaved (SOSIP.R6 G-MPER) or uncleaved (SOSIP.IEGR GMPER) gp140 trimers were immunoprecipitated with various MAbs, and then analyzed by SDS-PAGE and western blotting. (A) Anti-gp120 MAbs and the CD4-IgG2 protein were tested. (B) MAb 17b to a CD4-induced epitope was tested in the presence or absence of sCD4. (C) Anti-gp41 MAbs were tested. (D) Several of the anti-gp41 MAbs were re-tested for binding to cleaved gp140 trimers, but now in the presence of SDS.
DISCUSSION
Does cleavage matter? We considered it something worth looking into, from the perspective of HIV-1 vaccine development. Our overall conclusion is that cleavage does affect the conformation of the gp41 component of trimeric gp140 proteins, as judged by the binding of several non-neutralizing anti-gp41 MAbs to uncleaved but not cleaved gp140s. The addition of SDS reveals these epitopes on the cleaved trimers, showing that they are present on the protein but are normally occluded. As both Cluster I (residues 579 to 613) and Cluster II (residues 644 to 667) epitopes are affected, severing the link between gp120 and gp41 must significantly reduce the exposure of a significant fraction of the total gp41 surface. The likeliest explanation is that the non-neutralizing gp41 MAb epitopes are shielded by other components of the trimer, either by the gp120 subunits or by intermolecular interactions between the gp41 subunits. Another possibility is that the cleaved trimers might be fixed in a conformation in which non-neutralizing cluster 1 and cluster 2 epitopes are occluded, but other gp41 regions are exposed for which no antibody probes exist. Structures of cleaved and uncleaved trimers would be needed to discriminate between these and other possibilities.
That cluster 1 and cluster 2 MAbs do not neutralize infectious viruses implies that their epitopes are also occluded on functional, native, cleaved trimers, a view supported by recent electron micrographs of the spike structures (Le Gall et al., 1998). Moreover, the binding of soluble CD4 to Env on the surface of HIV-1 LAI-infected cells increases the exposure of several non-neutralizing MAb epitopes located within gp41 residues 521–663, but does not affect NAb 2F5 binding to its MPER epitope at residues 662–667 (Montefiori et al., 2007). The strong reactivity of the non-neutralizing gp41 MAbs with the uncleaved trimers therefore implies that the gp41 components of these proteins are in a non-native configuration in which a major fraction of the gp41 surface is abnormally exposed. As NAbs 2F5 and 4E10 bind with comparable efficiencies to the cleaved and uncleaved gp140s, the structural effects of cleavage on gp140 conformation do not extend to the extreme C-terminal region of gp41. Overall, cleaved gp140 trimers may be of particular use for structural studies, in that they appear to be better mimics of the native Env spike.
Previous studies from various groups, including our own, have also suggested that cleaved and uncleaved forms of HIV-1 Env differ in their antigenic structure, particularly in respect of the gp41 components. However, all the earlier reports on soluble gp140s compared proteins with sequence differences that were not limited to the cleavage site, thereby complicating the nature of any conclusions (Binley et al., 2000; Binley et al., 2002; Sanders et al., 2002; Stamatatos, Lim, and Cheng-Mayer, 2000; Yang et al., 2002; Yang et al., 2000). In the present experiments the cleaved and uncleaved KNH1144 SOSIP Env proteins have identical sequences outside the cleavage site, which eliminates other possible variables. MAb reactivity assays using Env proteins expressed on the surface of transfected cells have also shown significant differences between cleaved and uncleaved proteins, including differences in how neutralizing and non-neutralizing MAbs bind to the gp120 subunits (Pancera and Wyatt, 2005). Thus, only gp120-directed neutralizing MAbs (and soluble CD4) bound efficiently to properly cleaved glycoproteins, whereas both non-neutralizing and neutralizing MAbs bound the uncleaved Env complex. This again implies that significant changes in Env spike structure arise when the gp160 precursor is cleaved (Pancera and Wyatt, 2005). We did not see differences in gp120 MAb reactivity with the soluble gp140s; various neutralizing and non-neutralizing anti-gp120 MAbs bound comparably to both forms. An explanation of the difference between our results and those seen with the full-length Env glycoproteins is that, in the latter, the transmembrane and cytoplasmic domains influence the conformation of the gp120 subunits, and in a cleavage-dependent manner. Modulations of Env conformation by these domains are not, of course, relevant to soluble gp140s. An alternative explanation is that the intermolecular SOS bond and the I559P substitution that are common to both the uncleaved and cleaved proteins also act to minimize any influences of cleavage on the gp120 component of soluble gp140s.
The marked conformational difference between the cleaved and uncleaved forms of HIV-1 gp140s stands in contrast with what has been learned from studies of other viral fusion proteins, notably influenza HA (Stevens et al., 2004), Ebola virus glycoprotein (Wool-Lewis and Bates, 1999), mouse hepatitis virus (MHV) A59 glycoprotein (Bos, Luytjes, and Spaan, 1997; Hingley, Leparc-Goffart, and Weiss, 1998) and Sindbis virus E2 glycoprotein (Russell, Dalrymple, and Johnston, 1989). Of particular note is that the crystal structure of the uncleaved form of the human H1 hemagglutinin (HA) from Influenza virus showed that the cleavage event only minimally alters the local structure of the transmembrane glycoprotein and its antigenic sites (Stevens et al., 2004). In addition, in vitro and in vivo studies on several viruses have shown that Env glycoprotein cleavage is not always necessary for virus maturation/assembly or infectivity, implying that cleavage has little effect on function (Bos, Luytjes, and Spaan, 1997; Hingley, Leparc-Goffart, and Weiss, 1998; Russell, Dalrymple, and Johnston, 1989; Watanabe et al., 1995). However, these conclusions are often derived from one-step infection assays; it is possible that additional selection pressures are exerted in multiple replication rounds, and/or in vivo, that result in the retention of the highly conserved cleavage motif. Semliki Forest Virus (SFV) Env may, however, more closely resemble HIV-1 gp160. Thus, cleavage of the SFV Env precursor causes a dramatic, but localized, rearrangement of the trimeric spike on the surface of the virion, an event that does not happen with a mutant (uncleaved) form variant (Ferlenghi et al., 1998).
Structural studies on cleaved and uncleaved trimers may eventually enable the present observations to be understood at a molecular level. Whether the differences between these different types of trimer truly matter for the design of HIV-1 vaccines is hard to judge, because of the difficulty in predicting how antigenic structural variation affects the generation of immune responses. Both forms of trimer express the epitopes for the kinds of NAbs that a vaccine should induce, and to comparable extents. However, there has been speculation that the development of immune responses to non-neutralization epitopes is a hindrance to the generation of Nabs (Binley et al., 2004; Burton, Stanfield, and Wilson, 2005; Eggink, Melchers, and Sanders, 2007; Moore et al., 2006; Pantophlet, Wilson, and Burton, 2003; Selvarajah et al., 2005). If this truly is the case, then using cleaved proteins would seem the better approach, particularly if other non-neutralization epitopes can be eliminated by additional engineering. In the end, only comparative immunization studies would resolve this point and determine whether cleavage is important.
MATERIALS AND METHODS
Plasmids for expression of cleaved and uncleaved gp140s from HIV-1 KNH1144
We have previously described the stable expression of gp140 trimers from the subtype A strain KNH1144, using pPPI4 eukaryotic expression vectors (Beddows et al., 2007). A cleaved gp140 (designated KNH1144 SOSIP.R6) was generated by inserting a disulphide bond (SOS) between gp120 and gp41 (Binley et al., 2000), making a single point substitution in gp41 (I559P) (Sanders et al., 2002), and introducing a cleavage-enhancing hexa-arginine (R6) motif in place of the natural REKR sequence at the cleavage site between gp120 and gp41 (Binley et al., 2002). As the epitopes for MAbs 2G12, 2F5 and 4E10 are either absent or not optimal in the primary sequence of KNH1144, they were created via mutagenesis of the KNH1144 SOSIP.R6 sequence. For 2G12, this was achieved via a S295N change in gp120; for 2F5 and 4E10, we altered the gp41 sequence ALGKWANLWT to ELDKWASLWN. The specific amino acid substitutions were made using the QuikChange® II XL site-directed mutagenesis kit (Stratagene Inc., La Jolla, California) and the appropriate primers. The introduced mutations were verified by sequencing. The 2G12, 2F5 and 4E10 epitope-modified protein was designated KNH1144 SOSIP.R6 G-MPER, where G refers to the glycan-modification and MPER refers to the substitutions in the membrane proximal external region. The uncleaved gp140 (called KNH1144 SOSIP.IEGR G-MPER) contains the same SOS, I559P and G-MPER substitutions but the REKR sequence was mutated to IEGR to prevent cleavage (Schulke et al., 2002). Hence other than at the cleavage site, the cleaved and uncleaved KNH1144 Env proteins have identical sequences. The furin gene was expressed from plasmid pcDNA3.1furin (Binley et al., 2000).
MAbs and CD4-based proteins
The following anti-gp120 monoclonal antibodies (MAbs) were used: IgG1b12 (against the CD4 binding site (Burton et al., 1991)); 2G12 (against a mannose-dependent epitope (Scanlan et al., 2002; Trkola et al., 1996)); 17b (against a CD4-inducible epitope (Thali et al., 1993)); F425-B4e8 (against the V3 loop (Cavacini et al., 2003)). All the above antibodies were obtained from the IAVI NAC repository. Soluble CD4 (sCD4) and the CD4-based molecule CD4-IgG2 were obtained from Progenics Pharmaceuticals (Allaway et al., 1995).
The anti-gp41 MAbs used were: 2F5 (against an epitope centered on the sequence LELDKWANL (Zwick et al., 2005)); 4E10 (against an epitope centered on the sequence NWFDIS (Zwick et al., 2005); D50 (642–665) (Earl et al., 1997); 126–7 (Zhou et al., 2007); F240 (Cavacini et al., 1998; Duval, Posner, and Cavacini, 2008); 2.2B, 7B2. MAbs F240 (aa 598 to 604) (Duval, Posner, and Cavacini, 2008) and 7B2 (Haynes et al., 2005) were to gp41 Cluster I, MAbs D50, 2.2B (Haynes et al., 2005) and 126-7 were to gp41 Cluster II. All the anti-gp41 MAbs were obtained via NIH the AIDS Research & Reference Reagent Program, Division of AIDS, NIAID, NIH, except for 2.2B and 7B2 that were kindly provided by James Robinson. MAb CA13 (ARP3119), which binds to C1 region of gp120, was obtained from Ms C. Arnold via the EU Programme EVA Centralized Facility for AIDS Reagents, NIBSC, UK (AVIP Contract Number LSHP-CT-2004-503487). This MAb was used for all Western Blotting assays described in this study.
Expression and purification of cleaved and uncleaved gp140 glycoproteins
Cleaved and uncleaved trimers were transiently expressed in adherent HEK293T cells by transfection with gp140- and furin-expressing plasmids, using linear 25K Polyethylenimine (PEI) (Polysciences Inc., Warrington, PA), as described previously (Beddows et al., 2007; Binley et al., 2000; Dey et al., 2007). Culture supernatants that contained Env glycoproteins were concentrated by >20-fold and then subjected to Lectin-affinity chromatography. The column eluate was then size-fractionated using an analytical SuperoseTM 6 column (GE Amersham Pharmacia, Piscataway, NJ) equilibrated with phosphate-buffered saline (PBS; 100mM NaCl, 50mM sodium phosphate, pH 7.0). The column was calibrated with protein standards of known molecular weights (HMW Gel Filtration Calibration Kit; Amersham Pharmacia, Piscataway, NJ). Fractions (200μl) were collected and analyzed using Blue-native polyacrylamide electrophoresis (BN-PAGE) and SDS-PAGE. Quantification of proteins was carried out using the BCA quantification kit (Pierce) with known BSA standards, as described previously (Dey et al., 2007; Schulke et al., 2002).
SDS-PAGE, BN-PAGE, immunoprecipitation and western blot analyses
SDS-PAGE analyses were performed as described elsewhere (Binley et al., 2000). Reduced and non-reduced samples were prepared in Laemmli sample buffer (62.5mM Tris-HCl, pH 6.8, 2% SDS, 25% glycerol, 0.01% DTT) and boiled for 5 min in the presence or absence of 50 mM dithiothreitol (DTT), respectively. BN-PAGE was carried out as described previously (Schulke et al., 2002). Gel electrophoresis was performed for 2h at 150V (~0.07A) using 50 mM MOPS, 50mM Tris, pH 7.7, 0.002% Coomassie blue as the cathode buffer, and 50mM MOPS, 50mM Tris, pH 7.7 as the anode buffer. When purified proteins were analyzed, the gel was destained with several changes of 50mM MOPS, 50mM Tris (pH 7.7) buffer, subsequent to the electrophoresis step.
Immunoprecipitation was carried out by incubating known concentrations of cleaved and uncleaved gp140s overnight at 4°C with CD4-IgG2 or various MAbs (250ng) in a buffer containing 50mM Tris-HCl (pH 7.0), 150mM NaCl and 1M EDTA. The gp140-MAb/CD4-IgG2 complexes were then precipitated using ImmunoPure® immobilized Protein G agarose beads (Pierce Biotechnology, Rockford, IL) and analyzed by reducing SDS-PAGE.
The western blot procedure has been described elsewhere (Schulke et al., 2002). Following transfer, the polyvinylidene difluoride (PVDF) membrane was destained, then probed using anti-Env MAb CA13 (ARP3119), followed by horseradish peroxidase-labeled anti-mouse immunoglobulin G (IgG) (Kirkegaard & Perry Labs), at a final concentration of 0.2μg/ml. The bound MAbs were detected using the Western Blot Chemiluminescence Reagent Plus system (Perkin-Elmer Life Sciences, Boston, MA). Protein mixtures containing Thyroglobulin (669 kDa), Ferritin (440 kDa), Catalase (232 kDa), Lactate dehydrogenase (140 kDa) and BSA (66 kDa) (Amersham Biosciences) were used as standard markers for native gels; the MultiMark® multi-colored standard set (Invitrogen) was used for denaturing gels.
Sedimentation equilibrium experiments
Analytical ultracentrifugation measurements were performed on a Beckman XL-A Optima analytical ultracentrifuge with an An-60 Ti rotor at 20°C. Protein samples were dialyzed overnight into PBS buffer (50 mM sodium phosphate, pH 7.0, 150 mM NaCl), loaded at initial concentrations of 0.1, 0.2 and 0.4 mg/ml, and analyzed at rotor speeds of 3,500 and 5,000 rpm. Data were acquired at two wavelengths per rotor speed and were fit using the program NONLIN to a single species model of the natural logarithm of the absorbance versus radial distance squared (Johnson et al., 1981). Solvent density and protein partial specific volume parameters were calculated taking into account the solvent and protein composition, respectively (Laue et al., 1992).
ACKNOWLEDGEMENTS
We thank William Olson, Dennis Burton, Ralph Pantophlet, James Robinson, Hermann Katinger, Lisa Cavacini, Marshall Posner, Patricia Earl and Susan Zolla-Pazner for the gifts of important reagents, directly or via Repositories. We thank Rogier Sanders and Kaustuv Banerjee for critical reading of the manuscript. We appreciate receiving advice and support from William Olson and from past and present members of our group. This work was supported by NIH grants and contracts AI 45463 and AI 30030, and by the International AIDS Vaccine Initiative.
REFERENCES
- Allaway GP, Davis-Bruno KL, Beaudry GA, Garcia EB, Wong EL, Ryder AM, Hasel KW, Gauduin MC, Koup RA, McDougal JS, et al. Expression and characterization of CD4-IgG2, a novel heterotetramer that neutralizes primary HIV type 1 isolates. AIDS Res Hum Retroviruses. 1995;11(5):533–539. doi: 10.1089/aid.1995.11.533. [DOI] [PubMed] [Google Scholar]
- Beddows S, Franti M, Dey AK, Kirschner M, Iyer SP, Fisch DC, Ketas T, Yuste E, Desrosiers RC, Klasse PJ, Maddon PJ, Olson WC, Moore JP. A comparative immunogenicity study in rabbits of disulfide-stabilized, proteolytically cleaved, soluble trimeric human immunodeficiency virus type 1 gp140, trimeric cleavage-defective gp140 and monomeric gp120. Virology. 2007;360(2):329–340. doi: 10.1016/j.virol.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Binley JM, Sanders RW, Clas B, Schuelke N, Master A, Guo Y, Kajumo F, Anselma DJ, Maddon PJ, Olson WC, Moore JP. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J Virol. 2000;74(2):627–643. doi: 10.1128/jvi.74.2.627-643.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binley JM, Sanders RW, Master A, Cayanan CS, Wiley CL, Schiffner L, Travis B, Kuhmann S, Burton DR, Hu SL, Olson WC, Moore JP. Enhancing the proteolytic maturation of human immunodeficiency virus type 1 envelope glycoproteins. J Virol. 2002;76(6):2606–2616. doi: 10.1128/JVI.76.6.2606-2616.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binley JM, Wrin T, Korber B, Zwick MB, Wang M, Chappey C, Stiegler G, Kunert R, Zolla-Pazner S, Katinger H, Petropoulos CJ, Burton DR. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J Virol. 2004;78(23):13232–13252. doi: 10.1128/JVI.78.23.13232-13252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos EC, Luytjes W, Spaan WJ. The function of the spike protein of mouse hepatitis virus strain A59 can be studied on virus-like particles: cleavage is not required for infectivity. J Virol. 1997;71(12):9427–9433. doi: 10.1128/jvi.71.12.9427-9433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DR, Barbas CF, 3rd, Persson MA, Koenig S, Chanock RM, Lerner RA. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc Natl Acad Sci U S A. 1991;88(22):10134–10137. doi: 10.1073/pnas.88.22.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DR, Stanfield RL, Wilson IA. Antibody vs. HIV in a clash of evolutionary titans. Proc Natl Acad Sci U S A. 2005;102(42):14943–14948. doi: 10.1073/pnas.0505126102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavacini L, Duval M, Song L, Sangster R, Xiang SH, Sodroski J, Posner M. Conformational changes in env oligomer induced by an antibody dependent on the V3 loop base. Aids. 2003;17(5):685–689. doi: 10.1097/00002030-200303280-00006. [DOI] [PubMed] [Google Scholar]
- Cavacini LA, Emes CL, Wisnewski AV, Power J, Lewis G, Montefiori D, Posner MR. Functional and molecular characterization of human monoclonal antibody reactive with the immunodominant region of HIV type 1 glycoprotein 41. AIDS Res Hum Retroviruses. 1998;14(14):1271–1280. doi: 10.1089/aid.1998.14.1271. [DOI] [PubMed] [Google Scholar]
- Dey AK, David KB, Klasse PJ, Moore JP. Specific amino acids in the N-terminus of the gp41 ectodomain contribute to the stabilization of a soluble, cleaved gp140 envelope glycoprotein from human immunodeficiency virus type 1. Virology. 2007;360(1):199–208. doi: 10.1016/j.virol.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval M, Posner MR, Cavacini LA. A bispecific antibody composed of a nonneutralizing antibody to the gp41 immunodominant region and an anti-CD89 antibody directs broad human immunodeficiency virus destruction by neutrophils. J Virol. 2008;82(9):4671–4674. doi: 10.1128/JVI.02499-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl PL, Broder CC, Doms RW, Moss B. Epitope map of human immunodeficiency virus type 1 gp41 derived from 47 monoclonal antibodies produced by immunization with oligomeric envelope protein. J Virol. 1997;71(4):2674–2684. doi: 10.1128/jvi.71.4.2674-2684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggink D, Melchers M, Sanders RW. Antibodies to HIV-1: aiming at the right target. Trends Microbiol. 2007;15(7):291–294. doi: 10.1016/j.tim.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Ferlenghi I, Gowen B, de Haas F, Mancini EJ, Garoff H, Sjoberg M, Fuller SD. The first step: activation of the Semliki Forest virus spike protein precursor causes a localized conformational change in the trimeric spike. J Mol Biol. 1998;283(1):71–81. doi: 10.1006/jmbi.1998.2066. [DOI] [PubMed] [Google Scholar]
- Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, Ortel TL, Liao HX, Alam SM. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308(5730):1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- Hingley ST, Leparc-Goffart I, Weiss SR. The spike protein of murine coronavirus mouse hepatitis virus strain A59 is not cleaved in primary glial cells and primary hepatocytes. J Virol. 1998;72(2):1606–1609. doi: 10.1128/jvi.72.2.1606-1609.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffs SA, Goriup S, Kebble B, Crane D, Bolgiano B, Sattentau Q, Jones S, Holmes H. Expression and characterisation of recombinant oligomeric envelope glycoproteins derived from primary isolates of HIV-1. Vaccine. 2004;22(8):1032–1046. doi: 10.1016/j.vaccine.2003.08.042. [DOI] [PubMed] [Google Scholar]
- Johnson ML, Correia JJ, Yphantis DA, Halvorson HR. Analysis of data from the analytical ultracentrifuge by nonlinear least-squares techniques. Biophys J. 1981;36(3):575–588. doi: 10.1016/S0006-3495(81)84753-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laue TM, Shah BD, Ridgeway TM, Pelletier SL. Computer-aided interpretation of analytical sedimentation data for proteins. In: Harding SE, Rowe AJ, Horton JC, editors. Analytical ultracentrifugation in biochemistry and polymer science. Cambridge, United Kingdom: Royal Society of Chemistry; 1992. [Google Scholar]
- Le Gall S, Erdtmann L, Benichou S, Berlioz-Torrent C, Liu L, Benarous R, Heard JM, Schwartz O. Nef interacts with the mu subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC I molecules. Immunity. 1998;8(4):483–495. doi: 10.1016/s1074-7613(00)80553-1. [DOI] [PubMed] [Google Scholar]
- Montefiori D, Sattentau Q, Flores J, Esparza J, Mascola J. Antibodybased HIV-1 vaccines: recent developments and future directions. PLoS Med. 2007;4(12):e348. doi: 10.1371/journal.pmed.0040348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PL, Crooks ET, Porter L, Zhu P, Cayanan CS, Grise H, Corcoran P, Zwick MB, Franti M, Morris L, Roux KH, Burton DR, Binley JM. Nature of nonfunctional envelope proteins on the surface of human immunodeficiency virus type 1. J Virol. 2006;80(5):2515–2528. doi: 10.1128/JVI.80.5.2515-2528.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancera M, Wyatt R. Selective recognition of oligomeric HIV-1 primary isolate envelope glycoproteins by potently neutralizing ligands requires efficient precursor cleavage. Virology. 2005;332(1):145–156. doi: 10.1016/j.virol.2004.10.042. [DOI] [PubMed] [Google Scholar]
- Pantophlet R, Wilson IA, Burton DR. Hyperglycosylated mutants of human immunodeficiency virus (HIV) type 1 monomeric gp120 as novel antigens for HIV vaccine design. J Virol. 2003;77(10):5889–5901. doi: 10.1128/JVI.77.10.5889-5901.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DL, Dalrymple JM, Johnston RE. Sindbis virus mutations which coordinately affect glycoprotein processing, penetration, and virulence in mice. J Virol. 1989;63(4):1619–1629. doi: 10.1128/jvi.63.4.1619-1629.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders RW, Vesanen M, Schuelke N, Master A, Schiffner L, Kalyanaraman R, Paluch M, Berkhout B, Maddon PJ, Olson WC, Lu M, Moore JP. Stabilization of the soluble, cleaved, trimeric form of the envelope glycoprotein complex of human immunodeficiency virus type 1. J Virol. 2002;76(17):8875–8889. doi: 10.1128/JVI.76.17.8875-8889.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan CN, Pantophlet R, Wormald MR, Ollmann Saphire E, Stanfield R, Wilson IA, Katinger H, Dwek RA, Rudd PM, Burton DR. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1-->2 mannose residues on the outer face of gp120. J Virol. 2002;76(14):7306–7321. doi: 10.1128/JVI.76.14.7306-7321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulke N, Vesanen MS, Sanders RW, Zhu P, Lu M, Anselma DJ, Villa AR, Parren PW, Binley JM, Roux KH, Maddon PJ, Moore JP, Olson WC. Oligomeric and conformational properties of a proteolytically mature, disulfide-stabilized human immunodeficiency virus type 1 gp140 envelope glycoprotein. J Virol. 2002;76(15):7760–7776. doi: 10.1128/JVI.76.15.7760-7776.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvarajah S, Puffer B, Pantophlet R, Law M, Doms RW, Burton DR. Comparing antigenicity and immunogenicity of engineered gp120. J Virol. 2005;79(19):12148–12163. doi: 10.1128/JVI.79.19.12148-12163.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit JM, Klimstra WB, Ryman KD, Bittman R, Johnston RE, Wilschut J. PE2 cleavage mutants of Sindbis virus: correlation between viral infectivity and pH-dependent membrane fusion activation of the spike heterodimer. J Virol. 2001;75(22):11196–11204. doi: 10.1128/JVI.75.22.11196-11204.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatatos L, Lim M, Cheng-Mayer C. Generation and structural analysis of soluble oligomeric gp140 envelope proteins derived from neutralization-resistant and neutralization-susceptible primary HIV type 1 isolates. AIDS Res Hum Retroviruses. 2000;16(10):981–994. doi: 10.1089/08892220050058407. [DOI] [PubMed] [Google Scholar]
- Stevens J, Corper AL, Basler CF, Taubenberger JK, Palese P, Wilson IA. Structure of the uncleaved human H1 hemagglutinin from the extinct 1918 influenza virus. Science. 2004;303(5665):1866–1870. doi: 10.1126/science.1093373. [DOI] [PubMed] [Google Scholar]
- Thali M, Moore JP, Furman C, Charles M, Ho DD, Robinson J, Sodroski J. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J Virol. 1993;67(7):3978–3988. doi: 10.1128/jvi.67.7.3978-3988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore JP, Katinger H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol. 1996;70(2):1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Hirano A, Stenglein S, Nelson J, Thomas G, Wong TC. Engineered serine protease inhibitor prevents furin-catalyzed activation of the fusion glycoprotein and production of infectious measles virus. J Virol. 1995;69(5):3206–3210. doi: 10.1128/jvi.69.5.3206-3210.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wool-Lewis RJ, Bates P. Endoproteolytic processing of the ebola virus envelope glycoprotein: cleavage is not required for function. J Virol. 1999;73(2):1419–1426. doi: 10.1128/jvi.73.2.1419-1426.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang OO, Nguyen PT, Kalams SA, Dorfman T, Gottlinger HG, Stewart S, Chen IS, Threlkeld S, Walker BD. Nef-mediated resistance of human immunodeficiency virus type 1 to antiviral cytotoxic T lymphocytes. J Virol. 2002;76(4):1626–1631. doi: 10.1128/JVI.76.4.1626-1631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Florin L, Farzan M, Kolchinsky P, Kwong PD, Sodroski J, Wyatt R. Modifications that stabilize human immunodeficiency virus envelope glycoprotein trimers in solution. J Virol. 2000;74(10):4746–4754. doi: 10.1128/jvi.74.10.4746-4754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Xu L, Dey B, Hessell AJ, Van Ryk D, Xiang SH, Yang X, Zhang MY, Zwick MB, Arthos J, Burton DR, Dimitrov DS, Sodroski J, Wyatt R, Nabel GJ, Kwong PD. Structural definition of a conserved neutralization epitope on HIV-1 gp120. Nature. 2007;445(7129):732–737. doi: 10.1038/nature05580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwick MB, Jensen R, Church S, Wang M, Stiegler G, Kunert R, Katinger H, Burton DR. Anti-human immunodeficiency virus type 1 (HIV-1) antibodies 2F5 and 4E10 require surprisingly few crucial residues in the membraneproximal external region of glycoprotein gp41 to neutralize HIV-1. J Virol. 2005;79(2):1252–1261. doi: 10.1128/JVI.79.2.1252-1261.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]