Abstract
A central principle in life-history theory is that reproductive effort negatively affects survival. Costs of reproduction are thought to be physiologically-based, but the underlying mechanisms remain poorly understood. Using female North American red squirrels (Tamiasciurus hudsonicus), we test the hypothesis that energetic investment in reproduction overwhelms investment in antioxidant protection, leading to oxidative damage. In support of this hypothesis we found that the highest levels of plasma protein oxidative damage in squirrels occurred during the energetically-demanding period of lactation. Moreover, plasma protein oxidative damage was also elevated in squirrels that expended the most energy and had the lowest antioxidant protection. Finally, we found that squirrels that were food-supplemented during lactation and winter had increased antioxidant protection and reduced plasma protein oxidative damage providing the first experimental evidence in the wild that access to abundant resources can reduce this physiological cost.
Keywords: antioxidant protection, doubly-labeled water, daily energy expenditure, energetics, food-supplementation, life-history theory
INTRODUCTION
Life-history theory assumes that individuals maximize fitness by balancing the allocation of limited energy or resources to the competing demands of growth, reproduction, and somatic maintenance (Roff 1992; Stearns 1992). One of the best-studied trade-offs is how allocation toward reproduction compromises survival (Reznick 1992; Daan et al. 1996). In line with the theory that costs of reproduction are mediated by allocation trade-offs, theoreticians have hypothesized (van Noordwijk and de Jong 1986; McNamara and Houston 1996; Reznick et al. 2000) and empiricists have demonstrated (Tuomi et al. 1983; Reznick et al. 2000; Bonnet et al. 2002) that the negative effects of reproduction on survival are reduced when animals have access to abundant energy/resources. The reduction of survival costs of reproduction in these circumstances is thought to result because animals with abundant resources can allocate more energy/resources to somatic maintenance (Reznick et al. 2000). However, the physiological mechanisms that underlie how costs of reproduction are incurred, or can be reduced, remain a mystery (Zera and Harshman 2001; Speakman 2008).
Much recent interest has focused on oxidative damage as an important physiological mechanism responsible for costs of reproductive effort in free-ranging animals (Costantini 2008; Dowling and Simmons 2009; Monaghan et al. 2009; Selman et al. 2012). Lactation in mammals and chick rearing in birds are associated with high levels of daily energy expenditure (DEE) that may approach physiological limits (reviewed in McNab 2002). Experimental increases in the number of offspring supported leads to increases in parental DEE in free-ranging birds (e.g. Daan et al. 1996) and mammals (Humphries and Boutin 2000) suggesting a mechanistic link between DEE and reproductive effort. Previous research examining reproductive effort has used a variety of indicator variables (e.g. litter size, offspring mass and growth; e.g. Clutton-Brock 1984), but a more integrative measure of reproductive effort, such as DEE, might be more reflective of parental care costs than traditional indicators. Reactive oxygen species (ROS) are primarily by-products of aerobic energy expenditure that can cause oxidative damage to proteins, lipids, and nucleic acids (Beckman and Ames 1998; Finkel and Holbrook 2000). A suite of enzymatic and non-enzymatic antioxidants function to neutralize ROS before they cause damage (Beckman and Ames 1998); however, if there is an imbalance between the production of ROS from energy expenditure on one hand, and antioxidant protection and repair mechanisms on the other, oxidative damage occurs (Selman et al. 2012).
Comprehensive examinations of the oxidative damage hypothesis in free-living animals are currently required. Most tests of the oxidative damage hypothesis on wild-captured animals have been conducted in captivity (Alonso-Alvarez et al. 2004; Wiersma et al. 2004; Alonso-Alvarez et al. 2006; Bertrand et al. 2006; Selman et al. 2008a, b; Garratt et al. 2011; Garratt et al. 2012; Ołdakowski et al. 2012), with studies on free-ranging animals appearing only more recently (Bize et al. 2008; Nussey et al. 2009; Bergeron et al. 2011; Christe et al. 2011; Markó et al. 2011; Losdat et al. 2011; Heiss and Schoech 2012). These studies have produced equivocal results potentially because they have not obtained measures of DEE, antioxidant protection, and oxidative damage within the same individual (Selman et al. 2012). Moreover, experimental studies examining the effect of energy/resource availability on oxidative damage are rare in wild animals (Losdat et al. 2011; for captive studies see Bertrand et al. 2006; Costantini 2010). Experimentally increased energy/resource availability is expected to reduce oxidative damage if the balance between the production of ROS from energy expenditure on one hand, and antioxidant protection and repair mechanisms on the other, is in favor of the later. However, how this balance may be resolved in response to increased energy/resource availability is complicated for two reasons. First, the direction of the relationship between energy/resource availability and DEE is currently unresolved (Tinbergen and Verhulst 2000; Thomas et al. 2001; Speakman et al. 2003; Welcker et al. 2010); thus, it is unknown how energy/resource availability influences ROS production. Second, it is generally unknown whether levels of antioxidant protection are influenced by energy/resource availability in the wild (Losdat et al. 2011).
Here, we provide a comprehensive test of the oxidative damage hypothesis in a free-ranging population of North American red squirrels (Tamiasciurus hudsonicus; hereafter red squirrels). We quantified the DEE, antioxidant protection, and oxidative damage of female squirrels during three seasonal and reproductive stages: 1) lactation, 2) non-breeding during the summer, and 3) winter. To investigate the role of energy/resource availability in mediating oxidative damage, squirrels were examined on study areas where individuals only had access to natural food, as well as on experimental food-supplementation study areas where individuals were provided with supplemental peanut butter. To our knowledge, our study is the first in the wild to combine measures of DEE, antioxidant protection, and oxidative damage into a single study.
MATERIALS AND METHODS
Study region and squirrel life-stages
Female squirrels were studied during the spring/summer of 2006 and the winter of 2006–2007 in the boreal forest of Yukon, Canada (61° N, 138° W). The average temperature in the coldest and warmest months ranges between −22°C and 12.8°C (Environment Canada: 1967–2006; Burwash, Yukon; ~70 km from study area). Squirrels were examined on six study areas (see below). The minimum and maximum distances between study areas were ~ 200 m and ~ 8 km, respectively. Study areas were dominated by white spruce (Picea glauca) with an understory of willow (Salix spp.).
Following long-term data collection protocols, we tracked the reproductive output of all adult females living on our study areas during the spring/summer (for further details see Boutin et al. 2006; McAdam et al. 2007). All adult females living on our study areas were ear-tagged and pregnancy and parturition was tracked by palpating their abdomens and attempting to express milk from their nipples following repeated live-trapping. When a female was determined to have given birth, her pups were temporarily removed from the nest to be counted and weighed. The parturition date of each female was estimated based on her live-trapping records and the masses of her pups when they were removed from the nest. Pups were removed from the nest a second time at ~ 25 days postpartum to be ear-tagged. We ascertained that some females had lost their litters during lactation when we could no longer express milk from their nipples prior to the estimated date of weaning (~70 d post-partum; McAdam et al. 2007; S. Boutin, A. G. McAdam, M. M. Humphries unpublished data).
Oxidative damage and antioxidant protection were sampled in female squirrels during three seasonal and reproductive stages (hereafter, life-stages): (1) lactation, (2) non-breeding during summer (henceforth, non-breeding), and (3) winter. Lactating females were sampled between 2-May and 30-June 2006 during a consistent period of lactation (90% of females were sampled when they were 35–42 d postpartum; range = 35–49). Energetic demands are likely high during this period of lactation because this is just prior to when pups emerge from the nest at ~42 d postpartum and start transitioning to natural food items (pups are weaned at ~70 d postpartum; McAdam et al. 2007; S. Boutin, A. G. McAdam, M. M. Humphries unpublished data). All lactating females were attempting to raise their first litter of the year. Non-breeding females were sampled over a calendar period that approximately overlapped when lactating females were sampled (14-Jun to 4-Jul 2006). Two types of females were included in the non-breeding life-stage: females that lost their litter at least 29 days previously (n = 3), and females that did not attempt to breed in 2006 (n = 7). There was no significant difference in either the DEE (P = 0.32), oxidative damage (P = 0.13), or antioxidant protection (P = 0.43) between the two types of non-breeding females. Females during the winter were sampled between 17-Jan and 1-Apr 2007. None of the females in winter life-stage that we sampled were pregnant. We knew this based on four types of information: (1) autopsy after sacrifice (n = 11; S. B. Woods unpub. data), (2) known parturition dates (n = 8), (3) trapping records when females were known not to be pregnant (n = 4), and (4) the female disappeared from the study area, and thus was presumed dead (Descamps et al. 2009) prior to becoming being pregnant (n = 1). The females in this study were at least one year old (S. Boutin, A. G. McAdam, M. M. Humphries unpublished data); however, we did not investigate the effect of age in this study because we only knew the birth year of 50% of the females.
Natural-food and food-supplementation study areas
We examined squirrels on four study areas where individuals only had access to natural food (~40 ha each). Two of the natural-food study areas have been the subject of consistent long-term research on completely enumerated ear-tagged squirrels since 1987 (Boutin et al. 2006; McAdam et al. 2007). Red squirrels at this site hoard white spruce cones while they are mature during the autumn (Fletcher et al. 2010; Archibald et al. 2012) to help meet the energetic demands of winter and reproduction the following spring (Boutin et al. 2006). Past research at this site demonstrates that the production of white spruce cones in the previous year is the key factor shaping the ecology of squirrels. Large cone crops in the previous year are correlated with increased juvenile survival (Humphries and Boutin 2000; McAdam and Boutin 2003), increased adult overwinter survival (LaMontagne 2007), earlier parturition dates, and faster pup growth rates (Boutin et al. 2006). Lactating and non-breeding squirrels on three of the natural-food study areas experienced high levels of spruce cones, whereas lactating and non-breeding squirrels on the fourth natural-food study area experienced low levels of spruce cones (see Table 1). Spruce cone levels were low on the natural-food study area during winter. For further details on the availability of cones in this study and the effect of spruce cone abundance on squirrel ecology, see Supporting Information S1.
Table 1.
Sample sizes of oxidative damage and antioxidant protection samples collected from female squirrels within the three life-stages (lactation, non-breeding, and winter), on natural-food and food-supplementation study areas. Females within the lactation and non-breeding life-stages living on the natural-food study areas either experienced high or low natural-food levels. A proportion of the squirrels with oxidative damage/antioxidant protection samples were associated with and without paired DEE estimates.
| Lactation | Non-breeding | Winter | |||||
|---|---|---|---|---|---|---|---|
| Natural-food | Food-suppl. | Natural-food | Natural-food | Food-suppl. | |||
| High | Low | High | Low | ||||
| with DEE measurement | 7 | 5 | 4 | 7 | 2 | 11 | 2 |
| without DEE measurement | 1 | 2 | 1 | 0 | 1 | 6 | 5 |
Squirrels were also examined on two food-supplementation study areas where every individual was provided with supplemental natural peanut butter. We are confident that target squirrels from these food-supplemented populations had additional food resources because: 1) peanut butter was placed in closed plastic feeders near the center of each squirrel’s territory and the feeders were actively defended, 2) squirrels were frequently observed inside the feeders, and 3) the peanut butter was depleted. Continued ownership of the peanut butter feeders by the target squirrels was confirmed with live-trapping. It is possible that some peanut butter was eaten by non-target individuals or species but based on our field observations, it seems likely that the vast majority of the peanut butter was eaten by the intended recipients. The food-supplementation study area where squirrels were sampled during lactation (~45 ha) was different from the food-supplementation study area where squirrels were sampled during winter (~3.2 ha). On the food-supplementation study area where lactating squirrels were sampled, approximately 1 kg of natural peanut butter was provided to every resident squirrel (all squirrels on the study area were marked with ear-tags) every six weeks starting in October and continuing until the pups were weaned the following year (May–June). One kilogram of peanut butter has the approximate nutritional composition of the seed contained in 5000 spruce cones (Health Canada 1990; LaMontagne 2007), which is sufficient to meet the resting metabolic rate requirements of female red squirrels in this population for ~70 d (M. M. Humphries unpublished data). This food-supplementation started in 2004, and the reproductive output of females on this study area was also tracked following the same long-term data collection protocols as on the natural-food study areas. The squirrels that were food-supplemented during winter were provided with 1 kg of peanut butter at least every six weeks (Woods 2009). This study area was only provided with peanut butter during the winter of 2007 for winter energetics research (Woods 2009). Study animals on the winter food-supplementation study area were sacrificed at the end of winter (Woods 2009); therefore, it was not possible to conduct this research on the study area where lactating squirrels were sampled because destructive sampling would have interfered with the long-term data collection protocols.
We chose to spatially separate the natural-food and food-supplementation study areas instead of interspersing the food-supplemented squirrels amongst individuals that were not food-supplemented (i.e. natural-food squirrels). This design presumably minimized peanut butter pilfering and intruder pressure for ownership of the peanut butter feeders from the squirrels that were not food-supplemented. Moreover, we do not think that the food-supplementation results were confounded by location because of the similarity of squirrel habitat and densities on the study areas (Boonstra et al. 2001; S. Boutin and C. J. Krebs unpublished data).
Average parturition dates for lactating females in this study were earliest on the high natural-food study area (9-Apr) and were later on the low natural-food study area (4-May) and the food- supplementation study area (7-May). We did not sample all females attempting to raise their first litter of the year on these study areas; however, the lactating females we sampled on the high natural, low natural, and food-supplementation study areas ranged from having early to late parturition dates with respect to the other squirrels on their respective study areas (percentile range of parturition dates from sampled females: high natural = 31–100, low natural = 5–93, food-supplementation = 15–83). Parturition dates on the food-supplementation study area are generally earlier than on control study areas, which is consistent with the intent of the food-supplement to mimic naturally high levels of spruce cones (Boutin et al. 2006; S. Boutin, A. G. McAdam, M. M. Humphries unpublished data). However, parturition dates on the food- supplementation study area were later than on high natural-food study areas in this study presumably because in the previous year (2005) there was the fourth largest spruce cone crop on the high natural-food study areas (range of years examined = 1988–2005; see Boutin et al. 2006 for the correlation between earlier parturition dates and abundant cone production in the previous year).
Doubly-labelled water technique
We quantified DEE using the doubly-labeled water (DLW) technique (Nagy 1983; Speakman 1997) using the methodology presented in Fletcher et al. (2012). Eleven lactation, seven non-breeding, and nine female winter DEE values were initially presented in Fletcher et al. (2012). Briefly, CO2 production was calculated based on the differential washout of the hydrogen (2H) and oxygen (18O) isotopes in the DLW over a period of 72h–120h, and this value was converted to an estimate of DEE in kJ/day. For individuals with DEE measures, the average body mass of squirrels at the initial and final blood samples of the DLW technique was used in all analyses. See Supporting Information S2 for further details on DLW methodology.
Oxidative damage and antioxidant protection
All oxidative damage samples were paired with antioxidant protection samples. Blood samples used to quantify oxidative damage and antioxidant protection were collected in live-trapped animals not exposed the DLW technique, at the same time as the initial or final samples of the DLW technique, or from animals following sacrifice. Supporting Information S3 provides more details on how blood samples were collected in the three life-stages and demonstrates that our major conclusions are robust to the influence of these different types of blood collection. All squirrel oxidative damage/antioxidant protection blood samples were collected into heparanized glass capillary tubes, and kept on ice for ≤ 4 hrs before spinning. Samples were spun for 10 minutes at 5000 rpm, stored at −20°C, and then subsequently at −80°C until they were analyzed. The number of oxidative damage/antioxidant protection samples collected within the three life-stages, on the natural-food and food-supplementation study areas, with and without paired DEE estimates are presented in Table 1.
Plasma protein oxidative damage was determined by measuring protein carbonyls using the 2,4-dinitrophenylhydrazine (DNPH) method (nmol protein carbonyls/mg protein; BIOCELL Corporation Ltd, New Zealand). This marker reflects the oxidation of plasma proteins by ROS (Stadtman 1992; Berlett and Stadtman 1997) and it has been used as a marker of oxidative damage in a diversity of species (e.g. Selman et al. 2002; Salmon et al. 2009; Ołdakowski et al. 2012; Heiss and Schoech 2012; Archer et al. 2012). Protein levels were determined using the Bradford assay prior to the oxidative damage assay to determine the amount of sample required. We quantified total antioxidant capacity using a commercially available kit (mM Trolox; Cayman Chemical Company, USA). This technique provides an integrated measure of non-enzymatic antioxidant protection that takes into account the important interactions among antioxidants that determine protection against ROS (Cohen et al. 2007; Somogyi et al. 2007; Archer et al. 2012).
Statistical analyses
DEE, oxidative damage, antioxidant protection, and body mass values were log10 transformed in all analyses. Raw average values are presented in the text ± SE. Data are displayed in the figures as raw values on a log10 transformed scale. All analyses were performed using R (R Development Core Team 2011) with α = 0.05. Our analysis proceeded in three steps. First, we used three linear models to compare DEE, oxidative damage, and antioxidant protection between the three life-stages (lactation, non-breeding, and winter) for individuals living on natural-food study areas (the effect of mass and the mass by life-stage interaction were also included in these two models). Second, we used three linear models to examine the effect of food treatment (natural-food vs. food-supplementation), life-stage (lactation and winter), and their interaction, on DEE, antioxidant protection, and oxidative damage (the antioxidant protection and oxidative damage analyses incorporated females with and without DEE measurements; Table 1). Third, we used seven linear models to examine how DEE and antioxidant protection influenced oxidative damage. Life-stage and food-treatment were included in a subset of these models and a subset of models were restricted to single life-stages. These models allowed us to determine whether the effects of both DEE and antioxidant protection on oxidative damage were confounded by life-stage and food treatment effects. Because the seven linear models involved the testing of multiple hypotheses, we performed a false discovery rate (FDR) correction to control the expected proportion of falsely rejected null hypotheses (Benjamini and Hochberg 1995; for a review see García 2004).
RESULTS
DEE was greatest during lactation on the natural-food study areas (470 ± 44 kJ/day), exceeding non-breeding DEE by 48% (318 ± 40 kJ/day), and DEE during winter by 90% (247 ± 14 kJ/day; F2,29 = 12.6, P = 0.0001; Tukey’s HSD: P < 0.01). Plasma protein oxidative damage was also greatest during lactation on the natural-food study areas (0.71 ± 0.08 nmol/mg), exceeding non-breeding oxidative damage by 1.9 times (0.38 ± 0.05 nmol/mg), and oxidative damage during winter by 4.1 times (0.17 ± 0.02 nmol/mg; F2,39 = 30.4, P < 0.0001; Tukey’s HSD: P < 0.01). Antioxidant protection on the natural-food study areas during lactation (1.44 ± 0.03 mM Trolox) was greater than antioxidant protection during winter by 18% (1.22 ± 0.10 mM Trolox; F2,39 = 3.4, P = 0.04; Tukey’s HSD: P = 0.04); however, there was no difference in antioxidant protection between lactating and non-breeding females (1.33 ± 0.03 mM Trolox; Tukey’s HSD: P = 0.73). In all three of these analyses, the effects of mass, and the mass by life-stage interactions, were not significant (P > 0.13) and were removed from the models.
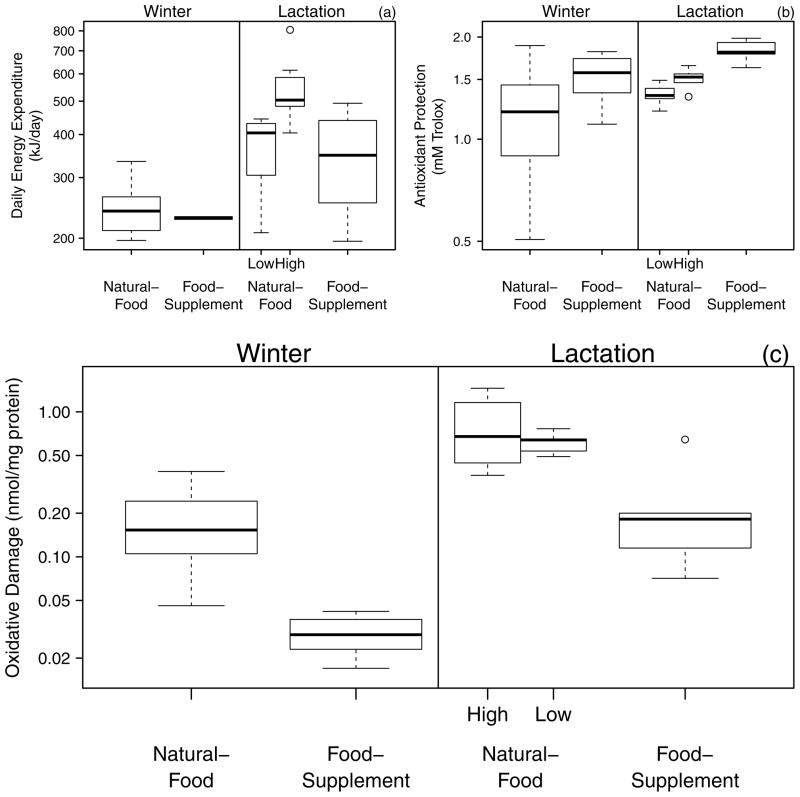
When squirrels exposed to high and low natural-food were compared, squirrels on high natural-food study areas had greater DEE (t10 = 2.9, P = 0.02; Fig. 1a) and antioxidant protection (t13 = 3.1, P = 0.008; Fig. 1b) compared to squirrels on low natural-food study areas. Plasma protein oxidative damage did not differ between the squirrels on high and low natural-food study areas (t13 = 0.8, P = 0.44; Fig. 1c).
Figure 1.
Effect of food-supplementation on (a) daily energy expenditure, (b) antioxidant protection, and (c) plasma protein oxidative damage during the life-stages of lactation and winter. Squirrels were examined on study areas where they only had access to only natural food sources (“Natural-Food”) and on study areas where all individuals had access to supplemental peanut butter (“Food-Supplement”). The natural food levels experienced by lactating females were either high or low depending on which natural-food study area the squirrels inhabited. Whiskers represent 95% confidence intervals and boxes bound the interquartile range including a median line. Life-stage influenced DEE (F1,26 = 27.5, P < 0.0001), antioxidant protection (F1,41 = 8.0, P = 0.007), and oxidative damage (F1,41 = 93.4, P < 0.0001); however, food-treatment did not interact with life-stage (P > 0.10), and was thus removed from these models.
There was a trend for squirrels on food-supplementation study areas to have lower DEE than squirrels on the natural-food study areas (Fig. 1a; F1,26 = 2.8, P = 0.11). Squirrels on food-supplementation study areas had greater antioxidant protection than squirrels on natural-food study areas (Fig. 1b; F1,41 = 9.1, P = 0.004). Food-supplemented squirrels had lower plasma protein oxidative damage than squirrels on natural-food study areas by 2.9 times during lactation and 5.9 times during winter (Fig. 1c; F1,41 = 65.8, P < 0.0001).
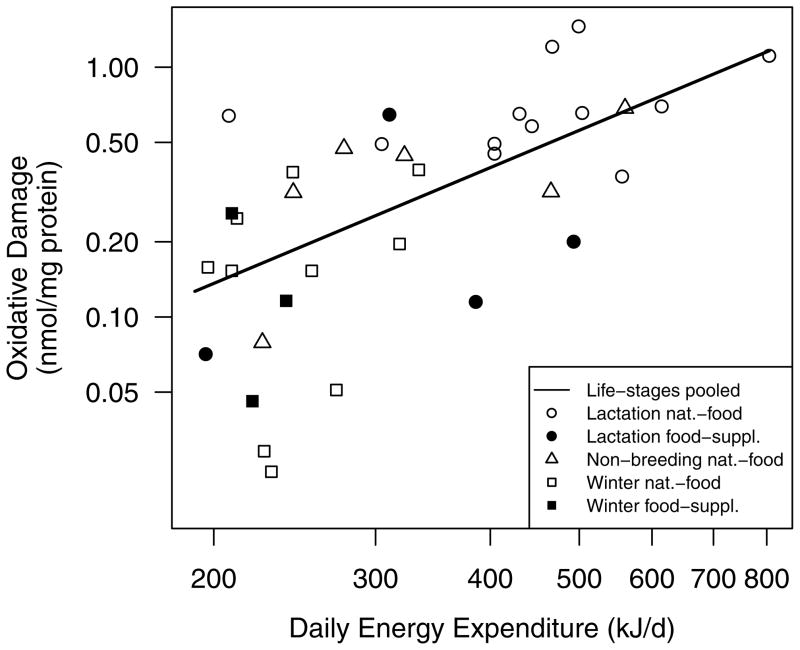
Oxidative damage was positively related with DEE (Fig. 2 – solid line) and negatively related with antioxidant protection when the effect of life-stage was controlled for (Table 2, Model number 1). However, the effects of DEE and antioxidant protection on plasma protein oxidative damage were confounded by food treatment because the effects of DEE and antioxidant protection on oxidative damage were reduced to trends (P ≤ 0.13) when the effect of food treatment was added to the model (Table 2, Model number 2). During lactation, plasma protein oxidative damage was positively related with DEE and negatively related with antioxidant protection (Table 2, Model number 3; note that the antioxidant protection effect was non-significant after the FDR correction); however, these effects were similarly confounded by food treatment (Table 2, Model number 4). Antioxidants were negatively related with oxidative damage during winter (Table 2, Model number 5; note that the antioxidant protection effect was non-significant after the FDR correction); however, this effect was also confounded by food treatment (Table 2, Model number 6). Plasma protein oxidative damage during the non-breeding life-stage was not related to either DEE or antioxidant protection (Table 2, Model number 7).
Figure 2.
The positive relationship between daily energy expenditure (DEE) and plasma protein oxidative damage pooling individuals on natural-food and food-supplementation study areas. The line-of best fit for all life-stages pooled (solid line; F1,36 = 18.8, P = 0.0001; R2 = 0.34) is displayed because this relationship was significant when the effects of life-stage and antioxidant protection were controlled for (Table 2; Model number 1).
Table 2.
Seven linear models examining the effects DEE, antioxidant protection, life-stage (winter, non-breeding, and lactation), and food-treatment (natural and food-supplement) on plasma protein oxidative damage. Individuals with DEE measurements (see Table 1) were included in each analysis. Filled cells within each model show the terms that were included in each initial model. Non-significant terms were sequentially backwards-dropped from the models. Coefficients and P-values are either those that were used to eliminate the terms from the model, or were those in the final model.
| Model Number | Life-stages included | DEE | Antioxidant protection | Life-stage | Food treatment |
|---|---|---|---|---|---|
| 1 | All | 0.9 ± 0.4 (0.03) | −1.9 ± 0.6 (0.002) | (0.004) | |
| 2 | All | 0.5 ± 0.3 (0.13) | −0.9 ± 0.8 (0.11) | (<0.0001) | (<0.0001) |
| 3 | Lactation | 1.2 ± 0.5 (0.02) | −3.0 ± 1.3 (0.04*) | ||
| 4 | Lactation | 0.03 ± 0.7 (0.96) | 1.9 ± 1.4 (0.19) | (0.0001) | |
| 5 | Winter | −0.9 ± 1.7 (0.62) | −1.9 ± 0.7 (0.03*) | ||
| 6 | Winter | −0.5 ± 1.4 (0.71) | −1.2 ± 0.6 (0.10) | (0.005) | |
| 7 | Non-breeding | 0.7 ± 0.6 (0.29) | −5.1 ± 3.4 (0.19) |
Significant variables remaining in the final models are bolded (coefficients ± SE with P-values in brackets). Bolded P-values that are starred were non-significant after a false discovery rate correction (see Materials and Methods).
DISCUSSION
Plasma protein oxidative damage was greatest during the energetically demanding life-stage of lactation as compared to the non-breeding and winter life-stages. Our results suggest that the oxidative damage experienced by animals varies considerably through the year in response to large life-stages differences in energy expenditure. DEE during lactation was nearly twice as high as DEE during winter, while plasma protein oxidative damage during lactation was more than four times greater than plasma protein oxidative damage during winter. Previous studies examining the role of oxidative damage as a cost of reproduction in captivity or in the wild have generally conducted their studies during or around the reproductive season (Alonso-Alvarez et al. 2004; Wiersma et al. 2004; Alonso-Alvarez et al. 2006; Bertrand et al. 2006; Bize et al. 2008; Garratt et al. 2011; Markó et al. 2011), when levels of energy expenditure are not likely to vary to the same extent as in our study that incorporated DEE measures of lactating, non-breeding, and winter females. DEE has been quantified in one other study that examined oxidative damage (Tamias striatus, Bergeron et al. 2011). In Bergeron et al. (2011), DEE was less variable than in our study (P. Bergeron pers. comm.: coefficient of variation = 31%; c.v. in our study = 42%). Moreover, they found that litter size was positively correlated with both DEE and oxidative damage, which suggested that there was a positive link between DEE and oxidative damage. However, the correlation between DEE and oxidative damage could not be tested directly by Bergeron et al. (2011) because DEE and oxidative damage were quantified in different individuals.
In addition to differing in DEE, squirrels in different life-stages presumably differed in hormone levels that may have influenced oxidative damage. It has been demonstrated in laboratory mammal studies conducted in vivo and in vitro that estrogens can induce antioxidant effects (Behl et al. 1997, Viña et al. 2003; Razmara et al. 2007; Miller et al. 2007; Persky et al. 2008); although other laboratory studies (reviewed in Cavalieri et al. 2000) and a study on captive Eurasian kestrels (Falco tinnunculus; Casagrande et al. 2012) reported the opposite effect.
Squirrels on the food-supplementation study areas had elevated antioxidant protection and reduced plasma protein oxidative damage during winter and lactation relative to squirrels on natural-food study areas. One possible explanation for this result is that antioxidants present in peanut butter were responsible for the reduction in oxidative damage. Peanut butter contains the polyphenolic compound resveratrol (Sobolev and Cole 1999; Ibern-Gómez et al. 2000), which is a known antioxidant (Murcia and Martinez-Tomé 2001). Moreover, roasted peanuts, which are used to make peanut butter, also have antioxidant properties that primarily result from the presence of other polyphenolics (especially p-coumaric acid; Talcott et al. 2005; Duncan et al. 2006). Considerable research has also demonstrated beneficial effects of specific antioxidants on, for example, growth, immunocompetence, and sexual selection signals (reviewed extensively in Catoni et al. 2008). For example, supplementing breeding female zebra finches (Taeniopygia guttata) with antioxidants (i.e. carotenoids) reduces the negative effect of reproductive effort on the ability to resist oxidative stress (Bertrand et al. 2006). Alternatively, squirrels on the food-supplementation study areas may have been able to increase allocation to antioxidant protection relative to squirrels on the natural-food study areas as a result of being in a better energetic “state” (sensu McNamara and Houston 1996). The greater antioxidant protection in squirrels on high natural-food versus low natural-food study areas is also consistent with the hypothesis that increased food availability increased oxidative protection as a result of an improved energetic state. This improved energetic state may involve improved body condition (fat and protein reserves), reduced activity requirements, and reduced thermoregulatory requirements resulting from favorable nutritional and energetic conditions created by access to more, higher quality food. Overall, our results comparing DEE, antioxidant protection, and oxidative damage between food treatments were based on small sample sizes (see Table 1). Thus, future research should follow up on our promising results by food-supplementing animals with natural diets and those rich in antioxidants.
Our results are consistent with the hypothesis that oxidative damage increases with DEE and decreases with antioxidant protection. However, the relationships between oxidative damage and both DEE and antioxidant protection primarily resulted due to the variation in DEE and antioxidant protection generated by the effect of food treatment (natural-food vs. food-supplementation). Specifically, food-supplementation decreased DEE (P = 0.11) and increased antioxidant protection (P = 0.004). As a result, the effects of DEE and antioxidant protection on oxidative damage were reduced to trends when the effect of food-supplementation was included in the model (compare Table 2, model numbers 1 and 2).
In conclusion, our results are consistent with the hypothesis that elevated oxidative damage results from energetic investment in reproduction that overwhelms antioxidant protection. This finding corroborates previous work on free ranging animals suggesting that oxidative damage mediates life-history trade-offs (Bergeron et al. 2011; Heiss and Schoech 2012; Wilson et al. 2012). Ironically, support for the oxidative damage hypothesis is emerging from field studies at a time when laboratory animal and biomedical researchers are turning away from this hypothesis because of equivocal and contradictory evidence and emerging alternative mechanisms (Speakman and Selman 2011). This difference may simply reflect a stronger publication bias against negative results in the wild than in the laboratory where the oxidative damage hypothesis has been tested for a longer period of time. However, a possible biological interpretation of this conflicting support is that oxidative damage may play a more important role in defining the costs of reproduction in the wild than in the laboratory. In accord with our finding that favorable nutritional and energetic conditions can reduce oxidative damage, it is possible that life-history trade-offs are not mediated by oxidative damage in the laboratory because oxidative damage is mitigated by the reduced activity requirements, increased food availability, and balanced diets that characterize many laboratory studies.
Supplementary Material
Acknowledgments
We thank all field technicians, especially Ainsley Sykes and Elizabeth Anderson, for field data collection and management. We are grateful to Paula Redman and Peter Thomson for technical assistance in isotope analyses for the DLW work. Thanks to Patrick Bergeron for providing the chipmunk DEE data. Thanks also to Neil Metcalfe, Bruce Lyon and two anonymous reviewers for helpful comments on the manuscript. Research support was provided by NSERC of Canada (A.G.M., S.B., M.M.H.), the National Science Foundation (DEB-0515849, A.G.M.), Aboriginal Affairs and Northern Development Canada Northern Scientific Training Program (Q.E.F., S.B.W.), the British Ecological Society (C.S.), and the National Institute on Aging (AG17994 and AG21042; C.L.) grants. Personal support was provided to Q.E.F. and S.B.W. by NSERC Postgraduate Graduate Scholarships, and to A.Y.S. by the American Heart Association (0615256B). This is paper number 66 of the Kluane Red Squirrel Project.
Contributor Information
Quinn E. Fletcher, Email: q.fletcher@gmail.com.
Colin Selman, Email: c.selman@abdn.ac.uk.
Stan Boutin, Email: sboutin@ualberta.ca.
Andrew G. McAdam, Email: amcadam@uoguelph.ca.
Sarah B. Woods, Email: sarah.b.woods@mail.mcgill.ca.
Arnold Y. Seo, Email: seoay@mail.nih.gov.
Christiaan Leeuwenburgh, Email: cleeuwen@aging.ufl.edu.
John R. Speakman, Email: j.speakman@abdn.ac.uk.
Murray M. Humphries, Email: murray.humphries@mcgill.ca.
Literature Cited
- Alonso-Alvarez C, Bertrand S, Devevey G, Prost J, Faivre B, Chastel O, Sorci G. An experimental manipulation of life-history trajectories and resistance to oxidative stress. Evolution. 2006;60:1913–1924. [PubMed] [Google Scholar]
- Alonso-Alvarez C, Bertrand S, Devevey G, Prost J, Faivre B, Sorci G. Increased susceptibility to oxidative stress as a proximate cost of reproduction. Ecol Lett. 2004;7:363–368. [Google Scholar]
- Archer CR, Sakaluk SK, Selman C, Royle NJ, Hunt J. Oxidative stress and the evolution of sex differences in lifespan and aging in the decorated cricket, Gryllodes sigillatus. Evolution. 2012 doi: 10.1111/j.1558-5646.2012.01805.x. In press. [DOI] [PubMed] [Google Scholar]
- Archibald DW, McAdam AG, Boutin S, Fletcher QE, Humphries MM. Within-season synchrony of a masting conifer enhances seed escape. Am Nat. 2012;179:536–544. doi: 10.1086/664623. [DOI] [PubMed] [Google Scholar]
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Behl C, Skutella T, Lezoualc’h F, Post A, Widmann M, Newton CJ, Holsboer F. Neuroprotection against oxidative stress by estrogens: structure-activity relationship. Mol Pharmacol. 1997;51:535–541. [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J Roy Stat Soc B Met. 1995;57:289–300. [Google Scholar]
- Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative Stress. J Biol Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- Bergeron P, Careau V, Humphries MM, Réale D, Speakman JR, Garant D. The energetic and oxidative costs of reproduction in a free-ranging rodent. Funct Ecol. 2011;25:1063–1071. [Google Scholar]
- Bertrand S, Alonso-Alvarez C, Devevey G, Faivre B, Prost J, Sorci G. Carotenoids modulate the trade-off between egg production and resistance to oxidative stress in zebra finches. Oecologia. 2006;147:576–584. doi: 10.1007/s00442-005-0317-8. [DOI] [PubMed] [Google Scholar]
- Bize P, Devevey G, Monaghan P, Doligez B, Christe P. Fecundity and survival in relation to resistance to oxidative stress in a free-living bird. Ecology. 2008;89:2584–2593. doi: 10.1890/07-1135.1. [DOI] [PubMed] [Google Scholar]
- Bonnet X, Lourdais O, Shine R, Naulleau G. Reproduction in a typical capital breeder: costs, currencies, and complications in the aspic viper. Ecology. 2002;83:2124–2135. [Google Scholar]
- Boonstra R, Boutin S, Byrom A, Karels T, Hubbs A, Stuart-Smith K, Blower M, Antpoehler S. The role of red squirrels and arctic ground squirrels. In: Krebs CJ, Boutin S, Boonstra R, editors. Ecosystem dynamics of the boreal forest. Oxford University Press; New York, USA: 2001. pp. 179–214. [Google Scholar]
- Boutin S, Wauters LA, McAdam AG, Humphries MM, Tosi G, Dhondt AA. Anticipatory reproduction and population growth in seed predators. Science. 2006;314:1928–1930. doi: 10.1126/science.1135520. [DOI] [PubMed] [Google Scholar]
- Casagrande S, Costantini D, Dell’Omo G, Tagliavini J, Groothuis TGG. Differential effects of testosterone metabolites oestradiol and dihydrotestosterone on oxidative stress and carotenoid-dependent colour expression in a bird. Behav Ecol Sociobiol. 2012;66:1319–1331. [Google Scholar]
- Catoni C, Peters A, Schaefer HM. Life history trade-offs are influenced by the diversity, availability and interactions of dietary antioxidants. Anim Behav. 2008;76:1107–1119. [Google Scholar]
- Cavalieri E, Frenkel K, Liehr JG, Rogan E, Roy D. Chapter 4: Estrogens as endogenous genotoxic agents - DNA adducts and mutations. J Natl Cancer Inst Monogr. 2000;2000:75–94. doi: 10.1093/oxfordjournals.jncimonographs.a024247. [DOI] [PubMed] [Google Scholar]
- Christe P, Glaizot O, Strepparava N, Devevey G, Fumagalli L. Twofold cost of reproduction: an increase in parental effort leads to higher malarial parasitaemia and to a decrease in resistance to oxidative stress. Proc R Soc Lond B Biol Sci. 2011;279:1142–1149. doi: 10.1098/rspb.2011.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock TH. Reproductive effort and terminal investment in iteroparous animals. Am Nat. 1984;123:212–229. [Google Scholar]
- Cohen A, Klasing K, Ricklefs R. Measuring circulating antioxidants in wild birds. Comp Biochem Phys B. 2007;147:110–121. doi: 10.1016/j.cbpb.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Costantini D. Oxidative stress in ecology and evolution: lessons from avian studies. Ecol Lett. 2008;11:1238–1251. doi: 10.1111/j.1461-0248.2008.01246.x. [DOI] [PubMed] [Google Scholar]
- Costantini Effects of diet quality on serum oxidative status and body mass in male and female pigeons during reproduction. Comp Biochem Physiol, A. 2010;156:294–299. doi: 10.1016/j.cbpa.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Daan S, Deerenberg C, Dijkstra C. Increased daily work precipitates natural death in the kestrel. J Anim Ecol. 1996;65:539–544. [Google Scholar]
- Descamps S, Boutin S, McAdam AG, Berteaux D, Gaillard JM. Survival costs of reproduction vary with age in North American red squirrels. Proc R Soc Lond B Biol Sci. 2009;276:1129–1135. doi: 10.1098/rspb.2008.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling DK, Simmons LW. Reactive oxygen species as universal constraints in life-history evolution. Proc R Soc Lond B Biol Sci. 2009;276:1737–1745. doi: 10.1098/rspb.2008.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan CE, Gorbet DW, Talcott ST. Phytochemical content and antioxidant capacity of water-soluble isolates from peanuts (Arachis hypogaea L.) Food Res Int. 2006;39:898–904. [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Fletcher QE, Boutin S, Lane JE, LaMontagne JM, McAdam AG, Krebs CJ, Humphries MM. The functional response of a hoarding seed predator to mast seeding. Ecology. 2010;91:2673–2683. doi: 10.1890/09-1816.1. [DOI] [PubMed] [Google Scholar]
- Fletcher QE, Speakman JR, Boutin S, McAdam AG, Woods SB, Humphries MM. Seasonal stage differences overwhelm environmental and individual factors as determinants of energy expenditure in free-ranging red squirrels. Funct Ecol. 2012;26:677–687. [Google Scholar]
- García LV. Escaping the Bonferroni iron claw in ecological studies. Oikos. 2004;105:657–663. [Google Scholar]
- Garratt M, McArdle F, Stockley P, Vasilaki A, Beynon RJ, Jackson MJ, Hurst JL. Tissue-dependent changes in oxidative damage with male reproductive effort in house mice. Funct Ecol. 2012;26:423–433. [Google Scholar]
- Garratt M, Vasilaki A, Stockley P, McArdle F, Jackson M, Hurst J. Is oxidative stress a physiological cost of reproduction? An experimental test in house mice. Proc R Soc Lond B Biol Sci. 2011;278:1098–1106. doi: 10.1098/rspb.2010.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Canada. The Nutrient Values of Some Common Foods. Canadian Government Publishing; Ottawa, Canada: 1990. [Google Scholar]
- Heiss RS, Schoech SJ. Oxidative cost of reproduction is sex specific and correlated with reproductive effort in a cooperatively breeding bird, the Florida Scrub Jay. Physiol Biochem Zool. 2012;85:499–503. doi: 10.1086/666840. [DOI] [PubMed] [Google Scholar]
- Humphries MM, Boutin S. The determinants of optimal litter size in free-ranging red squirrels. Ecology. 2000;81:2867–2877. [Google Scholar]
- Ibern-Gómez M, Roig-Pérez S, Lamuela-Raventós RM, de la Torre-Boronat MC. Resveratrol and piceid levels in natural and blended peanut butters. J Agric Food Chem. 2000;48:6352–6354. doi: 10.1021/jf000786k. [DOI] [PubMed] [Google Scholar]
- LaMontagne JM. PhD Thesis. University of Alberta; 2007. Spatial and temporal variability in white spruce (Picea glauca) cone production: individual and population responses of North American red squirrels (Tamiasciurus hudsonicus) [Google Scholar]
- Lane JE, Boutin S, Gunn MR, Slate J, Coltman DW. Genetic relatedness of mates does not predict patterns of parentage in North American red squirrels. Anim Behav. 2007;74:611–619. [Google Scholar]
- Losdat S, Helfenstein F, Gaude B, Richner H. Reproductive effort transiently reduces antioxidant capacity in a wild bird. Behav Ecol. 2011;22:1218–1226. [Google Scholar]
- Markó G, Costantini D, Michl G, Török J. Oxidative damage and plasma antioxidant capacity in relation to body size, age, male sexual traits and female reproductive performance in the collared flycatcher (Ficedula albicollis) J Comp Physiol B. 2011;181:73–81. doi: 10.1007/s00360-010-0502-x. [DOI] [PubMed] [Google Scholar]
- McAdam AG, Boutin S. Variation in viability selection among cohorts of juvenile red squirrels (Tamiasciurus hudsonicus) Evolution. 2003;57:1689–1697. doi: 10.1111/j.0014-3820.2003.tb00374.x. [DOI] [PubMed] [Google Scholar]
- McAdam AG, Boutin S, Sykes AK, Humphries MM. Life histories of female red squirrels and their contributions to population growth and lifetime fitness. Écoscience. 2007;14:362–369. [Google Scholar]
- McNab BK. The physiological ecology of vertebrates: a view from energetics. Cornell University Press; Ithaca, NY: 2002. [Google Scholar]
- McNamara JM, Houston AI. State-dependent life histories. Nature. 1996;380:215–221. doi: 10.1038/380215a0. [DOI] [PubMed] [Google Scholar]
- Miller AA, De Silva TM, Jackman KA, Sobey CG. Effect of gender and sex hormones on vascular oxidative stress. Clin Exp Pharmacol Physiol. 2007;34:1037–1043. doi: 10.1111/j.1440-1681.2007.04732.x. [DOI] [PubMed] [Google Scholar]
- Monaghan P, Metcalfe NB, Torres R. Oxidative stress as a mediator of life history tradeoffs: mechanisms, measurements and interpretation. Ecol Lett. 2009;12:75–92. doi: 10.1111/j.1461-0248.2008.01258.x. [DOI] [PubMed] [Google Scholar]
- Murcia MA, Martinez-Tomé M. Antioxidant activity of resveratrol compared with common food additives. J Food Prot. 2001;64:379–384. doi: 10.4315/0362-028x-64.3.379. [DOI] [PubMed] [Google Scholar]
- Nagy KA. The doubly labeled water (3HH18O) method: a guide to its use. University of California; Los Angeles: 1983. Publication No. 12-1417. [Google Scholar]
- van Noordwijk AJ, de Jong G. Acquisition and allocation of resources - their influence on variation in life-history tactics. Am Nat. 1986;128:137–142. [Google Scholar]
- Nussey D, Pemberton J, Pilkington J, Blount J. Life history correlates of oxidative damage in a free-living mammal population. Funct Ecol. 2009;23:809–817. [Google Scholar]
- Ołdakowski Ł, Piotrowska Ż, Chrząścik KM, Sadowska ET, Koteja P, Taylor JRE. Is reproduction costly? No increase of oxidative damage in breeding bank voles. J Exp Biol. 2012;215:1799–1805. doi: 10.1242/jeb.068452. [DOI] [PubMed] [Google Scholar]
- Persky AM, Green PS, Stubley L, Howell CO, Zaulyanov L, Brazeau GA, Simpkins JW. Protective effect of estrogens against oxidative damage to heart and skeletal muscle in vivo and in vitro. Proc Soc Exp Biol Med. 2000;223:59–66. doi: 10.1046/j.1525-1373.2000.22308.x. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. 2.13.1. R Foundation for Statistical Computing; Vienna, Austria: 2011. [Google Scholar]
- Razmara A, Duckles SP, Krause DN, Procaccio V. Estrogen suppresses brain mitochondrial oxidative stress in female and male rats. Brain Res. 2007;1176:71–81. doi: 10.1016/j.brainres.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick D. Measuring the costs of reproduction. Trends Ecol Evol. 1992;7:42–45. doi: 10.1016/0169-5347(92)90104-J. [DOI] [PubMed] [Google Scholar]
- Reznick D, Nunney L, Tessier A. Big houses, big cars, superfleas and the costs of reproduction. Trends Ecol Evol. 2000;15:421–425. doi: 10.1016/s0169-5347(00)01941-8. [DOI] [PubMed] [Google Scholar]
- Roff DA. The evolution of life histories: theory and analysis. Chapman and Hall; New York: 1992. [Google Scholar]
- Salmon AB, Leonard S, Masamsetti V, Pierce A, Podlutsky AJ, Podlutskaya N, Richardson A, Austad SN, Chaudhuri AR. The long lifespan of two bat species is correlated with resistance to protein oxidation and enhanced protein homeostasis. FASEB J. 2009;23:2317–2326. doi: 10.1096/fj.08-122523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman C, Grune T, Stolzing A, Jakstadt M, McLaren JS, Speakman JR. The consequences of acute cold exposure on protein oxidation and proteasome activity in short-tailed field voles, Microtus agrestis. Free Radic Biol Med. 2002;33:259–265. doi: 10.1016/s0891-5849(02)00874-2. [DOI] [PubMed] [Google Scholar]
- Selman C, McLaren J, Mayer C, Duncan J, Collins A, Duthie G, Redman P, Speakman J. Lifelong α-tocopherol supplementation increases the median life span of C57BL/6 mice in the cold but has only minor effects on oxidative damage. Rejuv Res. 2008a;11:83–96. doi: 10.1089/rej.2007.0586. [DOI] [PubMed] [Google Scholar]
- Selman C, McLaren JS, Collins AR, Duthie GG, Speakman JR. The impact of experimentally elevated energy expenditure on oxidative stress and lifespan in the short-tailed field vole Microtus agrestis. Proc R Soc Lond B Biol Sci. 2008b;275:1907–1916. doi: 10.1098/rspb.2008.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman C, Blount JD, Nussey DH, Speakman JR. Oxidative damage, ageing, and life-history evolution: where now? Trends Ecol. Evol. 2012;27:570–577. doi: 10.1016/j.tree.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Sobolev VS, Cole RJ. trans-Resveratrol content in commercial peanuts and peanut products. J Agric Food Chem. 1999;47:1435–1439. doi: 10.1021/jf9809885. [DOI] [PubMed] [Google Scholar]
- Somogyi A, Rosta K, Pusztai P, Tulassay Z, Nagy G. Antioxidant measurements. Physiol Meas. 2007;28:R41–R55. doi: 10.1088/0967-3334/28/4/R01. [DOI] [PubMed] [Google Scholar]
- Speakman JR. Doubly-labelled water: theory and practice. Chapman and Hall; London: 1997. [Google Scholar]
- Speakman JR. The physiological costs of reproduction in small mammals. Philos T Roy Soc B. 2008;363:375–398. doi: 10.1098/rstb.2007.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR, Ergon T, Cavanagh R, Reid K, Scantlebury D, Lambin X. Resting and daily energy expenditures of free-living field voles are positively correlated but reflect extrinsic rather than intrinsic effects. P Natl Acad Sci USA. 2003;100:14057–14062. doi: 10.1073/pnas.2235671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR, Selman C. The free-radical damage theory: accumulating evidence against a simple link of oxidative stress to ageing and lifespan. Bioessays. 2011;33:255–259. doi: 10.1002/bies.201000132. [DOI] [PubMed] [Google Scholar]
- Stadtman ER. Protein Oxidation and Aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- Stearns SC. The evolution of life histories. Oxford University Press; New York: 1992. [Google Scholar]
- Talcott ST, Passeretti S, Duncan CE, Gorbet DW. Polyphenolic content and sensory properties of normal and high oleic acid peanuts. Food Chem. 2005;90:379–388. [Google Scholar]
- Thomas DW, Blondel J, Perret P, Lambrechts MM, Speakman JR. Energetic and fitness costs of mismatching resource supply and demand in seasonally breeding birds. Science. 2001;291:2598–2600. doi: 10.1126/science.1057487. [DOI] [PubMed] [Google Scholar]
- Tinbergen JM, Verhulst S. A fixed energetic ceiling to parental effort in the great tit? J Anim Ecol. 2000;69:323–334. [Google Scholar]
- Tuomi J, Hakala T, Haukioja E. Alternative concepts of reproductive effort, costs of reproduction, and selection in life-history evolution. Am Zool. 1983;23:25–34. [Google Scholar]
- Viña J, Sastre J, Pallardó F, Borrás C. Mitochondrial theory of aging: importance to explain why females live longer than males. Antioxid Redox Signal. 2003;5:549–556. doi: 10.1089/152308603770310194. [DOI] [PubMed] [Google Scholar]
- Welcker J, Moe B, Bech C, Fyhn M, Schultner J, Speakman JR, Gabrielsen GW. Evidence for an intrinsic energetic ceiling in free-ranging kittiwakes Rissa tridactyla. J Anim Ecol. 2010;79:205–213. doi: 10.1111/j.1365-2656.2009.01626.x. [DOI] [PubMed] [Google Scholar]
- Wiersma P, Selman C, Speakman JR, Verhulst S. Birds sacrifice oxidative protection for reproduction. Proc R Soc Lond B Biol Sci. 2004;271:S360–S363. doi: 10.1098/rsbl.2004.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Gravel MA, Mackie TA, Willmore WG, Cooke SJ. Oxidative stress associated with paternal care in smallmouth bass (Micropterus dolomieu) Comp Biochem Physiol, A. 2012;162:212–218. doi: 10.1016/j.cbpa.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Woods SB. MSc Thesis. McGill University; 2009. Resource-mediated thermoregulation and energetics of a northern population of red squirrels (Tamiasciurus hudsonicus) in winter. [Google Scholar]
- Zera AJ, Harshman LG. The physiology of life history trade-offs in animals. Ann Rev Ecol Syst. 2001;32:95–126. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.