Abstract
Chronic venous leg ulcers (CVLUs) affect approximately 600,000 people annually in the United States and accrue yearly treatment costs of US$2.5–5 billion. As the population ages, demands on health care resources for CVLU treatments are predicted to drastically increase because the incidence of CVLUs is highest in those ≥65 years of age. Furthermore, regardless of current standards of care, healing complications and high recurrence rates prevail. Thus, it is critical that factors leading to or exacerbating CVLUs be discerned and more effective, adjuvant, evidence-based treatment strategies be utilized. Previous studies have suggested that CVLUs’ pathogenesis is related to the prolonged presence of high numbers of activated neutrophils secreting proteases in the wound bed that destroy growth factors, receptors, and the extracellular matrix that are essential for healing. These events are believed to contribute to a chronically inflamed wound that fails to heal. Therefore, the purpose of this project was to review studies from the past 15 years (1996–2011) that characterized neutrophil activity in the microenvironment of human CVLUs for new evidence that could explicate the proposed relationship between excessive, sustained neutrophil activity and CVLUs. We also appraised the strength of evidence for current and potential therapeutics that target excessive neutrophil activity.
Chronic venous leg ulcers (CVLUs) pose a significant health and economic burden due to their high prevalence and recurrence rates. They comprise the largest single group of leg ulcers treated in wound care clinics in the United States.1 Moreover, it has been estimated that up to 1 in 20 adults in all westernized countries are affected by venous ulcerations, either open or healed.2–4 CVLUs can be traumatic for patients because of pain, reduced mobility, decreased quality of life, and health care costs related to protracted treatments. Current statistics show that approximately 15% of venous ulcers never heal and that recurrence occurs once or numerous times in up to 71% of cases,5,6 which contributes to annual US treatment cost estimations of US$2.5–5 billion.7–11 In addition, these costs are predicted to escalate because the incidence of CVLUs increases in persons aged ≥65 years, which is a US population segment expected to grow to approximately 71 million by 2030.12,13 These dramatic health care statistics beg for a clearer understanding of the microprocesses that contribute to the development of CVLUs in patients with chronic venous insufficiency (CVI). Elucidating the pathobiology of CVLUs can inform the development of adjunct therapies to facilitate the healing of these recalcitrant wounds or help prevent their recurrence.
Although the definitive link between CVI and CVLU is unclear, many acknowledge that a consistent feature of venous ulcer formation is chronic inflammation associated with the trapping of activated leukocytes in limbs with venous dysfunction.14–18 Studies have shown increased neutrophil degranulation in all clinical stages of venous disease evident by enzyme-linked immunosorbent assay (ELISA) testing for plasma neutrophil elastase19,20 and lactoferrin.20 In addition, it has been showed that high numbers of activated neutrophils exist in the microenvironment of chronically inflamed ulcers secreting excessive amounts of proteases that can cause tissue destruction and persistent inflammation that delay advancement to subsequent healing stages.2,21–23 Findings from collective studies suggest that both a sustained systemic and local inflammatory response involving prolonged neutrophil activation is occurring in patients with CVI. This article briefly reviews (1) the function of neutrophils in wound healing; (2) studies in the past 15 years (1996–2011) that have characterized neutrophil activity in the microenvironment of human CVLUs; and (3) therapeutics to target excessive neutrophil activity.
NEUTROPHIL FUNCTION IN WOUND HEALING
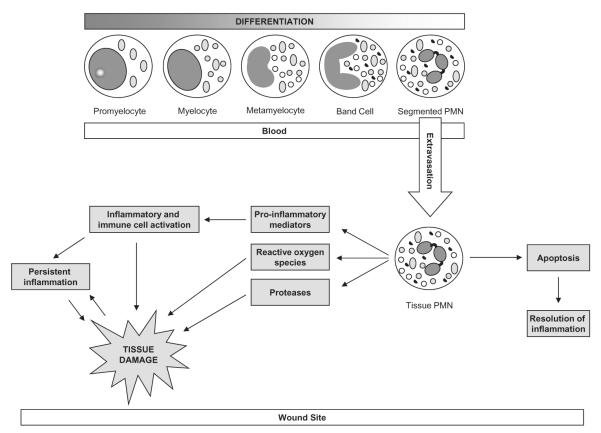
In the normal wound healing process, neutrophils are one of first cells to arrive at the site of tissue injury and have three primary actions: (1) sterilizing microbes; (2) producing molecular signals to limit the amassing of additional neutrophils; and (3) initiating an anti-inflammatory, tissue restorative process involving macrophages and epithelial cells. However, these actions require that neutrophils first migrate from the circulatory system to the wound site, a process that occurs within minutes of injury.24 After moving into the tissues, there is a burst of transcriptional activity that results in the neutrophilic generation of chemokines and cytokines important for chemotaxis of additional neutrophils, macrophages and T cells to the injured site, and for the control of their inflammatory responses.24,25 Interestingly, findings have differed among some studies examining the role of neutrophils in acute wound repair using animal models. For example, Simpson and Ross tested an antineutrophil serum in guinea pigs and reported findings suggesting that a neutrophil response is not necessary for normal wound healing.26 Conversely, Dovi et al. reported accelerated wound closure in neutrophil-depleted mice and suggested that neutrophils may delay normal wound closure.27 In studies of human wounds that fail to heal, such as CVLUs, it has been proposed that when signals from the inflammatory microenvironment are aberrant, and neutrophil influx continues unabated, neutrophils become resistant to apoptosis, the normal tightly regulated system of inflammation resolution fails, and tissue destruction occurs (Figure 1).28
Figure 1.
Unregulated neutrophil activation associated with chronic venous leg ulcers.
Neutrophils are phagocytes that engulf microorganisms present in the wound and kill them using a variety of antimicrobial substances such as oxidants and proteases. These substances are meant to be protective; however, they can contribute to the development of CVLUs by leading to further tissue damage when produced at high levels (Figure 1). During phagocytosis, neutrophils generate copious amounts of reactive oxygen species (ROS) by way of respiratory burst to kill pathogens.29 Apart from killing pathogens, ROS in the wound fluid help drive redox signaling.30 Excessive ROS are deleterious for the regenerating host tissue. This is particularly apparent in chronic wound situations, and might as well underlie the persistent tissue-destroying nature of such wounds. Neutrophils in patients with chronic venous disease inappropriately produce more oxygen free radicals (ROS) as a result of amplification of a calcium-dependent signal pathway.31 In addition, increasing evidence of high iron levels in venous leg ulcer tissue suggests that iron is playing an active role in perpetuating tissue damage in venous ulceration by augmenting local inflammation.31,32 The iron-mediated Fenton reaction may be another mechanism of excessive tissue damage in CVLU.
In addition to producing high levels of ROS, neutrophils also release many types of proteases during the repair process. Although the primary function of the proteases is to help kill and degrade microbes, they can also be used by the neutrophil to degrade components of the endothelial basement membrane. This can aid in neutrophil movement from the circulation, into the tissue, and to the site of injury. The proteases also contribute to the debridement of necrotic tissue at the wound site. There are two main classes of proteases released by neutrophils that play a role in wound healing: serine proteases and matrix metalloproteinases (MMPs). Neutrophil-derived serine proteases include cathepsin G, elastase, and proteinase 3. Of the MMPs present in neutrophil granules, MMP-2, MMP-8, and MMP-9 have been studied the most frequently in the context of wound repair. Clinically, these proteases are important because they are responsible for the added damage to host tissues that can be caused by neutrophils when released into the extracellular space at high levels. While neutrophil-derived proteases have important functions during the normal repair process, there is a great deal of evidence suggesting that high levels of proteases can be detrimental to the repair process.
STUDIES PROFILING NEUTROPHILS, PROTEASES, AND PROTEASE INHIBITORS IN HUMAN CVLUs
Over the years, numerous studies have evaluated the microenvironment of human chronic wounds of various origins in an effort to characterize the molecular and cellular components associated with poor healing. A vigorous neutrophil response and high levels of neutrophil-derived enzymes were frequently reported.21,33–41 However, to provide a more succinct, current analysis focusing specifically on CVLUs, this brief review will include observational or intervention studies within the past 15 years that have analyzed human CVLU wound fluid or tissue for neutrophil activity and/or other proteolytic enzymes. A search was conducted with PubMed, Medline, and Cochrane databases using combinations of the terms “neutrophils,” “enzymes,” “leukocytes,” “matrix metalloproteinases,” “proteases,” “proteinases,” “proteolytic activity,” “MMPs,” “microenvironment,” “human chronic wounds,” and “chronic venous leg ulcers.” Review articles were also searched.
Of the 24 studies identified using the chosen criteria, several evaluated the expression and activity of various MMP classes, including gelatinases (MMP-2, MMP-9), collagenases (MMP-1, MMP-8), stromelysins (MMP-3, MMP-10, and MMP-11) and membrane type MMPs (MT1-MMP, MT2-MMP) (Table 1). Elevated MMP levels have been associated with chronic wound types in previous studies.33,41–43 In general agreement, some researchers included in this review reported that select MMPs were up-regulated in the microenvironment of CVLUs.22,38,44–51 For example, levels of MMP-9, which is thought to be either neutrophil- or macrophage-derived in open non-healing ulcers,52 were found to be significantly higher in CVLUs than acute wounds and higher levels were associated with a clinically worse wound.53,54 Other studies reported similar data regarding MMP-9 activity45,55 and that diminishing MMP-9 expression coincided with healing.41,54,56 Mirastschijski et al. found that although MMP-9 activity did not differ significantly between acute and chronic wounds, MMP-9 was concentrated in inflammatory cells of the chronic ulcer bed, while in acute wounds, it was predominantly expressed by the advancing epithelium.40 They posit that a persistence of MMP-9 in the ulcer bed may be degrading the extracellular matrix (ECM) and depriving keratinocytes at the wounds edges from stimulatory cell-matrix interactions.
Table 1.
Trials characterizing proteolytic environment of human chronic venous leg ulcers from 1996–2011
| Reference, year | Number of patients |
Wound types | Samples | Sample collection method |
Results |
|---|---|---|---|---|---|
| Grinnell and Zhu, 199637 |
3 | CVLUs—3 Acute—? (mastectomy) |
Fluid | CVLUs—occlusive film (Tegaderm)—aspiration Acute—surgical drainages |
↑ Levels of HNE associated with cleavage of α2-M and α1-PI and fibronectin degradation in CVLUs ↑ Levels of MMP-9 in CVLUs |
| Weckroth et al., 199648 |
16 | CVLUs—10 Acute—6 (donor sites: skin grafting) |
Fluid | Blunt-end glass microcapillaries |
↑ Activity of gelatinase and MMP-1 Low activity of elastase and capthepsin G (neutrophil origin) |
| Herrick et al., 199769 | 28 | CVLUs—5 Acute—72 (created) |
Tissue | CVLU—excisional biopsies Acute—punch biopsies |
Up-regulation of HNE in acute wounds of aged subjects and CVLUs associated with ECM degradation |
| Vaalamo et al., 199751 | 22 | CVLUs—11 Acute—11 |
Tissue | CVLUs—excisional biopsies Acute—biopsies of healing donor areas |
↑ MMP-13 expression in CVLUs, but not in normally healing wounds |
| Yager et al., 199633 | 20 | CVLUs—3 Pressure ulcers-7 Acute—10 (mastectomy) |
Fluid | CVLUs—occlusive film (Tegaderm)—aspiration Acute—surgical drainages |
Levels of HNE > in chronic wounds than acute wounds—associated with degradation of growth factors |
| Latijnhouwers et al., 199871 |
6 | CVLUs—6 | Fluid | CVLUs—occlusive film—aspiration |
Degradation of tenascin-C (ECM glycoprotein) correlated with elastase and MMP activity |
| Nwomeh et al., 199939 |
37 | CVLUs—3 Pressure ulcers-10 Acute—4 (mastectomy) Controls—20 |
Fluid Tissue |
CVLUs—occlusive film (Tegaderm)—aspiration Acute—surgical drainages Controls—biopsy, occlusive dressing—aspiration |
↑ Levels of MMP-1 and MMP-8 and ↓ levels of TIMP-1 in chronic ulcers compared to healing wounds MMP-8 predominant collagenase in healing and nonhealing ulcers, but almost exclusively in inactive form in healing wounds |
| Trengove et al., 199938 |
33 | CVLUs—5 Mixed chronic—20 Acute—8 (mastectomy) |
Fluid | CVLUs—occlusive film (Opsite)—aspiration Acute—surgical drainages |
MMPs ↑ ×30-fold in chronic wounds compared to acute wounds Lower levels of TIMP-1 in chronic wounds; ↑ Levels of MMPs ↓ with healing of CVLUs |
| Tarlton et al., 199954 | 35 | CVLUs-25 Acute—10 |
Fluid | CVLUs—collection filters Acute—surgical drainages |
Higher expression of pro- and activated MMP-2 and 9 and HNE in CVLUS than acute wounds Higher expression of MMP-2 and 9 and HNE with increased severity of wound site ↑ MMP-9 at ulcer edge associated with least evidence of healing ↓ Expression of MMP-9 where ulcers showed evidence of healing |
| Mirastschijski et al., 200240 |
52 | CVLUs-10 Mixed chronic—24 Acute—18 |
Fluid Tissue |
Foam disks CVLUs—excisional biopsies Acute—dermatome wounds or punch biopsies |
MMP-9 activity did not differ significantly between acute and chronic wounds, but localization did. MMP-9 concentrated in inflammatory cells of ulcer bed in chronic wounds, but predominantly in advancing epithelium of acute wounds.40 |
| Norgauer et al., 200244 |
23 | CVLUs—12 Controls—11 |
Tissue | Punch biopsies | Higher expression of EMMPRIN, MMP-2, MT1-MMP, and MT2-MMP in CVLUs than controls |
| Ulrich et al., 200550 | 40 | CVLUs—20 Acute—20 (donor sites: skin grafting) |
Fluid | Absorption to release dressing |
CVLU fluid inhibited angiogenesis significantly thought to be effect of MMP-2 and MMP-9 MMP-2/9 inhibition associated with angiogenesis stimulation |
| Mwaura et al., 200647 | 40 | CVLUs—40 (20 healing, 20 nonhealing) |
Fluid Tissue |
Aspirated from dressing with micropipettes Punch biopsies |
↑ MMP-2 levels and ↓ TIMP-2 levels in nonhealing ulcers compared with healing ulcers Expression of EMMPRIN in all CVLUs |
| Pirila et al., 200761 | 16 | CVLUs—4 Mixed chronic—8 Acute—4 |
Tissue | Biopsies | MMP-8 predominantly expressed in all chronic wounds, but not detected in acute wounds MMP-26 expressed in most chronic wounds except for two most prolonged ulcers |
| Beidler et al., 200845 | 29 | CVLUs | Tissue— ulcers compared to healthy tissue |
Punch biopsies | ↑ MMP-1, 2, 3, 8, 9, 12, and 13 levels in ulcerated tissue MMP-8 and MMP-9 most highly expressed in ulcer tissue prior to compression treatment ↓ Levels of MMP-1, 2, and 3 associated with significantly improved healing rates |
| Lundqvist et al., 200872 |
24 | CVLUs—15 Acute—9 (mastectomy) |
Fluid Tissue |
CVLUs—occlusive film (Opsite)—aspiration Acute—surgical drainages 4 mm tissue biopsies |
↑ Levels neutrophil α-defensins in CVLU fluid and tissue than in acute wounds Neutrophil counts significantly higher in CVLUs than in acute wounds |
| Meyer et al., 200862 | 27 | CVLUs—27 Acute—15 |
Tissue | Punch biopsies | MMP-1 > in healing ulcers than nonhealing ulcers Total MMPs significantly > in CVLUs than acute wounds |
| Rayment et al., 200853 |
12 | CVLUs—9 Acute—3 (subepidermal blisters on feet) |
Fluid | CVLUs—occlusive film—aspiration Acute—aspiration |
Significantly > levels of MMP-9 in CVLUs than acute wounds Higher levels of MMP-9 in CVLUs associated with clinically worse wound |
| Smeets et al., 200846 | 27 | CVLUs—27 | Fluid | Absorption onto Release dressing |
↑ Activity of HNE, gelatinase, plasmin and MMP-2 |
| Subramaniam et al., 200864 |
17 | CVLUs—9 Acute—8 (mastectomy) |
Fluid | CVLUs—occlusive film (Opsite)—aspiration Acute—surgical drainages |
↑ MMP-1, ↑ MMP-3, ↓ TIMP-1 significantly induced by CVLU fluid |
| Moor et al., 200922 | 4 | CVLUs—4 | Fluid Tissue |
Capillary wicking (Tegapore, Whatman 54 filter papers) 4 mm tissue biopsies |
MMP-9 > in fluid than tissue MMP-8 in both fluid and tissue ↑ MPO, HNE and pro-inflammatory cytokines IL-6, IL-8, IL-1β in fluid |
| Wiegand et al., 201070 |
38 | CVLUs—11 Mixed chronic—17 Acute—10 (ablation of seborrheic warts) |
Fluid | Saline rinse—glass washing chamber |
HNE 10× > in chronic than acute wounds MMP-2, MMP-13 significantly > in CVLUs than acute wounds; IL-1β, IL-6, IL-8 significantly > in chronic than acute wounds ↓ Levels of HNE, MMP-2, MMP-13, IL-1β, IL-8 associated with initiation of CVLU healing |
| Eming et al., 201055 | 28 | CVLUs—19 Acute—9 (cutaneous wounds) |
Fluid | Occlusive film (Hyalofilm)- aspiration |
MMP-9, HNE, proteinase 3 exclusively detected in CVLUs MMP-9 22× > in CVLUs than acute wounds MPO markedly ↑ in CVLUs |
| Trostrup et al., 201176 | 33 | CVLUs—8 Acute—25 |
Fluid Tissue |
CVLUs—Polyurethane foam covered with occlusive film (Tegaderm)– aspiration Acute—occlusive film-aspiration CVLUs—excisional biopsies Acute—punch biopsies |
No differences in levels of proteinases/inhibitors between chronic and acute fluid |
α1-PI, alpha1-proteinase inhibitor; α2-M, alpha2-macroglobulin; CVI, chronic venous insufficiency; CVLU, chronic venous leg ulcer; ECM, extracellular matrix; EMMPRIN, extracellular MMP inducer; HNE, human neutrophil elastase; IL, interleukin; MMP, matrix metalloproteinases; MPO, myeloperoxidase; MT, membrane type; TIMP, tissue inhibitor of metalloproteinases.
Neutrophils contain large amounts of MMP-8 and MMP-9 in their granules57 and are hypothesized to be principal contributors of MMP-2 to the wound environment.41,42,58 Although MMP-9 and MMP-2 are believed to be essential for wound healing to occur when inflammation has subsided,59 some suggest that if significantly increased levels of MMP-2 and MMP-9 are present before inflammation has subsided, the excessive proteolytic milieu will continue to degrade key elements for normal healing to ensue, such as protein compounds of the ECM.53,60
Studies in this review that evaluated MMP-8 reported increased levels and expression in tissues biopsied from CVLUs,45 significantly higher levels in fluid from chronic ulcers compared with healing wounds,39,54 and that MMP-8 was strongly expressed in all chronic wounds evaluated, but not in acute wounds.61 Somewhat to the contrary, Nwomeh et al.39 found a preponderance of MMP-8 in both healing wounds and nonhealing ulcers.
Four studies quantified total MMPs and found higher levels in ulcerated tissue compared with adjacent tissue,45 higher levels in chronic wound fluid compare with acute wound fluid,38 higher levels in tissue from ulcers that failed to heal when compared with those that healed,62 and higher total MMP activity in ulcers than in normal skin.62
In addition to quantifying MMPs, three studies assessed tissue inhibitors of MMPs (TIMPs) because an imbalance between MMPs and TIMPs has been associated with ECM breakdown and thus, the pathogenesis of chronic wounds.60,63 These studies reported lower levels of TIMP-1 in chronic wound fluid compared with acute wound fluid,38 significantly lower levels in chronic ulcers compared with healing wounds39 and that CVLU fluid reduced TIMP-1 levels in vitro significantly more than acute wound fluid.64
Along with MMPs, human neutrophil elastase (HNE) was measured in several of the studies reviewed. Neutrophil elastase has broad specificity and has been found to be present in several chronic wound types.65–67 If uncontrolled, HNE can have damaging effects on a number of ECM proteins, cytokines and growth factors, and cell surface receptors.66,68 Studies included in this review evaluating HNE reported high levels in CVLU fluid,37,46,54 an up-regulation of HNE associated with ECM degradation in acute wounds of aged subjects and CVLUs,69 and higher levels in chronic wounds compared with acute wounds54,70 that were associated with degradation of growth factors68 and ECM glycoproteins.71 Furthermore, lower levels of HNE, along with MMP2, MMP12, and the proinflammatory cytokines IL-1β and IL-8 were associated with initiation of CVLU healing.70 One study by Weckroth et al.,48 however, reported low activity of HNE and cathepsin G of neutrophil origin in CVLU fluid.
Two of the studies reviewed quantifying neutrophil activity in wound fluid, reported strikingly elevated levels of a neutrophil marker of infiltration, myeloperoxidase (MPO), in CVLU fluid,22 and markedly increased MPO levels in CVLU fluid compared with acute wound fluid.55 Moreover, Lundqvist et al. determined that there were significantly higher absolute numbers of neutrophils and higher levels of α-defensins, an antimicrobial peptide in the azurophil granules of neutrophils in CVLU fluid and tissues when compared with acute wound fluid.72 Although defensins are secreted into the phagolysosome following phagocytosis of bacteria, they may be released extracellularly from neutrophils. Importantly, recent findings reveal that excessive levels of defensins initiate proinflammatory,73 and possibly cytotoxic actions74 because of their participation in chemotaxis and activation of antigen-presenting cells.75
One of the studies included in the present review used multiplexed antibody microarray profiling to compare 48 different proteins in wound fluid from CVLUs and acute wounds.76 No significant differences in levels of proteinases and antiproteinases, growth factors, angiogenic factors, or inflammatory cytokines were detected, contradicting the majority of earlier studies, but the injury-induced protein S100A8/A9 was lower in CVLU wound fluid compared with healing wounds. Although the implications of this finding are unclear, the authors suggest that it may reflect a difference in inflammatory cell composition between CVLUs and healing wounds.76
Determining if there are parallel findings across studies examining neutrophil activity in the microenvironment of human CVLUs can be challenging because of variations in design and methodology. For example, different wound fluid collection methods may not produce comparable outcomes.77 Similarly, variations in inclusion/exclusion criteria and CVLU diagnostic criteria complicate comparisons. Additionally, attaining a sample size adequate enough to generate statistically sound results continues to be a challenge for all human wound studies. However, the collective findings from the majority of studies reviewed for this paper provide evidence that imbalances in the expression and control of proteolytic enzymes are involved in the pathogenesis of CVLUs and that high levels of invading neutrophils are primary sources of several key proteases identified in these problematic wounds. So, how should these data be translated to clinical practice, or should they?
DIAGNOSTIC PROTEASE TOOLS
Several advisory panels assigned the task of creating evidence-based guidelines for treating chronic wounds such as CVLUs have recognized that a growing body of evidence supports a relationship between high wound fluid protease levels and nonhealing wounds.78–80 Some have endorsed using protease levels as biochemical markers to guide treatment.78,79,81,82 However, because clinical observations alone cannot detect high protease activity in the microenvironment of CVLUs, a simple diagnostic tool to quantify protease levels within the wound would help clinicians determine if appropriate management strategies designed to dampen excessive protease activity are indicated. One such tool (WoundChek Protease Status—Systagenix) has recently been brought to the market. It is a visually read immunochromatographic test that quantifies neutrophil-derived protease (MMPs and elastase) activity within 15 minutes using wound fluid swab samples. In addition, the World Union of Wound Healing Societies consensus document regarding the role of proteases in wound diagnostics has proposed an algorithm to determine when to use the point-of-care protease test in clinical practice.79 Together, the point-of-care diagnostic tool and the proposed algorithm are cost-effective, personalized treatment approaches to CVLU care because they help select the patients who are most likely to benefit from protease inhibiting therapies and they help determine when to start and stop their use.
PERSPECTIVES FOR THERAPY
The mounting data showing high neutrophil concentrations and excessive levels of active neutrophil-derived proteases in the microenvironment of CVLUs are guiding the development of novel therapeutics for treatment. (Table 2) Interestingly, the benefits of compression therapy, the gold standard for CVLU treatment, have been linked to its ability to reduce excessive levels of MMP-3, MMP-8, and MMP-9 in CVLU tissue.45 Additionally, an effect of negative pressure therapy to stimulate wound healing may be to diminish protease and proinflammatory mediator activity.83–85 However, adjunct therapies to conventional care are needed to improve healing rates, especially for the many patients who cannot tolerate or do not respond well to compression therapy.
Table 2.
Nonexhaustive list of therapeutics to reduce excessive neutrophil activity
| Therapeutics | Principle | Level strength of evidence* |
References |
|---|---|---|---|
| Topical | |||
| Lower extremity compression | ↓ Edema and venous hypertension ↓ Protease levels in CVLU tissue |
Level I | (43) |
| Negative pressure therapy | Removes exudates containing excessive proteases and proinflammatory mediators |
Level II | (81–83) |
| Mechanism-based dressings | To sequester, remove or inactivate excessive proteases |
Level I | (22,49,68,97–101) |
| Doxycycline | Inhibits MMP | Level II | (84–87,92,93) |
| Systemic | |||
| Pentoxifylline | Improves microcirculation of leg ↓ Neutrophil adhesion to endothelium ↓ Proinflammatory cytokine synthesis ↓ Free oxygen radical formation by neutrophils |
Level I | (102–106) |
| MPFF | ↓ Synthesis of prostaglandins, free oxygen radicals, and inhibits leukocyte trapping and activation |
Level I | (107–110) |
| EPA + DHA supplementation | ↑ Endogenous production of lipid mediators that reduce neutrophil influx ↓ Free oxygen radicals by leukocytes ↑ Macrophage clearance of apoptotic neutrophils |
Level II | (26,112–117) |
Level I: Meta-analysis of multiple randomized clinical trials (RCTs) or at least two RCTs support the intervention. Level II: Less than level I, but at least one RCT and at least two significant clinical series or expert opinion papers with literature reviews supporting the intervention. Experimental evidence convincing, but not yet supported by adequate human experience is included. Level III: Suggestive data of proof of principle, but lacking sufficient data such as meta-analysis, RCT, or multiple clinical series. MMP, matrix metalloproteinase; MPFF, micronized purified flavonoid fraction; EPA + DHA, eicosapentanoic acid and docosahexanoic acid—polyunsaturated fatty acids.
The testing of therapies to quell the damaging effects of high proteolytic activity has included topical doxycycline, which has been shown to be an inhibitor of MMPs.86–89 The inhibitory action of doxycycline on these zinc-dependent proteases is likely due to its ability to disrupt the active site, and not through degradation of the proteases.89 Studies of human and animal disease models characterized by high MMP activity such as osteoarthritis and periodontitis report that doxycycline and other tetracyclines not only reduce MMP catalytic activity, but suppress MMP synthesis when administered topically or systemically.87,90–94 Although there are only a few studies that have examined the effect of tetracycline derivatives on elevated levels of neutrophil-derived MMPs in chronic wounds, the findings are compelling. For example, when a topical nonantimicrobial tetracycline (chemically modified tetracycline) was applied to the wounds of diabetic rats, a dose-dependent effect was noted; there was decreased activity of MMP-9 and MMP-8 and increased granulation tissue when compared with untreated diabetic rat wounds.94 In a more recent study, Chin et al.95 reported that doxycycline reduced MMPs (collagenases: MMP-1, MMP-8, and MMP-13; gelatinases: MMP-2 and MMP-9) in fluid from human diabetic foot ulcers in a dose-dependent manner. The data also revealed that topical doxycycline therapy (1% gel) decreased levels of HNE and significantly increased ulcer healing when compared to ulcers treated with hydrogel with no adverse effects. Topical therapies containing tetracycline derivatives are associated with fewer complications when compared with oral forms, which can elicit allergic reactions, systemic reactions, and photosensitivity.96 Therefore, topical doxycycline may be indicated for chronic wounds such as CVLUs to inhibit select MMP activity, but its effectiveness for this purpose must first be tested in larger randomized clinical trials.
Other topical approaches for sequestering, removing, or inactivating excessive proteases involve mechanism-based wound dressings that have showed variable degrees of effectiveness.97,98 A few of the current designs include oxidized regenerated cellulose and collagen acting as a competitive substrate for wound fluid proteases,52 nonocrystalline silver-coated high density polyethylene,99 peptide,100 and ionically derivatized dressings of cotton,101 bacterial cellulose and collagen70 and oleic acid, a potent, nontoxic selective inhibitor of HNE, bound to cotton.102 Additionally, wound dressings composed of novel sulfonated hydrogel composites have been shown to be successful at appropriating and/or reducing excessive neutrophil-derived proteases. Sulfonated polymer is capable of binding proteases through electrostatic interactions.22 In a 2005 study, sulfonated styrene-ethylene-butylenes-styrene triblock polymer (S-SEBS) was exposed to several cations such as silver (Ag+), sodium (Na+), and doxycycline H+. The S-SEBS material containing docycycline H+ was found to be superior to a commercial dressing at inhibiting neutrophil-derived MMP-8 and HNE in chronic wound fluids when tested under the same conditions.103 As expected, the dressing also inhibited bacteria growth. This study showed that the S-SEBS polymer could be tailored to sequester specific proteases. In a more recent study, another sulfonated polymer dressing was also associated with a reduction in the activity of both MMP-8 and HNE.22 Novel wound dressings that sequester proteases or deliver inhibitors of neutrophil-derived proteases directly in the biochemical environment of CVLUs may be costly, but are reasonable approaches to consider.
In addition to applying specialized dressings to chronic wounds such as CVLUs to modulate neutrophil activity, there are systemic approaches. Two systemic agents that are included in the Guidelines for the Treatment of Venous Ulcers (Level I evidence) supported by the Wound Healing Society and recommended to be used in conjunction with compression therapy are pentoxifylline and micronized purified flavonoid fraction (MPFF).80 The beneficial action of pentoxifylline in the treatment of CVLUs is associated with improvement to the microcirculation of the legs.104–106 Specifically, studies have shown a diminishing of leukocyte adhesion to the endothelium, inhibition of synthesis of proinflammatory cytokines, decreasing platelet aggregation,107 and the attenuation of oxygen free radical formation by leukocytes.108 In a systematic review to answer the clinical question, “Does pentoxifylline aid the healing of venous ulcers?” Jull et al. concluded that “pentoxifylline is an effective adjunct to compression bandaging for treating venous ulcers and may be effective in the absence of compression.”107 All six randomized controlled trials reviewed showed increased healing in the pentoxifylline group (400 mg) with no benefit shown for higher doses.
MPFF is a semisynthetic micronized preparation of the γ-benzopyrone family that has been found to have several beneficial protective actions in vein tissue including inhibiting leukocyte trapping and activation, decreasing microvascular leakage and impeding the synthesis of prostaglandins and free oxygen radicals without causing neutropenia.109–112 The MPFF that has increased the most rapidly in popularity for CVLU treatment is Daflon. However, in spite of the various clinical trials already completed testing Daflon, some have suggested that additional redesigned trials should be implemented that include diabetes and infection as part of the exclusion criteria and larger samples sizes.113
Another promising systemic approach that is being explored to target excessive neutrophil activity is n-3 polyunsaturated fatty acid supplementation with eicosapentanoic acid (EPA) and docosahexanoic acid (DHA). Scientific evidence involving cell cultures and animal models of inflammation indicates that metabolism of EPA + DHA generates lipid mediators of inflammation, such as resolvins and protectins, that inhibit transendothelial and transepithelial migration of neutrophils to inflamed tissue sites and reduce cell synthesis and secretion of proinflammatory cytokines that are involved in recruiting and activating neutrophils.28,114–116 Importantly, resolvins and protectins have also been shown to be key players in the active process of inflammation resolution by enhancing clearance of apoptotic neutrophils by macrophages.28,114,116,117 Relative to wound healing, new evidence has emerged that oral EPA + DHA supplementation can push an EPA + DHA lipid mediator profile in the acute human wound microenvironment that is associated with decreased levels of MPO, a biomarker of neutrophil activity.118 However, there have been no studies examining the effects of EPA + DHA-derived lipid mediators such as the resolvins and protectins on excessive neutrophil activity associated with human CVLUs. Therefore, additional studies are needed before EPA + DHA supplementation or molecular mimetics of resolvins and protectins119 can be considered as adjunct therapeutics to current standard care regimens for CVLUs.
CONCLUSION
Although neutrophil-derived proteases such as HNE and select MMPs are important regulators of efficient wound healing,120 findings from numerous studies continue to suggest that excessive, prolonged activity is associated with degradation of the ECM, receptors and growth factors that can impede CVLU healing by thwarting cellular migration and attachment. Adjunct treatment strategies that target excessive neutrophil activity may assist with resolving the persistent inflammation associated with CVLUs and improve healing outcomes for patients with these tenacious wounds.
ACKNOWLEDGMENTS
The authors are supported in part by funding from the following sources: National Institutes of Health Clinical and Translational Science Award to The Ohio State University UL1RR025755 (JM); NIDDK R01 DK076566 (SR); National Institutes of Health grants CA127109 (TAW), ES020462 (TAW).
Footnotes
Conflict of Interest: The authors declare that they have no competing financial interests.
REFERENCES
- 1.Kistner RL, Shafritz R, Stark KR, Warriner RA., 3rd Emerging treatment options for venous ulceration in today’s wound care practice. Ostomy Wound Manage. 2010;56:E1–11. [PubMed] [Google Scholar]
- 2.Smith PC. The causes of skin damage and leg ulceration in chronic venous disease. Int J Low Extrem Wounds. 2006;5:160–8. doi: 10.1177/1534734606292429. [DOI] [PubMed] [Google Scholar]
- 3.Ruckley CV. Socioeconomic impact of chronic venous insufficiency and leg ulcers. Angiology. 1997;48:67–9. doi: 10.1177/000331979704800111. [DOI] [PubMed] [Google Scholar]
- 4.Vanhoutte PM, Corcaud S, de Montrion C. Venous disease: from pathophysiology to quality of life. Angiology. 1997;48:559–67. doi: 10.1177/000331979704800702. [DOI] [PubMed] [Google Scholar]
- 5.Abenhaim L, Kurz X. The VEINES study (VEnous Insufficiency Epidemiologic and Economic Study): an international cohort study on chronic venous disorders of the leg. VEINES group. Angiology. 1997;48:59–66. doi: 10.1177/000331979704800110. [DOI] [PubMed] [Google Scholar]
- 6.Nelzen O, Bergqvist D, Lindhagen A. Venous and non-venous leg ulcers: clinical history and appearance in a population study. Br J Surg. 1994;81:182–7. doi: 10.1002/bjs.1800810206. [DOI] [PubMed] [Google Scholar]
- 7.Valencia IC, Falabella A, Kirsner RS, Eaglstein WH. Chronic venous insufficiency and venous leg ulceration. J Am Acad Dermatol. 2001;44:401–21. doi: 10.1067/mjd.2001.111633. quiz 422-4. [DOI] [PubMed] [Google Scholar]
- 8.Abbade LP, Lastoria S. Venous ulcer: epidemiology, physiopathology, diagnosis and treatment. Int J Dermatol. 2005;44:449–56. doi: 10.1111/j.1365-4632.2004.02456.x. [DOI] [PubMed] [Google Scholar]
- 9.McGuckin M, Kerstein MD. Venous leg ulcers and the family physician. Adv Wound Care. 1998;11:344–6. [PubMed] [Google Scholar]
- 10.Heit JA, Rooke TW, Silverstein MD, Mohr DN, Lohse CM, Petterson TM, et al. Trends in the incidence of venous stasis syndrome and venous ulcer: a 25-year population-based study. J Vasc Surg. 2001;33:1022–7. doi: 10.1067/mva.2001.113308. [DOI] [PubMed] [Google Scholar]
- 11.Tran NT, Meissner MH. The epidemiology, pathophysiology, and natural history of chronic venous disease. Semin Vasc Surg. 2002;15:5–12. [PubMed] [Google Scholar]
- 12.Margolis DJ, Bilker W, Santanna J, Baumgarten M. Venous leg ulcer: incidence and prevalence in the elderly. J Am Acad Dermatol. 2002;46:381–6. doi: 10.1067/mjd.2002.121739. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Census Bureau Table 094. Midyear population, by age and sex. International database. 2012.
- 14.Moyses C, Cederholm-Williams SA, Michel CC. Haemoconcentration and accumulation of white cells in the feet during venous stasis. Int J Microcirc Clin Exp. 1987;5:311–20. [PubMed] [Google Scholar]
- 15.Thomas PR, Dormandy JA. White cell and platelet trapping in patients with chronic venous insufficiency. Phlebologie. 1988;41:771–6. [PubMed] [Google Scholar]
- 16.Coleridge Smith PD, Thomas P, Scurr JH, Dormandy JA. Causes of venous ulceration: a new hypothesis. Br Med J (Clin Res Ed) 1988;296:1726–7. doi: 10.1136/bmj.296.6638.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shields DA, Andaz S, Sarin S, Scurr JH. Coleridge-Smith PD. Neutrophil activation in experimental venous hypertension. Phlebologie. 1993;46:687–9. [PubMed] [Google Scholar]
- 18.Saharay M, Shields D, Porter J, Scurr J, Coleridge Smith P. Leukocyte activity in the microcirculation of the leg in patients with chronic venous disease. J Vasc Surg. 1997;26:265–73. doi: 10.1016/s0741-5214(97)70188-5. [DOI] [PubMed] [Google Scholar]
- 19.Shields DA, Andaz SK, Sarin S, Scurr JH, Coleridge Smith PD. Plasma elastase in venous disease. Br J Surg. 1994;81:1496–9. doi: 10.1002/bjs.1800811033. [DOI] [PubMed] [Google Scholar]
- 20.Shields DA, Andaz S, Abeysinghe RD, Porter JB, Scurr JH, Coleridge Smith PD. Plasma lactoferrin as a marker of white cell degranulation in venous disease. Phlebology. 1994;9:55–8. [Google Scholar]
- 21.Yager DR, Nwomeh BC. The proteolytic environment of chronic wounds. Wound Repair Regen. 1999;7:433–41. doi: 10.1046/j.1524-475x.1999.00433.x. [DOI] [PubMed] [Google Scholar]
- 22.Moor AN, Vachon DJ, Gould LJ. Proteolytic activity in wound fluids and tissues derived from chronic venous leg ulcers. Wound Repair Regen. 2009;17:832–9. doi: 10.1111/j.1524-475X.2009.00547.x. [DOI] [PubMed] [Google Scholar]
- 23.Nicolaides AN. Chronic venous disease and the leukocyte-endothelium interaction: from symptoms to ulceration. Angiology. 2005;56(Suppl. 1):S11. doi: 10.1177/00033197050560i103. [DOI] [PubMed] [Google Scholar]
- 24.Theilgaard-Monch K, Knudsen S, Follin P, Borregaard N. The transcriptional activation program of human neutrophils in skin lesions supports their important role in wound healing. J Immunol. 2004;172:7684–93. doi: 10.4049/jimmunol.172.12.7684. [DOI] [PubMed] [Google Scholar]
- 25.Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657–70. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Simpson DM, Ross R. The neutrophilic leukocyte in wound repair a study with antineutrophil serum. doi: 10.1172/JCI107007. [DOI] [PMC free article] [PubMed]
- 27.Dovi JV, He L, DiPietro LA. Accelerated wound closure in neutrophil-depleted mice. J Leukoc Biol. 2003;73:448–55. doi: 10.1189/jlb.0802406. [DOI] [PubMed] [Google Scholar]
- 28.Norling LV, Serhan CN. Profiling in resolving inflammatory exudates identifies novel anti-inflammatory and pro-resolving mediators and signals for termination. J Intern Med. 2010;268:15–24. doi: 10.1111/j.1365-2796.2010.02235.x. [DOI] [PubMed] [Google Scholar]
- 29.Babior BM. Activation of the respiratory burst oxidase. Environ Health Perspect. 1994;102(Suppl. 10):53–6. doi: 10.1289/ehp.94102s1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sen CK, Roy S. Redox signals in wound healing. Biochim Biophys Acta. 2008;1780:1348–61. doi: 10.1016/j.bbagen.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whiston RJ, Hallett MB, Davies EV, Harding KG, Lane IF. Inappropriate neutrophil activation in venous disease. Br J Surg. 1994;81:695–8. doi: 10.1002/bjs.1800810522. [DOI] [PubMed] [Google Scholar]
- 32.Yeoh-Ellerton S, Stacey MC. Iron and 8-isoprostane levels in acute and chronic wounds. J Invest Dermatol. 2003;121:918–25. doi: 10.1046/j.1523-1747.2003.12471.x. [DOI] [PubMed] [Google Scholar]
- 33.Yager DR, Zhang LY, Liang HX, Diegelmann RF, Cohen IK. Wound fluids from human pressure ulcers contain elevated matrix metalloproteinase levels and activity compared to surgical wound fluids. J Invest Dermatol. 1996;107:743–8. doi: 10.1111/1523-1747.ep12365637. [DOI] [PubMed] [Google Scholar]
- 34.Diegelmann RF. Excessive neutrophils characterize chronic pressure ulcers. Wound Repair Regen. 2003;11:490–5. doi: 10.1046/j.1524-475x.2003.11617.x. [DOI] [PubMed] [Google Scholar]
- 35.Galkowska H, Wojewodzka U, Olszewski WL. Chemokines, cytokines, and growth factors in keratinocytes and dermal endothelial cells in the margin of chronic diabetic foot ulcers. Wound Repair Regen. 2006;14:558–65. doi: 10.1111/j.1743-6109.2006.00155.x. [DOI] [PubMed] [Google Scholar]
- 36.James TJ, Hughes MA, Cherry GW, Taylor RP. Evidence of oxidative stress in chronic venous ulcers. Wound Repair Regen. 2003;11:172–6. doi: 10.1046/j.1524-475x.2003.11304.x. [DOI] [PubMed] [Google Scholar]
- 37.Grinnell F, Zhu M. Fibronectin degradation in chronic wounds depends on the relative levels of elastase, alpha1-proteinase inhibitor, and alpha2-macroglobulin. J Invest Dermatol. 1996;106:335–41. doi: 10.1111/1523-1747.ep12342990. [DOI] [PubMed] [Google Scholar]
- 38.Trengove NJ, Stacey MC, MacAuley S, Bennett N, Gibson J, Burslem F, et al. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen. 1999;7:442–52. doi: 10.1046/j.1524-475x.1999.00442.x. [DOI] [PubMed] [Google Scholar]
- 39.Nwomeh BC, Liang HX, Cohen IK, Yager DR. MMP-8 is the predominant collagenase in healing wounds and nonhealing ulcers. J Surg Res. 1999;81:189–95. doi: 10.1006/jsre.1998.5495. [DOI] [PubMed] [Google Scholar]
- 40.Mirastschijski U, Impola U, Jahkola T, Karlsmark T, AGren MS, Saarialho-Kere U. Ectopic localization of matrix metalloproteinase-9 in chronic cutaneous wounds. Hum Pathol. 2002;33:355–64. doi: 10.1053/hupa.2002.32221. [DOI] [PubMed] [Google Scholar]
- 41.Wysocki AB, Staiano-Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J Invest Dermatol. 1993;101:64–8. doi: 10.1111/1523-1747.ep12359590. [DOI] [PubMed] [Google Scholar]
- 42.Bullen EC, Longaker MT, Updike DL, Benton R, Ladin D, Hou Z, Howard EW. Tissue inhibitor of metalloproteinases-1 is decreased and activated gelatinases are increased in chronic wounds. J Invest Dermatol. 1995;104:236–40. doi: 10.1111/1523-1747.ep12612786. [DOI] [PubMed] [Google Scholar]
- 43.Saarialho-Kere UK, Pentland AP, Birkedal-Hansen H, Parks WC, Welgus HG. Distinct populations of basal keratinocytes express stromelysin-1 and stromelysin-2 in chronic wounds. J Clin Invest. 1994;94:79–88. doi: 10.1172/JCI117351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Norgauer J, Hildenbrand T, Idzko M, Panther E, Bandemir E, Hartmann M, et al. Elevated expression of extracellular matrix metalloproteinase inducer (CD147) and membrane-type matrix metalloproteinases in venous leg ulcers. Br J Dermatol. 2002;147:1180–6. doi: 10.1046/j.1365-2133.2002.05025.x. [DOI] [PubMed] [Google Scholar]
- 45.Beidler SK, Douillet CD, Berndt DF, Keagy BA, Rich PB, Marston WA. Multiplexed analysis of matrix metalloproteinases in leg ulcer tissue of patients with chronic venous insufficiency before and after compression therapy. Wound Repair Regen. 2008;16:642–8. doi: 10.1111/j.1524-475X.2008.00415.x. [DOI] [PubMed] [Google Scholar]
- 46.Smeets R, Ulrich D, Unglaub F, Woltje M, Pallua N. Effect of oxidised regenerated cellulose/collagen matrix on proteases in wound exudate of patients with chronic venous ulceration. Int Wound J. 2008;5:195–203. doi: 10.1111/j.1742-481X.2007.00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mwaura B, Mahendran B, Hynes N, Defreitas D, Avalos G, Adegbola T, et al. The impact of differential expression of extracellular matrix metalloproteinase inducer, matrix metalloproteinase-2, tissue inhibitor of matrix metalloproteinase-2 and PDGF-AA on the chronicity of venous leg ulcers. Eur J Vasc Endovasc Surg. 2006;31:306–10. doi: 10.1016/j.ejvs.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 48.Weckroth M, Vaheri A, Lauharanta J, Sorsa T, Konttinen YT. Matrix metalloproteinases, gelatinase and collagenase, in chronic leg ulcers. J Invest Dermatol. 1996;106:1119–24. doi: 10.1111/1523-1747.ep12340167. [DOI] [PubMed] [Google Scholar]
- 49.Nwomeh BC, Liang HX, Diegelmann RF, Cohen IK, Yager DR. Dynamics of the matrix metalloproteinases MMP-1 and MMP-8 in acute open human dermal wounds. Wound Repair Regen. 1998;6:127–34. doi: 10.1046/j.1524-475x.1998.60206.x. [DOI] [PubMed] [Google Scholar]
- 50.Ulrich D, Lichtenegger F, Unglaub F, Smeets R, Pallua N. Effect of chronic wound exudates and MMP-2/-9 inhibitor on angiogenesis in vitro. Plast Reconstr Surg. 2005;116:539–45. doi: 10.1097/01.prs.0000173447.81513.7a. [DOI] [PubMed] [Google Scholar]
- 51.Vaalamo M, Mattila L, Johansson N, Kariniemi AL, Karjalainen-Lindsberg ML, Kahari VM, Saarialho-Kere U. Distinct populations of stromal cells express collagenase-3 (MMP-13) and collagenase-1 (MMP-1) in chronic ulcers but not in normally healing wounds. J Invest Dermatol. 1997;109:96–101. doi: 10.1111/1523-1747.ep12276722. [DOI] [PubMed] [Google Scholar]
- 52.Cullen B, Smith R, McCulloch E, Silcock D, Morrison L. Mechanism of action of PROMOGRAN, a protease modulating matrix, for the treatment of diabetic foot ulcers. Wound Repair Regen. 2002;10:16–25. doi: 10.1046/j.1524-475x.2002.10703.x. [DOI] [PubMed] [Google Scholar]
- 53.Rayment EA, Upton Z, Shooter GK. Increased matrix metalloproteinase-9 (MMP-9) activity observed in chronic wound fluid is related to the clinical severity of the ulcer. Br J Dermatol. 2008;158:951–61. doi: 10.1111/j.1365-2133.2008.08462.x. [DOI] [PubMed] [Google Scholar]
- 54.Tarlton JF, Bailey AJ, Crawford E, Jones D, Moore K, Harding KD. Prognostic value of markers of collagen remodeling in venous ulcers. Wound Repair Regen. 1999;7:347–55. doi: 10.1046/j.1524-475x.1999.00347.x. [DOI] [PubMed] [Google Scholar]
- 55.Eming SA, Koch M, Krieger A, Brachvogel B, Kreft S, Bruckner-Tuderman L, et al. Differential proteomic analysis distinguishes tissue repair biomarker signatures in wound exudates obtained from normal healing and chronic wounds. J Proteome Res. 2010;9:4758–66. doi: 10.1021/pr100456d. [DOI] [PubMed] [Google Scholar]
- 56.Wysocki AB, Kusakabe AO, Chang S, Tuan TL. Temporal expression of urokinase plasminogen activator, plasminogen activator inhibitor and gelatinase-B in chronic wound fluid switches from a chronic to acute wound profile with progression to healing. Wound Repair Regen. 1999;7:154–65. doi: 10.1046/j.1524-475x.1999.00154.x. [DOI] [PubMed] [Google Scholar]
- 57.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–76. doi: 10.1056/NEJM198902093200606. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 58.Ladwig GP, Robson MC, Liu R, Kuhn MA, Muir DF, Schultz GS. Ratios of activated matrix metalloproteinase-9 to tissue inhibitor of matrix metalloproteinase-1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair Regen. 2002;10:26–37. doi: 10.1046/j.1524-475x.2002.10903.x. [DOI] [PubMed] [Google Scholar]
- 59.Fray MJ, Dickinson RP, Huggins JP, Occleston NL. A potent, selective inhibitor of matrix metalloproteinase-3 for the topical treatment of chronic dermal ulcers. J Med Chem. 2003;46:3514–25. doi: 10.1021/jm0308038. [DOI] [PubMed] [Google Scholar]
- 60.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 61.Pirila E, Korpi JT, Korkiamaki T, Jahkola T, Gutierrez-Fernandez A, Lopez-Otin C, et al. Collagenase-2 (MMP-8) and matrilysin-2 (MMP-26) expression in human wounds of different etiologies. Wound Repair Regen. 2007;15:47–57. doi: 10.1111/j.1524-475X.2006.00184.x. [DOI] [PubMed] [Google Scholar]
- 62.Meyer FJ, Burnand KG, Abisi S, Tekoppele JM, van Els B, Smith A. Effect of collagen turnover and matrix metalloproteinase activity on healing of venous leg uclers. doi: 10.1002/bjs.5946. [DOI] [PubMed]
- 63.Chen C, Schultz GS, Bloch M, Edwards PD, Tebes S, Mast BA. Molecular and mechanistic validation of delayed healing rat wounds as a model for human chronic wounds. Wound Repair Regen. 1999;7:486–94. doi: 10.1046/j.1524-475x.1999.00486.x. [DOI] [PubMed] [Google Scholar]
- 64.Subramaniam K, Pech CM, Stacey MC, Wallace HJ. Induction of MMP-1, MMP-3 and TIMP-1 in normal dermal fibroblasts by chronic venous leg ulcer wound fluid*. Int Wound J. 2008;5:79–86. doi: 10.1111/j.1742-481X.2007.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stacey MC, Burnand KG, Mahmoud-Alexandroni M, Gaffney PJ, Bhogal BS. Tissue and urokinase plasminogen activators in the environs of venous and ischaemic leg ulcers. Br J Surg. 1993;80:596–9. doi: 10.1002/bjs.1800800515. [DOI] [PubMed] [Google Scholar]
- 66.Rao CN, Ladin DA, Liu YY, Chilukuri K, Hou ZZ, Woodley DT. Alpha 1-antitrypsin is degraded and non-functional in chronic wounds but intact and functional in acute wounds: the inhibitor protects fibronectin from degradation by chronic wound fluid enzymes. J Invest Dermatol. 1995;105:572–8. doi: 10.1111/1523-1747.ep12323503. [DOI] [PubMed] [Google Scholar]
- 67.Rogers AA, Burnett S, Moore JC, Shakespeare PG, Chen WY. Involvement of proteolytic enzymes—plasminogen activators and matrix metalloproteinases—in the pathophysiology of pressure ulcers. Wound Repair Regen. 1995;3:273–83. doi: 10.1046/j.1524-475X.1995.30307.x. [DOI] [PubMed] [Google Scholar]
- 68.Chen SM, Ward SI, Olutoye OO, Diegelmann RF, Kelman Cohen I. Ability of chronic wound fluids to degrade peptide growth factors is associated with increased levels of elastase activity and diminished levels of proteinase inhibitors. Wound Repair Regen. 1997;5:23–32. doi: 10.1046/j.1524-475X.1997.50108.x. [DOI] [PubMed] [Google Scholar]
- 69.Herrick S, Ashcroft G, Ireland G, Horan M, McCollum C, Ferguson M. Up-regulation of elastase in acute wounds of healthy aged humans and chronic venous leg ulcers are associated with matrix degradation. Lab Invest. 1997;77:281–8. [PubMed] [Google Scholar]
- 70.Wiegand C, Schonfelder U, Abel M, Ruth P, Kaatz M, Hipler UC. Protease and pro-inflammatory cytokine concentrations are elevated in chronic compared to acute wounds and can be modulated by collagen type I in vitro. Arch Dermatol Res. 2010;302:419–28. doi: 10.1007/s00403-009-1011-1. [DOI] [PubMed] [Google Scholar]
- 71.Latijnhouwers MA, Bergers M, Veenhuis RT, Beekman B, Ankersmit-Ter Horst MF, Schalkwijk J. Tenascin-C degradation in chronic wounds is dependent on serine proteinase activity. Arch Dermatol Res. 1998;290:490–6. doi: 10.1007/s004030050341. [DOI] [PubMed] [Google Scholar]
- 72.Lundqvist K, Sørensen OE, Schmidtchen A. Increased levels of human neutrophil alpha-defensins in chronic venous leg ulcers. J Dermatol Sci. 2008;51:131–4. doi: 10.1016/j.jdermsci.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 73.Yang D, Biragyn A, Hoover DM, Lubkowski J, Oppenheim JJ. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu Rev Immunol. 2004;22:181–215. doi: 10.1146/annurev.immunol.22.012703.104603. [DOI] [PubMed] [Google Scholar]
- 74.Nishimura M, Abiko Y, Kurashige Y, Takeshima M, Yamazaki M, Kusano K, et al. Effect of defensin peptides on eukaryotic cells: primary epithelial cells, fibroblasts and squamous cell carcinoma cell lines. J Dermatol Sci. 2004;36:87–95. doi: 10.1016/j.jdermsci.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 75.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359–65. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 76.Trostrup H, Lundquist R, Christensen LH, Jorgensen LN, Karlsmark T, Haab BB, Agren MS. S100A8/A9 deficiency in nonhealing venous leg ulcers uncovered by multiplexed antibody microarray profiling. Br J Dermatol. 2011;165:292–301. doi: 10.1111/j.1365-2133.2011.10384.x. [DOI] [PubMed] [Google Scholar]
- 77.Yager DR, Kulina RA, Gilman LA. Wound fluids: a window into the wound environment? Int J Low Extrem Wounds. 2007;6:262–72. doi: 10.1177/1534734607307035. [DOI] [PubMed] [Google Scholar]
- 78.Snyder RJ, Driver V, Fife CE, Lantis J, Peirce B, Serena T, Weir D. Using a diagnostic tool to identify elevated protease activity levels in chronic and stalled wounds: a consensus panel discussion. Ostomy Wound Manage. 2011;57:36–46. [PubMed] [Google Scholar]
- 79.International Consensus . An Expert Working Group Review. 2011. The role of proteases in wound diagnostics. [Google Scholar]
- 80.Robson MC, Cooper DM, Aslam R, Gould LJ, Harding KG, Margolis DJ, et al. Guidelines for the treatment of venous ulcers. Wound Repair Regen. 2006;14:649–62. doi: 10.1111/j.1524-475X.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- 81.Moore K, Huddleston E, Stacey MC, Harding KG. Venous leg ulcers—the search for a prognostic indicator. Int Wound J. 2007;4:163–72. doi: 10.1111/j.1742-481X.2007.00335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.World Union of Wound Healing Societies (WUWHS) A Consensus Document. Principles of best practice: diagnostics and wounds. 2008.
- 83.Shi B, Zhang P, Li WZ, Chen SZ, Li JQ. Effect of vacuum assisted closure on collagenase activity in human chronic wound. Zhonghua Zheng Xing Wai Ke Za Zhi. 2006;22:465–7. [PubMed] [Google Scholar]
- 84.Stechmiller JK, Kilpadi DV, Childress B, Schultz GS. Effect of vacuum-assisted closure therapy on the expression of cytokines and proteases in wound fluid of adults with pressure ulcers. Wound Repair Regen. 2006;14:371–4. doi: 10.1111/j.1743-6109.2006.00134.x. [DOI] [PubMed] [Google Scholar]
- 85.Vuerstaek JD, Vainas T, Wuite J, Nelemans P, Neumann MH, Veraart JC. State-of-the-art treatment of chronic leg ulcers: a randomized controlled trial comparing vacuum-assisted closure (V.A.C.) with modern wound dressings. J Vasc Surg. 2006;44:1029–37. doi: 10.1016/j.jvs.2006.07.030. discussion 1038. [DOI] [PubMed] [Google Scholar]
- 86.Golub LM, Ciancio S, Ramamamurthy NS, Leung M, McNamara TF. Low-dose doxycycline therapy: effect on gingival and crevicular fluid collagenase activity in humans. J Periodontal Res. 1990;25:321–30. doi: 10.1111/j.1600-0765.1990.tb00923.x. [DOI] [PubMed] [Google Scholar]
- 87.Golub LM, Sorsa T, Lee HM, Ciancio S, Sorbi D, Ramamurthy NS, et al. Doxycycline inhibits neutrophil (PMN)-type matrix metalloproteinases in human adult periodontitis gingiva. J Clin Periodontol. 1995;22:100–9. doi: 10.1111/j.1600-051x.1995.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 88.Sheridan P. From the NIH. Tetracyclines block collagenase activity. JAMA. 1984;252:1989–90. doi: 10.1001/jama.252.15.1989. [DOI] [PubMed] [Google Scholar]
- 89.Nordstrom D, Lindy O, Lauhio A, Sorsa T, Santavirta S, Konttinen YT. Anti-collagenolytic mechanism of action of doxycycline treatment in rheumatoid arthritis. Rheumatol Int. 1998;17:175–80. doi: 10.1007/s002960050030. [DOI] [PubMed] [Google Scholar]
- 90.Shlopov BV, Smith GN, Jr, Cole AA, Hasty KA. Differential patterns of response to doxycycline and transforming growth factor beta1 in the down-regulation of collagenases in osteoarthritic and normal human chondrocytes. Arthritis Rheum. 1999;42:719–27. doi: 10.1002/1529-0131(199904)42:4<719::AID-ANR15>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 91.Yu LP, Jr, Smith GN, Jr, Hasty KA, Brandt KD. Doxycycline inhibits type XI collagenolytic activity of extracts from human osteoarthritic cartilage and of gelatinase. J Rheumatol. 1991;18:1450–2. [PubMed] [Google Scholar]
- 92.Lindy O, Konttinen YT, Sorsa T, Ding Y, Santavirta S, Ceponis A, López-Otín C. Matrix metalloproteinase 13 (collagenase 3) in human rheumatoid synovium. Arthritis Rheum. 1997;40:1391–9. doi: 10.1002/art.1780400806. [DOI] [PubMed] [Google Scholar]
- 93.Perry HD, Golub LM. Systemic tetracyclines in the treatment of noninfected corneal ulcers: a case report and proposed new mechanism of action. Ann Ophthalmol. 1985;17:742–4. [PubMed] [Google Scholar]
- 94.Ramamurthy NS, Kucine AJ, McClain SA, McNamara TF, Golub LM. Topically applied CMT-2 enhances wound healing in streptozotocin diabetic rat skin. Adv Dent Res. 1998;12:144–8. doi: 10.1177/08959374980120011001. [DOI] [PubMed] [Google Scholar]
- 95.Chin GA, Thigpin TG, Perrin KJ, Moldawer LL, Schultz G. Treatment of chronic ulcers in diabetic patients with a topical metalloproteinase inhibitor, doxycycline. Wounds. 2003;15:315–25. [Google Scholar]
- 96.Stechmiller J, Cowan L, Schultz G. The role of doxycycline as a matrix metalloproteinase inhibitor for the treatment of chronic wounds. Biol Res Nurs. 2010;11:336–44. doi: 10.1177/1099800409346333. [DOI] [PubMed] [Google Scholar]
- 97.Edwards JV, Buschle-Diller G, Goheen SC. Future structure and properties of mechanism-based wound dressings. In: Edwards JV, Buschle-Diller G, Goheen SC, editors. Modified fibers with medical and specialty applications. Springer; Dordecht: 2006. pp. 11–33. [Google Scholar]
- 98.Lobmann R, Schultz G, Lehnert H. Proteases and the diabetic foot syndrome: mechanisms and therapeutic implications. Diabetes Care. 2005;28:461–71. doi: 10.2337/diacare.28.2.461. [DOI] [PubMed] [Google Scholar]
- 99.Wright JB, Lam K, Buret AG, Olson ME, Burrell RE. Early healing events in a porcine model of contaminated wounds: effects of nanocrystalline silver on matrix metalloproteinases, cell apoptosis, and healing. Wound Repair Regen. 2002;10:141–51. doi: 10.1046/j.1524-475x.2002.10308.x. [DOI] [PubMed] [Google Scholar]
- 100.Edwards JV, Bopp AF, Batiste S, Ullah AJ, Cohen IK, Diegelmann RF, Montante SJ. Inhibition of elastase by a synthetic cotton-bound serine protease inhibitor: in vitro kinetics and inhibitor release. Wound Repair Regen. 1999;7:106–18. doi: 10.1046/j.1524-475x.1999.00106.x. [DOI] [PubMed] [Google Scholar]
- 101.Edwards JV, Yager DR, Cohen IK, Diegelmann RF, Montante S, Bertoniere N, Bopp AF. Modified cotton gauze dressings that selectively absorb neutrophil elastase activity in solution. Wound Repair Regen. 2001;9:50–8. doi: 10.1046/j.1524-475x.2001.00050.x. [DOI] [PubMed] [Google Scholar]
- 102.Edwards JV, Howley P, Davis R, Mashchak A, Goheen SC. Protease inhibition by oleic acid transfer from chronic wound dressings to albumin. Int J Pharm. 2007;340:42–51. doi: 10.1016/j.ijpharm.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 103.Vachon DJ, Yager DR. Novel sulfonated hydrogel composite with the ability to inhibit proteases and bacterial growth. J Biomed Mater Res A. 2006;76:35–43. doi: 10.1002/jbm.a.30440. [DOI] [PubMed] [Google Scholar]
- 104.Falanga V, Fujitani RM, Diaz C, Hunter G, Jorizzo J, Lawrence PF, et al. Systemic treatment of venous leg ulcers with high doses of pentoxifylline: efficacy in a randomized, placebo-controlled trial. Wound Repair Regen. 1999;7:208–13. doi: 10.1046/j.1524-475x.1999.00208.x. [DOI] [PubMed] [Google Scholar]
- 105.Colgan MP, Dormandy JA, Jones PW, Schraibman IG, Shanik DG, Young RA. Oxpentifylline treatment of venous ulcers of the leg. BMJ. 1990;300:972–5. doi: 10.1136/bmj.300.6730.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dale JJ, Ruckley CV, Harper DR, Gibson B, Nelson EA, Prescott RJ. Randomised, double blind placebo controlled trial of pentoxifylline in the treatment of venous leg ulcers. BMJ. 1999;319:875–8. doi: 10.1136/bmj.319.7214.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jull A, Arroll B, Parag V, Waters J. Pentoxifylline for treating venous leg ulcers. Cochrane Database Syst Rev. 2007;(3):CD001733. doi: 10.1002/14651858.CD001733.pub2. [DOI] [PubMed] [Google Scholar]
- 108.Barroso-Aranda J, Schmid-Schonbein GW. Pentoxifylline pretreatment decreases the pool of circulating activated neutrophils, in-vivo adhesion to endothelium, and improves survival from hemorrhagic shock. Biorheology. 1990;27:401–18. doi: 10.3233/bir-1990-273-417. [DOI] [PubMed] [Google Scholar]
- 109.Coleridge-Smith P, Lok C, Ramelet AA. Venous leg ulcer: a meta-analysis of adjunctive therapy with micronized purified flavonoid fraction. Eur J Vasc Endovasc Surg. 2005;30:198–208. doi: 10.1016/j.ejvs.2005.04.017. doi: 10.1002/14651858.CD001733.pub2. [DOI] [PubMed] [Google Scholar]
- 110.Bergan JJ, Schmid-Schonbein GW, Takase S. Therapeutic approach to chronic venous insufficiency and its complications: place of Daflon 500 mg. Angiology. 2001;52(Suppl. 1):S43–7. doi: 10.1177/0003319701052001S06. [DOI] [PubMed] [Google Scholar]
- 111.Ramelet AA. Clinical benefits of Daflon 500 mg in the most severe stages of chronic venous insufficiency. Angiology. 2001;52(Suppl. 1):S49–56. doi: 10.1177/0003319701052001S07. [DOI] [PubMed] [Google Scholar]
- 112.Boisseau MR. Leukocyte involvement in the signs and symptoms of chronic venous disease. Perspectives for therapy. Clin Hemorheol Microcirc. 2007;37:277–90. [PubMed] [Google Scholar]
- 113.Liu YC, Margolis DJ, Isseroff RR. Does inflammation have a role in the pathogenesis of venous ulcers? A critical review of the evidence. J Invest Dermatol. 2011;131:818–27. doi: 10.1038/jid.2010.428. [DOI] [PubMed] [Google Scholar]
- 114.Dona M, Fredman G, Schwab JM, Chiang N, Arita M, Goodarzi A, et al. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood. 2008;112:848–55. doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Arita M, Clish CB, Serhan CN. The contributions of aspirin and microbial oxygenase to the biosynthesis of anti-inflammatory resolvins: novel oxygenase products from omega-3 polyunsaturated fatty acids. Biochem Biophys Res Commun. 2005;338:149–57. doi: 10.1016/j.bbrc.2005.07.181. [DOI] [PubMed] [Google Scholar]
- 116.Kasuga K, Yang R, Porter TF, Agrawal N, Petasis NA, Irimia D, et al. Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J Immunol. 2008;181:8677–87. doi: 10.4049/jimmunol.181.12.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–74. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McDaniel J, Massey K, Nicolaou A. Fish oil supplementation alters levels of lipid mediators of inflammation in microenvironment of acute human wounds. Wound Repair Regen. 2011;19:189–200. doi: 10.1111/j.1524-475X.2010.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kohli P, Levy BD. Resolvins and protectins: mediating solutions to inflammation. Br J Pharmacol. 2009;158:960–71. doi: 10.1111/j.1476-5381.2009.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Broughton G, 2nd, Janis J. Attinger C. The basic science of wound healing. Plast Reconstr Surg. 2006;117:12S. doi: 10.1097/01.prs.0000225430.42531.c2. [DOI] [PubMed] [Google Scholar]